Evolution of Flavanol Glycosides during Red Grape Fermentation
Abstract
1. Introduction
2. Results and Discussion
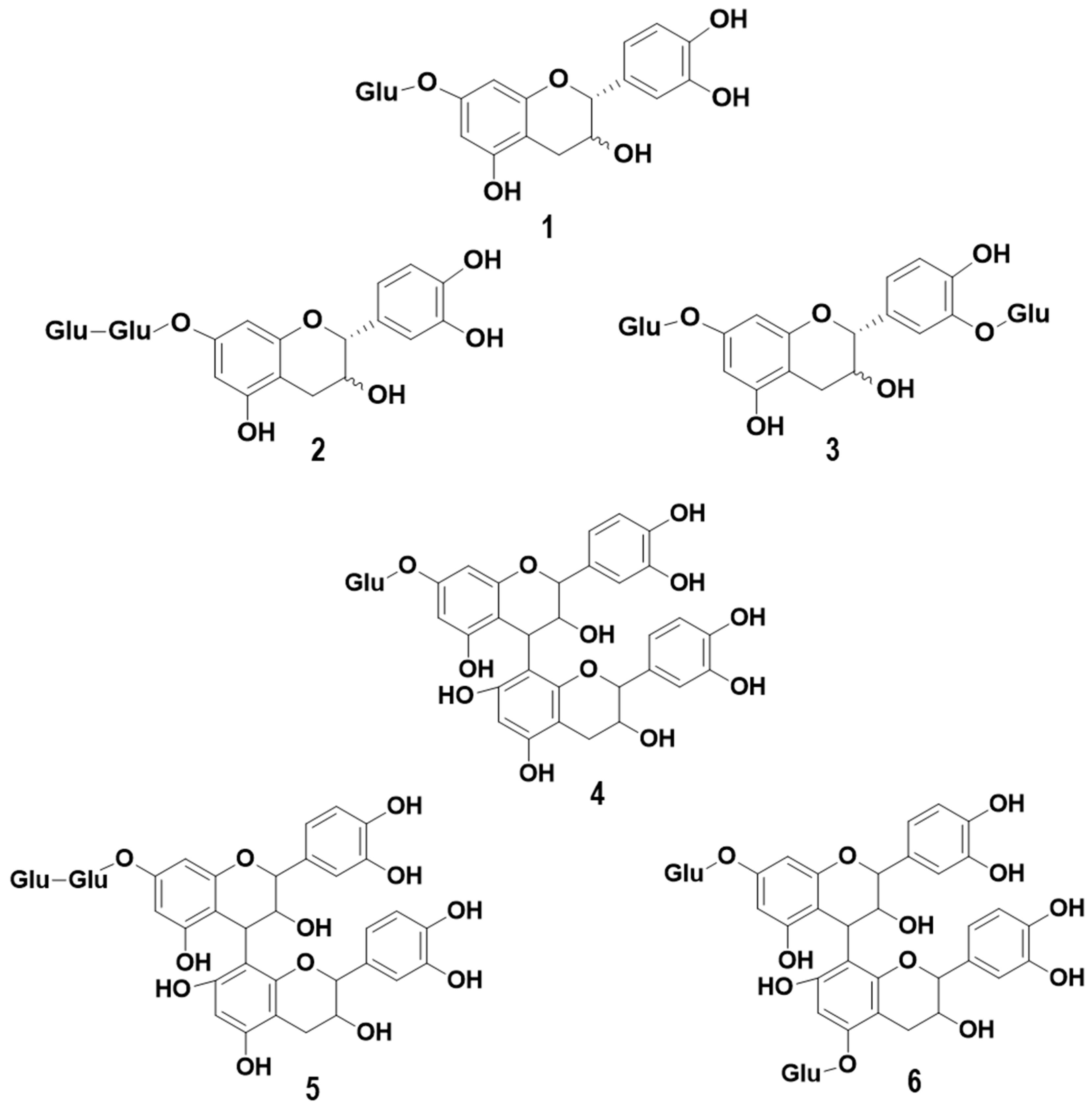
2.1. Monomers of (epi) Catechin Monoglycosides (MMG) and Dimers of (epi) Catechin Monoglycosides (DMG)
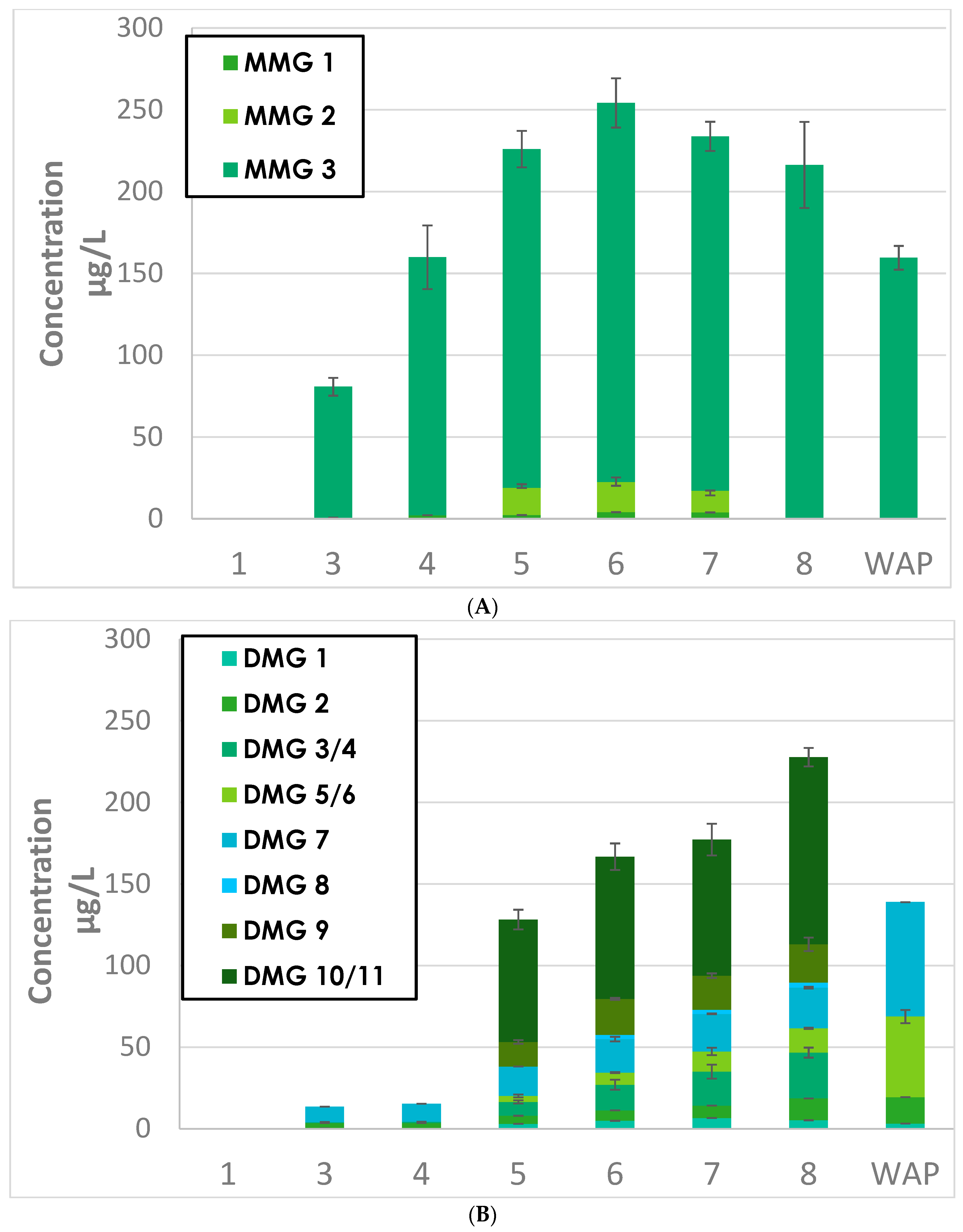
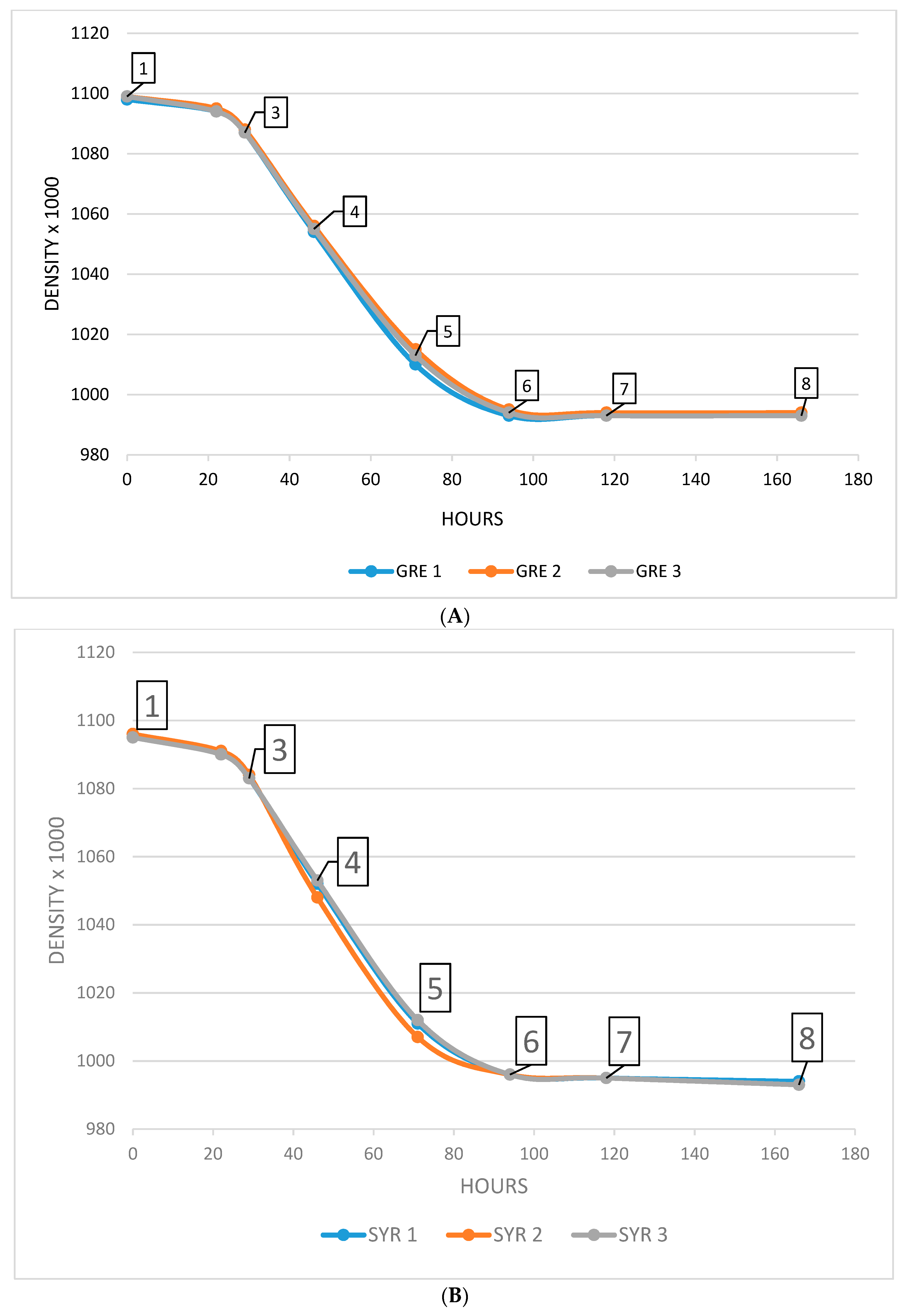
2.1.1. Extractions Kinetics of MMG and MDG during Grenache and Syrah Fermentation
2.1.2. Grape Origins of MMG and DMG in Wine
2.2. Monomers of (epi) Catechin Diglycosides (MDG) and Dimers of (epi) Catechin Diglycosides (DDG)
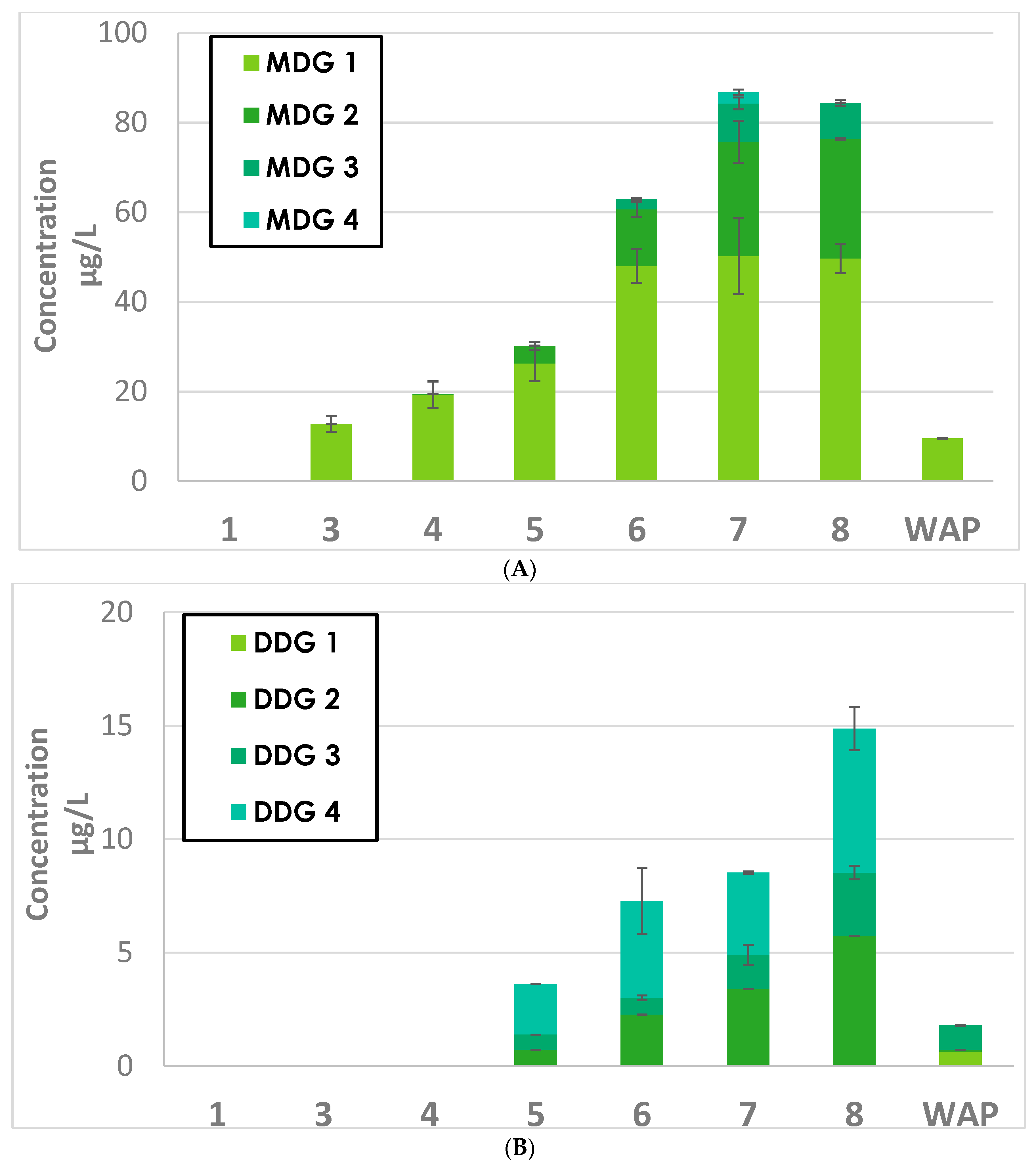
2.2.1. Extractions Kinetics of MDG and DDG during GRE and SYR Fermentation
2.2.2. Grape Origins of MDG and DDG in Wines
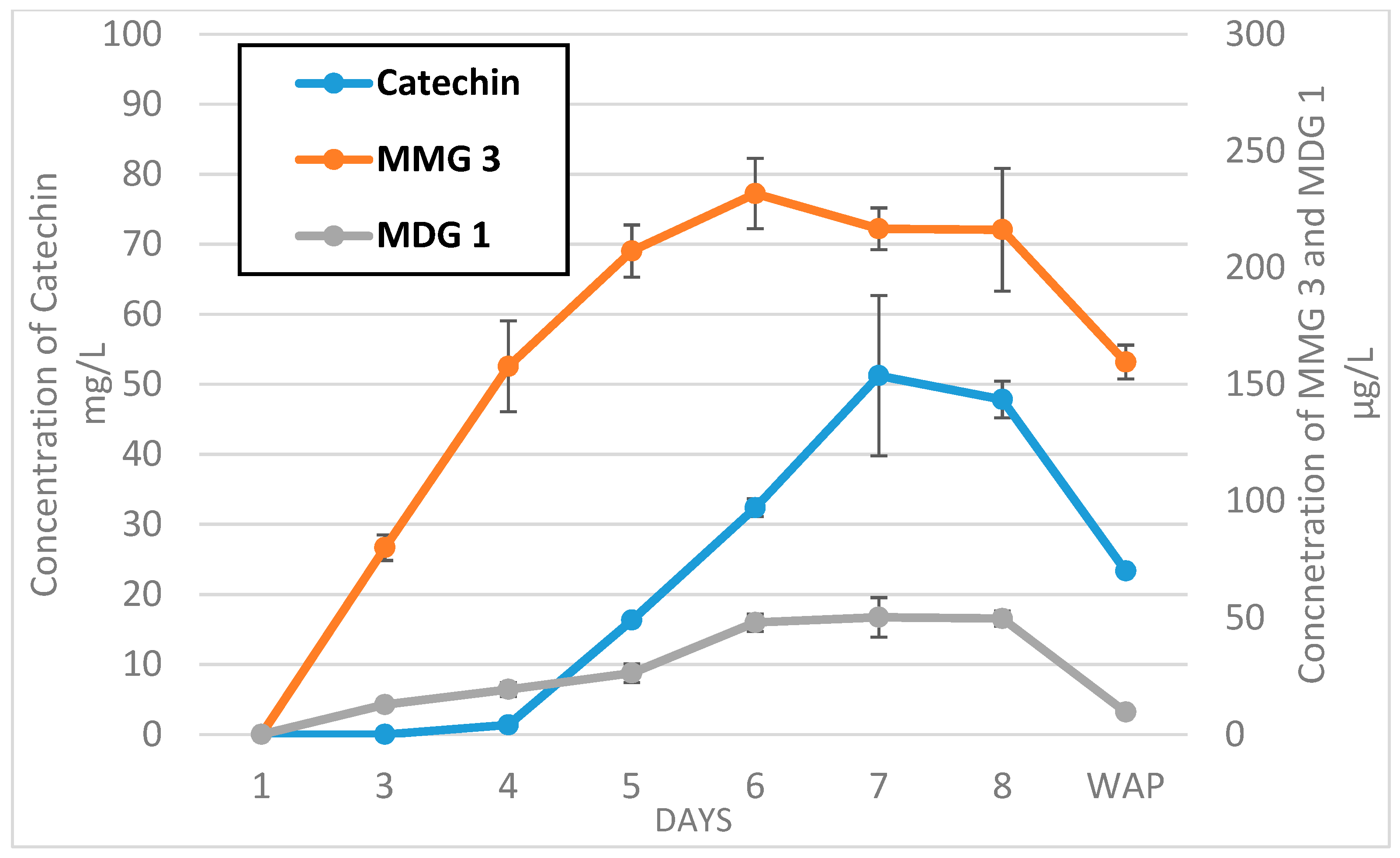
2.3. Evolution between Monomer and Dimer Concentrations during Fermentation
3. Materials and Methods
3.1. Reagents, Standards and Calibration
3.2. Grape and Wine Samples
3.2.1. Grape Samples
3.2.2. Winemaking and Samples
3.3. UHPLC-MS/MS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Grape Variety | Berry Weight (g) | Brix | Sugars g·L−1 | Potential Alcohol by Volume %vol |
|---|---|---|---|---|
| GRE 1 | 584 | 23.2 | 228.7 | 13.59 |
| GRE 2 | 586 | 23.2 | 228.7 | 13.59 |
| GRE 3 | 578 | 23.2 | 228.7 | 13.59 |
| SYR 1 | 561 | 22.0 | 214.8 | 12.76 |
| SYR 2 | 550 | 22.0 | 214.8 | 12.76 |
| SYR 3 | 536 | 22.0 | 214.8 | 12.76 |
Appendix B
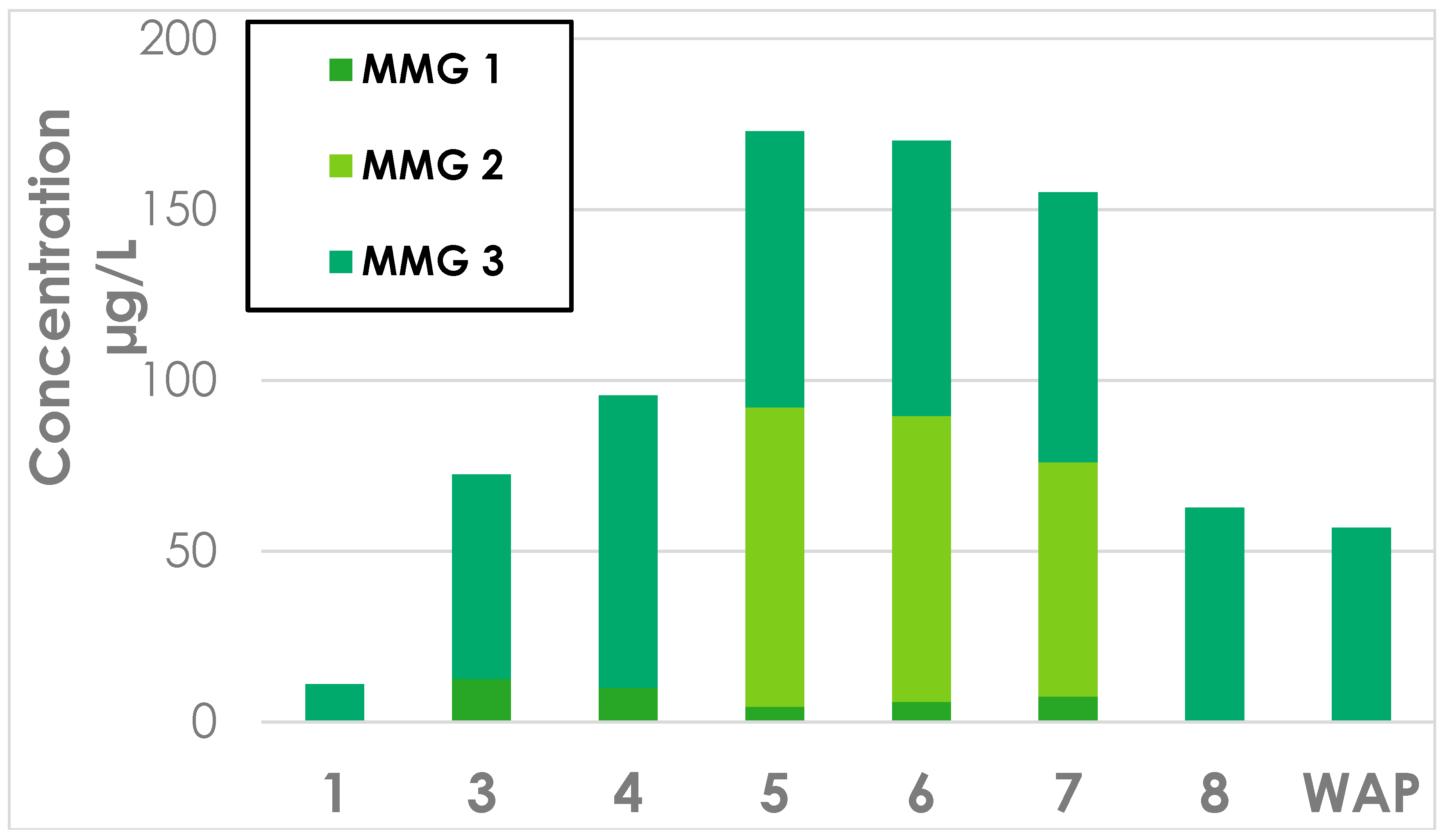
Appendix C
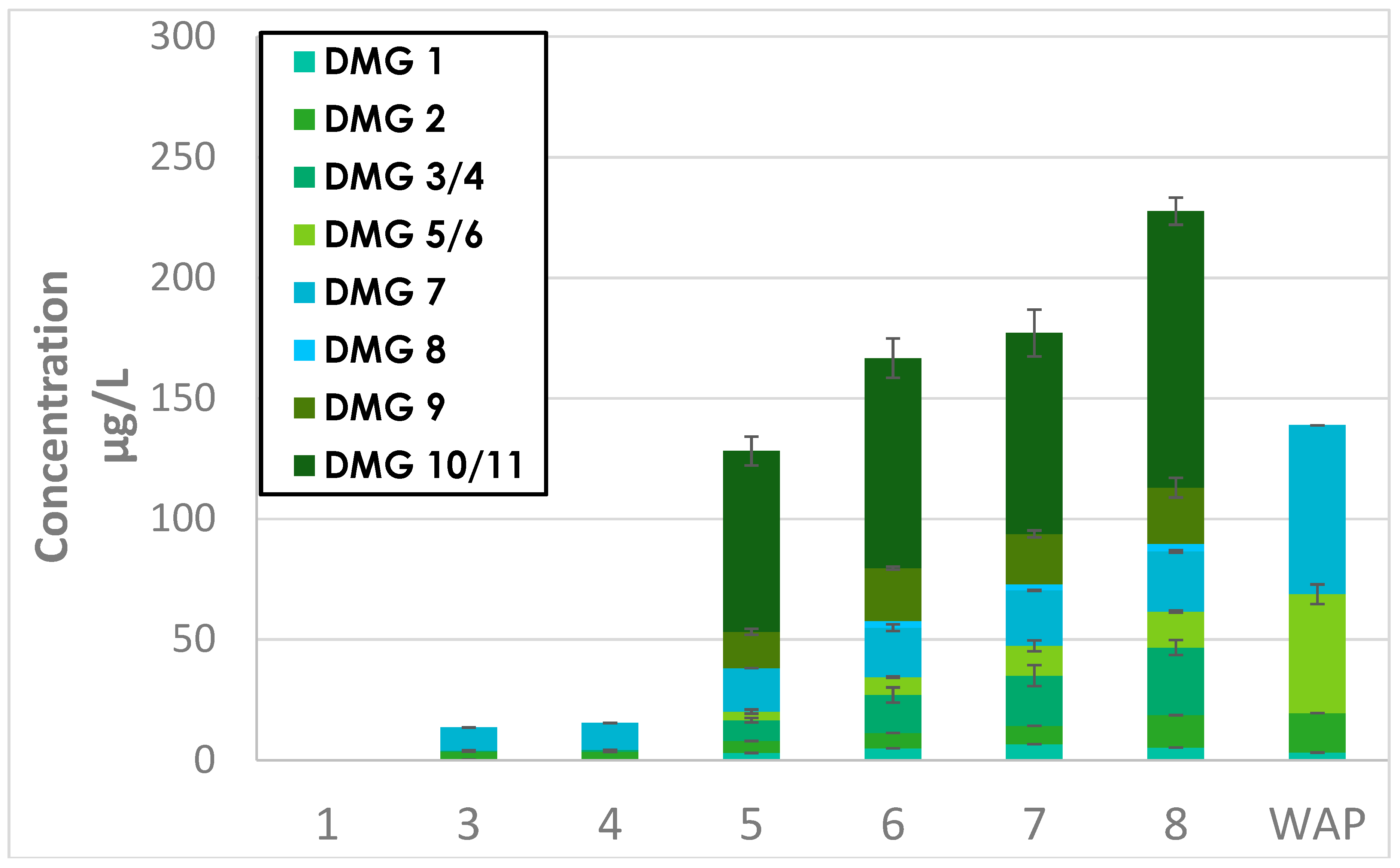
Appendix D
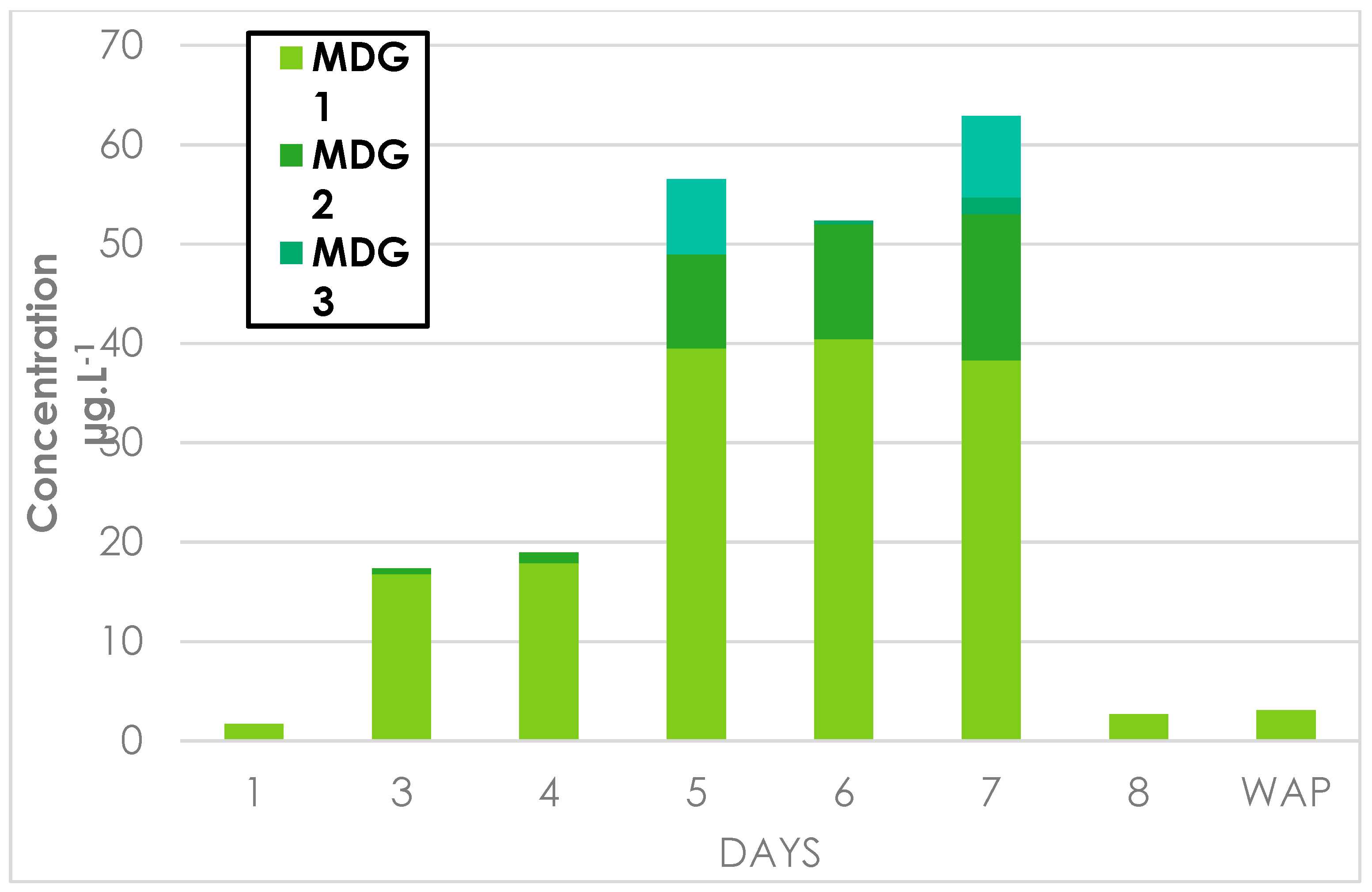
Appendix E
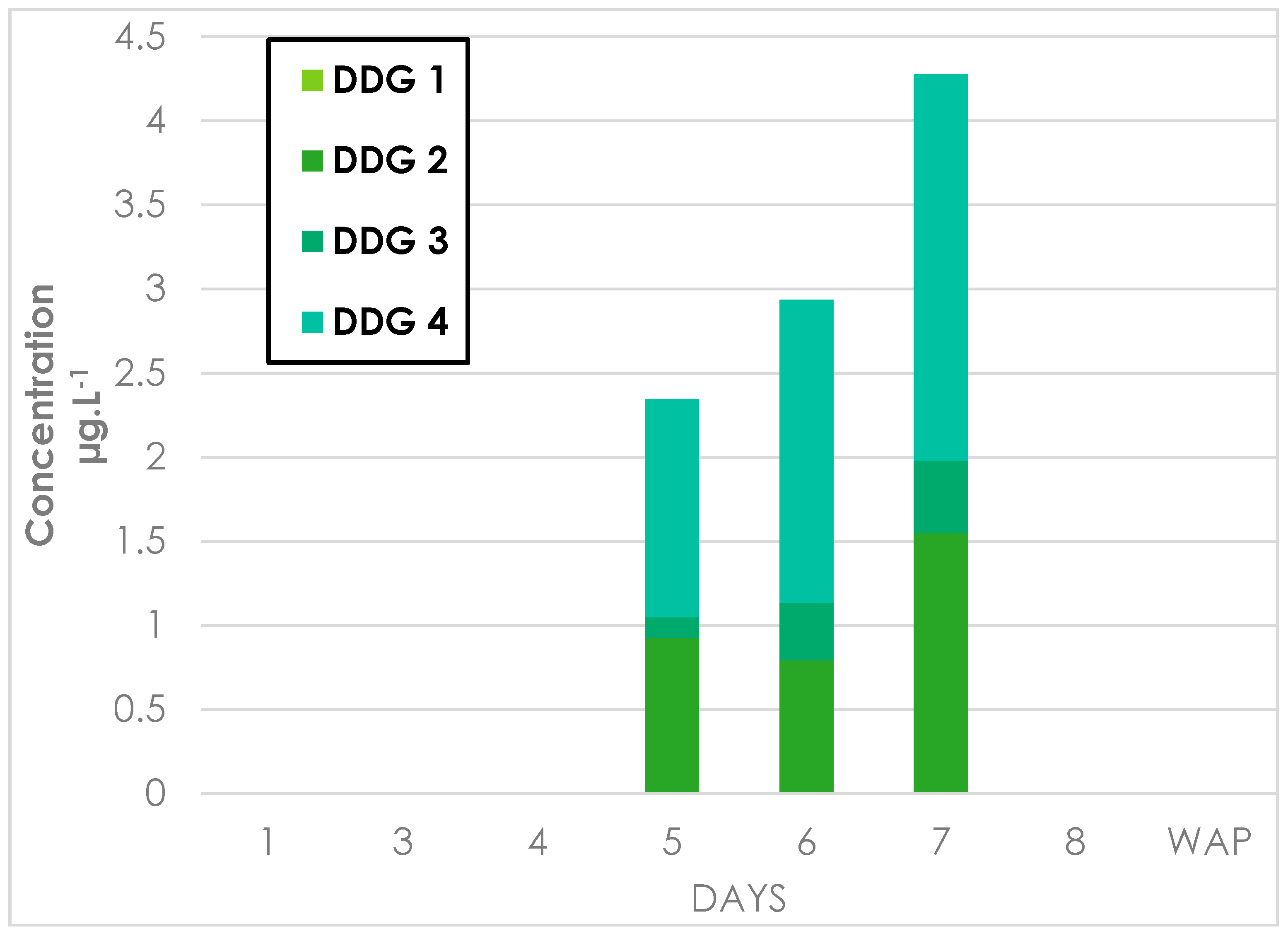
References
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. J. Ges. Dtsch. Chem. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharm. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Corder, R.; Mullen, W.; Khan, N.Q.; Marks, S.C.; Wood, E.G.; Carrier, M.J.; Crozier, A. Oenology: Red wine procyanidins and vascular health. Nature 2006, 444, 566. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol. Nat. Rev. Drug Dis. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Popović, B.M.; Štajner, D.; Ždero-Pavlović, R.; Tumbas-Šaponjac, V.; Čanadanović-Brunet, J.; Orlović, S. Water stress induces changes in polyphenol profile and antioxidant capacity in poplar plants (Populus spp.). Plant Physiol. Biochem. 2016, 105, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Brouillard, R. The mechanism of co-pigmentation of anthocyanins in aqueous solutions. Phytochemistry 1990, 29, 1097–1102. [Google Scholar] [CrossRef]
- Brouillard, R.; Dangles, O. Anthocyanin molecular interactions: The first step in the formation of new pigments during wine aging? Food Chem. 1994, 51, 365–371. [Google Scholar] [CrossRef]
- Rinaldi, A.; Jourdes, M.; Teissedre, P.L.; Moio, L. A preliminary characterization of Aglianico (Vitis vinifera L. cv.) grape proanthocyanidins and evaluation of their reactivity towards salivary proteins. Food Chem. 2014, 164, 142–149. [Google Scholar] [CrossRef]
- Vidal, S.; Cartalade, D.; Souquet, J.-M.; Fulcrand, H.; Cheynier, V. Changes in proanthocyanidin chain length in winelike model solutions. J. Agric. Food Chem. 2002, 50, 2261–2266. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Gómez-Plaza, E.; Martínez, A.; López-Roca, J.M. Evolution of phenolic compounds during wine fermentation and post-fermentation: Influence of grape temperature. J. Food Compos. Anal. 1999, 12, 259–272. [Google Scholar] [CrossRef]
- González-Manzano, S.; Dueñas, M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Santos-Buelga, C. Studies on the copigmentation between anthocyanins and flavan-3-ols and their influence in the colour expression of red wine. Food Chem. 2009, 114, 649–656. [Google Scholar] [CrossRef]
- Pinasseau, L.; Verbaere, A.; Roques, M.; Meudec, E.; Vallverdú-Queralt, A.; Terrier, N.; Boulet, J.C.; Cheynier, V.; Sommerer, N. A Fast and Robust UHPLC-MRM-MS method to characterize and quantify grape skin tannins after chemical depolymerization. Molecules 2016, 21, 1409. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Troup, G.J.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Ristic, R.; Iland, P.G.; Jones, G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2008, 6, 244–254. [Google Scholar] [CrossRef]
- Di Lecce, G.; Arranz, S.; Jáureguic, O.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Lamuela-Raventós, R.M. Phenolic profiling of the skin, pulp and seeds of Albariño grapes using hybrid quadrupole time-of-flight and triple-quadrupole mass spectrometry. Food Chem. 2014, 145, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Delcambre, A.; Saucier, C. Identification of new flavan-3-ol monoglycosides by UHPLC-ESI-Q-TOF in grapes and wine. J. Mass Spectrom. 2012, 47, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Zerbib, M.; Mazauric, J.-P.; Meudec, E.; Le Guernevé, C.; Lepak, A.; Nidetzky, B.; Cheynier, V.; Terrier, N.; Saucier, C. New flavanol O-glycosides in grape and wine. Food Chem. 2018, 266, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Raab, T.; Barron, D.; Arce Vera, F.; Crespy, V.; Oliveira, M.; Williamson, G. Catechin Glucosides: Occurrence, Synthesis, and Stability. Food Chem. 2010, 58, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Kitao, S.; Ariga, T.; Matsudo, T.; Sekine, H. The syntheses of catechin-glucosides by transglycosylation with leuconostoc mesenteroides sucrose phosphorylase. Biosci. Biotechnol. Biochem. 1993, 57, 2010–2015. [Google Scholar] [CrossRef]
- Pang, Y.; Peel, G.J.; Sharma, S.B.; Tang, Y.; Dixon, R.A. A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula. Proc. Natl. Acad. Sci. USA 2008, 105, 14210–14215. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, Z.; Ayestaran, B. Changes in the color components and phenolic content of red wines from Vitis vinifera L. Cv. “Tempranillo” during vinification and aging. Eur. Food Res. Technol. 2008, 228, 29–38. [Google Scholar] [CrossRef]
- Zerbib, M.; Cazals, G.; Enjalbal, C.; Saucier, C. Identification and quantification of flavanol glycosides in vitis vinifera grape seeds and skins during ripening. Molecules 2018, 23, 2745. [Google Scholar] [CrossRef] [PubMed]
- Dambergs, R.G.; Sparrow, A. The “Bodum French Press”: A simple, reliable small-lot red wine fermentation method. In Proceedings of the 14th Australian Wine Industry Technical Conference, Adelaide, Australia, 3–8 July 2010; p. 353. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| MMG | Grenache | Syrah | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seed a | Skin a | WFD4 b | WFD7 b | WAP b | Seed a | Skin a | WFD4 b | WFD7 b | WAP b | |
| 1 | 0.002 ± 4.10 × 10−4 | n.d. | 2.27 ± 0.475 | n.d. | n.d. | 0.002 ± 7.10 × 10−4 | 0.007 ± 0.02 | 4.44 ± 0.739 | n.d. | n.d. |
| 2 | n.d. | n.d. | 16.6 ± 2.30 | n.d. | n.d. | n.d. | n.d. | 87.7 ± 2.46 | n.d. | n.d. |
| 3 | 0.205 ± 0.039 | 0.643 ± 0.102 | 207 ± 11.1 | 216 ± 26.3 | 160 ± 7.27 | 0.045 ± 0.005 | 0.307 ± 0.056 | 80.7 ± 4.40 | 62.7 ± 2.17 | 56.8 ± 1.57 |
| DMG | Grenache | Syrah | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seed a | Skin a | WFD4 b | WFD7 b | WAP b | Seed a | Skin a | WFD4 b | WFD7 b | WAP b | |
| 1 | n.d. | 0.015 ± 0.006 | 2.99 ± 1.04 | 5.24 ± 2.06 | 3.17 ± 0.831 | n.d. | 0.028 ± 0.004 | 6.41 ± 0.136 | 3.79 ± 0.480 | 3.90 ± 0.378 |
| 2 | n.d. | 7.10 × 10−4 ± 2.10 × 10−4 | 5.00 ± 1.53 | 13.4 ± 6.36 | 16.3 ± 0.529 | n.d. | 0.004 ± 0 | 3.03 ± 0.390 | 8.15 ± 1.83 | 7.18 ± 1.66 |
| 3/4 | n.d. | 0.008 ± 0.003 | 8.50 ± 0.943 | 28.0 ± 3.08 | n.d. | n.d. | 0.015 ± 0.004 | 6.80 ± 2.14 | 4.86 ± 0.934 | 6.85 ± 1.09 |
| 5/6 | n.d. | 0.009 ± 0.003 | 3.62 ± 0.852 | 15.0 ± 0.395 | 49.4 ± 4.05 | n.d. | 0.015 ± 0.002 | 4.78 ± 0.317 | 15.2 ± 1.82 | 16.0 ± 0.791 |
| 7 | n.d. | 0.022 ± 0.001 | 18.1 ± 1.60 | 24.9 ± 1.2 | 70.1 ± 2.87 | n.d. | 0.027 ± 0.009 | 10.9 ± 1.05 | 21.8 ± 0.962 | 24.5 ± 0.106 |
| 8 | n.d. | 0.024 ± 0.004 | n.d. | 3.12 ± 0.569 | n.d. | n.d. | 0.078 ± 0.012 | 8.06 ± 2.37 | n.d. | n.d. |
| 9 | n.d. | 0.041 ± 0.019 | 15.0 ± 1.19 | 23.4 ± 4.09 | n.d. | n.d. | 0.031 ± 0.004 | 6.67 ± 2.08 | n.d. | n.d. |
| 10/11 | n.d. | 0.345 ± 0.114 | 75.0 ± 6.02 | 114.6 ± 5.62 | n.d. | n.d. | 0.250 ± 0.033 | 34.4 ± 3.30 | n.d. | n.d. |
| MDG | Grenache | Syrah | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seed a | Skin a | WFD4 b | WFD7 b | WAP b | Seed a | Skin a | WFD4 b | WFD7 b | WAP b | |
| 1 | 0.172 ± 0.014 | 0.038 ± 0.014 | 26.31 ± 3.97 | 49.7 ± 3.28 | 9.55 ± 0.014 | 0.122 ± 0.009 | 0.048 ± 0.013 | 39.5 ± 4.83 | 2.66 ± 0.959 | 3.09 ± 0.016 |
| 2 | 0.243 ± 0.014 | n.d. | 3.84 ± 0.947 | 26.6± 0.197 | n.d. | 0.136 ± 0.007 | n.d. | 9.48 ± 0.680 | n.d. | n.d. |
| 3 | 0.342 ± 0.026 | n.d. | n.d. | 8.10 ± 0.696 | n.d. | 0.178 ± 0.018 | n.d. | n.d. | n.d. | n.d. |
| 4 | 0.250 ± 0.058 | n.d. | n.d. | n.d. | n.d. | 0.074 ± 0.010 | n.d. | 7.54 ± 2.77 | n.d. | n.d. |
| DDG | Grenache | Syrah | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seed a | Skin a | WFD4 b | WFD7 b | WAP b | Seed a | Skin a | WFD4 b | WFD7 b | WAP b | |
| 1 | 0.032 ± 0.002 | n.d. | n.d. | n.d. | 0.610 ± 0.061 | 0.015 ± 0.005 | n.d. | n.d. | n.d. | n.d. |
| 2 | 0.079 ± 0.006 | n.d. | 0.716 ± 0.15 | 5.74 ± 0.951 | 0.110 ± 0.018 | 0.041 ± 0.005 | n.d. | 0.926 ± 0.107 | n.d. | n.d. |
| 3 | 0.043 ± 0.003 | n.d. | 0.672 ± 0 | 2.790 ± 0.297 | 1.070 ± 0.035 | 0.029 ± 0.005 | n.d. | 0.123 ± 0 | n.d. | n.d. |
| 4 | 0.080 ± 0.018 | n.d. | 2.23 ± 0.004 | 6.350 ± 0.953 | n.d. | 0.042 ± 0.005 | n.d. | 1.300 ± 0.336 | n.d. | n.d. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerbib, M.; Cazals, G.; Ducasse, M.-A.; Enjalbal, C.; Saucier, C. Evolution of Flavanol Glycosides during Red Grape Fermentation. Molecules 2018, 23, 3300. https://doi.org/10.3390/molecules23123300
Zerbib M, Cazals G, Ducasse M-A, Enjalbal C, Saucier C. Evolution of Flavanol Glycosides during Red Grape Fermentation. Molecules. 2018; 23(12):3300. https://doi.org/10.3390/molecules23123300
Chicago/Turabian StyleZerbib, Marie, Guillaume Cazals, Marie-Agnès Ducasse, Christine Enjalbal, and Cédric Saucier. 2018. "Evolution of Flavanol Glycosides during Red Grape Fermentation" Molecules 23, no. 12: 3300. https://doi.org/10.3390/molecules23123300
APA StyleZerbib, M., Cazals, G., Ducasse, M.-A., Enjalbal, C., & Saucier, C. (2018). Evolution of Flavanol Glycosides during Red Grape Fermentation. Molecules, 23(12), 3300. https://doi.org/10.3390/molecules23123300






