Alpinia zerumbet (Pers.): Food and Medicinal Plant with Potential In Vitro and In Vivo Anti-Cancer Activities
Abstract
1. Introduction
2. Results
2.1. Cytotoxicity of Methanol Extracts of Fifteen Egyptian Medicinal Plants Species
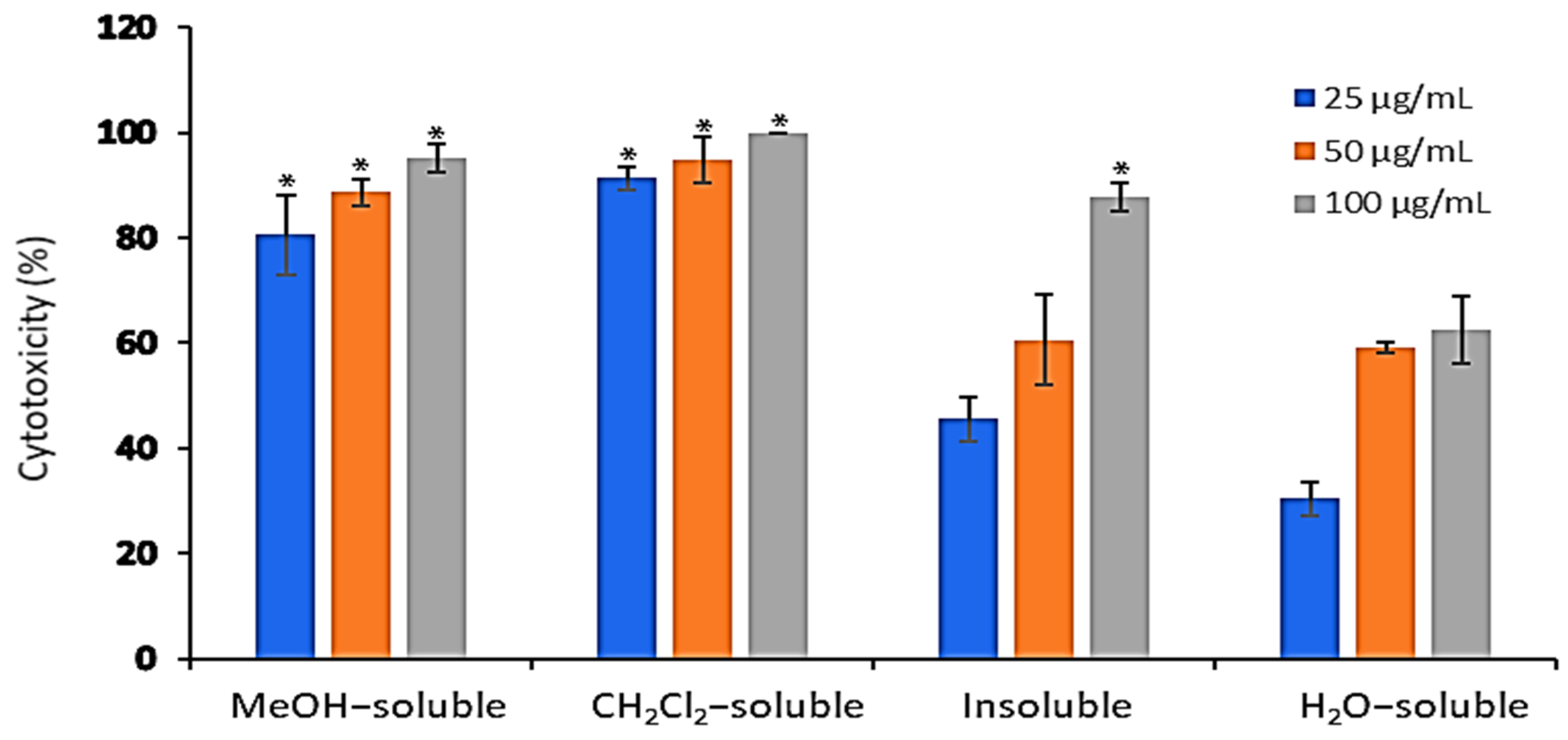
2.2. Cytotoxicity of A. zerumbet Extracts/Fractions
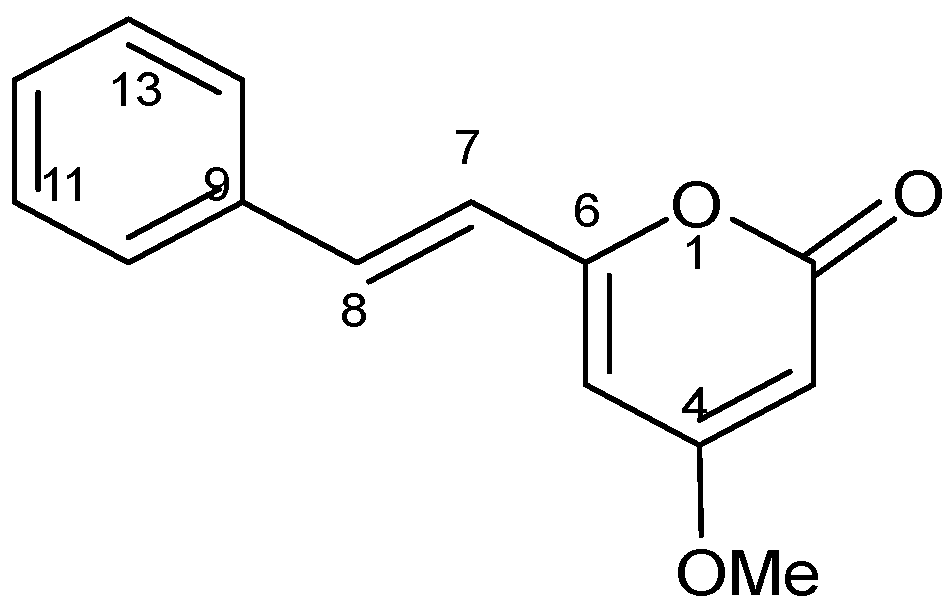
2.3. Structure Elucidation of DK
2.4. In Vitro Cytotoxic Activity of A. zerumbet Extracts Against Ehrlich Ascities Carcinoma (EAC) Cells
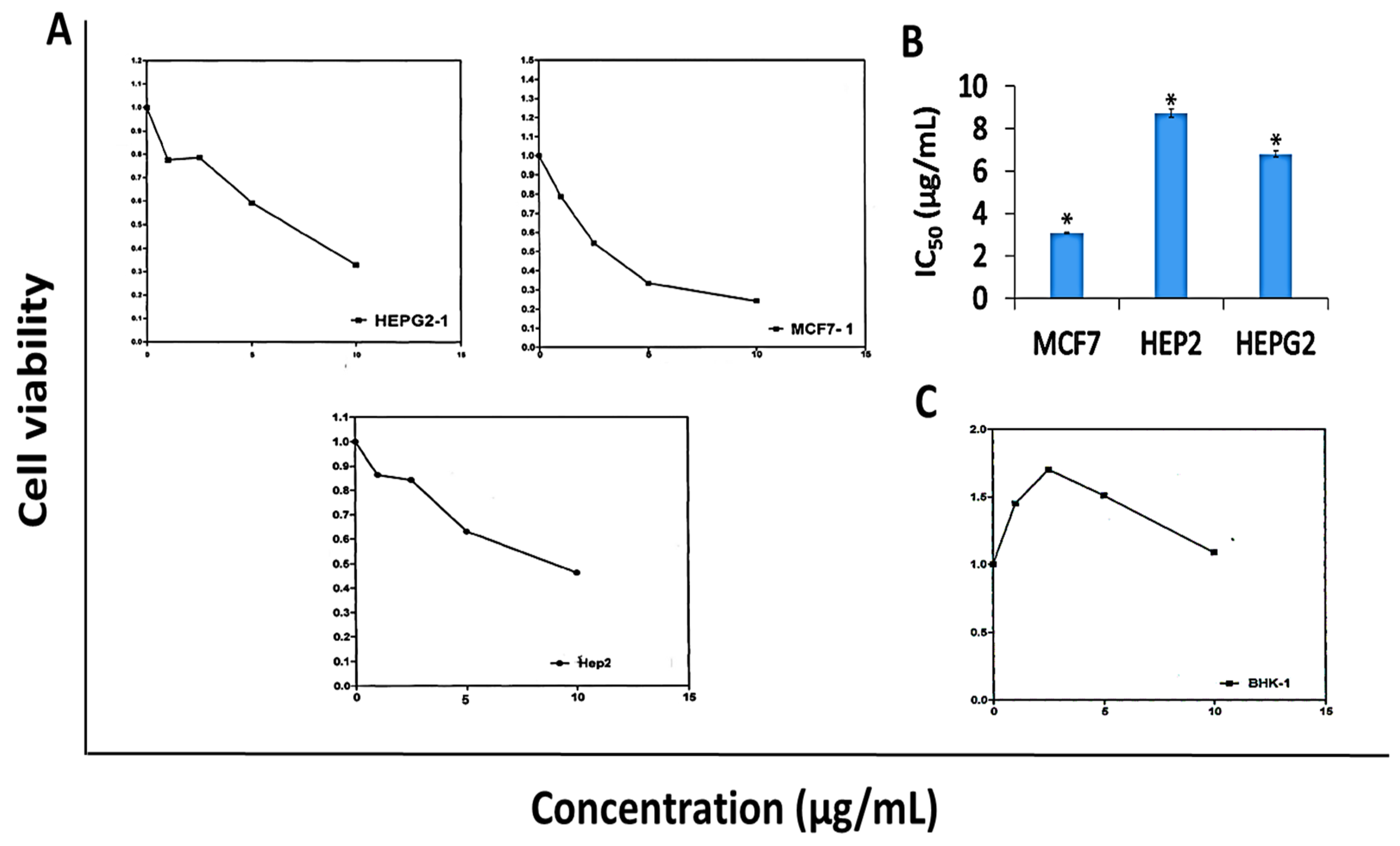
2.5. Anti-Proliferative Activity of DK
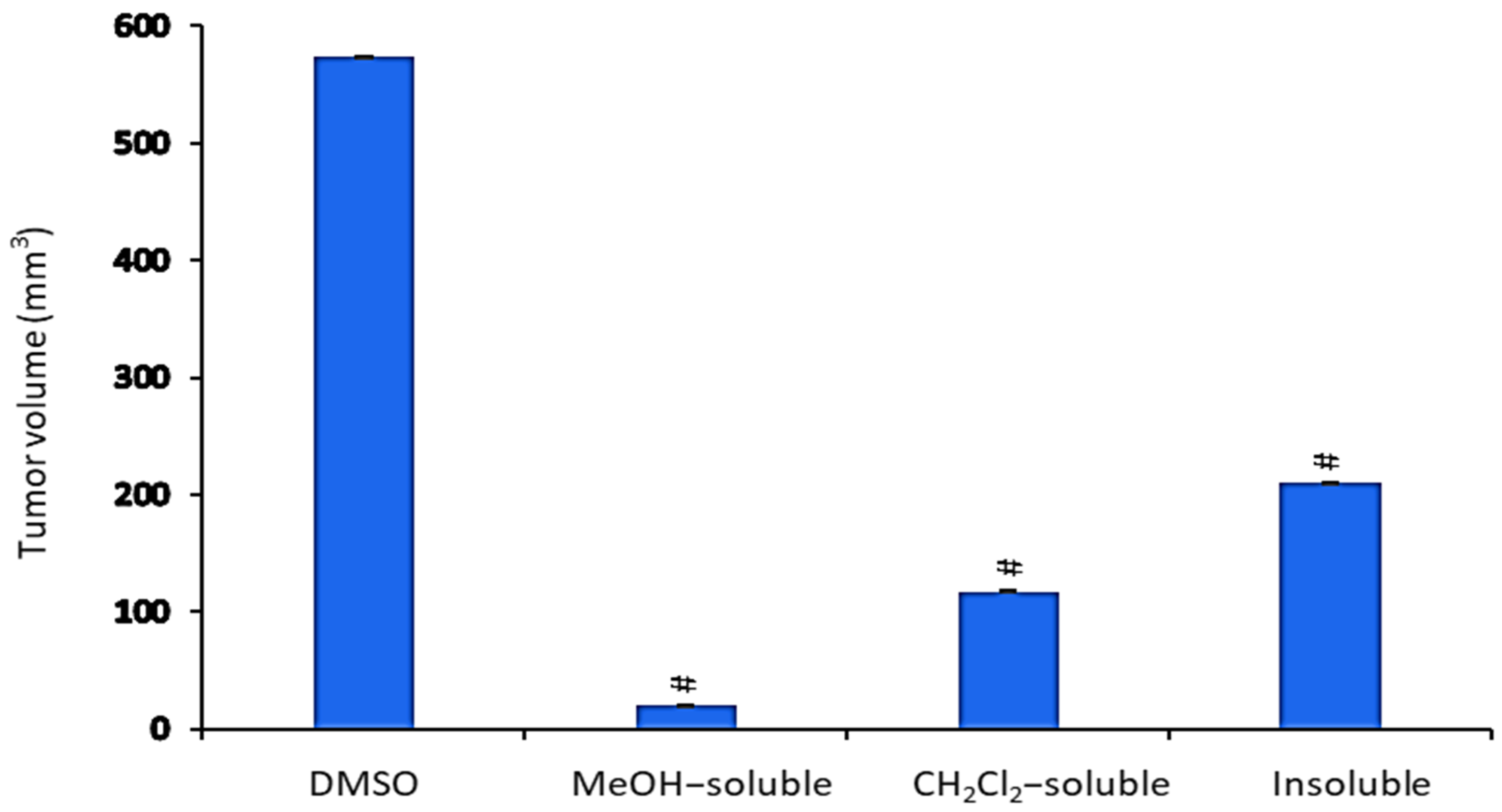
2.6. Effect of A. zerumbet Extracts on Tumor Volume (TV)
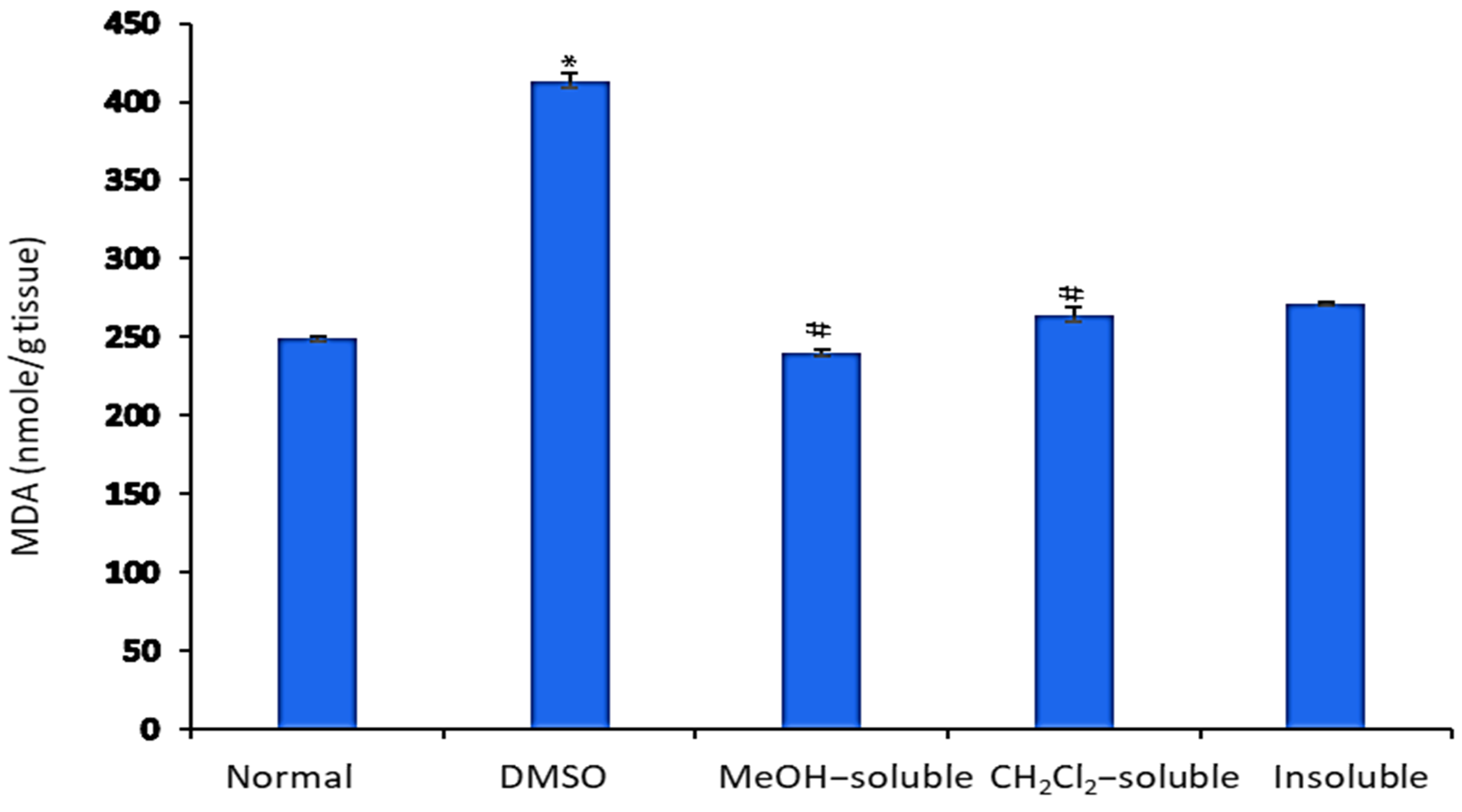
2.7. Effect of A. zerumbet Extracts on Malonaldehyde (MDA) Level
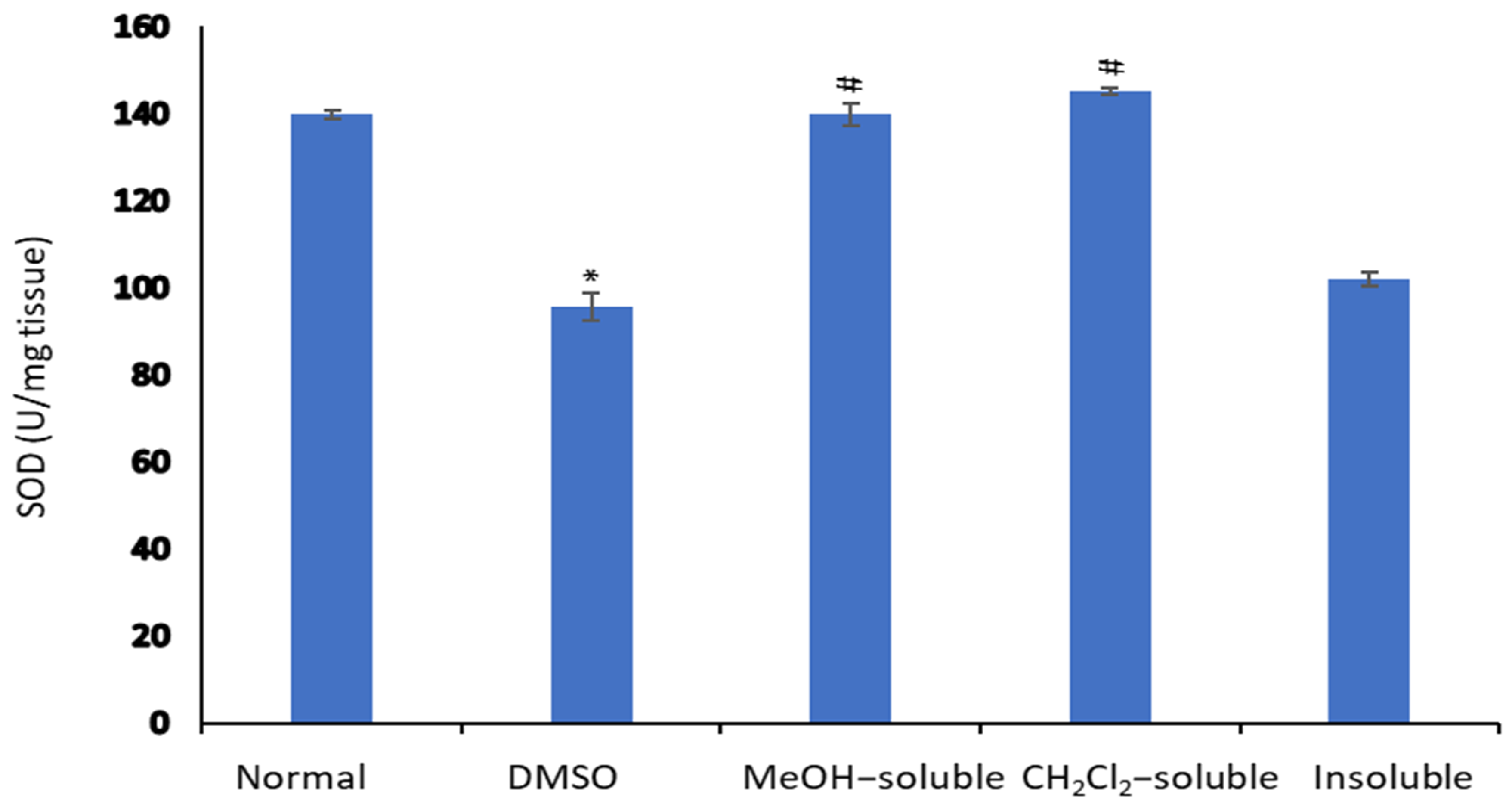
2.8. Effect of A. zerumbet Extracts on Superoxide Dismutase (SOD) Activity
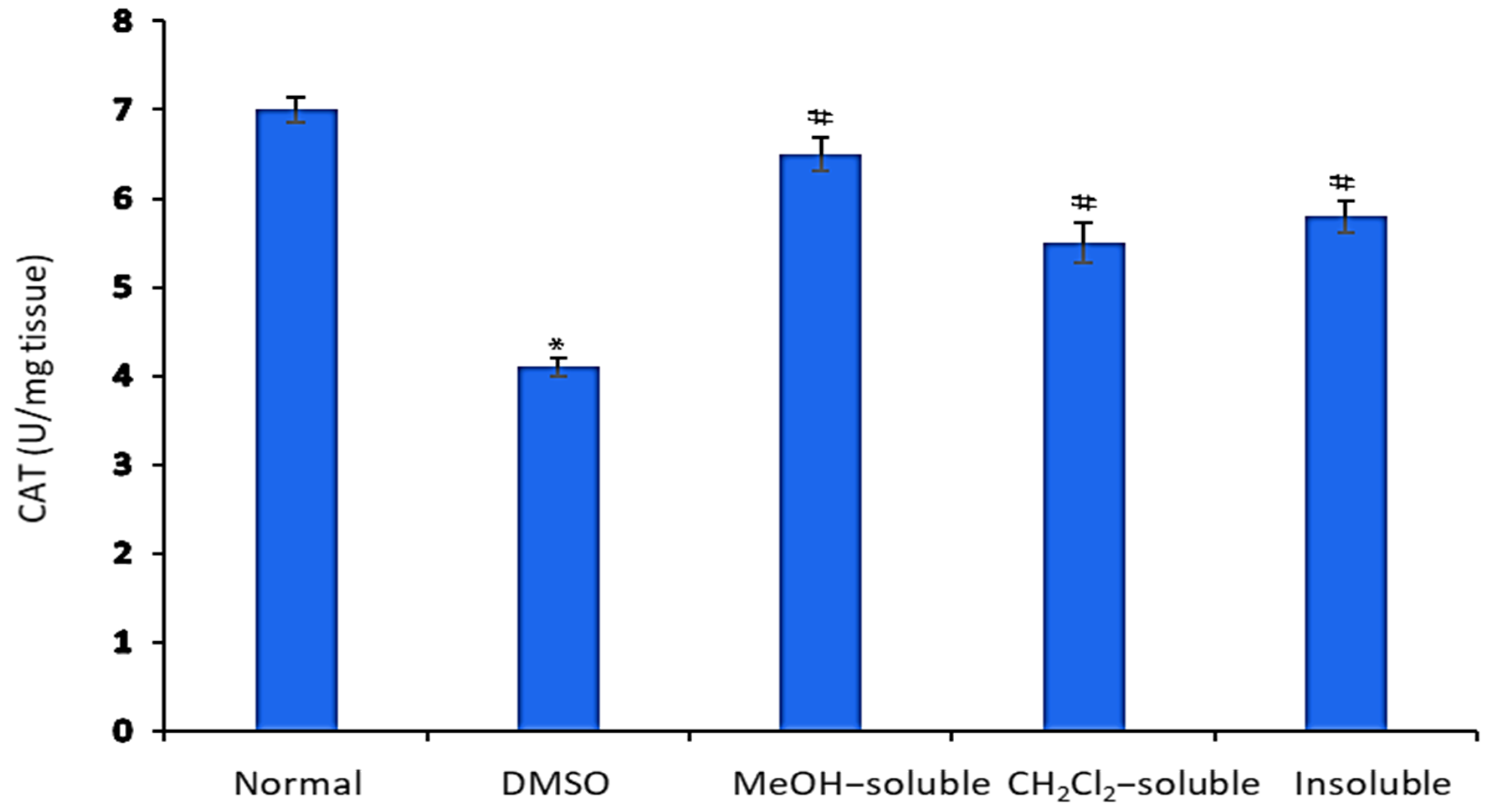
2.9. Effect of A. zerumbet Extracts on Catalase (CAT) Activity
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plants Material
4.3. Extraction and Isolation
4.4. Detection of the Cytotoxicity of Egyptian Medicinal Plants
4.5. Determination of the Cytotoxicity of A. zerumbet Extracts
4.6. Anti-Proliferative Activity of DK
4.6.1. Cell Lines and Cells Culture
4.6.2. Proliferation Assay
4.7. In Vivo Studies
4.7.1. Tumor Cells
4.7.2. Animals
4.7.3. Mouse Xenograft Model
4.7.4. Estimation of MDA Levels in Liver Homogenate
4.7.5. Estimation of SOD Levels in Liver Homogenate
4.7.6. Estimation of CAT Levels in Liver Homogenate
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Testino, G.; Borro, P.; Ancarani, O.; Sumberaz, A. Human carcinogenesis and alcohol in hepato-gastroenterology. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 512–518. [Google Scholar] [PubMed]
- Bravi, F.; Bosetti, C.; Filomeno, M.; Levi, F.; Garavello, W.; Galimberti, S.; Negri, E.; La Vecchia, C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br. J. Cancer 2013, 109, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Thanan, R.; Oikawa, S.; Hiraku, Y.; Ohnishi, S.; Ma, N.; Pinlaor, S.; Yongvanit, P.; Kawanishi, S.; Murata, M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2014, 16, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Polesel, J.; Gini, A.; Dal Maso, L.; Stocco, C.; Birri, S.; Taborelli, M.; Serraino, D.; Zucchetto, A. The negative impact of tobacco smoking on survival after prostate cancer diagnosis. Cancer Causes Control 2015, 26, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 29. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed]
- Simone, R.; Subash, C.G.; Madan, M.C.; Bharat, B.A. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA A Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- He, Z.; Tan, J.S.; Abbasiliasi, S.; Lai, O.M.; Tam, Y.J.; Ariff, A.B. Phytochemicals, nutritionals and antioxidant properties of miracle fruit Synsepalum dulcificum. Ind. Crops Prod. 2016, 86, 87–94. [Google Scholar] [CrossRef]
- Chakraverty, R.; Debnath, T.; Saha, N.; Ghosh, A.; Pati, A. Evaluation of the safety and anti-ulcerative potential of a polyherabal ayurvedic formulation (ASCO1): An exploratory study. J. Pharm. Res. 2016, 6, 4865–4869. [Google Scholar]
- El-Aarag, B.; Magdy, M.; AlAjmi, M.F.; Khalifa, S.A.; El-Seedi, H.R. Melittin Exerts Beneficial Effects on Paraquat-Induced Lung Injuries in Mice by Modifying Oxidative Stress and Apoptosis. Molecules 2019, 24, 1498. [Google Scholar] [CrossRef] [PubMed]
- Akhilesh, K.; Vimala, B. Alpinia zerumbet an essential medicinal herb. MOJ Toxicol. 2018, 4, 316–318. [Google Scholar]
- UNIDO. Herbs, Spices and Essential Oils- Post-Harvest Operations in Developing Countries; United Nations Industrial Development Organization (UNIDO) and the Food and Agriculture Organization of the United Nations (FAO): Vienna, Austria, 2005. [Google Scholar]
- Tawata, S.; Fukuta, M.; Xuan, T.D.; Deba, F. Total utilization of tropical plants Leucaena leucocephala and Alpinia zerumbet. J. Pestic. Sci. 2008, 33, 40–43. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Chong, K.L.; Tan, J.B.; Wong, S.K. Antioxidant properties of tropical and temperate herbal teas. J. Food Comp. Anal. 2010, 23, 185–189. [Google Scholar] [CrossRef]
- Victório, C.P. Therapeutic value of the genus Alpinia, Zingiberaceae. Rev. Bras. Farmacogn. 2011, 21, 194–201. [Google Scholar] [CrossRef]
- Tao, L.; Hu, H.S.; Shen, X.C. Endothelium-dependent vasodilatation effects of the essential oil from Fructus Alpiniae zerumbet (EOFAZ) on rat thoracic aortic rings in vitro. Phytomedicine 2013, 20, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Elzaawely, A.; Xuan, T.; Koyama, H.; Tawata, S. Antioxidant activity and contents of essential oil and phenolic compounds in flowers and seeds of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. Food Chem. 2007, 104, 1648–1653. [Google Scholar]
- Upadhyay, A.; Chompoo, J.; Taira, N.; Fukuta, M.; Tawata, S. Significant longevity-extending effects of Alpinia zerumbet leaf extract on the life span of Caenorhabditis elegans. Biosci. Biotechnol. Biochem. 2013, 77, 217–223. [Google Scholar] [CrossRef]
- Ghareeb, M.; Sobeh, M.; Rezq, S.; El-Shazly, A.; Mahmoud, M.; Wink, M. HPLC-ESI-MS/MS Profiling of Polyphenolics of a Leaf Extract from Alpinia zerumbet (Zingiberaceae) and Its Anti-Inflammatory, Anti-Nociceptive, and Antipyretic Activities In Vivo. Molecules 2018, 23, 3238. [Google Scholar] [CrossRef]
- Wu, M.; Li, Q.; Hu, Z.; Li, X.; Chen, S. The Complete Amomum kravanh Chloroplast Genome Sequence and Phylogenetic Analysis of the Commelinids. Molecules 2017, 22, 1875. [Google Scholar] [CrossRef]
- Mpalantinos, M.A.; de Moura, R.S.; Parente, J.P.; Kuster, R.M. Biologically active flavonoids and kava pyrones from the aqueous extract of Alpinia zerumbet. Phytother. Res. 1998, 12, 442–444. [Google Scholar] [CrossRef]
- Upadhyay, A.; Chompoo, J.; Kishimoto, W.; Makise, T.; Tawata, S. HIV-1 integrase and neuraminidase inhibitors from Alpinia zerumbet. J. Agric. Food Chem. 2011, 59, 2857–2862. [Google Scholar] [CrossRef] [PubMed]
- Tawata, S.; Taira, S.; Kobamoto, N.; Ishihara, M.; Toyama, S. Syntheses and biological activities of dihydro-5,6-dehydrokawain derivatives. Biosci. Biotechnol. Biochem. 1996, 60, 1643–1645. [Google Scholar] [CrossRef] [PubMed]
- Sirat, H.M.; Rahman, A.A.; Itokawa, H.; Morita, H. Constituents of the rhizomes of two Alpinia species of Malaysia. Planta Med. 1996, 62, 188–189. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, R.; Karthigayan, S.; Balasubashini, M.S.; Vijayalakshmi, S.; Somasundaram, S.T.; Balasubramanian, T. Antitumor effect of snake venom (Hydrophis spiralis) on Ehrlich ascites carcinoma bearing mice. Int. J. Cancer Res. 2007, 3, 167–173. [Google Scholar]
- Chandrashekhar, G.; Joshi, M.; Gopal, N.; Kumari, S. Antitumor activity of hexane and ethyl acetate extracts of Tragia involucrata. Int. J. Cancer Res. 2011, 7, 267–277. [Google Scholar]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Larsen, K. Distribution patterns and diversity centres of Zingiberaceae in SE Asia. Biol. Skr. 2005, 55, 219–228. [Google Scholar]
- Dolai, N.; Karmakar, I.; Kumar, R.S.; Kar, B.; Bala, A.; Haldar, P.K. Evaluation of antitumor activity and in vivo antioxidant status of Anthocephalus cadamba on Ehrlich ascites carcinoma treated mice. J. Ethnopharmacol. 2012, 142, 865–870. [Google Scholar] [CrossRef]
- Haldar, P.K.; Kar, B.; Bala, A.; Bhattacharya, S.; Mazumder, U.K. Antitumor activity of Sansevieria roxburghiana rhizome against Ehrlich ascites carcinoma in mice. Pharm. Biol. 2010, 48, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- El-Far, M.; El-Motwally, A.E.-G.; Hashem, I.A.; Bakry, N. Biochemical role of intravaginal sildenafil citrate as a novel antiabortive agent in unexplained recurrent spontaneous miscarriage: First clinical study of four case reports from Egypt. Clin. Chem. Lab. Med. 2009, 47, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- El-Far, M.; El-Sayed, I.; El-Motwally, A.; Hashem, I.; Bakry, N. Serum levels of TNF-α and antioxidant enzymes and placental TNF-α expression in unexplained recurrent spontaneous miscarriage. J. Physiol. Biochem. 2009, 65, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Lipid peroxidation—DNA damage by malondialdehyde. Mutat. Res. Fund. Mol. Mutagen. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Seven, A.; Civelek, S.; Inci, E.; Inci, F.; Korkut, N.; Burc¸ak, G. Evaluation of oxidative stress parameters in blood of patients with laryngeal carcinoma. Clin. Biochem. 1999, 32, 369–373. [Google Scholar] [CrossRef]
- Valdivia, A.; Perez-Alvarez, S.; Aroca-Aguilar, J.; Ikuta, I.; Jordan, J. Superoxide dismutases: A physiopharmacological update. J. Physiol. Biochem. 2009, 65, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K. Lipid peroxides and human diseases. Chem. Phys. Lipids 1987, 45, 337–351. [Google Scholar] [CrossRef]
- Hamid, A.; Ibrahim, F.; Ming, T.; Nasrom, M.; Eusoff, N.; Husain, K. Zingiber zerumbet L. (Smith) extract alleviates the ethanol-induced brain damage via its antioxidant activity. BMC Complement. Altern. Med. 2018, 18, 101. [Google Scholar] [CrossRef]
- Chompoo, J.; Upadhyay, A.; Fukuta, M.; Tawata, S. Effect of Alpinia zerumbet components on antioxidant and skin diseases-related enzymes. ISCMR 2012, 12, 106. [Google Scholar] [CrossRef]
- Tu, T.; Chompoo, J.; Tawata, S. Hispidin and related herbal compounds from Alpinia zerumbet inhibit both PAK1-dependent melanogenesis in melanocytes and reactive oxygen species (ROS) production in adipocytes. Drug Discov. Ther. 2015, 9, 197–204. [Google Scholar]
- Gupta, M.; Mazumder, U.K.; Rath, N.; Mukhopadhyay, D.K. Antitumor activity of methanolic extract of Cassia fistula L. seed against Ehrlich ascites carcinoma. J. Ethnopharmacol. 2000, 72, 151–156. [Google Scholar] [CrossRef]
- Saroja, M.; Santhi, R.; Annapoorani, S. Evaluation of antitumor and antioxidant activity of flavonoid fraction of Terminalia Catappa against Ehrlich Ascites carcinoma in mice. Int. J. Drug Dev. Res. 2012, 4, 180–187. [Google Scholar]
- Karmakar, I.; Dolai, N.; Suresh Kumar, R.; Kar, B.; Roy, S.N.; Haldar, P.K. Antitumor activity and antioxidant property of Curcuma caesia against Ehrlich’s ascites carcinoma bearing mice. Pharm. Biol. 2013, 51, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mazumder, U.K.; Kumar, R.S.; Kumar, T.S. Antitumor activity and antioxidant role of Bauhinia racemosa against Ehrlich ascites carcinoma in Swiss albino mice. Acta Pharmacol. Sin. 2004, 25, 1070–1076. [Google Scholar]
- El-Seedi, H.R. Antimicrobial arylcoumarins from Asphodelus microcarpus. J. Nat. Prod. 2007, 70, 118–120. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Shabasy, R.; Sakr, H.; Zayed, M.; El-Said, A.; Helmy, K.M.; Gaara, A.H.; Turki, Z.; Azeem, M.; Ahmed, A.M.; et al. Anti-schistosomiasis triterpene glycoside from the Egyptian medicinal plant Asparagus stipularis. Revista Brasileira Farmacognosia 2012, 22, 314–318. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Ullah, M.O.; Haque, M.; Urmi, K.F.; Zulfiker, A.H.M.; Anita, E.S.; Begum, M.; Hamid, K. Anti-bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac. J. Trop. Biomed. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Muthuraman, M.S.; Dorairaj, S.; Rangarajan, P.; Pemaiah, B. Antitumor and antioxidant potential of Tragia Plukenetii, R. Smith on Ehrlich ascites carcinoma in mice. Afr. J. Biotechnol. 2008, 7, 3527–3530. [Google Scholar]
- Sunila, E.S.; Kuttan, R.; Preethi, K.C.; Kuttan, G. Dynamized preparations in cell culture. Evid.-Based Complement. Altern. Med. 2009, 6, 257–263. [Google Scholar] [CrossRef]
- Guirgis, A.A.; Zahran, M.; Mohamed, A.S.; Talaat, R.M.; Abdou, B.Y.; Agwa, H.S. Effect of thalidomide dithiocarbamate analogs on the intercellular adhesion molecule-1 expression. Int. Immunopharmacol. 2010, 10, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, D.; Kimler, B.F.; Estes, N.C.; Durham, F.J. Growth delay effect of combined interstitial hyperthermia and brachytherapy in a rat solid tumor model. Anticancer Res. 1989, 9, 45–47. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| No. | Plant Scientific Name | Vernacular Name | Part Used | % Mortality |
|---|---|---|---|---|
| 1 | Alpinia zerumbet | Variegated ginger | Flowers | 93.33 |
| 2 | Anabasis setifera | Agram, Asal, Glew عجرم, عسل، جلو | Flowers | 43.33 |
| 3 | Ballota undulata | Ghassa, Zafra غصة، زفرة | Flowers | 50.00 |
| 4 | Calathea metallica | ND | Flowers | 23.33 |
| 5 | Caylusea hexagyna | Danaban دنبان | Flowers | 10.00 |
| 6 | Echinops glaberrimus | Khashir خشير | Flowers | 56.66 |
| 7 | E. spinosus | Qatad, Gorreih قتاد، جريح | Flowers | 86.66 |
| 8 | Globularia arabica | Handaqouq, zorreiqa حندوق، زُريقة | Flowers | 46.66 |
| 9 | Lavandula pubescens | Atan عطن | Fruits | 63.33 |
| 10 | Psoralea mutisii | ND | Flowers | 13.33 |
| 11 | P. pubescens | ND | Flowers | 6.66 |
| 12 | Reseda arabica | ND | Flowers | 30.00 |
| 13 | Salvadora persica | Araak, Siwaak, Miswaak أراك، سواك، مسواك | Flowers | 0.00 |
| 14 | Salvia aegyptiaca | Ra‘alah رعلة | Flowers | 63.33 |
| 15 | Senecio reflexun | Morrar, Umm Lonein مررار, أم لونين | Flowers | 36.66 |
| Substance | Mortality % |
|---|---|
| Concentration 100 (µg/mL) | |
| CH2Cl2 ex. | 100.00 |
| MeOH-H2O (90%) ex. | 80.00 |
| H2O ex. | 50.00 |
| Hexane ex. | 50.00 |
| Insoluble ex. | 13.33 |
| F1 | 100.00 |
| F2 | 30.00 |
| F3 | 20.00 |
| F4 | 63.33 |
| F5 | 86.66 |
| DK | 100.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahra, M.H.; Salem, T.A.R.; El-Aarag, B.; Yosri, N.; EL-Ghlban, S.; Zaki, K.; Marei, A.H.; Abd El-Wahed, A.; Saeed, A.; Khatib, A.; et al. Alpinia zerumbet (Pers.): Food and Medicinal Plant with Potential In Vitro and In Vivo Anti-Cancer Activities. Molecules 2019, 24, 2495. https://doi.org/10.3390/molecules24132495
Zahra MH, Salem TAR, El-Aarag B, Yosri N, EL-Ghlban S, Zaki K, Marei AH, Abd El-Wahed A, Saeed A, Khatib A, et al. Alpinia zerumbet (Pers.): Food and Medicinal Plant with Potential In Vitro and In Vivo Anti-Cancer Activities. Molecules. 2019; 24(13):2495. https://doi.org/10.3390/molecules24132495
Chicago/Turabian StyleZahra, Maram Hussein, Tarek A.R. Salem, Bishoy El-Aarag, Nermeen Yosri, Samah EL-Ghlban, Kholoud Zaki, Amel H. Marei, Aida Abd El-Wahed, Aamer Saeed, Alfi Khatib, and et al. 2019. "Alpinia zerumbet (Pers.): Food and Medicinal Plant with Potential In Vitro and In Vivo Anti-Cancer Activities" Molecules 24, no. 13: 2495. https://doi.org/10.3390/molecules24132495
APA StyleZahra, M. H., Salem, T. A. R., El-Aarag, B., Yosri, N., EL-Ghlban, S., Zaki, K., Marei, A. H., Abd El-Wahed, A., Saeed, A., Khatib, A., AlAjmi, M. F., Shathili, A. M., Xiao, J., Khalifa, S. A. M., & El-Seedi, H. R. (2019). Alpinia zerumbet (Pers.): Food and Medicinal Plant with Potential In Vitro and In Vivo Anti-Cancer Activities. Molecules, 24(13), 2495. https://doi.org/10.3390/molecules24132495









