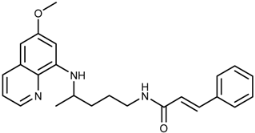
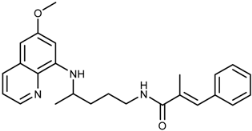
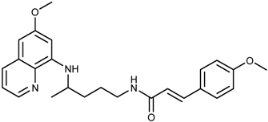
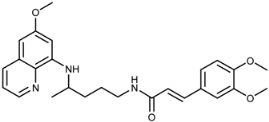
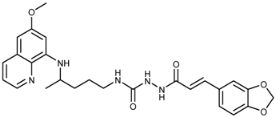
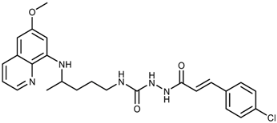
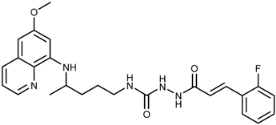
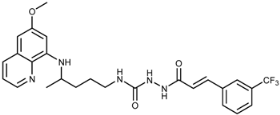
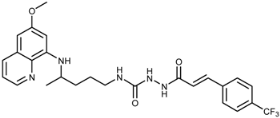
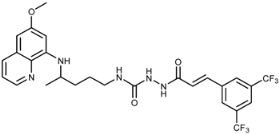
Abstract
In this paper design and synthesis of a scaffold comprising primaquine (PQ) motif and cinnamic acid derivatives (CADs) bound directly (compounds 3a–k) or via a spacer (compounds 7a–k) are reported. In the first series of compounds, PQ and various CADs were connected by amide bonds and in the second series by acylsemicarbazide functional groups built from the PQ amino group, CONHNH spacer and the carbonyl group originating from the CADs. PQ-CAD amides 3a–k were prepared by a simple one-step condensation reaction of PQ with a series of CAD chlorides (method A) or benzotriazolides 2 (method B). The synthesis of acylsemicarbazides 7a–k included activation of PQ with benzotriazole, preparation of PQ-semicarbazide 6 and its condensation with CAD chlorides 4. All synthesized PQ-CAD conjugates were evaluated for their anticancer, antiviral and antioxidative activities. Almost all compounds from series 3 were selective towards the MCF-7 cell line and active at micromolar concentrations. The o-fluoro derivative 3h showed high activity against HeLa, MCF-7 and in particular against the SW 620 cell line, while acylsemicarbazide 7f with a benzodioxole ring and 7c, 7g and especially 7j with methoxy-, chloro- or trifluoromethyl-substituents in the para position showed high selectivity and high inhibitory activity against MCF-7 cell line at micromolar (7c, 7f, 7g) and nanomolar (7j) levels. Acylsemicarbazide derivatives with trifluoromethyl group(s) 7i, 7j and 7k showed specific activity against human coronavirus (229E) at concentrations which did not alter the normal cell morphology. The same compounds exerted the most potent reducing activity in the DPPH test, together with 7d and 7g, while methoxy (compounds 7c–e), benzodioxole (7f), p-Cl (7g) and m-CF3 (7i) acylsemicarbazides and amide 3f presented the highest LP inhibition (83%–89%). The dimethoxy derivative 7d was the most potent LOX inhibitor (IC50 = 10 μΜ). The performed biological tests gave evidence of acylsemicarbazide functional group as superior binding group in PQ-CAD conjugates.
1. Introduction
The molecular hybridization approach based on the combination of the pharmacophoric moieties of different compounds was used to produce new hybrid molecules with cinnamic acid derivatives (CADs) and primaquine (PQ) motifs. Cinnamic acid (CA, trans-3-phenylacrylic acid, trans-3-phenyl-2-propenoic acid) and its derivatives are naturally occurring substances found in various plants. They are important intermediates in biosynthetic pathways of secondary metabolites, which play key roles in plant growth, development, reproduction and disease resistance [1]. Numerous research papers report various pharmacological activities of CADs [2,3]. First of all, they exhibit strong antimicrobial, antifungal and antiviral activity [4,5,6,7,8]. A review on natural and synthetic CADs with antimicrobial activity has been recently published [1]. CADs show good to moderate antitubercular activity or synergistic activity with antitubercular drugs [9,10,11,12,13]. Many CADs, especially those with phenolic hydroxyl groups, are well-known antioxidants and are supposed to have several health benefits due to their strong free radical scavenging properties [14,15,16]. Methoxy substituted cinnamates play an important role in controlling inflammatory diseases [17], while methoxy substituted octylcinnamates are efficient sunscreen agents [18]. 3-Hydroxy, 4-hydroxy and 3,4-dihydroxy CADs possess hepatoprotective [19] and hypolipidemic activity [20,21], while halogenated derivatives have CNS depressant activity [22]. Some amides derived from CA are potential antimalarial leads [23]. N-cinnamoyl chloroquine, PQ, aminoacridines and 4-aminoquinoline derivatives are reported as dual-action antimalarials and antiproliferative agents [24,25,26,27]. Anticancer potential of piplartine, a CA alkaloid/imide derivative from peppers, and various CADs have been reported in numerous papers and reviewed by Bezerra [28] and De and co-workers [29]. Finally, CA motifs are present in several approved antiallergic drugs, such as cinnarizine, cinaserin and tranilast and antiplatelet agent ozagrel [30].
On the other hand, PQ is a well-known antimalarial drug with pronounced antiproliferative activity. Numerous reports corroborate anticancer properties of antimalarial drug classes and their usefulness in adjuvant chemotherapies [31,32,33,34,35,36,37,38,39,40,41]. Several antimalarials reached a clinical stage in anticancer research [42].
In several papers, we have shown that PQ derivatives of urea and acylsemicarbazide type possess strong antiproliferative effects against a number of tumor cell lines and/or high selectivity towards the breast cell line MCF-7 [43,44,45,46,47]. The most active compounds have one or more aromatic rings attached to a PQ scaffold via nitrogen- and oxygen-rich spacers (urea, carbamate, hydroxyurea, double urea). The literature survey showed that hydroxysemicarbazides are potential antitumor agents as well [48]. In the light of the above considerations, we have designed a series of hybrid molecules with cinnamoyl residue as the aromatic component bound by amide or acylsemicarbazide functionality to PQ pharmacophore. Our new series of PQ derivatives differ from the previous ones both in the aromatic region and in the type of spacers. The present study reports the synthesis and characterization of PQ-CAD conjugates and their evaluation as potential anticancer and antiviral agents. The chemopreventive potential on carcinogenesis, inhibition of lipoxygenase as a marker of anti-inflammatory activity and antioxidative ability of PQ-CAD were evaluated as well.
2. Results and Discussion
2.1. Chemistry
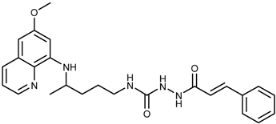
Two series of PQ-CAD conjugates 3a–k and 7a–k were prepared, with CA, α-methylcinnamic acid or methoxy, dimethoxy, trimethoxy, methylenedioxy, chloro, fluoro, trifluoromethyl and bis-trifluoromethylcinnamic acid as the CAD component. In series 3 PQ and CA Q moieties are directly bound by an amide bond, while in series 7 they are connected by the CONHNH spacer. Terminal amino group of PQ, the hydrazide spacer and CA carbonyl group together form a new acylsemicarbazide functional group. The synthetic route leading to PQ-CAD conjugates is outlined in Scheme 1.
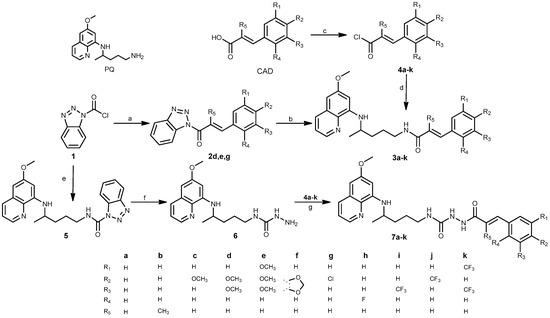
Scheme 1.
Synthetic route for preparation of compounds 3 and 7 and their precursors. Reagents and conditions: (a) CA or CAD, TEA, toluene, 0.5 h; (b) PQ, TEA, dioxane, 20 h; (c) SOCl2, toluene, DMF, 3 h; (d) PQ, TEA, CH2Cl2, 0.5–3 h; (e) PQ, TEA, toluene, 24 h; (f) NH2NH2 × H2O, Na2S2O4, dioxane, 4 days; (g) 4a–k, TEA, CH2Cl2, overnight. All reactions were performed at r.t. The reactions with PQ were run light protected.
Two methods for the preparation of amides 3a–k were applied: acid chlorides 4 (method A) and benzotriazolides 2 (method B) were used as the activated CAD intermediates. Both methods gave products in similar yields. CAD-benzotriazolides 2 were prepared following our procedure described previously: benzotriazolides of various carboxylic acids were synthesized and transformed to esters, amides or hydroxamic acids [49,50,51,52]. Benzotriazolides 2 (active amides) and PQ-benzotriazolide 5 (active urea) were prepared from the same starting compound BtcCl (1) and CAD or PQ, in the presence of triethylamine (TEA). Reaction of 5 with hydrazine gave semicarbazide 6, which reacted with chlorides 4 and afforded PQ-CAD conjugates of general formula 7. Compounds 5 and 6 are stable intermediates applied in our previous work for syntheses of numerous PQ derivatives [43,46].
All new compounds were fully characterized by the usual spectroscopic methods (IR, 1H-, 13C-NMR and MS) and elemental analyses. Spectral data are consistent with the proposed structures and are summarized in the Experimental section and in detail in the Supplementary Materials. Syntheses of compounds 3a, 3c and 3g were reported previously, but without any spectral and analytical data [26]. Compounds 2 and 4 were used in further reaction steps without purification and their structures were confirmed indirectly.
The presence of the PQ residue in compounds 3, 5, 6 and 7 was confirmed by NMR spectra: hydrogen atoms next to pyridine nitrogen occurred in 1H-NMR spectra at δ between 8.55 and 8.51, methoxy group at 3.81–3.80, CH of chiral carbon as a multiplet at 3.70–3.63, methyl group between 1.24 and 1.20 ppm, while in 13C spectra the corresponding carbon atoms appeared at 144.27–144.18, 55.18–54.91, 47.01–46.98 and 20.25–20.19 ppm, respectively. CAD benzene ring afforded additional hydrogen or carbon signals in aromatic region. Signals of methoxy groups in products 3c–e and 7c–e were located very close to the PQ methoxy group. CF3 groups present in 3i–k and 7i–k and adjacent aromatic carbon atoms split in 13C-NMR spectra and appeared as quartets at 132.28–118.69 and 131.14–128.44 ppm, respectively.
Amide NH signals in series 3 were visible in the 1H-NMR spectra at 8.24–7.95 and amine NH at 6.16–6.11 ppm (all D2O exchangeable). Compounds from series 7 showed four NH signals: the NH close to the CAD carbonyl appeared as a singlet between 9.85 and 9.62 ppm, the next NH as a singlet between 7.99 and 7.67, the PQ terminal amino group between 6.53 and 6.43 and PQ NH group next to chiral atom as doublet between 6.14 and 6.09 ppm. The presence of one (compounds 3a–k) and two carbonyl groups (compounds 7a–k) was indicated by the appearance of strong stretching vibration bands in the corresponding IR spectra between 1663 and 1651 cm−1 and signals between 168.71 and 164.07 ppm in the 13C-NMR spectra.
All PQ-CAD amides and almost all acylsemicarbazides are fully in agreement with the Lipinski rule of five and Gelovani rules for prospective small molecular drugs: MW ≤ 500, log P ≤ 5, number of H-bond donors ≤ 5, number of H-bond acceptors ≤ 10, molecular polar surface area (PSA) < 140 Å2 molar refractivity (MR) within the range of 40 and 130 cm3/mol, the number of atoms 20–70 [53]. The parameters were calculated with the Chemicalize.org program [54] and are presented in Table 1.

Table 1.
Properties of the PQ-CAD conjugates calculated with Chemicalize.org program [54]. The Lipinski and Gelovani parameters.
2.2. Biological Evaluation
Considering the individual biological and medicinal importance of PQ and CADs, we wanted to explore novel chemical entities based on PQ and CAD moieties with respect to their biological significance. The synthesized PQ-CAD conjugates were evaluated for their anticancer, antiviral, anti-inflammatory and antioxidative activities. The results are summarized in Table 2 and Table 3.

Table 2.
Growth inhibition of tumor cell lines in vitro: IC50 (μM) a.

Table 3.
DPPH-reducing ability (RA), in vitro inhibition of soybean lipoxygenase (LOX) and lipid peroxidation (LP).
2.2.1. Anticancer Activity
The newly prepared PQ-CAD derivatives were designed and prepared primarily as potential anticancer agents. Their antiproliferative activity was evaluated in vitro on five different types of human tumor cell lines: lymphoblastic leukemia (CEM), cervical carcinoma (HeLa), lung carcinoma (NCI-H460), colon carcinoma (SW 620), breast carcinoma (MCF-7) and murine lymphocytic leukemia (L1210) and compared with the standard anticancer drugs (sorafenib, cisplatin and 5-fluorouracil) and PQ. Amide derivatives 3a–k showed weak activity against L1210 and CEM, and no activity on H460 cell line. Most of the compounds of series 3 were inactive against SW 620. However, 3a and 3i showed mild and 3h very strong activity (IC50 0.3 ± 0.1 μM) against the same cell line. Activity against HeLa varied very much: derivatives 3a and 3h showed strong activity in low micromolar concentration (IC50 4.0 ± 0.9 and 2.1 ± 2.1 μM, respectively), while the other amide derivatives were either inactive (3c–e) or their activity was very weak (the rest of the compounds in series 3) (Table 2). MCF-7 was the most susceptible tumor cell line. Practically all compounds 3 were active in low micromolar concentrations. Again, the most active was the o-fluoro derivative 3h (IC50 1.1 ± 0.6 μM). Acylsemicarbazide derivatives 7a–k were more active than amide derivatives and more or less active against all the tested cell lines and, in general, very active against MCF-7. Four compounds, 7c, 7g, 7f and 7j, showed very strong activity against MCF-7 and variable activity against the other tested cell lines. It is interesting to note that three of the most active PQ-CAD derivatives bear substituent in para position (methoxy, chloro and trifluoromethyl, respectively). The most active compound was trifluoromethyl derivative 7j with IC50 in nanomolar scale (30 ± 20 nM). This derivative exerted 100-to 500-fold higher activity than the anticancer reference drugs and 900-fold higher activity than PQ.
The most interesting finding of the current research is the high sensitivity of MCF-7 tumor cells to this series of compounds. We have already noticed and discussed this phenomenon in our previous work with PQ derivatives [43,45,46]. The investigation of the underlying mechanism is in progress.
2.2.2. Antiviral Activity Assays
Compounds 3a–k and 7a–k were evaluated against a broad variety of viruses including herpes simplex virus type 1 (KOS), herpes simplex virus 2 (G), herpes simplex virus 1 TK–(KOS) ACVr, vaccinia virus, adeno virus 2 and human coronavirus (229E) in HEL cell cultures and their activities were compared with reference compounds such as brivudin, cidofovir, acyclovir, gancyclovir, zalcitabine, alovudine, Urtica dioica agglutinin (UDA) and ribavirin, respectively. Compounds of series 3 were inactive towards all tested viruses, while acylsemicarbazide derivatives 7b, 7f (EC50 = 15.0 and 12.5 μΜ, respectively), 7i, 7j and 7k (EC50 = 7.9–9.5 μΜ) showed moderate activity against human coronavirus (229E), but three or two fold lower activity than UDA (EC50 = 4.0 μΜ). These compounds did not alter normal cell morphology in confluent HEL cell cultures at 100 µM concentration (data not shown): selectivity ratio (SI) (MCC to EC50 ratio, e.g., ratio of minimum cytotoxic concentration (concentration that causes a microscopically detectable alteration of normal cell morphology) and concentration required to reduce virus-induced cytopathogenicity by 50%) was close to ten. It is worth mentioning that the three most active compounds contain trifluoromethyl group: 7i (m-CF3, EC50 = 7.9 μΜ), 7j (p-CF3, EC50 = 9.5 μΜ), 7k (two m-CF3, EC50 = 8.4 μΜ), while halogen and methoxy derivatives were inactive. Therefore, one can conclude that acylsemicarbazide functional group and the presence of CF3 motif may be responsible for enhancing antiviral activity against human coronavirus. These findings could be useful in further structural optimization.
2.2.3. Cellular Cytotoxicity Assays
Cytotoxicity measurements were based on the inhibition of HEL growth and the results were expressed as MCC. MCC values for all PQ-CAD derivatives were equal or higher than 100 μΜ, except for 7c and 7g, which was around 20 μΜ.
2.2.4. Antioxidative Activity
Antioxidant potentials of new PQ and CAD hybrids 3a–k and 7a–k were studied and compared to the well-known antioxidant agents such as nordihydroguaiaretic acid (NDGA) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox). Two different antioxidant assays were used: (i) interaction with the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical; and (ii) interaction with the water-soluble azo compound 2,2’-azobis(2-amidinopropane) dihydrochloride (AAPH), used as a source of peroxyl radicals [47].
DPPH interaction of the tested compounds was examined at 50 and 100 μΜ concentrations after 20 and 60 min. DPPH-reducing ability (RA) of the amide derivatives 3a–k was very low or missing at 50 µM. However, an increase was observed with the increase of their concentration to 100 μM. The highest activity was presented by the unsubstituted derivative 3a (53%) (Table 3). On the contrary, the acylsemicarbazide derivatives 7a–k exhibited stronger antioxidant activity in both concentrations and this ability was found to be concentration and time dependent for the majority of compounds. Trifluoromethyl derivatives 7j, 7k, 7i, and dimethoxy 7d and chloro derivative 7g showed the highest reducing activity within the dataset. Perusal of RA values (3a < 7a, 3b < 7b, etc.) supported the fact that keeping the rest structural characteristics the same, the presence of acylsemicarbazide functional group was correlated with higher antioxidant activity.
In the acylsemicarbazide series 7, the attachment of CF3 group(s) on the cinnamic ring enhanced the antioxidant ability. The presence of two vicinal methoxy groups (m, p) lead to higher activity, compared to the presence of single methoxy group (7d > 7c). On the contrary, the third methoxy group diminished the activity (7e), probably due to steric reasons (bulky radical DPPH could not approach and interact). Replacement of the phenyl ring by a benzo[d][1,3]dioxole increased activity (7f > 7a). The nature and/or position of the halogen atom on the cinnamic ring affected the antioxidant potency. Thus, p-Cl derivative 7g was found more potent than o-F 7h. For both groups of compounds no correlation between overall lipophilicity and activity was found (Table 1 and Table 3). However, hydrophilic contribution of the spacer seems to play a more significant role (log P 3a < 7a, 3b < 7b, etc.)
In our studies, AAPH was used as a free radical initiator to follow oxidative changes of linoleic acid to conjugated diene hydroperoxide. The results showed that all amide derivatives 3 significantly inhibited lipid peroxidation (LP) (32%–87%). Compound 3f with a benzodioxole ring exhibited the highest activity. Again, acylsemicarbazides 7 generally presented higher LP inhibition (50%–89%) than amides. Unsubstituted (7a), methoxy (7c–e), benzodioxole (7f), p-Cl (7g) and m-CF3 (7i) derivatives were the most potent (83%–89%). A correlation between lipophilicity and LP inhibition was not found.
LOX inhibitors have attracted attention initially as potential agents for the treatment of inflammatory and allergic diseases, certain types of cancer, and cardiovascular diseases. In order to diminish the pro-cancerous mechanisms, a key treatment strategy is to reduce the free radical load. Antioxidant activity by scavenging of reactive oxygen species is important in preventing potential damage of cellular compounds such as DNA, proteins and lipids. Moreover, it has been found that LOX inhibitors may have chemopreventive activity in lung carcinogenesis [55]. Reports published over the past three decades support a growth-regulatory role of arachidonic acid metabolites in the etiology of mammary carcinogenesis [56]. Studies have demonstrated that levels of several eicosanoids are increased in breast cancer in comparison to benign breast tumours [56]. In this context, we evaluated the newly prepared PQ-CAD conjugates for their ability to inhibit soybean lipoxygenase (LOX). From the tested compounds, all amides 3, with the exception of 3j, were inactive (10%–45% at 100 μΜ). However, acylsemicarbazides 7 exhibited significant activity in LOX inhibition assay (IC50 values 10–100 μΜ). Dimethoxy derivative 7d was the most potent inhibitor with an IC50 value of 10 μΜ (double as the concentration of the reference compound NDGA), followed by 7j and 7h. The compounds with high log P values were not active, so the idea that lipophilicity was an important physicochemical property for LOX inhibition was not supported. Our results indicated that LOX inhibition is accompanied and correlated with antilipid peroxidation and DPPH radical scavenging activity. In general, the presence of acylsemicarbazide moiety in PQ-CAD conjugates led to hybrids with better biological response.
3. Experimental Section
3.1. Chemistry
3.1.1. Materials and Methods
Melting points were measured on Stuart Melting Point (SMP3) apparatus (Barloworld Scientific, Staffordshire, UK) in open capillaries with uncorrected values. IR spectra were recorded on FTIR Perkin Elmer Paragon 500 and UV-Vis spectra on Lambda 20 double beam spectrophotometer (Perkin-Elmer, Waltham, MA, USA). All NMR (1H and 13C) spectra were recorded at 25 °C on NMR Avance 600 (Bruker, Rheinstetten, Germany) and Varian Inova 400 spectrometers (Varian, Palo-Alto, CA, USA) at 300, 400 and 600 MHz for 1H and 75, 100 and 150 MHz for 13C nuclei, respectively. Chemical shifts (δ) are reported in parts per million (ppm) using tetramethylsilane as reference in the 1H and the DMSO residual peak as reference in the 13C spectra (39.51 ppm). Coupling constants (J) are reported in Hertz (Hz). Mass spectra were recorded on HPLC-MS/MS (HPLC, Agilent Technologies 1200 Series; MS, Agilent Technologies 6410 Triple Quad, Santa Clara, CA, USA). Mass determination was realized using electron spray ionization (ESI) in positive mode. Elemental analyses were performed on a CHNS LECO analyzer (LECO Corporation, St. Joseph, MI, USA). All compounds were routinely checked by TLC with Merck silica gel 60F-254 glass plates using the following solvent systems: petrolether/ethyl acetate/methanol 30:10:5, dichloromethane/methanol 97:3 and 95:5. Spots were visualized by short-wave UV light and iodine vapor. Column chromatography was performed on silica gel 0.063–0.200 mm (Kemika, Zagreb, Croatia) and 0.040–0.063 mm (Merck, Darmstadt, Germany), with the same eluents used in TLC. All chemicals, solvents and biochemical reagents were of analytical grade and purchased from commercial sources. CA, α-methylcinnamic acid ((2-methyl-3-phenyl)acrylic acid), 4-methoxy-cinnamic acid (p-coumaric acid methyl ether), 3,4-dimethoxycinnamic acid (caffeic acid dimethyl ether), 3,4,5-trimethoxycinnamic acid, 3,4-(methylenedioxy)cinnamic acid (3-benzo[1,3]-dioxol-5-yl-acrylic acid), 4-chlorocinnamic acid, 2-fluorocinnamic acid, 4-(trifluoromethyl)cinnamic acid, 3-(trifluoromethyl)cinnamic acid and 3,5-bis(trifluoromethyl)cinnamic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA), predominantly as trans stereoisomers (≥99%). 1H-benzo[d][1,2,3] triazole (BtH), triphosgene, PQ diphosphate, TEA, hydrazine hydrate, LOX, linoleic acid sodium salt, DPPH, NDGA, AAPH and Trolox were purchased from Sigma-Aldrich as well. PQ was prepared from PQ diphosphate prior the use. All reactions with PQ were run light protected.
3.1.2. General Procedure for the Synthesis of Benzotriazolides 2d,e,g
To a solution of CA or CAD (1 equiv.) and TEA (1 equiv.) in dry toluene, solution of BtcCl (1) (1 equiv.) in dry toluene was added dropwise. The reaction mixture was stirred at room temperature for 30 min. Solvent was evaporated and the residue was dissolved in EtOAc/H2O mixture (1:1). The organic layer was extracted with water, dried over anhydrous sodium sulfate, filtered and evaporated.
(E)-1-(1H-Benzo[d][1,2,3]triazol-1-yl)-3-(3,4-dimethoxyphenyl)prop-2-en-1-one (2d): Compound 2d was synthesized according to the general procedure using 3,4-dimethoxycinnamic acid (0.208 g, 1 mmol) and BtcCl (0.181 g, 1 mmol). The crude product (0.207 g, 67%) was triturated with ether and used in further reactions without purification.
(E)-1-(1H-Benzo[d][1,2,3]triazol-1-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (2e): Compound 2e was synthesized according to the general procedure using 3,4,5-trimethoxycinnamic acid (0.155 g, 0.65 mmol) and BtcCl (0.118 g, 0.65 mmol). The crude product (0.128 g, 58%) was triturated with ether/petrolether and used in further reactions without purification.
(E)-1-(1H-Benzo[d][1,2,3]triazol-1-yl)-3-(4-chlorophenyl)prop-2-en-1-one (2g): Compound 2g was synthesized according to the general procedure using 4-chlorocinnamic acid (0.365 g, 2 mmol) and BtcCl (0.362 g, 2 mmol). The crude product (0.238 g, 42%) was triturated with ether and used in further reactions without purification.
3.1.3. General Procedure for the Synthesis of Chlorides 4a–k
A solution of CA or CAD (1 equiv.), thionyl chloride (5 equiv.) and two drops of DMF in dry toluene was stirred at room temperature for 3 h. Solvent was evaporated. The residue was dissolved in dry toluene, evaporated again and used in further reactions without purification.
3.1.4. General Procedure for the Synthesis of Amides 3a–k
Method A: A suspension of PQ diphosphate (1 equiv.) and TEA (3 equiv.) in dry dichloromethane was stirred at room temperature. After 15 min, a solution of chloride 4 (1.25 equiv.) in dry dichloromethane was added dropwise. The reaction mixture was stirred at room temperature, light protected. After 0.5–3 h, solvent was removed under reduced pressure and the residue was dissolved in ethyl acetate/5% NaOH mixture (1:1). The organic layer was extracted with 5% NaOH solution and water, dried over anhydrous sodium sulfate, filtered and evaporated. The crude product was purified by column chromatography.
Method B: NaOH solution was added to a solution of PQ diphosphate (1 equiv.) in water until pH 9-10 was reached and PQ base was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate, filtered and evaporated. PQ and TEA (1 equiv.) were dissolved in dry dioxane and added dropwise to a solution of 2 (1 equiv.) in dry dioxane. The reaction mixture was stirred at room temperature overnight and evaporated under reduced pressure. The residue was dissolved in ethyl acetate and extracted with 5% NaOH solution and water, dried over anhydrous sodium sulfate, filtered and evaporated. The crude product was purified by column chromatography.
(E)-N-(4-(6-Methoxyquinolin-8-ylamino)pentyl)-3-phenylacrylamide (3a): Compound 3a was synthesized according to the general procedure (method A) using chloride 4a (0.108 g, 0.65 mmol) and PQ diphosphate (0.228 g, 0.5 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5) and recrystallization from EtOAc/ether, pure compound 3a was obtained (0.126 g, 65%, m.p. = 72–74 °C). IR (KBr) (cm−1): 3387, 3282, 3061, 2927, 2852, 1660, 1614, 1576, 1556, 1516, 1454, 1424, 1387, 1338, 1220, 1203, 1160, 1052, 1031, 977, 902, 863, 822, 791, 766, 680, 626. 1H-NMR (δ/ppm): 8.55–8.53 (dd, 1H, J = 1.43, 4.13), 8.13 (t, 1H, J = 5.69), 8.09–8.06 (dd, 1H, J = 1.43, 8.28), 7.55 (d, 2H, J = 6.49), 7.45–7.34 (m, 5H), 6.61 (d, 1H, J = 15.82), 6.47 (s, 1H), 6.28 (s, 1H), 6.15 (d, 1H, J = 8.73), 3.81 (s, 3H), 3.66 (m, 1H), 3.21 (m, 2H), 1.64 (m, 4H), 1.22 (d, 3H, J = 6.25). 13C-NMR (δ/ppm): 164.78, 159.01, 144.64, 144.23, 138.40, 134.96, 134.80, 134.52, 129.58, 129.33, 128.89, 127.44, 122.32, 122.10, 96.12, 91.61, 54.96, 47.01, 39.02, 33.54, 26.03, 20.23. MS (ESI): m/z = 390.3 [M + H]+. Anal. Calcd. For C24H27N3O2: C, 74.31; H, 6.99; N, 10.79. Found: C, 74.49; H, 7.02; N, 10.50.
(E)-N-(4-(6-Methoxyquinolin-8-ylamino)pentyl)-2-methyl-3-phenylacrylamide (3b): Compound 3b was synthesized according to the general procedure (method A) using chloride 4b (0.117g, 0.65 mmol) and PQ diphosphate (0.228 g, 0.5 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5), oil compound 3b was obtained (0.157 g, 78%). IR (film) (cm−1): 3378, 3054, 2936, 2867, 1735, 1651, 1616, 1578, 1519, 1456, 1424, 1388, 1271, 1220, 1202, 1158, 1052, 1031, 926, 900, 822, 792, 763, 738, 703, 624. 1H-NMR (δ/ppm): 8.55–8.53 (dd, 1H, J = 1.63, 4.19), 8.09–8.03 (m, 2H), 7.45–7.28 (m, 6H), 7.18 (s, 1H), 6.48 (s, 1H), 6.28 (s, 1H), 6.16–6.14 (d, 1H, J = 8.75), 3.81 (s, 3H), 3.70–3.63 (m, 1H), 3.23–3.17 (m, 2H), 1.99 (s, 3H), 1.73–1.56 (m, 4H), 1.24–1.23 (d, 3H, J = 6.29). 13C-NMR (δ/ppm): 168.71, 159.02, 144.58, 144.27, 136.14, 134.80, 134.54, 132.68, 131.87, 129.58, 129.17, 128.35, 127.56, 122.10, 96.11, 91.58, 54.96, 47.04, 39.02, 33.43, 25.95, 20.19, 14.29. MS (ESI): m/z = 404.3 [M + H]+. Anal. Calcd. For C25H29N3O2: C, 74.41; H, 7.24; N, 10.41. Found: C, 74.20; H, 7.02; N, 10.66.
(E)-3-(4-Methoxyphenyl)-N-(4-(6-methoxyquinolin-8-ylamino)pentyl)acrylamide (3c): Compound 3c was synthesized according to the general procedure (method A) using chloride 4c (0.128 g, 0.65 mmol) and PQ diphosphate (0.228 g, 0.5 mmol). After purification by column chromatography (cyclohexane/EtOAc/MeOH = 30:10:7 and CH2Cl2:/MeOH = 95:5) and recrystallization from EtOAc/ether, pure compound 3c was obtained (0.133 g, 66%, m.p. = 78–80 °C). IR (KBr) (cm−1): 3255, 3070, 2962, 2930, 2856, 1738, 1652, 1610, 1571, 1517, 1455, 1424, 1385, 1304, 1229, 1168, 1032, 981, 823, 791, 678, 630. 1H-NMR (δ/ppm): 8.54–8.53 (dd, 1H, J = 1.5, 4.2), 8.09–8.05 (dd, 1H, J = 1.4, 8.3), 8.02–7.98 (t, 1H, J = 5.4), 7.50–7.47 (d, 2H, J = 8.69), 7.44–7.40 (m, 1H), 7.38–7.32 (d, 1H, J = 15.75), 6.98–6.95 (d, 2H, J = 8.70), 6.48–6.42 (m, 2H), 6.28 (s, 1H), 6.15–6.12 (d, 1H, J = 8.74), 3.81 (s, 3H), 3.78 (s, 3H), 3.65 (m, 1H), 3.21–3.19 (m, 2H), 1.7–1.56 (m, 4H), 1.23–1.21 (d, 3H, J = 6.27). 13C-NMR (δ/ppm): 165.05, 160.18, 158.98, 144.61, 144.18, 138.02, 134.74, 134.49, 129.53, 128.93, 127.50, 122.03, 119.85, 114.30, 96.08, 91.61, 55.18, 54.93, 47.00, 39.02, 33.54, 26.03, 20.19. MS (ESI): m/z = 420.3 [M + H]+. Anal. Calcd. For C25H29N3O3: C, 71.57; H, 6.97; N, 10.02. Found: C, 71.43; H, 7.02; N, 10.17.
(E)-3-(3,4-Dimethoxyphenyl)-N-(4-(6-methoxyquinolin-8-ylamino)pentyl)acrylamide (3d): Compound 3d was synthesized according to the general procedure using chloride 4d (0.147 g, 0.65 mmol) and PQ diphosphate (0.228 g, 0.5 mmol) (method A) or compound 2d (0.090 g, 0.29 mmol) and PQ diphosphate (0.132 g, 0.29 mmol) (method B). After purification by column chromatography (petroleum ether/EtOAc 1:2), pure compound 3d was obtained (0.157 g, 70%, method A; 0.098 g, 75%; method B, m.p. = 85–88 °C). IR (KBr) (cm−1): 3355, 3256, 3072, 2964, 2932, 1738, 1653, 1613, 1555, 1518, 1458, 1425, 1388, 1339, 1298, 1259, 1201, 1166, 1138, 1022, 980, 851, 819, 793, 765, 681, 627, 597. 1H-NMR (δ/ppm): 8.54–8.53 (dd, 1H, J = 1.62, 4.19), 8.09–8.05 (dd, 1H J = 1.56, 8.29), 8.00–7.96 (t, 1H, J = 5.52), 7.44–7.40 (m, 1H, J = 4.20, 8.25), 7.36–7.31 (d, 1H, J = 15.73), 7.14–7.08 (m, 2H), 6.98–6.96 (d, 1H, J = 8.32), 6.51–6.45 (m, 2H), 6.28 (s, 1H), 6.15–6.12 (d, 1H, J = 8.76), 3.81–3.78 (m, 9H), 3.65 (m, 1H), 3.23–3.19 (m, 2H), 1.70–1.56 (m, 4H), 1.23–1.21 (d, 2H, J = 6.29). 13C-NMR (δ/ppm): 165.05, 158.97, 149.98, 148.85, 144.61, 144.18, 138.36, 134.73, 134.49, 129.53, 127.75, 122.03, 121.20, 120.05, 111.74, 109.96, 96.08, 91.61, 55.49, 55.37, 54.92, 47.00, 39.02, 33.55, 26.02, 20.20. MS (ESI): m/z = 450.5 [M + H]+. Anal. Calcd. For C26H31N3O4: C, 69.47; H, 6.95; N, 9.35. Found: C, 69.80; H, 7.01; N, 9.57.
(E)-N-(4-(6-Methoxyquinolin-8-ylamino)pentyl)-3-(3,4,5-trimethoxyphenyl)acrylamide (3e): Compound 3e was synthesized according to the general procedure using chloride 4e (0.169 g, 0.65 mmol) and PQ diphosphate (0.228 g, 0.5 mmol) (method A) or compound 2e (0.109 g, 0.32 mmol) and PQ diphosphate (0.146 g, 0.32 mmol) (method B). After purification by column chromatography (petroleum ether/EtOAc/MeOH 30:10:5) and crystallization from EtOAc, pure compound 3e was obtained (0.125 g, 52%, method A; 0.106 g, 69%, method B, m.p. = 120–123.5 °C). IR (KBr) (cm−1): 3367, 3285, 3084, 2962, 1657, 1617, 1583, 1520, 1456, 1421, 1388, 1328, 1277, 1241, 1221, 1200, 1164, 1125, 1052, 1014, 978, 824, 792, 682, 622, 604. 1H-NMR (δ/ppm): 8.54–8.53 (d, 1H, J = 2.85), 8.09–8.03 (m, 2H), 7.44–7.40 (m, 1H), 7.37–7.32 (d, 1H, J = 15.68), 6.88 (s, 2H), 6.54–6.52 (d, 1H, J = 15.69), 6.47 (s, 1H), 6.28 (s, 1H), 6.15–6.12 (d, 1H, J = 8.69), 3.81 (s, 9H), 3.68 (s, 4H), 3.21–3.19 (m, 2H), 1.69–1.65 (m, 4H), 1.23–1.22 (d, 3H, J = 6.15). 13C-NMR (δ/ppm): 164.83, 158.97, 153.00, 144.62, 144.18, 138.57, 138.46, 134.74, 134.49, 130.55, 129.53, 122.04, 121.66, 104.87, 96.08, 91.61, 60.02, 55.82, 54.92, 46.99, 39.02, 33.56, 25.98, 20.20. MS (ESI): m/z = 480.3 [M + H]+. Anal. Calcd. For C27H33N3O5: C, 67.62; H, 6.94; N, 8.76. Found: C, 67.99; H, 7.06; N, 8.99.
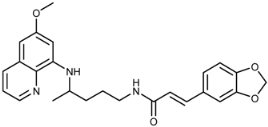
(E)-3-(Benzo[d][1,3]dioxo-5-yl)-N-(4-(6-methoxyquinolin-8-ylamino)pentyl)acrylamide (3f): Compound 3f was synthesized according to the general procedure (method A) using chloride 4f (0.137 g, 0.65 mmol) and PQ diphosphate (0.228 g, 0.5 mmol). After purification by column chromatography (CH2Cl2/MeOH = 97:3) and recrystallization from EtOAc/ether, pure compound 3f was obtained (0.124 g, 57%, m.p. = 77–79.5 °C). IR (KBr) (cm−1): 3350, 3253, 3069, 2961, 2934, 1738, 1655, 1620, 1576, 1557, 1520, 1491, 1454, 1425, 1387, 1356, 1334, 1281, 1247, 1200, 1167, 1124, 1096, 1041, 977, 927, 855, 819, 792, 752, 681, 624, 593. 1H-NMR (δ/ppm): 8.53-8.51 (dd, 1H, J = 1.61, 4.18), 8.07–8.04 (dd, 1H, J = 1.57, 8.29), 7.99–7.95 (t, 1H, J = 5.52), 7.43–7.39 (m, 1H), 7.33–7.28 (d, 1H), 7.11 (s, 1H), 7.05–7.02 (dd, 1H, J = 1.46, 8.11), 6.93–6.91 (d, 1H, J = 8.00), 6.46–6.40 (m, 2H), 6.27 (s, 1H), 6.14–6.11 (d, 1H, J = 8.77), 6.04 (s, 2H), 3.80 (s, 3H), 3.64 (m, 1H), 3.21–3.15 (m, 2H), 1.69–1.51 (m, 4H), 1.22–1.20 (d, 2H, J = 6.29). 13C-NMR (δ/ ppm): 164.93, 158.97, 148.29, 147.85, 144.61, 144.18, 138.13, 134.73, 134.49, 129.53, 129.33, 123.00, 122.05, 120.42, 108.49, 106.11, 101.33, 96.08, 91.61, 54.93, 46.99, 39.02, 33.53, 26.01, 20.19. MS (ESI): m/z = 434.2 [M + H]+. Anal. Calcd. For C25H27N3O4: C, 69.27; H, 6.28; N, 9.69. Found: C, 69.40; H, 6.69; N, 9.22.
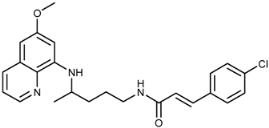
(E)-3-(4-Chlorophenyl)-N-(4-(6-methoxyquinolin-8-ylamino)pentyl)acrylamide (3g): Compound 3g was synthesized according to the general procedure using chloride 4g (0.131 g, 0.65 mmol) and PQ diphosphate (0.228 g, 0.5 mmol) (method A) or 2g (0.113 g, 0.4 mmol) and PQ diphosphate (0.182 g, 0.4 mmol) (method B). After purification by column chromatography (CH2Cl2/MeOH = 97:3), pure compound 3g was obtained (0.167 g, 79%, method A; 0.056 g, 33%, method B, m.p. = 85–87 °C). IR (KBr) (cm−1): 3358, 3275, 3078, 2958, 2327, 2857, 1740, 1655, 1615, 1558, 1520, 1492, 1457, 1426, 1388, 1343, 1227, 1202, 1168, 1097, 1052, 1013, 982, 903, 823, 791, 736, 709, 677, 628. 1H-NMR (δ/ppm): 8.54–8.53 (dd, 1H, J = 1.60, 4.19), 8.13–8.05 (m, 2H), 7.58–7.56 (d, 2H, J = 8.54), 7.47–7.37 (m, 4H), 7.63–7.58 (d, 1H, J = 15.82), 7.47 (s, 1H), 6.28 (s, 1H), 6.15–6.12 (d, 1H, J = 8.79), 3.81 (s, 3H), 3.65 (m, 1H), 3.22–3.20 (m, 2H), 1.72–1.53 (m, 4H), 1.23–1.21 (d, 3H, J = 6.28). 13C-NMR (δ/ ppm): 164.56, 158.97, 144.60, 144.18, 136.97, 134.73, 134.49, 133.91, 133.68, 129.53, 129.08), 128.85, 123.14, 122.03, 96.08, 91.61, 54.92, 46.98, 39.02, 33.51, 26.95, 20.19. MS (ESI): m/z = 424.2 [M + H]+. Anal. Calcd. For C24H26ClN3O2: C, 68.00; H, 6.18; N, 9.91. Found: C, 68.44; H, 6.35; N, 10.05.
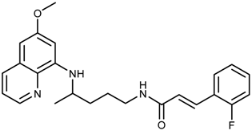
(E)-3-(2-Fluorophenyl)-N-(4-(6-methoxyquinolin-8-ylamino)pentyl)acrylamide (3h): Compound 3h was synthesized according to the general procedure (method A) using chloride 4h (0.120 g, 0.65 mmol) and PQ diphosphate (0.228 g, 0.5 mmol). After purification by column chromatography (CH2Cl2/MeOH = 97:3) and recrystallization from EtOAc/ether, pure compound 3h was obtained (0.120 g, 59%, m.p. = 65–67.5 °C). IR (KBr) (cm−1): 3357, 3271, 3078, 2960, 2927, 2857, 1739, 1655, 1616, 1578, 1557, 1520, 1487, 1458, 1426, 1388, 1343, 1283, 1226, 1201, 1167, 1093, 1052, 1032, 985, 903, 875, 822, 792, 758, 714, 678, 625. 1H-NMR (δ/ppm): 8.55–8.53 (dd, 1H, J = 1.60, 4.19), 8.23–8.19 (t, 1H, J = 5.50), 8.09–8.05 (dd, 1H, J = 1.55, 8.28), 7.66–7.61 (t, 1H, J = 6.49), 7.50–7.39 (m, 3H), 7.30–7.23 (m, 2H), 6.73–6.68 (d, 1H, J = 15.94), 6.47 (s, 1H), 6.28 (s, 1H), 6.15–6.13 (d, 1H, J = 8.79), 3.81 (s, 3H), 3.65 (m, 1H), 3.25–3.21 (m, 2H), 1.73–1.53 (m, 4H), 1.24–1.21 (d, 3H, J = 6.28). 13C-NMR (δ/ppm): 164.48, 162.02–158.71 (d, J = 250.43), 158.97, 144.61, 144.18, 134.73, 134.49, 131.14–131.02 (d, J = 8.58), 130.72, 129.53, 129.03–128.99 (d, J = 3.26), 125.17–125.09 (d, J = 6.18), 124.97, 122.61–122.41 (d, J = 12.66), 122.03, 116.12–115.83 (d, J = 23.47), 96.08, 91.61, 54.91, 46.98, 39.02, 33.51, 25.91, 20.19. MS (ESI): m/z = 408.1 [M + H]+. Anal. Calcd. For C24H26FN3O2: C, 70.74; H, 6.43; N, 10.31. Found: C, 70.60; H, 5.99; N, 10.39.
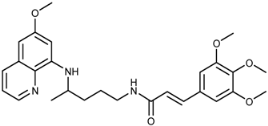
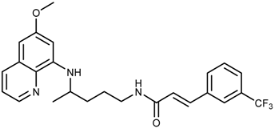
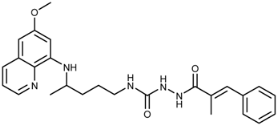
(E)-N-(4-(6-Methoxyquinolin-8-ylamino)pentyl)-3-(3-trifluoromethyl)phenyl)acrylamide (3i): Compound 3i was synthesized according to the general procedure (method A) using chloride 4i (0.152 g, 0.65 mmol) and PQ diphosphate (0.228 g, 0.5 mmol). After purification by column chromatography (CH2Cl2/MeOH = 97:3) and recrystallization from EtOAc/petroleum ether, pure compound 3i was obtained (0.162 g, 71%, m.p. = 48.5–49.5 °C). IR (KBr) (cm−1): 3262, 3075, 2964, 2861, 1661, 1618, 1561, 1520, 1454, 1387, 1335, 1221, 1166, 1126, 975, 803, 864, 820, 793, 735, 688, 625, 584, 513. 1H-NMR (δ/ppm): 8.55–8.53 (dd, 1H, J = 1.46, 4.10), 8.15–8.11 (t, 1H, J = 5.51), 8.08–8.05 (dd, 1H, J = 1.56, 8.27), 7.89–7.84 (m, 2H), 7.73–7.62 (m, 2H), 7.52–7.47 (d, 1H, J = 15.84), 7.44–7.40 (m, 1H), 6.77–6.72 (d, 1H, J = 15.85), 6.47 (s, 1H), 6.29 (s, 1H), 6.16–6.13 (d, 1H, J = 8.76), 3.81 (s, 3H), 3.66 (m, 1H), 3.23–3.21 (m, 2H), 1.72–1.57 (m, 4H), 1.24–1.22 (d, 3H, J = 6.25). 13C-NMR (δ/ppm): 164.34, 158.97, 144.61, 144.18, 136.62, 136.17, 134.73, 134.50, 132.28–122.55 (q, J = 244.60), 131.20, 130.28–128.83 (q, J = 31.73), 129.96, 129.44, 125.60–125.46 (q, J = 3.70), 124.48, 123.75–123.60 (q, J = 3.71), 122.03, 96.09, 91.60, 54.91, 46.98, 39.02, 33.50, 25.91, 20.20. MS (ESI): m/z = 458.3 [M + H]+. Anal. Calcd. For C25H26F3N3O2: C, 65.63; H, 5.73; N, 9.18. Found: C, 65.24; H, 6.00; N, 9.43.
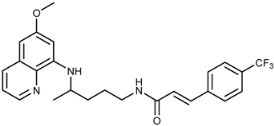
(E)-N-(4-(6-Methoxyquinolin-8-ylamino)pentyl)-3-(4-(trifluoromethyl)phenyl)acrylamide (3j): Compound 3j was synthesized according to the general procedure (method A) using chloride 4j (0.152 g, 0.65 mmol) and PQ diphosphate (0.228 g, 0.5 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5), pure compound 3j was obtained (0.167 g, 73%, m.p. = 82–84 °C). IR (KBr) (cm−1): 3359, 3262, 3075, 2963, 2931, 2856, 1738, 1655, 1615, 1577, 1560, 1519, 1456, 1424, 1386, 1326, 1227, 1203, 1163, 1130, 1110, 1069, 1050, 1015, 979, 957, 903, 880, 829, 792, 719, 681, 623, 596. 1H-NMR (δ/ppm): 8.55–8.53 (dd, 1H, J = 1.61, 4.19), 8.24–8.21 (t, 1H, J = 5.56), 8.09–8.06 (dd, 1H, J = 1.57, 8.30), 7.76 (s, 4H), 7.50–7.45 (d, 1H, J = 15.87), 7.45–7.40 (m, 1H), 6.76–6.71 (d, 1H, J = 15.84), 6.47 (s, 1H), 6.29 (s, 1H), 6.16–6.13 (d, 1H, J = 8.80), 3.81 (s, 3H), 3.66 (m, 1H), 3.25–3.21 (m, 2H), 1.73–1.53 (m, 4H), 1.24–1.21 (d, 3H, J = 6.28). 13C-NMR (δ/ppm): 164.31, 159.00, 144.63, 144.23, 139.07, 136.74, 134.79, 134.52, 129.57, 129.71–128.44 (q, J = 31.56), 129.51–118.69 (q, J = 272.14), 128.07, 125.82–125.67 (q, J = 3.80), 125.12, 122.09, 96.12, 91.60, 54.95, 46.99, 39.02, 33.52, 23.96, 20.23. MS (ESI): m/z = 458.2 [M + H]+. Anal. Calcd. For C25H26F3N3O2: C, 65.63; H, 5.73; N, 9.18. Found: C, 65.98; H, 6.01; N, 9.44.
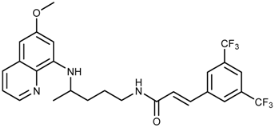
(E)-3-(3,5-Bis(trifluoromethyl)phenyl)-N-[4-(6-methoxyquinolin-8-ylamino)pentyl]acrylamide (3k): Compound 3k was synthesized according to the general procedure (method A) using chloride 4k (0.097 g, 0.32 mmol) and PQ diphosphate (0.114 g, 0.25 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5) and crystallization from ether, pure compound 3k was obtained (0.097 g, 74%, m.p. = 148–150 °C). IR (KBr) (cm−1): 3395, 3286, 3095, 2964, 2934, 2861, 1663, 1623, 1576, 1521, 1456, 1423, 1334, 1341, 1278, 1224, 1174, 1136, 1052, 1031, 978, 940, 899, 868, 845, 822, 792, 683, 624. 1H-NMR (δ/ppm): 8.54 (s, 1H), 8.26 (s, 2H), 8.18 (t, 1H, J = 5.21), 8.08–8.07 (m, 2H), 7.60–7.58 (d, 1H, J = 15.82), 7.44–7.42 (m, 1H), 6.91–6.88 (d, 1H, J = 15.90), 6.47 (s, 1H), 6.28 (s, 1H), 6.16–6.14 (d, 1H, J = 8.54), 3.80 (s, 3H), 3.66 (m, 1H), 3.23 (m, 2H), 1.70–1.58 (m, 4H), 1.23–1.22 (d, 3H, J = 5.99). 13C-NMR (δ/ppm): 164.07, 159.00, 144.64, 144.24, 137.97, 135.16, 134.81, 134.53, 131.14–130.48 (q, J = 33.83), 129.58, 127.84, 126.61, 125.94–120.54 (q, J = 272.30), 122.23, 122.11, 96.14, 91.59, 54.95, 46.99, 39.02, 33.51, 25.92, 20.25. MS (ESI): m/z = 526.4 [M + H]+. Anal. Calcd. For C26H25F6N3O2: C, 59.43; H, 4.80; N, 8.00. Found: C, 59.67; H, 5.02; N, 8.22.
3.1.5. Synthesis of N-(4-((6-Methoxyquinolin-8-yl)amino)pentyl)-1H-benzo[d][1,2,3]triazole-1-carboxamide (5)
Compound 5 was prepared according to our procedures published earlier [43].
3.1.6. Synthesis of N-(4-((6-Methoxyquinolin-8-yl)amino)pentyl)hydrazinecarboxamide (6)
The title compound was prepared following previously published procedure [46].
3.1.7. General Procedure for the Synthesis of Acylsemicarbazides 7a–k
To a solution of chloride 4a–k (1.1 equiv.) in dry dichloromethane, a solution of semicarbazide 6 (1 equiv.) and TEA (1 equiv.) in dry dichloromethane was added dropwise. The reaction mixture was stirred overnight at room temperature, light protected. The solvent was removed under reduced pressure and the residue was dissolved in ethyl acetate/5% NaOH mixture (1:1). The organic layer was extracted with 5% NaOH solution and water, dried over anhydrous sodium sulfate, filtered and evaporated. The crude product was purified by column chromatography.
(E)-1-Cinnamoyl-4-(4-(6-methoxyquinolin-8-ylamino)pentyl)semicarbazide (7a): Compound 7a was synthesized according to the general procedure using chloride 4a (0.183 g, 1.1 mmol) and compound 6 (0.317 g, 1 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5) and crystallization from ether/petroleum ether, pure compound 7a was obtained (0.251 g, 56%, m.p. = 188–190 °C, decomp.). IR (KBr) (cm−1): 3302, 3236, 3038, 2936, 1696, 1628, 1578, 1596, 1458, 1388, 1424, 1356, 1222, 1156, 1054, 978, 862, 822, 790, 762, 726, 678, 658, 632, 556, 488. 1H-NMR (δ/ppm): 9.75 (s, 1H), 8.54–8.53 (dd, 1H, J = 1.41), 8.09–8.06 (dd, 1H, J = 1.30, 8.25), 7.85 (s, 1H), 7.58–7.36 (m, 7H), 6.65–6.60 (d, 1H, J = 15.90), 6.48–6.44 (m, 2H), 6.28 (s, 1H), 6.13–6.10 (d, 1H, J = 8.62), 3.82 (s, 3H), 3.64 (m, 1H), 3.06–3.04 (m, 2H), 1.63–1.47 (m, 4H), 1.22–1.20 (d, 3H, J = 6.24). 13C-NMR (δ/ppm): 164.65, 158.98, 157.92, 144.61, 144.20, 139.46, 134.74, 134.67, 134.49, 129.60, 129.53, 128.92, 127.50, 122.04, 119.94, 96.07, 91.61, 54.95, 47.01, 39.02, 33.41, 26.65, 20.18. MS (ESI): m/z = 448.3 [M + H]+. Anal. Calcd. For C25H29N5O3: C, 67.09; H, 6.53; N, 15.65. Found: C, 67.37; H, 6.22; N, 15.66.
(E)-4-(4-(6-Methoxyquinolin-8-ylamino)pentyl)-1-(2-methyl-3-phenylacryloyl)semicarbazide (7b): Compound 7b was synthesized according to the general procedure using chloride 4b (0.197 g, 1.1 mmol) and compound 6 (0.317 g, 1 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5), oily product 7b was obtained, which crystallized after a long storage at low temperature (0.245 g, 53%, m.p. = 65.5 °C, decomp.). IR (KBr) (cm−1): 3265, 2961, 2935, 1652, 1617, 1576, 1520, 1454, 1423, 1387, 1336, 1258, 1239, 1220, 1203, 1158, 1051, 1031, 1004, 928, 910, 822, 791, 762, 709, 695, 625, 589, 515. 1H-NMR (δ/ppm): 9.67 (s, 1H, 2′′), 8.54–8.53 (dd, 1H, J = 1.57, 4.15), 8.08–8.07 (dd, 1H, J = 1.49, 8.28), 7.67 (s, 1H), 7.43–7.30 (m, 7H), 6.48–6.45 (m, 2H), 6.28 (s, 1H), 6.13–6.11 (d, 1H, J = 8.75), 4.05–3.82 (s, 3H), 3.65–3.63 (m, 1H), 3.08–3.02 (m, 2H), 2.02 (s, 3H), 1.66–1.47 (m, 4H), 1.22–1.21 (d, 3H, J = 6.29). 13C-NMR (δ/ppm): 168.89, 159.00, 158.36, 144.63, 144.24, 135.83, 134.78, 134.51, 133.23, 130.74, 129.56, 129.22, 128.42, 127.80, 122.08, 96.10, 91.61, 54.97, 47.02, 39.02, 33.43, 26.73, 20.21, 14.15. MS (ESI): m/z = 462.3 [M + H]+. Anal. Calcd. For C26H31N5O3: C, 67.66; H, 6.77; N, 15.17. Found: C, 67.51; H, 6.55; N, 15.00.
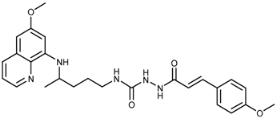
(E)-1-(3-(4-Methoxyphenyl)acryloyl)-4-(4-(6-methoxyquinolin-8-ylamino)pentyl)semicarbazide (7c): Compound 7c was synthesized according to the general procedure using chloride 4c (0.177 g, 0.9 mmol) and compound 6 (0.254 g, 0.8 mmol). After two purifications by column chromatography (first eluent: petroleum ether/EtOAc/MeOH = 30:10:5, second eluent CH2Cl2/MeOH = 95:5), pure compound 7c was obtained (0.229 g, 60%, m.p. = 89.5 °C, decomp.). IR (KBr) (cm−1): 3248, 2934, 1654, 1604, 1575, 1517, 1456, 1424, 1386, 1251, 1167, 1029, 980, 824, 791, 628, 521. 1H-NMR (δ/ppm): 9.64 (s, 1H), 8.54–8.52 (dd, 1H, J = 1.45, 4.10), 8.08–8.06 (d, 1H, J = 7.07), 7.80 (s, 1H), 7.53–7.42 (m, 4H), 6.99–6.97 (d, 2H, J = 8.66), 6.49–6.45 (m, 3H), 6.27 (s, 1H), 6.10 (s, 1H), 3.82 (s, 3H), 3.78 (s, 3H), 3.62 (m, 1H), 3.04 (m, 2H), 1.63–1.46 (m, 4H), 1.21–1.19 (d, 3H, J = 6.23). 13C-NMR (δ/ppm): 165.24, 160.56, 159.06, 158.17, 144.69, 144.35, 139.41, 134.90, 134.56, 129.65 (14), 129.28 (4′, 8′), 127.30 (3′), 122.19 (12), 117.37 (1′), 114.49 (5′, 7′), 96.19 (17), 91.71, 55.34, 55.07, 47.11, 39.02, 33.49, 26.77, 20.29. MS (ESI): m/z = 478.3 [M + H]+. Anal. Calcd. For C26H31N5O4: C, 65.39; H, 6.54; N, 14.66. Found: C, 65.51; H, 6.60; N, 14.48.
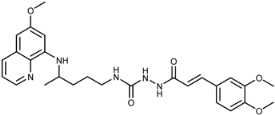
(E)-1-(3-(3,4-Dimethoxyphenyl)acryloyl)-4-(4-(6-methoxyquinolin-8-ylamino)pentyl)semicarbazide (7d): Compound 7d was synthesized according to the general procedure using chloride 4d (0.227 g, 1 mmol) and compound 6 (0.286 g, 0.9 mmol). After trituration with ethyl acetate, dichloromethane, hot acetone, ether and hot ethanol, pure compound 7d was obtained (0.260 g, 57%, m.p. = 200.5–201.5 °C, decomp.). IR (KBr) (cm−1): 3375, 3213, 3084, 3003, 2937, 1668, 1628, 1596, 1559, 1515, 1456, 1421, 1387, 1356, 1293, 1262, 1235, 1202, 1171, 1138, 1052, 1022, 977, 939, 856, 816, 788, 767, 710, 679, 622, 594, 560, 460. 1H-NMR (δ/ppm): 9.62–9.61 (d, 1H, J = 1.73), 8.54–8.52 (dd, 1H, J = 1.39, 4.06), 8.08–8.06 (dd, 1H, J = 1.29, 8.29), 7.81 (s, 1H), 7.44–7.40 (m, 2H), 7.15–7.12 (m, 2H), 7.00–6.98 (d, 1H, J = 8.28), 6.53–6.46 (m, 3H), 6.26 (s, 1H), 6.11–6.09 (d, 1H, J = 8.73), 3.81–3.78 (m, 9H), 3.62 (m, 1H), 3.04–3.02 (m, 2H), 1.54–1.51 (m, 4H), 1.21–1.19 (d, 3H, J = 6.25). 13C-NMR (δ/ppm): 165.27, 159.06, 158.19, 150.32, 148.93, 144.69, 144.36, 139.74, 134.91, 134.57, 129.66, 127.55, 122.21, 121.48, 117.64, 111.81, 110.20, 96.19, 91.69, 55.62, 55.52, 55.08, 47.11, 39.02, 33.50, 26.80, 20.30. MS (ESI): m/z = 508.3 [M + H]+. Anal. Calcd. For C27H33N5O5: C, 63.89; H, 6.55; N, 13.80. Found: C, 64.03; H, 6.68; N, 13.50.
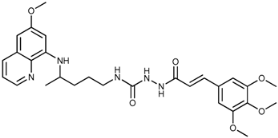
(E)-4-(4-(6-Methoxyquinolin-8-ylamino)pentyl)-1-(3-(3,4,5-trimethoxyphenyl)acryloyl)semicarbazide (7e): Compound 7e was synthesized according to the general procedure using chloride 4e (0.257 g, 1 mmol) and compound 6 (0.286 g, 0.9 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5) and crystallization from ether/petroleum ether, pure compound 7e was isolated (0.300 g, 62%, m.p. = 90.5 °C, decomp.). IR (KBr) (cm−1): 3250, 2937, 1654, 1618, 1582, 1508, 1454, 1420, 1388, 1326, 1266, 1240, 1221, 1203, 1155, 1126, 1051, 1031, 1003, 976, 822, 791, 677, 624, 585, 526, 512, 464. 1H-NMR (δ/ppm): 9.64–9.63 (d, 1H, J = 1.91), 8.53–8.52 (dd, 1H, J = 1.51, 4.16), 8.07–8.05 (dd, 1H, J = 1.41, 8.28), 7.83 (s, 1H), 7.45–7.40 (m, 2H), 6.90 (s, 2H), 6.59–6.55 (d, 1H, J = 15.80), 6.46–6.43 (m, 2H), 6.26 (s, 1H), 6.11–6.09 (d, 1H, J = 8.78), 3.81 (s, 3H), 3.80 (s, 6H), 3.68 (s, 3H), 3.63 (m, 1H), 3.04–3.02 (m, 2H), 1.61–1.51 (m, 4H), 1.21–1.19 (d, 3H, J = 6.25). 13C-NMR (δ/ppm): 164.92, 159.05, 158.05, 153.14, 144.69, 144.33, 139.82, 138.89, 134.88, 134.56, 130.38, 129.64, 122.17, 119.26, 105.09, 96.17, 91.69, 60.16, 55.96, 55.05, 47.10, 39.02, 33.49, 26.76, 20.28. MS (ESI): m/z = 538.3 [M + H]+. Anal. Calcd. For C28H35N5O6: C, 62.55; H, 6.56; N, 13.03. Found: C, 62.79; H, 6.86; N, 13.29.
(E)-1-(3-(Benzo[d][1,3]dioxo-5-yl)acryloyl)-4-(4-(6-methoxyquinolin-8-ylamino)pentyl)semicarbazide (7f): Compound 7f was synthesized according to the general procedure using chloride 4f (0.211 g, 1 mmol) and compound 6 (0.286 g, 0.9 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5) and crystallization from ether/petroleum ether, pure compound 7f was obtained (0.217 g, 49%, m.p. = 75.5 °C, decomp.). IR (KBr) (cm−1): 3250, 2335, 2361, 1654, 1618, 1577, 1560, 1521, 1490, 1448, 1388, 1252, 1202, 1158, 1100, 1037, 976, 929, 819, 791, 670, 625, 592, 518. 1H-NMR (δ/ppm): 9.64 (s, 1H), 8.53 (d, 1H, J = 2.84), 8.08–8.06 (d, 1H, J = 8.05), 7.82 (s, 1H), 7.43–7.39 (m, 2H), 7.15 (s, 1H), 7.09–7.07 (d, 1H, J = 8.05), 6.96–6.94 (d, 1H, J = 7.96), 6.48–6.44 (m, 3H), 6.26 (s, 1H), 6.11–6.09 (d, 1H, J = 7.59), 6.06 (s, 3H), 3.82 (s, 3H), 3.62 (m, 1H), 3.04 (m, 2H), 1.60–1.46 (m, 4H), 1.12–1.19 (d, 3H, J = 6.13). 13C-NMR (δ/ppm): 165.45, 159.43, 158.49, 149.08, 148.38, 145.05, 144.69, 139.84, 135.25, 134.93, 130.01, 129.50, 123.83, 122.54, 118.33, 109.05, 106.66, 101.92, 96.55, 92.06, 55.42, 47.47, 39.02, 33.86, 27.14, 20.65. MS (ESI): m/z = 492.3 [M + H]+. Anal. Calcd. For C26H29N5O5: C, 63.53; H, 5.95; N, 14.25. Found: C, 63.73; H, 5.84; N, 14.00.
(E)-1-(3-(4-Chlorophenyl)acryloyl)-4-(4-(6-methoxyquinolin-8-ylamino)pentyl)semicarbazide (7g): Compound 7g was synthesized according to the general procedure using chloride 4g (0.201 g, 1 mmol) and compound 6 (0.286 g, 0.9 mmol). After purification by column chromatography (petroleum ether/EtOAc/MeOH = 30:10:5) and trituration with ether, compound 7g was obtained (0.256 g, 59%, m.p. = 185–187.5 °C). IR (KBr) (cm−1): 3337, 3230, 3037, 2966, 2937, 2362, 2343, 1697, 1661, 1625, 1592, 1521, 1490, 1458, 1425, 1408, 1391, 1354, 1290, 1266, 1239, 1221, 1199, 1161, 1090, 1052, 1010, 982, 945, 899, 866, 819, 790, 726, 676, 628, 495. 1H-NMR (δ/ppm): 9.79 (s, 1H), 8.53–8.52 (dd, 1H, J = 1.06, 4.11), 8.08–8.05 (dd, 1H, J = 1.30, 8.25), 7.88 (s, 1H), 7.61–7.59 (d, 2H, J = 8.47), 7.50–7.45 (m, 3H), 7.43–7.40 (m, 1H), 6.64–6.60 (d, 1H, J = 15.89), 6.51–6.48 (t, 1H, J = 5.44), 6.46 (s, 1H), 6.25 (s, 1H), 6.11–6.09 (d, 1H, J = 8.71), 3.81 (s, 3H), 3.62 (m, 1H), 3.04 (m, 2H), 1.61–1.51 (m, 4H), 1.20–1.19 (d, 3H, J = 6.25). 13C-NMR (δ/ppm): 164.63, 159.05, 158.03, 144.68, 144.33, 138.28, 134.89, 134.56, 134.18, 133.68, 129.64, 129.35, 129.09, 122.19, 120.75, 96.17, 91.66, 55.06, 47.08, 39.02, 33.47, 26.77, 20.28. MS (ESI): m/z = 482.2 [M + H]+. Anal. Calcd. For C25H28ClN5O3: C, 62.30; H, 5.86; N, 14.53. Found: C, 62.69; H, 6.01; N, 14.30.
(E)-1-(3-(2-Fluorophenyl)acryloyl)-4-(4-(6-methoxyquinolin-8-ylamino)pentyl)semicarbazide (7h): Compound 7h was synthesized according to the general procedure using chloride 4h (0.185 g, 1 mmol) and compound 6 (0.286 g, 0.9 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5) and trituration with ether, pure compound 7h was obtained (0.372 g, 89%, m.p. = 91–93 °C, decomp.). IR (KBr) (cm−1): 3250, 2964, 1654, 1618, 1578, 1520, 1457, 1388, 1221, 1159, 1052, 982, 822, 791, 757. 1H-NMR (δ/ppm): 9.85 (s, 1H), 8.54–8.53 (dd, 1H, J = 1.50, 4.10), 8.08-8.06 (dd, 1H, J = 1.42, 8.22), 7.87 (s, 1H), 7.67–7.65 (t, 1H, J = 7.22), 7.57–7.55 (d, 1H, J = 16.02), 7.45–7.41 (m, 2H), 7.30–7.26 (m, 2H), 6.76–6.73 (d, 1H, J = 16.03), 6.48 (s, 2H), 6.28 (s, 1H), 6.12–6.11 (d, 1H, J = 8.70), 3.83 (s, 3H), 3.64 (m, 1H), 3.07 (m, 2H), 1.65–1.49 (m, 4H), 1.22–1.21 (d, 3H, J = 6.28). 13C-NMR (δ/ppm): 164.43, 161.30–159.64 (d, J = 250.54), 158.99, 157.86, 144.62, 144.21), 134.74, 134.50, 131.90, 131.47–131.41 (d, J = 8.67), 129.54, 129.22–129.20 (d, J = 2.69), 124.98, 122.90–122.86 (d, J = 6.35), 122.35–122.28 (d, J = 11.54), 122.04, 116.13–115.99 (d, J = 21.68), 96.09, 91.04, 54.95, 47.03, 39.02, 33.43, 26.64, 20.19. MS (ESI): m/z = 466.1 [M + H]+. Anal. Calcd. For C25H28FN5O3: C, 64.50; H, 6.06; N, 15.04. Found: C, 64.69; H, 6.17; N, 15.33.
(E)-4-(4-(6-Methoxyquinolin-8-ylamino)pentyl)-1-(3-(3-(trifluoromethyl)phenyl)acryloyl)semicarbazide (7i): Compound 7i was synthesized according to the general procedure using chloride 4i (0.235 g, 1 mmol) and compound 6 (0.286 g, 0.9 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5) and crystallization from dichloromethane, pure compound 7i was obtained (0.343 g, 74%, m.p. = 86–88 °C, decomp. IR (KBr) (cm−1): 3254, 2964, 1659, 1618, 1577, 1521, 1456, 1424, 1388, 1334, 1222, 1199, 1166, 1126, 1075, 976, 900, 822, 792, 694, 660, 625, 561, 514. 1H-NMR (δ/ppm): 9.77 (s, 1H), 8.54 (d, 1H, J = 2.58), 8.08–8.06 (d, 1H, J = 7.91), 7.92–7.88 (m, 3H), 7.75–7.73 (d, 1H, J = 7.53), 7.68–7.65 (t, 1H, J = 7.55), 7.61–7.59 (d, 1H, J = 15.88), 7.43–7.41 (m, 1H), 6.78–6.76 (d, 1H, J = 15.89), 6.47 (s, 2H), 6.28 (s, 1H), 6.12–6.11 (d, 1H, J = 8.50), 3.83 (s, 3H), 3.64 (m, 1H), 3.07–3.06 (m, 2H), 1.65–1.49 (m, 4H), 1.22–1.21 (d, 3H, J = 6.00). 13C-NMR (δ/ppm): 164.18, 158.99, 157.81, 144.62, 144.20, 137.75, 135.90, 134.74, 134.50, 132.14–127.87 (q, J = 214.50), 131.15, 130.07–129.41 (q, J = 30.02), 130.06, 129.53, 125.84, 123.98, 122.11, 122.03, 96.08, 91.64, 54.94, 47.03, 39.02, 33.43, 26.64, 20.18. MS (ESI): m/z = 516.1 [M + H]+. Anal. Calcd. For C26H28F3N5O3: C, 60.57; H, 5.47; N, 13.58. Found: C, 60.60; H, 5.81; N, 13.77.
(E)-4-(4-(6-Methoxyquinolin-8-ylamino)pentyl)-1-(3-(4-(trifluoromethyl)phenyl)acryloyl)semicarbazide (7j): Compound 7j was synthesized according to the general procedure using chloride 4j (0.235 g, 1 mmol) and compound 6 (0.286 g, 0.9 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5) and crystallization from ether/petroleum ether, pure compound 7j was obtained (m.p. = 123 °C, decomp.). A portion of product 7j crystallized from the reaction mixture. It was filtered off and washed with water. Overall yield: 0.311 g, 67%. IR (KBr) (cm−1): 3266, 1695, 1664, 1612, 1525, 1469, 1425, 1390, 1327, 1235, 1169, 1118, 1067, 834, 789, 728, 631, 592. 1H-NMR (δ/ ppm): 9.85 (s, 1H), 8.54–8.53 (d, 1H, J = 3.71), 8.08–8.06 (d, 1H, J = 8.07), 7.90 (s, 1H), 7.78 (s, 4H), 7.59–7.56 (d, 1H, J = 15.92), 7.43–7.41 (m, 1H), 6.77–6.74 (d, 1H, J = 15.90), 6.47 (s, 2H), 6.28 (s, 1H), 6.12–6.11 (d, 1H, J = 8.58), 3.83 (s, 3H), 3.64 (m, 1H), 3.07–3.05 (m, 2H), 1.65–1.47 (m, 4H), 1.22–1.21 (d, 3H, J = 6.06). 13C-NMR (δ/ ppm): 164.10, 158.98, 157.80, 144.61, 144.19, 138.73, 137.75, 134.73, 134.49, 129.64–129.01 (q, J = 31.19), 129.53, 128.13, 126.81–121.32 (q, J = 271.67), 125.76, 122.77, 122.02, 96.07, 91.63, 54.93, 47.01, 39.02, 33.42, 26.63, 20.17. MS (ESI): m/z = 516.2 [M + H]+. Anal. Calcd. For C26H28F3N5O3: C, 60.57; H, 5.47; N, 13.58. Found: C, 60.22; H, 5.24; N, 13.33.
(E)-1-(3-(3,5-Bis(trifluoromethyl)phenyl)acryloyl)-4-(4-(6-methoxyquinolin-8-ylamino)pentyl)semicarbazide (7k): Compound 7k was synthesized according to the general procedure using chloride 4k (0.303 g, 1 mmol) and compound 6 (0.286 g, 0.9 mmol). After purification by column chromatography (CH2Cl2/MeOH = 95:5) and crystallization from toluene, pure compound 7k was obtained (0.294 g, 56%, m.p. = 118–119.5 °C, decomp.). IR (KBr) (cm−1): 3393, 3335, 3220, 3020, 2942, 2363, 1647, 1617, 1579, 1521, 1459, 1425, 1384, 1340, 1280, 1224, 1175, 1135, 1053, 971, 942, 898, 847, 823, 792, 730, 683, 629, 599, 561, 519, 466. 1H-NMR (δ/ppm): 9.81 (s, 1H), 8.55–8.53 (dd, 1H, J = 1.62, 4.19), 8.29 (s, 2H), 8.12 (s, 1H), 8.09–8.07 (dd, 1H, J = 1.58, 8.31), 7.99 (s, 1H), 7.72–7.68 (d, 1H, J = 15.92), 7.77–7.41 (m, 1H), 6.94–6.90 (d, 1H, J = 15.97), 6.51–6.47 (m, 2H), 6.28 (s, 1H), 6.14–6.11 (d, 1H, J = 8.74), 3.82 (s, 3H), 3.64 (m, 1H), 3.06 (m, 2H), 1.66–1.48 (m, 4H), 1.22–1.21 (d, 3H, J = 6.28). 13C-NMR (δ/ppm): 163.86, 159.02, 157.78, 144.65, 144.27, 137.67, 136.28, 134.83, 134.53, 131.40–130.40 (q, J = 33.53), 129.60, 128.22, 127.28–119.14 (q, J = 272.61), 125.53, 124.23, 122.13, 96.13, 91.61, 55.01, 47.04, 39.02, 33.45, 26.74, 20.25. MS (ESI): m/z = 584.3 [M + H]+. Anal. Calcd. For C27H27F6N5O3: C, 55.57; H, 4.66; N, 12.00. Found: C, 55.60; H, 4.83; N, 12.29.
3.2. Biological Evaluation
3.2.1. Anticancer Activity
The experiments were carried out on a murine lymphocytic leukemia cell line (L1210) and five human tumor cell lines: CEM, HeLa, NCI-H460, SW 620 and MCF-7. The cells were cultured as monolayers and maintained in Dulbecco′s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere with 5% CO2 at 37 °C.
The growth inhibition activity of the compounds was assessed as described previously [46]. The cell lines were inoculated onto a series of standard 96-well microtiter plates on day 0, at 1.5 × 104 cells/mL (SW 620, H460) to 2.5 × 104 cells/mL (MCF-7, CEM and L1210), depending on the growth of the tumor cell line. Test agents were then added in ten-fold dilutions (10−8 to 10−4 M) and the cell cultures were then incubated for 3–4 days with 5% CO2 at 37 °C. Working dilutions of the compounds were freshly prepared on the day of testing.
After 3 (MCF-7, SW 620, NCI-H460) or 4 days (CEM, L1210), the cell growth rate was evaluated first light microscopically, then by viable cell counting and/or by performing the MTT assay, which detects dehydrogenase activity in live, not dead, cells. The absorbance (A) was measured on a microplate reader at 570 nm. The absorbance is directly proportional to the number of living, metabolically active cells. The results are expressed as IC50, which is the concentration necessary for 50% of inhibition. The IC50 values for each compound are calculated from concentration-response curves using linear regression analysis by fitting the test concentrations that give percentage of growth (PG) values above and below the reference value (i.e., 50%). If however, all of the tested concentrations produce PGs exceeding the respective reference level of effect (e.g., PG value of 50), then the highest tested concentration is assigned as the default value, which is preceded by a “>” sign. Minimum two individual experiments were carried out and each test point was performed in quadruplicate.
3.2.2. Antiviral Activity
Antiviral activity against herpes simplex virus type 1 (KOS), herpes simplex virus 2 (G), herpes simplex virus 1 TK− (KOS) ACVr, vaccinia virus, adeno virus 2 and human coronavirus (229E) was determined essentially as described previously [57,58]. After a 2 h incubation period, residual virus was removed and the infected cells were further incubated with the medium containing different concentration of the test compounds. After incubation for 3 days at 37 °C, virus-induced cytopathogenicity was monitored microscopically. Antiviral activity was expressed as the concentration required to reduce virus-induced cytopathogenicity by 50% (EC50).
3.2.3. Cytotoxicity Assays
Cytotoxicity measurements were based on the inhibition of HEL cell growth. Cells were seeded at 5 × 103 cells/well into 96-well microtiter plates. Then, medium containing different concentrations of the test compounds was added. After 3 days of incubation at 37 °C, the alteration of morphology of the cell cultures was recorded light microscopically. Cytotoxicity was expressed as MCC.
3.2.4. Interaction of the New Derivatives with the Stable Radical DPPH
To a solution of DPPH in absolute ethanol the appropriate volume of the compounds (50 or 100 µM final concentrations) dissolved in DMSO was added. The absorbance was recorded at 517 nm after 20 and 60 min at room temperature. The experiments were repeated at least in triplicate and the standard deviation of absorbance was less than 10% of the mean [47]. NDGA was used under the same experimental conditions as a reference compound.
3.2.5. Inhibition of Linoleic Acid Peroxidation
For initiating the lipid peroxidation free radical AAPH was used [47]. Final solution in the UV cuvette consisted of 10 μL linoleate sodium solution (c = 16 mM), 0.93 mL phosphate buffer (c = 0.05 M), pH 7.4, thermostated at 37 °C. 50 μL AAPH solution (c = 40 mM) and 10 μL of the tested compounds were added. The experiment was performed at 37 °C under air. The oxidation of linoleic acid sodium salt was monitored at 234 nm. The assays were repeated at least in triplicate and the standard deviation of absorbance was less than 10% of the mean. Trolox was used under the same experimental conditions as a reference compound.
3.2.6. Anti-inflammatory Activity: LOX Inhibition Study In Vitro
LOX inhibitory assay in vitro was accomplished as described previously [47]. The test compounds (stock solutions 10 mM in DMSO) were incubated at room temperature with sodium linoleate (c = 0.1 mM) and 0.2 mL of LOX enzyme solution (1/9 × 10−4 m/V in saline). The conversion of sodium linoleate to 13-hydroperoxylinoleic acid was measured at 234 nm and compared with the reference inhibitor. Several concentrations were used for the IC50 determination. The assays were repeated at least in triplicate and the standard deviation of absorbance was less than 10% of the mean. NDGA was used under the same experimental conditions as a reference compound.
4. Conclusions
We have designed and synthesized a new scaffold comprising CADs and PQ motifs bound by amide (compounds 3a–k) or acylsemicarbazide bonds (compounds 7a–k). Twenty two PQ-CAD conjugates were prepared and subjected to extensive biological evaluation. Anticancer screening in vitro against six selected cancer cell lines showed that acylsemicarbazide derivatives were, in general, more active than amide derivatives. Almost all compounds from series 3 were selective towards MCF-7 cell line and active in micromolar concentrations. The most selective compound from the amide series was the o-fluoro derivative 3h. It showed high activity against HeLa, MCF-7 and in particular against the SW 620 cell line. Four acylsemicarbazide derivatives, namely 7f with benzodioxole ring and 7c, 7g and especially 7j with methoxy, chloro and trifluoromethyl substituents in para position of cinnamic benzene ring, showed high selectivity and very strong activity against MCF-7 cell line in micromolar (7c, 7f and 7g) and nanomolar levels (7j). These compounds might provide fertile and much needed leads in anticancer drug discovery.
Trifluoromethyl acylsemicarbazide derivatives 7i, 7j and 7k showed selective antiviral activity against human coronavirus (strain 229E) at concentrations which did not alter normal cell morphology and could be, therefore, considered as potential drug leads for further structural optimization. The same compounds, together with 7d and 7g, exerted the most potent antioxidant abilities in interaction with the free stable radical DPPH, while unsubstituted (7a), methoxy (7c–e), benzodioxole (7f), p-Cl (7g) and m-CF3 (7i) acylsemicarbazides and amide 3f presented the highest anti-lipid peroxidation activity (83–89%). Dimethoxy derivative 7d was the most potent LOX inhibitor (IC50 = 10 μΜ). The above results support the principal idea of PQ-CADs hybridization leading to multifunctional agents.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/12/1629/s1.
Acknowledgments
Support for this study by the Croatian Science Foundation (project number IP-2014-09-1501), University of Zagreb (Projects numbers BM1.9, 13.11.2.1.12 and 13.11.1.1.11.), Croatian Science Foundation (project number IP-11-2013-5660, MultiCaST), and the KU Leuven, Belgium (GOA 10/014) are gratefully acknowledged. We sincerely thank Leentje Persoons and Lizette Van Berckelaer for their excellent help in the evaluation of these series of compounds.
Author Contributions
K.P. performed the majority of the synthetic experiments, analyzed the data in chemical part and prepared experimental data for publishing. I.P. analyzed the data in chemical part. P.G and F.K. performed the synthetic experiments (a part). K.E. performed antiproliferative experiments (a part). M.K. analyzed and discussed the antiproliferative experiments. D.S. performed antiproliferative experiments (a part) and antiviral experiments. E.P. screened the antioxidant potential and D.H.-L. analyzed the antioxidative data. B.Z. designed and wrote the paper, analyzed the data and coordinated the work.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AAPH | 2,2’-azobis(2-amidinopropane) dihydrochloride |
| BtcCl | 1-benzotriazole carboxylic acid chloride |
| BtH | 1H-benzo[d][1,2,3]triazole |
| CA | cinnamic acid |
| CAD | cinnamic acid derivative |
| CEM | acute lymphoblastic leukemia cell line |
| CIS | cisplatin |
| DMEM | Dulbecco′s modified Eagle′s medium |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EC50 | concentration required to reduce virus-induced cytopathogenicity by 50% |
| FBS | fetal bovine serum |
| 5-FU | 5-fluorouracil |
| H460 | lung carcinoma cell line |
| HEL | human erythroleukemia cell line |
| HeLa | cervical carcinoma cell line |
| IC50 | concentration that causes 50% growth inhibition |
| L1210 | murine lymphocytic leukemia cell line |
| LOX | soybean lipoxygenase |
| LP | lipid peroxidation |
| MCC | minimum cytotoxic concentration, concentration that causes a microscopically detectable alteration of normal cell morphology |
| MCF-7 | breast adenocarcinoma cell line |
| MR | molar refractivity |
| MTT | (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NDGA | nordihydroguaiaretic acid |
| PSA | molecular polar surface area |
| PG | percentage of growth |
| PQ | primaquine |
| RA | DPPH reducing ability |
| SI | selectivity ratio, MCC to EC50 ratio |
| SOR | sorafenib |
| SW 620 | colon cancer cell line |
| TEA | triethylamine |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
| UDA | Urtica dioica agglutinin |
References
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 292–349. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.J. Cinnamic acid derivatives: A new chapter of various pharmacological activities. Chem. Pharm. Res. 2011, 3, 403–423. [Google Scholar]
- Lone, R.; Shuab, R.; Koul, K.K. Role of cinnamate and cinnamate derivatives in pharmacology. Glob. J. Pharmacol. 2014, 8, 328–335. [Google Scholar]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, B.; Belsare, D.; Pharande, D.; Mourya, V.; Dhake, A. Esters, amides and substituted derivatives of cinnamic acid: Synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2004, 39, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Campbell, B.C.; Mahomey, N.E.; Chan, K.L.; Molyneux, R.J. Identification of phenolics for control of Aspergillus flavus using Saccharomyces cerevisiae in a model target-gene bioassay. J. Agric. Food Chem. 2004, 52, 7814–7821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, H.; Kobamoto, N.; Yasuda, M.; Tawata, S. Fungitoxic and phytotoxic activities of cinnamic acid esters and amides. J. Pestic. Sci. 2000, 25, 263–266. [Google Scholar] [CrossRef]
- Neogi, P.; Lakner, F.J.; Medicherla, S.; Cheng, J.; Dey, D.; Gowri, M.; Nag, B.; Sharma, S.D.; Pickford, L.B.; Gross, C. Synthesis and structure-activity relationship studies of cinnamic acid-based novel thiazolidinedione antihyperglycemic agents. Bioorg. Med. Chem. 2003, 11, 4059–4067. [Google Scholar] [CrossRef]
- Bairwa, R.; Kakwani, M.; Tawari, N.R.; Lalchandani, J.; Ray, M.K.; Rajan, M.G.R.; Degani, M.S. Novel molecular hybrids of cinnamic acids and guanylhydrazones as potential antitubecular agents. Bioorg. Med. Chem. 2010, 20, 1623–1625. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.M.; Nadadhur, G.; Daneluzzi, D.; Dimova, V.; Gangadharam, P.R. Antimycobacterial activity of a new rifamycin derivative, 3-(4-cinnamylpiperazinyl iminomethyl) rifamycin SV (T9). J. Antimicrob. Agents Chemother. 1995, 39, 2320–2324. [Google Scholar] [CrossRef]
- Carvalho, S.A.; da Silva, E.F.; de Souza, M.V.; Lourenco, M.C.; Vicente, F.R. Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Kakwani, M.D.; Suryavanshi, P.; Ray, M.; Rajan, M.G.R.; Majee, S.; Samad, A.; Devarajan, P.; Degani, M.S. Design, synthesis and antimycobacterial activity of cinnamide derivatives: A molecular hybridization approach. Bioorg. Med. Chem. Lett. 2011, 21, 1997–1999. [Google Scholar] [CrossRef] [PubMed]
- Yoya, G.K.; Bedos-Belval, F.; Constant, P.; Duran, H.; Daffé, M.; Baltas, M. Synthesis and evaluation of a novel series of pseudo-cinnamic derivatives as antituberculosis agents. Bioorg. Med. Chem. Lett. 2009, 19, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Bogdashev, N.N.; Tukhovskaya, N.A.; Pogrebnyak, A.V. Physicochemical characterisation of cinnamic acid derivatives. Part 1. Relationship between antioxidant activity and physicochemical properties. Pharm. Chem. J. 1998, 32, 31–33. [Google Scholar]
- Chen, J.H.; Ho, C.T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Arya, P.; Mukherjee, C.; Singh, B.K.; Singh, N.; Parmar, V.S.; Prasad, A.K.; Ghosh, B. Novel aromatic ester from Piper longum and its analogues inhibit expression of cell adhesion molecules on endothelial cells. Biochemistry 2005, 44, 15944–15952. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Choudhary, R.K. Process for Preparation of Sunscreen Agents. U.S. Patent 5,527,947, 19 December 1996. [Google Scholar]
- Fernandez-Martinez, E.; Bobadilla, R.A.; Morales-Rios, M.S.; Muriel, P.; Perez-Alvarez, V.M. Trans-3-phenyl-2-propenoic acid (cinnamic acid) derivatives: Structure-activity relationship as hepatoprotective agents. Med. Chem. 2007, 3, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Duchnowicz, P.; Broncel, M.; Podsędek, A.; Koter-Michalak, M. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). Eur. J. Nutr. 2012, 51, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Park, Y.B.; Moon, S.S.; Bok, S.H.; Kim, D.J.; Ha, T.Y.; Jeong, T.S.; Jeong, K.S.; Choi, M.S. Hypocholesterolemic and antioxidant properties of 3-(4-hydroxyl)propanoic acid derivatives in high-cholesterol fed rats. Chem. Biol. Interact. 2007, 170, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Yabe, T.; Hirahara, H.; Harada, N.; Ito, N.; Nagal, T.; Sangi, T.; Yamada, H. Ferulic acid induces neural progenitor cell proliferation in vitro and in vivo. Neuroscience 2010, 165, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Kanaani, J.; Ginsburg, H. Effects of cinnamic acid derivatives on in vitro growth of Plasmodium falciparum and on the permeability of the membrane of malaria-infected erythrocytes. Antimicrob. Agents Chemother. 1992, 36, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.C.; Fernandes, I.; Mateus, N.; Teixeira, C.; Gomes, P. Recycling antimalarial leads for cancer: Antiproliferative properties of N-cinnamoyl chloroquine analogues. Bioorg. Med. Chem. Lett. 2013, 23, 6769–6772. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.; Teixeira, C.; Albuquerque, I.S.; Gut, J.; Rosenthal, P.J.; Prudencio, M.; Gomes, P. PRIMACINS, N-cinnamoyl-primaquine conjugates, with improved liver-stage antimalarial activity. Med. Chem. Commun. 2012, 3, 1170–1172. [Google Scholar] [CrossRef]
- Pérez, B.; Teixeira, C.; Gomes, A.S.; Albuquerque, I.S.; Gut, J.; Rosenthal, P.J.; Prudęncio, M.; Gomes, P. In vitro efficiency of 9-(N-cinnamoylbutyl)aminoacridines against blood- and liver-stage malaria parasites. Bioorg. Med. Chem. Lett. 2013, 23, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.C.; Teixeira, C.; Figueiras, M.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.B.; Gomes, P. Novel cinnamic acid/4-aminoquinoline conjugates bearing non-proteinogenic amino acids: Towards the development of potential dual action antimalarials. Eur. J. Med. Chem. 2012, 54, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents-a review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.; Chang, X.; Zhang, C.; Zhou, H.; Liu, M. Ozagrel for acute ischemic stroke: A meta-analysis of data from randomized controlled trials. Neurol. Res. 2012, 34, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Lee, H. Chloroquine and its analogs: A new promise of an old drug for effective and safe cancer therapies. Eur. J. Pharmacol. 2009, 625, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Hu, C.; Lee, H. Design and synthesis of chloroquine analogs with anti-breast cancer property. Eur. J. Med. Chem. 2010, 45, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- Van Huijsduijnen, R.H.; Kiplin Guy, R.; Chibale, K.; Haynes, R.K.; Peitz, I.; Kelter, G.; Phillips, M.A.; Vennerstrom, J.L.; Yuthavong, Y.; Wells, T.N.C. Anticancer properties of distinct antimalarial drug classes. PLoS ONE 2013, 8, e82962. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takabatake, Y.; Takahashi, A.; Isaka, Y. Chloroquine in cancer therapy: A double-edged sword of autophagy. Cancer Res. 2013, 73, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.; Choudhury, D.; Datta, S.; Bhattacharya, S.; Chakrabarti, G. Inhibition of autophagy by chloroquine potentiates synergistically anti-cancer property of artemisinin by promoting ROS dependent apoptosis. Biochimie 2014, 107, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shang, Y.; Chen, S-Z. Chloroquine potentiates the anti-cancer effect of lidamycin on non-small cell lung cancer cells in vitro. Acta Pharmacol. Sin. 2014, 35, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Aziz, A.; Shouman, S.; El-Demerdash, E.; Elgendy, M.; Abdel-Naim, A.B. Chloroquine as a promising adjuvant chemotherapy together with sunitinib. Sci. Proc. 2014, 1, e384. [Google Scholar]
- Das, A.K. Anticancer effect of antimalarial artemisinin compounds. Ann. Med. Health Sci. Res. 2015, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Coulter, D.W.; Vennerstrom, J.; Sharp, J.G.; Dong, Y.; Wang, X.; McIntyre, E.; McGuire, T. Screening of investigational antimalarials for anticancer activity in high risk N-MYC amplified neuroblastoma (NB). Cancer Res. 2015, 75. [Google Scholar] [CrossRef]
- Xu, C.-C.; Deng, T.; Fan, M.-L.; Lv, W.-B.; Liu, J.-H.; Yu, B.-Y. Synthesis and in vitro antitumor evaluation of dihydroartemisinin-cinnamic acid ester derivatives. Eur. J. Med. Chem. 2016, 107, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Duffy, R.; Wade, C. Discovery of anticancer drugs from antimalarial natural products: A MEDLINE literature review. Drug Discov. Today 2012, 17, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Džimbeg, G.; Zorc, B.; Kralj, M.; Ester, K.; Pavelić, K.; Balzarini, J.; de Clercq, E.; Mintas, M. The novel primaquine derivatives of N-alkyl, cycloalkyl or aryl urea: Synthesis, cytostatic and antiviral activity evaluations. Eur. J. Med. Chem. 2008, 43, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Šimunović, M.; Perković, I.; Zorc, B.; Ester, K.; Kralj, M.; Hadjipavlou-Litina, D.; Pontiki, E. Urea and carbamate derivatives of primaquine: Synthesis, cytostatic and antioxidant activities. Bioorg. Med. Chem. 2009, 17, 5605–5613. [Google Scholar]
- Perković, I.; Tršinar, S.; Žanetić, J.; Kralj, M.; Martin-Kleiner, I.; Balzarini, J.; Hadjipavlou-Litina, D.; Katsori, A.M.; Zorc, B. Novel 1-acyl-4-substituted semicarbazide derivatives of primaquine—Synthesis, cytostatic, antiviral and antioxidative studies. J. Enzym. Inhib. Med. Chem. 2013, 28, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Pavić, K.; Perković, I.; Cindrić, M.; Pranjić, M.; Martin-Kleiner, I.; Kralj, M.; Schols, D.; Hadjipavlou-Litina, D.; Katsori, A.M.; Zorc, B. Novel semicarbazides and ureas of primaquine with bulky aryl or hydroxyalkyl substituents: Synthesis, cytostatic and antioxidative activity. Eur. J. Med. Chem. 2014, 86, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Perković, I.; Antunović, M.; Marijanović, I.; Pavić, K.; Ester, K.; Kralj, M.; Vlainić, J.; Kosalec, I.; Schols, D.; Hadjipavlou-Litina, D.; et al. Novel urea and bis-urea primaquine derivatives with hydroxyphenyl and halogenphenyl substituents: Synthesis and biological evaluation. Eur. J. Med. Chem. 2016, 124, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, R.; Komatsu, K.; Bonaz-Krause, P.; Zyrianov, Y.; McKenna, C.E.; Csipke, C.; Tokes, Z.A.; Lien, E.J. Synthesis, biological evaluation, and quantitative structure-activity relationship analysis of new Schiff Bases of hydroxysemicarbazide as potential antitumor agents. J. Med. Chem. 2002, 45, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Zovko, M.; Zorc, B.; Jadrijević-Mladar Takač, M.; Metelko, B.; Novak, P. The novel ketoprofenamides—Synthesis and spectroscopic characterization. Croat. Chem. Acta 2003, 76, 335–341. [Google Scholar]
- Barbarić, M.; Kralj, M.; Marjanović, M.; Husnjak, I.; Pavelić, K.; Filipović-Grčić, J.; Zorc, D.; Zorc, B. Synthesis and in vitro antitumor effect of diclofenac and fenoprofen thiolated and nonthiolated polyaspartamide-drug conjugates. Eur. J. Med. Chem. 2007, 42, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Rajić, Z.; Butula, I.; Zorc, B.; Kraljević Pavelić, S.; Hock, K.; Pavelić, K.; Naesens, L.; de Clercq, E.; Balzarini, J.; Przyborowska, M.; et al. Cytostatic and antiviral evaluations of NSAID hydroxamic acids. Chem. Biol. Drug Des. 2009, 73, 328–338. [Google Scholar] [PubMed]
- Rajić, Z.; Hadjipavlou-Litina, D.; Pontiki, E.; Kralj, M.; Šuman, L.; Zorc, B. The novel ketoprofen amides—Synthesis and biological evaluation as antioxidants, lipoxygenase inhibitors and cytostatic agents. Chem. Biol. Drug Des. 2010, 75, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Luzina, E.L.; Popov, A.V. Synthesis, evaluation of anticancer activity and COMPARE analysis of N-bis(tri fluoromethyl)alkyl-N’-substituted ureas with pharmacophoricmoieties. Eur. J. Med. Chem. 2012, 53, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Instant Cheminformatics Solutions. Available online: http://www.chemicalize.org/ (accessed on 1 September 2016).
- Rioux, N.; Castonguay, A. Inhibitors of lipoxygenase: A new class of cancer chemopreventive agents. Carcinogenesis (Lond.) 1998, 19, 1393–1400. [Google Scholar] [CrossRef]
- Kort, W.J.; Bijma, A.M.; van Dam, J.J.; van der Ham, A.C.; Hekking, J.M.; van der Ingh, H.F.; Meijer, W.S.; van Wilgenburg, M.G.; Zijlstra, F.J. Eicosanoids in breast cancer patients before and after mastectomy. Prostaglandin Leukot. Essent. 1992, 45, 319–327. [Google Scholar] [CrossRef]
- De Clercq, E.; Holý, A.; Rosenberg, I.; Sakuma, T.; Balzarini, J.; Maudgal, P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature 1986, 323, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Naesens, L.; Slachmuylders, J.; Niphuis, H.; Rosenberg, I.; Holý, A.; Schellekens, H.; de Clercq, E. 9-(2-Phosphonylmethoxyethyl)adenine (PMEA) effectively inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in rhesus monkeys. AIDS 1991, 5, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).