Abstract
(1) We document the invertebrate fauna collected from 24 oak canopies in east and west Norway as a contribution to the Norwegian Biodiversity Information Centre’s ‘The Norwegian Taxonomy Initiative’. (2) A snap-shot inventory of the canopies was recorded by means of emitting a mist of natural pyrethrum into the canopies at night using a petrol-driven fogger and collecting the specimens in butterfly nets spread on the ground under the canopy. (3) Almost the entire catch of more than 6800 specimens was identified to 722 species. Out of 92 species new to the Norwegian fauna, 21 were new to science and, additionally, 15 were new to the Nordic fauna. Diptera alone constituted nearly half of the species represented, with 61 new records (18 new species). Additionally, 24 Hymenoptera (one new species), six oribatid mites (two new species) and one Thysanoptera were new to the Norwegian fauna. (4) Our study emphasizes the importance of the oak tree as a habitat both for a specific fauna and occasional visitors, and it demonstrates that the canopy fogging technique is an efficient way to find the ‘hidden fauna’ of Norwegian forests. The low number of red listed species found reflects how poor the Norwegian insect fauna is still studied. Moreover, the implication of the IUCN red list criteria for newly described or newly observed species is discussed.
1. Introduction
Pedunculate oak (Quercus robur L.) and sessile oak (Q. petraea (Matt.) Liebl.) are regarded as a biodiversity hotspot in Northern Europe and have been the target of a wide variety of biodiversity studies on arthropods (e.g., [,,,,,,,,,]).
Relatively few studies have targeted oak canopy invertebrates sampled with canopy fogging methods in Europe but see, e.g., [,,] and chapters in []. Efraín Tovar-Sánchez with colleagues, together with a few others (e.g., [,,,,,,,,]), have been pioneers in the Americas on oak canopy studies.
Emitting insecticides into the forest canopy to sample invertebrates has opened up a new area of forest biodiversity research. Originally developed in the tropics, canopy fogging techniques are now being used increasingly in temperate forests to increase the knowledge of European arboreal fauna [,,,,,,,,,,,,,,,,]. Stork and colleagues [] discuss the efficiency of fogging as a method for sampling arthropods from the canopies. A larger spectrum of species is sampled compared with any other single method. This makes fogging a useful method for arthropod snapshot inventories. The major disadvantage is that external and internal feeders are underrepresented (phloem feeders, leaf miners and wood borers), non-obligate occasional by-passers (tourists) will be captured and that the method is sensitive to wind and precipitation [,].
This study presents empirical data and analyses of oak canopy invertebrate data from a survey of 24 oak canopies in Norway. We proposed the following hypotheses: 1) there are large geographical differences in species composition and 2) trees on cultivated lands (Berge and Mule Varde) have a different species composition than forest trees. Both hypotheses are related to climatic differences on macro- (H1) and microlevels (H2) (e.g., []) as well as the geography of Norway, where oaks are distributed along the coast, usually with scattered populations [,]. H2 is founded on the generally more uniform structure of managed lands and lack of a multi-layered canopy of such forest stands []. The project was granted by the Norwegian Biodiversity Information Centre as a part of the Norwegian Taxonomy Initiative to search for the hidden life and new species in Norway.
2. Materials and Methods
2.1. The Oaks
Quercus robur and Q. petraea have a sympatric distribution and often hybridize [], though Q. robur is claimed to be more widespread [,]. Thus, we have not distinguished between the two species of oak or their hybrids in this study.
2.2. Site Descriptions
The study was carried out at six sites in southern Norway in June–July 2011 and 2012 (Figure 1, Table 1). All sites were continuous oak-dominated forests, except Berge (site 1) and Mule Varde (site 5), which had oak trees scattered on managed land. Four oaks were treated at each site. The sites were carefully selected to represent a gradient from the inner fjords of West Norway, via known biodiversity hotspots inland Vestfold and Telemark to the coastal areas of SE Norway [,,,] aligned with the hypotheses.
Figure 1.
Site overview.

Table 1.
Site details.
Site 1 (Berge) is a protected landscape area and classified as IUCN category V []. It contains the largest assemblage of old and pruned oak trees in the country. This and the proximity to a lake with specialized swamp vegetation and several old buildings are the main reasons for its protection status [].
Site 2 (Skeianeset) is a steep slope facing south and has according to one of the highest concentrations of hollow, previously pruned oaks in Norway []. The area is characterized by having an unusually high proportion of red-listed species of plants, bryophytes and fungi and is considered to be one of the most important deciduous forests in West Norway [].
Site 3 (Steinknapp) is a nature reserve that is known to harbor many rare and threatened species (e.g., []). Its importance for biological diversity also explains its status as a nature reserve (IUCN category IA). Most likely, large parts of this area were clear-cut in the past as really old oaks are sparsely present and the more or less continuous oak forest is rather homogenous. The oaks treated in this study were just outside of the reserve.
Site 4 (Djupedal) is also a nature reserve protected according to the IUCN IA criteria. In contrast to the nearby site 3, there are several giant oaks in this area and the forest is characterized as old growth. Moreover, the forest is more closed and heterogeneous than at site 3.
Site 5 (Mule Varde) is a cultural heritage site and public park. Large oak trees are scattered throughout the property.
Site 6 (Skjærsjø) is a mixed deciduous forest with larger areas of conifer woods intermixed.
2.3. Data Collection
The trees were chosen to represent ‘typical’ trees in the areas. This implies that after traversing the site, the chosen trees were not at the edges, not standalone trees except for at Berge and Mule Varde where most trees were standalone. Furthermore, the biggest and smallest trees were also avoided. Arthropod sampling was performed by emitting a 1% concentration of natural pyrethrum, Py-Sekt, into the canopy using a Golden Eagle 2610E fogger for approximately 10 minutes in the period between 1 AM and 3 AM on a windless and dry night. Py-sekt contains 1–5% piperonyl butoxide and 0–1% pyrethrum []. It breaks down quickly in direct sunlight and is, therefore, relatively safe to use in natural environments []. The available space for arthropods will obviously vary both according to the breadth and height of the crown, but for practical reasons we preferred to collect knocked-down invertebrates from a fixed area. Twenty large butterfly nets (18 with Ø50 cm and 2 with Ø100 cm, mesh size from 0.3–0.5 mm) were mounted on the ground or on the lower branches beneath the crown to collect the knocked-down invertebrates, i.e., 5.11 m2 of the area beneath each tree was sampled. As so, the proportion of the crown projection area covered will vary slightly between individual trees but is assumed not to affect the qualitative data. The nets remained on the ground for approximately one hour after fogging before the collected material was transferred to 80% ethanol. The material was then sorted and shipped to the co-authors of this paper for identification, with the exception of Lepidoptera and cecidomyiid midges, which remain unidentified.
Most of the material is stored in the Natural History Museum at the University of Oslo and the Norwegian Institute of Bioeconomy Research’s entomological collection. The phorid flies are at the Zoological Museum at Cambridge University, England, and a part of sciarid material, including the holotype of Bradysia quercina Menzel and Köhler, 2014, is deposited at the Senckenberg Deutsches Entomologisches Institut, Müncheberg, Germany.
2.4. Species Records
Species designated as new records for Norway or the Nordic countries at the time of identification were based on the individual expert’s consideration, but also on published records in Fauna Europaea [] and records in the Norwegian Biodiversity Information Centre’s species record database accessed throughout the preparation of this manuscript at www.artsdatabanken.no.
Specimens fully identified to species level were included in the analyses and counted in addition to unidentified species with only one species collected in the respective higher taxon. Uncertain species identifications, i.e., denoted with confer (cf.) or near, were included when the species were not already identified with certainty from other specimens. In cases where the identity of the species was clear, yet undefined (i.e., denoted as sp., sp. 1, etc.), the species beyond the number of identified species were counted. When more unidentified species within the same genus were found, i.e., spp., they were not included in the counts except for counting 1 when no other species in that genus was found. Abundances of common species of spiders and collembolans were sometimes indicated as ‘few’, ‘some’ and ‘many’, and were thence given dummy numbers 5, 10 and 20, respectively.
2.5. Data Analyses
Rarefaction curves extrapolated to three times the sample size, i.e., 72 trees were carried out with EstimateS, version 9.1.0 []. The extrapolation relies on statistical sampling methods rather than modeling. Here, the bias corrected form of Chao1 is the asymptotic richness estimator for individual-based abundance data []. We chose to extrapolate because rarefaction curves of insect assemblages are usually steep and do not converge unless a massive sampling effort is conducted. However, extrapolation beyond three times the sample size is not recommended [] because the variance increases with the extrapolation.
Whittaker’s β was calculated as a measure of species turnover along the sampling gradient. It is insensitive to species richness and is calculated as follows:
where S = total number of species, αmax = highest number of species in any one locality and N = the number of localities []. It ranges from zero (no turnover) to 100 (every locality has a unique set of species). These calculations were performed to complement multivariate analysis using detrended correspondence analysis (DCA) with Canoco, version 4.56 [] to relate species composition and site characteristics along the sampling gradient. The aim was to investigate whether the species composition within a site differed from the composition of species at the other sites and relate that to environmental characteristics. DCA assumes unimodal species responses to environmental factors in contrast to principal components analysis, or its detrended equivalent, where linear responses are assumed []. Therefore, over a longer geographic gradient with different climatic or other underlying environmental factors, DCA is to be preferred. The multivariate analysis was performed on untransformed species abundances with downweighing rare species.
3. Results
3.1. Faunistics
Combined, more than 6800 specimens were identified to 722 species. Ninety-two species (12.7%) were new to the Norwegian fauna upon sampling (Table A1), 61 Diptera, 24 Hymenoptera, one Thysanoptera and six oribatid mites. Of these, the following 21 species (2.9%) were new to science: 16 phorid flies (13 described in []), one sciarid midge [], one chironomid midge [], one aphelinid wasp [] and two oribatid mites awaiting description. Additionally, of the 92 new Norwegian records, 15 were found in the Nordic countries for the first time (Table A1). Diptera was the most species-rich order of invertebrates with 334 species (46.3%), followed by Hymenoptera with 117 (16.2%) and Coleoptera with 84 (11.6%). Additionally, Diptera was represented with the highest number of specimens with 1339 (19.5%), followed by Hemiptera with 1108 (16.1%) and Coleoptera with 821 (12.0%). Collembola and Araneae were not included in the specimen calculations as their abundances were ranked for the common species. These figures correspond well with other inventories from canopies.
Amongst the sites, the six most species-rich orders were represented in stable proportions with respect to the number of species present (Figure 2), with Diptera being the clearly most species rich at all the sites (29% in Skjærsjø to 47% in Berge). The proportion of specimens for the six most abundant orders, however, showed a varied pattern in that Isopoda constituted 23% of the specimens collected at Djupedal, Hemiptera almost 45% at Mule Varde and Coleoptera 25% at Skjærsjø (Figure 2). Moreover, the number of collected species ranged from 166 in Berge to 370 in Steinknapp, and the number of specimens collected was 4.6 times higher in Steinknapp (2440) than at Berge (536) (Table 2). Steinknapp contained 1.8 times as many species as the second most species-rich site, Djupedal (just a few kilometers away). Although species new to science were found in all the localities, 14 of the 21 new species were found in Steinknapp (25 specimens) with five species as the second highest number in any of the other localities (Skjærsjø, 37 specimens). In addition, 45 species new to Norway (134 specimens) were found in Steinknapp, followed by 20 species (60 specimens) in Djupedal.
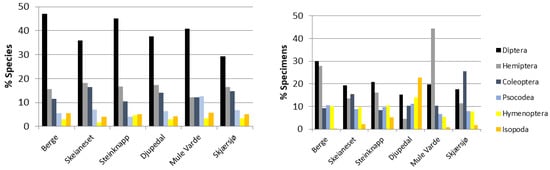
Figure 2.
(Left) Percentage distribution of species (top six orders). (Right) Percentage distribution of specimens (top six orders).

Table 2.
Site diversity data. NSpecies = Number of species collected from the site. NSpecimens = Number of specimens collected from the site. Rα = Range of species numbers collected from any tree within the site. RSpecimens = Range of specimens collected from any tree within the site. NSingletons = Number of species represented by one specimen only. Turnover = Whittaker’s β within the site.
Even though 50.6% of the species (358 species) were represented by singletons and 56.1% (397 species) were found in only one tree (uniques), the turnover along the entire sampling gradient (all 24 trees) was as low as β = 13.34. Rejecting H1, this means that the species communities along the gradient are comparably similar. Between-site turnover showed the same with β = 18.27. Within the sites, however, turnover was higher (Table 2), ranging from 31.01 (Berge) to 44.30 (Skjærsjø). Thus, despite the high turnover within each site (Table 2), the shift in species composition throughout the sampling gradient was comparably lower, indicating that a similar set of species appear in low numbers in geographically disjunct locations.
This separation of sites is also reflected in the DCA ordination diagram (Figure 3), as the two sites on cultivated land (Berge and Mule Varde) were nicely grouped separately from the other sites indicating similar within-site composition of species but different from each other (except tree 11 from Steinknapp), and thus supporting H2. At the opposite side of the gradient, the Djupedal site also indicates a similar species composition within the site, but different from the other sites. The strong explanatory powers of the DCA axes one and two (Eigenvalues = 0.51 and 0.32, respectively), as well as the long gradient (3.98 SD), corroborate this.
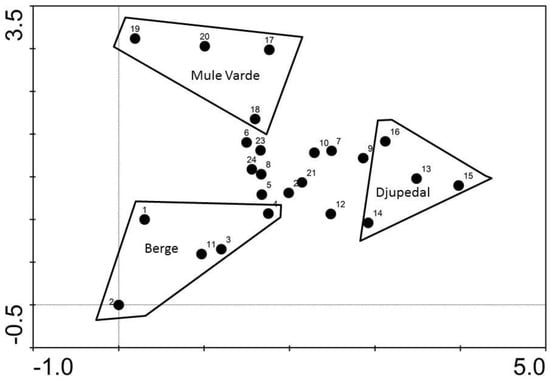
Figure 3.
DCA ordination diagram. Eigenvalue 1 = 0.51. Eigenvalue 2 = 0.32. Dummy values 5, 10 and 20 for Araneae and Collembola included as described in the material and methods chapter.
3.2. Species Records
3.2.1. Araneae
Spiders are all predators and are usually more associated with their prey than with tree species. Noteworthy though, among the 28 species collected, one threatened species was found (Dipoena braccata (C. L. Koch, 1841), see Table 3). Diplocephalus picinus (Blackwall, 1841) is a species normally found in broadleaf forests, while Moebelia penicillata (Westring, 1851), Paidiscura pallens (Blackwall, 1834), Neriene peltata (Wider, 1834) and Theridion mystaceum L. Koch, 1870 are all known to climb trees [,].

Table 3.
Red listed species []. Categories: VU = vulnerable, NT = near threatened.
3.2.2. Acari
Two oribatid mites new to science were found. Damaeus sp. n. was abundant, with 51 specimens and was present at all the sites except Djupedal, while Phthiracarus sp. n. was found with five geographically disjunct specimens (Table A1). In addition, the following four oribatid species were recorded from the Nordic countries for the first time: Liacarus (Dorycranosus) splendens (Coggi, 1898) with one specimen from Steinknapp, Oribatella (Oribatella) quadricornuta (Michael, 1880) with 14 specimens from Steinknapp, Phauloppia nemoralis (Berlese, 1916) with one specimen from Skeianeset and two from Steinknapp, and Xenillus (Xenillus) discrepans Grandjean, 1936 with 14 specimens from Skeianeset, three from Mule Varde and one from Skjærsjø, respectively.
Among the arboreal species of oribatid mites inhabiting the oak canopies, we can include the following species living in the growths of mosses and lichens therein: Camisia (C.) horrida (Hermann, 1804), Carabodes (C.) areolatus Berlese, 1916, Carabodes (C.) labyrinthicus (Michael, 1879), Cymberemaeus cymba (Nicolet, 1855), Eupelps acromios (Hermann, 1804) and Oribatula (Zygoribatula) exilis (Nicolet, 1855). The following specialized lichenophagous species were also common in the treetops, feeding on the lichen thalluses: Phauloppia lucorum (C. L. Koch, 1841) and Phauloppia nemoralis (Berlese, 1916). The following oribatid species, preferring decaying wood, were also frequent in tree canopies: Caleremaeus monilipes (Michael, 1882), Carabodes (C.) rugosior Berlese, 1916 and Euphthiracarus (E.) cribrarius (Berlese, 1904). Arboreal species are usually bigger (length of body 600–1000 µm), dark brown or black, with a heavily sclerotized cuticle and a thick layer of waxy cerotegument on the body surface, protecting them from desiccation. Forest litter and soil species, on the other hand, are characteristically smaller, lighter in color, with a weaker sclerotized cuticle and a thinner layer of cerotegument (families Tectocepheidae, Oppiidae, Suctobelbidae, Brachychthoniidae, etc.). They were not found in the tree canopies.
3.2.3. Isopoda
Trachelipus ratzeburgii (Brandt, 1833) is categorized as near threatened on the Norwegian red list []. It appeared with 16 specimens in Djupedal and three in Skjærsjø (Table 3).
3.2.4. Collembola
Being scavengers for most, springtails are common in trees []. All of the 23 species found in the oak canopies can be considered as common species, with Entomobrya nivalis (Linnaeus, 1758) as the most abundant species in this study by far. This species, together with E. albocincta (Templeton, 1835), E. corticalis (Nicolet, 1842), E. marginata (Tullberg, 1871) and Sminthurinus alpinus Gisin, 1953, are known arboreal species being associated with the lichens growing on bark.
3.2.5. Hemiptera
Altogether, 35 species of Hemiptera were collected–21 Heteroptera and 14 Auchenorrhyncha–most of them are oak associates [,,]. Temnostethus gracilis Horváth, 1907 and Phylus melanocephalus (Linnaeus, 1767) were the two most common species of Heteroptera and were found in almost all the sites. Other oak dwellers worth mentioning are, for example, Cyllecoris histrionicus (Linnaeus, 1767), Psallus varians (Herrich-Schaeffer, 1841), P. mollis (Mulsant and Rey, 1852), P. variabilis (Fallén, 1807) and P. wagneri Ossiannilsson, 1953.
3.2.6. Psocodea
Twenty-four species of the order Psocodea were collected from the oak canopies, all belonging to families formerly referred to as the paraphyletic «order Psocoptera» [,]. Most Psocodea feed on algae, microfungi and lichens, or decomposing stages of these, as well as pollen. Most of the foliage-living species are associated with either conifers or broadleaved trees, whereas bark-living species (on trunks as well as branches and twigs) are less discriminate. For most Psocodea, the character of the foodstuff itself, which may be dependent on physical factors such as moisture, light and exposure, is probably more important than the tree species. No Psocodea species was found at all the sites, but Reuterella helvimacula (Enderlein, 1901), Valenzuela flavidus (Stephens, 1836) and Mesopsocus unipunctatus (Müller, 1764) were the most common species (see Table A1). Almost all of the collected species are arboreal on a variety of tree species; Lachesilla quercus (Kolbe, 1880) has been believed to be confined to oak [], but may also be found on other tree species, and outside the distribution of oak. Its apparent association with oak may rather be an expression of its preference [,] for dead leaves lingering on the tree, as commonly found on oaks, or on cut-off branches on the ground. Valenzuela flavidus and Graphopsocus cruciatus (Linnaeus, 1768) are associated with foliage of various deciduous trees [,].
3.2.7. Thysanoptera
Five specimens of Poecilothrips albopictus Uzel, 1895 were found at the two sites in Drangedal and in Larvik. This species was taken for the first time in Norway and its distribution indicates that it is fairly common. The biology of Thysanoptera is generally poorly known and it cannot be claimed that any of the 14 species in this study are associated with oaks—they are more likely to be associated with substrates offered by the tree, such as fungal spores, algae, etc.
3.2.8. Diptera
This was by far the most species rich group, with 334 species collected, 18 species new to science, 7 species new to the Nordic fauna and an additional 52 species caught in Norway for the first time (Table A1). Phoridae was the family with the largest number of specimens collected (212 specimens), followed by Ceratopogonidae (203) and Chironomidae (123). Phoridae was also the most species rich family by far, with 76 species, of which 16 species were new to science (all of them in the genus Megaselia); in addition, four species were new to the Nordic countries and 23 were new to Norway []. Borophaga agilis (Meigen, 1830) was reported new to Norway in [], but was later found to have been reported in []. Sciaridae was the second most species-rich group, with 43 species (one species new to science and eleven new records for Norway) [,], followed by Chironomidae with 42 species (one species new to science [], and two new to Norway). In addition, the following families were represented by new records: Limoniidae and Lauxaniidae (one new to the Nordic countries and one new to Norway, respectively), Ceratopogonidae (one new to the Nordic countries) and Fanniidae (one new to Norway).
The ecology of Diptera is mostly poorly known, and the abundant families in this study, e.g., Phoridae, Ceratopogonidae and Chironomidae, are usually neglected in general faunistic surveys. Only adults were identified, while habitat requirements are a characteristic of the larvae of most species in these families. Nonetheless, most of the species in the sciarid genera Bradysia, Corynoptera and Scatopsciara in this study (see Table A1) might have a connection with oak trees beyond accidental visits, as they are mentioned as deciduous forest species in the literature [,]. Other species of Sciaridae are also mentioned as deciduous forest associates (see Table A1). Additionally, Phyllodromia melanocephala (Fabricius, 1794) (Empididae) and Systenus bipartitus (Loew, 1850) (Dolichopodidae) are species known to inhabit deciduous forests. The first was one of the most common species, with 77 specimens collected and from all the sites.
Many species of Diptera are known to be trunk dwelling, fungivores or associated with rotting wood, habitats that are present abundantly in old oak trees. A rather high proportion of the collected species, where ecological information is available, can be assigned to either of these categories, most of them with few specimens. One exception was Forcipomyia titillans (Winnertz, 1852), a rotting matter associate [], which was found with 22 individuals.
Other individual species accounts worth mentioning are those being abundant at all the sites or aggregated at any one site. Culicoides impunctatus Goetghebuer, 1920 (Ceratopogonidae) is a haematophagous parasite on vertebrates and is also known to aggregate close to the breeding sites, which are humid areas, preferably peat bogs []. It was abundant in Steinknapp and Skjærsjø in particular, with 36 and 30 specimens collected, respectively. Phora edentata Schmitz, 1920 (Phoridae), a species new to Norway, was fairly abundant at most of the sites, which indicates that it is a rather common species. Two other species, Rhagio lineola Fabricius, 1794 (Rhagionidae) and Lyciella platycephala (Loew, 1847) (Lauxaniidae) were abundant in most sites. Both of these species are common and occupy many habitats. Twelve specimens of Anapausis helvetica Haenni, 1984 (Scatopsidae) were collected from Mule Varde and not from elsewhere. This species is rarely collected, but present knowledge may indicate an association with open areas, farmlands and parks []. Platypalpus ecalceatus (Zetterstedt, 1838) (Hybotidae) was collected with 13 individuals and only in Djupedal. This species is most likely a predator, as are nearly all Empidoidea (Terje Jonassen, pers. comm), but we cannot readily explain why it appears aggregated at only one site. We can see a similar pattern for two other Empidoidea, the dolichopodids Chrysotimus flaviventris (von Roser, 1840) and Dolichopus plumipes (Scopoli, 1763), being represented with 21 and 66 specimens in the Drangedal samples, respectively, and almost absent from all the other sites (see Table A1). Ten specimens of Megaselia robertsoni Disney, 2008 (Phoridae), a species new to Norway, were found only at Steinknapp.
3.2.9. Hymenoptera
A total of 117 species of Hymenoptera were collected, with one species new to science, four species new to the Nordic countries and 21 additional species new to Norway (Table A1). Many of the specimens could only be identified to genera or ‘near to’ designated species. Thus, we cannot rule out that there are additional undescribed species in this material. Of the two suborders, Symphyta and Apocrita were represented only by Apocrita. Of the 118 species, 12 Aculeata, i.e., nine Formicidae and three Crabronidae, were found, with the remaining 106 species all belonging to the ‘Parasitica infraorder’. Ceraphronoidea with 22 species (68 specimens); Chalcidoidea, 55 species (160); Cynipoidea, nine species (31); Diaprioidea, 11 species (15); Platygastroidea, 21 species (56). The Ichneumonoidea superfamily was not processed, only one species of Gelis sp. (1) has been added to the list. Ants in the mound building Formica rufa group, namely F. polyctena (Förster, 1850) were, not surprisingly, the most abundant species. They were all collected in Drangedal and from all the treated trees at Djupedal. None of the remaining species were abundant in any of the sites, but 30 specimens of Tamarixia pubescens (Nees, 1834) (Eulophidae), a new species to the Nordic fauna, were collected and taken at all the sites. This is a parasitoid of psyllids known to parasitize Trioza remota Förster, 1848 [], which, as nymph, is an oak obligate. T. remota was, however, not found in this study. Seladerma tarsale (Walker, 1833) (Pteromalidae) was also rather common with 24 specimens, whereof 14 were collected in Steinknapp. This species is a primary parasitoid of Agromyzidae flies []. No Agromyzidae were present in the material, however.
The representation of species shows a well-defined association with oak-galls. The oak-galls living inquilins are Ceroptres clavicornis Hartig, 1840, Neuroterus nr. politus Hartig, 1840, Saphonecrus connatus (Hartig, 1840), Synergus apicalis Hartig, 1841, S. crassicornis (Curtis, 1838), S. gallaepomiformis (Fonscolombe, 1832) and S. pallipes Hartig, 1840, all of which are in the Cynipidae family. Of the large number of oak-gall parasitoids the following are worth mentioning: Aulogymnus gallarum (Linnaeus, 1761) (Eulophidae), Eupelmus annulatus Nees, 1834 (Eupelmidae), Ormyrus pomaceus (Geoffroy, 1785) (Ormyridae) and the pteromalids Cecidostiba semifascia (Walker, 1835), Mesopolobus dubius (Walker, 1834), M. fasciiventris Westwood, 1833, M. tarsatus (Nees, 1834), M. tibialis (Westwood, 1833), M. xanthocerus (Thomson, 1878), Megastigmus dorsalis (Fabricius, 1798) and Torymus flavipes (Walker, 1833).
3.2.10. Coleoptera
Of the 84 species of beetles found, the following three are on the Norwegian red list: Malthinus seriepunctatus Kiesenwetter, 1851 (Cantharidae), Prionocyphon serricornis (Müller, 1821) (Scirtidae) and Dasytes aeratus Stephens, 1830 (Dasytidae) (Table 3), all of which are categorized as near threatened in [].
Several of the following species are associated with oak or oak habitats: the curculionid Archarius pyrrhoceras (Marsham, 1802), Coeliodes rana (Fabricius, 1787), Orchestes quercus (Linnaeus, 1758), the already-mentioned cantharid M. seriepunctatus, the ciid Cis vestitus (Mellié, 1848), the melandryid Conopalpus testaceus (Olivier, 1790), the chrysomelid Cryptocephalus labiatus (Linnaeus, 1761) and the cerambycid Leiopus linnei Wallin, Nylander and Kvamme, 2009 [,,]. Furthermore, many species are known to be arboreal (see Table A1) but being rare in this material was common for most of them. A common, arboreal species was Otiorhynchus singularis (Linnaeus, 1767) (Curculionidae), which is a species found almost everywhere. Thirty-one specimens were found at all the sites but Skjærsjø. Another weevil, Strophosoma capitatum (De Geer, 1775), a common herbivore on broadleaf trees, was found with 86 specimens at all but the two sites in Western Norway. The predacious Cantharidae Malthodes guttifer Kiesenwetter, 1852 was collected at all the sites, except for Berge, with a total of 61 specimens. This is a common species associated with shrubs and often found climbing trees []. Eleven specimens of Orchesia micans (Panzer, 1793) (Melandryidae) were taken in Skjærsjø, its only appearance in the study. It has a close association with polypore fungi in the genus Inonotus []. The throscid Trixagus dermestoides (Linnaeus, 1767) was found with 11 specimens, ten of them from Steinknapp. This species is known as a generalist pollen and mold feeder (e.g., []), with habitats plentiful in oaks.
3.2.11. Species Accumulation
The number of invertebrate species collected was 722 and with an overall turnover of 13.34, suggesting a rather homogenous species pool along the sampling gradient, thus rejecting H1. Despite the apparent homogeneity, there is a logarithmic relationship between the number of specimens collected and the number of species found (Figure 4), suggesting that a much more profound sampling effort needs to be performed before the accumulation curve starts to converge. A steep species accumulation curve is to be expected, as the sample size was low and there was a high number of singletons and uniques.
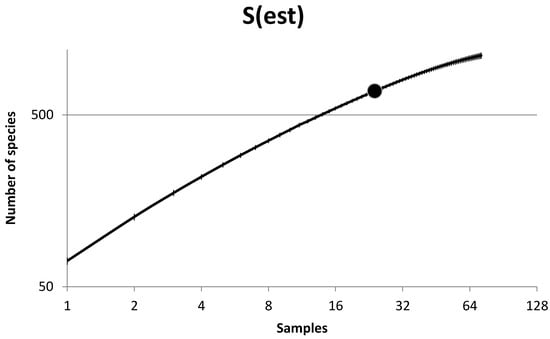
Figure 4.
Rarefaction curves of oak diversity extrapolated beyond the dot (i.e., 24 trees) to yield 72 treatments (i.e., trees). The dot shows the number of species sampled by the number of trees treated. Note the log2 x-axis and the log10 y-axis.
4. Discussion
4.1. Invertebrate Samples
The number of collected specimens in this study was very low compared with the material collected from a comparable study of 24 pine trees over a geographic gradient from west to east Norway, and where nearly 30,000 specimens were collected using the same methodology []. One explanation is fairly obvious, as the weather in both sampling periods (June/July 2011/2012) was generally cool and wet. The monthly temperature in 2011 was, on average, slightly higher than the normal temperature (ranging from −0.1 °C below (Kvam, June) to +1.7 °C above (Kvam, July)), but the precipitation ranged from 104% (Kvam, July) to 270% (Drangedal, July) of the normal [,]. For the year 2012, the monthly temperature was lower than the normal temperature (from −1.8 °C (Drangedal, June) to −0.2 °C (Kvam, July) below), and these months were also generally wetter than the normal (from 69% (Kvam, June) to 169% (Kvam, July)) [,]. Other reasons for the low catch may be related to the structure and complexity of the oak canopy compared with the more open canopy of, for example, pine, in that a larger proportion of the invertebrates remain in the tree—either stuck in the dense foliage or on the branches [].
4.2. Faunistics
Despite the fact that the ecology is unknown for many species (see Table A1), a large proportion of the species found in this study must be assumed to be occasional visitors (i.e., the oak canopy is not their primary habitat). As oaks offer a wide selection of sites to rest, swarm and feed, an abundance of generalists is to be assumed, as well as opportunists taking advantage of the secondary habitats in the trees, for example, the ant Camponotus ligniperda (Latreille, 1802) living in dead parts of the tree or the numerous species associated with deposited leaf litter or soils. Yet, a few other species are likely to be accidental visitors from the surroundings, e.g., species associated with grasses and Calluna (see Table A1). The presence of the marine chironomid Halocladius variablis (Stæger, 1839) in Steinknapp is surprising, as the distance to the ocean is about 30 km. Its presence in Skeianeset and Mule Varde makes sense, however, as both sites are close to the sea.
Even though neither the psyllid Trioza nor agromyzid flies were found as adults, we must believe them to be present, as parasitoids of both were common—Tamarixia pubescens (Eulophidae) and Seladerma tarsale (Pteromalidae), respectively. Both host groups are known to live on oaks [,]. Another fact to note is that no species of the egg parasitoid family Mymaridae (Chalcidoidea) were collected. Mymaridae are among the smallest insects in the world and, regarding the number of species and specimens collected, it is inconceivable that Mymaridae species would not be present in larger numbers as well. Unfortunately, due to their size and fragility, they are likely to remain in the canopy foliage after fogging.
Correspondence in the presence of species over a broader selection of the literature shows that 80 of the species collected in this study were also present in other European studies on oak canopy or oak tree faunas [,,,,,,,,,,,].
4.3. Conservation and Distribution of Invertebrates
Some paradoxes arise when comparing the number of red-listed species with the number of species new to science or new occurrences. Only five red-listed species were found, while the number of new occurrences, including new species, were 92 altogether, most of them with very few specimens. This demonstrates how poorly known the Norwegian arboreal invertebrate fauna still is. One of the criteria for inclusion on the Red List is that a species should be known to reproduce for more than 10 years in the period 1800–2015 []. Moreover, rarity is not a criterion for inclusion as such, but reduced population sizes, reduced habitats or reduced distributions are. Thus, the value of the red list category for a species is based on the changes in the intermediate-term development of its population and no new species or species observations will qualify for considerations into the list, but it should incentivize the monitoring of those species. Inasmuch, a new species does not necessarily have to be rare, it may just have been overlooked. Several new species or occurrences were widespread and with intermediate numbers, e.g., Damaeus n. sp. (50 specimens, five localities), Xenillus (Xenillus) discrepans (18 specimens, three localities), Tamarixia pubescens (30 specimens, all localities), Megaselia ignobilis (19 specimens, four localities) and Phora edentata (40 specimens, four localities) (Table A1). Canopy specialists may well have been overlooked, as some are, apparently, rarely collected using conventional techniques and the obvious inaccessibility to the canopy complicates sampling.
Oaks used to be evenly distributed within its distributional range in Norway, and fragmentation was caused by overexploitation and a colder climate in the beginning of the sub-Atlantic era []. The rejection of H1 can be a response to a historically continuous distribution of oaks by the remaining relic populations of invertebrates. Additionally, compared with the more diverse forest sites, the poorer community of plants, homogeneous canopy structure [] and different microclimate [,] in the actively managed sites, Mule Varde and Berge, are likely to source a different fauna to the oak trees on these sites, thus, supporting H2.
Author Contributions
Conceptualization, K.H.T. and G.E.E.S.; methodology, K.H.T.; formal analysis, K.H.T.; investigation, G.E.E.S., C.T., A.F., S.O., S.R., C.-C.C., R.H.L.D., J.S., G.V., T.J., J.A., A.K., F.M., R.S., E.S., W.A., K.M.O., T.K., A.E., S.P., S.K., L.O.H., G.M.K., J.-P.H. and L.B.; data curation, G.E.E.S. and T.K.; writing—original draft preparation, K.H.T.; writing—review and editing, G.E.E.S., C.T., A.F., S.O., S.R., C.-C.C., R.H.L.D., J.S., G.V., T.J., J.A., A.K., F.M., R.S., E.S., W.A., K.M.O., T.K., A.E., S.P., S.K., L.O.H., G.M.K., J.-P.H. and L.B.; project administration, K.H.T. and G.E.E.S.; funding acquisition, K.H.T. and G.E.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Norwegian Biodiversity Information Centre, grant numbers 70184219 and 70184228.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Input data and result files to the numerical analyses can be acquired by contacting K.H.T. They are also available by consulting post@nibio.no.
Acknowledgments
We are indebted to Hans Nyeggen, Adrian Rasmussen, Jon Peder Lindemann and Vebjørn G. Thunes for assistance during the field work. We would also like to thank Tibor Bukovinszky for valuable comments on the manuscript and to Belachew Gizachew Zeleke for preparing Figure 1. Finally, we would like to thank the landowners in question for giving permission to use their land.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Complete list of species with numbers per locality. Literature used for the table: [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,], relevant volumes of Die Käfer Mitteleuropas, Danmarks Fauna, Svensk Insektfauna, Fauna Entomologica Scandinavica, and personal comments from the authors. The (B) and (S) in the heading under Kvam are Berge and Skeianeset, respectively, while the (S) and (D) under Drangedal are Steinknapp and Djupedal, respectively. An ¤, * or ** in front of the species name depicts a new record for either science, Norway or Nordic countries upon sampling, respectively. x, xx and xxx represent dummy numbers 5, 10 and 20, respectively.
Table A1.
Complete list of species with numbers per locality. Literature used for the table: [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,], relevant volumes of Die Käfer Mitteleuropas, Danmarks Fauna, Svensk Insektfauna, Fauna Entomologica Scandinavica, and personal comments from the authors. The (B) and (S) in the heading under Kvam are Berge and Skeianeset, respectively, while the (S) and (D) under Drangedal are Steinknapp and Djupedal, respectively. An ¤, * or ** in front of the species name depicts a new record for either science, Norway or Nordic countries upon sampling, respectively. x, xx and xxx represent dummy numbers 5, 10 and 20, respectively.
| Kvam | Drangedal | Porsgrunn | Larvik | |||||
|---|---|---|---|---|---|---|---|---|
| Higher Taxon | Species | Habitat | 1(B) | 2(S) | 3(S) | 4(D) | 5 | 6 |
| ARANEAE | ||||||||
| Anyphaenidae | Anyphaena accentuata (Walckenaer, 1802) | Varies | x | x | ||||
| Araneidae | Araneus sturmi (Hahn, 1831) | Conifer forests | x | x | ||||
| Araniella displicata (Hentz, 1847) | x | |||||||
| Clubionidae | Clubiona brevipes Blackwall, 1841 | x | x | |||||
| Dictynidae | Dictyna pusilla Thorell, 1856 | x | ||||||
| Linyphiidae | Agyneta conigera (Cambridge, 1863) | x | ||||||
| Diplocephalus picinus Blackwall, 1841 | Broadleaf forest | x | ||||||
| Entelecara acuminata (Wider, 1834) | x | x | ||||||
| Erigone atra Blackwall, 1833 | Varies | x | ||||||
| Maso sundevalli (Westring, 1851) | x | |||||||
| Moebelia penicillata (Westring, 1851) | Crevices, forests, arboreal | x | ||||||
| Neriene peltata (Wider, 1834) | Branches, bushes | x | x | x | x | x | ||
| N. radiata (Walckenaer, 1842) | x | |||||||
| Pelecopsis elongata (Wider, 1834) | Vegetation, dry | x | ||||||
| Mimetidae | Ero furcata (Villers, 1789) | Varies | x | |||||
| Philodromidae | Philodromus cespitum (Walckenaer, 1802) | Conifer forests | x | x | ||||
| Pisauridae | Pisaura mirabilis (Clerck, 1757) | Heath, dry | x | |||||
| Segestriidae | Segestria senoculata (Linnaeus, 1758) | Holes in wall and bark | x | |||||
| Tetragnathidae | Tetragnatha montana Simon, 1874 | x | ||||||
| Theridiidae | Selimus vittatus (C. L. Koch, 1836) | x | x | |||||
| Dipoena braccata (C. L. Koch, 1841) | Thermoph., branches | x | ||||||
| Paidiscura pallens (Blackwall, 1834) | Varies, oak | x | x | x | x | x | x | |
| Parasteatoda tepidariorum (C. L. Koch, 1841) | x | |||||||
| Platnickina tincta (Walckenaer, 1802) | Conifer forests | x | ||||||
| Robertus neglectus (Cambridge, 1871) | x | |||||||
| Theridion hemerobium Simon, 1914 | x | |||||||
| T. mystaceum L. Koch, 1870 | Synantrop, bark, bush | x | ||||||
| Uloboridae | Hyptiotes paradoxus (C. L. Koch, 1834) | Spruce forest | x | |||||
| Sum species: 28 | 5 | 3 | 17 | 6 | 6 | 6 | ||
| OPILIONES | ||||||||
| Phalangiidae | Lacinius ephippiatus (C. L. Koch, 1835) | 12 | 1 | 3 | ||||
| Mitopus morio (Fabricius, 1799) | 1 | 1 | ||||||
| Sclerosomatidae | Leiobunum gracile Thorell, 1876 | 2 | 1 | |||||
| Nelima gothica Lohmander, 1945 | 2 | |||||||
| Sum species: 4 | 2 | 2 | 1 | 1 | 2 | |||
| Sum specimens: 23 | 13 | 3 | 3 | 2 | 2 | |||
| ACARI | ||||||||
| Anystidae | Anystis baccarum (Linnaeus, 1758) | Predator, woody plants | 7 | 1 | 272 | 120 | 8 | |
| Ascidae | Neojordensia sinuata Athias-Henrlot, 1973 | Predator | 1 | |||||
| Bdellidae | Bdella iconica Berlese, 1923 | Predator | 1 | |||||
| B. muscorum Ewing, 1909 | Predator | 4 | ||||||
| Biscirus silvaticus (Kramer, 1881) | Predator | 6 | 8 | |||||
| Erythraeidae | cf. Abrolophus sp. | 1 | ||||||
| Eupodidae | Eupodes voxencollinus Thor, 1934 | 1 | ||||||
| Ixodidae | Ixodes ricinus (Linnaeus, 1758) | Mammal parasite | 4 | 4 | 2 | 3 | ||
| Parasitidae | Holoparasitus calcaratus (C. L. Koch, 1839) | Predator | 1 | 2 | ||||
| Parasitus sp. | Predator | 2 | ||||||
| Phytoseiidae | Euseius finlandicus (Oudemans, 1915) | Predator, woody plants | 1 | 1 | ||||
| Zerconidae | Zercon spatulatus (C. L. Koch, 1839) | Predator, dry habitats | 1 | 1 | ||||
| Achipteriidae | Achipteria (A.) coleoptrata (Linnaeus, 1758) | Forest litter, meadows, | 1 | |||||
| Caleremaeidae | Caleremaeus monilipes (Michael, 1822) | Decaying wood, stumps | 1 | |||||
| Camisiidae | Camisia (C.) horrida (Hermann, 1804) | Mosses on trees | 2 | 12 | 7 | 1 | 5 | |
| Heminothrus (Platynothrus) peltifer (C. L. Koch, 1839) | Forest litter, mosses | 1 | 2 | |||||
| Carabodidae | Carabodes (C.) areolatus Berlese, 1916 | Lichens, mosses on trees | 1 | |||||
| C. (C.) labyrinthicus (Michael, 1879) | Lichens, mosses on trees | 3 | 1 | 4 | ||||
| C. (C.) ornatus Štorkán, 1925 | Coniferous forest litter | 1 | ||||||
| C. (C.) rugosior Berlese, 1916 | Forest litter, stumps, | 1 | ||||||
| Odontocepheus (O.) elongates (Michael, 1879) | Forest litter, mosses | 1 | ||||||
| Cepheidae | Cepheus cepheiformis (Nicolet, 1855) | Forest leaf litter | 1 | |||||
| Metrioppiidae | Ceratoppia bipilis (Hermann, 1804) | Forest leaf litter | 1 | |||||
| Cymberemaeidae | Cymbaeremaeus cymba (Nicolet, 1855) | Lichens, mosses on trees | 1 | 4 | 1 | |||
| Damaeidae | ¤Damaeus n.sp. | 2 | 7 | 9 | 2 | 31 | ||
| Ceratozetidae | Diapterobates humeralis (Hermann, 1804) | Forest litter, mosses | 47 | 2 | 12 | |||
| Trichoribates (T.) trimaculatus (C. L. Koch, 1836) | Forest litter | 1 | ||||||
| Eremaeidae | Eueremaeus oblongus silvestris Forsslund, 1956 | Mosses, leaf litter | 5 | 1 | 1 | |||
| Phenopelopodidae | Eupelops acromios (Hermann, 1804) | Mosses, lichens on trees | 8 | 16 | 4 | |||
| Euphthiracaridae | Euphthiracarus (E.) cribrarius (Berlese, 1904) | Forest litter, decaying wood | 1 | |||||
| Galumnidae | Galumna (G.) lanceata (Oudemans, 1900) | Forest litter | 1 | |||||
| Oribatulidae | Hemileius (H.) initialis (Berlese, 1908) | Forest litter, meadows | 1 | 2 | 1 | 1 | ||
| Oribatula (Zygoribatula) exilis (Nicolet, 1855) | Mosses, lichens on trees | 1 | 13 | 1 | ||||
| Phauloppia lucorum (C. L. Koch, 1841) | Lichens on trees | 5 | 6 | 87 | 43 | 6 | 23 | |
| **P. nemoralis (Berlese, 1916) | Lichens on trees | 1 | 2 | |||||
| Chamobatidae | Chamobates (C.) borealis (Trägårdh, 1902) | Forest litter | 1 | 1 | 2 | |||
| C. (C.) pusillus (Berlese, 1895) | Forest litter | 3 | 2 | 4 | 4 | 1 | ||
| Liacaridae | **Liacarus (Dorycranosus) splendens (Coggi, 1898) | Decaying wood, | 1 | |||||
| L. (Liacarus) coracinus (C. L. Koch, 1841) | Decaying wood, litter | 1 | ||||||
| Mycobatidae | Mycobates (M.) parmeliae (Michael, 1884) | Forest litter | 2 | |||||
| Oribatellidae | **Oribatella (Oribatella) quadricornuta (Michael, 1880) | Forest litter | 14 | |||||
| Phthiracaridae | ¤Phthiracarus n. sp. | 1 | 1 | 3 | ||||
| Steganacaridae | Steganacarus (Tropacarus) carinatus (C. L. Koch, 1841) | Leaf litter in forests | 1 | |||||
| Xenillidae | **Xenillus (Xenillus) discrepans Grandjean, 1936 | Deciduous forest litter | 14 | 3 | 1 | |||
| Sum species: 44 | 11 | 12 | 28 | 14 | 8 | 22 | ||
| Sum specimens: 907 | 28 | 42 | 500 | 212 | 24 | 101 | ||
| ISOPODA | ||||||||
| Armadillidiidae | Armadillidium pictum Brandt, 1833 | 14 | 127 | 366 | 8 | |||
| A. pulchellum (Zencker, 1799) | 2 | |||||||
| Oniscidae | Oniscus asellus Linnaeus, 1758 | 2 | 2 | 1 | ||||
| Philosciidae | Philoscia muscorum (Scopoli, 1763) | 6 | ||||||
| Trachelipodidae | Trachelipus ratzeburgii (Brandt, 1833) | Broadleaf forest | 16 | 3 | ||||
| Sum species: 5 | 1 | 2 | 1 | 2 | 1 | 4 | ||
| Sum specimens: 547 | 2 | 16 | 127 | 382 | 6 | 14 | ||
| MYRIAPODA | ||||||||
| Chilopoda | ||||||||
| Lithobiidae | Lithobius borealis Meinert, 1868 | 2 | 3 | 7 | ||||
| Diplopoda | ||||||||
| Julidae | Cylindroiulus punctatus (Leach, 1815) | 1 | ||||||
| Sum species: 2 | 1 | 2 | 1 | |||||
| Sum specimens: 13 | 3 | 6 | 8 | |||||
| COLLEMBOLA | ||||||||
| Bourletiellidae | Bourletiella hortensis (Fitch, 1863) | Vegetation | 1 | |||||
| Deuterosminthurus bicinctus (Koch, 1840) | Vegetation, bushes | 8 | ||||||
| Dicyrtomidae | Dicyrtomina minuta (O. Fabricius, 1783) | Forest floor | 2 | 3 | ||||
| Entomobryidae | Entomobrya albocincta (Templeton, 1835) | Bark, lichens | 3 | |||||
| E. corticalis (Nicolet, 1842) | Bark, lichens | xx | xx | 1 | ||||
| E. marginata (Tullberg, 1871) | Bark, lichens | x | xx | |||||
| E. nicoleti (Lubbock, 1868) | Forest floor | 9 | ||||||
| E. nivalis (Linnaeus, 1758) | Bark, lichens | 1 | 90 | xxx | xxx | 20 | xxx | |
| Lepidocyrtus lignorum (Fabricius, 1793) | Litter | 1 | xx | x | 6 | 5 | ||
| L. violaceus (Geoffroy, 1762) | Litter | 1 | ||||||
| Orchesella bifasciata Bourlet, 1839 | Moss, rocks, trunks | 11 | x | xx | 3 | |||
| O. cincta (Linnaeus, 1758) | Forest floor | 3 | xx | 3 | ||||
| O. flavescens (Bourlet, 1839) | Forest floor | x | 1 | 1 | ||||
| Willowsia buskii (Lubbock, 1870) | Xero- thermoph, trunks | x | ||||||
| Hypogastruridae | Xenylla maritima Tullberg, 1869 | Xerophilous | 1 | xx | x | |||
| Isotomidae | Isotoma anglicana Lubbock, 1862 | Litter | 1 | |||||
| Isotomurus graminis Fjellberg, 2007 | Hygrophilous | 1 | ||||||
| Pseudisotoma sensibilis (Tullberg, 1876) | Moss, forest floor | 2 | ||||||
| Katiannidae | Sminthurinus aureus (Lubbock, 1836) | Litter | 2 | |||||
| S. alpinus Gisin, 1953 | Bark, dead trees | x | ||||||
| Sminthuridae | Allacma fusca (Linnaeus, 1758) | Forest floor | 4 | 26 | 9 | xx | ||
| Lipothrix lubbocki (Tullberg, 1872) | Forest floor | x | 1 | |||||
| Sminthuridae (juveniles) | Litter | 2 | ||||||
| Tomoceridae | Pogonognathellus flavescens (Tullberg, 1871) | Forest floor | 7 | |||||
| Sum species: 23 | 6 | 9 | 7 | 9 | 10 | 8 | ||
| DICTYOPTERA | ||||||||
| Blattelidae | Ectobius lapponicus (Linnaeus, 1758) | 8 | 2 | |||||
| DERMAPTERA | ||||||||
| Forficulidae | Chelidura guentheri (Galvagni, 1994) | 7 | 4 | 2 | 2 | |||
| Sum species: 2 | 1 | 2 | 2 | 1 | ||||
| Sum specimens: 25 | 7 | 12 | 4 | 2 | ||||
| EPHEMEROPTERA | ||||||||
| Baetidae | Cloeon inscriptum Bengtsson, 1914 | 1 | ||||||
| PLECOPTERA | ||||||||
| Nemouridae | Amphinemura borealis (Morton, 1894) | Streams | 1 | |||||
| A. sulcicollis (Stephens, 1836) | Streams | 1 | ||||||
| Nemoura cinerea (Retzius, 1783) | Streams | 3 | 10 | 2 | ||||
| Sum species: 4 | 1 | 2 | 1 | 1 | 1 | |||
| Sum specimens: 18 | 1 | 4 | 10 | 1 | 2 | |||
| HEMIPTERA | ||||||||
| Heteroptera | ||||||||
| Anthocoridae | Anthocoris nemoralis (Fabricius, 1794) | Predator, arboreal, deciduous | 6 | |||||
| A. nemorum (Linnaeus, 1761) | Predator, vegetation | 1 | 1 | |||||
| Orius minutus (Linnaeus, 1758) | Predator, varies | 1 | ||||||
| Temnostethus gracilis Horváth, 1907 | Predator, varies | 54 | 1 | 81 | 20 | |||
| T. cf. gracilis Horváth, 1907 | 40 | 1 | 52 | 1 | 18 | |||
| Microphysidae | Loricula elegantula (Baerensprung, 1858) | Predator, lichens, trunk | 2 | 2 | 71 | 4 | 19 | 1 |
| L. pselaphiformis Curtis, 1833 | Predator, lichens, trunk | 2 | 1 | 3 | 1 | |||
| Indet. (Anthocoridae or Microphysidae) | 50 | 80 | 2 | 1 | 37 | |||
| Miridae | Blepharidopterus angulatus (Fallén, 1807) | Predator, arboreal, deciduous | 1 | |||||
| Cyllecoris histrionicus (Linnaeus, 1767) | Predator, oak | 3 | 6 | 3 | ||||
| Dichrooscytus rufipennis (Fallén, 1807) | Pine flowers and cones | 1 | ||||||
| Orthotylus tenellus (Fallén, 1807) | Predator, arboreal, deciduous | 2 | 1 | |||||
| Phoenicocoris obscurellus (Fallén, 1829) | Pine | 3 | ||||||
| Phylus melanocephalus (Linnaeus, 1767) | Predator, oak | 15 | 16 | 10 | 36 | 7 | ||
| Phytocoris intricatus Flor, 1861 | Conifers | 2 | 1 | |||||
| Ph. sp. | 1 | 9 | 1 | 1 | ||||
| Psallus confusus Rieger, 1981 | Predator, oak | 2 | ||||||
| Ps. mollis (Mulsant and Rey, 1852) | Predator, oak | 17 | 4 | 5 | 1 | |||
| Ps. variabilis (Fallén, 1807) | Predator, oak | 1 | 10 | |||||
| Ps. varians (Herrich-Schaeffer, 1841) | Predator, arboreal, deciduous | 9 | 27 | 1 | 6 | 1 | ||
| Ps. wagneri Ossiannilsson, 1953 | Predator, oak | 2 | 1 | |||||
| Ps. spp. | 23 | 3 | 84 | 31 | 104 | 1 | ||
| Rhabdomiris striatellus (Fabricius, 1794) | Oak | 1 | 5 | |||||
| Miridae indet. | 1 | 1 | ||||||
| Lygaeidae | Scolopostethus thomsoni Reuter, 1875 | Varies | 1 | |||||
| Pentatomidae | Pentatoma rufipes (Linnaeus, 1758) | Predator, arboreal, deciduous | 3 | 7 | 5 | 1 | ||
| Sum species: 21 | 9 | 7 | 11 | 9 | 16 | 8 | ||
| Sum specimens:1030 | 149 | 71 | 377 | 59 | 284 | 90 | ||
| Auchenorrhyncha | ||||||||
| Cixiidae | Cixius cunicularius (Linnaeus, 1767) | Deciduous wood plants | 1 | |||||
| Delphacidae | Javesella forcipata (Boheman, 1847) | Grass, open habitats | 1 | |||||
| Stiroma affinis Fieber, 1866 | Grass, forest | 3 | ||||||
| Xanthodelphax flaveolus (Flor, 1861) | Grass | 1 | ||||||
| Issidae | Issus muscaeformis (Schrank, 1781) | Deciduous wood plants | 7 | 7 | 15 | 2 | ||
| Aphrophoridae | Neophilaenus lineatus (Linnaeus, 1758) | Grass | 1 | 1 | ||||
| Cicadellidae | Alebra albostriella (Fallén, 1826) | Quercus | 10 | 2 | 7 | 1 | ||
| Edwardsiana frustrator (Edwards, 1908) | Deciduous wood plants | 1 | 1 | |||||
| E. sp. | 1 | 1 | ||||||
| Eupteryx sp. | 1 | |||||||
| Eurhadina concinna (Germar, 1831) | Quercus | 5 | ||||||
| Iassus lanio (Linnaeus, 1761) | Quercus | 1 | ||||||
| Populicerus populi (Linnaeus, 1761) | Populus tremula | 6 | ||||||
| Ribautiana scalaris (Ribaut, 1931) | Quercus | 1 | ||||||
| Typhlocyba quercus (Fabricius, 1777) | Prunus / Quercus | 1 | ||||||
| Sum species: 14 | 5 | 4 | 4 | 6 | 4 | |||
| Sum specimens: 78 | 26 | 15 | 19 | 13 | 5 | |||
| PSOCODEA | ||||||||
| Trogiidae | Cerobasis guestfalica (Kolbe, 1880) | Bark | 2 | 3 | 1 | 1 | ||
| Caeciliusidae | Valenzuela burmeisteri (Brauer, 1876) | Conifers, arboreal | 1 | 1 | ||||
| V. despaxi (Badonnel, 1936) | Conifers, arboreal | 1 | 1 | |||||
| V. flavidus (Stephens, 1836) | Deciduous, arboreal | 11 | 4 | 79 | 31 | 2 | ||
| V. sp. | 10 | |||||||
| Elipsocidae | Cuneopalpus cyanops (Rostock, 1876) | Conifers, arboreal | 1 | |||||
| Elipsocus abdominalis Reuter, 1904 | Bark, lichens | 1 | ||||||
| E. moebiusi Tetens, 1891 | Bark | 11 | 7 | |||||
| E. pumilis (Hagen, 1861) | Bark | 14 | 41 | 1 | ||||
| E. sp. (moebiusi or pumilis) | 1 | |||||||
| Reuterella helvimacula (Enderlein, 1901) | Bark, lichens | 1 | 15 | 88 | 18 | 11 | ||
| Mesopsocidae | Mesopsocus immunis (Stephens, 1836) | Bark | 3 | 4 | ||||
| M. laticeps (Kolbe, 1880) | Bark | 7 | ||||||
| M. unipunctatus (Müller, 1764) | Bark | 16 | 34 | 20 | 18 | |||
| Psocidae | Amphigerontia bifasciata (Latreille, 1799) | Bark | 2 | 1 | ||||
| Blaste conspurcata (Rambur, 1842) | Bark, xerophilous | 1 | ||||||
| Loensia fasciata (Fabricius, 1787) | Bark | 1 | 4 | 1 | ||||
| L. sp. (variegata or pearmani) | 1 | 20 | 1 | |||||
| Metylophorus nebulosus (Stephens, 1836) | Bark | 6 | 7 | 6 | ||||
| Psococerastis gibbosa (Sulzer, 1776) | Bark | 9 | 22 | 3 | 1 | 47 | ||
| Trichadenotecnum sexpunctatum (Linnaeus, 1758) | Bark | 3 | 4 | |||||
| Lachesillidae | Lachesilla quercus (Kolbe, 1880) | Dead branches, leaves | 1 | |||||
| Stenopsocidae | Graphopsocus cruciatus (Linnaeus, 1768) | Deciduous, arboreal | 3 | 19 | 12 | 1 | ||
| Stenopsocus lachlani Kolbe, 1880 | Conifers, arboreal | 1 | 2 | |||||
| Philotarsidae | Philotarsus parviceps Roesler, 1954 | Bark | 2 | |||||
| Peripsocidae | Peripsocus phaeopterus (Stephens, 1836) | Bark | 1 | |||||
| P. subfasciatus (Rambur, 1842) | Bark | 6 | 9 | |||||
| P. sp. (didymus or phaeopterus) | 2 | |||||||
| Sum species: 24 | 9 | 7 | 19 | 9 | 10 | 9 | ||
| Sum specimens: 655 | 57 | 64 | 235 | 187 | 45 | 67 | ||
| THYSANOPTERA | ||||||||
| Aeolothripidae | Aeolothrips melaleucus Haliday, 1852 | Predator | 1 | |||||
| A. versicolor Uzel, 1895 | Predator | 1 | ||||||
| Thripidae | Ceratothrips ericae (Haliday, 1836) | Calluna, heath | 1 | 1 | ||||
| Oxythrips ajugae Uzel, 1895 | Pine cones | 6 | ||||||
| Taeniothrips picipes (Zetterstedt, 1828) | Herb flowers | 1 | ||||||
| Thrips major Uzel, 1895 | Herb flowers | 4 | ||||||
| T. pini (Uzel, 1895) | Pine | 1 | ||||||
| Phlaeothripidae | Acanthothrips nodicornis (Reuter, 1880) | Dead branches, bark | 1 | |||||
| Haplothrips sp. | 1 | 1 | ||||||
| Hoplothrips pedicularius (Haliday, 1836) | Stereum rugosus | 2 | 4 | 1 | ||||
| H. ulmi (Fabricius, 1781) | Dead wood, fungivore | 2 | ||||||
| Phlaeothrips coriaceus Haliday, 1836 | Dead wood, fungivore | 10 | ||||||
| *Poecilothrips albopictus Uzel, 1895 | ? Dead wood, fungivore | 2 | 2 | 1 | ||||
| Xylaplothrips fuliginosus (Schille, 1911) | Buds, bark, predator | 1 | ||||||
| Sum species: 14 | 1 | 2 | 7 | 5 | 2 | 3 | ||
| Sum specimens: 44 | 6 | 6 | 20 | 7 | 2 | 3 | ||
| TRICHOPTERA | ||||||||
| Hydropsychidae | Hydropsyche siltalai Doehler, 1963 | 2 | ||||||
| Limnephilidae | Limnephilus centralis Curtis, 1834 | 5 | ||||||
| Sum species: 2 | 1 | 1 | ||||||
| Sum specimens: 7 | 5 | 2 | ||||||
| DIPTERA | ||||||||
| Nematocera | ||||||||
| Tipulidae | Tipula irrorata Macquart, 1826 | Rotten wood, mosses | 1 | |||||
| T. lunata Linnaeus, 1758 | Shredder, leaf litter, soil | 1 | ||||||
| T. scripta Meigen, 1830 | Shredder, leaf litter, green mosses | 1 | 1 | |||||
| Nephrotoma analis (Schummel, 1833) | Shredder, leaf litter, soil, exposed riverine sediments | 1 | ||||||
| Limoniidae | **Achyrolimonia neonebulosa (Alexander, 1924) | Rotten wood, fungi, wood sap | 1 | 1 | ||||
| Austrolimnophila ochracea (Meigen, 1804) | Rotten wood, fungi | 1 | ||||||
| Dicranomyia didyma (Meigen, 1804) | Aquatic, semiaquatic, aquatic mosses, algae in waterfalls, shredder | 1 | ||||||
| D. mitis (Meigen, 1830) | Leaf litter, soil, exposed riverine sediments, shredder | 1 | 1 | |||||
| D. modesta (Meigen, 1818) | Leaf litter, soil, exposed riverine sediments, shredder | 1 | 5 | |||||
| Dicranophragma separatum (Walker, 1848) | Predator, semi-aquatic | 1 | 1 | 1 | ||||
| Epiphragma ocellare (Linnaeus, 1761) | Rotten wood | 1 | ||||||
| Erioptera lutea Meigen, 1804 | Collector, semi-aquatic | 1 | ||||||
| Euphylidorea phaeostigma (Schummel, 1829) | Predator, semi-aquatic | 1 | ||||||
| Limonia flavipes (Fabricius, 1787) | Leaf litter, soil, under bark, shredder | 2 | ||||||
| L. phragmitidis (Schrank, 1781) | Leaf litter, soil, under bark, riverside mud, shredder | 1 | ||||||
| Molophilus appendiculatus (Staeger, 1840) | Collector, semi-aquatic | 2 | 3 | 1 | ||||
| M. bifidus Goetghebuer, 1920 | Collector, semi-aquatic | 1 | ||||||
| M. medius de Meijere, 1918 | Collector, semi-aquatic | 1 | ||||||
| M. ochraceus (Meigen, 1818) | Collector, semi-aquatic | 1 | ||||||
| Neolimonia dumetorum (Meigen, 1804) | Rotten wood, fungi | 1 | ||||||
| Ormosia lineata (Meigen, 1804) | Collector, semi-aquatic | 2 | ||||||
| O. ruficauda (Zetterstedt, 1838) | Collector, semi-aquatic | 1 | 1 | |||||
| Pilaria discicollis (Meigen, 1818) | Predator, semi-aquatic | 2 | ||||||
| *Tasiocera fuscescens (Lackschewitz, 1940) | Collector, semi-aquatic | 1 | 3 | |||||
| Bibionidae | Bibio nigriventris Haliday, 1833 | Eurytop, soil | 1 | 1 | ||||
| Psychodidae | Pericoma cf. albomaculata Wahlgren, 1904 | Likely saprophagous | 1 | 3 | ||||
| Psychoda gemina (Eaton, 1904) | Saprophag, semiaquatic | 4 | ||||||
| P. phalaenoides (Linnaeus, 1758) | Coprophagous | 4 | 14 | 1 | ||||
| P. sp. | 2 | 1 | 1 | |||||
| Trichopsychoda hirtella (Tonnoir, 1919) | Saprophagous | 2 | ||||||
| Anisopodidae | Sylvicola cinctus (Fabricius, 1787) | Rotten wood, fungi | 1 | 6 | 1 | |||
| Keroplatidae | Neoplatyura nigricauda (Strobl, 1893) | 2 | ||||||
| Orfelia unicolor (Staeger, 1840) | 1 | |||||||
| Mycetophilidae | Boletina nigricans Dziedzicki, 1885 | Mycetophagous | 1 | |||||
| B. sp. | Mycetophagous | 1 | ||||||
| Coelosia flava (Staeger, 1840) | Mycetophagous | 1 | ||||||
| Ectrepesthoneura sp. | Mycetophagous | 1 | ||||||
| Mycetophila sp. | Mycetophagous | 1 | 1 | |||||
| Mycoma sp. | Mycetophagous | 1 | ||||||
| Neuratelia nemoralis (Meigen, 1818) | Mycetophagous | 1 | ||||||
| Sceptonia sp. | Mycetophagous | 1 | ||||||
| Zygomyia semifusca (Meigen, 1818) | Mycetophagous | 1 | ||||||
| Sciaridae | Bradysia affinis (Zetterstedt, 1838) | Woodland, wetlands, meadows, gardens, saprophagous | 2 | 2 | ||||
| B. alpicola (Winnertz, 1867) | Woodland, bogs, grasslands, dunes, saprophagous | 1 | ||||||
| *B. fenestralis (Zetterstedt, 1838) | Woodland (oak, hazel, pine), heathland, grassland, water meadows, gardens, saprophagous | 1 | ||||||
| B. hilariformis Tuomikoski, 1960 | Woodland, wetlands (mires, bogs), saprophagous | 1 | ||||||
| B. nitidicollis (Meigen, 1818) | Woodland, heathland, wetlands (water meadows, fens, mires, bogs), grassland, dunes, saltmarsh, gardens, saprophagous | 1 | ||||||
| ¤B. quercina Menzel and Köhler, 2014 | Woodland (oak, ash, aspen, spruce), saprophagous | 2 | ||||||
| B. sp. 1 | saprophagous | 1 | ||||||
| B. sp. 2 | saprophagous | 1 | ||||||
| B. sp. 3 | saprophagous | 1 | ||||||
| B. sp. 4 | saprophagous | 1 | 1 | 1 | ||||
| *Corynoptera forcipata (Winnertz, 1867) | Woodland, heathland, wetlands (incl. water meadows, fens, bogs, basin mires), grassland, coastal landslips, saprophagous | 2 | 1 | 8 | ||||
| C. hypopygialis (Lengersdorf, 1926) | Woodland (oak, beech, hazel), calcareous grassland, heathland, wetlands (incl. fens, bogs), open montane habitats, saprophagous | 2 | 3 | |||||
| *C. irmgardis (Lengersdorf, 1930) | Woodland, heathland, wetlands (incl. water meadows, fens, bogs, reed beds, mires, bogs), grazed grassland, saprophagous | 1 | ||||||
| *C. membranigera (Kieffer, 1903) | Woodland (oak, beech, poplar, pine, spruce, conifers), grassland, saprophagous | 2 | 2 | 2 | 2 | |||
| C. sp. 1 | saprophagous | 2 | ||||||
| C. sp. 2 | saprophagous | 1 | ||||||
| C. sp. 3 | saprophagous | 1 | ||||||
| C. sp. 4 | saprophagous | 1 | ||||||
| C. sp. 5 | saprophagous | 1 | ||||||
| C. sp. 6 | saprophagous | 1 | ||||||
| C. sp. 7 | saprophagous | 1 | ||||||
| C. sp. 8 | saprophagous | 1 | ||||||
| C. sp. 9 | saprophagous | 1 | ||||||
| C. sp. 10 | saprophagous | 1 | ||||||
| *Cratyna (C.) ambigua (Lengersdorf, 1934) | Woodland (oak, beech, poplar, pine, spruce), calcareous grassland, water meadows, saprophagous | 1 | ||||||
| C. sp. 1 | saprophagous | 1 | ||||||
| Epidapus gracilis (Walker, 1848) | Woodland (oak, beech, maple, larch, pine, spruce, conifers), heathland, bogs, saprophagous | 1 | ||||||
| Leptosciarella sp. 1 | Xylobiont | 1 | ||||||
| Lycoriella ingenua (Dufour, 1839) | Woodland (oak, hazel, poplar), heathland, wetlands (fens, sedge beds, water meadows), parkland, gardens, greenhouses, mycetophagous | 1 | ||||||
| *Pseudolycoriella paludum (Frey, 1948) | Woodland (oak, beech, elm), bogs, saprophagous | 4 | ||||||
| Scatopsciara atomaria (Zetterstedt, 1851) | Woodland, heathland, wetlands (fens, bogs, mires, water meadows), marshland, grassland, parkland, gardens, saprophagous | 4 | 12 | 1 | ||||
| *S. calamophila Frey, 1948 | Woodland, grassland, heathland, marshland, gardens, saprophagous | 6 | 2 | 1 | ||||
| *S. multispina (Bukowski and Lengersdorf, 1936) | Woodland, grassland, heathland, wetlands (dump meadows, sedge beds), parkland, gardens, saprophagous | 3 | 6 | |||||
| *S. neglecta Menzel and Mohrig, 1998 | Woodland, grassland, heathland, wetlands (water meadows, sedge beds), parkland, gardens, saprophagous | 1 | ||||||
| S. pusilla (Meigen, 1818) | Woodland, grassland, heathland, wetlands (bogs, dump meadows), saprophagous | 1 | ||||||
| S. vitripennis (Meigen, 1818) | Woodland, grassland, heathland, wetlands (water meadows, fens), parkland, sand dunes, saprophagous | 3 | 8 | |||||
| S. sp. 1 | saprophagous | 1 | ||||||
| S. sp. 2 | saprophagous | 1 | ||||||
| *Trichosia (T.) flavicoxa Tuomikoski, 1960 | Woodland, parkland (oak, alder, beech), Xylobiont | 1 | ||||||
| T. sp. 1 | Xylobiont | 1 | 1 | |||||
| T. sp. 2 | Xylobiont | 1 | ||||||
| *Xylosciara trimera Tuomikoski, 1960 | Woodland, parkland (oak, beech), xylobiont | 1 | ||||||
| X. sp. 1 | Xylobiont | 1 | ||||||
| Ceratopogonidae | Atrichopogon griseolus (Zetterstedt, 1855) | 1 | ||||||
| A. minutus (Meigen, 1830) | Rotting material | 1 | ||||||
| A. muelleri (Müller, 1905) | Aquatic larvae | 1 | ||||||
| A. sp. | 1 | |||||||
| Bezzia flavicornis (Staeger, 1839) | 1 | |||||||
| Be. ornata (Meigen, 1830) | 1 | |||||||
| Brachypogon perpusillus (Edwards, 1921) | 1 | |||||||
| Br. sociabilis (Goetghebuer, 1920) | 1 | |||||||
| Culicoides chiopterus (Meigen, 1830) | Dung / saprophagous | 1 | ||||||
| C. clintoni Boorman, 1984 | Peat bogs | 2 | ||||||
| C. impunctatus Goetghebuer, 1920 | Peat bogs | 4 | 16 | 36 | 1 | 1 | 30 | |
| C. kibunensis Tokunaga, 1937 | 1 | 1 | 2 | 1 | ||||
| C. obsoletus (Meigen, 1818) | 1 | 9 | 11 | 2 | 4 | |||
| C. pallidicornis Kieffer, 1919 | 1 | 1 | ||||||
| C. pictipennis (Staeger, 1839) | 1 | |||||||
| C. scoticus Downes and Kettle, 1952 | Dung / saprophagous | 2 | ||||||
| C. segnis Campbell and Pelham-Clinton, 1960 | 3 | 3 | 3 | 1 | 1 | |||
| Dasyhelea spp. | 1 | 1 | ||||||
| **Forcipomyia dichromata Remm, 1968 | 1 | |||||||
| F. tibialis Remm, 1961 | 1 | |||||||
| F. titillans (Winnertz, 1852) | Rotting material | 6 | 8 | 3 | 1 | 4 | ||
| F. spp. | 2 | 1 | 1 | 1 | 1 | |||
| Kolenohelea calcarata (Goetghebuer, 1920) | 4 | |||||||
| Palpomyia pubescens Kieffer, 1919 | 10 | 1 | 4 | 1 | ||||
| Serromyia femorata (Meigen, 1804) | 1 | 1 | 1 | |||||
| Stilobezzia ochracea (Winnertz, 1852) | 1 | |||||||
| Scatopsidae | Anapausis helvetica Haenni, 1984 | 12 | ||||||
| A. rectinervis Duda, 1928 | Eurytop | 1 | ||||||
| Efcookella albitarsis (Zetterstedt, 1850) | Saprophagous | 1 | ||||||
| Holoplagia bullata (Edwards, 1925) | Rotting wood, ants (?) | 1 | ||||||
| Swammerdamella acuta Cook, 1956 | 4 | 1 | ||||||
| Chironomidae | ||||||||
| Chironominae | Chironomus (Chaetolabis) macani Freeman, 1948 | 1 | ||||||
| *Chironomus (Lobochironomus) pseudomendax Wülker, 1998 | 1 | |||||||
| Glyptotendipes (G.) cauliginellus (Kieffer, 1913) | 5 | |||||||
| Microspectra nana (Meigen, 1818) | 1 | 2 | ||||||
| M. pallidula (Meigen, 1830) | 1 | |||||||
| Parachironomus tenuicaudatus (Malloch, 1915) | 1 | |||||||
| Paratendipes albimanus (Meigen, 1818) | 2 | |||||||
| Stempellinella brevis (Edwards, 1929) | 3 | |||||||
| Tanytarsus medius Reiss and Fittkau, 1971 | 1 | |||||||
| T. signatus (van der Wulp, 1859) | 1 | |||||||
| Orthocladiinae | Bryophaenocladius ictericus (Meigen, 1830) | 1 | ||||||
| B. cf. vernalis (Goetghebuer, 1921) | 2 | 2 | 1 | |||||
| B. sp. 4ES | 4 | 1 | ||||||
| *B. sp. 10ES | 1 | |||||||
| Corynoneura lacustris Edwards, 1924 | 2 | |||||||
| Co. sp. 16ES | 1 | |||||||
| Cricotopus glacialis Edwards, 1922 | 1 | |||||||
| Cr. tibialis (Meigen, 1804) | 1 | |||||||
| Eukiefferiella brevicalcar (Kieffer, 1911) | 1 | |||||||
| ¤Gymnometriocnemus (Gymnometriocnemus) pallidus Stur and Ekrem, 2015 | 3 | 1 | ||||||
| Halocladius variabilis (Staeger, 1839) | Marine, intertidal | 1 | 1 | 4 | ||||
| Limnophyes asquamatus Søgaard Andersen, 1937 | 1 | 1 | ||||||
| L. habilis (Walker, 1856) | 1 | |||||||
| L. minimus (Meigen, 1818) | 5 | 1 | 7 | 5 | 2 | |||
| L. natalensis (Kieffer, 1914) | 2 | |||||||
| L. sp. 3ES | 1 | |||||||
| L. sp. 14ES | Parthenogenetic? | 2 | ||||||
| Metriocnemus albolineatus (Meigen, 1818) | 5 | 2 | ||||||
| M. fuscipes (Meigen, 1818) | 1 | |||||||
| M. picipes (Meigen, 1818) | 2 | 1 | 1 | |||||
| M. sp. 3ES | 1 | |||||||
| Parametriocnemus stylatus adzharicus Kownacki and Zosidze, 1973 | 1 | |||||||
| Paraphaenocladius impensus (Walker, 1856) | 1 | 1 | ||||||
| Pseudorthocladius sp. (curtistylus or uniserratus) | 2 | 1 | 1 | |||||
| Pseudosmittia albipennis (Goetghebuer, 1921) | 2 | 5 | 1 | 3 | ||||
| P. forcipata (Goetghebuer, 1921) | 3 | 2 | ||||||
| Smittia sp. 8ES | 2 | 1 | ||||||
| S. sp. 16ES | 1 | |||||||
| S. sp. 19ES | 1 | |||||||
| Tvetenia calvescens (Edwards, 1929) | 1 | |||||||
| Tanypodinae | Krenopelopia spp. | 2 | 1 | 1 | 1 | |||
| Zavrelimyia divisa (Walker, 1856) | 1 | |||||||
| Sum species: 153 | 47 | 28 | 76 | 41 | 30 | 20 | ||
| Sum specimens: 564 | 98 | 75 | 195 | 81 | 55 | 60 | ||
| Brachycera | ||||||||
| Hybotidae | Bicellaria nigra (Meigen, 1824) | Several habitats | 1 | 1 | 1 | |||
| Drapetis pusilla Loew, 1859 | 1 | |||||||
| Euthyneura gyllenhali (Zetterstedt, 1838) | 1 | 1 | ||||||
| E. myrtilli Macquart, 1836 | Several habitats | 5 | ||||||
| Hybos grossipes (Linnaeus, 1767) | Vegetation, predator | 2 | 1 | 2 | ||||
| Oedalea stigmatella Zetterstedt, 1842 | 1 | |||||||
| O. zetterstedti Collin, 1926 | 1 | |||||||
| Platypalpus calceatus (Meigen, 1822) | 1 | |||||||
| P. candicans (Fallén, 1815) | 1 | 3 | ||||||
| P. ciliaris (Fallén, 1816) | 1 | |||||||
| P. cothurnatus Macquart, 1827 | 1 | |||||||
| P. cursitans (Fabricius, 1775) | 3 | 6 | ||||||
| P. ecalceatus (Zetterstedt, 1838) | 13 | |||||||
| P. exilis (Meigen, 1822) | 2 | 1 | ||||||
| P. longiseta (Zetterstedt, 1842) | 1 | 4 | ||||||
| P. luteus (Meigen, 1804) | 1 | 1 | 2 | |||||
| P. major (Zetterstedt, 1842) | 1 | 2 | ||||||
| P. nigritarsis (Fallén, 1816) | Ground vegetation | 1 | 1 | 1 | ||||
| P. pectoralis (Fallén, 1815) | 1 | 1 | 1 | |||||
| P. pseudofulvipes (Frey, 1909) | 1 | |||||||
| P. verralli (Collin, 1926) | 1 | |||||||
| Tachydromia umbrarum Haliday, 1833 | Tree trunks, predator | 1 | 2 | |||||
| Tachypeza fuscipennis (Fallén, 1815) | Tree trunks, predator | 1 | 3 | 1 | ||||
| T. nubila (Meigen, 1804) | Tree trunks | 1 | 1 | 1 | ||||
| Trichina clavipes Meigen, 1830 | Vegetation, predator | 3 | 11 | 2 | 3 | |||
| Empididae | Chelifera trapezina (Zetterstedt, 1838) | Aquatic larvae | 1 | 1 | ||||
| Empis stercorea Linnaeus, 1761 | 2 | 6 | ||||||
| Gloma fuscipennis Meigen, 1822 | 1 | |||||||
| Hilara canescens Zetterstedt, 1849 | 1 | |||||||
| H. intermedia (Fallén, 1816) | 1 | |||||||
| H. platyura Loew, 1873 | 1 | |||||||
| Phyllodromia melanocephala (Fabricius, 1794) | Deciduous trees, predator | 15 | 12 | 9 | 23 | 1 | 17 | |
| Rhamphomyia crassirostris (Fallén, 1816) | 1 | |||||||
| R. flava (Fallén, 1816) | 1 | |||||||
| Trichopeza longicornis (Meigen, 1822) | 1 | |||||||
| Atelestidae | Atelestus pulicarius (Fallén, 1816) | 2 | ||||||
| Dolichopodidae | Chrysotimus flaviventris (von Roser, 1840) | 1 | 21 | 6 | ||||
| C. molliculus (Fallén, 1823) | 4 | |||||||
| Chrysotus cilipes Meigen, 1824 | 1 | |||||||
| Dolichopus nigricornis Meigen, 1824 | 1 | 1 | 1 | |||||
| D. plumipes (Scopoli, 1763) | 2 | 66 | ||||||
| D. popularis Wiedemann, 1817 | 2 | 3 | ||||||
| D. simplex Meigen, 1824 | 3 | 13 | ||||||
| Gymnopternus aerosus (Fallén, 1823) | 1 | |||||||
| G. celer (Meigen, 1824) | 1 | |||||||
| Medetera abstrusa Thunberg, 1955 | Tree trunks, predator | 1 | ||||||
| M. belgica Parent, 1936 | Tree trunks, predator | 1 | 1 | |||||
| Neurigona pallida (Fallén, 1823) | 1 | 1 | ||||||
| N. suturalis (Fallén, 1823) | 1 | |||||||
| Sciapus platypterus (Fabricius, 1805) | 1 | |||||||
| cf. Sympycnus pulicarius (Fallén, 1823) | 3 | |||||||
| Systenus bipartitus (Loew, 1850) | Sap, deciduous trees | 1 | 1 | |||||
| Xanthochlorus ornatus (Haliday, 1832) | 1 | |||||||
| X. tenellus (Wiedemann, 1817) | 4 | |||||||
| Phoridae | ||||||||
| Borophaga agilis (Meigen, 1830) | 1 | |||||||
| *Megaselia albiclava (Schmitz, 1926) | 2 | |||||||
| ¤M. aliusmyia Disney, 2015 | 1 | |||||||
| ¤M. alphamyia Disney, 2015 | 2 | 3 | ||||||
| *M. basispinata (Lundbeck, 1920) | 1 | 1 | ||||||
| ¤M. chimyia Disney, 2015 | 1 | |||||||
| M. ciliata (Zetterstedt, 1848) | Predacious larvae | 2 | 1 | |||||
| M. conformis (Wood, 1909) | 1 | |||||||
| M. cothurnata (Schmitz, 1919) | 2 | 3 | ||||||
| *M. crassipes (Wood, 1909) | 1 | |||||||
| ¤M. deltamyia Disney, 2015 | 1 | |||||||
| *M. differens Schmitz, 1948 | 2 | 4 | 1 | 1 | ||||
| M. discreta (Wood, 1909) | Fungi | 2 | 2 | |||||
| M. diversa (Wood, 1909) | 1 | |||||||
| ¤M. etamyia Disney, 2015 | 1 | |||||||
| M. fuscovariana Schmitz, 1933 | 4 | |||||||
| ¤M. geiri Disney, 2015 | 1 | |||||||
| M. giraudii (Egger, 1862) | Decaying material | 2 | 3 | |||||
| *M. gregaria (Wood, 1910) | 2 | |||||||
| *M. hirticrus (Schmitz, 1918) | 1 | 3 | 1 | |||||
| *M. hortensis (Wood, 1909) | 1 | |||||||
| *M. ignobilis (Schmitz, 1919) | 2 | 14 | 2 | 1 | ||||
| *M. immodensior Disney, 2001 | 1 | |||||||
| M. insons (Lundbeck, 1920) | 1 | 2 | 1 | |||||
| *M. intercostata (Lundbeck, 1921) | 3 | 1 | ||||||
| ¤M. karli Disney, 2015 | 1 | |||||||
| **M. kozlovi Disney, 2013 | 1 | 1 | ||||||
| ¤M. lambdamyia Disney, 2015 | 2 | |||||||
| M. lata (Wood, 1910) | Fungi | 1 | ||||||
| M. longicostalis (Wood, 1912) | Decaying material | 1 | ||||||
| *M. longifurca (Lundbeck, 1921) | Predacious larvae | 1 | ||||||
| M. lutea (Meigen, 1830) | Fungi | 1 | ||||||
| **M. malhamensis Disney, 1986 | 1 | 6 | 1 | |||||
| *M. mixta (Schmitz, 1918) | Fungi | 1 | ||||||
| **M. nigrescens (Wood, 1910) | Fungi | 1 | ||||||
| M. nigriceps (Loew, 1866) | Necrophagous | 1 | 1 | 2 | ||||
| ¤M. numyia Disney, 2015 | 2 | |||||||
| ¤M. omicronmyia Disney, 2015 | 1 | |||||||
| M. pectorella Schmitz, 1929 | 2 | 1 | ||||||
| *M. protarsalis Schmitz, 1927 | 1 | |||||||
| M. pusilla (Meigen, 1830) | Polysaprophagous | 2 | ||||||
| *M. quadriseta Schmitz, 1918 | 2 | |||||||
| ¤M. rhomyia Disney, 2015 | 1 | |||||||
| *M. robertsoni Disney, 2008 | 10 | |||||||
| M. ruficornis (Meigen, 1830) | Decaying materials | 1 | 1 | 1 | ||||
| ¤M. solii Disney, 2015 | 1 | |||||||
| *M. speiseri Schmitz, 1929 | 1 | |||||||
| *M. spinicincta (Wood, 1910) | Fungi | 1 | ||||||
| *M. surdifrons (Wood, 1909) | 1 | |||||||
| ¤M. thunesi Disney, 2015 | 1 | |||||||
| *M. wickenensis Disney, 2000 | 1 | |||||||
| ¤M. sp. n. H | 1 | |||||||
| ¤M. sp. n. I | 1 | |||||||
| ¤M. sp. n. T(5) | 1 | 4 | 1 | 1 | ||||
| M. sp. U | 1 | |||||||
| M. sp. 2 | 1 | |||||||
| M. sp. 3 | 1 | 2 | ||||||
| M. sp. 4 | 1 | |||||||
| M. sp. 6 | 1 | 1 | ||||||
| M. sp. 7 | 1 | |||||||
| M. sp. 8 | 1 | |||||||
| M. sp. 9 | 1 | |||||||
| M. sp. 11 | 1 | |||||||
| M. sp. 12 | 1 | |||||||
| M. sp. 14 | 1 | |||||||
| M. sp. 15 | 1 | |||||||
| M. sp. 17 | 1 | 1 | 1 | |||||
| M. sp. 18 | 1 | |||||||
| M. sp. 20 | 1 | |||||||
| M. sp. 21 | 1 | |||||||
| Menozziola obscuripes (Schmitz, 1927) | Ant parasitoid | 1 | ||||||
| *Phalactrophora fasciata (Fallén, 1823) | Coccinellidae parasitoid | 1 | ||||||
| Phora edentata Schmitz, 1920 | 2 | 5 | 21 | 12 | ||||
| P. holosericea Schmitz, 1920 | Root aphid predator | 1 | ||||||
| P. tincta Schmitz, 1920 | 1 | |||||||
| **Pseudacteon formicarum (Verrall, 1827) | Ant parasitoid | 1 | ||||||
| Rhagionidae | Ptiolina obscura (Fallén, 1814) | 1 | 1 | |||||
| Rhagio lineola Fabricius, 1794 | 4 | 4 | 12 | 5 | 19 | |||
| R. maculatus (DeGeer, 1776) | 1 | |||||||
| R. scolopaceus (Linnaeus, 1758) | 1 | |||||||
| Symphoromyia crassicornis (Panzer, 1806) | 1 | |||||||
| Tanypezidae | Tanypeza longimana Fallén, 1820 | 1 | ||||||
| Stratiomyidae | Beris chalybata (Forster, 1771) | 1 | ||||||
| B. clavipes (Linnaeus, 1767) | 2 | 14 | ||||||
| Tabanidae | Hematopogon sp. | 1 | ||||||
| Opomyzidae | Opomyza germinationis (Linnaeus, 1758) | 1 | ||||||
| SciomyzidaE | Pherbellia annulipes (Zetterstedt, 1846) | 1 | ||||||
| P. dubia (Fallén, 1820) | 3 | |||||||
| P. sp. (rozkosnyi or scutellaris) | 1 | |||||||
| Lonchopteridae | Lonchoptera sp. | 2 | ||||||
| Clusiidae | Clusiodes verticalis (Collin, 1912) | 1 | ||||||
| Lonchaeidae | Lonchaea sp. | 1 | ||||||
| Milichidae | Phyllomyza sp. | 1 | ||||||
| Lauxaniidae | *Homoneura lamellata (Becker, 1895) | 1 | ||||||
| **H. thalhammeri Papp, 1978 | 1 | |||||||
| Lyciella decempunctata (Fallén, 1820) | 2 | 7 | 5 | 1 | ||||
| L. platycephala (Loew, 1847) | 6 | 4 | 17 | 24 | 7 | |||
| L. rorida (Fallén, 1820) | 1 | 3 | 1 | |||||
| L. vittata (Walker, 1849) | 1 | |||||||
| Pseudolyciella pallidiventris (Fallén, 1820) | 1 | 1 | ||||||
| P. stylata (Papp, 1978) | 2 | |||||||
| P. spp. | 2 | 5 | ||||||
| Sapromyza basalis Zetterstedt, 1847 | 2 | |||||||
| S. hyalinata (Meigen, 1826) | 1 | 3 | 1 | |||||
| Sapromyzosoma quadricincta (Becker, 1895) | 1 | 1 | ||||||
| Tricholauxania praeusta (Fallén, 1820) | 2 | |||||||
| Drosophilidae | Drosophila sp. (melanogaster or simulans) | 1 | ||||||
| Scaptomyza pallida Zetterstedt, 1847 | 1 | |||||||
| Ephydridae | Athyroglossa glabra (Meigen, 1830) | 1 | ||||||
| Fanniidae | *Fannia pauli Pont, 1997 | 1 | ||||||
| F. polychaeta (Stein, 1895) | 2 | 1 | ||||||
| F. cf. polychaeta (Stein, 1895) | 1 | |||||||
| F. tuberculata (Zetterstedt, 1849) | 1 | |||||||
| F. spp. | 2 | 2 | 1 | |||||
| Piezura pardalina Rondani, 1866 | 1 | |||||||
| Heleomyzidae | Suillia bicolor (Zetterstedt, 1838) | 1 | ||||||
| Anthomyiidae | Mycophaga testacea (Gimmerthal, 1834) | 1 | ||||||
| Muscidae | Coenosia pudorosa Collin, 1953 | 3 | ||||||
| Helina depuncta (Fallén, 1825) | 2 | 1 | 1 | 1 | 1 | |||
| H. impuncta (Fallén, 1825) | 1 | |||||||
| Hydrotaea irritans (Fallén, 1823) | 1 | |||||||
| cf. Hydrotaea sp. | 2 | |||||||
| Muscina levida (Harris, 1780) | 1 | |||||||
| Phaonia laeta (Fallén, 1823) | 1 | |||||||
| Thricops semicinereus (Wiedemann, 1817) | 1 | |||||||
| Rhinophoridae | Paykullia brevicornis (Zetterstedt, 1844) | 1 | ||||||
| Stevenia atramentaria (Meigen, 1824) | 2 | |||||||
| Sarcophagidae | Sarcophaga depressifrons Zetterstedt, 1845 | 1 | ||||||
| S. variegata (Scopoli, 1763) | 1 | |||||||
| Sepsidae | Sepsis cynipsea (Linnaeus, 1758) | 1 | ||||||
| Tachinidae | Cinochira atra Zetterstedt, 1845 | 1 | ||||||
| Sum species: 181 | 31 | 33 | 91 | 37 | 32 | 41 | ||
| Sum specimens: 775 | 63 | 64 | 311 | 174 | 86 | 77 | ||
| HYMENOPTERA | ||||||||
| Formicidae | Camponotus ligniperda (Latreille, 1802) | Woodlands, dead wood | 11 | 35 | 2 | 2 | 6 | |
| Formica fusca Linnaeus, 1758 | Xerophilous | 2 | 15 | 1 | 2 | |||
| F. polyctena Förster, 1850 | 10 | 172 | ||||||
| F. rufa Linnaeus, 1761 | 56 | |||||||
| Lasius brunneus (Latreille, 1798) | Decidous | 1 | 1 | 1 | ||||
| L. platythorax Seifert, 1991 | Forests | 2 | 25 | 1 | ||||
| Myrmica rubra (Linnaeus, 1758) | Forests | 7 | ||||||
| M. ruginodis Nylander, 1846 | Forests | 2 | 2 | 2 | 11 | |||
| Temnothorax cf. tuberum (Fabricius, 1775) | 3 | |||||||
| Crabronidae | Crossocerus tarsatus (Shuckard, 1837) | 1 | ||||||
| Passaloecus sp. | 1 | |||||||
| Stigmus solskyi Morawitz, 1864 | 1 | 1 | ||||||
| Ichneumonidae | Gelis sp. | Parasitoid | 1 | |||||
| Ceraphronidae | Aphanogmus apicalis Szelenyi, 1938 | Parasitoid | 1 | |||||
| A. clavicornis Thomson, 1859 | Parasitoid | 2 | ||||||
| A. cf. clavicornis sp. 1 | Parasitoid | 3 | 3 | 1 | ||||
| A. cf. clavicornis sp. 2 | Parasitoid | 1 | ||||||
| A. cf. clavicornis sp. 3 | Parasitoid | 1 | ||||||
| A. compressus (Ratzeburg, 1852) | Parasitoid | 1 | 2 | 4 | 3 | 1 | ||
| A. nr. compressus (Ratzeburg, 1852) | Parasitoid | 1 | 2 | 1 | ||||
| A. nr. dessarti Hellen, 1966 | Parasitoid | 1 | ||||||
| A. nigrifornicatus Pschom-Walker, 1956 | Parasitoid | 1 | ||||||
| A. steinitzi Priesner, 1936 | Parasitoid | 3 | 1 | |||||
| A. tenuicornis Thomson, 1859 | Parasitoid | 1 | 1 | 2 | ||||
| A. nr. tenuicornis Thomson, 1859 | Parasitoid | 2 | ||||||
| A. spp. | Parasitoid | 5 | 2 | 3 | ||||
| *Ceraphron pedes Förster, 1861 | Parasitoid | 2 | ||||||
| *C. trissacantha Kieffer, 1907 | Parasitoid | 1 | ||||||
| Megaspilidae | Conostigmus sp. 1 | Parasitoid | 2 | 3 | ||||
| C. sp. 2 | Parasitoid | 1 | ||||||
| Dendrocerus laevis (Ratzeburg, 1852) | Parasitoid | 1 | ||||||
| D. sp. 1 | Parasitoid | 1 | 1 | |||||
| D. sp. 2 | Parasitoid | 1 | 1 | |||||
| D. sp. 3 | Parasitoid | 1 | ||||||
| D. spp. | Parasitoid | 1 | 3 | |||||
| Aphelinidae | *Aphelinus mali (Haldeman, 1851) | Parasitoid, Hemiptera | 1 | |||||
| ¤A. quercus Japoshvili and Hansen, 2015 | Parasitoid, Quercus | 1 | ||||||
| **A. subflavescens (Westwood, 1837) | Parasitoid, Aphidiidae | 1 | ||||||
| Eulophidae | *Achrysocharoides butus (Walker, 1839) | Parasitoid, Gracillariidae | 1 | |||||
| *A. latreillii (Curtis, 1826) | Parasitoid, Gracillariidae | 2 | 2 | |||||
| Aprostocetus spp. | Parasitoid | 3 | 1 | 1 | ||||
| *Asecodes erxias (Walker, 1848) | Parasitoid, polyphagous | 1 | 1 | |||||
| Aulogymnus gallarum (Linnaeus, 1761) | Parasitoid, oak-galls | 3 | 1 | |||||
| Chrysocharis cf. prodice (Walker, 1839) | Parasitoid | 1 | ||||||
| C. sp. | Parasitoid, | 1 | ||||||
| Cirrospilus diallus Walker, 1838 | Parasitoid, | 1 | 2 | |||||
| Closterocerus trifasciatus Westwood, 1833 | Parasitoid, polyphagous | 1 | ||||||
| Elachertus sp. | Parasitoid, | 1 | ||||||
| Entedon ergias Walker, 1839 | Parasitoid, polyphagous | 1 | ||||||
| E. sp. | Parasitoid | 1 | ||||||
| Eulophus larvarum (Linnaeus, 1758) | Parasitoid, polyphagous | 2 | ||||||
| Omphale acamas (Walker, 1839) | Parasitoid | 3 | ||||||
| Pediobius eubius (Walker, 1839) | Parasitoid, polyphagous | 1 | ||||||
| P. saulius (Walker, 1839) | Parasitoid, polyphagous | 1 | ||||||
| P. spp. | Parasitoid | 2 | ||||||
| Sympiesis gordius (Walker, 1839) | Parasitoid, polyphagous | 2 | ||||||
| S. sericeicornis (Nees, 1834) | Parasitoid, polyphagous | 1 | 1 | |||||
| **Tamarixia pubescens (Nees, 1834) | Parasitoid, Trioza | 4 | 3 | 16 | 4 | 2 | 1 | |
| Tetrstichus paululus Graham, 1991 | Parasitoid | 1 | ||||||
| Eupelmidae | Eupelmus annulatus Nees, 1834 | Parasitoid, polyphagous | 1 | |||||
| Mymaridae | Anagrus sp. | Parasitoid | 1 | |||||
| Ormyridae | Ormyrus pomaceus (Geoffroy, 1785) | Parasitoid, oak-galls | 1 | 1 | ||||
| Pteromalidae | Ablaxia parviclava (Thomson, 1878) | Parasitoid, polyphagous | 1 | |||||
| A. sp. | Parasitoid, | 1 | ||||||
| Cecidostiba semifascia (Walker, 1835) | Parasitoid, oak-galls | 1 | ||||||
| Cyrtogaster vulgaris Walker, 1833 | Parasitoid, polyphagous | 1 | 1 | 1 | ||||
| Holcaeus stenogaster (Walker, 1836) | Parasitoid | 2 | ||||||
| *Hyperimerus pusillus (Walker, 1833) | Parasitoid, Hemiptera | 1 | 1 | |||||
| Merismus megapterus Walker, 1833 | Parasitoid, polyhgagous | 1 | ||||||
| Mesopolobus dubius (Walker, 1834) | Parasitoid, oak-galls | 1 | ||||||
| M. fasciiventris Westwood, 1833 | Parasitoid, oak-galls | 1 | 1 | |||||
| M. tarsatus (Nees, 1834) | Parasitoid, oak-galls | 1 | 1 | |||||
| M. tibialis (Westwood, 1833) | Parasitoid, oak-galls | 1 | ||||||
| *M. xanthocerus (Thomson, 1878) | Parasitoid, oak-galls | 1 | 1 | 1 | ||||
| Miscogaster maculata Walker, 1833 | Parasitoid, Agromyzidae | 1 | ||||||
| Plutothrix bicolorata (Spinola, 1808) | Parasitoid, Anobiidae | 1 | 1 | 1 | ||||
| Pteromalinae sp. | Parasitoid | 1 | ||||||
| Seladerma tarsale (Walker, 1833) | Parasitoid, Agromyzidae | 6 | 14 | 3 | 1 | |||
| Spalangiopelta sp. | Parasitoid | 1 | ||||||
| Stenomalina epistena (Walker, 1835) | Parasitoid | 1 | ||||||
| S. gracilis (Walker, 1834) | Parasitoid, polyphagous | 1 | ||||||
| *Syntomopus thoracicus Walker, 1833 | Parasitoid, Agromyzidae | 1 | ||||||
| Trigonoderus princeps Westwood, 1832 | Parasitoid, Coleoptera | 1 | ||||||
| Torymidae | Megastigmus dorsalis (Fabricius, 1798) | Parasitoid, oak-galls | 9 | 2 | 3 | 1 | 5 | |
| Torymus flavipes (Walker, 1833) | Parasitoid, oak-galls | 5 | 1 | |||||
| T. nr. microcerus (Walker, 1833) | Parasitoid | 2 | ||||||
| Trichogrammatidae | Trichogramma spp. | Parasitoid | 1 | 1 | ||||
| Cynipidae | Ceroptres clavicornis Hartig, 1840 | Inquilin in oak-gall | 2 | 5 | 2 | |||
| Neuroterus nr. politus Hartig, 1840 | Gall-maker on oak | 1 | ||||||
| Saphonecrus connatus (Hartig, 1840) | Inquilin in oak-gall | 2 | 4 | |||||
| Synergus apicalis Hartig, 1841 | Inquilin in oak-gall | 6 | 1 | 1 | ||||
| S. crassicornis (Curtis, 1838) | Inquilin in oak-gall | 1 | ||||||
| S. gallaepomiformis (Fonscolombe, 1832) | Inquilin in oak-gall | 2 | ||||||
| S. pallipes Hartig, 1840 | Inquilin in oak-gall | 1 | ||||||
| Figitidae | *Alloxysta brachyptera (Hartig, 1840) | Parasitoid | 1 | |||||
| A. spp. | Parasitoid | 2 | ||||||
| Platygastridae | *Amblyaspis angustula Thomson, 1859 | Parasitoid | 1 | |||||
| A. tritici (Walker, 1835) | Parasitoid | 1 | ||||||
| Euxestonotus spp. | Parasitoid | 2 | 1 | 3 | 1 | 3 | ||
| Inostemma hispo Walker, 1838 | Parasitoid | 2 | 1 | |||||
| Platygaster cf. sp. 1 | Parasitoid | 2 | ||||||
| P. sp. 1 | Parasitoid | 1 | 1 | 1 | ||||
| P. sp. 2 | Parasitoid | 1 | ||||||
| P. sp. 3 | Parasitoid | 1 | ||||||
| P. sp. 4 | Parasitoid | 1 | ||||||
| P. sp. 5 | Parasitoid | 1 | 1 | |||||
| P. spp. | Parasitoid | 1 | 2 | 1 | ||||
| Prosactogaster sp. | Parasitoid | 1 | ||||||
| Synopeas sp. 1 | Parasitoid | 1 | ||||||
| S. sp. 2 | Parasitoid | 1 | ||||||
| Scelionidae | *Telenomus angustatus (Thomson, 1861) | Parasitoid | 1 | 3 | 1 | 7 | ||
| *T. kolbei Mayr, 1879 | Parasitoid | 1 | ||||||
| *T. lineolatus Kozlov, 1967 | Parasitoid | 1 | ||||||
| **T. punctatissimus (Ratzeburg, 1844) | Parasitoid | 3 | ||||||
| T. sp. 1 | Parasitoid | 2 | ||||||
| T. spp. | Parasitoid | 3 | 1 | |||||
| Trimorus sp. | Parasitoid | 1 | ||||||
| Diapriidae | Aclista sp. 1 | Parasitoid | 1 | 1 | 1 | |||
| A. sp. 2 | Parasitoid | 1 | ||||||
| *Cinetus piceus Thomson, 1859 | Parasitoid | 1 | ||||||
| Diapriidae spp. | Parasitoid | 1 | 1 | |||||
| Diphora sp. | Parasitoid | 1 | ||||||
| Entomacis perplexa (Haliday, 1857) | Parasitoid | 1 | ||||||
| *Ismarus halidayi Förster, 1850 | Parasitoid | 1 | ||||||
| *Trichopria aptera (Rhute, 1859) | Parasitoid | 1 | ||||||
| *Zygota ruficornis (Curtis, 1831) | Parasitoid | 1 | ||||||
| Z. sp. 1 | Parasitoid | 1 | ||||||
| cf. Zygota sp. | Parasitoid | 1 | 1 | |||||
| Encyrtidae | *Copidosoma floridanum (Ashmead, 1900) | Parasitoid, polyphag | 1 | |||||
| **Habrolepis italicus Delucchi, 1965 | Parasitoid, Hemiptera | 1 | ||||||
| Sum species: 117 | 26 | 31 | 62 | 36 | 21 | 29 | ||
| Sum specimens: 713 | 54 | 69 | 258 | 232 | 36 | 64 | ||
| COLEOPTERA | ||||||||
| Ptiliidae | Acrotrichis intermedia (Gillmeister, 1845) | Humus, mycetophagous | 2 | |||||
| Coccinellidae | Adalia decempunctata (Linnaeus, 1758) | Eurytop, carnivore | 1 | |||||
| Halyzia sedecimguttata (Linnaeus, 1758) | Arboreal, mycetoph | 1 | ||||||
| Staphylinidae | Atheta vaga (Heer, 1839) | Eurytop, carnivorous | 1 | |||||
| Dexiogyia forticornis (Strand, 1939) | Carnivorous | 4 | ||||||
| Eusphalerum luteum (Marsham, 1802) | Eurytop, phytophagous | 1 | ||||||
| Haploglossa villosula (Stephens, 1832) | Humus, carnivorous | 1 | ||||||
| Holobus flavicornis (Lacordaire, 1835) | Humus, detritivorous | 2 | ||||||
| Leptusa fumida (Erichson, 1839) | Bark, carnivorous | 4 | 1 | |||||
| L. ruficollis (Erichson, 1839) | Bark, carnivorous | 7 | 47 | 57 | 30 | 12 | 144 | |
| Oxypoda arborea Zerche, 1994 | Carnivorous | 1 | ||||||
| Phloeocharis subtilissima Mannerheim, 1830 | Bark, dead trees | 1 | 1 | |||||
| Scraptiidae | Anaspis marginicollis Lindberg, 1925 | Eurytop, phytoph, carniv | 4 | 1 | 1 | |||
| A. rufilabris (Gyllenhal, 1827) | Lignicolous, carnivorous | 2 | 2 | |||||
| A. thoracica (Linnaeus, 1758) | Eurytop, phytoph, carniv | 1 | ||||||
| Aderidae | Anidorus nigrinus (Germar, 1842) | Xylophagous, mycetoph | 1 | |||||
| Curculionidae | Archarius pyrrhoceras (Marsham, 1802) | Arboreal, Quercus | 1 | |||||
| Brachysomus echinatus (Bonsdorff, 1785) | Humicolous, polyph | 1 | 1 | |||||
| Coeliodes rana (Fabricius, 1787) | Arboreal, Quercus | 1 | 1 | |||||
| Hylobius abietis (Linnaeus, 1758) | Conifers | 1 | ||||||
| Micrelus ericae (Gyllenhal, 1813) | Calluna, Erica | 1 | ||||||
| Orchestes quercus (Linnaeus, 1758) | Arboreal, Quercus | 6 | 1 | 1 | 2 | 1 | 1 | |
| Otiorhynchus scaber (Linnaeus, 1758) | Arboreal, polyphagous | 2 | 2 | 1 | ||||
| O. singularis (Linnaeus, 1767) | Arboreal, polyphagous | 2 | 13 | 4 | 11 | 1 | ||
| Polydrusus cervinus (Linnaeus, 1758) | Arboreal, polyphagous | 1 | ||||||
| P. tereticollis (De Geer, 1775) | Arboreal, polyphagous | 2 | ||||||
| Strophosoma capitatum (De Geer, 1775) | Arboreal, polyphagous | 39 | 34 | 2 | 11 | |||
| S. melanogrammum (Forster, 1771) | Arboreal, polyphagous | 1 | ||||||
| Elateridae | Athous haemorrhoidalis (Fabricius, 1801) | Herbs, phytophagous | 2 | |||||
| A. subfuscus (Müller, 1764) | Herbs, carnivorous | 3 | 2 | 2 | 1 | 4 | ||
| Dalopius marginatus (Linnaeus, 1758) | Arboreal, polyphagous | 1 | 2 | 1 | ||||
| Paraphotistus impressus (Fabricius, 1792) | Arboreal, phytophagous | 1 | ||||||
| Cryptophagidae | Atomaria fuscata (Schönherr, 1808) | Eurytop, saproph, mycetoph | 2 | |||||
| A. turgida Erichson, 1846 | Eurytop, saproph, mycetoph | 1 | ||||||
| Cryptophagus setulosus Sturm, 1845 | Xerophil, saproph, mycetoph | 1 | 1 | |||||
| Byturidae | Byturus tomentosus (De Geer, 1774) | Arboreal, Rosaceae | 1 | |||||
| Cantharidae | Cantharis figurata Mannerheim, 1843 | Eurytop, carnivorous | 2 | |||||
| Malthinus flaveolus (Herbst, 1786) | Eurytop, carnivorous | 6 | 6 | 1 | 5 | |||
| M. seriepunctatus Kiesenwetter, 1851 | Thermoph, carnivorous, Quercus | 1 | 3 | 32 | ||||
| Malthodes brevicollis (Paykull, 1798) | Carnivorous | 2 | 2 | 1 | 3 | |||
| M. crassicornis (Mäklin, 1846) | Xerophilous, carniv | 1 | ||||||
| M. fuscus (Waltl, 1838) | Eurytop, carnivorous | 1 | 1 | 2 | 2 | 6 | ||
| M. guttifer Kiesenwetter, 1852 | Arboreal, Salix, carniv | 12 | 1 | 27 | 19 | 2 | ||
| M. marginatus (Latreille, 1806) | Arboreal, carnivorous | 1 | 1 | 3 | ||||
| M. pumilus (Brebisson, 1835) | Xerophilous, carniv | 1 | 1 | 1 | 1 | |||
| M. spathifer Kiesenwetter, 1852 | Eurytop, carnivorous | 5 | 4 | 13 | 5 | 3 | ||
| Podistra rufotestacea (Letzner, 1845) | Eurytop, carnivorous | 1 | ||||||
| Rhagonycha lignosa (Müller, 1764) | Eurytop, carnivorous | 2 | 9 | |||||
| R. lutea (Müller, 1764) | Eurytop, carnivorous | 1 | ||||||
| R. nigriventris Motschulsky, 1860 | Eurytop, carnivorous | 2 | 4 | 1 | ||||
| Ciidae | Cis festivus (Panzer, 1793) | Eurytop, mycetoph | 1 | |||||
| C. glabratus Mellié, 1848 | Polypor, mycetoph | 1 | ||||||
| C. vestitus (Mellié, 1848) | Polypor, mycetoph, Quercus | 2 | 2 | |||||
| Orthocis alni (Gyllenhal, 1813) | Polypor, mycetoph | 1 | 1 | |||||
| Melandryidae | Conopalpus testaceus (Olivier, 1790) | Xylophagous, mycetoph, Quercus | 1 | 1 | ||||
| Orchesia micans (Panzer, 1793) | Polypor, mycetoph | 11 | ||||||
| Latridiidae | Corticarina minuta (Fabricius, 1792) | Eurytop, mycetoph | 1 | |||||
| C. similata (Gyllenhal, 1827) | Eurytop, mycetoph | 3 | 5 | 2 | 1 | |||
| Cortinicara gibbosa (Herbst, 1793) | Eurytop, mycetoph | 2 | 1 | |||||
| Chrysomelidae | Cryptocephalus labiatus (Linnaeus, 1761) | Arboreal, Quercus | 1 | |||||
| Phratora laticollis (Suffrian, 1851) | Arboreal, Populus | 1 | ||||||
| Nitidulidae | Cychramus variegatus (Herbst, 1792) | Eurytop, mycetoph | 2 | |||||
| Epuraea unicolor (Olivier, 1790) | Eurytop, saproph | 6 | ||||||
| Scirtidae | Cyphon coarctatus Paykull, 1799 | Hygroph, phytoph | 2 | 6 | ||||
| C. padi (Linnaeus, 1758) | Hygroph, phytoph, Sphagnum | 2 | ||||||
| Prionocyphon serricornis (Müller, 1821) | Eurytop, saproph | 2 | ||||||
| Dasytidae | Dasytes aeratus Stephens, 1829 | Eurytop, carnivorous | 1 | |||||
| D. plumbeus (Müller, 1776) | Eurytop, carnivorous | 1 | ||||||
| Carabidae | Dromius agilis (Fabricius, 1787) | Arboreal, carnivorous | 1 | 5 | ||||
| D. angustus Brullé, 1834 | Arboreal, carniv, Pinus | 2 | ||||||
| D. quadrimaculatus (Linnaeus, 1758) | Arboreal, carnivorous | 1 | 7 | |||||
| Philorhizus notatus (Stephens, 1827) | Humus, xeroph, carniv | 1 | ||||||
| Ptinidae | Dryophilus pusillus (Gyllenhal, 1808) | Xylophagous, Pinus | 1 | |||||
| Grynobius planus (Fabricius, 1787) | Xylophagous | 1 | ||||||
| Ptinus subpillosus Sturm, 1837 | Phytop, saprophagous | 1 | 3 | 1 | ||||
| P. villiger (Reitter, 1884) | Humus, xylophagous | 1 | ||||||
| Histeridae | Gnathoncus buyssoni Auzat, 1917 | Eurytop, carnivorous | 1 | |||||
| Cerambycidae | Leiopus linnei Wallin, Nylander and Kvamme, 2009 | Xylophagous, Quercus | 3 | 1 | ||||
| Pogonocherus hispidulus (Piller and Mitterpacher, 1783) | Xylophagous, Fagaceae | 1 | ||||||
| Salpingidae | Salpingus planirostris (Fabricius, 1787) | Bark, carnivorous | 4 | 2 | 2 | |||
| Silvanidae | Silvanoprus fagi (Guérin-Ménéville, 1844) | Eurytop, omnivorous | 1 | |||||
| Throscidae | Trixagus carinifrons (Bonvouloir, 1859) | Eurytop, arboreal | 5 | |||||
| T. dermestoides (Linnaeus, 1766) | Eurytop, arboreal | 10 | 1 | |||||
| T. leseigneuri Muona, 2002 | Eurytop, arboreal | 8 | ||||||
| Sum species: 84 | 19 | 28 | 39 | 29 | 21 | 26 | ||
| Sum specimens 821 | 50 | 111 | 206 | 173 | 69 | 212 | ||
References
- Birtele, D.; Hardersen, S. Analysis of Vertical Stratification of Syrphidae (Diptera) in an Oak-Horn-Beam Forest in Northern Italy. Ecol. Res. 2012, 27, 755–763. [Google Scholar] [CrossRef]
- Bouget, C.; Brin, A.; Brustel, H. Exploring the «last Biotic Frontier»: Are Temperate Forest Canopies Special for Saproxylic Beetles? For. Ecol. Manag. 2011, 261, 211–220. [Google Scholar] [CrossRef]
- Engen, S.; Sæther, B.E.; Sverdrup-Thygeson, A.; Grøtan, V.; Ødegaard, F. Assessment of Species Diversity from Species Abundance Distributions at Different Localities. Oikos 2008, 117, 738–748. [Google Scholar] [CrossRef]
- Gossner, M.; Liston, A.; Späth, J. Sawflies in the Crowns of Native and Exotic Trees, Sampled with Flight-Intercept Traps in Southern Germany. Entomol. Gen. 2007, 30, 273–282. [Google Scholar] [CrossRef]
- Gossner, M.; Engel, K.; Jessel, B. Plant and Arthropod Communities in Young Oak Stands: Are They Determined by Site History? Biodivers. Conserv. 2008, 17, 3165–3180. [Google Scholar] [CrossRef]
- Koponen, S.; Rinne, V.; Clayhills, T. Arthropods on Oak Branches in SW Finland, Collected by a New Trap Type. Entomol. Fenn. 1997, 8, 177–183. [Google Scholar] [CrossRef]
- Saure, C.; Kielhorn, K.-H. Netzflügler als Bewohner der Kronenregion von Eiche und Kiefer (Neuroptera: Coniopterygidae, Hemerobiidae, Chrysopidae). Faun. Ökol. Mitt. 1993, 6, 391–402. [Google Scholar]
- Sverdrup-Thygeson, A. Oaks in Norway: Hotspots for red-listed beetles (Coleoptera). In Proceedings of the 5th Symposium and Workshop on the Conservation of Saproxylic Beetles, Luneberg, Germany, 14–16 June 2008. [Google Scholar]
- Vodka, S.; Konvička, M.; Čižek, L. Habitat Preferences of Oak-Feeding Xylophagous Beetles in a Temperate Woodland: Implications for Forest History and Management. J. Insect Conserv. 2009, 13, 553–562. [Google Scholar] [CrossRef]
- Widerberg, M.K.; Ranius, T.; Drobyshev, I.; Nilsson, U.; Lindbladh, M. Increased Openness around Retained Oaks Increases Species Richness of Saproxylic Beetles. Biodivers. Conserv. 2012, 21, 3035–3059. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Wint, G.R.E.; Kennedy, C.E.J.; Greenwood, S.R. The Composition of the Arthropod Fauna of the Canopies of Some Species of Oak (Quercus). Eur. J. Entomol. 2005, 102, 65–72. [Google Scholar] [CrossRef]
- Disney, R.H.L. Scuttle Flies: The Phoridae; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Mupepele, A.C.; Müller, T.; Dittrich, M.; Floren, A. Are Temperate Canopy Spiders Tree-Species Specific? PLoS ONE 2014, 9, e86571. [Google Scholar]
- Floren, A.; Schmidl, J. (Eds.) Canopy Arthropod Research in Europe; Bioform: Nuremberg, Germany, 2008. [Google Scholar]
- Gering, J.C.; Crist, T.O. Patterns of Beetle (Coleoptera) Diversity in Crowns of Representative Tree Species in an Old-Growth Temperate Deciduous Forest. Selbyana 2000, 21, 38–47. [Google Scholar]
- Palacios-Vargas, J.G.; Iglesias, R.; Castaño-Meneses, G. Mites from Mexican Oak Canopies. Insect Sci. Its Appl. 2003, 23, 287–292. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E. Canopy Arthropods Community within and among Oak Species in Central Mexico. Curr. Zool. 2009, 55, 132–144. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E.; Cano-Santana, Z.; Oyama, K. Canopy Arthropod Communities on Mexican Oaks at Sites with Different Disturbance Regimes. Biol. Conserv. 2003, 115, 79–87. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E.; Valencia-Cuevas, L.; Castillo-Mendoza, E.; Mussali-Galante, P.; Pérez-Ruiz, R.V.; Mendoza, A. Association between Individual Genetic Diversity of Two Oak Host Species and Canopy Arthropod Community Structure. Eur. J. For. Res. 2013, 132, 165–179. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E.; Valencia-Cuevas, L.; Mussali-Galante, P.; Ramírez-Rodríguez, R.; Castillo-Mendoza, E. Effect of Host-Plant Genetic Diversity on Oak Canopy Arthropod Community Structure in Central Mexico. Rev. Chil. Hist. Nat. 2015, 88, 12. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E.; Oyama, K. Community Structure of Canopy Arthropods Associated to Quercus crassifolia x Quercus crassipes Complex. Oikos 2006, 112, 370–381. [Google Scholar] [CrossRef]
- Valencia-Cuevas, L.; Tovar-Sánchez, E. Oak Canopy Arthropod Communities: Which Factors Shape its Structure? Rev. Chil. Hist. Nat. 2015, 88, 15. [Google Scholar] [CrossRef]
- Maldonado-Lopez, Y.; Vaca-Sanchez, M.S.; Gonzalez-Rodriguez, A.; Oyama, K.; Lopez-Barbosa, E.; Fagundes, M.; Cuevas-Reyes, P. Hybridization Increases Canopy Arthropod Diversity in the Quercus affinis x Quercus laurina Complex. J. Insect Conserv. 2018, 22, 781–793. [Google Scholar] [CrossRef]
- Barnard, P.C.; Brooks, S.J.; Stork, N.E. The Seasonality and Distribution of Neuroptera, Raphidioptea and Mecoptera on Oaks in Richmond Park, Surrey, as Revealed by Insecticide Knock-down Sampling. J. Nat. Hist. 1986, 20, 1321–1331. [Google Scholar] [CrossRef]
- Disney, R.H.L. Scuttle Flies (Diptera: Phoridae) from the Canopies of Oak Trees (Fagaceae) in Norway, Including 13 New Species. Nor. J. Entomol. 2015, 62, 20–52. [Google Scholar]
- Dolek, M.; Freese-Hager, A.; Bussler, H.; Floren, A.; Liegl, A.; Schmidl, J. Ants on Oaks: Effects of Forest Structure on Species Composition. J. Insect Conserv. 2009, 13, 367–375. [Google Scholar] [CrossRef]
- Köhler, A.; Menzel, F.; Thunes, K.H.; Søli, G.E.E. Black Fungus Gnats (Diptera: Sciaridae) in Oak Canopies: Description of Bradysia quercina Menzel & Köhler spec. nov. and New Records from Norway. Stud. Dipterol. 2014, 20, 325–331. [Google Scholar]
- Ozanne, C.M.P. A Comparison of the Canopy Arthropod Communities of Coniferous and Broad-Leaved Trees in the United Kingdom. Selbyana 1999, 20, 290–298. [Google Scholar]
- Ozanne, C.M.P.; Speight, M.R.; Hambler, C.; Evans, H.F. Isolated Trees and Forest Patches: Patterns in Canopy Arthropod Abundance and Diversity in Pinus sylvestris (Scots Pine). For. Ecol. Manag. 2000, 137, 53–63. [Google Scholar] [CrossRef]
- Pedley, S.M.; Martin, R.D.; Oxbrough, A.; Irwin, S.; Kelly, T.C.; O’Halloran, J. Commercial Spruce Plantations Support a Limited Canopy Fauna: Evidence from a Multi Taxa Comparison of Native and Plantation Forests. For. Ecol. Manag. 2014, 314, 172–182. [Google Scholar] [CrossRef]
- Simandl, J. Canopy Arthropods on Scots Pine: Influence of Season and Stand Age on Community Structure and the Position of Sawflies (Diprionidae) in the Community. For. Ecol. Manag. 1993, 62, 85–98. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Moran, V.C.; Kennedy, C.E.J. The Assessment of Arboreal Insect Fauna: Comparisons of Knockdown Sampling and Faunal Lists. Ecol. Entomol. 1982, 7, 331–340. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Wint, G.R.E.; Kennedy, C.E.J.; Greenwood, S.R. Seasonality, Abundance, Species Richness and Specificity of the Phytophagous Guild of Insects on Oak (Quercus) Canopies. Eur. J. Entomol. 2004, 101, 43–50. [Google Scholar] [CrossRef]
- Stork, N.E.; Adis, J.; Didham, R.K. (Eds.) Canopy Arthropods; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Stork, N.E.; Hammond, P.M.; Russell, B.L.; Hadwen, W.L. The Spatial Distribution of Beetles within the Canopies of Oak Trees in Richmond Park, U.K. Ecol. Entomol. 2001, 26, 302–311. [Google Scholar] [CrossRef]
- Stork, N.E.; Hammond, P.M. Species Richness and Temporal Partitioning in the Beetle Fauna of Oak Trees (Quercus robur, L.) in Richmond Park, UK. Insect Conserv. Divers. 2013, 6, 67–81. [Google Scholar] [CrossRef]
- Thunes, K.H.; Skartveit, J.; Gjerde, I. The Canopy Arthropods of Old and Mature Pine Pinus sylvestris in Norway. Ecography 2003, 26, 490–502. [Google Scholar] [CrossRef]
- Thunes, K.H.; Skartveit, J.; Gjerde, I.; Stary, J.; Solhøy, T.; Fjellberg, A.; Kobro, S.; Nakahara, S.; Zur Strassen, R.; Szadziewski, R.; et al. The Arthropod Community of Scots Pine (Pinus sylvestris, L.) Canopies in Norway. Entomol. Fenn. 2004, 15, 65–90. [Google Scholar]
- Stork, N.E.; Hammond, P.M. Sampling Arthropods from Tree-Crowns by Fogging with Knockdown Insecticides: Lessons from Studies of Oak Tree Beetle Assemblages in Richmond Park (UK); Chapman & Hall: London, UK, 1997; pp. 3–26. [Google Scholar]
- Ozanne, C.M.P. Techniques and methods for sampling canopy insects. In Insect Sampling in Forest Ecosystems; Leather, S., Ed.; Blackwell Science Ltd.: Oxford, UK, 2005; pp. 146–167. [Google Scholar]
- Schmidt, M.; Jochheim, H.; Kersebaum, K.-C.; Lischeid, G.; Nendel, C. Gradients of Microclimate, Carbon and Nitrogen in Transition Zones of Fragmented Landscapes—a Review. Agric. For. Meteorol. 2017, 232, 659–671. [Google Scholar] [CrossRef]
- EUFORGEN Distribution Map of Quercus Robur. Available online: http://www.euforgen.org/species/quercus-robur/ (accessed on 9 March 2021).
- EUFORGEN Distribution Map of Quercus Petraea. Available online: http://www.euforgen.org/species/quercus-petraea/ (accessed on 9 March 2021).
- Gao, T.; Hedblom, M.; Emilsson, T.; Nielsen, A.B. The Role of Forest Stand Structure as Biodiversity Indicator. For. Ecol. Manag. 2014, 330, 82–93. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publ. Off. EU: Luxembourg, 2016; pp. 160–163. [Google Scholar]
- Holtan, D. Kartlegging og Verdisetting av Naturtypar i Kvam; Kvam Herad og Fylkesmannen i Hordaland; MVA-Rapport: Kvam Municipality, Norway, 2009; p. 103. [Google Scholar]
- Fylkesmannen i Hordaland. Forvaltningsplan for Berge Landskapsvernområde: Naturkvalitetar, Bevaringsmål og Forvaltningstiltak; Fylkesmannen i Hordaland: Bergen, Norway, 2013; p. 64. [Google Scholar]
- Sverdrup-Thygeson, A.; Laugsand, A.; Olberg, S. Oppfølging av Handlingsplan for Sinoberbille 2009. Kartlegging i Froland og Drangedal Kommuner; NINA Rapport: Lillehamme, Noway, 2009; p. 22. [Google Scholar]
- IUCN. Categories, Objectives and Criteria: Final Report of the Committee and Criteria of the CNPPA/IUCN; IUCN: Morges, Switzerland, 1978. [Google Scholar]
- Birkemoe, T.; Mehl, R.; Ottesen, P.; Riddervold, K.W.; Soleng, A.; Aak, A. Kjemiske og Biologiske Bekjempelsesmidler Mot Skadedyr i Norge; Skadedyr; Nasjonalt Folkehelseinstitutt: Oslo, Norway, 2005; pp. 1–82. [Google Scholar]
- Schowalter, T.; Chao, J.-T. Canopy insect sampling. In Measuring Arthropod Biodiversity; Santos, J.C., Fernandes, G.W., Eds.; Springer: Cham, Switzerland, 2021; pp. 467–493. [Google Scholar]
- De Jong, Y.; Verbeek, M.; Michelsen, V.; de Place Bjørn, P.; Los, W.; Steeman, F.; Bailly, N.; Basire, C.; Chylarecki, P.; Stloukal, E.; et al. Fauna Europaea—All European Animal Species on the Web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Available online: http://purl.oclc.org/estimates 2013 (accessed on 4 June 2021).
- Chao, A. Estimating the Population Size for Capture-Recapture Data with Unequal Catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Ross, S.J.; Lawton, J.J. Beta Diversity on Geographic Gradients in Britain. J. Anim. Ecol. 1992, 61, 151–158. [Google Scholar] [CrossRef]
- ter Braak, C.J.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5). Available online: https://research.wur.nl/en/publications/canoco-reference-manual-and-canodraw-for-windows-users-guide-soft (accessed on 4 June 2021).
- ter Braak, C.J. Ordination. In Data Analysis in Community and Landscape Ecology; Jongman, R.H., Ter Braak, C.J., van Tongeren, O.F.R., Eds.; Pudoc: Wageningen, The Netherlands, 1987; pp. 91–173. [Google Scholar]
- Stur, E.; Ekrem, T. A Review of Norwegian Gymnometriocnemus (Diptera, Chironomidae) Including the Description of Two New Species and a New Name for Gymnometriocnemus volitans (Goetghebuer) Sensu Brundin. ZooKeys 2015, 508, 127–142. [Google Scholar] [CrossRef][Green Version]
- Japoshvili, G.; Hansen, L.O.; Sørlibråten, O. New Records of Aphelinidae (Hymenoptera, Chalcidoidea) from Norway, with Additional Information on Host Associations and Description of a New Species. Nor. J. Entomol. 2015, 62, 110–116. [Google Scholar]
- Korenko, S.; Kula, E.; Simon, V.; Michálková, V.; Pekár, S. Are Arboreal Spiders Associated with Particular Tree Canopies? North West. J. Zool. 2011, 7, 261–269. [Google Scholar]
- Henriksen, S.; Hilmo, O. (Eds.) Norsk Rødliste for Arter 2015; Artsdatabanken: Trondheim, Norway, 2015. [Google Scholar]
- Hopkin, S.P. Biology of the Springtails (Insecta: Collembola); Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Müller, J.; Goßner, M. Single Host Trees in a Closed Forest Canopy Matrix: A Highly Fragmented Landscape? J. Appl. Entomol. 2007, 131, 613–620. [Google Scholar] [CrossRef]
- Sobek, S.; Goßner, M.M.; Scherber, C.; Steffan-Dewenter, I.; Tscharntke, T. Tree Diversity Drives Abundance and Spatiotemporal SS-Diversity of True Bugs (Heteroptera). Ecol. Entomol. 2009, 34, 772–782. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Lienhard, C. In Search of the Sister Group of the True Lice: A Systematic Review of Booklice and Their Relatives, with an Updated Checklist of Liposcelididae (Insecta: Psocodea). Arthropod Syst. Phylogeny 2010, 68, 181–195. [Google Scholar]
- Mockford, E.L. A New Species of Belaphopsocus Badonnel (Psocodea: ‘Psocoptera’: Troctomorpha: Liposcelididae: Embidopsocinae) from Costa Rican Amber. Life Excit. Biol. 2015, 3, 207–212. [Google Scholar] [CrossRef]
- Lienhard, C. Psocoptères euro-méditerranéens. Faune Fr. 1998, 83, 1–517. [Google Scholar]
- Svensson, B.W.; Hall, K. Nationalnyckeln till Sveriges flora och fauna. Stövsländor, Psocoptera; ArtDatabanken, SLU: Uppsala, Sweden, 2010. [Google Scholar]
- Kanervo, J.; Kozlov, M.V. Diversity and Abundance of Arboreal Psocids (Psocoptera) along Latitudinal Gradients in Northern Europe. Eur. J. Entomol. 2014, 111, 51–58. [Google Scholar] [CrossRef]
- Gammelmo, Ø.; Søli, G. Notes on New and Interesting Diptera from Norway. Nor. J. Entomol. 2011, 58, 189–195. [Google Scholar]
- Menzel, F.; Gammelmo, Ø.; Olsen, K.M.; Köhler, A. The Black Fungus Gnats (Diptera, Sciaridae) of Norway—Part I: Species Records Published until December 2019, with an Updated Checklist. ZooKeys 2020, 957, 17–104. [Google Scholar] [CrossRef]
- Menzel, F.; Schulz, U. Die Trauermücken in Deutschland—Ökosystemare Bedeutung, zönologische Koinzidenzen und bioindikatorisches Potential (Diptera: Sciaridae). Beitr. Zur Entomol. 2007, 57, 9–36. [Google Scholar] [CrossRef]
- Hagan, D.V.; Hassold, E.; Kynde, B.; Szadziewski, R.; Thunes, K.H.; Skartveit, J.; Grogan, W.L., Jr. Ceratopogonidae from Forest Habitats in Norway. Pol. J. Entomol. 2000, 69, 465–476. [Google Scholar]
- Hendry, G. Midges in Scotland, 4th ed.; Mercat Press: Edinburgh, Scotland, 2003. [Google Scholar]
- Haenni, J.-P.; Greve, L. Anapausis helvetica Haenni, 1984 (Diptera; Scatopsidae), a Species New to Fennoscandia and Denmark. Nor. J. Entomol. 2005, 52, 115–116. [Google Scholar]
- Zuparko, R.L.; De Queiroz, D.L.; La Salle, J. Two New Species of Tamarixia (Hymenoptera: Eulophidae) from Chile and Australia, Established as Biological Control Agents of Invasive Psyllids (Hemiptera: Calophyidae, Triozidae) in California. Zootaxa 2011, 2921, 13–27. [Google Scholar] [CrossRef]
- Dan Mitroui, M. Pteromalidae (Hymenoptera: Chalcidoidea) New to Romania (I). An. Stiintifice Ale Univ. AlICuza Iasi Biol. Anim. 2004, 50, 85–88. [Google Scholar]
- Sverdrup-Thygeson, A.; Skarpaas, O.; Ødegaard, F. Hollow Oaks and Beetle Conservation: The Significance of the Surroundings. Biodivers. Conserv. 2010, 19, 837–852. [Google Scholar] [CrossRef]
- Leschen, R.A.B.; Lawrence, J.F.; Beutel, R.G.; Slipinski, A. (Eds.) Handbook of Zoology. Arthropoda: Insecta. Coleoptera, Beetles Volume 2: Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia Partim); Walter de Gruyter GmbH: Berlin, Germany; New York, NY, USA, 2010. [Google Scholar]
- Johnson, P.J. Throscidae Laporte 1840. In American Beetles Polyphaga: Scarabaeoidea through Curculionoidea; Arnett, R.H.J., Thomas, M.C., Skelly, P.E., Frank, J.H., Eds.; CRC Press: London, UK; New York, NY, USA; Washington, DC, USA, 2002; pp. 158–160. [Google Scholar]
- Iden, K.; Isaksen, K.; Kristiansen, S.; Mamen, J.; Szewczyk-Bartnicka, H. Været i Norge, Klimatologisk Månedsoversikt Juni 2011; Met.no Info; Norwegian Meteorological Institute: Oslo, Norway, 2011. [Google Scholar]
- Iden, K.; Isaksen, K.; Kristiansen, S.; Mamen, J.; Szewczyk-Bartnicka, H. Været i Norge, Klimatologisk Månedsoversikt Juli 2011; Met.no Info; Norwegian Meteorological Institute: Oslo, Norway, 2011. [Google Scholar]
- Iden, K.; Kristiansen, S.; Mamen, J.; Szewczyk-Bartnicka, H.; Tilley Tajet, H.T. Været i Norge, Klimatologisk Månedsoversikt Juni 2012; Met.no Info; Norwegian Meteorological Institute: Oslo, Norway, 2012. [Google Scholar]
- Gangstø, R.; Iden, K.; Kristiansen, S.; Mamen, J.; Szewczyk-Bartnicka, H.; Tilley Tajet, H.T. Været i Norge, Klimatologisk Månedsoversikt Juli 2012; Met.no Info; Norwegian Meteorological Institute: Oslo, Norway, 2012. [Google Scholar]
- Butin, H. Effect of Endophytic Fungi from Oak (Quercus robur L) on Mortality of Leaf Inhabiting Insects. Eur. J. For. Pathol. 1992, 22, 237–246. [Google Scholar] [CrossRef]
- Faeth, S.E.; Hammond, K.E. Fungal Endophytes and Phytochemistry of Oak Foliage: Determinants of Oviposition Preference of Leafminers? Oecologia 1996, 108, 728–736. [Google Scholar] [CrossRef]
- Finch, O.-D. Evaluation of Mature Conifer Plantations as Secondary Habitat for Epigeic Forest Arthropods (Coleoptera: Carabidae; Araneae). For. Ecol. Manag. 2005, 204, 21–34. [Google Scholar] [CrossRef]
- Milberg, P.; Bergman, K.-O.; Johansson, H. Low Host-Tree Preferences among Saproxylic Beetles: A Comparison of Four Deciduous Species. Insect Conserv. Divers. 2014, 7, 508–522. [Google Scholar] [CrossRef]
- Sunding, P.; Grindeland, J.M.; Foslie, M. Eik. Store Nor. Leks. 2021. Available online: https://snl.no/vegetasjonsperiode (accessed on 4 June 2021).
- Wright, T.E.; Kasel, S.; Tausz, M.; Bennett, L.T. Edge Microclimate of Temperate Woodlands as Affected by Adjoining Land Use. Agric. For. Meteorol. 2010, 150, 1138–1146. [Google Scholar] [CrossRef]
- Biedermann, R.; Niedringhaus, R. Die Zikaden Deutschlands. Bestimmungstafeln für alle Arten; Wissenschaftlich Akademischer Buchvertrieb: Berlin, Germany, 2009. [Google Scholar]
- Böhme, J. Katalog (Faunistische Übersicht); Die Käfer Mitteleuropas; Elsevier GmbH: Berlin, Germany, 2005. [Google Scholar]
- Brown, N.E.; Mitchell, S.C.; Garbary, D.J. Host Selection by Larvae of a Marine Insect Halocladius variabilis: Nutritional Dependency or Escape from Predation? J. Mar. Biol. Assoc. UK 2013, 93, 1373–1379. [Google Scholar] [CrossRef]
- Chvála, M. Swarming, Mating and Feeding Habits in Empididae (Diptera), and Their Significance in Evolution of the Family. Acta Entomol. Bohemoslov. 1976, 73, 353–366. [Google Scholar]
- Haenni, J.-P.; Greve, L. Faunistic Note about Norwegian Scatopsidae (Diptera), with Description of a New Species. Fauna Nor. Ser. B 1995, 42, 71–82. [Google Scholar]
- Haenni, J.-P.; Greve, L. New Records of Norwegian Scatopsidae (Diptera). Nor. J. Entomol. 2000, 47, 65–71. [Google Scholar]
- Hirvenoja, M.; Palmén, E.; Hirvenoja, E. The Emergence of Halocladius variabilis (Staeger) (Diptera: Chironomidae) in the Surroundings of the Tvärminne Biological Station in the Northern Baltic Sea. Entomol. Fenn. 2006, 17, 87–89. [Google Scholar] [CrossRef]
- Kvamme, T.; Wallin, H. Biological Notes and Distribution of Leiopus Audinet-Serville, 1835 (Coleoptera, Cerambycidae) in Norway. Nor. J. Entomol. 2013, 60, 119–125. [Google Scholar]
- Menzel, F.; Smith, J.E.; Chandler, P.J. The Sciarid Fauna of the British Isles (Diptera: Sciaridae), Including Descriptions of Six New Species. Zool. J. Linn. Soc. 2006, 146, 1–147. [Google Scholar] [CrossRef]
- Negrobov, O. Family Dolichopodidae. In Catalogue of the Palaerarctic Diptera; Soós, A., Papp, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 7, pp. 11–139. [Google Scholar]
- Niesiolowski, S. Empididae aquatic wodne wujkowate (Insecta Diptera). Fauna Pol. 1992, 14, 1–127. [Google Scholar]
- Satchell, G.H. The Larvae of the British Species of Psychoda (Diptera: Psychodidae). Parasitology 1947, 38, 51–69. [Google Scholar] [CrossRef]
- Skartveit, J. Distribution and Flight Periods of Bibio Geoffroy, 1762 Species (Diptera, Bibionidae) in Norway, with a Key to the Species. Fauna Nor. Ser. B 1995, 42, 83–112. [Google Scholar]
- Svensson, B.W. Fjärilsmyggfaunan i ett hagmarksområde och en ladugård i östra Blekinges skogsland. Med en översikt av familjen Psychodidaes morfologi, systematik och utforskande, samt särskilt de svenska Psychoda s.l. -arternas biologi. Entomol. Tidskr. 2009, 130, 185–208. [Google Scholar]
- Withers, P. Moth Flies (Diptera: Psychodidae). Dipter. Dig. 1989, 4, 1–83. [Google Scholar]
- Japoshvili, G.; Hansen, L.O. New Records of Encyrtidae (Hymenoptera, Chalcidoidea) from Norway II. Nor. J. Entomol. 2013, 60, 68–72. [Google Scholar]
- Japoshvili, G.; Hansen, L.O. New Records of Encyrtidae (Hymenoptera, Chalcidoidea) from Norway IV. Nor. J. Entomol. 2014, 61, 180–185. [Google Scholar]
- Japoshvili, G.; Hansen, L.O. Revision of the Genus Aphelinus Dalman (Hymenoptera: Chalcidoidea: Aphelinidae). Turk. J. Zool. 2014, 38, 552–558. [Google Scholar] [CrossRef]
- Oliver, P.G.; Meechan, C.J. Woodlice—Synopses of the British Fauna (New Series); Field Studies Council: Shrewsbury, UK, 1993; Volume 49. [Google Scholar]
- Gregor, F.; Rozkošny, R.; Barták, M.; Vaňhara, J. The Muscidae (Diptera) of Central Europe. Folia Fac. Sci. Nat. Univ. Masaryk. Brun. Biol. 2002, 107, 1–280. [Google Scholar]
- Hennig, W. Anthomyiidae. In Die Fliegen der Palaearktischen Region; Lindner, E., Ed.; E. Schweizerbartsche Verlagsbuchhandlung: Stuttgart, Germany, 1976; Volume 1, pp. 1–974. [Google Scholar]
- Hennig, W. Muscidae. In Die Fliegen der Palaearktischen Region; Lindner, E., Ed.; E. Schweizerbartsche Verlagsbuchhandlung: Stuttgart, Germany, 1964; Volume 2, pp. 1–1110. [Google Scholar]
- Papp, L. Families of Heleomyzoidea. In Contributions to a Manual of Palaearctic Diptera (with Special Reference to Flies of Economic Importance)– 3. Higher Brachycera; Papp, L., Darvas, B., Eds.; Science Herald: Hameln, Germany, 1998; pp. 425–455. [Google Scholar]
- Rozkošný, R.; Gregor, F.; Pont, A.C. The European Fanniidae (Diptera); Institute of Landscape Ecology: Münster, Germany, 1997. [Google Scholar]
- Shatalkin, A.I. Keys to the palaeartic flies of the family Lauxaniidae (Diptera). Zool. Issled. 2000, 5, 1–102. [Google Scholar]
- Schacht, W.; Kurina, O.; Merz, B.; Gaimari, S. Zweiflügler aus Bayern XXIII (Diptera: Lauxaniidae, Chamaemyiidae). Z. Für Entomol. 2004, 25, 41–80. [Google Scholar]
- Freeman, P. Sciarid Flies. Diptera, Sciaridae. Handb. Identif. Br. Insects 1983, 9, 1–68. [Google Scholar]
- Hippa, H.; Vilkamaa, P.; Heller, K. Review of the Holarctic Corynoptera Winnertz, 1867, s. str. (Diptera, Sciaridae). Zootaxa 2010, 2695, 1–197. [Google Scholar] [CrossRef]
- Frey, R. Entwurf einer neuen Klassifikation der Mückenfamilie Sciaridae (Lycoriidae). II. Die nordeuropäischen Arten. Not. Entomol. 1948, 27, 33–112. [Google Scholar]
- Menzel, F.; Heller, K. Sechs neue Arten aus den Gattungen Bradysia, Camptochaeta und Corynoptera (Diptera: Sciaridae) nebst einigen Bemerkungen zur Nomenklatur europäischer Trauermücken. Stud. Dipterol. 2005, 11, 335–357. [Google Scholar]
- Menzel, F.; Mohrig, W. Revision der Paläarktischen Arten von Trichosia Winnertz sensu Tuomikoski, 1960 (Diptera, Sciaridae)—Teil, I. Gattung Trichosia Winnertz, 1867. Stud. Dipterol. 1997, 4, 3–40. [Google Scholar]
- Menzel, F.; Mohrig, W. Beiträge Zur Taxonomie und Faunistik der Paläarktischen Trauermücken (Diptera, Sciaridae). Teil VI—Neue Ergebnisse aus Typenuntersuchungen und die daraus resultierenden taxonomisch-nomenklatorischen Konsequenzen. Stud. Dipterol. 1998, 5, 351–378. [Google Scholar]
- Menzel, F.; Mohrig, W. Revision der Paläarktischen Trauermücken (Diptera: Sciaridae). Stud. Dipterol. Suppl. 2000, 6, 1–761. [Google Scholar]
- Mohrig, W. Zur Kenntnis flügelreduzierter Dipteren der Bodenstreu—I. Beitrag. Wiss. Z. Ernst Moritz Arndt Univ. Greifswald Math. Nat. Reihe 1969, 18, 53–59. [Google Scholar]
- Mohrig, W.; Heller, K.; Hippa, H.; Vilkamaa, P.; Menzel, F. Revision of Black Fungus Gnats (Diptera: Sciaridae) of North America. Stud. Dipterol. 2013, 19, 141–286. [Google Scholar]
- Shin, S.; Menzel, F.; Heller, K.; Lee, H.; Lee, S. Review of the Genus Cratyna Winnertz (Diptera: Sciaridae) in Korea, Including the Description of a New Species. Zootaxa 2014, 3794, 344–354. [Google Scholar] [CrossRef]
- Tuomikoski, R. Zur Kenntnis der Sciariden (Dipt.) Finnlands. Ann. Zool. Soc. Zool. Bot. Fenn. Vanamo 1960, 21, 1–164. [Google Scholar]
- Wagner, E. Blindwanzen oder Miriden. In Die Tierwelt Deutschlands und der Angrenzenden Meeresteile; Dahl, F., Dahl, M., Bischoff, H., Eds.; Nature: Jena, Germany, 1952; Volume I–IV, pp. 1–218. [Google Scholar]
- Wagner, E. Wanzen oder Heteropteren—I Pentatomorpha. In Die Tierwelt Deutschlands und der angrenzenden Meeresteile; Dahl, F., Dahl, M., Peus, F., Eds.; Nature: Jena, Germany, 1966; Volume I–VI, pp. 1–235. [Google Scholar]
- Wagner, E. Wanzen oder Heteropteren—II Cimicomorpha. In Die Tierwelt Deutschlands und der angrenzenden Meeresteile; Dahl, F., Dahl, M., Peus, F., Eds.; Nature: Jena, Germany, 1967; Volume I–VI, pp. 1–179. [Google Scholar]
- Wyniger, D. Taxonomy and Phylogeny of the Central European Bug Genus Psallus (Hemiptera, Miridae) and Faunistics of the Terrestrial Heteroptera of Basel and Surroundings (Hemiptera). Ph.D. Thesis, University of Basel, Basel, Switzerland, 2004. [Google Scholar]
- Giljarov, M.S. (Ed.) Opredelitel Obitajuščich v Počve Kleščej. Sarcoptiformes. [Key of Soil Mites. Sarcoptiformes]; Nauka: Moscow, Russia, 1975. [Google Scholar]
- Kunst, M. Nadkohorta pancířníci—Oribatei [Supercohort moss mites—Oribatei]. In Klíč Zvířeny ČSSR [Key of the Fauna of Czechoslovakia]; Daniel, M., Černý, V., Eds.; Academia: Prague, Czech Republic, 1971; Volume IV, pp. 531–580. [Google Scholar]
- Weigmann, G. Hornmilben (Oribatida). Acari, Actinotrichida. In Die Tierwelt Deutschlands; Goecke & Evers: Keltern, Germany, 2006; Volume 76, pp. 1–520. [Google Scholar]
- Assis Fonseca, E.C.M. Diptera: Dolichopodidae. Handb. Identif. Br. Insects 1978, IX, 1–90. [Google Scholar]
- Grichanov, I.Y. A Checklist and Keys to North. European Genera and Species of Dolichopodidae (Diptera); Plant Protection News Supplement 2006; All-Russian Institute of Plant Protection RAAS: St. Petersburg, Russia, 2006; pp. 1–61. [Google Scholar]
- Negrobov, O.P. 29. Dolichopodidae. In Die Fliegen der palaearktischen Region; Lindner, E., Ed.; E. Schweizerbart: Stuttgart, Germany, 1974; Volume 4, pp. 273–353. [Google Scholar]
- Douwes, P.; Abenius, J.; Cederberg, B.; Wahlstedt, U.; Hall, K.; Starkenberg, M.; Reisborg, C.; Östman, T. Nationalnyckeln till Sveriges flora och fauna. Steklar: Myror—getingar. Hymenoptera: Formicidae—Vespidae; ArtDatabanken, SLU: Uppsala, Sweden, 2012. [Google Scholar]
- Kvamme, T.; Wetås, Å. Revidert Liste Over Norske Maur. Inkludert Dialektale Navn og Forslag til Nye Norske navn; Norsk Institutt for Skog og Landskap: Ås, Norway, 2010; pp. 1–127. [Google Scholar]
- Seifert, B. The Ants of Central and North. Europe; Lutra Verlags- und Vertriebsgesellschaft: Tauer, Germany, 2018. [Google Scholar]
- Contreras-Lichtenberg, R. Revision der Westpaläarktischen Arten des Genus Glyptotendipes Kieffer, 1913 (Insecta: Diptera, Nematocera, Chironomidae), Teil 2: Sg. Glyptotendipes s. str. Kieffer, 1913 Und Sg. Trichotendipes Heyn, 1993. Ann. Nat. Mus. Wien. 2001, 103B, 417–451. [Google Scholar]
- Ekrem, T. A Taxonomic Review of the Genus Stempellinella (Diptera: Chironomidae). J. Nat. Hist. 2007, 41, 1367–1465. [Google Scholar] [CrossRef]
- Ferrington, L.C.J.; Sæther, O.A. A Revision of the Genera Pseudosmittia Edwards, 1932, Allocladius Kieffer, 1913, and Hydrosmittia gen. n. (Diptera, Chironomidae, Orthocladiinae). Zootaxa 2011, 2849, 1–314. [Google Scholar] [CrossRef]
- Fittkau, E.J. Die Tanypodinae (Diptera, Chironomidae). Die Tribus Anatopyniini, Macropeloplini und Pentaneurini. Abh. Zur Larvalsystematik Insekten 1962, 6, 1–453. [Google Scholar]
- Freeman, P. Two New Species of Chironomidae (Dipt.) from Britain. Entomol. Mon. Mag. 1948, 84, 49–50. [Google Scholar]
- Reiss, F. Die neue, europäisch verbreitete Chironomidengattung Parapsectra mit einem brachypteren Artvertreter aus Mooren (Diptera). Arch. Für Hydrobiol. 1969, 66, 192–211. [Google Scholar]
- Hirvenoja, M. Revision der Gattung Cricotopus van der Wulp und ihrer Verwandten (Diptera, Chironomidae). Ann. Zool. Fenn. 1973, 10, 1–363. [Google Scholar]
- Kownacki, P.H.; Zosidze, R. Parametriocnemus stylatus adzharicus n. ssp. (Chironomidae, Diptera). Bull. Acad. Pol. Sci. 1973, 21, 127–130. [Google Scholar]
- Langdon, P.H.; Pinder, L.C.V. Keys to the Adult Male Chironomidae of Britain and Ireland. Freshw. Biol. Assoc. Sci. Publ. 2007, 64, 168–239. [Google Scholar]
- Langdon, P.H.; Vallenduuk, H.J. The Karyotype and Morphology of All Stages of Chironomus (Chaetolabis) macani Freeman, 1948 and Chironomus (Chaetolabis) bitumineus nom. nov. for C. (C.) macani Wiederholm, 1997 nec Freeman. Lauterbornia 2013, 76, 11–18. [Google Scholar]
- Lehmann, J. Revision der europäischen Arten der Gattung Parachironomus Lenz (Diptera, Chironomidae). Hydrobiologia 1970, 36, 129–158. [Google Scholar] [CrossRef]
- Lehmann, J. Revision der europäischen Arten der Gattung Eukiefferiella Thienemann (Diptera: Chironomidae). Beitr. Zur Entomol. 1972, 22, 347–405. [Google Scholar]
- Schlee, D. Vergleichende Merksmalanalyse zur Morphologie und Phylogenie der Corynoneura-Gruppe (Diptera, Chironomidae). Zugleich eine allgemeine Morphologie der Chironomiden-Imago. Stuttg. Beitr. Zur Naturkunde 1968, 180, 1–150. [Google Scholar]
- Stur, E.; Ekrem, T. A Revision of West Palaearctic Species of the Micropsectra atrofasciata Species Group (Diptera: Chironomidae). Zool. J. Linn. Soc. 2006, 146, 165–225. [Google Scholar] [CrossRef]
- Sæther, O.A. Metriocnemus van Der Wulp: Seven New Species, Revision of Species, and New Records (Diptera: Chironomidae). Ann. Limnol. 1995, 31, 35–64. [Google Scholar] [CrossRef]
- Sæther, O.A. Metriocnemus van Der Wulp: A New Species and a Revision of Species Described by Meigen, Zetterstedt, Stæger, Holmgren, Lundström and Strenzke (Diptera: Chironomidae). Entomol. Scand. 1989, 19, 393–430. [Google Scholar] [CrossRef]
- Sæther, O.A. A Review of the Genus Limnophyes Eaton from the Holarctic and Afrotropical Regions (Diptera: Chironomidae, Orthocladiinae). Entomol. Scand. Suppl. 1990, 35, 1–135. [Google Scholar]
- Sæther, O.A.; Halvorsen, G.A. Diagnoses of Tvetenia Kieff. emend., Dratnalia n. gen., and Eukiefferiella Thien. emend., with a Phylogeny of the Cardiocladius Group (Diptera: Chironomidae). Entomol. Scand. Suppl. 1981, 15, 269–285. [Google Scholar]
- Sæther, O.A.; Sublette, J.E. A Review of the Genera Doithrix n. gen., Georthocladius Strenzke, Parachaetocladius Wülker and Pseudorthocladius Goetghebuer (Diptera: Chironomidae, Orthocladiinae). Entomol. Scand. Suppl. 1983, 20, 1–100. [Google Scholar]
- Sæther, O.A.; Wang, X. Revision of the Genus Paraphaenocladius Thienemann, 1924 of the World (Diptera: Chironomidae, Orthocladiinae). Entomol. Scand. Suppl. 1995, 48, 1–69. [Google Scholar]
- Chironomidae of the Holarctic Region. Keys and Diagnoses. Part 3. Adult Males. Entomol. Scand. Suppl. 1989, 34, 1–532. [Google Scholar]
- Wülker, W. A Lobochironomus-species with 3 chromosomes (2n = 6)−the true Chironomus (Lobochironomus) mendax Storå (Diptera, Chironomidae). In A festschrift Honoring Mary and Jim Sublette. Part 1: Taxonomy and Systematics of Chironomidae; Berg, M.B., Ferrington, L.C.J., Hayford, B.L., Eds.; Kansas Entomological Society: Lawrence, KS, USA, 1999; Volume 71, pp. 304–314. [Google Scholar]
- Podenas, S.; Byun, H.-W. New Limoniinae Crane Flies (Diptera: Limoniidae) of Korea. J. Species Res. 2014, 3, 167–182. [Google Scholar] [CrossRef][Green Version]
- Podenas, S.; Geiger, W.; Haenni, J.-P.; Gonseth, Y. Limoniidae & Pediciidae de Suisse. Fauna Helv. 2006, 14, 1–375. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).