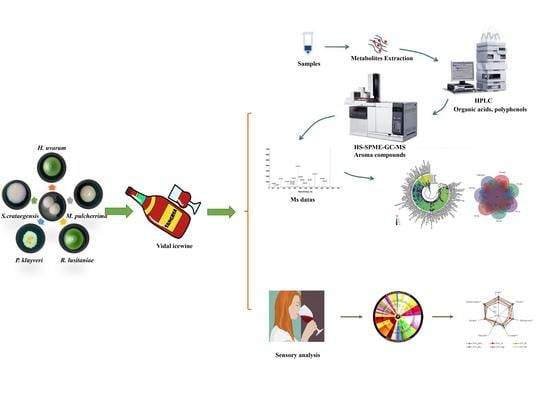
Effects of Simultaneous Co-Fermentation of Five Indigenous Non-Saccharomyces Strains with S. cerevisiae on Vidal Icewine Aroma Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Reagents and Standards
2.3. Grape Juice
2.4. Fermentation Strategies
2.5. Assay for Organic Acids and Polyphenol Compounds
2.6. Quantification of Volatile Compounds
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
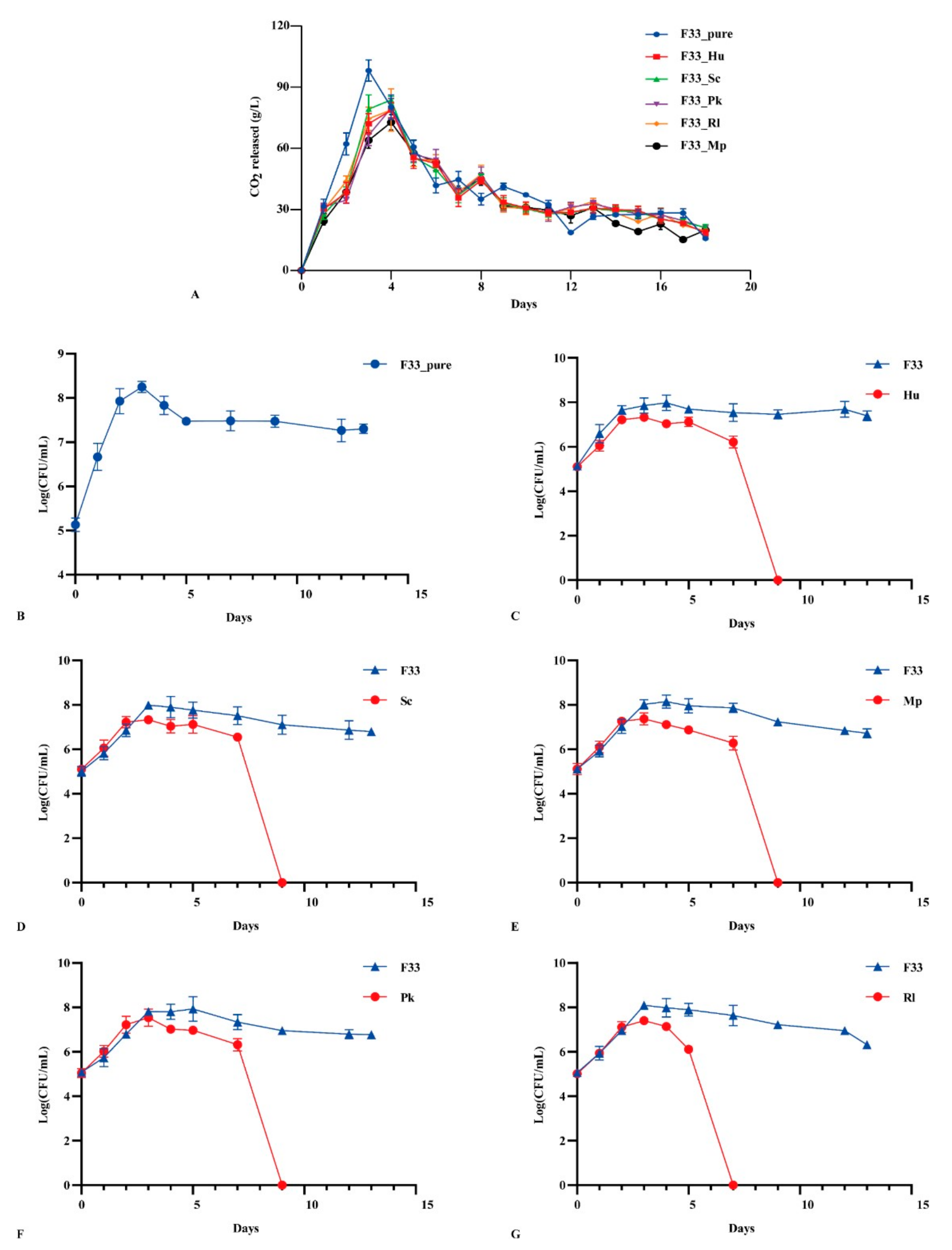
3.1. Fermentation Performance of Yeasts
3.2. Effect of Fermentation Strategies on Physicochemical Characteristics in Vidal Icewine
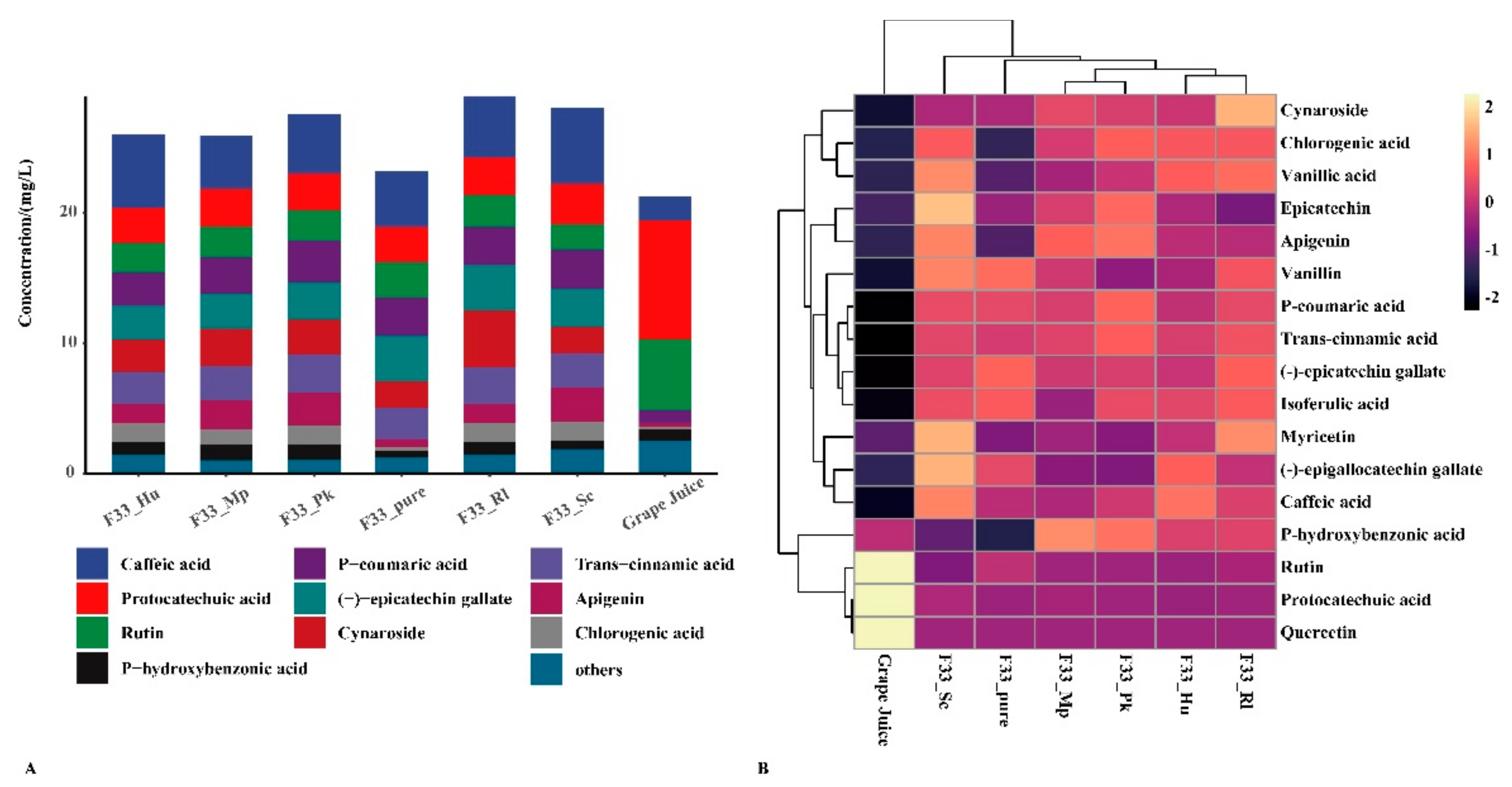
3.3. Effect of Fermentation Strategies on Polyphenols in Vidal Icewine
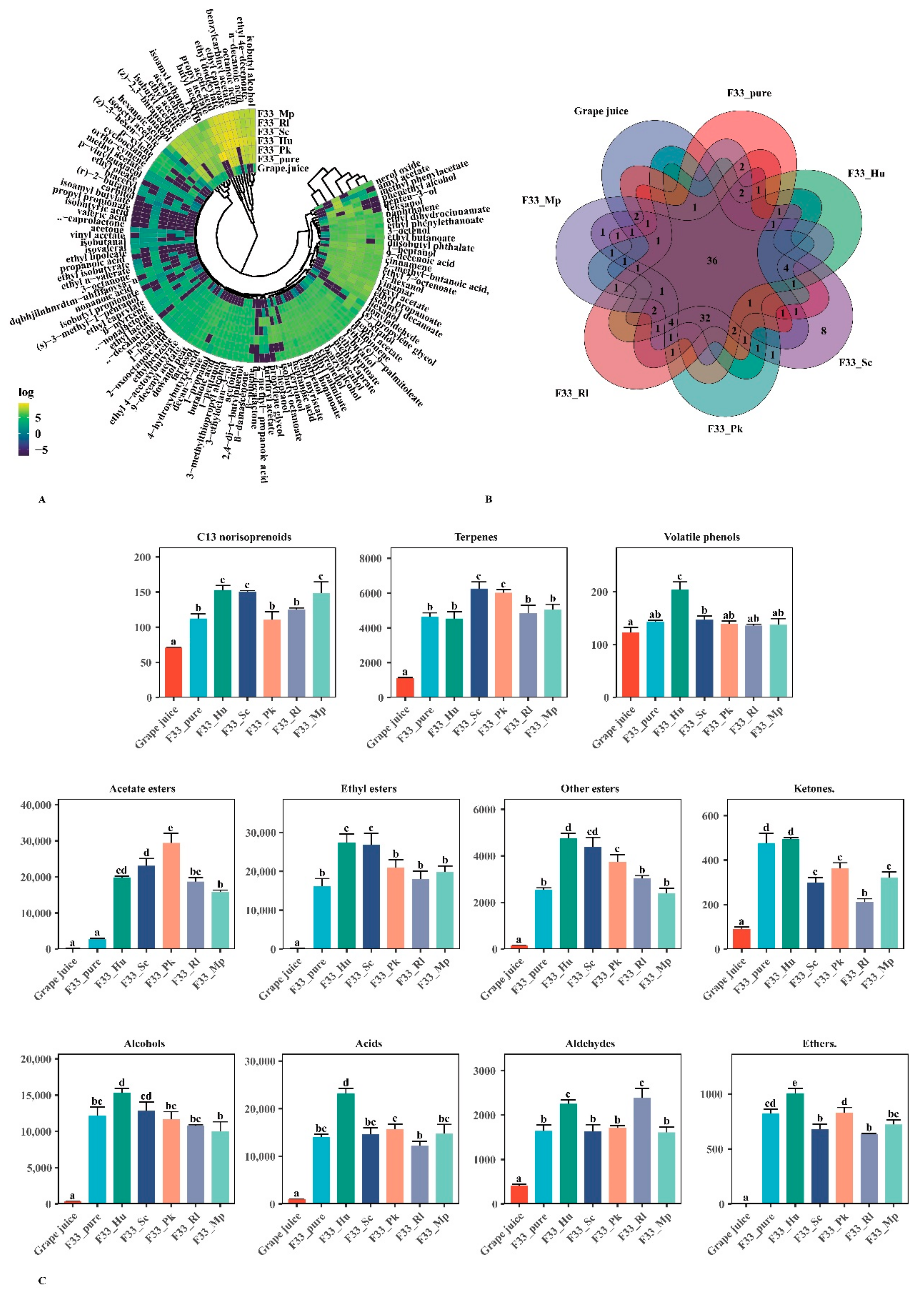
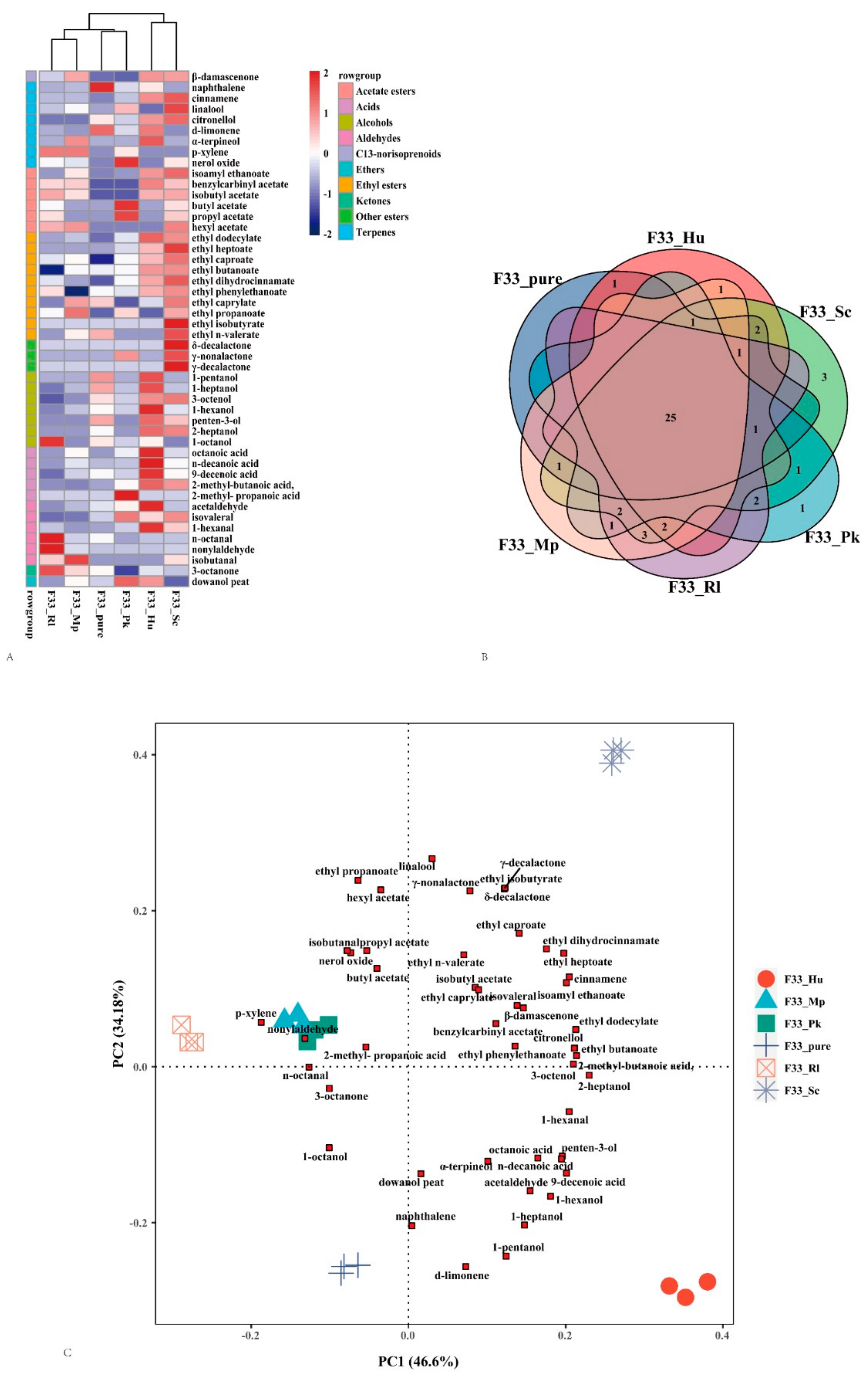
3.4. Effect of Fermentation Strategies on Volatile Aroma Substances in Vidal Icewine
3.4.1. Varietal and Aroma Substances
3.4.2. Fermentative Aroma Compounds
Esters
Alcohols
Acids
Aldehydes
3.5. Relative Odor Activity Values
3.6. PCA Analysis
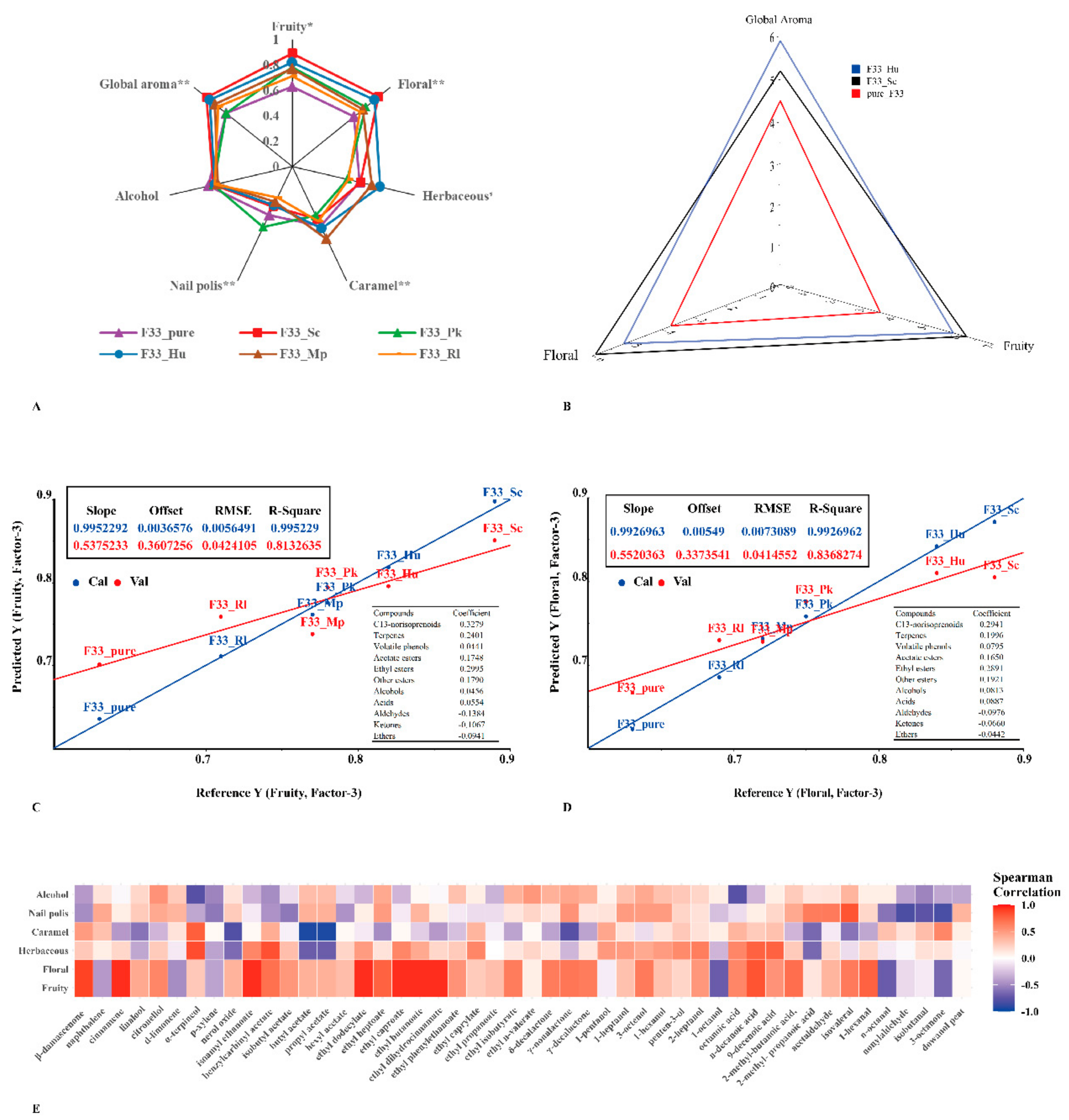
3.7. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Hu, W.Z.; Xu, Y.P. Diversity and dynamics of yeasts during vidal blanc icewine fermentation: A strategy of the combination of culture-dependent and high-throughput sequencing approaches. Front. Microbiol. 2019, 10, 1588. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, J.; Ye, D.; Song, Y.; Qin, Y.; Liu, Y. Yeast population dynamics during spontaneous fermentation of icewine and selection of indigenous Saccharomyces cerevisiae strains for the winemaking in Qilian, China. J. Food Sci. Technol. 2020, 100, 5385–5394. [Google Scholar]
- Huang, L.; Ma, Y.; Tian, X.; Li, J.M.; Li, L.X.; Tang, K.; Xu, Y. Chemosensory characteristics of regional Vidal icewines from China and Canada. Food Chem. 2018, 261, 66–74. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Giacosa, S.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profiles and chromatic characteristics of red wines produced with Starmerella bacillaris and Saccharomyces cerevisiae. Food Res. Int. 2018, 109, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Li, A.H.; Dizy, M.; Ullah, N.; Sun, W.X.; Tao, Y.S. Evaluation of aroma enhancement for “Ecolly” dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017, 228, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aroma evolution throughout alcoholic fermentation sequentially inoculated with non- Saccharomyces/Saccharomyces yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef]
- Morales, M.L.; Fierro-Risco, J.; Ríos-Reina, R.; Ubeda, C.; Paneque, P. Influence of Saccharomyces cerevisiae and Lachancea thermotolerans co-inoculation on volatile profile in fermentations of a must with a high sugar content. Food Chem. 2019, 276, 427–435. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.Q.; Xu, Y.H.; Li, A.H.; Tao, Y.S. Increased glycosidase activities improved the production of wine varietal odorants in mixed fermentation of P. fermentans and high antagonistic S. cerevisiae. Food Chem. 2020, 332, 127426. [Google Scholar] [CrossRef]
- Kong, C.; Li, A.; Jin, G.; Zhu, X.; Tao, Y. Evolution of volatile compounds treated with selected non-Saccharomyces extracellular extract during Pinot noir winemaking in monsoon climate. Food Res. Int. 2019, 119, 177–186. [Google Scholar] [CrossRef]
- Ruiz, J.; Ortega, N.; Martín-Santamaría, M.; Acedo, A.; Marquina, D.; Pascual, O.; Rozès, N.; Zamora, F.; Santos, A.; Belda, I. Occurrence and enological properties of two new non-conventional yeasts (Nakazawaea ishiwadae and Lodderomyces elongisporus) in wine fermentations. Int. J. Food Microbiol. 2019, 305, 108255. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yan, X.; Wang, Q.; Zhang, Y.; Tao, Y. Performance of selected P. fermentans and its excellular enzyme in co-inoculation with S. cerevisiae for wine aroma enhancement. LWT Food Sci. Technol. 2017, 86, 361–370. [Google Scholar] [CrossRef]
- Englezos, V.; Pollon, M.; Rantsiou, K.; Ortiz-Julien, A.; Botto, R.; Segade, R.S.; Giacosa, S.; Rolle, L.; Cocolin, L. Saccharomyces cerevisiae-Starmerella bacillaris strains interaction modulates chemical and volatile profile in red wine mixed fermentations. Food Res. Int. 2019, 122, 392–401. [Google Scholar] [CrossRef]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High Potential of Pichia kluyveri and Other Pichia Species in Wine Technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Hu, K.; Xu, Y.-H.; Mei, W.-C.; Tao, Y.-S. Biomass suppression of Hanseniaspora uvarum by killer Saccharomyces cerevisiae highly increased fruity esters in mixed culture fermentation. LWT 2020, 132, 109839. [Google Scholar] [CrossRef]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Parapouli, M.; Hatziloukas, E.; Drainas, C.; Perisynakis, A. The effect of Debina grapevine indigenous yeast strains of Metschnikowia and Saccharomyces on wine flavour. J. Microbiol. Biotechnol. 2010, 37, 85–93. [Google Scholar] [CrossRef]
- Mencher, A.; Morales, P.; Curiel, J.A.; Gonzalez, R.; Tronchoni, J. Metschnikowia pulcherrima represses aerobic respiration in Saccharomyces cerevisiae suggesting a direct response to co-cultivation. Food Microbiol. 2021, 94, 103670. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2014, 240, 999–1012. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Qiu, Y.; Guo, H.; Ju, H.; Wang, Y.; Yuan, Y.; Yue, T. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co- and sequential fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef] [PubMed]
- Analytical Methods of Wine and Fruit Wine. Available online: http://down.foodmate.net/standard/sort/3/11619.html (accessed on 20 March 2019).
- Chen, K.; Escott, C.; Loira, I.; Del Fresno, J.M.; Morata, A.; Tesfaye, W.; Calderon, F.; Suarez-Lepe, J.A.; Han, S.; Benito, S. Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: Influence on colour, aroma and sensorial properties of young wines. Food Microbiol. 2018, 69, 51–63. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Effects of sequential mixed cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on apple cider fermentation. FEMS Yeast Res. 2014, 14, 873–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhao, Q.; Lan, T.; Geng, T.; Gao, C.; Yuan, Q.; Zhang, Q.; Xu, P.; Sun, X.; Liu, X.; et al. Comparative analysis of physicochemical characteristics, nutritional and functional components and antioxidant capacity of fifteen Kiwifruit (Actinidia) cultivars-Comparative analysis of fifteen Kiwifruit (Actinidia) cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Zhang, Y.; Zhang, H.; Sun, B.; Ren, F.; Chen, H.; Tang, Y. Characterization of the Key Aroma Compounds in Traditional Hunan Smoke-Cured Pork Leg (Larou, THSL) by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV), and Sensory Evaluation Experiments. Foods 2020, 9, 413. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Wang, J.; Wang, H.; Lan, T.; Liu, R.; Gao, T.; Yang, W.; Zhou, Y.; Ge, Q.; Fang, Y.; et al. Is overnight fresh juice drinkable? The shelf life prediction of non-industrial fresh watermelon juice based on the nutritional quality, microbial safety quality, and sensory quality. Food Nutr. Res. 2020, 64, 4237. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Luan, Y.; Duan, C.Q.; Yan, G.L. Use of Torulaspora delbrueckii co-fermentation with two Saccharomyces cerevisiae strains with different aromatic characteristic to improve the diversity of red wine aroma profile. Front. Microbiol. 2018, 9, 606. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Giacosa, S.; Río Segade, S.; Rolle, L.; Cocolin, L. Cell-to-cell contact mechanism modulates Starmerella bacillaris death in mixed culture fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2019, 289, 106–114. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Xu, Y.H.; Tao, Y.S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2017, 24, 166–180. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Farina, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lan, Y.; Zhu, B.; Xiang, X.; Duan, C.; Shi, Y. Changes in monosaccharides, organic acids and amino acids during Cabernet Sauvignon wine ageing based on a simultaneous analysis using gas chromatography-mass spectrometry. J. Sci. Food Agric. 2018, 98, 104–112. [Google Scholar] [CrossRef]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and identification of organic acids in wine samples. Problems and challenges. TrAC 2019, 120, 115630. [Google Scholar] [CrossRef]
- Coelho, E.M.; da Silva Padilha, C.V.; Miskinis, G.A.; de Sá, A.G.B.; Pereira, G.E.; de Azevêdo, L.C.; dos Santos Lima, M. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [Green Version]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res. Int. 2019, 119, 378–389. [Google Scholar] [CrossRef]
- Kanter, J.-P.; Benito, S.; Brezina, S.; Beisert, B.; Fritsch, S.; Patz, C.-D.; Rauhut, D. The impact of hybrid yeasts on the aroma profile of cool climate Riesling wines. Food Chem. X 2020, 5, 100072. [Google Scholar] [CrossRef]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolin, L. Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Tofalo, R.; Schirone, M.; Torriani, S.; Rantsiou, K.; Cocolin, L.; Perpetuini, G.; Suzzi, G. Diversity of Candida zemplinina strains from grapes and Italian wines. Food Microbiol. 2012, 29, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Yuan, Y.; Dai, L.; Yue, T. Characteristic fruit wine production via reciprocal selection of juice and non-Saccharomyces species. Food Microbiol. 2019, 79, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Voon, M.K.W.; Chua, J.Y.; Huang, D.; Lee, P.R.; Liu, S.Q. The effects of co- and sequential inoculation of Torulaspora delbrueckii and Pichia kluyveri on chemical compositions of durian wine. Appl. Microbiol. Biotechnol. 2017, 101, 7853–7863. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Cui, Y.; Zhang, S.; Li, L.; Suo, H.; Sun, B. Detailed phenolic composition of Vidal grape pomace by ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2017, 1068–1069, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.J.; White, K.P.; Edwards, C.G. Growth and metabolism of non-Saccharomyces yeasts isolated from Washington state vineyards in media and high sugar grape musts. Food Microbiol. 2019, 77, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.K.; Wang, J.; Chen, F.S.; Zhang, X.Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT Food Sci. Technol. 2019, 116, 108477. [Google Scholar] [CrossRef]
- Whitener, M.E.B.; Stanstrup, J.; Carlin, S.; Divol, B.; Dutoit, M.; Vrhovsek, U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Garijo, P.; Berlanas, C.; López-Alfaro, I.; López, R.; Gutiérrez, A.R.; Santamaría, P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017, 54, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J. Agric. Food Chem. 2007, 55, 6674–6684. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Zini, C.A. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 53. [Google Scholar] [CrossRef]
- Liu, J.; Arneborg, N.; Toldam-Andersen, T.B.; Petersen, M.A.; Bredie, W.L.P. Effect of sequential fermentations and grape cultivars on volatile compounds and sensory profiles of Danish wines. J. Sci. Food Agric. 2017, 97, 3594–3602. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Wang, X.; Xie, K.; Zhuang, H.; Ye, R.; Fang, Z.; Feng, T. Volatile flavor compounds, total polyphenolic contents and antioxidant activities of a China gingko wine. Food Chem. 2015, 182, 41–46. [Google Scholar] [CrossRef]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Procopio, S.; Krause, D.; Hofmann, T.; Becker, T. Significant amino acids in aroma compound profiling during yeast fermentation analyzed by PLS regression. LWT Food Sci. Technol. 2013, 51, 423–432. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, X.; Niu, Y.; Yu, D.; Zhu, J.; Zhu, G. Optimization and application of headspace-solid-phase micro-extraction coupled with gas chromatography-mass spectrometry for the determination of volatile compounds in Cherry wines. J. Chromatogr. B 2015, 978–979, 122–130. [Google Scholar] [CrossRef]
- Lukić, I.; Radeka, S.; Grozaj, N.; Staver, M.; Peršurić, Đ. Changes in physico-chemical and volatile aroma compound composition of Gewürztraminer wine as a result of late and ice harvest. Food Chem. 2016, 196, 104–1057. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-Q.; Wang, X.-C.; Tao, N.-P.; Wu, N. Characterization of volatile compounds in different edible parts of steamed Chinese mitten crab (Eriocheir sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar] [CrossRef]
- Dorado, A.D.; Husni, S.; Pascual, G.; Puigdellivol, C.; Gabriel, D. Inventory and treatment of compost maturation emissions in a municipal solid waste treatment facility. J. Waste Manag. 2014, 34, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile composition of Merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Aroma compounds in Ontario Vidal and Riesling icewines. II. Effects of crop level. Food Res. Int. 2015, 76, 55–560. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache Rose´ Wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Tronchoni, J.; Curiel, J.A.; Sáenz-Navajas, M.P.; Morales, P.; de-la-Fuente-Blanco, A.; Fernández-Zurbano, P.; Gonzalez, R. Aroma profiling of an aerated fermentation of natural grape must with selected yeast strains at pilot scale. Food Microbiol. 2018, 70, 214–223. [Google Scholar] [CrossRef]
- Bueno, M.; Zapata, J.; Ferreira, V. Simultaneous determination of free and bonded forms of odor-active carbonyls in wine using a headspace solid phase microextraction strategy. J. Chromatogr. A 2014, 1369, 33–42. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Jiang, D.; Zhang, Y.; Zhang, S.; Sun, S. Effect of Saccharomyces cerevisiae, Torulaspora delbrueckii and malolactic fermentation on fermentation kinetics and sensory property of black raspberry wines. Food Microbiol. 2020, 91, 103551. [Google Scholar] [CrossRef] [PubMed]

| Grape Juice | F33_Pure | F33_Hu | F33_Sc | F33_Mp | F33_Pk | F33_Rl | |
|---|---|---|---|---|---|---|---|
| Ethanol (%) | ND | 10.12 ± 0.93 c | 8.66 ± 0.53 bc | 8.46 ± 0.17 b | 8.21 ± 0.08 b | 7.35 ± 0.70 b | 7.82 ± 0.56 b |
| TS (g/L) ** | 71.93 ± 6.86 c | 344.0 ± 6.19 d | 36.76 ± 3.01 ab | 36.76 ± 3.62 ab | 46.65 ± 4.07 b | 32.36 ± 2.53 a | 45.55 ± 6.03 ab |
| TA (g/L) | 6.14 ± 0.37 a | 6.09 ± 0.52 a | 5.97 ± 0.21 a | 6.49 ± 0.11 a | 6.66 ± 0.23 a | 6.31 ± 0.39 a | 6.14 ± 0.11 a |
| PH | 3.77 ± 0.44 a | 3.64 ± 0.28 a | 3.87 ± 0.39 a | 3.84 ± 0.23 a | 3.90 ± 0.08 a | 3.89 ± 0.35 a | 3.89 ± 0.22 a |
| Organic acids (g/L) | |||||||
| Citric acid ** | 0.358 ± 0.033 b | 0.274 ± 0.003 a | 0.299 ± 0.024 a | 0.279 ± 0.022 a | 0.284 ± 0.005 a | 0.276 ± 0.006 a | 0.293 ± 0.023 a |
| Pyruvic acid ** | ND | 0.047 ± 0.003 bc | 0.051 ± 0.005 c | 0.065 ± 0.006 d | 0.041 ± 0.002 b | 0.069 ± 0.002 d | 0.055 ± 0.002 c |
| Oxalic acid ** | ND | 0.41 ± 0.03 bc | 0.403 ± 0.05 bc | 0.423 ± 0.018 bc | 0.418 ± 0.029 bc | 0.348 ± 0.032 b | 0.47 ± 0.033 c |
| Fumaric acid ** | 0.007 ± 0.001 a | 0.024 ± 0.001 b | 0.022 ± 0.002 b | 0.021 ± 0.002 b | 0.021 ± 0.001 b | 0.021 ± 0.001 b | 0.022 ± 0.001 b |
| Ascorbic acid ** | 0.022 ± 0.001 a | 0.057 ± 0.005 bd | 0.052 ± 0.002 b | 0.063 ± 0.002 cd | 0.054 ± 0.004 bc | 0.048 ± 0.004 b | 0.066 ± 0.006 d |
| Tartaric acid ** | 4.938 ± 0.198 c | 4.258 ± 0.043 ab | 4.102 ± 0.142 a | 4.654 ± 0.123 bc | 4.319 ± 0 ab | 4.125 ± 0.23 a | 4.554 ± 0.164 bc |
| Malic acid ** | 3.412 ± 0.329 c | 2.035 ± 0.113 a | 2.548 ± 0.234 ab | 2.296 ± 0.204 ab | 2.419 ± 0.211 ab | 2.699 ± 0.282 b | 2.296 ± 0.143 ab |
| Malonic acid ** | ND | 0.062 ± 0.003 bc | 0.07 ± 0.004 cd | 0.058 ± 0.004 b | 0.072 ± 0.006 cd | 0.064 ± 0.001 bd | 0.073 ± 0.005 d |
| Succinic acid ** | 0.045 ± 0.003 a | 1.064 ± 0.092 de | 0.842 ± 0.03 bc | 0.76 ± 0.03 b | 1.156 ± 0.064 ef | 1.287 ± 0.089 f | 0.943 ± 0.081 cd |
| Shikimic acid ** | 0.048 ± 0.003 a | 0.124 ± 0.003 ab | 0.16 ± 0.009 b | 1.347 ± 0.036 e | 0.854 ± 0.043 c | 1.378 ± 0.024 e | 0.992 ± 0.075 d |
| Lactic acid ** | ND | 0.519 ± 0 b | 0.538 ± 0.034 b | 0.641 ± 0.061 c | 0.542 ± 0.03 b | 0.569 ± 0.025 bc | 0.527 ± 0.011 b |
| Acetic acid ** | ND | 0.492 ± 0.026 bd | 0.425 ± 0.038 b | 0.468 ± 0.012 bc | 0.543 ± 0.042 cd | 0.564 ± 0.041 d | 0.512 ± 0.022 cd |
| Grape Juice | F33_pure | F33_Hu | F33_Sc | F33_Mp | F33_Pk | F33_Rl | |
|---|---|---|---|---|---|---|---|
| Protocatechuic acid ** | 9.124 ± 0.790 b | 2.715 ± 0.144 a | 2.667 ± 0.148 a | 3.124 ± 0.143 a | 2.908 ± 0.324 a | 2.869 ± 0.224 a | 2.897 ± 0.161 a |
| P-hydroxybenzonic acid * | 0.867 ± 0.065 b | 0.522 ± 0.019 a | 0.956 ± 0.066 b | 0.663 ± 0.023 a | 1.186 ± 0.059 c | 1.122 ± 0.074 c | 0.966 ± 0.017 b |
| Chlorogenic acid ** | 0.200 ± 0.017 a | 0.308 ± 0.033 a | 1.434 ± 0.112 bc | 1.456 ± 0.064 c | 1.175 ± 0.082 b | 1.506 ± 0.153 c | 1.434 ± 0.129 bc |
| Vanillic acid ** | 0.176 ± 0.006 a | 0.233 ± 0.012 a | 0.548 ± 0.019 c | 0.630 ± 0.069 c | 0.365 ± 0.048 b | 0.426 ± 0.041 b | 0.576 ± 0.027 c |
| (-)-epigallocatechin gallate ** | ND | 0.295 ± 0.023 d | 0.352 ± 0.010 e | 0.488 ± 0.021 f | 0.125 ± 0.011 b | 0.114 ± 0.011 b | 0.221 ± 0.013 c |
| Epicatechin ** | 0.017 ± 0.001 a | 0.029 ± 0.001 bc | 0.033 ± 0.001 cd | 0.066 ± 0.005 f | 0.041 ± 0.004 d | 0.052 ± 0.004 e | 0.024 ± 0.002 ab |
| Vanillin ** | 0.028 ± 0.001 a | 0.285 ± 0.024 de | 0.170 ± 0.015 b | 0.304 ± 0.014 e | 0.211 ± 0.015 c | 0.148 ± 0.012 b | 0.254 ± 0.009 d |
| P-coumaric acid ** | 0.966 ± 0.029 a | 2.941 ± 0.284 bc | 2.580 ± 0.129 b | 2.985 ± 0.136 bc | 2.810 ± 0.296 bc | 3.261 ± 0.000 c | 2.947 ± 0.179 bc |
| (-)-epicatechin gallate ** | ND | 3.497 ± 0.334 c | 2.610 ± 0.188 b | 2.908 ± 0.126 bc | 2.708 ± 0.097 b | 2.838 ± 0.327 b | 3.436 ± 0.206 c |
| Isoferulic acid ** | 0.038 ± 0.002 a | 0.294 ± 0.039 c | 0.262 ± 0.011 c | 0.276 ± 0.000 c | 0.185 ± 0.021 b | 0.272 ± 0.026 c | 0.292 ± 0.005 c |
| Rutin ** | 5.400 ± 0.389 c | 2.700 ± 0.258 b | 2.256 ± 0.135 ab | 1.924 ± 0.126 a | 2.317 ± 0.184 ab | 2.279 ± 0.046 ab | 2.428 ± 0.170 ab |
| Trans-cinnamic acid ** | 0.012 ± 0.001 a | 2.427 ± 0.146 b | 2.487 ± 0.149 bc | 2.637 ± 0.147 bc | 2.577 ± 0.220 bc | 2.966 ± 0.136 c | 2.787 ± 0.348 bc |
| Apigenin ** | 0.300 ± 0.019 a | 0.582 ± 0.047 b | 1.477 ± 0.074 c | 2.615 ± 0.069 f | 2.278 ± 0.060 d | 2.466 ± 0.025 e | 1.469 ± 0.025 c |
| Myricetin ** | 0.032 ± 0.001 a | 0.036 ± 0.001 ab | 0.046 ± 0.001 b | 0.068 ± 0.004 c | 0.041 ± 0.005 ab | 0.037 ± 0.003 ab | 0.063 ± 0.007 c |
| Cynaroside * | ND | 2.048 ± 0.182 b | 2.461 ± 0.186 bc | 2.044 ± 0.108 b | 2.895 ± 0.181 c | 2.659 ± 0.027 bc | 4.422 ± 0.585 d |
| Caffeic acid * | 1.904 ± 0.148 a | 4.267 ± 0.128 b | 5.632 ± 0.627 c | 5.873 ± 0.411 c | 4.114 ± 0.179 b | 4.568 ± 0.121 b | 4.741 ± 0.246 b |
| Quercetin ** | 2.228 ± 0.194 b | 0.021 ± 0.001 a | 0.021 ± 0.001 a | 0.023 ± 0.001 a | 0.019 ± 0.002 a | 0.022 ± 0.002 a | 0.022 ± 0.002 a |
| Compounds | CAS | RI | Formula | Grape Juice | F33_Hu | F33_Sc | F33_Mp | F33_Pk | F33_pure | F33_Rl |
|---|---|---|---|---|---|---|---|---|---|---|
| C13-norisoprenoids | ||||||||||
| β-damascenone | 23726-93-4 | 1440 | C13H18O | 70.742 ± 6.288 a | 152.303 ± 4.030 c | 150.536 ± 12.322 c | 148.687 ± 7.868 c | 110.502 ± 9.045 b | 112.345 ± 2.972 b | 125.335 ± 9.948 b |
| Terpenes | ||||||||||
| ethylbenzene | 100-41-4 | 893 | C8H10 | 2.435 ± 0.199 a | 112.692 ± 9.224 d | 85.223 ± 2.557 bc | 89.754 ± 7.180 bc | 74.833 ± 9.196 b | 101.783 ± 9.160 cd | 74.109 ± 6.792 b |
| naphthalene | 91-20-3 | 1231 | C10H8 | 148.192 ± 5.343 a | 416.705 ± 30.049 c | 288.905 ± 23.648 b | 305.452 ± 21.382 b | 342.680 ± 13.707 bc | 686.057 ± 74.208 d | 295.368 ± 36.891 b |
| cinnamene | 100-42-5 | 883 | C8H8 | 383.869 ± 36.619 a | 752.324 ± 71.767 c | 837.172 ± 52.281 c | 501.109 ± 13.258 b | 516.951 ± 18.639 b | 433.728 ± 11.475 ab | 510.919 ± 18.421 b |
| linalool | 78-70-6 | 1082 | C10H18O | 182.614 ± 15.920 a | 1922.437 ± 183.389 b | 3306.532 ± 288.257 e | 2469.939 ± 154.248 cd | 2771.142 ± 264.350 d | 2102.666 ± 55.631 bc | 2255.023 ± 112.751 bc |
| citronellol | 106-22-9 | 1179 | C10H20O | ND | 392.406 ± 20.390 de | 423.152 ± 29.621 e | 248.001 ± 19.684 b | 296.777 ± 20.774 bc | 339.634 ± 27.800 cd | 250.362 ± 27.879 b |
| farnesol | 4602-84-0 | 1710 | C15H26O | ND | 177.063 ± 11.611 cd | 143.529 ± 2.486 b | 138.131 ± 16.285 b | 197.755 ± 8.620 d | 198.741 ± 5.962 d | 163.259 ± 13.363 bc |
| d-limonene | 5989-27-5 | 1018 | C10H16 | 99.847 ± 4.992 a | 336.024 ± 36.347 c | 190.037 ± 10.581 b | 198.335 ± 17.176 b | 232.202 ± 16.744 b | 346.862 ± 9.177 c | 209.016 ± 9.578 b |
| β-myrcene | 123-35-3 | 958 | C10H16 | 14.564 ± 1.137 b | ND | 52.174 ± 3.421 c | 52.005 ± 4.766 c | 58.626 ± 5.111 cd | 63.110 ± 5.466 d | 52.099 ± 2.271 c |
| β-pinene | 127-91-3 | 943 | C10H16 | ND | 80.024 ± 6.550 b | 135.067 ± 8.104 c | 126.075 ± 14.540 c | 134.699 ± 8.193 c | 147.793 ± 12.097 c | ND |
| α-terpineol | 98-55-5 | 1143 | C10H18O | 58.715 ± 3.667 b | 346.417 ± 18.331 d | 248.798 ± 32.913 c | 264.095 ± 13.205 c | ND | ND | 245.392 ± 13.663 c |
| p-xylene | 106-42-3 | 907 | C8H10 | ND | ND | ND | 200.713 ± 14.050 c | 104.695 ± 3.775 b | ND | 205.178 ± 11.424 c |
| ortho-cymene | 527-84-4 | 134 | C10H14 | ND | ND | ND | 104.903 ± 6.551 b | ND | 180.368 ± 10.822 c | 100.813 ± 2.667 b |
| 2,6-Di(tert-butyl)-4-hydroxy-4 -methyl-2,5-cyclohexadien-1-one | 10396-80-2 | 236 | C15H24O2 | 12.057 ± 0.435 b | ND | 44.632 ± 2.715 d | ND | ND | 36.560 ± 1.900 c | 62.032 ± 1.861 e |
| nerol oxide | 1786-08-9 | 1125 | C10H16O | 200.123 ± 9.171 b | ND | 506.272 ± 43.256 e | 346.800 ± 15.892 c | 1289.712 ± 38.691 f | ND | 420.144 ± 19.253 d |
| Volatile phenols | ||||||||||
| 2,4-di-t-butylphenol | 96-76-4 | 1555 | C14H22O | 122.009 ± 4.399 a | 168.209 ± 8.901 b | 118.566 ± 1.186 a | 113.006 ± 4.926 a | 107.611 ± 6.546 a | 110.456 ± 7.653 a | 110.801 ± 3.995 a |
| p-vinylguaiacol | 7786-61-0 | 1293 | C9H10O2 | 0.760 ± 0.042 a | 35.550 ± 2.220 d | 28.858 ± 2.020 bc | 24.896 ± 1.743 b | 31.405 ± 1.570 cd | 32.540 ± 1.127 cd | 24.964 ± 2.176 b |
| Acetate esters | ||||||||||
| ethyl acetate | 141-78-6 | 586 | C4H8O2 | 36.591 ± 3.354 a | 1793.153 ± 111.982 d | 1147.773 ± 79.520 bc | 1239.243 ± 54.017 bc | 1218.622 ± 76.103 bc | 1319.687 ± 117.296 c | 1043.714 ± 83.497 b |
| isoamyl ethanoate | 123-92-2 | 820 | C7H14O2 | 36.070 ± 1.443 a | 1653.245 ± 103.245 d | 1856.324 ± 115.927 d | 1255.537 ± 45.269 c | 789.373 ± 91.035 b | 704.055 ± 30.689 b | 779.904 ± 71.479 b |
| methyl acetate | 79-20-9 | 487 | C3H6O2 | ND | 29.858 ± 1.368 c | 18.378 ± 1.753 b | 17.119 ± 1.522 b | 17.138 ± 0.453 b | 18.705 ± 0.857 b | 16.041 ± 0.976 b |
| 9-decenyl acetate | 50816-18-7 | 1371 | C12H22O2 | ND | 85.289 ± 7.386 c | 62.297 ± 3.469 b | 62.817 ± 4.743 b | 88.262 ± 2.335 c | 60.878 ± 4.983 b | 55.911 ± 4.438 b |
| heptyl acetate | 112-06-1 | 1086 | C9H18O2 | 23.569 ± 1.650 a | 565.351 ± 46.276 d | 220.310 ± 11.015 c | 198.468 ± 1.985 bc | 192.093 ± 16.412 bc | 209.375 ± 6.281 c | 154.255 ± 8.589 b |
| benzylcarbinyl acetate | 103-45-7 | 1259 | C10H12O2 | ND | 8355.253 ± 521.785 c | 6552.383 ± 472.499 b | 6272.270 ± 287.432 b | ND | ND | 5981.076 ± 467.137 b |
| isobutyl acetate | 110-19-0 | 721 | C6H12O2 | 55.399 ± 2.216 a | 2066.293 ± 94.689 c | 2166.231 ± 156.209 c | 1666.309 ± 88.173 b | ND | ND | 2123.640 ± 212.364 c |
| butyl acetate | 123-86-4 | 774 | C6H12O2 | ND | 1027.800 ± 17.802 b | 3346.875 ± 57.970 d | 712.800 ± 72.341 b | 8517.500 ± 596.225 e | ND | 2125.800 ± 221.940 c |
| propyl acetate | 109-60-4 | 666 | C5H10O2 | ND | 3471.600 ± 125.170 b | 6046.400 ± 742.994 c | 3319.200 ± 87.818 b | 12370.400 ± 1237.040 d | ND | 5502.000 ± 524.857 c |
| hexyl acetate | 142-92-7 | 984 | C8H16O2 | ND | 668.210 ± 78.781 c | 993.560 ± 69.549 e | 892.314 ± 70.825 de | 663.210 ± 56.665 c | 332.569 ± 25.974 b | 801.792 ± 28.909 cd |
| amyl acetate | 628-63-7 | 884 | ND | ND | 6.789 ± 0.413 a | 11.352 ± 0.341 a | ND | 2411.600 ± 63.805 b | ND | 24.650 ± 2.219 a |
| isooctyl acetate | 31565-19-2 | 1523 | C10H20O2 | ND | 9.365 ± 0.487 a | 12.457 ± 0.374 a | 12.540 ± 0.783 a | 325.652 ± 34.310 b | 2.366 ± 0.103 a | 10.325 ± 0.723 a |
| methyl phenylacetate | 101-41-7 | 1160 | ND | ND | 124.560 ± 5.708 a | 532.140 ± 24.386 b | 121.250 ± 7.951 a | 2258.650 ± 148.110 c | 33.456 ± 1.004 a | 55.410 ± 4.831 a |
| furfuryl acetate | 623-17-6 | 1009 | ND | ND | 12.365 ± 0.567 a | 114.021 ± 12.697 b | ND | 563.256 ± 29.805 c | 9.632 ± 0.674 a | 25.364 ± 1.342 a |
| Ethyl esters | ||||||||||
| ethyl dodecylate | 106-33-2 | 1580 | C14H28O2 | 123.400 ± 3.265 a | 9751.865 ± 258.010 e | 8865.334 ± 386.431 e | 5740.305 ± 604.779 cd | 6228.243 ± 186.847 d | 4468.127 ± 389.522 b | 5271.596 ± 139.473 bc |
| ethyl heptoate | 106-30-9 | 1099 | C9H18O2 | ND | 342.760 ± 17.810 c | 456.321 ± 31.615 d | 213.880 ± 18.274 b | 256.669 ± 27.042 b | 217.832 ± 27.207 b | 217.230 ± 16.966 b |
| ethyl caproate | 123-66-0 | 987 | C8H16O2 | 14.016 ± 0.853 a | 7896.235 ± 753.253 cd | 8865.336 ± 845.699 d | 6961.214 ± 638.006 c | 6831.520 ± 361.490 c | 4447.635 ± 117.673 b | 6608.752 ± 302.851 c |
| ethyl butanoate | 105-54-4 | 785 | C6H12O2 | 3.078 ± 0.268 a | 800.858 ± 52.516 d | 819.353 ± 40.968 d | 506.172 ± 22.064 c | 510.596 ± 22.256 c | 476.131 ± 50.164 c | 217.327 ± 23.508 b |
| ethyl myristate | 124-06-1 | 1779 | C16H32O2 | ND | 331.083 ± 14.432 d | 426.836 ± 50.323 e | 262.372 ± 15.959 c | 398.082 ± 31.091 de | 167.334 ± 6.693 b | 146.116 ± 6.369 b |
| ethyl nonanoate | 123-29-5 | 1282 | C11H22O2 | ND | 386.235 ± 17.699 c | 456.321 ± 31.942 d | 316.691 ± 27.426 b | 319.467 ± 8.452 b | 277.837 ± 24.221 b | 322.431 ± 30.758 bc |
| ethyl palmitate | 628-97-7 | 1978 | C18H36O2 | ND | 398.775 ± 3.988 de | 445.478 ± 16.062 e | 283.855 ± 22.170 b | 342.940 ± 30.481 c | 355.733 ± 17.787 cd | 273.505 ± 9.861 b |
| ethyl lactate | 97-64-3 | 848 | C5H10O3 | ND | 59.112 ± 4.617 c | 69.336 ± 2.773 d | 36.924 ± 1.692 b | 38.662 ± 2.679 b | 38.125 ± 4.124 b | 40.215 ± 3.836 b |
| ethyl dihydrocinnamate | 2021-28-5 | 1359 | C11H14O2 | ND | 642.765 ± 17.006 e | 782.340 ± 28.208 f | 347.334 ± 26.223 c | 477.504 ± 21.882 d | 225.021 ± 25.951 b | 443.101 ± 13.293 d |
| ethyl 4e-decenoate | 76649-16-6 | 1389 | C12H22O2 | ND | 4446.576 ± 320.647 e | 3007.235 ± 104.174 bc | 2807.420 ± 202.446 bc | 3355.207 ± 146.250 cd | 3792.373 ± 330.611 d | 2687.685 ± 123.165 b |
| ethyl (e)-9-palmitoleate | 54546-22-4 | 1966 | C18H34O2 | 12.300 ± 0.325 a | 392.378 ± 23.867 d | 266.860 ± 25.457 c | 210.950 ± 12.832 b | 277.847 ± 15.470 c | 267.319 ± 21.881 c | 190.610 ± 7.624 b |
| ethyl 4-acetoxybutyrate | 25560-91-2 | 1151 | ND | ND | 92.067 ± 4.013 d | 74.297 ± 7.087 bc | 63.795 ± 5.847 b | 65.518 ± 2.362 b | 85.816 ± 7.332 cd | 65.822 ± 3.665 b |
| ethyl 7-octenoate | 35194-38-8 | 1173 | C10H18O2 | ND | 794.572 ± 78.256 e | 649.356 ± 19.481 cd | 519.541 ± 22.646 b | 742.910 ± 26.786 de | 701.225 ± 61.131 de | 561.630 ± 48.962 bc |
| ethyl phenylethanoate | 101-97-3 | 1259 | C10H12O2 | ND | 489.700 ± 17.656 e | 556.300 ± 47.530 f | 206.764 ± 4.135 b | 322.129 ± 31.726 c | 352.771 ± 14.111 cd | 413.200 ± 4.132 d |
| ethyl caprylate | 106-32-1 | 1085 | C10H20O2 | 0.210 ± 0.018 a | 38.304 ± 2.762 d | 77.526 ± 6.346 g | 67.944 ± 4.243 f | 12.332 ± 1.176 b | 56.771 ± 1.502 e | 22.354 ± 0.387 c |
| ethyl propanoate | 105-37-3 | 686 | C5H10O2 | ND | 517.560 ± 22.560 b | 981.120 ± 35.375 d | 1226.400 ± 97.342 e | 774.213 ± 55.829 c | 112.356 ± 3.892 a | 580.800 ± 43.849 b |
| ethyl isobutyrate | 97-62-1 | 728 | C6H12O2 | ND | ND | 55.188 ± 1.104 d | 3.552 ± 0.249 b | 6.876 ± 0.563 c | ND | ND |
| ethyl n-valerate | 539-82-2 | 874 | C7H14O2 | ND | ND | 32.280 ± 2.260 d | 12.912 ± 0.258 b | ND | 20.733 ± 0.415 c | ND |
| ethyl oleate | 111-62-6 | 2185 | C20H38O2 | 2.451 ± 0.209 b | ND | 11.448 ± 0.637 c | ND | ND | 50.295 ± 2.012 d | ND |
| ethyl linoleate | 544-35-4 | 2193 | C20H36O2 | ND | ND | 8.208 ± 0.701 b | 40.776 ± 2.480 c | ND | ND | ND |
| Other esters | ||||||||||
| methyl caprate | 110-42-9 | 1282 | C11H22O2 | ND | 384.104 ± 6.653 d | 423.658 ± 52.915 d | 230.150 ± 22.667 bc | 259.881 ± 11.909 c | 191.094 ± 6.890 b | 237.603 ± 14.453 bc |
| 4-hydroxybutyric acid | 591-81-1 | 1018 | C4H7O3 | ND | 228.427 ± 7.913 d | 221.913 ± 11.743 cd | 158.713 ± 9.523 b | 198.682 ± 7.164 c | 205.574 ± 14.390 cd | 169.733 ± 11.881 b |
| txib | 6846-50-0 | 1605 | C16H30O4 | 112.412 ± 8.487 a | 1165.722 ± 143.246 d | 1178.762 ± 42.501 d | 504.371 ± 36.371 b | 1165.017 ± 90.991 d | 857.818 ± 61.858 c | 1207.867 ± 67.251 d |
| isoamyl decanoate | 2306-91-4 | 1615 | C15H30O2 | ND | 807.121 ± 44.939 d | 574.914 ± 15.211 b | 811.419 ± 72.120 d | 766.740 ± 40.572 cd | ND | 684.864 ± 13.697 c |
| diisobutyl phthalate | 84-69-5 | 1908 | C16H22O4 | ND | 662.163 ± 69.132 d | 503.222 ± 5.032 c | 446.305 ± 16.092 bc | 478.597 ± 36.133 bc | 404.190 ± 22.504 b | ND |
| teksanol | 77-68-9 | 1331 | ND | 39.739 ± 1.051 a | 942.490 ± 18.850 e | 221.283 ± 19.668 b | ND | 640.200 ± 75.478 cd | 551.147 ± 16.534 c | 708.996 ± 58.034 d |
| vinyl acetate | 108-05-4 | 576 | ND | ND | 20.565 ± 2.167 d | 16.672 ± 0.167 c | ND | ND | 14.152 ± 1.158 bc | 12.396 ± 0.496 b |
| isobutyl octanoate | 5461-06-3 | 1317 | C12H24O2 | ND | 516.499 ± 17.892 e | 485.671 ± 22.256 e | 221.345 ± 21.115 c | 112.356 ± 9.730 b | 324.568 ± 16.865 d | ND |
| δ-decalactone | 705-86-2 | 1404 | C10H18O2 | ND | ND | 489.300 ± 16.950 b | ND | ND | ND | ND |
| γ-nonalactone | 104-61-0 | 1284 | C9H16O2 | ND | 16.512 ± 0.858 b | 88.244 ± 2.335 e | 29.244 ± 1.520 c | 60.264 ± 4.346 d | ND | 19.704 ± 1.718 b |
| γ-decalactone | 706-14-9 | 1383 | C10H18O2 | ND | ND | 112.325 ± 7.863 b | ND | ND | ND | ND |
| γ-caprolactone | 695-06-7 | 986 | C6H10O2 | ND | ND | 22.346 ± 0.387 b | ND | ND | ND | ND |
| isobutyl propionate | 540-42-1 | 826 | C7H14O2 | ND | ND | 14.880 ± 1.181 b | ND | 60.432 ± 3.626 c | ND | ND |
| isobutyric acid | 2445-69-4 | 955 | C9H18O2 | ND | ND | 13.464 ± 0.539 b | ND | ND | ND | ND |
| propyl propionate | 106-36-5 | 789 | C6H12O2 | ND | ND | 8.208 ± 0.376 b | ND | ND | ND | ND |
| isoamyl butylate | 106-27-4 | 1019 | C9H18O2 | ND | ND | 5.088 ± 0.353 b | ND | ND | ND | ND |
| Alcohols | ||||||||||
| 1-pentanol | 71-41-0 | 761 | C5H12O | ND | 205.830 ± 10.292 c | 147.275 ± 8.200 b | 137.478 ± 10.737 b | 141.817 ± 5.113 b | 151.472 ± 2.624 b | 137.523 ± 5.994 b |
| 1-dodecanol | 112-53-8 | 1457 | C12H26O | 0.573 ± 0.035 a | 334.224 ± 8.843 e | 307.108 ± 16.251 e | 191.411 ± 8.343 c | 227.642 ± 12.046 d | 155.982 ± 9.741 b | 208.998 ± 10.450 cd |
| 1-butanol | 71-36-3 | 662 | C4H10O | 2.257 ± 0.195 a | 430.340 ± 29.815 c | 289.039 ± 5.006 b | 299.995 ± 21.633 b | 294.046 ± 17.886 b | 293.252 ± 20.317 b | 279.417 ± 15.557 b |
| isobutyl alcohol | 78-83-1 | 597 | C4H10O | 105.581 ± 11.023 a | 4433.994 ± 406.382 c | 3327.766 ± 295.778 b | 3155.172 ± 166.956 b | 3187.708 ± 307.411 b | 3280.948 ± 56.828 b | 2806.563 ± 97.222 b |
| 1-heptanol | 111-70-6 | 960 | C7H16O | 32.072 ± 1.156 a | 998.475 ± 39.939 d | 738.863 ± 33.859 b | 728.334 ± 40.552 b | 733.172 ± 31.958 b | 893.890 ± 35.756 c | 659.920 ± 54.017 b |
| 3-octenol | 3391-86-4 | 969 | C8H16O | 25.770 ± 1.690 a | 542.349 ± 27.117 de | 563.245 ± 31.360 e | 378.817 ± 36.137 b | 420.158 ± 36.629 bc | 479.423 ± 37.444 cd | 341.961 ± 11.846 b |
| 1-hexanol | 111-27-3 | 860 | C6H14O | 19.236 ± 1.763 a | 935.539 ± 16.204 d | 652.266 ± 59.781 c | 619.475 ± 6.195 bc | 635.212 ± 16.806 bc | 693.327 ± 18.344 c | 560.650 ± 49.832 b |
| 3-methylthiopropyl alcohol | 505-10-2 | 912 | C4H10OS | ND | 201.238 ± 12.567 e | 164.832 ± 13.083 d | 118.321 ± 12.798 bc | 128.177 ± 5.874 c | 122.202 ± 10.583 bc | 97.817 ± 2.588 b |
| benzyl alcohol | 100-51-6 | 1036 | C7H8O | 4.170 ± 0.232 a | 324.222 ± 8.578 e | 356.234 ± 10.687 e | 249.895 ± 13.914 cd | 262.505 ± 22.428 d | 199.869 ± 15.864 b | 215.898 ± 16.300 bc |
| (z)-2,3-butanediol | 24347-58-8 | 743 | C4H10O2 | 101.777 ± 2.036 a | 1774.534 ± 93.900 b | 2533.951 ± 87.779 cd | 2158.838 ± 208.191 bc | 2623.290 ± 78.699 de | 1811.684 ± 118.800 b | 2985.573 ± 273.632 e |
| hotrienol | 29957-43-5 | 1072 | C10H16O | 13.637 ± 0.625 a | 392.499 ± 23.875 c | 382.457 ± 26.497 c | 271.597 ± 24.140 b | 284.811 ± 5.696 b | 253.654 ± 11.057 b | 269.831 ± 28.171 b |
| 3-ethyloctan-3-ol | 2051-32-3 | 1107 | C10H22O | ND | 165.414 ± 17.892 d | 108.020 ± 1.080 bc | 98.871 ± 10.417 bc | 103.338 ± 10.789 bc | 122.511 ± 6.483 c | 96.114 ± 4.404 b |
| hexanol | 104-76-7 | 995 | C8H18O | 0.512 ± 0.040 a | 624.766 ± 39.017 c | 499.458 ± 4.995 b | 477.362 ± 25.260 b | 496.366 ± 37.475 b | ND | 467.178 ± 30.635 b |
| cyclooctanol | 696-71-9 | 1147 | C8H16O | 8.865 ± 0.320 b | ND | 125.682 ± 5.027 d | 113.488 ± 0.000 c | ND | 134.935 ± 1.349 e | 116.765 ± 3.089 c |
| penten-3-ol | 616-25-1 | 671 | C5H10O | 1.206 ± 0.024 a | 1375.887 ± 148.825 c | 878.405 ± 83.795 b | ND | ND | 1030.670 ± 30.920 b | ND |
| (s)-propylene glycol | 4254-15-3 | 724 | C3H8O2 | ND | ND | ND | 427.920 ± 32.307 c | ND | 287.644 ± 25.888 b | 654.712 ± 51.966 d |
| propylene glycol | 57-55-6 | 725 | C3H8O2 | ND | 394.080 ± 14.209 b | 520.038 ± 5.200 c | ND | 508.569 ± 45.203 c | ND | ND |
| (s)-3-methyl-1-pentanol | 42072-39-9 | 796 | C6H14O | 0.074 ± 0.005 a | ND | 71.546 ± 3.279 c | 52.434 ± 3.633 b | ND | 66.027 ± 5.241 c | ND |
| 2-heptanol | 543-49-7 | 879 | C7H16O | 15.703 ± 1.030 a | 629.160 ± 43.589 d | 552.364 ± 14.614 c | 198.720 ± 19.164 b | ND | 218.160 ± 12.147 b | ND |
| (z)-3-hexen-1-ol | 928-96-1(正) | 868 | C6H12O | ND | 96.084 ± 3.328 c | 98.832 ± 2.615 c | 200.520 ± 19.128 e | ND | 60.325 ± 2.413 b | 125.100 ± 8.203 d |
| phenethyl alcohol | 60-12-8 | 1136 | C8H10O | ND | 1122.288 ± 73.593 c | 248.928 ± 7.468 b | ND | 1259.352 ± 90.813 cd | 1284.624 ± 96.987 d | ND |
| 1-octanol | 111-87-5 | 1059 | C8H18O | 1.011 ± 0.062 a | 294.912 ± 17.939 d | 74.256 ± 3.403 b | 82.488 ± 5.151 b | 185.424 ± 12.159 c | 303.432 ± 9.103 d | 646.200 ± 39.307 e |
| 1-propanol | 71-23-8 | 562 | C3H8O | ND | 93.240 ± 9.324 c | 185.616 ± 12.993 e | 33.288 ± 1.153 b | 171.096 ± 2.963 e | 349.584 ± 15.238 f | 131.352 ± 12.667 d |
| (r)-2-butanol | 14898-79-4 | 584 | ND | ND | ND | 8.112 ± 0.354 b | 14.760 ± 1.172 c | 34.080 ± 2.128 e | ND | 21.384 ± 1.697 d |
| Acids | ||||||||||
| octanoic acid | 124-07-2 | 1173 | C8H16O2 | 55.346 ± 1.996 a | 6868.409 ± 629.500 d | 4031.541 ± 358.331 bc | 4501.788 ± 400.128 c | 4232.770 ± 193.970 bc | 3456.568 ± 307.226 b | 3477.012 ± 104.310 b |
| n-decanoic acid | 334-48-5 | 1372 | C10H20O2 | ND | 6043.870 ± 516.388 d | 2966.528 ± 207.657 c | 2696.471 ± 260.038 c | 2417.799 ± 274.609 bc | 2262.857 ± 45.257 bc | 1848.797 ± 161.174 b |
| butanoic acid | 107-92-6 | 775 | C4H8O2 | ND | 183.842 ± 3.184 c | 134.668 ± 6.733 b | 132.010 ± 10.805 b | 129.043 ± 9.305 b | 139.062 ± 7.358 b | 122.402 ± 13.240 b |
| 9-decenoic acid | 14436-32-9 | 1367 | C10H18O2 | ND | 1224.469 ± 76.468 d | 808.850 ± 37.066 c | 718.849 ± 43.726 c | 717.956 ± 31.295 c | 799.379 ± 27.691 c | 553.493 ± 53.377 b |
| acetic acid | 64-19-7 | 576 | C2H4O2 | 739.225 ± 29.569 a | 7510.545 ± 327.377 d | 5260.826 ± 368.258 b | 5393.834 ± 494.353 b | 6059.155 ± 277.665 bc | 6512.075 ± 586.087 cd | 5143.817 ± 224.214 b |
| 2-methyl-butanoic acid | 116-53-0 | 817 | C5H10O2 | 155.086 ± 9.685 a | 782.741 ± 20.709 d | 728.649 ± 66.782 cd | 534.598 ± 33.386 b | 632.755 ± 38.489 bc | 555.536 ± 27.777 b | 542.009 ± 21.680 b |
| heptanoic acid | 111-14-8 | 1073 | C7H14O2 | ND | 485.917 ± 39.774 e | 408.038 ± 4.080 d | 280.068 ± 19.605 c | 394.192 ± 33.680 d | 150.860 ± 10.560 b | 288.175 ± 15.249 c |
| 2-oxooctanoic acid | 328-51-8 | 1309 | C8H14O3 | ND | 114.051 ± 10.999 e | 83.348 ± 5.834 bc | 67.059 ± 5.489 b | 81.520 ± 5.706 bc | 86.314 ± 1.726 cd | 100.692 ± 4.389 de |
| 2-methyl- propanoic acid | 79-31-2 | 711 | C4H8O2 | ND | ND | 142.968 ± 11.437 c | ND | 686.160 ± 38.204 d | ND | 58.260 ± 6.302 b |
| hexanoic acid | 142-62-1 | 974 | C6H12O2 | 2.789 ± 0.170 a | 10.764 ± 1.124 a | ND | 361.812 ± 23.726 d | 224.880 ± 17.849 c | 21.528 ± 0.776 ab | 53.052 ± 4.625 b |
| propanoic acid | 79-09-4 | 676 | C3H6O2 | ND | ND | 51.660 ± 3.725 b | ND | ND | ND | ND |
| nonanoic acid | 112-05-0 | 1272 | C9H18O2 | ND | 13.680 ± 1.068 bc | 6.864 ± 0.247 ab | 44.880 ± 2.244 d | 92.640 ± 7.411 e | 19.260 ± 1.668 c | 37.296 ± 1.345 d |
| valeric acid | 109-52-4 | 875 | C5H10O2 | ND | ND | 25.656 ± 0.513 b | ND | ND | ND | ND |
| Aldehydes | ||||||||||
| acetaldehyde | 75-07-0 | 408 | C2H4O | 114.563 ± 8.948 a | 2089.726 ± 165.867 c | 1446.705 ± 146.825 b | 1418.946 ± 116.146 b | 1598.697 ± 162.250 b | 1519.862 ± 105.299 b | 1335.899 ± 23.138 b |
| isovaleral | 590-86-3 | 643 | C5H10O | 35.654 ± 2.785 d | 17.387 ± 0.968 a c | 19.246 ± 1.711 bc | 13.470 ± 0.486 a | 19.648 ± 1.874 c | 15.322 ± 0.668 ab | 13.159 ± 0.696 a |
| 1-hexanal | 66-25-1 | 806 | C6H12O | 88.623 ± 1.772 c | 86.230 ± 6.844 c | 58.712 ± 1.553 b | 35.430 ± 2.455 a | 42.741 ± 4.274 a | 36.610 ± 3.857 a | 42.360 ± 1.527 a |
| n-octanal | 124-13-0 | 1005 | C8H16O | 83.372 ± 3.335 c | 59.549 ± 5.955 ab | 60.233 ± 2.087 ab | 60.536 ± 1.049 ab | 53.042 ± 4.005 a | 69.814 ± 5.453 b | 122.397 ± 6.815 d |
| nonylaldehyde | 124-19-6 | 1104 | C9H18O | 60.392 ± 1.046 a | ND | 42.480 ± 2.653 a | 64.912 ± 3.246 a | ND | ND | 872.538 ± 79.969 b |
| isobutanal | 78-84-2 | 543 | C4H8O | 25.360 ± 2.076 d | ND | 7.080 ± 0.511 b | 15.816 ± 0.791 c | ND | ND | 7.920 ± 0.792 b |
| Ketones | ||||||||||
| acetone | 67-64-1 | 455 | C3H6O | ND | 13.451 ± 0.485 d | 8.852 ± 0.708 c | 6.943 ± 0.551 b | 7.490 ± 0.225 b | 8.234 ± 0.594 bc | 7.426 ± 0.324 b |
| acetoine | 513-86-0 | 717 | C4H8O2 | 86.230 ± 7.664 a | 158.089 ± 5.476 c | 114.843 ± 6.986 b | 122.897 ± 5.632 b | 131.471 ± 2.629 bc | 157.308 ± 19.330 c | 127.983 ± 11.084 b |
| decan-3-one | 928-80-3 | 1151 | C10H20O | ND | 290.986 ± 21.969 d | 140.271 ± 2.430 b | 128.400 ± 3.852 b | 206.693 ± 24.369 c | 273.184 ± 19.123 d | ND |
| biacetyl | 431-03-8 | 691 | C4H6O2 | 4.083 ± 0.071 b | ND | 10.112 ± 0.763 c | 16.611 ± 1.734 d | 16.430 ± 0.999 d | ND | ND |
| 3-octanone | 106-68-3 | 958 | C8H16O | ND | 30.912 ± 2.695 bc | 24.600 ± 0.852 b | 46.080 ± 0.461 d | ND | 37.932 ± 2.007 c | 76.032 ± 6.758 e |
| Ethers | ||||||||||
| dowanol peat | 111-35-3 | 837 | C5H12O2 | 6.007 ± 0.334 a | 180.186 ± 15.395 de | 104.944 ± 2.777 b | 149.404 ± 14.715 cd | 198.431 ± 24.384 e | 133.791 ± 3.540 bc | 120.211 ± 10.819 bc |
| vinamar | 109-92-2 | 485 | C4H8O | ND | 821.468 ± 42.685 e | 571.485 ± 22.859 bc | 573.201 ± 29.784 bc | 609.315 ± 38.052 cd | 671.467 ± 40.844 d | 503.150 ± 10.063 b |
| carbitol | 111-90-0 | 1012 | C6H14O3 | ND | 4.944 ± 0.396 c | 1.752 ± 0.109 b | ND | 23.736 ± 0.856 f | 18.192 ± 0.364 e | 12.144 ± 0.421 d |
| Compounds | CAS | RI * | Odor Threshold # | Odor Description | Grape Juice | F33_pure | F33_Hu | F33_Mp | F33_Pk | F33_Sc | F33_Rl | Identification Methods | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C13-norisoprenoids | |||||||||||||
| 1 | β-damascenone | 23726-93-4 | 1440 | 0.05 [63] | apple, rose, honey, tobacco, sweet | 1414.84 ± 61.67 a | 2246.90 ± 178.34 b | 3046.07 ± 213.23 c | 2973.74 ± 107.22 c | 2210.04 ± 192.67 b | 3010.71 ± 137.97 c | 2506.70 ± 130.25 b | MS, RI, S |
| Terpenes | |||||||||||||
| 1 | naphthalene | 91-20-3 | 1231 | 60 [64] | pungent, dry, tarry | 2.47 ± 0.17 a | 11.43 ± 0.79 d | 6.95 ± 0.49 c | 5.09 ± 0.18 b | 5.71 ± 0.60 bc | 4.82 ± 0.34 b | 4.92 ± 0.22 b | MS, RI, S |
| 2 | cinnamene | 100-42-5 | 883 | 65 [64] | sweet, balsam, floral, plastic | 5.91 ± 0.31 a | 6.67 ± 0.24 ab | 11.57 ± 0.35 d | 7.71 ± 0.43 bc | 7.95 ± 0.56 c | 12.88 ± 0.56 e | 7.86 ± 0.61 bc | MS, RI, S |
| 3 | linalool | 78-70-6 | 1082 | 15 [52] | citrus, orange, lemon, floral, waxy | 12.17 ± 0.97 a | 140.18 ± 5.05 bc | 128.16 ± 7.14 b | 164.66 ± 6.59 cd | 184.74 ± 14.66 d | 220.44 ± 17.50 e | 150.34 ± 6.55 bc | MS, RI, S |
| 4 | citronellol | 106-22-9 | 1179 | 100 [52] | floral, rose, sweet, green, fruity | ND | 3.40 ± 0.38 cd | 3.92 ± 0.31 de | 2.48 ± 0.07 b | 2.97 ± 0.24 bc | 4.23 ± 0.11 e | 2.50 ± 0.14 b | MS, RI, S |
| 5 | d-limonene | 5989-27-5 | 1018 | 10 [64] | sweet, orange, citrus, terpy | 9.99 ± 0.61 a | 34.69 ± 2.96 c | 33.60 ± 2.87 c | 19.83 ± 1.39 b | 23.22 ± 1.81 b | 19.00 ± 0.83 b | 20.90 ± 0.72 b | MS, RI, S |
| 6 | α-terpineol | 98-55-5 | 1143 | 250 [10] | citrus, woody, lemon, nuance | ND | ND | 1.39 ± 0.08 c | 1.06 ± 0.10 b | ND | ND | ND | MS, RI, S |
| 7 | p-xylene | 106-42-3 | 907 | 58 [65] | ND | ND | ND | 3.46 ± 0.07 c | 1.81 ± 0.15 b | ND | 3.54 ± 0.16 c | MS, RI | |
| 8 | nerol oxide | 1786-08-9 | 1125 | 80 [15] | green, vegetative, floral, leafy | 2.50 ± 0.25 b | ND | ND | 4.34 ± 0.20 c | 16.12 ± 1.32 e | 6.33 ± 0.60 d | 5.25 ± 0.18 cd | MS, RI, S |
| Acetate esters | |||||||||||||
| 1 | isoamyl ethanoate | 123-92-2 | 820 | 30 [66] | sweet, fruity, banana | 1.20 ± 0.07 a | 23.47 ± 0.94 b | 55.11 ± 0.96 d | 41.85 ± 2.51 c | 26.31 ± 2.41 b | 61.88 ± 6.09 d | 26.00 ± 1.80 b | MS, RI, S |
| 2 | benzylcarbinyl acetate | 103-45-7 | 1259 | 250 [66] | sweet, honey, floral, rosy | ND | ND | 33.42 ± 2.89 c | 25.09 ± 2.71 b | ND | 26.21 ± 1.84 b | 23.92 ± 1.68 b | MS, RI, S |
| 3 | isobutyl acetate | 110-19-0 | 721 | 1600 [10] | sweet, fruity, banana | ND | ND | 1.29 ± 0.10 c | 1.04 ± 0.10 b | ND | 1.35 ± 0.08 c | 1.33 ± 0.17 c | MS, RI, S |
| 4 | butyl acetate | 123-86-4 | 774 | 1800 [52] | sweet, ripe, banana | ND | ND | ND | ND | 4.73 ± 0.30 d | 1.86 ± 0.05 c | 1.18 ± 0.12 b | MS, RI, S |
| 5 | propyl acetate | 109-60-4 | 666 | 4740 [15] | fruity, banana, honey | ND | ND | ND | ND | 2.61 ± 0.23 c | 1.28 ± 0.11 b | 1.16 ± 0.08 b | MS, RI, S |
| 6 | hexyl acetate | 142-92-7 | 984 | 670 [14] | fruity, green, fresh, sweet, banana | ND | ND | ND | 1.33 ± 0.14 bc | ND | 1.48 ± 0.12 c | 1.20 ± 0.10 b | MS, RI, S |
| Ethyl esters | |||||||||||||
| 1 | ethyl dodecylate | 106-33-2 | 1580 | 500 [10] | waxy, soapy, floral | ND | 8.94 ± 0.32 b | 19.50 ± 1.35 d | 11.48 ± 0.23 bc | 12.46 ± 1.25 c | 17.73 ± 1.52 d | 10.54 ± 0.38 bc | MS, RI, S |
| 2 | ethyl heptoate | 106-30-9 | 1099 | 2.2 [52] | fruity, pineapple, banana | ND | 99.01 ± 5.94 b | 155.80 ± 6.23 c | 97.22 ± 6.07 b | 116.67 ± 12.99 b | 207.42 ± 12.95 d | 98.74 ± 5.50 b | MS, RI, S |
| 3 | ethyl caproate | 123-66-0 | 987 | 8 [52] | sweet, pineapple, fruity, waxy | 1.75 ± 0.09 a | 555.95 ± 33.82 b | 987.03 ± 84.33 cd | 870.15 ± 62.75 c | 853.94 ± 74.45 c | 1108.17 ± 106.87 d | 826.09 ± 37.86 c | MS, RI, S |
| 4 | ethyl butanoate | 105-54-4 | 785 | 400 [52] | fruity, sweet, frutti, apple | ND | 1.19 ± 0.01 b | 2.00 ± 0.04 c | 1.27 ± 0.08 b | 1.28 ± 0.05 b | 2.05 ± 0.02 c | ND | MS, RI, S |
| 5 | ethyl dihydrocinnamate | 2021-28-5 | 1359 | 1.6 [10] | rose, honey, fruity | ND | 140.64 ± 16.58 b | 401.73 ± 17.51 e | 217.08 ± 13.21 c | 298.44 ± 7.90 d | 488.96 ± 9.78 f | 276.94 ± 12.07 d | MS, RI |
| 6 | ethyl phenylethanoate | 101-97-3 | 1259 | 250 [67] | strong, sweet, rosy, honey | ND | 1.41 ± 0.08 b | 1.96 ± 0.07 d | ND | 1.29 ± 0.07 b | 2.23 ± 0.06 e | 1.65 ± 0.06 c | MS, RI |
| 7 | ethyl caprylate | 106-32-1 | 1085 | 5 [68] | sweet, waxy, fruity, pineapple | ND | 11.35 ± 0.41 d | 7.66 ± 0.38 c | 13.59 ± 1.80 de | 2.47 ± 0.19 b | 15.51 ± 1.21 e | 4.47 ± 0.36 b | MS, RI, S |
| 8 | ethyl propanoate | 105-37-3 | 686 | 550 [52] | fruity, sweet, winey | ND | ND | ND | 2.23 ± 0.14 e | 1.41 ± 0.13 c | 1.78 ± 0.08 d | 1.06 ± 0.05 b | MS, RI, S |
| 9 | ethyl isobutyrate | 97-62-1 | 728 | 15 [69] | pungent, etherial, fruity | ND | ND | ND | ND | ND | 3.68 ± 0.07 b | ND | MS, RI, S |
| 10 | ethyl n-valerate | 539-82-2 | 874 | 1.5 [12] | fruity, strawberry, sweet, pineapple | ND | 13.82 ± 1.46 c | ND | 8.61 ± 0.54 b | ND | 21.52 ± 1.88 d | ND | MS, RI |
| Other esters | |||||||||||||
| 1 | δ-decalactone | 705-86-2 | 1404 | 386 [57] | coconut, creamy, fatty, milky | ND | ND | ND | ND | ND | 1.27 ± 0.05 b | ND | MS, RI, S |
| 2 | γ-nonalactone | 104-61-0 | 1284 | 30 [57] | coconut, creamy, waxy | ND | ND | ND | ND | 2.01 ± 0.16 b | 2.94 ± 0.19 c | ND | MS, RI, S |
| 3 | γ-decalactone | 706-14-9 | 1383 | 88 [57] | fruity, creamy, peach | ND | ND | ND | ND | ND | 1.28 ± 0.09 b | ND | MS, RI, S |
| Alcohols | |||||||||||||
| 1 | 1-pentanol | 71-41-0 | 761 | 150.2 [64] | fermented, bready | ND | 1.01 ± 0.09 b | 1.37 ± 0.05 c | ND | ND | ND | ND | MS, RI, S |
| 2 | 1-heptanol | 111-70-6 | 960 | 250 [10] | oily, nutty, fatty | ND | 3.58 ± 0.38 cd | 3.99 ± 0.21 d | 2.91 ± 0.15 b | 2.93 ± 0.35 bc | 2.96 ± 0.17 bc | 2.64 ± 0.14 b | MS, RI, S |
| 3 | 3-octenol | 3391-86-4 | 969 | 1 [52] | mushroom, earthy, vegetative | 25.77 ± 1.95 a | 479.42 ± 26.69 d | 542.35 ± 24.85 e | 378.82 ± 32.37 bc | 420.16 ± 16.81 cd | 563.25 ± 14.90 e | 341.96 ± 21.36 b | MS, RI, S |
| 4 | 1-hexanol | 111-27-3 | 860 | 110 [52] | green, fruity, apple-skin, oily | ND | 6.30 ± 0.55 c | 8.51 ± 0.31 d | 5.63 ± 0.49 bc | 5.78 ± 0.61 bc | 5.93 ± 0.26 bc | 5.10 ± 0.27 b | MS, RI, S |
| 5 | penten-3-ol | 616-25-1 | 671 | 400 [52] | whiskey, green, apple | ND | 2.58 ± 0.22 c | 3.44 ± 0.14 d | ND | ND | 2.20 ± 0.14 b | ND | MS, RI, S |
| 6 | 2-heptanol | 543-49-7 | 879 | 200 [52] | fruity, green, earthy, bitter | ND | 1.09 ± 0.05 b | 3.15 ± 0.17 c | ND | ND | 2.76 ± 0.35 c | ND | MS, RI, S |
| 7 | 1-octanol | 111-87-5 | 1059 | 40 [5] | floral, sweet, rosey, bready | ND | 7.59 ± 0.55 d | 7.37 ± 0.34 d | 2.06 ± 0.09 b | 4.64 ± 0.46 c | 1.86 ± 0.10 b | 16.16 ± 1.17 e | MS, RI, S |
| Acids | |||||||||||||
| 1 | octanoic acid | 124-07-2 | 1173 | 500 [66] | rancid, soapy, cheesy, fatty | ND | 6.91 ± 0.21 b | 13.74 ± 0.63 e | 9.00 ± 0.72 d | 8.47 ± 0.52 cd | 8.06 ± 0.74 bd | 6.95 ± 0.61 bc | MS, RI, S |
| 2 | n-decanoic acid | 334-48-5 | 1372 | 1000 [68] | soapy, waxy, fruity | ND | 2.26 ± 0.12 bc | 6.04 ± 0.52 e | 2.70 ± 0.10 cd | 2.42 ± 0.23 bd | 2.97 ± 0.19 d | 1.85 ± 0.10 b | MS, RI, S |
| 3 | 9-decenoic acid | 14436-32-9 | 1367 | 40 [52] | waxy, creamy, fatty | ND | 19.98 ± 0.80 c | 30.61 ± 2.92 d | 17.97 ± 2.00 bc | 17.95 ± 0.72 bc | 20.22 ± 0.54 c | 13.84 ± 1.27 b | MS, RI, S |
| 4 | 2-methyl-butanoic acid | 116-53-0 | 817 | 33 [70] | fruity | 4.70 ± 0.25 a | 16.83 ± 0.17 bc | 23.72 ± 0.63 d | 16.20 ± 1.60 b | 19.17 ± 1.17 c | 22.08 ± 0.80 d | 16.43 ± 1.08 b | MS, RI |
| 5 | 2-methyl- propanoic acid | 79-31-2 | 711 | 230 [68] | acidic, sour, cheesy | ND | ND | ND | ND | 2.98 ± 0.21 b | ND | ND | MS, RI |
| Aldehydes | |||||||||||||
| 1 | acetaldehyde | 75-07-0 | 408 | 186 [64] | pungent, fresh | ND | 8.17 ± 0.78 b | 11.24 ± 0.88 c | 7.63 ± 0.20 b | 8.60 ± 0.34 b | 7.78 ± 0.54 b | 7.18 ± 0.88 b | MS, RI, S |
| 2 | isovaleral | 590-86-3 | 643 | 0.35 [52] | fruity, dry, green, chocolate | 101.87 ± 7.35 c | 43.78 ± 4.22 ab | 49.68 ± 4.33 ab | 38.49 ± 3.08 a | 56.14 ± 6.47 b | 54.99 ± 5.25 b | 37.60 ± 3.28 a | MS, RI, S |
| 3 | 1-hexanal | 66-25-1 | 806 | 4.5 [52] | green, woody, vegetative | 19.69 ± 0.52 c | 8.14 ± 0.67 a | 19.16 ± 0.96 c | 7.87 ± 0.47 a | 9.50 ± 0.19 a | 13.05 ± 1.14 b | 9.41 ± 0.82 a | MS, RI, S |
| 4 | n-octanal | 124-13-0 | 1005 | 15 [52] | aldehyde, green | 5.56 ± 0.31 b | 4.65 ± 0.23 ab | 3.97 ± 0.12 a | 4.04 ± 0.50 a | 3.54 ± 0.18 a | 4.02 ± 0.20 a | 8.16 ± 0.83 c | MS, RI, S |
| 5 | nonylaldehyde | 124-19-6 | 1104 | 15 [52] | cucumber, melon, rindy | 4.03 ± 0.34 b | ND | ND | 4.33 ± 0.35 b | ND | 2.83 ± 0.10 ab | 58.17 ± 3.63 c | MS, RI |
| 6 | isobutanal | 78-84-2 | 543 | 6 [71] | fresh, herbal, green | 4.23 ± 0.40 d | ND | ND | 2.64 ± 0.07 c | ND | 1.18 ± 0.04 b | 1.32 ± 0.04 b | MS, RI, S |
| Ketones | |||||||||||||
| 1 | 3-octanone | 106-68-3 | 958 | 21.4 [52] | mushroom, ketonic, cheesy | ND | 1.77 ± 0.19 cd | 1.44 ± 0.01 bc | 2.15 ± 0.21 d | ND | 1.15 ± 0.11 b | 3.55 ± 0.34 e | MS, RI, S |
| Ethers | |||||||||||||
| 1 | dowanol peat | 111-35-3 | 837 | 100 [52] | fruit | ND | 1.34 ± 0.07 cd | 1.80 ± 0.08 e | 1.49 ± 0.08 d | 1.98 ± 0.16 e | 1.05 ± 0.04 b | 1.20 ± 0.12 bc | MS, RI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Q.; Guo, C.; Zhang, J.; Yan, Y.; Zhao, D.; Li, C.; Sun, X.; Ma, T.; Yue, T.; Yuan, Y. Effects of Simultaneous Co-Fermentation of Five Indigenous Non-Saccharomyces Strains with S. cerevisiae on Vidal Icewine Aroma Quality. Foods 2021, 10, 1452. https://doi.org/10.3390/foods10071452
Ge Q, Guo C, Zhang J, Yan Y, Zhao D, Li C, Sun X, Ma T, Yue T, Yuan Y. Effects of Simultaneous Co-Fermentation of Five Indigenous Non-Saccharomyces Strains with S. cerevisiae on Vidal Icewine Aroma Quality. Foods. 2021; 10(7):1452. https://doi.org/10.3390/foods10071452
Chicago/Turabian StyleGe, Qian, Chunfeng Guo, Jing Zhang, Yue Yan, Danqing Zhao, Caihong Li, Xiangyu Sun, Tingting Ma, Tianli Yue, and Yahong Yuan. 2021. "Effects of Simultaneous Co-Fermentation of Five Indigenous Non-Saccharomyces Strains with S. cerevisiae on Vidal Icewine Aroma Quality" Foods 10, no. 7: 1452. https://doi.org/10.3390/foods10071452
APA StyleGe, Q., Guo, C., Zhang, J., Yan, Y., Zhao, D., Li, C., Sun, X., Ma, T., Yue, T., & Yuan, Y. (2021). Effects of Simultaneous Co-Fermentation of Five Indigenous Non-Saccharomyces Strains with S. cerevisiae on Vidal Icewine Aroma Quality. Foods, 10(7), 1452. https://doi.org/10.3390/foods10071452