1-Carbomethoxy-β-Carboline, Derived from Portulaca oleracea L., Ameliorates LPS-Mediated Inflammatory Response Associated with MAPK Signaling and Nuclear Translocation of NF-κB
Abstract
1. Introduction
2. Results and Discussion
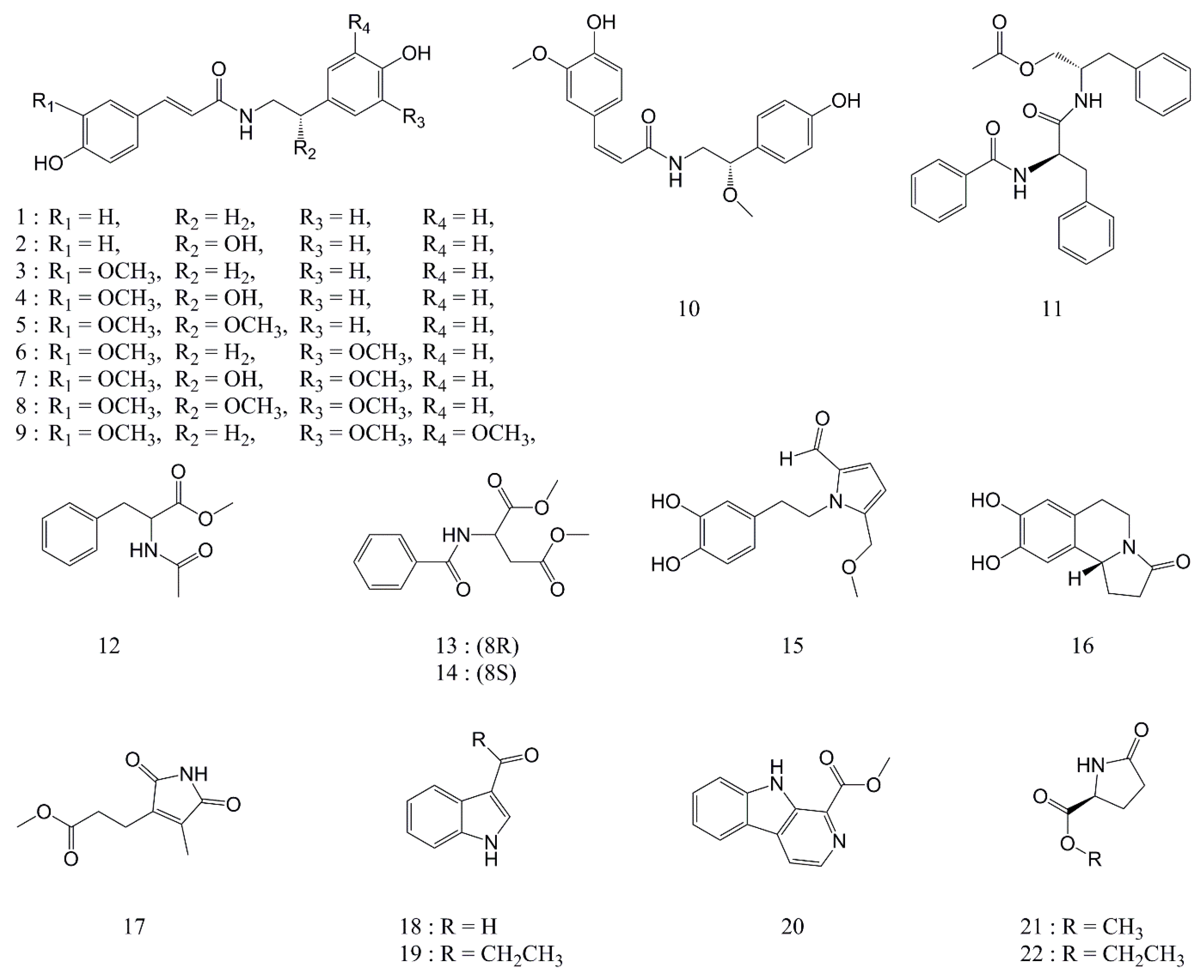
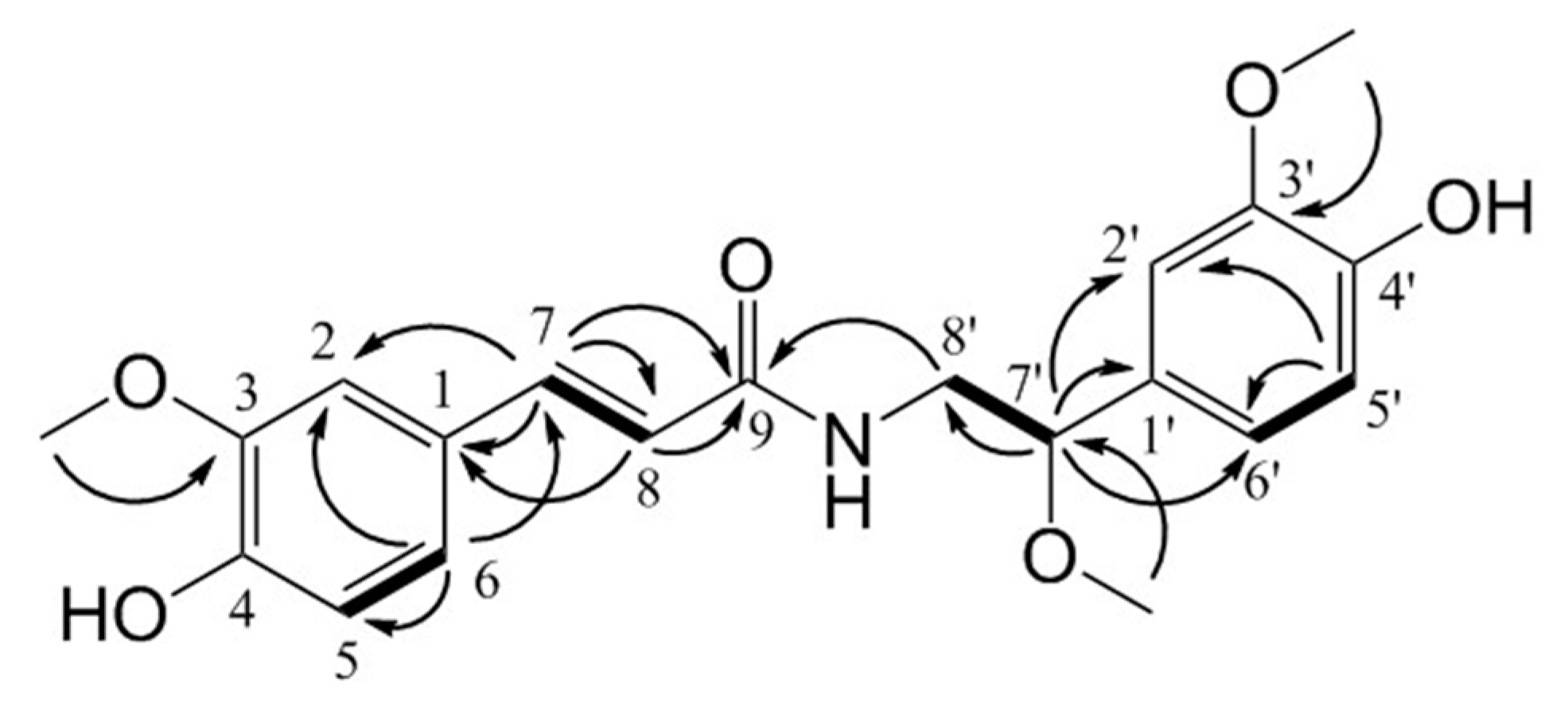
2.1. Isolation and Structural Elucidation of Compounds
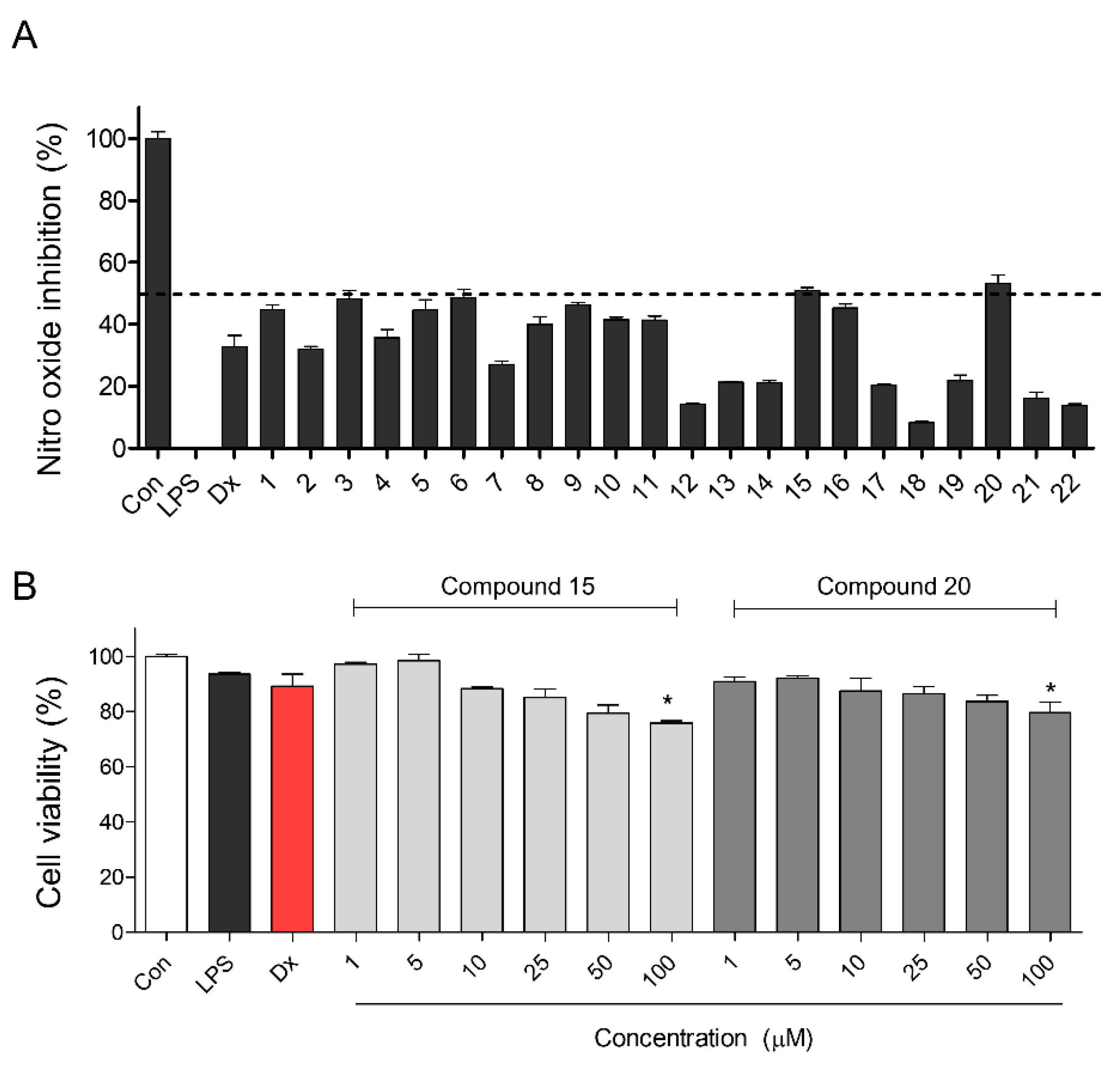
2.2. Nitric Oxide Production Screening Using Twenty-Two Compounds Isolated from Portulaca oleracea
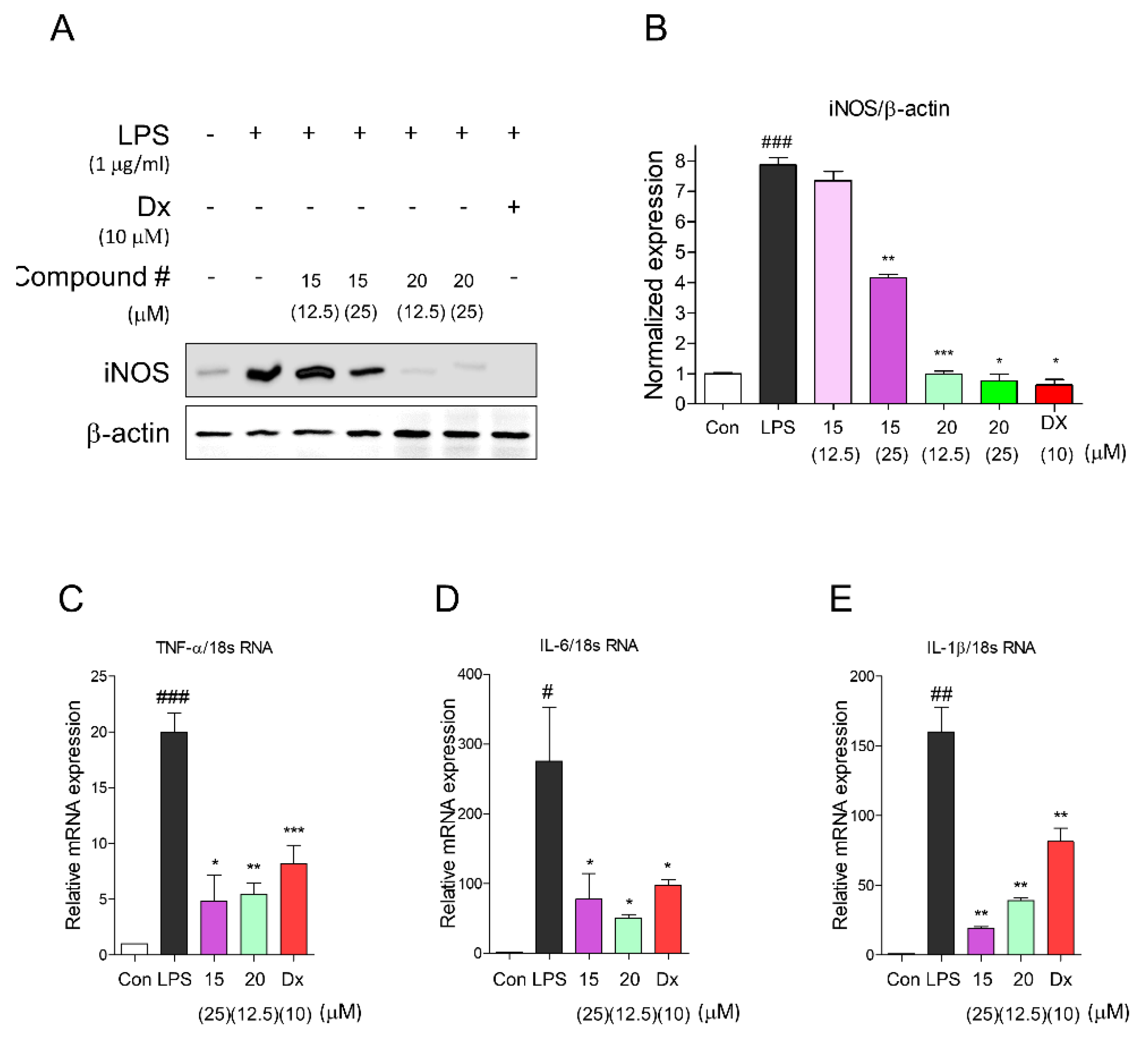
2.3. Inhibitory Activities of Proinflammatory Mediators by Compounds 15 and 20
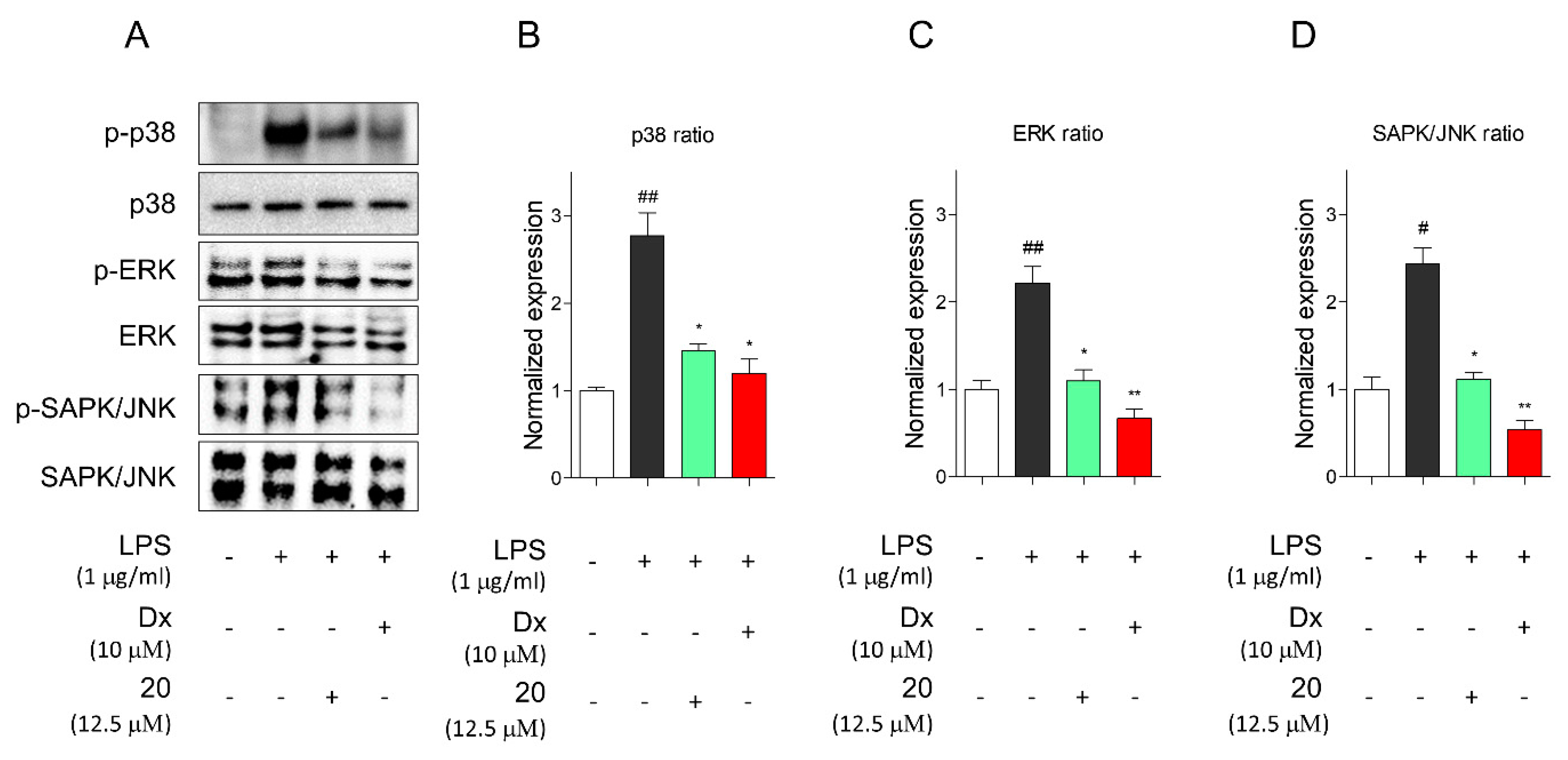
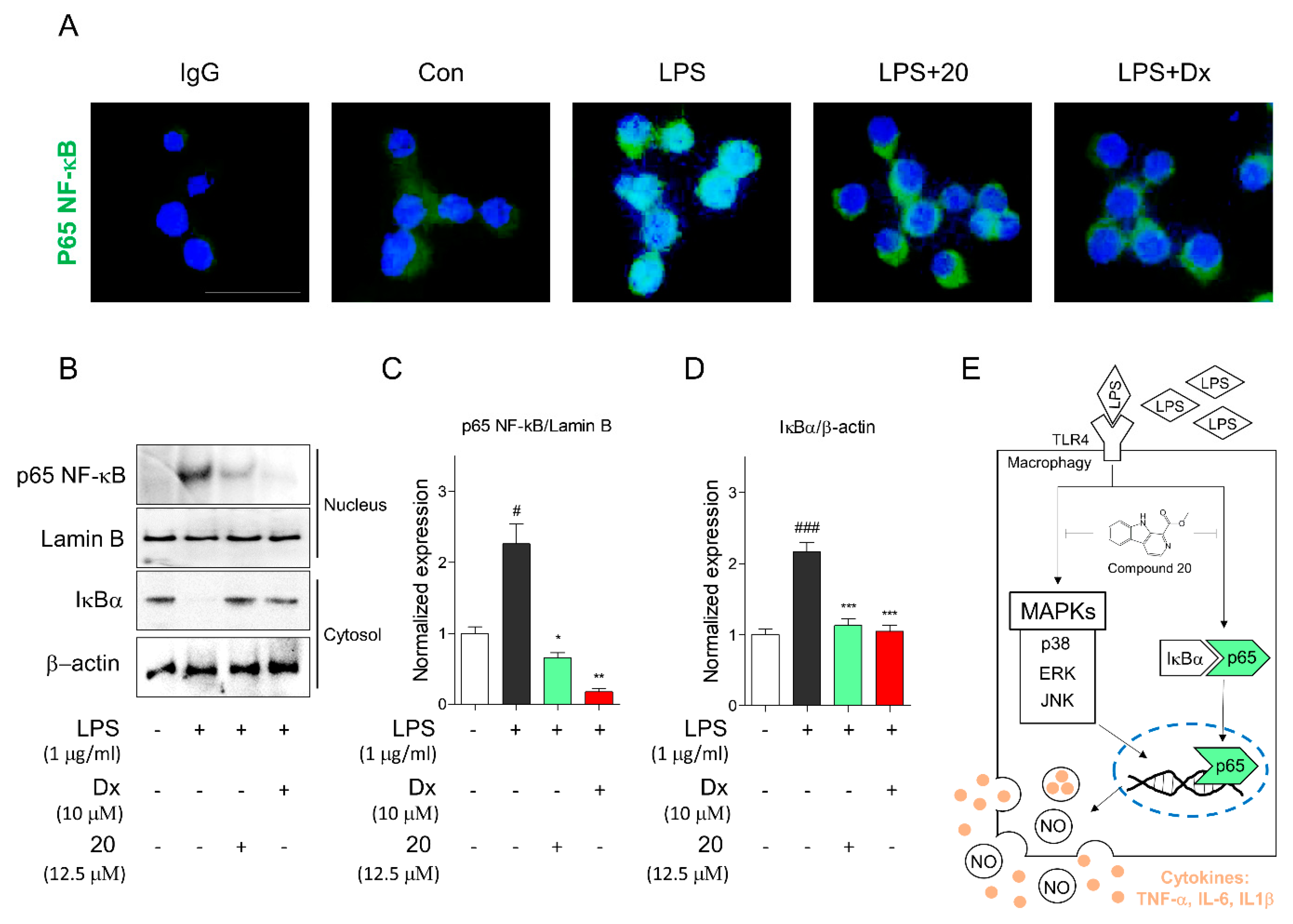
2.4. Portulaca oleracea Inhibits Proinflammatory and Inflammatory Signaling
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cell Culture
3.5. Measurement of NO Contents and Cell Cytotoxicity
3.6. Immunoblot Analysis
3.7. Real-Time PCR Using TaqMan Probe
3.8. Immunocytochemistry
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, X.; Zhang, W.; Ying, X.; Stien, D. New flavonoids from Portulaca oleracea L. and their activities. Fitoterapia 2018, 127, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Cakilcioglu, U.; Turkoglu, I. An ethnobotanical survey of medicinal plants in Sivrice (Elazig-Turkey). J. Ethnopharmacol. 2010, 132, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Khan, M.A.; Shah, M.H.; Shah, M.M.; Pervez, A.; Ahmad, M. Ethnobotanical appraisal and cultural values of medicinally important wild edible vegetables of Lesser Himalayas-Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.; Fatima, N.; Kabeer, G. Portulaca oleracea L.: A mini review on phytochemistry and phramacology. Int. J. Biol. Biotechnol. 2016, 13, 637–641. [Google Scholar]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. Biomed. Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef]
- Gallo, M.; Conte, E.; Naviglio, D. Analysis and Comparison of the Antioxidant Component of Portulaca Oleracea Leaves Obtained by Different Solid-Liquid Extraction Techniques. Antioxidants 2017, 6, 64. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, H.J.; Jang, H.-J.; Lee, S.; Jung, K.; Lee, S.W.; Lee, S.-J.; Rho, M.-C. Portulaca oleracea extracts and their active compounds ameliorate inflammatory bowel diseases in vitro and in vivo by modulating TNF-α, IL-6 and IL-1β signalling. Food Res. Int. 2018, 106, 335–343. [Google Scholar] [CrossRef]
- Xiu, F.; Li, X.; Zhang, W.; He, F.; Ying, X.; Stien, D. A new alkaloid from Portulaca oleracea L. and its antiacetylcholinesterase activity. Nat. Prod. Res. 2019, 33, 2583–2590. [Google Scholar] [CrossRef]
- Liang, X.; Tian, J.; Li, L.; Gao, J.; Zhang, Q.; Gao, P.; Song, S. Rapid determination of eight bioactive alkaloids in Portulaca oleracea L. by the optimal microwave extraction combined with positive–negative conversion multiple reaction monitor (+/−MRM) technology. Talanta 2014, 120, 167–172. [Google Scholar] [CrossRef]
- Lajide, L.; Escoubas, P.; Mizutani, J. Termite Antifeedant Activity in Tropical Plants. Part 1. Termite Antifeedant Activity in Xylopia aethiopica. Phytochemistry 1995, 40, 1105–1112. [Google Scholar] [CrossRef]
- Joo Lee, J.; Oh, C.-H.; Heon Yang, J.; Baek, N.-I.; Kim, S.-H.; Hyeon Cho, C.; Keun Kim, D. Cytotoxic Alkaloids from the Wood of Picrasma quassioides. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 663–667. [Google Scholar]
- Souto, A.L.; Tavares, J.F.; da Silva, M.S.; Diniz Mde, F.; de Athayde-Filho, P.F.; Barbosa Filho, J.M. Anti-inflammatory activity of alkaloids: An update from 2000 to 2010. Molecules 2011, 16, 8515–8534. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, S.C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Al-Taweel, A.M.; Perveen, S.; El-Shafae, A.M.; Fawzy, G.A.; Malik, A.; Afza, N.; Iqbal, L.; Latif, M. Bioactive phenolic amides from Celtis africana. Molecules 2012, 17, 2675–2682. [Google Scholar] [CrossRef]
- Chao, J.-B.; Tong, H.; Huang, S.-P.; Liu, D.-S. Preparation and study on the solid inclusion complex of sparfloxacin with β-cyclodextrin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 161–166. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kim, Y.; Jang, H.J.; Oh, H.M.; Lim, C.H.; Lee, S.W.; Rho, M.C. Study of the UV Light Conversion of Feruloyl Amides from Portulaca oleracea and Their Inhibitory Effect on IL-6-Induced STAT3 Activation. Molecules 2016, 21, 865. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Ho, P.; Bourquin, G.; Yeh, L.-A.; Cuny, G.D. Synthesis, stereochemistry confirmation and biological activity evaluation of a constituent from Isodon excisus. Tetrahedron 2003, 59, 9961–9969. [Google Scholar] [CrossRef]
- Wan Woo, K.; Young Han, J.; Suh, W.S.; Hyun Lee, J.; Ro Lee, K. ChemInform Abstract: Two New Chemical Constituents from Leaves of Perilla frutescens var. acuta. Bull. Korean Chem. Soc. 2014, 35, 2151–2154. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, Z.; Feng, Q.; Liang, X.; Li, J.; Zanin, M.; Jiang, Z.; Zhong, N. Aurantiamide acetate from Baphicacanthus cusia root exhibits anti-inflammatory and anti-viral effects via inhibition of the NF-κB signaling pathway in Influenza A virus-infected cells. J. Ethnopharmacol. 2017, 199, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Moulin, D.; Darcel, C.; Jugé, S. Versatile synthesis of P-chiral (ephedrine) AMPP ligands via their borane complexes. Structural consequences in Rh-catalyzed hydrogenation of methyl α-acetamidocinnamate. Tetrahedron Asymmetry 1999, 10, 4729–4743. [Google Scholar] [CrossRef]
- Lee, S.-H.; Bok, J.; Qi, X.; Kim, S.K.; Lee, Y.-S.; Yoon, J. Ring-opening of oxazolines derived from l-serine: A short and efficient stereoselective synthesis of all four diastereomers of 3-mercaptoaspartic acid derivatives. Tetrahedron Lett. 2007, 48, 7309–7312. [Google Scholar] [CrossRef]
- Yu, J.; Song, X.; Yang, P.; Wang, X.; Wang, X. Alkaloids from Scindapsus officinalis (Roxb.) Schott. and their biological activities. Fitoterapia 2018, 129, 54–61. [Google Scholar] [CrossRef]
- Xiang, L.; Xing, D.; Wang, W.; Wang, R.-F.; Ding, Y.; Du, L. Alkaloids from Portulaca oleracea L. Phytochemistry 2005, 66, 2595–2601. [Google Scholar] [CrossRef]
- Jao, C.-W.; Hung, T.-H.; Chang, C.; Chuang, T.-H. Chemical Constituents of Phaius mishmensis. Molecules 2016, 21, 1605. [Google Scholar] [CrossRef]
- Xu, H.; Bin Yang, W.; Wang, Q. Antifungal Agents. Part 3: Synthesis and Antifungal Activities of 3-Acylindole Analogs against Phytopathogenic Fungi In Vitro. Chem. Biol. Drug Des. 2011, 78, 864–868. [Google Scholar] [CrossRef]
- Yang, Y.B.; Tan, N.H.; Zhang, F.; Lu, Y.Q.; He, M.; Zhou, J. Cyclopeptides and Amides from Pseudostellaria heterophylla (Caryophyllaceae). Helv. Chim. Acta 2003, 86, 3376–3379. [Google Scholar] [CrossRef]
- Takaya, Y.; Furukawa, T.; Miura, S.; Akutagawa, T.; Hotta, Y.; Ishikawa, N.; Niwa, M. Antioxidant Constituents in Distillation Residue of Awamori Spirits. J. Agric. Food Chem. 2007, 55, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar] [PubMed]
- Gayeong, H. Bioactive effects of a Herbal Formula KDC16-2 Consisting Portulaca leracea L. Extracts. Korea. J. Pharmacogn. 2019, 50, 37–45. [Google Scholar]
- Miao, L.; Tao, H.; Peng, Y.; Wang, S.; Zhong, Z.; El-Seedi, H.; Dragan, S.; Zengin, G.; Cheang, W.S.; Wang, Y.; et al. The anti-inflammatory potential of Portulaca oleracea L. (purslane) extract by partial suppression on NF-κB and MAPK activation. Food Chem. 2019, 290, 239–245. [Google Scholar] [CrossRef]
- Cho, J.Y. Immunomodulatory effect of nonsteroidal anti-inflammatory drugs (NSAIDs) at the clinically available doses. Arch. Pharmacal Res. 2007, 30, 64–74. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, L.; Wang, M.H. N-trans-feruloyltyramine inhibits LPS-induced NO and PGE2 production in RAW 264.7 macrophage: Involvement of AP-1 and MAP kinase signaling pathways. Chem. Bio. Interact. 2015, 235, 56–62. [Google Scholar] [CrossRef]
- Wang, S.; Suh, J.H.; Zheng, X.; Wang, Y.; Ho, C.-T. Identification and Quantification of Potential Anti-inflammatory Hydroxycinnamic Acid Amides from Wolfberry. J. Agric. Food Chem. 2017, 65, 364–372. [Google Scholar] [CrossRef]
- Cai, X.F.; Chin, Y.W.; Oh, S.R.; Kwon, O.K.; Ahn, K.S.; Lee, H.K. Anti-inflammatory constituents from solanum nigrum. Bull. Korean Chem. Soc. 2010, 31, 199–201. [Google Scholar] [CrossRef][Green Version]
- Sun, H.; He, X.; Liu, C.; Li, L.; Zhou, R.; Jin, T.; Yue, S.; Feng, D.; Gong, J.; Sun, J.; et al. Effect of Oleracein E, a Neuroprotective Tetrahydroisoquinoline, on Rotenone-Induced Parkinson’s Disease Cell and Animal Models. ACS Chem. Neurosci. 2017, 8, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Huang, S.Y.; Duh, C.Y.; Chen, I.S.; Wang, T.C.; Fang, H.Y. A new cytotoxic amide from the stem wood of Hibiscus tiliaceus. Planta Med. 2006, 72, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Liggett, J.L.; Zhang, X.; Eling, T.E.; Baek, S.J. Anti-tumor activity of non-steroidal anti-inflammatory drugs: Cyclooxygenase-independent targets. Cancer Lett. 2014, 346, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Su, X.D.; Jang, H.J.; Wang, C.Y.; Lee, S.W.; Rho, M.C.; Kim, Y.H.; Yang, S.Y. Anti-inflammatory Potential of Saponins from Aster tataricus via NF-κB/MAPK Activation. J. Nat. Prod. 2019, 82, 1139–1148. [Google Scholar] [CrossRef]
- Lim, H.J.; Jang, H.J.; Bak, S.G.; Lee, S.; Lee, S.W.; Lee, K.M.; Lee, S.J.; Rho, M.C. In vitro inhibitory effects of cirsiliol on IL-6-induced STAT3 activation through anti-inflammatory activity. Bioorganic Med. Chem. Lett. 2019, 29, 1586–1592. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, S.; Lee, S.J.; Lim, H.J.; Jung, K.; Kim, Y.H.; Lee, S.W.; Rho, M.C. Anti-inflammatory Activity of Eudesmane-Type Sesquiterpenoids from Salvia plebeia. J. Nat. Prod. 2017, 80, 2666–2676. [Google Scholar] [CrossRef]
- Bertucci, A.; Kim, K.H.; Kang, J.; Zuidema, J.M.; Lee, S.H.; Kwon, E.J.; Kim, D.; Howell, S.B.; Ricci, F.; Ruoslahti, E.; et al. Tumor-Targeting, MicroRNA-Silencing Porous Silicon Nanoparticles for Ovarian Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 23926–23937. [Google Scholar] [CrossRef]
| Positions | δC c | Type | δH (J in Hz) b |
|---|---|---|---|
| 1 | 127.0 | C | |
| 2 | 110.2 | CH | 7.13 (1H, d, 1.8) |
| 3 | 148.8 | C | |
| 4 | 148.5 | C | |
| 5 | 115.1 | CH | 6.80 (1H, overlap) |
| 6 | 121.9 | CH | 7.03 (1H, dd, 8.4, 1.8) |
| 7 | 140.9 | CH | 7.44 (1H, d, 15.6) |
| 8 | 117.3 | CH | 6.45 (1H, d, 15.6) |
| 9 | 167.9 | C | |
| OCH3-3 | 55.6 | CH3 | 3.89 (3H, s) |
| 1’ | 130.9 | C | |
| 2’ | 109.8 | CH3 | 6.92 (3H, s) |
| 3’ | 147.9 | C | |
| 4’ | 146.2 | C | |
| 5’ | 114.8 | CH | 6.80 (1H, overlap) |
| 6’ | 119.5 | CH | 6.80 (1H, overlap) |
| 7’ | 82.2 | CH | 4.26 (1H, dd, 8.4, 4.8) |
| 8’α | 48.2 | CH2 | 3.53 (1H, dd, 13.8, 4.8) |
| 8’β | 3.42 (1H, dd, 13.8, 8.4) | ||
| OCH3-3’ | 55.1 | CH3 | 3.86 (3H, s) |
| OCH3-7’ | 45.7 | CH3 | 3.24 (3H, s) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-H.; Park, E.-J.; Jang, H.-J.; Lee, S.-J.; Park, C.S.; Yun, B.-S.; Lee, S.W.; Rho, M.-C. 1-Carbomethoxy-β-Carboline, Derived from Portulaca oleracea L., Ameliorates LPS-Mediated Inflammatory Response Associated with MAPK Signaling and Nuclear Translocation of NF-κB. Molecules 2019, 24, 4042. https://doi.org/10.3390/molecules24224042
Kim K-H, Park E-J, Jang H-J, Lee S-J, Park CS, Yun B-S, Lee SW, Rho M-C. 1-Carbomethoxy-β-Carboline, Derived from Portulaca oleracea L., Ameliorates LPS-Mediated Inflammatory Response Associated with MAPK Signaling and Nuclear Translocation of NF-κB. Molecules. 2019; 24(22):4042. https://doi.org/10.3390/molecules24224042
Chicago/Turabian StyleKim, Kang-Hoon, Eun-Jae Park, Hyun-Jae Jang, Seung-Jae Lee, Chan Sun Park, Bong-Sik Yun, Seung Woong Lee, and Mun-Chual Rho. 2019. "1-Carbomethoxy-β-Carboline, Derived from Portulaca oleracea L., Ameliorates LPS-Mediated Inflammatory Response Associated with MAPK Signaling and Nuclear Translocation of NF-κB" Molecules 24, no. 22: 4042. https://doi.org/10.3390/molecules24224042
APA StyleKim, K.-H., Park, E.-J., Jang, H.-J., Lee, S.-J., Park, C. S., Yun, B.-S., Lee, S. W., & Rho, M.-C. (2019). 1-Carbomethoxy-β-Carboline, Derived from Portulaca oleracea L., Ameliorates LPS-Mediated Inflammatory Response Associated with MAPK Signaling and Nuclear Translocation of NF-κB. Molecules, 24(22), 4042. https://doi.org/10.3390/molecules24224042





