Abstract
Pneumoviruses include pathogenic human and animal viruses, the most known and studied being the human respiratory syncytial virus (hRSV) and the metapneumovirus (hMPV), which are the major cause of severe acute respiratory tract illness in young children worldwide, and main pathogens infecting elderly and immune-compromised people. The transcription and replication of these viruses take place in specific cytoplasmic inclusions called inclusion bodies (IBs). These activities depend on viral polymerase L, associated with its cofactor phosphoprotein P, for the recognition of the viral RNA genome encapsidated by the nucleoprotein N, forming the nucleocapsid (NC). The polymerase activities rely on diverse transient protein-protein interactions orchestrated by P playing the hub role. Among these interactions, P interacts with the NC to recruit L to the genome. The P protein also plays the role of chaperone to maintain the neosynthesized N monomeric and RNA-free (called N0) before specific encapsidation of the viral genome and antigenome. This review aims at giving an overview of recent structural information obtained for hRSV and hMPV P, N, and more specifically for P-NC and N0-P complexes that pave the way for the rational design of new antivirals against those viruses.
1. The Pneumoviridae Family
Pneumoviruses belong to the Mononegavirales order that includes many pathogenic human or animal viruses in 11 families, such as respiratory syncytial virus (RSV), metapneumovirus (MPV), Measles, Mumps, Rabies, Nipah, Ebola, and Vesicular stomatitis viruses (VSV) []. Mononegavirales have a non-segmented negative-sense RNA genome ranging from 13.2 to 15.3 kb. They form a large group exhibiting common genome organization and sharing similar replication mechanisms. Recently, the former paramyxoviral subfamily Pneumovirinae was elevated to family status Pneumoviridae []. This “new” family is composed of the two genera, Metapneumovirus and Orthopneumovirus (Table 1) [].

Table 1.
Phylogeny of Pneumoviridae.
The Metapneumovirus genus includes human metapneumovirus (hMPV) and avian metapneumovirus (aMPV). The Orthopneumovirus genus groups human respiratory syncytial virus (hRSV), bovine respiratory syncytial virus (bRSV), and pneumonia virus of mice (PVM). Although unclassified by the International Committee on Taxonomy of Viruses (ICTV), this taxon also includes ovine respiratory syncytial virus (ORV) and canine pneumovirus (CPV). More recently, an eighth pneumovirus was identified by metagenomic sequencing of pooled nasal swabs in feral swine in the USA []. This newly identified Orthopneumovirus shows 93% and 91% protein identities with PVM and CPV, respectively, and was named swine orthopneumovirus (SOV). Amino acid sequence identities between nucleoproteins of SOV and other pneumoviruses are 59.8% for hRSV, 60% for bRSV, 45.7% for hMPV, and 43.3% for aMPV, respectively, indicating that the PVM/SOV group is distinct from Meta- and Ortho-pneumoviruses and could constitute a third genus.
2. Impact of Infections by Pneumoviridae
Viruses belonging to the Pneumoviridae family cause severe respiratory diseases in humans and animals. Among them, hRSV and hMPV are the main cause of bronchiolitis and pneumonia in young children (<5 years) [,,,]. hRSV infects nearly 100% of children in the first three years of life and is one of the principal causes of child hospitalizations. Worldwide, hRSV is estimated to be responsible for ~33 million acute lower respiratory infections (ALRI), resulting in more than 3.2 million ALRI-related hospitalizations and 118,200 deaths in children under 5 years []. In a recent systemic multisite study, hRSV was shown to be the first etiological agent responsible for severe pneumonia (more than 30%) in hospitalized children in Asia and Africa []. It is noteworthy that hRSV is also a frequent cause of otitis in infants [] and that children who suffer from severe hRSV infection are at risk of developing further respiratory complications such as asthma [].
After hRSV, hMPV is considered the second most common cause of ALRI in young children [,,]. Isolated in 2001 in the Netherlands [], it is thought to have derived from avian metapneumovirus (aMPV) subgroup C, 200 years ago []. The peak age of hospitalization for infants infected by hMPV occurs between 6–12 months, slightly later than the peak of hRSV, which is around 2–3 months. The clinical features and severity of hMPV are similar to those of hRSV. Furthermore, hRSV and hMPV are now recognized as being responsible for significant morbidity and mortality in elderly and immunocompromised persons, such as bone marrow transplant patients (with comparable disease burden to influenza) [,,,,]. These viruses are seasonal, the peak of infection typically extending from early fall to early spring. In 2020, the emergence of coronavirus disease (COVID-19) triggered the large-scale implementation of non-pharmaceutical interventions such as confinement, mask-wearing, and extensive handwashing []. These preventive public health measures have had an impact on the circulation of diverse pathogens, specifically hRSV, as evidenced by the interseasonal epidemics of hRSV in several countries of the southern hemisphere and late epidemics of hRSV in the USA, Japan, and several European countries [,,,]. For example, the 2020–2021 bronchiolitis epidemic in mainland France lasted 15 weeks, comparable to the previous season, but with a delayed peak, 13 weeks later than that of the previous season, with a much lower amplitude. The proportion of hospitalizations for bronchiolitis has been comparable to that of recent seasons, but notable features of the 2020/21 season were a decrease in the proportion of cases over 65 years of age and an increase in the proportion of cases in children over 3 months and up to 5 years. In addition to the resurgence of hRSV and hMPV infections since March 2021, data indicate more severe illness in younger infants, possibly because of reduced immunity due to lack of exposure to these viruses in the previous season.
Finally, the Pneumoviridae family is also an important threat for livestock farming and has a strong economic impact, bRSV, and aMPV causing severe respiratory diseases in calves and poultry, respectively [,]. These infections are responsible for important animals‘ morbidity, leading to high mortality rates, mainly due to opportunistic infections by other viruses or bacteria [,]. To limit this, the current care consists of antibiotic administration during epidemics, which represents an indirect risk for animal and human health due to the emergence of resistant bacteria. In addition, the discovery of SOV in the USA suggests that yet unknown pneumoviruses could be responsible for respiratory diseases in other animal species. A recent study suggested a high prevalence of this virus in France []. However, further studies are required to determine whether this virus is pathogenic for pigs.
3. Treatments against Pneumoviruses
No vaccine is available against hRSV and hMPV. Although several vaccines against bRSV and aMPV are commercialized, their efficacy remains limited [,,,,]. In this context, the development of antiviral drugs with a wide spectrum represents an alternative to human vaccination. This is especially true because vaccine development against hRSV and hMPV is hampered by the fact that these viruses mostly infect infants who have an immature immune system. Furthermore, as hRSV is 80% fatal to immunocompromised and transplanted patients and a significant cause of death in the elderly, the elaboration of antiviral strategies is a recognized necessity. So far, no specific inhibitors are commercially available against these viruses, ribavirin being used only exceptionally because of its toxicity and poor efficiency. A humanized monoclonal antibody directed against the surface fusion F glycoprotein (palivizumab Synagis®) is also available as a preventive treatment, but its efficiency is limited (≈50%), and its high cost restricts its use to high-risk infants []. Approximately 6000 children are treated with Synagis each year in France, with a cost of EUR 8000 per child (five injections). There is, therefore, a need for new and cheaper treatments, which implies the critical necessity to better understand the molecular mechanisms of virus replication. To date, most developed antiviral strategies aim at targeting the F protein to impair virus entry [,,,]. The viral polymerase L that is responsible for the enzymatic activities required for viral replication and transcription is the second main target of interest [,]. Among the developed compounds, the two fusion inhibitors, GS-5806 and JNJ-53718678, and the polymerase inhibitor ALS-008176, have been tested in humans [,,]. However, the results of phase 2b trials of GS-5806 were disappointing [,], and clinical trials of ALS-008176 have recently been halted. The emergence of escape mutants upon treatment represents the main restriction and highlights the necessity to identify new targets and to associate different compounds. The functioning of the viral polymerase depends on different highly conserved transient protein-protein interactions (PPIs) that have no counterparts in cells. These viral PPIs being transient and of low affinity, molecules of high affinity that could compete with them may represent a new class of inhibitors. Furthermore, these interactions are now structurally well-characterized, allowing the rational structure-based design of antivirals.
4. Virions and Viral Cycle
Pneumoviruses are enveloped viruses, the virions having pleomorphic but mostly filamentous shapes [,,]. Their genomes contain 8 to 10 genes that encode 9 and 11 proteins in the case of MPV and RSV, respectively (Figure 1A). The two non-structural proteins NS1 and NS2 of RSV, which are involved in the control of antiviral pathways during infection [,], have no counterparts in MPV. The virions present three transmembrane proteins: the glycoprotein (G) involved in virion attachment to the cell surface, the fusion (F) protein responsible for receptor binding and fusion between viral and cellular membranes, and the small hydrophobic protein (SH), a viroporin whose immunomodulatory role still remains unclear [,,] (Figure 1B). The inner side of the viral membrane is lined by the matrix (M) protein. The viral particles contain the genomic RNA encapsidated by the N protein, forming the nucleocapsid (NC), which is associated with the P-L-M2-1 proteins.
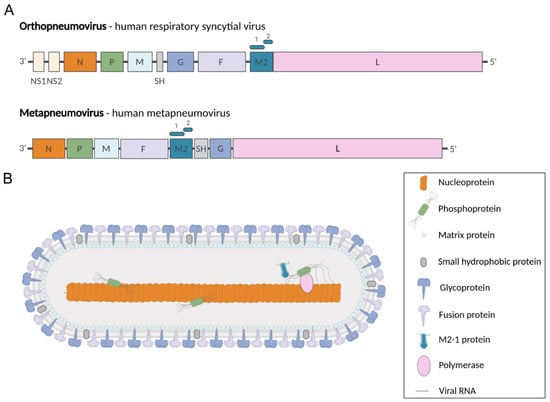
Figure 1.
Pneumoviridae genomes and virion. (A) Genome organization of representative members of the Pneumoviridae family. Genomic RNAs are presented in sense (coding) orientation (3′-to-5′), with each box representing a gene encoding a separate mRNA drawn approximately to scale. The M2 gene encodes M1-2 and M2-2 proteins (represented by rectangles above M2 gene). (B) Representation of pneumovirus viral particle showing the structural proteins. Created with BioRender.com.
After fusion of the viral envelope with the cell membrane, the viral NC penetrates into the cytoplasm, where viral RNA transcription and replication occur (Figure 2). The viral RNA-dependent RNA polymerase (RdRp) L, associated with its cofactor P, is responsible for both activities [,]. Transcription of RSV also requires the viral protein M2-1, which acts as an anti-terminator/elongation factor [,], whereas MPV M2-1 is not essential for virus replication in cell culture []. During transcription, the RdRp has all the activities to transcribe, cap, and poly-adenylate mRNAs. Amplification of the viral genome by the RdRp necessitates the synthesis of an antigenome, which is also encapsidated by N. At the final stage of the viral cycle, NCs assemble with the other structural viral proteins at the cell surface to generate new virions (Figure 2).
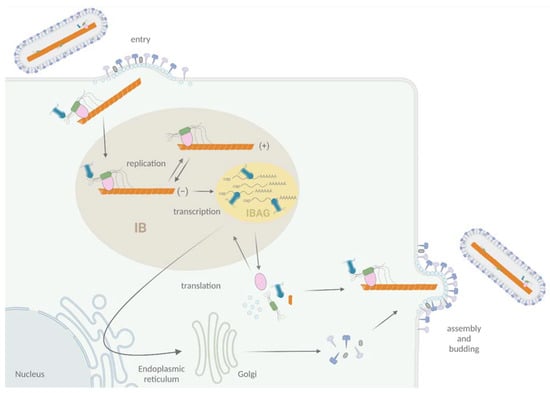
Figure 2.
Schematic representation of the viral cycle of Pneumoviruses. Virion attachment to the cell is mediated by F and G proteins. The F protein is responsible for the fusion of viral and cell membranes, leading to the delivery in the cytoplasm of the NC complexed with L, P, and M2-1 proteins. Transcription and replication occur in membrane-less organelles called cytoplasmic inclusions bodies (IBs, light brown). Within IBs, M2-1 and viral mRNAs accumulate into sub-structures called inclusion body-associated granules (IBAGs, yellow). After viral protein production and genome replication, assembly and budding of new viral particles take place at the plasma membrane. Adapted from “Replication Cycle”, by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates (30 November 2021).
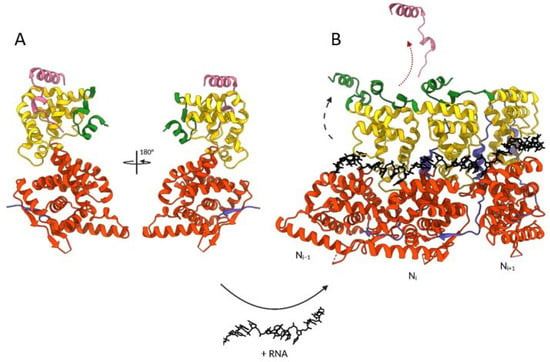
More specifically, viral replication and transcription take place in cytoplasmic inclusions bodies (IBs), where all the proteins required for the activities of the RdRp concentrate [] (Figure 2). These structures, also observed for others Mononegavirales, are membrane-less organelles that present liquid-like properties [,], and expression of N and P was shown to be sufficient to induce the formation of pseudo-IBs [,]. These IBs contain dynamic sub-compartments called IBAGs (IB-associated granules), where viral mRNA and the transcription factor M2-1 specifically accumulate [] (Figure 2). It is noteworthy that hRSV proteins NS2 and M were also shown to localize to IBs [,,]. Furthermore, different cellular proteins such as HSP70, actin, actin-associated proteins, translation initiation factors PABP, and eIF4G, as well as the phosphatase PP1, were shown to be recruited to IBs [,,]. In particular, N was shown to interact with proteins involved in innate immune pathways such as MAVS, MDA5, and more recently, the subunit p65 of NF-κB, leading to their sequestration into IBs [,]. Thus, there is accumulating evidence that IBs are complex organelles that play a central role in the viral cycle, not only for viral RNA synthesis but also as platforms for the traffic of NCs from IBs to the plasma membrane and for assembly, as well as in the regulation of cellular innate immune responses to infection.
5. The Replication/Transcription Machinery of Pneumoviruses
The RdRp functioning depends on different PPIs, with the phosphoprotein P acting as a hub to recruit many partners, and more particularly by interacting with NC, L, M2-1, and the neosynthesized N (N0) (Figure 3).
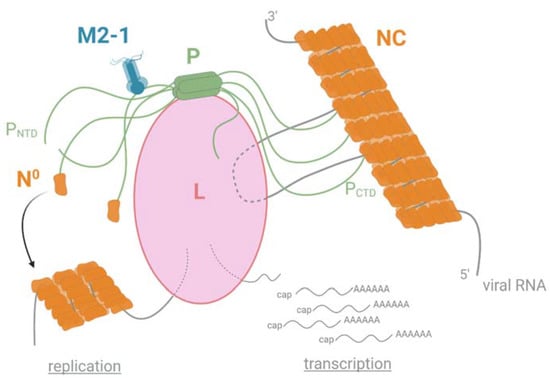
Figure 3.
Schematic representation of the polymerase functioning of pneumoviruses. The polymerase L is responsible for both viral replication and transcription. The P protein plays a role in the hub by interacting with L and NC through its C-terminal PCTD domain and with M2-1 and the monomeric and RNA-free N (N0) through its N-terminal PNTD domain. Created with BioRender.com (30 November 2021).
The last decades were marked by the accumulation of structural and functional information on the pneumoviral RdRp. The main achievement was the recent determination of the 3D structure, although partial, of the L-P complexes of hRSV and hMPV by cryo-electron microscopy [,]. These structures revealed strong structural conservation between these two complexes, with a particular mode of P binding to L (see Section 6.1). They allowed establishing a model for the spatial functioning of L []. The structure and activities of the L protein will not be discussed extensively here. Briefly, the RdRp recognizes and uses the viral RNA genome as a template exclusively when it is encapsidated by N inside a flexible helical NC (Figure 3). This recognition is mediated by P, which is essential for loading the L polymerase onto the NC template and for keeping it bound to its template in a dynamic fashion during RNA synthesis.
The L protein embeds all enzymatic activities required for replication and transcription. The L protein contains an RdRp domain followed by a polyribonucleotidyl-transferase domain (PRNTase or capping domain) and a methyltransferase (MTase) domain. During transcription, the L protein scans the viral RNA, and mRNA synthesis begins at a conserved gene start sequence (GS). When the RNA is about 30 nucleotides long, a GMP moiety, covalently linked to the PRNTase domain of L, is transferred to the 5′ end of the nascent viral RNA, forming a cap structure (GpppG-RNA). The cap is subsequently methylated on its 2′ O and N7 position (N7GpppGm-RNA) by the MTase activity of L []. It is noteworthy that, although the mechanism still remains poorly understood, efficient hRSV transcription requires the recruitment of the M2-1 protein by P [,]. During replication, the RdRp synthetises antigenomes and genomes that are concomitantly encapsidated by the N protein. The assembly of new functional viral genomes requires a continuous supply of unassembled N molecules (N0). The P protein is an essential co-factor in this process by forming an N0-P complex to maintain N in a competent form for the encapsidation of new viral genomes (Figure 3).
6. Insights into P and N Protein Structures
The P and N proteins are the two main actors of the polymerase complex. Besides their direct role in viral RNA synthesis, they were shown to be the scaffold proteins responsible for IB morphogenesis [,]. This architectural role of P and N for IB formation was recently shown to depend on a liquid-liquid phase separation (LLPS) mechanism, requiring N-P interaction []. The LLPS mechanism is now well characterized. It is initiated by scaffold molecules that form condensates through the establishment of a network of interactions, more frequently, proteins and RNA. The archetype of protein architecture sustaining the formation of the LLPS relies on proteins with intrinsically disordered regions (IDRs) presenting multiple interacting motifs of low affinity [,,] and RNA-interacting domains. The pneumoviruses P proteins, which present different IDRs and interact with NC, appear to be the pivotal element for IB morphogenesis [].
6.1. The Modular Structure of the Phosphoprotein P
Pneumoviral P proteins play a central role during the virus cycle, their high plasticity allowing the establishment of transient and complex interactions with various partners. Both hRSV and hMPV P proteins (of 241 and 294 residues, respectively) form parallel tetramers with a central oligomerization domain (POD) consisting of a helical coiled-coil core, flanked by two intrinsically disordered regions (PNTD and PCTD) (Figure 4A) [,,,,]. Sequence alignment of the hRSV and hMPV phosphoproteins indicates a sequence identity and similarity of 28% and 38%, respectively, as calculated with the Sequence Manipulation Suite using an alignment made on the T-coffee server []. POD displays very high conservation with 65% identity and 80% similarity between hRSV and hMPV. PCα, a subdomain of PCTD with a high helical propensity, has 41% identity and 52% similarity. The PNTD domain is longer in hMPV P than in hRSV P, but the N-terminus and the region proximal to the oligomerization domain also present conserved motifs that are likely molecular recognition elements (Figure 4A).
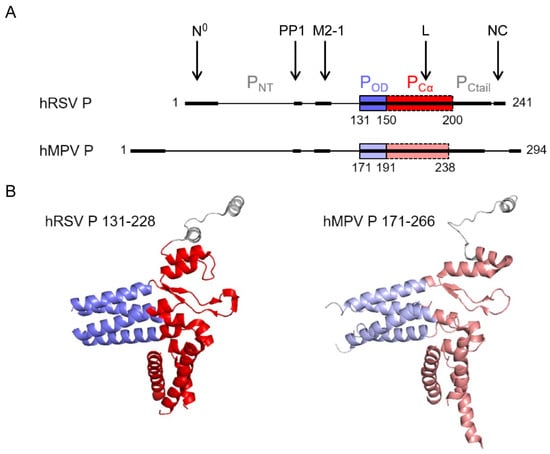
Figure 4.
Structure of Pneumovirus P proteins. (A) Domain architecture of hRSV and hMPV P proteins, with a fully disordered N-terminal domain, PNTD, a short tetrameric coiled-coil oligomerization domain, POD (blue), and a C-terminal domain, PCTD, consisting of a domain with a high α-helical propensity, PCα (red), and a highly disordered C-terminal tail, PCtail. The interaction regions of hRSV P with RdRp, or associated proteins like PP1, are indicated by arrows and bold lines. The corresponding regions in hMPV are also in bold lines. (B) High-resolution cryo-EM structures of the tetrameric L-associated hRSV and hMPV P proteins. Only the POD and PCα domains are observed in the L-P complex structures. Neither PNTD nor PCtail, except for a single protomer, could be observed due to high disorder. Created with Pymol (https://pymol.org, 30 November 2021).
NMR proved to be a well-suited tool to obtain structural data of hRSV P alone in solution, and of its intrinsically disordered domains, in particular. This revealed that although these domains were not stably folded, several regions of PNTD and PCTD presented a propensity to form transient α-helices, likely to be involved in various PPIs [,,]. These results were confirmed by interaction studies between P and N or M2-1 [,,], but also by resolution of the 3D structures of P fragments in the complex with N0 [], M2-1 [], and L [,]. Whereas the interactions with N0 and M2-1 were shown to involve short linear motifs of the PNTD domain that fold into helices upon binding, the recent structures of L-P complexes revealed that both POD and PCTD, the latter mostly through PCα, interacts with L []. The location of these binding sites is indicated in Figure 4A. Interestingly, each PCTD in the P tetramer was shown to adopt a specific and different conformation in contact with L (Figure 4B). However, the conformation of the L-bound hRSV and hMPV PCTD tetramers is strikingly similar: structural alignment yields an RMSD of 1.224 Å. Of note, a recent study revealed that the hRSV P-M interaction involves both PNTD and POD []. In contrast to these extended binding regions, the linear sequence corresponding to the last C-terminal residues of P was shown to be sufficient for binding to NC [,,].
It is noteworthy that major and minor sites of phosphorylation were identified on hRSV P, with two main clusters of phosphorylated serines S116/S117/S119 and S232/S237 [,]. The phosphorylation status of P was shown to depend on cellular casein kinase II [] and phosphatases PP1 and PP2A []. Although the role of these post-translational modifications during replication and transcription remains unclear [,,], phosphorylation of hRSV P was shown to regulate its interaction with N and M2-1 [,,,]. hRSV P protein was also shown to recruit the phosphatase PP1 to IBs []. This interaction involves an RVxF-like motif of P located nearby and upstream of the M2-1 binding region (Figure 4A), which is conserved in hMPV P. Through its interaction with hRSV P, PP1 is involved in M2-1 dephosphorylation required for the efficient functioning of M2-1 and viral transcription. Therefore, phosphorylation is critical for the regulation of PPIs within the polymerase complex and efficient functioning.
6.2. Structure of Nucleoproteins
The N protein, which is responsible for genome and antigenome encapsidation, is composed of 391 and 394 residues for hRSV and hMPV, respectively. This protein has a high binding affinity for RNA coupled with a strong tendency to oligomerize. The 3D crystal structure of the hRSV N expressed in E. coli and purified as annular ribonucleoprotein complexes composed of 10 N proteins bound to RNA (N-RNA rings) was first obtained in 2009 (Figure 5A) []. More recently, the structure of oligomeric N of hMPV, also purified as N-RNA rings, was also obtained []. These structures revealed strong structural conservation: N proteins have N- and C-terminal globular domains (NNTD and NCTD, respectively) separated by a hinge region that forms the RNA-binding groove. Two flexible arms located at the N- and C-terminus of the protein bind to adjacent N protomers and rigidify the structure (Figure 5A).
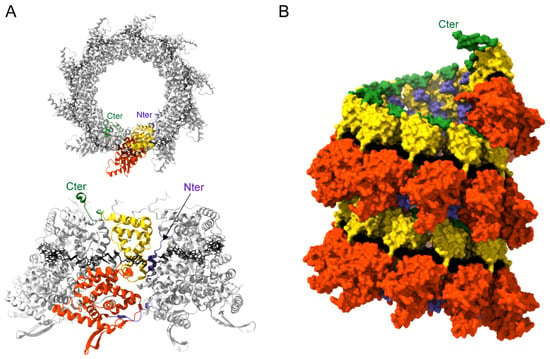
Figure 5.
Structure of Pneumovirus N proteins. (A) Top (upper panel) and side (lower panel) views of hRSV N-RNA rings composed of 10 N proteins bound to RNA, purified from E. coli (PDB: 2WJ8 and 5FVC, respectively). N monomers are represented in ribbons; one N subunit is colored with the NNTD in orange, NCTD in yellow, and the N- and C-terminal parts in blue and green, respectively. The RNA is represented with the bases in black. N- and C-terminal extremities are indicated. (B) Left-handed N-RNA helix model (PDB: 4BKK). N monomers and RNA (black) atoms are shown as surfaces. One N subunit is colored with the NNTD in orange, NCTD in yellow, and the N- and C-terminal parts in blue and green, respectively. The C-terminal extremity of the N monomer at the top of the helix model is annotated. Created with UCSF ChimeraX [].
The RNA wraps around the N protein ring in a basic groove, with seven nucleotides contacting each N monomer. The RNA is constrained and twisted by the N proteins, alternating rows of four and three stacked bases that are exposed and buried within the protein groove, respectively. Surprisingly, N-RNA rings, which were considered artifacts of production/purification, were recently found together with NCs in viral particles []. This raises the question of the potential role of these oligomers during the viral cycle. Electron microscopy analysis of hRSV NCs expressed in insect cells as well as cryotomography performed on viral particles revealed that these are left-handed helices [,]. Although the resolution of the helix was low, an atomic model of a left-handed RSV NC was generated (Figure 5B). These data allowed us to gain information on the interactions between N protomers of successive helix turns, and more importantly, to reveal that the 3′ end of the RSV genome is located at the pointed end of the NC. The structure of NC at high resolution still remains to be established to gain information on the mechanism sustaining the encapsidation of the viral genome and those required to allow genome accessibility to the polymerase.
Finally, during viral replication, the neo-synthesized N is maintained monomeric and RNA-free (N0) by P, which acts as a chaperone (see Section 7.2). Compared to the oligomeric form, N0 is characterized by a weak rotation of the NNTD relative to the NCTD and by the interaction of the N C-arm with the RNA groove, thereby preventing RNA binding.
8. Conclusions
The recent advances in the structure of the viral proteins associated with the L polymerase of pneumoviruses pave the way for the development of new antiviral strategies. Of particular interest, the PPIs required for the polymerase functioning represent potential targets for the design of new classes of antivirals. Indeed, these viral interactions are highly specific and have no cellular counterparts, suggesting that inhibitors should have limited off-target activity. Furthermore, because these interactions are transient and of low affinity, molecules of higher affinity should efficiently compete with the native mimicked sequence. Among these PPIs, the two modes of N-P interactions, which are now well characterized and relatively conserved between hRSV and hMPV, can be targeted using rational structure-based approaches. As described here, the design of potent inhibitors will depend on the nature of the PPIs: whereas small molecules seem promising to target the P binding site on oligomeric N and N-RNA complexes, such as the helical NCs, small peptides seem more adapted to inhibit the N0-P interaction. Similar approaches could be used to target L-P or M2-1-P interactions. It is noteworthy that these antiviral strategies could be applied to other Mononegavirales. Given the emergence of resistant escape viruses upon treatment with both anti-F and anti-L inhibitors, combinations of molecules directed against different viral targets may be required for efficient and long-term treatment.
Author Contributions
Writing original draft preparation, H.D., L.G., C.S., and M.G.; writing—review and editing, C.S., I.G., J.-F.E., and M.G.; supervision, M.G.; funding acquisition, C.S., J.-F.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the French Agence Nationale de la Recherche, generic ANR Antibronchio n° ANR-19-CE18-0012-01 and ANR DecRisP n° ANR-19-CE11-0017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L.F. Problems of classification in the family Paramyxoviridae. Arch. Virol. 2018, 163, 1395–1404. [Google Scholar] [CrossRef]
- Afonso, C.L.; Amarasinghe, G.K.; Banyai, K.; Bao, Y.; Basler, C.F.; Bavari, S.; Bejerman, N.; Blasdell, K.R.; Briand, F.X.; Briese, T.; et al. Taxonomy of the order Mononegavirales: Update 2016. Arch. Virol. 2016, 161, 2351–2360. [Google Scholar] [CrossRef]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L.; et al. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef]
- Hause, B.M.; Padmanabhan, A.; Pedersen, K.; Gidlewski, T. Feral swine virome is dominated by single-stranded DNA viruses and contains a novel Orthopneumovirus which circulates both in feral and domestic swine. J. Gen. Virol. 2016, 97, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Crowe, J.E. Respiratory Syncytial Virus and Metapneumovirus. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1601–1646. [Google Scholar]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Deloria-Knoll, M.; Madhi, S.A.; Cohen, C.; Arguelles, V.L.; Basnet, S.; Bassat, Q.; Brooks, W.A.; Echavarria, M.; et al. Global burden of acute lower respiratory infection associated with human parainfluenza virus in children younger than 5 years for 2018: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e1077–e1087. [Google Scholar] [CrossRef]
- van den Hoogen, B.G.; Osterhaus, D.M.; Fouchier, R.A. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr. Infect. Dis. J. 2004, 23 (Suppl. S1), 25–32. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet 2019, 394, 757–779. [Google Scholar]
- Phillips, M.; Finelli, L.; Saiman, L.; Wang, C.; Choi, Y.; Patel, J. Respiratory Syncytial Virus-associated Acute Otitis Media in Infants and Children. J. Pediatric Infect. Dis. Soc. 2020, 9, 544–550. [Google Scholar] [CrossRef]
- Shi, T.; Ooi, Y.; Zaw, E.M.; Utjesanovic, N.; Campbell, H.; Cunningham, S.; Bont, L.; Nair, H.; Investigators, R. Association Between Respiratory Syncytial Virus-Associated Acute Lower Respiratory Infection in Early Life and Recurrent Wheeze and Asthma in Later Childhood. J. Infect. Dis. 2020, 222 (Suppl. 7), 628-–633. [Google Scholar] [CrossRef]
- Papenburg, J.; Alghounaim, M. Unraveling the Pneumonia Burden Associated with Human Metapneumovirus Infection. Clin. Infect. Dis. 2021, 72, 118–120. [Google Scholar] [CrossRef]
- van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001, 7, 719–724. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.; Osterhaus, A.; Fouchier, R.A.M.; Holmes, E.C. Evolutionary dynamics of human and avian metapneumoviruses. J. Gen. Virol. 2008, 89, 2933–2942. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Asner, S.; Stephens, D.; Pedulla, P.; Richardson, S.E.; Robinson, J.; Allen, U. Risk factors and outcomes for respiratory syncytial virus-related infections in immunocompromised children. Pediatr. Infect. Dis. J. 2013, 32, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.N.; Chemaly, R.F. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011, 117, 2755–2763. [Google Scholar] [CrossRef]
- Uddin, S.; Thomas, M. Human Metapneumovirus; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cowling, B.J.; Ali, S.T.; Ng, T.W.Y.; Tsang, T.K.; Li, J.C.M.; Fong, M.W.; Liao, Q.; Kwan, M.Y.; Lee, S.L.; Chiu, S.S.; et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: An observational study. Lancet Public Health 2020, 5, e279–e288. [Google Scholar] [CrossRef]
- Foley, D.A.; Phuong, L.K.; Peplinski, J.; Lim, S.M.; Lee, W.H.; Farhat, A.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; et al. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch. Dis. Child. 2021, 1–7. [Google Scholar] [CrossRef]
- Agha, R.; Avner, J.R. Delayed Seasonal RSV Surge Observed During the COVID-19 Pandemic. Pediatrics 2021, 148, e2021052089. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M.; Tsuzuki, S.; Nakamoto, T.; Iwamoto, N. Resurgence of Respiratory Syncytial Virus Infections during COVID-19 Pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021, 27, 2969–2970. [Google Scholar] [CrossRef]
- Di Mattia, G.; Nenna, R.; Mancino, E.; Rizzo, V.; Pierangeli, A.; Villani, A.; Midulla, F. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr. Pulmonol. 2021, 56, 3106–3109. [Google Scholar] [CrossRef] [PubMed]
- Easton, A.J.; Domachowske, J.B.; Rosenberg, H.F. Animal pneumoviruses: Molecular genetics and pathogenesis. Clin. Microbiol. Rev 2004, 17, 390–412. [Google Scholar] [CrossRef]
- Valarcher, J.F.; Taylor, G. Bovine respiratory syncytial virus infection. Vet. Res. 2007, 38, 153–180. [Google Scholar] [CrossRef]
- Giovanardi, D.; Lupini, C.; Pesente, P.; Rossi, G.; Ortali, G.; Catelli, E. Longitudinal field studies of avian metapneumovirus and turkey hemorrhagic enteritis virus in turkeys suffering from colibacillosis associated mortality. Vet. Res. Commun. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Makoschey, B.; Berge, A.C. Review on bovine respiratory syncytial virus and bovine parainfluenza—Usual suspects in bovine respiratory disease—A narrative review. BMC Vet. Res. 2021, 17, 261. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.A.; Hervet, C.; Menard, D.; Gutsche, I.; Normand, V.; Renois, F.; Meurens, F.; Eleouet, J.F. First demonstration of the circulation of a pneumovirus in French pigs by detection of anti-swine orthopneumovirus nucleoprotein antibodies. Vet. Res. 2018, 49, 118. [Google Scholar] [CrossRef]
- Ellis, J.A. How efficacious are vaccines against bovine respiratory syncytial virus in cattle? Vet. Microbiol. 2017, 206, 59–68. [Google Scholar] [CrossRef]
- Valarcher, J.F.; Hagglund, S.; Naslund, K.; Jouneau, L.; Malmstrom, E.; Boulesteix, O.; Pinard, A.; Leguere, D.; Deslis, A.; Gauthier, D.; et al. Single-Shot Vaccines against Bovine Respiratory Syncytial Virus (BRSV): Comparative Evaluation of Long-Term Protection after Immunization in the Presence of BRSV-Specific Maternal Antibodies. Vaccines 2021, 9, 236. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Y.; Rauf, A.; Zhang, Y.; Ma, Y.; Zhang, X.; Shilo, K.; Yu, Q.; Saif, Y.M.; Lu, X.; et al. Methyltransferase-defective avian metapneumovirus vaccines provide complete protection against challenge with the homologous Colorado strain and the heterologous Minnesota strain. J. Virol. 2014, 88, 12348–12363. [Google Scholar] [CrossRef]
- Ball, C.; Forrester, A.; Herrmann, A.; Lemiere, S.; Ganapathy, K. Comparative protective immunity provided by live vaccines of Newcastle disease virus or avian metapneumovirus when co-administered alongside classical and variant strains of infectious bronchitis virus in day-old broiler chicks. Vaccine 2019, 37, 7566–7575. [Google Scholar] [CrossRef]
- Collins, P.L.; Melero, J.A. Progress in understanding and controlling respiratory syncytial virus: Still crazy after all these years. Virus Res. 2011, 162, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, G.S.; Good, J.A.D.; Mathews, N. State of the Art in Respiratory Syncytial Virus Drug Discovery and Development. J. Med. Chem. 2019, 62, 3206–3227. [Google Scholar] [CrossRef]
- Detalle, L.; Stohr, T.; Palomo, C.; Piedra, P.A.; Gilbert, B.E.; Mas, V.; Millar, A.; Power, U.F.; Stortelers, C.; Allosery, K.; et al. Generation and Characterization of ALX-0171, a Potent Novel Therapeutic Nanobody for the Treatment of Respiratory Syncytial Virus Infection. Antimicrob. Agents Chemother. 2015, 60, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Rusch, S.; DeVincenzo, J.; Kim, Y.I.; Harrison, L.; Meals, E.A.; Boyers, A.; Fok-Seang, J.; Huntjens, D.; Lounis, N.; et al. Antiviral Activity of Oral JNJ-53718678 in Healthy Adult Volunteers Challenged With Respiratory Syncytial Virus: A Placebo-Controlled Study. J. Infect. Dis. 2018, 218, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, G.S. JNJ-5371678, Defining a Role for Fusion Inhibitors in the Treatment of Respiratory Syncytial Virus. J. Med. Chem. 2020, 63, 8043–8045. [Google Scholar] [CrossRef] [PubMed]
- DeVincenzo, J.P.; Whitley, R.J.; Mackman, R.L.; Scaglioni-Weinlich, C.; Harrison, L.; Farrell, E.; McBride, S.; Lambkin-Williams, R.; Jordan, R.; Xin, Y.; et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. New Engl. J. Med. 2014, 371, 711–722. [Google Scholar] [CrossRef]
- DeVincenzo, J.P.; McClure, M.W.; Symons, J.A.; Fathi, H.; Westland, C.; Chanda, S.; Lambkin-Williams, R.; Smith, P.; Zhang, Q.; Beigelman, L.; et al. Activity of Oral ALS-008176 in a Respiratory Syncytial Virus Challenge Study. New Engl. J. Med. 2015, 373, 2048–2058. [Google Scholar] [CrossRef]
- Wang, G.; Deval, J.; Hong, J.; Dyatkina, N.; Prhavc, M.; Taylor, J.; Fung, A.; Jin, Z.; Stevens, S.K.; Serebryany, V.; et al. Discovery of 4’-chloromethyl-2’-deoxy-3’,5’-di-O-isobutyryl-2’-fluorocytidine (ALS-8176), a first-in-class RSV polymerase inhibitor for treatment of human respiratory syncytial virus infection. J. Med. Chem. 2015, 58, 1862–1878. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Dadwal, S.S.; Bergeron, A.; Ljungman, P.; Kim, Y.J.; Cheng, G.S.; Pipavath, S.N.; Limaye, A.P.; Blanchard, E.; Winston, D.J.; et al. A Phase 2, Randomized, Double-blind, Placebo-Controlled Trial of Presatovir for the Treatment of Respiratory Syncytial Virus Upper Respiratory Tract Infection in Hematopoietic-Cell Transplant Recipients. Clin. Infect. Dis. 2020, 71, 2777–2786. [Google Scholar] [CrossRef]
- Marty, F.M.; Chemaly, R.F.; Mullane, K.M.; Lee, D.G.; Hirsch, H.H.; Small, C.B.; Bergeron, A.; Shoham, S.; Ljungman, P.; Waghmare, A.; et al. A Phase 2b, Randomized, Double-blind, Placebo-Controlled Multicenter Study Evaluating Antiviral Effects, Pharmacokinetics, Safety, and Tolerability of Presatovir in Hematopoietic Cell Transplant Recipients with Respiratory Syncytial Virus Infection of the Lower Respiratory Tract. Clin. Infect. Dis. 2020, 71, 2787–2795. [Google Scholar] [PubMed]
- Kiss, G.; Holl, J.M.; Williams, G.M.; Alonas, E.; Vanover, D.; Lifland, A.W.; Gudheti, M.; Guerrero-Ferreira, R.C.; Nair, V.; Yi, H.; et al. Structural analysis of respiratory syncytial virus reveals the position of M2-1 between the matrix protein and the ribonucleoprotein complex. J. Virol. 2014, 88, 7602–7617. [Google Scholar] [CrossRef]
- Jeffree, C.E.; Rixon, H.W.; Brown, G.; Aitken, J.; Sugrue, R.J. Distribution of the attachment (G) glycoprotein and GM1 within the envelope of mature respiratory syncytial virus filaments revealed using field emission scanning electron microscopy. Virology 2003, 306, 254–267. [Google Scholar] [CrossRef]
- Ke, Z.; Dillard, R.S.; Chirkova, T.; Leon, F.; Stobart, C.C.; Hampton, C.M.; Strauss, J.D.; Rajan, D.; Rostad, C.A.; Taylor, J.V.; et al. The Morphology and Assembly of Respiratory Syncytial Virus Revealed by Cryo-Electron Tomography. Viruses 2018, 10, 446. [Google Scholar] [CrossRef]
- Thornhill, E.M.; Verhoeven, D. Respiratory Syncytial Virus’s Non-structural Proteins: Masters of Interference. Front. Cell Infect. Microbiol. 2020, 10, 225. [Google Scholar] [CrossRef]
- Sedeyn, K.; Schepens, B.; Saelens, X. Respiratory syncytial virus nonstructural proteins 1 and 2: Exceptional disrupters of innate immune responses. PLoS Pathog. 2019, 15, e1007984. [Google Scholar] [CrossRef]
- Carter, S.D.; Dent, K.C.; Atkins, E.; Foster, T.L.; Verow, M.; Gorny, P.; Harris, M.; Hiscox, J.A.; Ranson, N.A.; Griffin, S.; et al. Direct visualization of the small hydrophobic protein of human respiratory syncytial virus reveals the structural basis for membrane permeability. FEBS Lett. 2010, 584, 2786–2790. [Google Scholar] [CrossRef]
- Russell, R.F.; McDonald, J.U.; Ivanova, M.; Zhong, Z.; Bukreyev, A.; Tregoning, J.S. Partial Attenuation of Respiratory Syncytial Virus with a Deletion of a Small Hydrophobic Gene Is Associated with Elevated Interleukin-1beta Responses. J. Virol. 2015, 89, 8974–8981. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The Human Immune Response to Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef]
- Fearns, R.; Plemper, R.K. Polymerases of paramyxoviruses and pneumoviruses. Virus Res. 2017, 234, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Hill, M.G.; Camargo, E.; Grosfeld, H.; Chanock, R.M.; Murphy, B.R. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5’ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. USA 1995, 92, 11563–11567. [Google Scholar]
- Fearns, R.; Collins, P.L. Role of the M2-1 transcription antitermination protein of respiratory syncytial virus in sequential transcription. J. Virol. 1999, 73, 5852–5864. [Google Scholar] [CrossRef]
- Buchholz, U.J.; Biacchesi, S.; Pham, Q.N.; Tran, K.C.; Yang, L.; Luongo, C.L.; Skiadopoulos, M.H.; Murphy, B.R.; Collins, P.L. Deletion of M2 gene open reading frames 1 and 2 of human metapneumovirus: Effects on RNA synthesis, attenuation, and immunogenicity. J. Virol. 2005, 79, 6588–6597. [Google Scholar] [CrossRef] [PubMed]
- Rincheval, V.; Lelek, M.; Gault, E.; Bouillier, C.; Sitterlin, D.; Blouquit-Laye, S.; Galloux, M.; Zimmer, C.; Eleouet, J.F.; Rameix-Welti, M.A. Functional organization of cytoplasmic inclusion bodies in cells infected by respiratory syncytial virus. Nat. Commun. 2017, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Dolnik, O.; Gerresheim, G.K.; Biedenkopf, N. New Perspectives on the Biogenesis of Viral Inclusion Bodies in Negative-Sense RNA Virus Infections. Cells 2021, 10, 1460. [Google Scholar] [CrossRef]
- Lopez, N.; Camporeale, G.; Salgueiro, M.; Borkosky, S.S.; Visentin, A.; Peralta-Martinez, R.; Loureiro, M.E.; de Prat-Gay, G. Deconstructing virus condensation. PLoS Pathog. 2021, 17, e1009926. [Google Scholar] [CrossRef]
- Garcia, J.; Garcia-Barreno, B.; Vivo, A.; Melero, J.A. Cytoplasmic inclusions of respiratory syncytial virus-infected cells: Formation of inclusion bodies in transfected cells that coexpress the nucleoprotein, the phosphoprotein, and the 22K protein. Virology 1993, 195, 243–247. [Google Scholar] [CrossRef]
- Derdowski, A.; Peters, T.R.; Glover, N.; Qian, R.; Utley, T.J.; Burnett, A.; Williams, J.V.; Spearman, P.; Crowe, J.E. Human metapneumovirus nucleoprotein and phosphoprotein interact and provide the minimal requirements for inclusion body formation. J. Gen. Virol. 2008, 89, 2698–2708. [Google Scholar] [CrossRef]
- Weber, E.; Humbert, B.; Streckert, H.J.; Werchau, H. Nonstructural protein 2 (NS2) of respiratory syncytial virus (RSV) detected by an antipeptide serum. Respiration 1995, 62, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ghildyal, R.; Mills, J.; Murray, M.; Vardaxis, N.; Meanger, J. Respiratory syncytial virus matrix protein associates with nucleocapsids in infected cells. J. Gen. Virol. 2002, 83, 753–757. [Google Scholar] [CrossRef]
- Bajorek, M.; Galloux, M.; Richard, C.A.; Szekely, O.; Rosenzweig, R.; Sizun, C.; Eleouet, J.F. Tetramerization of Phosphoprotein is Essential for Respiratory Syncytial Virus Budding while its N Terminal Region Mediates Direct Interactions with the Matrix Protein. J. Virol. 2021, 95, e02217-20. [Google Scholar] [CrossRef]
- Richard, C.A.; Rincheval, V.; Lassoued, S.; Fix, J.; Cardone, C.; Esneau, C.; Nekhai, S.; Galloux, M.; Rameix-Welti, M.A.; Sizun, C.; et al. RSV hijacks cellular protein phosphatase 1 to regulate M2-1 phosphorylation and viral transcription. PLoS Pathog. 2018, 14, e1006920. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J.; Koo, L.Y.; Brown, C.R.; Collins, P.L. p38 and OGT sequestration into viral inclusion bodies in cells infected with human respiratory syncytial virus suppresses MK2 activities and stress granule assembly. J. Virol. 2013, 87, 1333–1347. [Google Scholar] [CrossRef]
- Lifland, A.W.; Jung, J.; Alonas, E.; Zurla, C.; Crowe, J.E., Jr.; Santangelo, P.J. Human respiratory syncytial virus nucleoprotein and inclusion bodies antagonize the innate immune response mediated by MDA5 and MAVS. J. Virol. 2012, 86, 8245–8258. [Google Scholar] [CrossRef]
- Jobe, F.; Simpson, J.; Hawes, P.; Guzman, E.; Bailey, D. Respiratory Syncytial Virus Sequesters NF-kappaB Subunit p65 to Cytoplasmic Inclusion Bodies To Inhibit Innate Immune Signaling. J. Virol. 2020, 94, e01380-20. [Google Scholar] [CrossRef] [PubMed]
- Gilman, M.S.A.; Liu, C.; Fung, A.; Behera, I.; Jordan, P.; Rigaux, P.; Ysebaert, N.; Tcherniuk, S.; Sourimant, J.; Eleouet, J.F.; et al. Structure of the Respiratory Syncytial Virus Polymerase Complex. Cell 2019, 179, 193–204 e14. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Qian, X.; Lattmann, S.; El Sahili, A.; Yeo, T.H.; Jia, H.; Cressey, T.; Ludeke, B.; Noton, S.; Kalocsay, M.; et al. Structure of the human metapneumovirus polymerase phosphoprotein complex. Nature 2020, 577, 275–279. [Google Scholar] [CrossRef]
- Sutto-Ortiz, P.; Tcherniuk, S.; Ysebaert, N.; Abeywickrema, P.; Noel, M.; Decombe, A.; Debart, F.; Vasseur, J.J.; Canard, B.; Roymans, D.; et al. The methyltransferase domain of the Respiratory Syncytial Virus L protein catalyzes cap N7 and 2’-O-methylation. PLoS Pathog. 2021, 17, e1009562. [Google Scholar] [CrossRef]
- Garcia, J.; Garcia-Barreno, B.; Martinez, I.; Melero, J.A. Mapping of monoclonal antibody epitopes of the human respiratory syncytial virus p protein. Virology 1993, 195, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Galloux, M.; Risso-Ballester, J.; Richard, C.A.; Fix, J.; Rameix-Welti, M.A.; Eleouet, J.F. Minimal Elements Required for the Formation of Respiratory Syncytial Virus Cytoplasmic Inclusion Bodies In Vivo and In Vitro. mBio 2020, 11, e01202-20. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Elbaum-Garfinkle, S.; Kim, Y.; Szczepaniak, K.; Chen, C.C.; Eckmann, C.R.; Myong, S.; Brangwynne, C.P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA 2015, 112, 7189–7194. [Google Scholar] [CrossRef] [PubMed]
- Nott, T.J.; Petsalaki, E.; Farber, P.; Jervis, D.; Fussner, E.; Plochowietz, A.; Craggs, T.D.; Bazett-Jones, D.P.; Pawson, T.; Forman-Kay, J.D.; et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 2015, 57, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Castagne, N.; Barbier, A.; Bernard, J.; Rezaei, H.; Huet, J.C.; Henry, C.; Da Costa, B.; Eleouet, J.F. Biochemical characterization of the respiratory syncytial virus P-P and P-N protein complexes and localization of the P protein oligomerization domain. J. Gen. Virol. 2004, 85, 1643–1653. [Google Scholar] [CrossRef]
- Llorente, M.T.; Garcia-Barreno, B.; Calero, M.; Camafeita, E.; Lopez, J.A.; Longhi, S.; Ferron, F.; Varela, P.F.; Melero, J.A. Structural analysis of the human respiratory syncytial virus phosphoprotein: Characterization of an alpha-helical domain involved in oligomerization. J. Gen. Virol. 2006, 87, 159–169. [Google Scholar] [CrossRef]
- Llorente, M.T.; Taylor, I.A.; Lopez-Vinas, E.; Gomez-Puertas, P.; Calder, L.J.; Garcia-Barreno, B.; Melero, J.A. Structural properties of the human respiratory syncytial virus P protein: Evidence for an elongated homotetrameric molecule that is the smallest orthologue within the family of paramyxovirus polymerase cofactors. Proteins 2008, 72, 946–958. [Google Scholar] [CrossRef]
- Simabuco, F.M.; Asara, J.M.; Guerrero, M.C.; Libermann, T.A.; Zerbini, L.F.; Ventura, A.M. Structural analysis of human respiratory syncytial virus p protein: Identification of intrinsically disordered domains. Braz. J. Microbiol. 2011, 42, 340–345. [Google Scholar] [CrossRef]
- Renner, M.; Paesen, G.C.; Grison, C.M.; Granier, S.; Grimes, J.M.; Leyrat, C. Structural dissection of human metapneumovirus phosphoprotein using small angle x-ray scattering. Sci. Rep. 2017, 7, 14865. [Google Scholar] [CrossRef]
- Di Tommaso, P.; Moretti, S.; Xenarios, I.; Orobitg, M.; Montanyola, A.; Chang, J.M.; Taly, J.F.; Notredame, C. T-Coffee: A web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011, 39, W13–W17. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Cardone, C.; Lassoued, S.; Galloux, M.; Fix, J.; Assrir, N.; Lescop, E.; Bontems, F.; Eleouet, J.F.; Sizun, C. New Insights into Structural Disorder in Human Respiratory Syncytial Virus Phosphoprotein and Implications for Binding of Protein Partners. J. Biol. Chem. 2017, 292, 2120–2131. [Google Scholar] [CrossRef]
- Cardone, C.; Caseau, C.M.; Bardiaux, B.; Thureaux, A.; Galloux, M.; Bajorek, M.; Eleouet, J.F.; Litaudon, M.; Bontems, F.; Sizun, C. A Structural and Dynamic Analysis of the Partially Disordered Polymerase-Binding Domain in RSV Phosphoprotein. Biomolecules 2021, 11, 1225. [Google Scholar] [CrossRef]
- Cardone, C.; Caseau, C.M.; Pereira, N.; Sizun, C. Pneumoviral Phosphoprotein, a Multidomain Adaptor-Like Protein of Apparent Low Structural Complexity and High Conformational Versatility. Int. J. Mol. Sci. 2021, 22, 1537. [Google Scholar] [CrossRef]
- Esperante, S.A.; Paris, G.; de Prat-Gay, G. Modular unfolding and dissociation of the human respiratory syncytial virus phosphoprotein p and its interaction with the m(2-1) antiterminator: A singular tetramer-tetramer interface arrangement. Biochemistry 2012, 51, 8100–8110. [Google Scholar] [CrossRef] [PubMed]
- Renner, M.; Bertinelli, M.; Leyrat, C.; Paesen, G.C.; Saraiva de Oliveira, L.F.; Huiskonen, J.T.; Grimes, J.M. Nucleocapsid assembly in pneumoviruses is regulated by conformational switching of the N protein. eLife 2016, 5, e12627. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.; Yegambaram, K.; Todd, E.; Richard, C.A.; Dods, R.L.; Pangratiou, G.M.; Trinh, C.H.; Moul, S.L.; Murphy, J.C.; Mankouri, J.; et al. The Structure of the Human Respiratory Syncytial Virus M2-1 Protein Bound to the Interaction Domain of the Phosphoprotein P Defines the Orientation of the Complex. mBio 2018, 9, e01554-18. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.L.; Castagne, N.; Bhella, D.; Varela, P.F.; Bernard, J.; Chilmonczyk, S.; Berkenkamp, S.; Benhamo, V.; Grznarova, K.; Grosclaude, J.; et al. The nine C-terminal amino acids of the respiratory syncytial virus protein P are necessary and sufficient for binding to ribonucleoprotein complexes in which six ribonucleotides are contacted per N protein protomer. J. Gen. Virol. 2007, 88, 196–206. [Google Scholar] [CrossRef]
- Galloux, M.; Tarus, B.; Blazevic, I.; Fix, J.; Duquerroy, S.; Eleouet, J.F. Characterization of a viral phosphoprotein binding site on the surface of the respiratory syncytial nucleoprotein. J. Virol. 2012, 86, 8375–8387. [Google Scholar] [CrossRef] [PubMed]
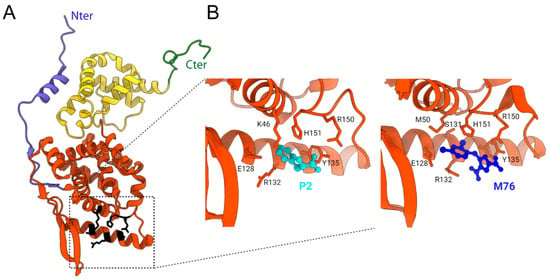
- Ouizougun-Oubari, M.; Pereira, N.; Tarus, B.; Galloux, M.; Lassoued, S.; Fix, J.; Tortorici, M.A.; Hoos, S.; Baron, B.; England, P.; et al. A Druggable Pocket at the Nucleocapsid/Phosphoprotein Interaction Site of Human Respiratory Syncytial Virus. J. Virol. 2015, 89, 11129–11143. [Google Scholar] [CrossRef]
- Navarro, J.; Lopez-Otin, C.; Villanueva, N. Location of phosphorylated residues in human respiratory syncytial virus phosphoprotein. J. Gen. Virol. 1991, 72, 1455–1459. [Google Scholar] [CrossRef]
- Asenjo, A.; Rodriguez, L.; Villanueva, N. Determination of phosphorylated residues from human respiratory syncytial virus P protein that are dynamically dephosphorylated by cellular phosphatases: A possible role for serine 54. J. Gen. Virol. 2005, 86, 1109–1120. [Google Scholar] [CrossRef]
- Mazumder, B.; Barik, S. Requirement of casein kinase II-mediated phosphorylation for the transcriptional activity of human respiratory syncytial viral phosphoprotein P: Transdominant negative phenotype of phosphorylation-defective P mutants. Virology 1994, 205, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, N.; Hardy, R.; Asenjo, A.; Yu, Q.; Wertz, G. The bulk of the phosphorylation of human respiratory syncytial virus phosphoprotein is not essential but modulates viral RNA transcription and replication. J. Gen. Virol. 2000, 81, 129–133. [Google Scholar] [CrossRef]
- Lu, B.; Ma, C.H.; Brazas, R.; Jin, H. The major phosphorylation sites of the respiratory syncytial virus phosphoprotein are dispensable for virus replication in vitro. J. Virol. 2002, 76, 10776–10784. [Google Scholar] [CrossRef] [PubMed]
- Asenjo, A.; Gonzalez-Armas, J.C.; Villanueva, N. Phosphorylation of human respiratory syncytial virus P protein at serine 54 regulates viral uncoating. Virology 2008, 380, 26–33. [Google Scholar] [CrossRef]
- Shapiro, A.B.; Gao, N.; O’Connell, N.; Hu, J.; Thresher, J.; Gu, R.F.; Overman, R.; Hardern, I.M.; Sproat, G.G. Quantitative investigation of the affinity of human respiratory syncytial virus phosphoprotein C-terminus binding to nucleocapsid protein. Virol. J. 2014, 11, 191. [Google Scholar] [CrossRef][Green Version]
- Tawar, R.G.; Duquerroy, S.; Vonrhein, C.; Varela, P.F.; Damier-Piolle, L.; Castagne, N.; MacLellan, K.; Bedouelle, H.; Bricogne, G.; Bhella, D.; et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science 2009, 326, 1279–1283. [Google Scholar] [CrossRef]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018, 27, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Conley, M.J.; Short, J.M.; Hutchings, J.; Burns, A.M.; Streetley, J.; Bakker, S.E.; Jaffery, H.; Stewart, M.; Power, J.; Zanetti, G.; et al. Helical Ordering of Envelope Associated Proteins and Glycoproteins in Respiratory Syncytial Virus Filamentous Virions. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bakker, S.E.; Duquerroy, S.; Galloux, M.; Loney, C.; Conner, E.; Eleouet, J.F.; Rey, F.A.; Bhella, D. The respiratory syncytial virus nucleoprotein-RNA complex forms a left-handed helical nucleocapsid. J. Gen. Virol. 2013, 94, 1734–1738. [Google Scholar] [CrossRef]
- Liljeroos, L.; Krzyzaniak, M.A.; Helenius, A.; Butcher, S.J. Architecture of respiratory syncytial virus revealed by electron cryotomography. Proc. Natl. Acad. Sci. USA 2013, 110, 11133–11138. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Kaul, T.N.; Middleton, E., Jr.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef]
- Sa, J.M.; Piloto, J.V.; Cilli, E.M.; Tasic, L.; Fossey, M.A.; Almeida, F.C.L.; Souza, F.P.; Caruso, I.P. Hesperetin targets the hydrophobic pocket of the nucleoprotein/phosphoprotein binding site of human respiratory syncytial virus. J. Biomol. Struct. Dyn. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Chapman, J.; Abbott, E.; Alber, D.G.; Baxter, R.C.; Bithell, S.K.; Henderson, E.A.; Carter, M.C.; Chambers, P.; Chubb, A.; Cockerill, G.S.; et al. RSV604, a novel inhibitor of respiratory syncytial virus replication. Antimicrob. Agents Chemother. 2007, 51, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.A.; Alber, D.G.; Baxter, R.C.; Bithell, S.K.; Budworth, J.; Carter, M.C.; Chubb, A.; Cockerill, G.S.; Dowdell, V.C.; Fraser, I.J.; et al. 1,4-benzodiazepines as inhibitors of respiratory syncytial virus. The identification of a clinical candidate. J. Med. Chem 2007, 50, 1685–1692. [Google Scholar] [CrossRef]
- Challa, S.; Scott, A.D.; Yuzhakov, O.; Zhou, Y.; Tiong-Yip, C.L.; Gao, N.; Thresher, J.; Yu, Q. Mechanism of Action for Respiratory Syncytial Virus Inhibitor RSV604. Antimicrob. Agents Chemother. 2015, 59, 1080–1087. [Google Scholar] [CrossRef]
- Rhodin, M.H.J.; McAllister, N.V.; Castillo, J.; Noton, S.L.; Fearns, R.; Kim, I.J.; Yu, J.M.; Blaisdell, T.P.; Panarese, J.; Shook, B.C.; et al. EDP-938, a novel nucleoprotein inhibitor of respiratory syncytial virus, demonstrates potent antiviral activities in vitro and in a non-human primate model. PloS Pathog. 2021, 17, e1009428. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Yaita, K.; Khamrin, P.; Kumthip, K.; Kashiwagi, T.; Eleouet, J.F.; Rameix-Welti, M.A.; Watanabe, H. A small fragmented P protein of respiratory syncytial virus inhibits virus infection by targeting P protein. J. Gen. Virol. 2020, 101, 21–32. [Google Scholar] [CrossRef]
- Decool, H.; Bardiaux, B.; Checa Ruano, L.; Sperandio, O.; Fix, J.; Gutsche, I.; Richard, C.A.; Bajorek, M.; Eleouet, J.F.; Galloux, M. Characterization of the interaction domains between the phosphoprotein and the nucleoprotein of human Metapneumovirus. J. Virol. 2021, JVI0090921. [Google Scholar] [CrossRef] [PubMed]
- El Omari, K.; Scott, K.; Dhaliwal, B.; Ren, J.; Abrescia, N.G.A.; Budworth, J.; Lockyer, M.; Powell, K.L.; Hawkins, A.R.; Stammers, D.K. Crystallization and preliminary X-ray analysis of the human respiratory syncytial virus nucleocapsid protein. Acta Crystallogr. Sect. F-Struct. Biol. Cryst. Comm. 2008, 64, 1019–1023. [Google Scholar] [CrossRef]
- Esneau, C.; Raynal, B.; Roblin, P.; Brule, S.; Richard, C.A.; Fix, J.; Eleouet, J.F.; Galloux, M. Biochemical characterization of the respiratory syncytial virus N(0)-P complex in solution. J. Biol. Chem. 2019, 294, 3647–3660. [Google Scholar] [CrossRef] [PubMed]
- Galloux, M.; Gabiane, G.; Sourimant, J.; Richard, C.A.; England, P.; Moudjou, M.; Aumont-Nicaise, M.; Fix, J.; Rameix-Welti, M.A.; Eleouet, J.F. Identification and Characterization of the Binding Site of the Respiratory Syncytial Virus Phosphoprotein to RNA-Free Nucleoprotein. J. Virol. 2015, 89, 3484–3496. [Google Scholar] [CrossRef] [PubMed]
- Karlin, D.; Belshaw, R. Detecting remote sequence homology in disordered proteins: Discovery of conserved motifs in the N-termini of Mononegavirales phosphoproteins. PLoS ONE 2012, 7, e31719. [Google Scholar]
- Mallipeddi, S.K.; Lupiani, B.; Samal, S.K. Mapping the domains on the phosphoprotein of bovine respiratory syncytial virus required for N-P interaction using a two-hybrid system. J. Gen. Virol. 1996, 77, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Khattar, S.K.; Yunus, A.S.; Samal, S.K. Mapping the domains on the phosphoprotein of bovine respiratory syncytial virus required for N-P and P-L interactions using a minigenome system. J. Gen. Virol. 2001, 82, 775–779. [Google Scholar] [CrossRef]
- Jamin, M.; Yabukarski, F. Nonsegmented Negative-Sense RNA Viruses-Structural Data Bring New Insights Into Nucleocapsid Assembly. Adv. Virus Res. 2017, 97, 143–185. [Google Scholar]
- Leyrat, C.; Yabukarski, F.; Tarbouriech, N.; Ribeiro, E.A., Jr.; Jensen, M.R.; Blackledge, M.; Ruigrok, R.W.; Jamin, M. Structure of the vesicular stomatitis virus N(0)-P complex. PLoS Pathog. 2011, 7, e1002248. [Google Scholar] [CrossRef]
- Yabukarski, F.; Lawrence, P.; Tarbouriech, N.; Bourhis, J.M.; Delaforge, E.; Jensen, M.R.; Ruigrok, R.W.; Blackledge, M.; Volchkov, V.; Jamin, M. Structure of Nipah virus unassembled nucleoprotein in complex with its viral chaperone. Nat. Struct. Mol. Biol. 2014, 21, 754–759. [Google Scholar] [CrossRef]
- Guryanov, S.G.; Liljeroos, L.; Kasaragod, P.; Kajander, T.; Butcher, S.J. Crystal Structure of the Measles Virus Nucleoprotein Core in Complex with an N-Terminal Region of Phosphoprotein. J. Virol. 2015, 90, 2849–2857. [Google Scholar] [CrossRef]
- Kirchdoerfer, R.N.; Abelson, D.M.; Li, S.; Wood, M.R.; Saphire, E.O. Assembly of the Ebola Virus Nucleoprotein from a Chaperoned VP35 Complex. Cell Rep. 2015, 12, 140–149. [Google Scholar] [CrossRef]
- Zhu, T.; Song, H.; Peng, R.; Shi, Y.; Qi, J.; Gao, G.F. Crystal Structure of the Marburg Virus Nucleoprotein Core Domain Chaperoned by a VP35 Peptide Reveals a Conserved Drug Target for Filovirus. J. Virol. 2017, 91, e00996-17. [Google Scholar] [CrossRef]
- Aggarwal, M.; Leser, G.P.; Kors, C.A.; Lamb, R.A. Structure of the Paramyxovirus Parainfluenza Virus 5 Nucleoprotein in Complex with an Amino-Terminal Peptide of the Phosphoprotein. J. Virol. 2018, 92, e01304-17. [Google Scholar] [CrossRef] [PubMed]
- Galloux, M.; Gsponer, N.; Gaillard, V.; Fenner, B.; Larcher, T.; Vilotte, M.; Riviere, J.; Richard, C.A.; Eleouet, J.F.; Le Goffic, R.; et al. Targeting the Respiratory Syncytial Virus N-0-P Complex with Constrained alpha-Helical Peptides in Cells and Mice. Antimicrob. Agents Chemother. 2020, 64, e00717-20. [Google Scholar] [CrossRef] [PubMed]
- Verdine, G.L.; Hilinski, G.J. Stapled peptides for intracellular drug targets. Methods Enzymol. 2012, 503, 3–33. [Google Scholar]
- Kim, Y.W.; Grossmann, T.N.; Verdine, G.L. Synthesis of all-hydrocarbon stapled alpha-helical peptides by ring-closing olefin metathesis. Nat. Protoc. 2011, 6, 761–771. [Google Scholar] [CrossRef]
- Bird, G.H.; Madani, N.; Perry, A.F.; Princiotto, A.M.; Supko, J.G.; He, X.; Gavathiotis, E.; Sodroski, J.G.; Walensky, L.D. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc. Natl. Acad. Sci. USA 2010, 107, 14093–14098. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.H.; Boyapalle, S.; Wong, T.; Opoku-Nsiah, K.; Bedi, R.; Crannell, W.C.; Perry, A.F.; Nguyen, H.; Sampayo, V.; Devareddy, A.; et al. Mucosal delivery of a double-stapled RSV peptide prevents nasopulmonary infection. J. Clin. Investig. 2014, 124, 2113–2124. [Google Scholar] [CrossRef]
- Gaillard, V.; Galloux, M.; Garcin, D.; Eleouet, J.F.; Le Goffic, R.; Larcher, T.; Rameix-Welti, M.A.; Boukadiri, A.; Heritier, J.; Segura, J.M.; et al. A Short Double-Stapled Peptide Inhibits Respiratory Syncytial Virus Entry and Spreading. Antimicrob. Agents Chemother. 2017, 61, e02241-16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).