Abstract
In this study, Vidal grape must was fermented using commercial Saccharomyces cerevisiae F33 in pure culture as a control and in mixed culture with five indigenous non-Saccharomyces yeast strains (Hanseniaspora uvarum QTX22, Saccharomycopsis crataegensis YC30, Pichia kluyveri HSP14, Metschnikowia pulcherrima YC12, and Rhodosporidiobolus lusitaniae QTX15) through simultaneous fermentation in a 1:1 ratio. Simultaneous fermentation inhibited the growth of S. cerevisiae F33 and delayed the time to reach the maximum biomass. Compared with pure fermentation, the contents of polyphenols, acetic esters, ethyl esters, other esters, and terpenes were increased by R. lusitaniae QTX15, S. crataegensis YC30, and P. kluyveri HSP14 through simultaneous fermentation. S. crataegensis YC30 produced the highest total aroma activity and the most abundant aroma substances of all the wine samples. The odor activity values of 1 C13-norisoprenoid, 3 terpenes, 6 acetic esters, and 10 ethyl esters improved significantly, and three lactones (δ-decalactone, γ-nonalactone, and γ-decalactone) related to coconut and creamy flavor were only found in this wine. Moreover, this sample showed obvious “floral” and “fruity” note odor due to having the highest amount of ethyl ester aromatic substances and cinnamene, linalool, citronellol, β-damascenone, isoamyl ethanoate, benzylcarbinyl acetate, isobutyl acetate, etc. We suggest that simultaneous fermentation of S. crataegensis YC30 with S. cerevisiae might represent a novel strategy for the future production of Vidal icewine.
1. Introduction
Icewine is a kind of sweet wine that is made by delaying the harvest, allowing the grapes to hang on the vine for a certain period of time, followed by freezing, harvesting, and pressing the grapes at low temperature, and brewing []. Delaying harvesting increases the concentrations of sugars, acids, aroma compounds, and some non-volatile substances in the grapes, intensifying the flavor characteristics. The methods of freezing grapes, low-temperature maceration, and fermentation technology better maintain the aroma substances in the wine []. The current research on icewine has mainly focused on the identification of characteristic flavor substances [] and the analysis of the dynamic changes in yeast populations during fermentation []. However, relatively few results have been reported on the influence of mixed fermentation with non-Saccharomyces and Saccharomyces yeasts on the enhancement in icewine flavor quality.
Wine fermentation is a complex biochemical process in which yeasts play a critical role by converting sugar into ethanol, carbon dioxide, and thousands of other secondary metabolites []. Scientific research showed that the quality of wine is highly dependent on the metabolic activities and fermentation behavior of different yeasts, which significantly contribute to the chemical composition, and sensory and flavor characteristics of wine []. To date, S. cerevisiae is the most widely used strain for wine production in the industry [], mainly due to its ability to control the risk of deterioration and its good fermentation power [], but its use is associated with problems regarding the singularity and homogeneity of wine flavor characteristics []. The fermentation strategy of pure starter cultures has drastically reduced the diversity of the yeast species and clones involved in winemaking, resulting in a uniformly plain flavor of the product []. As such, using mixed S. cerevisiae and non-Saccharomyces yeast fermentation has become a strategy pursued by winemakers, especially using some indigenous yeasts that have strong adaptability and representativeness, to obtain a unique style of wine that has representative, diverse, and complex aroma characteristics, thereby improving and enhancing wine flavor quality [].
Non-Saccharomyces yeasts have become a choice to improve wine quality []. Numerous studies have demonstrated that these yeast strains can be used to achieve specific objectives such as producing some expected secondary metabolites, lowering the ethanol content, preventing the growth of some undesirable strains, and increasing the production of specific enzymes, but the disadvantage is their insufficient fermentation power []. Mixed fermentation of non-Saccharomyces and S. cerevisiae yeasts can not only improve the diversity and complexity of wine aroma, but also compensate for the lack of fermentation power of non-Saccharomyces yeasts, which is an effective method to improve wine aroma quality []. Li et al. [] found that highly antagonistic S. cerevisiae co-inoculation with P. fermentans significantly improved the production of glycosidase activity and wine varietal odorants. Morales et al. [] confirmed that L. thermotolerans co-inoculated with S. cerevisiae at the ratios of 50:1 and 20:1 enhances wine flavor quality and aromatic complexity. S. cerevisiae sequential inoculation fermentation with Metschnikowia pulcherrima, Torulaspora delbrueckii and Zygosaccharomyces bailii under 0.025 VVM aeration conditions reduced the ethanol concentration of chardonnay wine by 1.6%, 0.9%, and 1.0% (v/v), and the chemical volatile profiles of the wine were acceptable []. Using Starmerella bacillaris mixed with S. cerevisiae produced significantly lower levels of acetic acid, ethanol, and ethyl acetate, and higher amounts of glycerol, higher alcohols, and esters []. P. kluyveri improves wine quality parameters such as thiol, fruity ester, and terpene concentrations, mainly in sequential fermentation []. Higher concentration of medium-chain fatty acids was associated with the higher biomass suppression of H. uvarum in co-inoculation with killer S. cerevisiae, which resulted in the increased formation of fruity esters, but effectively restricted the production of ethyl acetate []. H. uvarum, in mixed fermentation with commercial SC F5, increased the medium-chain fatty acid ethyl ester content in both the synthetic media and grape must of cabernet Gernischt grapes []. M. pulcherrima has also been shown to increase wine flavour and aroma in Debina wines [,], and/or enhance positive sensory attributes, such as “citrus/grape fruit”, “pear” and “flowery” in Riesling [] and in base wine for sparkling wine production [].
Therefore, in this study, using the Vidal icewine as an example, we evaluated the influence of the mixed fermentation of non-Saccharomyces and Saccharomyces yeasts on the flavor characteristics of wine, explored the potential non-Saccharomyces yeasts that can improve Vidal icewine flavor quality, and then analyzed the potential association between characteristic aroma substances and their contribution to wine aroma. Our findings provide a new strategy for the industrial production of icewine.
2. Materials and Methods
2.1. Yeast Strains
The fermentations were performed with one commercial S. cerevisiae Actiflore® F33 strain (Laffort) as control and five representative indigenous non-Saccharomyces yeast strains isolated from Ningxia province in China: Hanseniaspora uvarum QTX22 (Hu, MT505668), Saccharomycopsis crataegensis YC30 (Sc, MT505672), Pichia kluyveri HSP14 (Pk, MT505679), Metschnikowia pulcherrima YC12 (Mp, MT505675) and Rhodosporidiobolus lusitaniae QTX15 (Rl, MT505670). S. crataegensis YC30 grew on the WL medium as with a white flocculent convex colony morphology (Figure 1).
Figure 1.
The colony morphology diagram of S. crataegensis YC30.
2.2. Reagents and Standards
The organic acid standards (L-tartaric acid, lactic acid, malic acid, acetic acid, etc.), as well as polyphenol standards (protocatechuic acid, p-hydroxybenzonic acid, chlorogenic acid, vanillic acid, (-)-epigallocatechin gallate, epicatechin, vanillin, p-coumaric acid, (-)-epicatechin gallate, isoferulic acid, etc.), were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Water was purified using a Milli-Q system from Millipore (Bedford, MA, USA).
Isoamyl ethanoate, benzylcarbinyl acetate, isobutyl acetate, butyl acetate, propyl acetate, hexyl acetate β-damascenone, naphthalene, cinnamene, linalool, citronellol, d-limonene, α-terpineol, nerol oxide, ethyl dodecylate, ethyl heptoate, ethyl caproate, ethyl butanoate, ethyl caprylate, ethyl propanoate, ethyl isobutyrate, ethyl n-valerate, etc., were provided by Sigma-Aldrich (Shanghai, China). The internal standard of 4-methyl-1-pentanol was obtained from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). The calculation of retention indices (RIs) was used with n-alkanes (C8-C40) purchased from Supelco (Bellefonte, PA, USA).
2.3. Grape Juice
Vitis vinifera L. cv. Vidal grape was harvested in December 2017 with temperature at −12 °C and obtained from the Chateau barges vineyard (Yinchuan, Ningxia Province, China, 106.02° E, 38.24° N), with a sugar content of 344.0 g/dm3, acidity of 4.2 g/dm3 (as tartaric acid) and pH 3.58. The total sugar content was analyzed by the reduction method using Fehling’s reagent []. Total acid content was determined by titration of samples with 0.1 mol/L NaOH (tartaric acid equivalent) and ethanol content was assayed using the gas chromatographic method (GB/T 15038-2006, 2006) []. The agronomic practices of the region were applied to manage the vineyard and the vines were planted in 2013 with a vine row spacing of 1.0 × 2.0 m. About 80 kg of grapes were destemmed, crushed, and treated with sulfur dioxide (50 mg/L K2S2O5) and 20 mg/L pectinase (≥500 U/mg) purchased from Laffort Co. (Bordeaux, France), and macerated at 4 °C for 72 h to inhibit bacterial growth and increase the juice yield. The obtained grape juice was stored at −20 °C until use.
2.4. Fermentation Strategies
For fermentation, we followed a microfermentation method according to Wei et al. [], with modifications. The yeast strains were stored at −80 °C with 20% sterile glycerol before use. The cryogenically preserved yeasts were propagated by two successive transfers in sterile yeast extract peptone dextrose (YPD) medium (yeast extract, peptone and glucose were 1%, 2% and 2% (w/v), respectively) for 24 h each time. Subsequently, the yeast strains were pre-cultured in pasteurized grape juice (100 °C, 10 min) for 12 h (28 °C, 2.5 g). After the yeast strains were activated to viable counts of approximately 106 CFU/mL, they were inoculated into 350 mL of sterile grape juice in 500-mL Erlenmeyer flasks. The flasks were fitted with sterile glass air locks that contain sulfuric acid to allow the CO2 produced during the fermentation to escape to avoid microbial contamination []. Fermentation trials were performed using commercial S. cerevisiae F33 in pure culture as a control (pure_F33), and simultaneous co-fermentation with H. uvarum QTX22 (F33_Hu), S. crataegensis YC30, P. kluyveri HSP14 (F33_Pk), M. pulcherrima YC12 (F33_Mp), and R. lusitaniae QTX15 (F33_Rl) in a ratio of 1:1, separately. The quantity of CO2 released was monitored every 24 h by weighing the bottles during fermentation. Fermentations were controlled at 18 °C and continued until no more weight loss was quantified for three consecutive days, regardless of the residual sugar level. This was performed in triplicate: three flasks were inoculated with the same culture at the same time. The grape juice uninoculated with yeast strains was used as a control and incubated alongside the fermentations. One milliliter of fermenting grape juice was collected periodically (Day 0, 1, 2, 3, 4, 5, 7, 9, 12 and 13) and diluted to a suitable concentration, and then 100 μL of these cultures were plated on WL nutrient agar to facilitate yeast population counts. The colonies of S. cerevisiae F33 were smooth and creamy white, whereas those of H. uvarum QTX22 and R. lusitaniae QTX15 were smooth and green, those of S. crataegensis YC30 and P. kluyveri HSP14 were white with flocculent folds, and that of M. pulcherrima YC12 was milky white, and could be easily discriminated from S. cerevisiae F33 in mixed cultures on WL media. Fermentation rate was calculated as the loss of CO2 (g/L) within 24 h during the fermentation []. After fermentation, all wine samples (350 mL) were centrifuged for 8 min (4000× g, 4 °C), and the cell-free supernatants were stored at −20 °C and used within 6 months for analysis.
2.5. Assay for Organic Acids and Polyphenol Compounds
The organic acids and polyphenols samples were analyzed following the method described by Ye et al. [], with modifications. The organic acids and polyphenolic compounds were determined by high-performance liquid chromatography (HPLC) on an LC-15C HPLC system equipped with a photodiode array detection and a SIL-10AF automatic sampler (Shimadzu, Kyoto, Japan). For the analysis of organic acids, wine samples were diluted with ultrapure water to an appropriate concentration and filtered through a 0.25 μm Waters membrane filter. The target compounds were separated on a Waters XSelect® HSS T3 reversed-phase column (250 × 4.6 mm, particle size of 5.0 μm) at a total flow rate of 1 mL/min with gradient elution. Mobile phases A and B were methanol and 0.01 mol/L ammonium phosphate aqueous solution, respectively. The mobile phase A increased linearly within 12 min from 0% to 2% and then held for 13 min. For the analyses of polyphenolic compounds, 50 mL samples were adjusted to pH 7.0 and pH 2.0 with 1 mol/L NaOH and HCl, respectively, and were extracted three times with 100 mL ethyl acetate. The organic phase was combined and evaporated to dryness on a vacuum rotary evaporator at 35 °C, and then dissolved in 25 mL of methanol. The resultant solution was filtered through a 0.25 μm Waters membrane. The mobile phase A was 2% acetic acid in water (v/v), and the mobile phase B was 0.5% acetic acid in water and acetonitrile (50:50, v/v). The gradient elution was 10–55% solvent B (50 min), 55–100% B (10 min), and 100–10% B (5 min), with a flow rate of 0.8 mL/min on a Waters xTerra MS C18 reverse-phase column (250 × 4.6 mm, particle size of 5.0 μm) at 40 °C, with a 75 min total run time. The absorbance signal was read at 280 nm for dihydrochalcones and flavan-3-ol, 320 nm for hydroxycinnamic acid, and 360 nm for flavonols. Both organic acids and polyphenolic compounds quantification methods were performed using standards with the external standard and repeated three times.
2.6. Quantification of Volatile Compounds
Volatile compounds were analyzed by headspace solid-phase microextraction combined with gas chromatography-mass spectrometry (HS-SPME-GC-MS) on a GC-MS TQ8050 NX system (Shimadzu) equipped with an InertCap WAX chromatographic column (30 m × 0.25 mm × 0.25 μm, GL Sciences Inc., Tokyo, Japan), as described previously []. We placed 5 mL wine samples were placed in 20 mL headspace bottles containing 1.5 g NaCl, and 4-methyl-1-pentanol internal standard solution was added to each sample. SPME fiber (50/30 μm, DVB/CAR/PDMS, Supelco, Inc., Bellefonte, PA, USA) was inserted into the headspace bottles containing the sample solution, equilibrated in a 40 °C water bath with stirring for 15 min, extracted for 35 min, and then desorbed in the GC injector at 250 °C for 3 min. Mass spectra were acquired over m/z 50–450 in EI mode at 70 eV. The retention index (RI) based on the mixture of n-alkanes (C8-C40), retention time, mass spectra, and 85% similarity, per the NIST 14 library, were used to tentatively qualitatively analyze the compounds. Where possible, the identification of compounds was confirmed by comparing an external standard method with authentic standards. Each experiment was repeated three times with three replicates in each experiment.
The odor activity value (OAV), defined as the ratio between the concentration of the individual chemical compound and its sensory detection threshold in the literature, was calculated for all the identified volatiles to evaluate the contribution of volatiles to wine aroma [].
2.7. Sensory Analysis
For our sensory analysis method, we followed a method described in the literature with modifications []. About 18 sensory panel students of 9 women and 9 men were trained using a 54 aroma kit (Le Nez du Vin®, Jean Lenoir, Provence, France), according to the wine industry, for 45 days. During the training, each aroma type was identified by the panel every six days until the panel identification deviation for each aroma item was less than 5%. Wine samples were placed in a clean black glass in a random order at a 25 °C room temperature and identified in duplicate. The wine aroma profile was described by each sensory panelist according to the terms of Le Nez du Vin aroma kit. A five-point scale was used for wine aroma profile scores: 0, no perception; 1, very weak; 2, weak; 3, medium; 4, strong; and 5, very strong. The computational formula was as follows:
where F% is the average detection frequency of the terms described in an aroma group by the panel, and I% is the average intensity of the described terms in the group expressed as the percentage of maximum intensity.
Triangular tests were performed to determine whether there was a perceptible difference between the two samples. During the tasting, two samples were poured into three glasses at a time. Two of them contained the same sample. Sensory panel students were asked to identify which glass was different from the other two samples. The wine samples were presented in a random order, including all possible combinations. The results of all of the triangular tests were statistically analyzed according to the tables reported in the literature [] and based on the binomial law corresponding to the distribution of answers in this type of test.
2.8. Statistical Analysis
The correlation analysis between sensory analysis and aroma compounds was performed using PLSR via Unscrambler 9.7 (CAMO ASA, Trondheim, Norway). One-way analysis of variance (ANOVA) and Duncan’s multiple-range test (p < 0.05), column graphs, PCA, and pheatmaps were implemented using R version 3.6.1.
3. Results and Discussion
3.1. Fermentation Performance of Yeasts
Parameters including fermentation rate, along with yeasts biomass, cell numbers, etc., were found to have a critical influence on the final flavor quality of wine during fermentation []. Figure 2A shows the growth kinetic characteristics of pure culture of S. cerevisiae F33 and the mixed fermentation with five non-Saccharomyces yeasts. The results showed that the maximum CO2 production rate in the pure culture was reached on day 3 (98.2 g/L); when with non-Saccharomyces yeast strains, the maximum was reached on day 4 (72.8–83.7 g/L). The final CO2 release in pure fermentation was almost perfectly aligned with simultaneous fermentation, indicating that the fermentation of Vidal icewine fermented well under the two fermentation strategies.
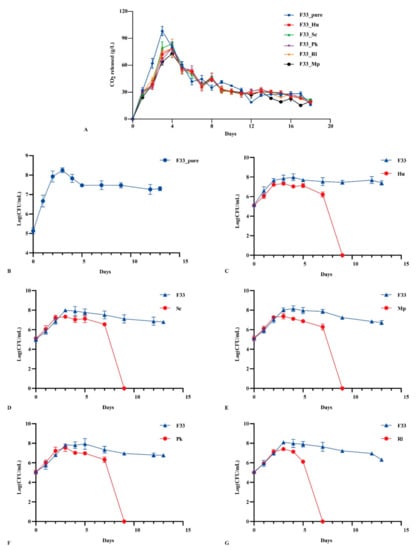
Figure 2.
The fermentation kinetics characteristics and yeast population dynamics in Vidal icewine during fermentation with six fermentation strategies. (A) CO2 release in pure and co-mixed fermentation. pure_F33: S. cerevisiae F33 monoculture. F33_Hu: co-inoculation of S. cerevisiae F33 and H. uvarum QTX22. F33_Sc: co-inoculation of S. cerevisiae F33 and S. crataegensis Y30. F33_Mp: co-inoculation of S. cerevisiae F33 and M. pulcherrima YC15. F33_Pk: co-inoculation of S. cerevisiae F33 and P. kluyveri HSP11. F33_Rl: co-inoculation of S. cerevisiae F33 and R. lusitaniae QTX26. (B–G) Yeast population dynamics in different fermentations. Hu: H. uvarum QTX22. Sc: S. crataegensis. Mp: M. pulcherrima YC15. Pk: P. kluyveri HSP11. Rl: R. lusitaniae QTX26.
The growth kinetics of S. cerevisiae F33 in pure and mixed fermentation with five non-Saccharomyces yeasts are shown in Figure 2B–G. All yeast strains propagated sharply within 1 day after inoculation; pure S. cerevisiae F33 reached a maximum on day 3 (8.25 log CFU/mL), and then continually decreased until it stabilized on day 7 (7.48 log CFU/mL). However, the simultaneous fermentation of five non-Saccharomyces yeast strains delayed the time for S. cerevisiae F33 to reach the maximum biomass. S. cerevisiae F33 reached the maximum biomass on day 3 when mixed with S. crataegensis YC30 (7.99 log CFU/mL) and R. lusitaniae QTX15 (7.99, 8.10 log CFU/mL), on day 4 when mixed with H. uvarum QTX22 (7.98 log CFU/mL) and M. pulcherrima YC12 (8.14 log CFU/mL), and on day 5 when mixed with P. kluyveri HSP14 (7.93 log CFU/mL). The mixed fermentation strategy inhibited and delayed the growth and development of S. cerevisiae F33 to different degrees, which is consistent with a previous report []. The wine fermented with S. cerevisiae F33 and R. lusitaniae QTX15 could not be detected after day 7, whereas S. cerevisiae F33 mix-fermented with other non-Saccharomyces yeast strains could not be detected after day 5 under this competitive relationship. Binati et al. [] reported that the biomass of non-Saccharomyces yeast strains decreased sharply after inoculation with S. cerevisiae, especially Metschnikowia spp. Non-Saccharomyces yeast strains could not be detected after day 3 during the fermentation of pinot grigio wines, which may related to the antimicrobial peptide secreted by S. cerevisiae and the contact between cells []. In addition, although non-Saccharomyces yeast strains reached the maximum biomass (7.33–7.53 log CFU/mL) on day 3, the maximum biomass was all lower than that of S. cerevisiae F33 and inhibited the biomass of S. cerevisiae F33, especially P. kluyveri HSP14 and H. uvarum QTX22, suggesting that weak competition occurs between S. cerevisiae and non-Saccharomyces yeast strains. Canonico et al. reported that the mixed fermentation of Torulaspora delbrueckii and S. cerevisiae in a ratio of 1:1 can inhibit the biomass of S. cerevisiae and control the entire fermentation process, which plays a dominant role when increasing the inoculation amount. Hu et al. (2018b) [] reported that H. uvarum has a weak competitive relationship with S. cerevisiae, and controlling the amount of inoculation can indirectly affect the production of wine aroma. In our study, we found that the biomass of and CO2 released by yeast strains were inhibited and delayed by the addition of non-Saccharomyces yeast strains; though timely and reliable completion of fermentation is of primary importance in the wine industry [], low-speed fermentation can be considered a benefit for the production and retention of aroma substances in wine and a reduction in the demand for the energy requirement of yeast, which may improve the wine aroma quality [].
3.2. Effect of Fermentation Strategies on Physicochemical Characteristics in Vidal Icewine
The physicochemical characteristics of grape juice and Vidal icewine are shown in Table 1 and Figure 3. The ANOVA revealed significant (p < 0.0001) effects for all the wine samples in terms of physicochemical data and organic acid concentration, except for pH and total acid. Compared with grape juice, the contents of total sugar in wine decreased by 79.09–90.59% and ethanol increased by 7.35–10.12%, indicating that the fermentation progressed smoothly and thoroughly. Compared with the fermentation in pure culture, simultaneous fermentation reduced the ethanol concentration by 14.42–27.27%, which might be related to the weak competition among the yeast strains [].

Table 1.
Physicochemical parameters of Vidal grape juice and icewines fermented with six fermentation strategies.
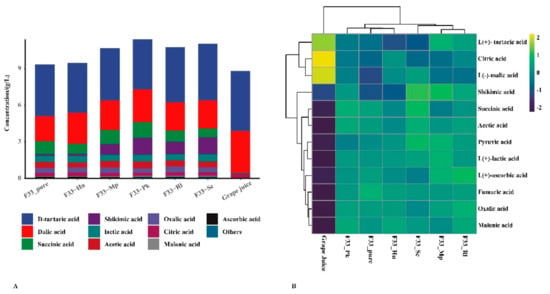
Figure 3.
The distribution of organic acids content under different fermentation strategies. (A) Stacked graph. (B) Clustering pheatmap.
The type and composition of organic acids significantly affect the sensory and chemical properties of wine, including pH, total acid, microbiological stability, etc. []. Some organic acids can produce more complex odors through esterification, which enhances the fruit notes of the wine []. Therefore, it was necessary to evaluate the effects of different fermentation strategies on the organic acids of Vidal icewine. The main organic acids in grape juice were tartaric, malic, and citric acid, accounting for 98.6% of all the individual organic acids, which is similar to the values in a previous report []. Pyruvate, oxalic, malonic, acetic, and lactic acid were not detected in grape juice, which are all produced by yeast metabolism during fermentation; malic, citric, and tartaric acid decreased during fermentation []. In the wine samples, the highest concentrations were tartaric and malic acids, followed by succinic, lactic, and acetic acids. Lactic acid, providing roundness and balanced acidity to the taste of the wine, is mainly affected by different strains []. Lachancea thermotolerans can produce more lactic acid to modify the wine’s acidity []. Acetic acid is a fermentation coproduct with a bitter and sour taste, which is related to the decrease in the malic acid concentration [], and its concentration should be controlled within 0.9 g/L []. Rantsiou et al. [] reported that the concentration of acetic acid in sweet wine fermented by C. zemplinina and S. cerevisiae can be reduced to 0.30 g/L due to the osmotic stress response of the yeast. In our study, simultaneous fermentation produced a higher concentration of malic acid than pure fermentation, but it had little effect on citric, oxalic, and fumaric acids. Simultaneous fermentation of S. cerevisiae F33 and the Pk strain produced the highest levels of pyruvate, malic, succinic, and acetic acids (0.564 g/L), whereas the lowest concentrations of acetic, succinic, and pyruvate acids were produced by Hu, Sc, and Mp strains. Candida zemplinina can increase malic acid content [], whereas Lachancea thermotolerans can limit the production of malic acid [,]. In addition, Pichia kluyveri can increase oxalic, lactic, and succinic acids contents and malic acid degradation in wine [,], modifying the acidity of wine using various yeast strains.
3.3. Effect of Fermentation Strategies on Polyphenols in Vidal Icewine
Polyphenol, a critical quality parameter of the secondary metabolites of the grapes and wine, is not only related to wine color, astringency, bitterness, and other flavor quality parameters, but also has good biological and antioxidant properties []. Table 2 and Figure 4 describe the polyphenol contents of Vidal icewine produced using different fermentation strategies. Protocatechuic acid, rutin, and quercetin were the main phenolic substances in grape juice, whereas (-)-epigallocatechin gallate, (-)-epicatechin gallate, and cynaroside were not detected. The total polyphenol concentrations in mixed fermentation increased by approximately 4.70–7.69 mg/L, whereas that in the wine produced with S. cerevisiae F33 through pure fermentation increased by 1.91 mg/L. The main phenolic substances in wine samples produced by mixed fermentation were caffeic acid, (-)-epicatechin gallate, and p-coumaric acid, followed by protocatechuic acid, cynaroside, trans-cinnamic acid, etc. Compared with S. cerevisiae F33 produced by pure culturing, mixed fermentation of F33 and H. uvarum QTX22 significantly increased the contents of caffeic acid, chlorogenic acid, apigenin, p-hydroxybenzonic acid, etc.; S. crataegensis YC30 significantly increased myricetin, chlorogenic acid, quercetin, vanillic acid, p-hydroxybenzonic acid, etc., contents; M. pulcherrima YC12 significantly increased myricetin, vanillic acid, caffeic acid, chlorogenic acid, etc., contents. Medina et al. [] found that the mixed fermentation of commercial S. cerevisiae with Hanseniaspora vinea increases the phenolic concentrations of chardonnay wine and improves some sensory characteristics of the wine, which is consistent with our experimental conclusions. However, Hranilovic et al. [] reported that the mixed fermentation of S. cerevisiae and M. pulcherrima led to a decrease in the content of flavan-3-ols and anthocyanins compared with S. cerevisiae in pure in Syrah wines, which may be related to the raw material of grape juice.

Table 2.
Concentrations of polyphenols (mg/L) of Vidal grape juice and icewines fermented with six fermentation strategies.
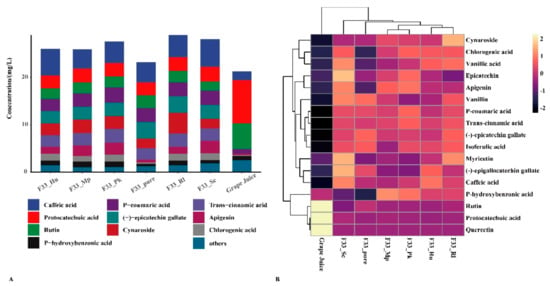
Figure 4.
The distribution of polyphenols content under different fermentation strategies. (A) Stacked graph. (B) Clustering pheatmap.
3.4. Effect of Fermentation Strategies on Volatile Aroma Substances in Vidal Icewine
A total of 118 aroma compounds were detected by HS-SPME-GC-MS (Table 3 and Figure 5A,C). There were 36 common aroma substances to grape juice and wine samples under the six fermentation strategies, among which only simultaneous fermentation of S. cerevisiae F33 and S. crataegensis YC30 produced eight unique aroma substances including ethyl linoleate, δ-decalactone, γ-decalactone, γ-caprolactone, isobutyric acid, propyl propionate, isoamyl butylate, and valeric acid (Figure 5B). The main aroma substances in grape juice were terpenes, acids, and aldehydes, accounting for 74.9%; those in the wine samples were mainly acetate esters, ethyl esters, alcohols, and acids, accounting for 84.8%, which is similar to previously reported values []. The total content of aroma substances in wine was 24.3 times that in grape juice, which is mainly attributed to the glucosidase, protease, and pectinase secreted by yeasts through sugar and amino acid metabolism during fermentation, so that the varietal aroma substances mainly exist in grape in the form of odorless binding glycosides hydrolyzed to free volatile aroma substances, which improve the aroma quality of wine []. Additionally, non-Saccharomyces yeast strains significantly increased the total content of volatile aroma substances and the contents of acetate esters, ethyl esters, other esters, and terpenes in Vidal icewine, especially the co-culture of S. crataegensis YC30 and H. uvarum QTX22.

Table 3.
All the individual volatile compounds (μg/L) from Vidal icewine fermented with six fermentation strategies.
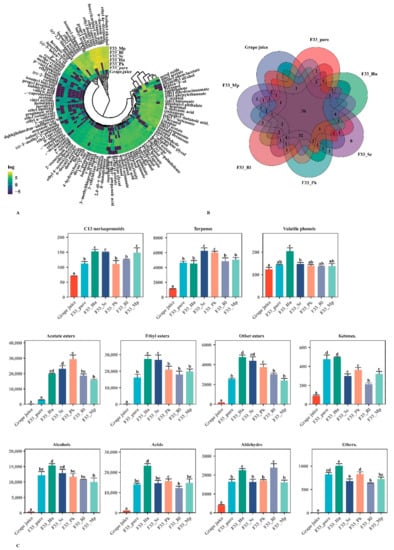
Figure 5.
Volatile compounds from Vidal grape juice and icewines fermented with six fermentation strategies. pure_F33: S. cerevisiae F33 monoculture. F33_Hu: co-inoculation of S. cerevisiae F33 and H. uvarum QTX22. F33_Sc: co-inoculation of S. cerevisiae F33 and S. crataegensis Y30. F33_Mp: co-inoculation of S. cerevisiae F33 and M. pulcherrima YC15. F33_Pk: co-inoculation of S. cerevisiae F33 and P. kluyveri HSP11. F33_Rl: co-inoculation of S. cerevisiae F33 and R. lusitaniae QTX26. (A) Pheatmap clustering graph of all the individual volatile substances. Yellow: relatively high production; Black: relatively low production. Count represents the standardized content aroma-active compounds. (B) Venn diagram showing the distribution of the numbers of the aroma compounds. (C) Column diagram showing the content distribution of the aroma categories. Different letters indicate statistically significant differences.
3.4.1. Varietal and Aroma Substances
A total of 17 varieties of aroma substances were detected, including 1 C13-norisoprenoid (β-damascenone), 14 terpenes, and 2 volatile phenols (Table 3, Figure 5A,C) through the qualitative and quantitative analysis of the aroma substances of Vidal icewine. Compared with grape juice, C13-norisoprenoid (β-damascenone) and terpenes were significantly increased during the fermentation, in agreement with a previous study []. Terpenes and C13-norisoprenoid (β-damascenone) aroma substances are a highly important category of aroma compounds in wine that provide essential contributions to wine’s floral and fruity aroma notes []. Compared with pure fermentation, mixed fermentation of S. cerevisiae F33 with S. crataegensis YC30 and P. kluyveri HSP14 significantly increased the content of terpenes substances, which may be related to a higher β-glucosidase activity produced by P. kluyveri [,]; mixed fermentation with H. uvarum QTX22, S. crataegensis YC30, and M. pulcherrima YC12 significantly increased C13-norisoprenoids substances (β-damascenone); mixed fermentation with H. uvarum QTX22 significantly improved the content of volatile phenolic compounds by about 29.8%. Hu et al. (2018b) [] reported that mixed fermentation of H. uvaum and S. cerevisiae increased the content of terpenes in cabernet sauvignon wine, but it also increased the volatile phenols by 53%. Although research reports are scarce on the effects of non-Saccharomyces yeast strains on volatile phenols, their improvement may lead to unpleasant odors such as a medicinal taste of the wine [], which should be a focus in future production. With simultaneous fermentation, S. crataegensis YC30 significantly increased the concentration of β-damascenone, cinnamene, linalool, citronellol, α-terpineol, and nerol oxide contents; H. uvarum QTX22 significantly increased β-damascenone, cinnamene, α-terpineol, and 2,4-di-t-butylphenol contents; and P. kluyveri HSP14 significantly increased the contents of linalool, p-xylene, and nerol oxide. β-damascenone, cinnamene, linalool, citronellol, and nerol oxide are mainly related to floral and fruit flavors [], which contribute to enhancing the sensory characteristics, the floral and fruity aroma notes, of wine. Beckner et al. [] confirmed that simultaneous fermentation of S. cerevisiae and non-Saccharomyces yeast strains can increase the contents of linalool, geraniol, nerol, and ocimene in sauvignon blanc wine, and the increase in these terpenes is mainly related to some active enzymes such as β-glycosidase.
3.4.2. Fermentative Aroma Compounds
Esters
Esters are one of the main fermentation products second only to alcohols in wine, and include acetate esters, ethyl esters, and other esters []. A total of 50 esters were detected, including 14 acetate esters, 20 ethyl esters, and 16 other esters (Table 3, Figure 5A,C). Among acetate ester aromatic compounds, only four acetate aromatic compounds were detected in grape juice, and most of them were produced during fermentation by the yeast strains []. In the wine samples, the main aroma substances were propyl acetate, benzylcarbinyl acetate, butyl acetate, isobutyl acetate, and ethyl acetate, and all five non-Saccharomyces yeast strains significantly increased the acetate ester aromatic compound contents, especially P. kluyveri HSP14, H. uvarum QTX22, and S. crataegensis YC30, all of which increased the contents of butyl acetate, propyl acetate, and hexyl acetate. Additionally, both S. crataegensis YC30 and P. kluyveri HSP14 increased the contents of methyl phenylacetate and furfuryl acetate, P. kluyveri HSP14 and H. uvarum QTX22 increased the content of 9-decenyl acetate, and S. crataegensis YC30 and H. uvarum QTX22 significantly increased the contents of benzylcarbinyl acetate, isobutyl acetate, and isoamyl ethanoate. In particular, among the five non-Saccharomyces yeast strains, only P. kluyveri HSP14 significantly increased the contents of amyl acetate and isooctyl acetate, and only H. uvarum QTX22 increased the contents of ethyl acetate, methyl acetate, and heptyl acetate. M. pulcherrima YC12 and R. lusitaniae QTX15 showed a weaker ability to enhance the acetate esters aroma substances content of wine. The ability of H. uvarum QTX22 to increase the content of acetate ester substances in wine has been reported [,]. Wei et al. [] also confirmed that P. kluyveri HSP14 can significantly increase the aroma substances of cider acetate. In this study, we found that in addition to H. uvarum QTX22 and P. kluyveri HSP14, S. crataegensis YC30 also has a good ability to produce acetate substances.
Among ethyl ester substances, there were six ethyl ester compounds; about 0.155 mg/L was detected in grape juice. We detected 18 ethyl ester compounds, about 12.57–17.58 mg/L, in wine samples, and the main aroma substances were ethyl caproate, ethyl dodecylate, ethyl 4e−decenoate, ethyl propanoate, an ethyl 7−octenoate. S. cerevisiae F33 mixed-fermented with H. uvarum QTX22, S. crataegensis YC30, and P. kluyveri HSP14 significantly increased ethyl ester aroma substances. Among them, S. crataegensis YC30 significantly increased the concentration of 17 aroma substances including ethyl dodecylate, ethyl heptoate, ethyl caproate, ethyl butanoate, ethyl myristate, ethyl nonanoate, ethyl palmitate, ethyl lactate, and ethyl dihydrocinnamate; H. uvarum QTX22 significantly increased 12 kinds of aroma substance concentration, including ethyl dodecylate, ethyl heptoate, and ethyl caproate, ethyl butanoate, ethyl myristate, ethyl nonanoate, etc.; and P. kluyveri HSP14 significantly increased the concentration of six kinds of aroma substances: ethyl propanoate, ethyl isobutyrate, ethyl dihydrocinnamate, ethyl myristate, ethyl dodecylate, and ethyl caproate. The reported results of ethyl ester aroma substances from mixed fermentation vary; Ye et al. [] reported that mixed fermentation of S. cerevisiae and Wickerhamomyces anomalus increased the content of ethyl ester aroma substances, whereas Renault et al. [] reported that mixed fermentation with Torulaspora delbrueckii had little effect on the content of ethyl ester aroma substances. Varela et al. [] suggested that mixed fermentation with M. pulcherrima resulted in a 16% reduction in ethyl ester aroma substances; Hu et al. [] found that mixed fermentation with H. uvarum can increase the content of ethyl esters, especially the medium-chain fatty acid ethyl ester content. In our study, only H. uvarum QTX22, S. crataegensis YC30, and P. kluyveri HSP14 strains mixed with S. cerevisiae F33 increased the content of ethyl ester aroma substances in Vidal icewine, whereas R. lusitaniae QTX15 and M. pulcherrima YC12 had a non-significant main effect on the increase in ethyl ester aroma substances.
For other esters, H. uvarum QTX22, S. crataegensis YC30, and P. kluyveri HSP14 produced a significant effect. S. crataegensis YC30 significantly enhanced 14 other ester aroma substances, of which 3 lactone aroma substances, including δ-decalactone, γ-decalactone, and γ-caprolactone, were not detected in other grape juice and wine samples nor were isobutyric acid, propyl propionate, and isoamyl butylate. In addition, all five non-Saccharomyces yeast strains significantly increased the concentration of γ-nonalactone and isoamyl decanoate; H. uvarum QTX22, S. crataegensis YC30, and P. kluyveri HSP14 significantly increased methyl caprate and isobutyl octanoate; and S. crataegensis YC30, H. uvarum QTX22, and R. lusitaniae QTX15 significantly increased the concentration of vinyl acetate. Some lactones, such as δ-decalactone, γ-nonalactone, γ-decalactone, and γ-caprolactone, are mainly related to coconut, creamy, milky, fruity, and sweet odor characteristics [], and are produced by the corresponding hydroxy acids [], so they further improve the complexity of the aroma quality of wine.
Alcohols
A total of 24 alcohols were detected in the grape juice and wine samples (Table 3, Figure 5A,C). Although the highest alcohol content was detected in grape juice, it significantly increased during fermentation, and the alcohol contents in the wine samples were approximately 30–46 times higher than in grape juice. Wei et al. [] reported that the alcohol content of cider was 16–30 times higher than that of apple juice. A large amount of phenylethyl alcohol was detected in cider, but not in apple juice, which is consistent with our findings. The main alcohol compounds found in our wine samples were isobutyl alcohol, (z)-2,3-butanediol, 1-heptanol, 1-hexanol, phenethyl alcohol, etc. Compared with pure fermentation, only mixed fermentation with H. uvarum QTX22 increased the total alcohol aroma compounds content, mainly including those of 13 aroma compounds: 1-pentanol, 1-dodecanol, 1-butanol, isobutyl alcohol, benzyl alcohol, hotrienol, hexanol, penten-3-ol, 2-heptanol, (z)-3-hexen-1-ol, etc. Additionally, S. crataegensis YC30 significantly increased the contents of 11 alcohol aroma compounds, such as 1-dodecanol, benzyl alcohol, hexanol, penten-3-ol, 2-heptanol, (z)-2,3-butanediol, (z)-3-hexen-1-o, etc.; P. kluyveri HSP14 and R. lusitaniae QTX15 significantly increased the contents of six and seven alcohol aroma compounds, respectively. Benzyl alcohol, hexanol, penten-3-ol, 2-heptanol, and (z)-3-hexen-1-ol are mainly responsible for fruity, floral, rose and other aroma characteristics of wine, which are desired in wine aroma []. The complexity and the fruity, floral, and rose aroma notes were further enhanced by the mixed fermentation of H. uvarum QTX22 and S. crataegensis YC30 with S. cerevisiae F33. The higher alcohol content is related to the enzymatic activity of alcohol acetyltransferase, which is esterified under the action of acetyltransferase []. The alcohol aroma compounds content produced by the fermentation of S. cerevisiae F33 mixed with H. uvarum QTX22 was the highest. Among them, isobutyl alcohol is mainly produced from the catabolism of valine [].
Acids
Acids are mainly produced during fermentation, and the concentrations of acids in the wine were 12–24 times higher than in grape juice (Table 3, Figure 5A,C). A total of 13 acids were detected, mainly including acetic acid, octanoic acid, n-decanoic acid, 9-decenoic acid, heptanoic acid, etc. Compared with pure fermentation, only mixed fermentation with H. uvarum QTX22 significantly increased the content of acids, mainly including octanoic acid, n-decanoic acid, butanoic acid, 9-decenoic acid, 2-methyl-butanoic acid, heptanoic acid, and 2-oxooctanoic acid. In addition, mixed fermentation with S. crataegensis YC30 significantly increased the concentrations of acetic acid, 2-methyl-butanoic acid, heptanoic acid, 2-methyl-propanoic acid, propanoic acid, and valeric acid. P. kluyveri HSP14 significantly increased the concentrations of 2-methyl-butanoic acid, heptanoic acid, 2-methyl-propanoic acid, hexanoic acid, and nonanoic acid. Both M. pulcherrima YC12 and R. lusitaniae QTX15 significantly increased the concentrations of heptanoic acid, hexanoic acid, and nonanoic acid; and R. lusitaniae QTX15 also significantly increased the concentration of acetic acid. A high concentration of volatile acetic acid can produce a cat urine taste in wine, which deleteriously affects the aroma quality []. Canonico et al. [] reported that the concentration of acetic acid in beer produced by mixed fermentation was significantly higher than that produced by pure culture with T. delbrueckii. However, Li et al. [] reported that C. zemplinina mixed-fermented with S. cerevisiae reduced the ethanol and acetic acid concentrations. In our study, only the acetic acid concentration of wines mixed-fermented with F33 and S. crataegensis YC30 and R. lusitaniae QTX15 increased, whereas it was reduced in other wine samples.
Aldehydes
Aldehydes are produced during wine fermentation through alcohol oxidation or acid decarboxylation []. A total of six aldehydes were detected in the grape juice and wine samples: acetaldehyde, nonylaldehyde, n-octanal, 1-hexanal, isovaleral, and isobutanal (Table 3, Figure 5A,C). Most aldehydes compounds were found in grape juice, but the contents of aldehyde compounds in wine were much higher than in grape juice, which was mainly attributed to acetaldehyde. The concentration of acetaldehyde in wines was 12–18 times higher than that in grape juice.
Compared with pure fermentation, mixed fermentation with H. uvarum QTX22 and R. lusitaniae QTX15 significantly increased the content of aldehydes; S. crataegensis YC30, M. pulcherrima YC12, and R. lusitaniae QTX15 significantly increased the contents of nonylaldehyde and isobutanal, which were not detected with pure fermentation. In addition, P. kluyveri HSP14 and R. lusitaniae QTX15 significantly increased isovaleral and n-octanal concentrations, and S. crataegensis YC30 and H. uvarum QTX22 significantly increased the concentrations of isovaleral and 1-hexanal.
3.5. Relative Odor Activity Values
These substances have the possibility of contributing to the wine’s aroma when the concentrations of aroma compounds in the wine are greater than its odor threshold []. The odor activity values (OAVs) of different volatile compounds are shown in Table 4, Figure 6A according to the odor threshold reported in the literature. We report 48 odor activity values, including 1 C13-norisoprenoid (β-damascenone), 8 terpenes, 6 acetate esters, 10 ethyl esters, 3 other esters, 7 alcohols, 5 acids, 6 aldehydes, 1 ketone, and 1 ether compounds. The total OAV of the volatile aroma compounds in wine samples was 2–4 times higher than that in grape juice. Compared with pure fermentation, mixed fermentation with non-Saccharomyces yeast strains increased the aroma activity value of acetate esters and ethyl ester substances. Among them, the wine fermented with S. cerevisiae F33 and S. crataegensis YC30 produced the highest value and the largest amount of active aroma substances, indicated by the increased the OAV values of 3 terpenes (cinnamene, linalool, citronellol), 1 C13-norisoprenoid (β-damascenone), 6 acetate esters (isoamyl ethanoate, benzylcarbinyl acetate, isobutyl acetate, butyl acetate, propyl acetate, and hexyl acetate), 10 ethyl esters (ethyl dodecylate, ethyl heptoate, ethyl caproate, ethyl isobutyrate, etc.), and 3 alcohols (3-octenol, penten-3-ol, 2-heptanol). Notably, the wine produced by mixed fermentation of S. cerevisiae F33 and S. crataegensis YC30 also showed three lactones (δ-decalactone, γ-nonalactone, and γ-decalactone), which are related to coconut and creamy odors [], which were not found in other non-Saccharomyces strains (Figure 6B). In addition, the mixed fermentation of S. cerevisiae F33 and H. uvarum QTX22 wine also resulted in a higher total OAV and better aroma characteristics. We found that the aroma activity values of 4 terpenes (naphthalene, cinnamene, citronellol, d-limonene, and α-terpineol), 1 C13-norisoprenoid (β-damascenone), 3 acetate esters (isoamyl ethanoate, benzylcarbinyl acetate, and isobutyl acetate), 6 ethyl ester aroma substances (ethyl dodecylate, ethyl heptoate, ethyl caproate, ethyl butanoate, ethyl phenylethanoate, and ethyl dihydrocinnamate), and 6 alcohols (1-pentanol, 1-heptanol, 1-hexanol, etc.) significantly increased. Simultaneous fermentation with S. cerevisiae F33 and P. kluyveri HSP14 significantly increased the OAVs of 2-methyl-propanoic acid, butyl acetate, propyl acetate, etc.; and R. lusitaniae QTX15 significantly increase the OAVs of n-octanal, nonylaldehyde, p-xylene, benzylcarbinyl acetate, isobutyl acetate, etc. β-damascenone, cinnamene, linalool, citronellol, nerol oxide, benzylcarbinyl acetate, ethyl dodecylate, ethyl dihydrocinnamate, and 1-octanol are mainly associated with floral-like aromas, whereas isoamyl ethanoate, benzylcarbinyl acetate, isobutyl acetate, 1-hexanol, penten-3-ol, 2-heptanol ,and most ethyl esters are related to fruity odors, which contribute to the complexity and typical qualities of wine.

Table 4.
Mean values of OAV for volatile compounds which presented OAV > 1 in Vidal icewine fermented with six fermentation strategies.
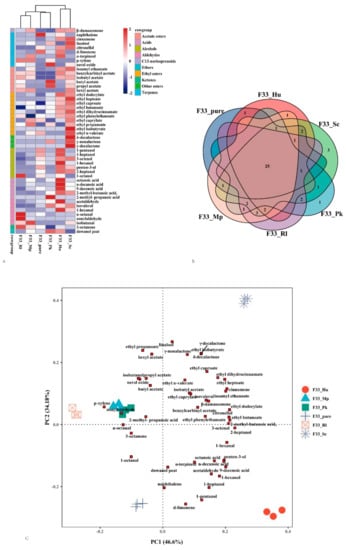
Figure 6.
Aroma-active compounds from Vidal icewine fermented with six fermentation strategies. pure_F33: S. cerevisiae F33 monoculture. F33_Hu: co-inoculation of S. cerevisiae F33 and H. uvarum QTX22. F33_Sc: co-inoculation of S. cerevisiae F33 and S. crataegensis Y30. F33_Mp: co-inoculation of S. cerevisiae F33 and M. pulcherrima YC15. F33_Pk: co-inoculation of S. cerevisiae F33 and P. kluyveri HSP11. F33_Rl: co-inoculation of S. cerevisiae F33 and R. lusitaniae QTX26. (A) Pheatmap clustering graph of aroma-active compounds. Red: relatively high production; blue: relatively low production. Count represents the standardized content aroma-active compounds. (B) Venn diagrams of the aroma-active compounds. (C) Principal component analysis of aroma-active compounds.
3.6. PCA Analysis
To further understand the influence of different fermentation strategies on the aroma compounds of Vidal icewine, principal component analysis was performed on aroma-active substances with an OAV > 1 [] (Figure 6C). In a 48 × 6 data matrix, the generated data explained 80.78% of the total variance. The Vidal icewine purely fermented with S. cerevisiae F33 was located in the third quadrant, which was well-distinguished from the mixed fermented wine, indicating that mixed fermentation with non-Saccharomyces yeast strains had a significant effect on improving the aroma substances of Vidal icewine. Wines produced by the mixed fermentation of S. cerevisiae F33 with S. crataegensis YC30 and H. uvarum QTX22 were located in the first and fourth quadrants, respectively, and could also be well-distinguished due to their unique aroma characteristics. Finally, the wine purely fermented with S. cerevisiae F33 was located on the farthest negative end of the Y-axis, and was positively correlated with d-limonene, naphthalene, 1-pentanol, and 1-heptanol; the wine produced by mixed fermentation of S. cerevisiae F33 with S. crataegensis YC30 was located at the farthest positive end of the Y-axis, and was positively correlated with linalool, γ-decalactone, γ-nonalactone, ethyl isobutyrate, δ-decalactone, ethyl propanoate, hexyl acetate, ethyl heptoate, isoamyl ethanoate, ethyl butanoate, and 2-heptanol, being significantly different from the wine produced by pure fermentation in terms of the aroma characteristics. The mixed fermentation with H. uvarum QTX22 was located at the farthest point on the positive end of the X-axis and the negative end of the Y-axis, related to d-limonene, 1-pentanol, ethyl heptoate, isoamyl ethanoate, ethyl butanoate, 2-heptanol, etc. Mixed fermentation with R. lusitaniae QTX15 was located farthest from the negative end of the X-axis, and was positively correlated with p-xylene, nonylaldehyde, n-octanal, etc.; mixed fermentation with M. pulcherrima YC12 and P. kluyveri HSP14 showed similar aroma characteristics, both located in the second quadrant on the negative end of the Y-axis. The main characteristic aromas of F33 purely fermented Vidal icewine were alcohols and terpenes, whereas those of mixed fermentation with S. crataegensis YC30 and H. uvarum QTX22 were esters, further indicating that non-Saccharomyces yeast strains can increase the content of ester aroma substances in Vidal icewine. Ester aroma substances are generally related to fruity and floral aroma notes; γ-decalactone and γ-nonalactone have fruity and ice cream odors []; hexyl acetate has banana and apple odors []; ethyl heptoate has pineapple, banana, and strawberry flavors []; and isoamyl ethanoate, ethyl propanoate, and ethyl butanoate [] all have sweet and fruity odors, which greatly enriches the complexity and quality of the Vidal icewine aroma.
3.7. Sensory Analysis
Figure 7A shows the MF value contributed by pure fermentation and mixed fermentation to the aroma of Vidal icewine, including fruity, floral, alcohol, nail polish, caramel, herbal, and overall aromas. In the six wine samples, except for the alcohol note, all others were significant or extremely significant (p < 0.05 or p < 0.001). Compared with pure fermentation, the overall, fruity, and floral aroma MF values of the wine produced by mixed fermentation with S. crataegensis YC30 were the highest, followed by H. uvarum QTX22. In addition, H. uvarum QTX22, M. pulcherrima YC12, and P. kluyveri HSP14 also showed strong herbal, caramel and nail polish odors, respectively. To determine whether there was a perceptible difference between the icewine fermented with S. crataegensis YC30, H. uvarum QTX22, and S. cerevisiae F33 (as a control) in the fruity and floral note aspects, triangular tests were also performed (Figure 7B). The wine simultaneously co-fermented with S. crataegensis YC30 and S. cerevisiae F33 was perceived as having a significantly intense fruity and floral odor compared with that produced by pure fermentation with S. cerevisiae F33. No significant differences were found in these odor characteristics with the simultaneous fermentation treatments (S. cerevisiae F33 and H. uvarum QTX22). Since the aromas of wine are perceived through the perceptual response to odorants, PLSR analysis was used to predict and reveal the correlation between the odor characteristics of wine aroma (fruit, floral, alcohol, nail polish, caramel, and herbal) and the amount of aroma compounds [] (Figure 7C,D). Only the floral (R2 cal/val = 0.993/0.837) and fruity (R2 cal/val = 0.995/0.813) aroma notes could be accurately predicted. Among them, the perception of fruity was influenced by the combination of the positive and negative contributions of volatiles. The presence of C13-norisoprenoid (β-damascenone +0.3279), terpenes (+0.2401), ethyl esters (+0.2995), acetate esters (+0.1748), and other esters (+0.1790) positively correlated with this aroma; whereas aldehydes (−0.1384), ketones (−0.1067), and ethers (−0.0941) showed negative correlations. C13-norisoprenoid (β-damascenone, +0.2941), terpenes (+0.1996), ethyl esters (+0.2891), acetate esters (+0.1650), and other esters (+0.1921) positively correlated with floral aroma notes, and aldehydes (−0.0976), ketones (−0.0660), and ethers (−0.0442) showed negative correlation, which is consistent with a previous study in which aroma characteristics were affected by the complex contributions of various aroma-active substances []. These results further illustrate that the aroma characteristics and quality of wine are affected by the type and diversity of volatile aroma substances, especially esters and terpenes [].
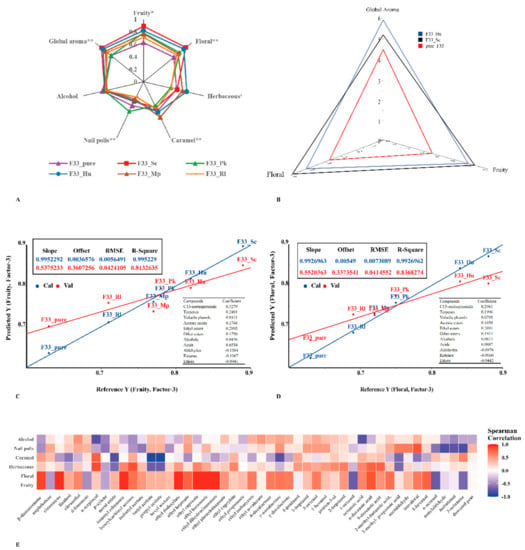
Figure 7.
The sensory evaluation of Vidal icewine fermented with six fermentation strategies. pure_F33: S. cerevisiae F33 monoculture. F33_Hu: co-inoculation of S. cerevisiae F33 and H. uvarum QTX22. F33_Sc: co-inoculation of S. cerevisiae F33 and S. crataegensis Y30. F33_Mp: co-inoculation of S. cerevisiae F33 and M. pulcherrima YC15. F33_Pk: co-inoculation of S. cerevisiae F33 and P. kluyveri HSP11. F33_Rl: co-inoculation of S. cerevisiae F33 and R. lusitaniae QTX26. (A) Modified frequency (MF) values of aroma characteristics in six fermentation strategies. * and **, significant at p < 0.05 and 0.01, respectively. (B) The analysis results of triangular tests. Grades ranked from 0 (poorly intense) to 7 (very intense). (C,D) PLS regression of fruity (C) and floral (D) aroma volatiles. Val, validation; Cal, calibration. (E) Correlation analysis between major aroma substances (OAV > 1) and sensory analysis.
The relationships between the 48 major aroma substances (OAV > 1) and sensory analysis are depicted in Figure 4E. Wine samples’ fruity and floral odors were positively correlated with β-damascenone, cinnamene, isoamyl ethanoate, ethyl dodecylate, ethyl caproate, ethyl butanoate, ethyl dihydrocinnamate, and n-decanoic acid (correlation coefficient > 0.8), which further verified that terpenes and esters were related to the production of floral and fruity odors in Vidal icewine.
4. Conclusions
We analyzed the effects of commercial S. cerevisiae F33 in pure culture as well as mixed culture with five indigenous non-Saccharomyces yeast strains to evaluate the effects on the aroma characteristics of Vidal icewine. In the process of pure fermentation with F33, the maximum biomass was reached on day 3, whereas co-inoculation with non-Saccharomyces yeast strains reached maximum biomass on day 4; the non-Saccharomyces yeast strains were undetectable on days 5 and 7. Simultaneous fermentation increased the concentrations of total polyphenol, especially the S. crataegensis YC30 strain, which significantly increased the concentrations of myricetin, chlorogenic acid, quercetin, vanillic acid, p-hydroxybenzonic acid, etc. A total of 118 aroma compounds were detected, mainly including acetate esters, ethyl esters, alcohols, and acids. Simultaneous fermentation markedly increased the total content of volatile aroma compounds and the contents of acetate, ethyl ester, other esters, and terpenes in Vidal icewine, especially with S. crataegensis YC30 and H. uvarum QTX22. S. crataegensis YC30 significantly increased the aroma activity values of 1 C13-norisoprenoid (β-damascenone), 6 acetate esters, 10 ethyl esters, 3 terpenes, and 3 alcohols, and produced obvious floral and fruity aroma notes. PLSR analysis showed that this was mainly related to C13-norisoprenoids (β-damascenone, r = 0.3279), terpenes (r = 0.2401), ethyl esters (r = 0.2995), acetate esters (r = 0.1748), and other esters (r = 0.1790). This study provides a new method for the development and production of icewine.
Author Contributions
Conceptualization, Q.G. and T.Y.; formal analysis, Q.G.; funding acquisition, Y.Y. (Yahong Yuan) and J.Z.; investigation, X.S. and C.L.; methodology, Q.G. and T.M.; project administration, Y.Y. (Yue Yan); software, C.G. and D.Z.; validation, Q.G.; writing—original draft, Q.G.; writing—review and editing, Y.Y. (Yue Yan) and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Key Research and Development Projects of the 13th Five Year Plan of China (No. 2017YFD0400105), the Science and Technology Innovation Project of Ningxia Agriculture and Forestry Academy (No. NKYJ-20-01 and NGSB-2021-5-04), Ningxia Youth Science and Technology Talent Project (No. NXKJTJGC2020140), Natural Science Foundation of Ningxia (No. 2021AAC03282).
Conflicts of Interest
The authors declare no conflict of interest in this work.
References
- Li, J.; Hu, W.Z.; Xu, Y.P. Diversity and dynamics of yeasts during vidal blanc icewine fermentation: A strategy of the combination of culture-dependent and high-throughput sequencing approaches. Front. Microbiol. 2019, 10, 1588. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, J.; Ye, D.; Song, Y.; Qin, Y.; Liu, Y. Yeast population dynamics during spontaneous fermentation of icewine and selection of indigenous Saccharomyces cerevisiae strains for the winemaking in Qilian, China. J. Food Sci. Technol. 2020, 100, 5385–5394. [Google Scholar]
- Huang, L.; Ma, Y.; Tian, X.; Li, J.M.; Li, L.X.; Tang, K.; Xu, Y. Chemosensory characteristics of regional Vidal icewines from China and Canada. Food Chem. 2018, 261, 66–74. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Giacosa, S.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profiles and chromatic characteristics of red wines produced with Starmerella bacillaris and Saccharomyces cerevisiae. Food Res. Int. 2018, 109, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Li, A.H.; Dizy, M.; Ullah, N.; Sun, W.X.; Tao, Y.S. Evaluation of aroma enhancement for “Ecolly” dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017, 228, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aroma evolution throughout alcoholic fermentation sequentially inoculated with non- Saccharomyces/Saccharomyces yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef]
- Morales, M.L.; Fierro-Risco, J.; Ríos-Reina, R.; Ubeda, C.; Paneque, P. Influence of Saccharomyces cerevisiae and Lachancea thermotolerans co-inoculation on volatile profile in fermentations of a must with a high sugar content. Food Chem. 2019, 276, 427–435. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.Q.; Xu, Y.H.; Li, A.H.; Tao, Y.S. Increased glycosidase activities improved the production of wine varietal odorants in mixed fermentation of P. fermentans and high antagonistic S. cerevisiae. Food Chem. 2020, 332, 127426. [Google Scholar] [CrossRef]
- Kong, C.; Li, A.; Jin, G.; Zhu, X.; Tao, Y. Evolution of volatile compounds treated with selected non-Saccharomyces extracellular extract during Pinot noir winemaking in monsoon climate. Food Res. Int. 2019, 119, 177–186. [Google Scholar] [CrossRef]
- Ruiz, J.; Ortega, N.; Martín-Santamaría, M.; Acedo, A.; Marquina, D.; Pascual, O.; Rozès, N.; Zamora, F.; Santos, A.; Belda, I. Occurrence and enological properties of two new non-conventional yeasts (Nakazawaea ishiwadae and Lodderomyces elongisporus) in wine fermentations. Int. J. Food Microbiol. 2019, 305, 108255. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yan, X.; Wang, Q.; Zhang, Y.; Tao, Y. Performance of selected P. fermentans and its excellular enzyme in co-inoculation with S. cerevisiae for wine aroma enhancement. LWT Food Sci. Technol. 2017, 86, 361–370. [Google Scholar] [CrossRef]
- Englezos, V.; Pollon, M.; Rantsiou, K.; Ortiz-Julien, A.; Botto, R.; Segade, R.S.; Giacosa, S.; Rolle, L.; Cocolin, L. Saccharomyces cerevisiae-Starmerella bacillaris strains interaction modulates chemical and volatile profile in red wine mixed fermentations. Food Res. Int. 2019, 122, 392–401. [Google Scholar] [CrossRef]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High Potential of Pichia kluyveri and Other Pichia Species in Wine Technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Hu, K.; Xu, Y.-H.; Mei, W.-C.; Tao, Y.-S. Biomass suppression of Hanseniaspora uvarum by killer Saccharomyces cerevisiae highly increased fruity esters in mixed culture fermentation. LWT 2020, 132, 109839. [Google Scholar] [CrossRef]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Parapouli, M.; Hatziloukas, E.; Drainas, C.; Perisynakis, A. The effect of Debina grapevine indigenous yeast strains of Metschnikowia and Saccharomyces on wine flavour. J. Microbiol. Biotechnol. 2010, 37, 85–93. [Google Scholar] [CrossRef]
- Mencher, A.; Morales, P.; Curiel, J.A.; Gonzalez, R.; Tronchoni, J. Metschnikowia pulcherrima represses aerobic respiration in Saccharomyces cerevisiae suggesting a direct response to co-cultivation. Food Microbiol. 2021, 94, 103670. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2014, 240, 999–1012. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Qiu, Y.; Guo, H.; Ju, H.; Wang, Y.; Yuan, Y.; Yue, T. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co- and sequential fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef] [PubMed]
- Analytical Methods of Wine and Fruit Wine. Available online: http://down.foodmate.net/standard/sort/3/11619.html (accessed on 20 March 2019).
- Chen, K.; Escott, C.; Loira, I.; Del Fresno, J.M.; Morata, A.; Tesfaye, W.; Calderon, F.; Suarez-Lepe, J.A.; Han, S.; Benito, S. Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: Influence on colour, aroma and sensorial properties of young wines. Food Microbiol. 2018, 69, 51–63. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Effects of sequential mixed cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on apple cider fermentation. FEMS Yeast Res. 2014, 14, 873–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhao, Q.; Lan, T.; Geng, T.; Gao, C.; Yuan, Q.; Zhang, Q.; Xu, P.; Sun, X.; Liu, X.; et al. Comparative analysis of physicochemical characteristics, nutritional and functional components and antioxidant capacity of fifteen Kiwifruit (Actinidia) cultivars-Comparative analysis of fifteen Kiwifruit (Actinidia) cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Zhang, Y.; Zhang, H.; Sun, B.; Ren, F.; Chen, H.; Tang, Y. Characterization of the Key Aroma Compounds in Traditional Hunan Smoke-Cured Pork Leg (Larou, THSL) by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV), and Sensory Evaluation Experiments. Foods 2020, 9, 413. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Wang, J.; Wang, H.; Lan, T.; Liu, R.; Gao, T.; Yang, W.; Zhou, Y.; Ge, Q.; Fang, Y.; et al. Is overnight fresh juice drinkable? The shelf life prediction of non-industrial fresh watermelon juice based on the nutritional quality, microbial safety quality, and sensory quality. Food Nutr. Res. 2020, 64, 4237. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Luan, Y.; Duan, C.Q.; Yan, G.L. Use of Torulaspora delbrueckii co-fermentation with two Saccharomyces cerevisiae strains with different aromatic characteristic to improve the diversity of red wine aroma profile. Front. Microbiol. 2018, 9, 606. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Giacosa, S.; Río Segade, S.; Rolle, L.; Cocolin, L. Cell-to-cell contact mechanism modulates Starmerella bacillaris death in mixed culture fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2019, 289, 106–114. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Xu, Y.H.; Tao, Y.S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2017, 24, 166–180. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Farina, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lan, Y.; Zhu, B.; Xiang, X.; Duan, C.; Shi, Y. Changes in monosaccharides, organic acids and amino acids during Cabernet Sauvignon wine ageing based on a simultaneous analysis using gas chromatography-mass spectrometry. J. Sci. Food Agric. 2018, 98, 104–112. [Google Scholar] [CrossRef]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and identification of organic acids in wine samples. Problems and challenges. TrAC 2019, 120, 115630. [Google Scholar] [CrossRef]
- Coelho, E.M.; da Silva Padilha, C.V.; Miskinis, G.A.; de Sá, A.G.B.; Pereira, G.E.; de Azevêdo, L.C.; dos Santos Lima, M. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [Green Version]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res. Int. 2019, 119, 378–389. [Google Scholar] [CrossRef]
- Kanter, J.-P.; Benito, S.; Brezina, S.; Beisert, B.; Fritsch, S.; Patz, C.-D.; Rauhut, D. The impact of hybrid yeasts on the aroma profile of cool climate Riesling wines. Food Chem. X 2020, 5, 100072. [Google Scholar] [CrossRef]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolin, L. Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Tofalo, R.; Schirone, M.; Torriani, S.; Rantsiou, K.; Cocolin, L.; Perpetuini, G.; Suzzi, G. Diversity of Candida zemplinina strains from grapes and Italian wines. Food Microbiol. 2012, 29, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Yuan, Y.; Dai, L.; Yue, T. Characteristic fruit wine production via reciprocal selection of juice and non-Saccharomyces species. Food Microbiol. 2019, 79, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Voon, M.K.W.; Chua, J.Y.; Huang, D.; Lee, P.R.; Liu, S.Q. The effects of co- and sequential inoculation of Torulaspora delbrueckii and Pichia kluyveri on chemical compositions of durian wine. Appl. Microbiol. Biotechnol. 2017, 101, 7853–7863. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Cui, Y.; Zhang, S.; Li, L.; Suo, H.; Sun, B. Detailed phenolic composition of Vidal grape pomace by ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2017, 1068–1069, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.J.; White, K.P.; Edwards, C.G. Growth and metabolism of non-Saccharomyces yeasts isolated from Washington state vineyards in media and high sugar grape musts. Food Microbiol. 2019, 77, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.K.; Wang, J.; Chen, F.S.; Zhang, X.Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT Food Sci. Technol. 2019, 116, 108477. [Google Scholar] [CrossRef]
- Whitener, M.E.B.; Stanstrup, J.; Carlin, S.; Divol, B.; Dutoit, M.; Vrhovsek, U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Garijo, P.; Berlanas, C.; López-Alfaro, I.; López, R.; Gutiérrez, A.R.; Santamaría, P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017, 54, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J. Agric. Food Chem. 2007, 55, 6674–6684. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Zini, C.A. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 53. [Google Scholar] [CrossRef]
- Liu, J.; Arneborg, N.; Toldam-Andersen, T.B.; Petersen, M.A.; Bredie, W.L.P. Effect of sequential fermentations and grape cultivars on volatile compounds and sensory profiles of Danish wines. J. Sci. Food Agric. 2017, 97, 3594–3602. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Wang, X.; Xie, K.; Zhuang, H.; Ye, R.; Fang, Z.; Feng, T. Volatile flavor compounds, total polyphenolic contents and antioxidant activities of a China gingko wine. Food Chem. 2015, 182, 41–46. [Google Scholar] [CrossRef]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Procopio, S.; Krause, D.; Hofmann, T.; Becker, T. Significant amino acids in aroma compound profiling during yeast fermentation analyzed by PLS regression. LWT Food Sci. Technol. 2013, 51, 423–432. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, X.; Niu, Y.; Yu, D.; Zhu, J.; Zhu, G. Optimization and application of headspace-solid-phase micro-extraction coupled with gas chromatography-mass spectrometry for the determination of volatile compounds in Cherry wines. J. Chromatogr. B 2015, 978–979, 122–130. [Google Scholar] [CrossRef]
- Lukić, I.; Radeka, S.; Grozaj, N.; Staver, M.; Peršurić, Đ. Changes in physico-chemical and volatile aroma compound composition of Gewürztraminer wine as a result of late and ice harvest. Food Chem. 2016, 196, 104–1057. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-Q.; Wang, X.-C.; Tao, N.-P.; Wu, N. Characterization of volatile compounds in different edible parts of steamed Chinese mitten crab (Eriocheir sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar] [CrossRef]
- Dorado, A.D.; Husni, S.; Pascual, G.; Puigdellivol, C.; Gabriel, D. Inventory and treatment of compost maturation emissions in a municipal solid waste treatment facility. J. Waste Manag. 2014, 34, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile composition of Merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Aroma compounds in Ontario Vidal and Riesling icewines. II. Effects of crop level. Food Res. Int. 2015, 76, 55–560. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache Rose´ Wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Tronchoni, J.; Curiel, J.A.; Sáenz-Navajas, M.P.; Morales, P.; de-la-Fuente-Blanco, A.; Fernández-Zurbano, P.; Gonzalez, R. Aroma profiling of an aerated fermentation of natural grape must with selected yeast strains at pilot scale. Food Microbiol. 2018, 70, 214–223. [Google Scholar] [CrossRef]
- Bueno, M.; Zapata, J.; Ferreira, V. Simultaneous determination of free and bonded forms of odor-active carbonyls in wine using a headspace solid phase microextraction strategy. J. Chromatogr. A 2014, 1369, 33–42. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Jiang, D.; Zhang, Y.; Zhang, S.; Sun, S. Effect of Saccharomyces cerevisiae, Torulaspora delbrueckii and malolactic fermentation on fermentation kinetics and sensory property of black raspberry wines. Food Microbiol. 2020, 91, 103551. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).