Abstract
Extraintestinal pathogenic Escherichia coli (ExPEC) strains are capable of causing various systemic infections in both humans and animals. In this study, we isolated and characterized 30 E. coli strains from the parenchymatic organs and brains of young (<3 months of age) camel calves which died in septicemia. Six of the strains showed hypermucoviscous phenotype. Based on minimum inhibitory concentration (MIC) values, seven of the strains were potentially multidrug resistant, with two additional showing colistin resistance. Four strains showed mixed pathotypes, as they carried characteristic virulence genes for intestinal pathotypes of E. coli: three strains carried cnf1, encoding cytotoxic necrotizing factor type 1, the key virulence gene of necrotoxigenic E. coli (NTEC), and one carried eae encoding intimin, the key virulence gene of enteropathogenic E. coli (EPEC). An investigation of the integration sites of pathogenicity islands (PAIs) and the presence of prophage-related sequences showed that the strains carry diverse arrays of mobile genetic elements, which may contribute to their antimicrobial resistance and virulence patterns. Our work is the first to describe ExPEC strains from camels, and points to their veterinary pathogenic as well as zoonotic potential in this important domestic animal.
1. Introduction
Escherichia coli, besides including numerous intestinal pathotypes, includes several lineages of strains capable of causing extraintestinal disease, collectively referred to as extraintestinal pathogenic E. coli (ExPEC) []. ExPEC are responsible for a range of urinary tract infections (UTI), in which case they are referred to as uropathogenic E. coli (UPEC) [,], newborn meningitis (NMEC), and other kinds of sepsis and systemic infections, in humans and animals alike [].
While intestinal pathotypes of E. coli are defined by the carriage of one or few key virulence genes (VGs), ExPEC are more heterogeneous in their virulence apparatus, and it seems that there is no single VG that enables an ExPEC strain to cause a site-specific infection []. These infections are more likely to be a multifactorial process, involving fitness genes broadly distributed among commensal E. coli, suggesting that there is no strong selective pressure on E. coli to become an ExPEC, and that many ExPEC are actually opportunistic pathogens, with no clear distinction between commensal E. coli and ExPEC [].
Another difference between ExPEC and intestinal pathotypes of E. coli is that the source of infection is often difficult to identify, as the pattern of infections does not follow the classic ‘outbreak’ scheme [].
Similarly to intestinal pathogenic E. coli, ExPEC are genetically quite heterogeneous, with mobile genetic elements (MGE) forming a significant part of their genome, and playing an important role in the dissemination of VGs and those related to antimicrobial resistance (AMR) []. Pathogenicity islands (PAIs) are considered significant MGEs in pathogenic E. coli, especially in the case of UPEC, where important VGs are encoded on PAIs, which make up a significant proportion of the whole genome [,,].
Camels are uniquely important domestic animals in many countries of North Africa and the Middle East, and the study of their infectious diseases is, therefore, of utmost importance from the perspective of food safety, public health and economy as well []. Various pathotypes of intestinal pathogenic E. coli, as well as E. coli of unspecified pathotypes carrying ARGs (antimicrobial resistance related genes), especially those encoding extended-spectrum beta-lactamase (ESBL) have been characterized from camels in the past decades [,,,].
In this study, our aim was the molecular and phenotypic characterization of 30 E. coli strains isolated from camel calves which died in sepsis. To our knowledge, no E. coli strains isolated from extraintestinal infections in camels have been reported to date; therefore, our findings could enrich our knowledge about the virulence and resistance characteristics, and thus, the zoonotic potential of such strains.
2. Materials and Methods
2.1. Bacterial Strains, Isolation and Culturing
Strains in the study originated from dead camel calves from two camel farms (F1 and F2) in the United Arab Emirates (UAE), involved in camel milk production. All animals were below three months of age, with some of them being younger than one month at the time of death. Bacteriological samples were taken from parenchymal organs during the post-mortem examination of the corpses transported to the diagnostic laboratory after death. After burning off the surface of the parenchymal organ samples with a heated flat metal instrument (decontamination), sampling was performed through the burnt surface using a sampling cotton swab. These swabs were then directly spotted onto the surface of solid media and streaked with bacteriological loop. The used media were sheep blood agar (Neogen, USA), brilliant green phenol red lactose sucrose agar (BPLS; Merck, Germany) and nutrient agar (Oxoid, UK); the streaked cultures were incubated for 37 °C overnight. Pure colonies were obtained by subculturing on sheep blood agar followed by identification with MALDI-TOF (see Section 2.2). Hemolysis was tested by growth with the same conditions on blood agar. The list of strains with their origin and the results of their preliminary characterization are presented in Table 1. For DNA isolation, strains were grown in 150 μL volume of lysogeny broth (LB) in 96-well plates and grown overnight at 37 °C as well. For long-term storage, overnight liquid LB cultures of the strains were supplemented with 30 v/v% sterile glycerol and stored at −70 °C. Hypermucoviscous (hmv) phenotype was demonstrated using the ‘string test’ [] by pulling mucous string from agar-grown colonies of the respective strains.

Table 1.
List of origin and phenotypical characteristics of the strains in the study; hmv: hypermucoviscous.
In addition to the strains of camel calf origin, E. coli reference strains were used as controls for PCR reactions; these are listed in Table 2.

Table 2.
List of reference strains used as positive or internal negative controls in PCR reactions for the detection of VGs. For the primers, see Table A1.
2.2. MALDI-TOF
Species-level identification of the strains was performed using mass spectrometry with the Bruker MALDI-TOF system (Bruker, Millerica, MA, USA) according to the manufacturer’s instructions. Briefly, cultures underwent a preparatory extraction with formic acid, and then they were analyzed with the instrument. Identification was conducted using the MALDI Biotyper database (Bruker, Millerica, MA, USA).
2.3. Determination of Antibiotic Resistance and Minimum Inhibitory Concentration (MIC) Values
MIC values for a set of thirteen antibiotics were determined using the standard method of serial dilution in Mueller–Hinton broth, according to the standard protocol of CLSI for bacterial isolates of animal origin []. The applied antibiotics, together with the results, are summarized in Table 3.

Table 3.
MIC values of the studied strains for different antimicrobials. Values are given in μg/mL. Abbreviations: AM: ampicillin; AMCL amoxicillin + clavulanic acid; CEFT: cefotaxime; CEFQ: cefquinome; GEN: gentamicin; NEO: neomycin; OTC: oxytetracycline; DOX: doxycycline; FLO: florfenicol; COL: colistin; ENR: enrofloxacin; MAR: marbofloxacin; TMP-SMX: trimethoprim + sulfamethoxazole.
2.4. DNA Isolation and PCR-Based Investigations
The presence of significant VGs associated with pathogenic E. coli was checked by PCR using the primers listed in Table A1. Phylogenetic relations were mapped by the extended Clermont PCR-scheme [].
The presence of MGEs was checked by two schemes. The Sakai prophage (Sp) typing utilizes the marker genes of twelve prophages carried by the enterohemorrhagic E. coli (EHEC) O157:H7 Sakai strain, described earlier [], and the DNA isolated from this prototypic strain was used as the control (Table 2). The intact or occupied state of the integration sites of typical UPEC PAIs was also checked with targeted PCR using the primers listed in Table A1, with the strains listed in Table 2 used as controls for the reactions indicated.
For the reactions, cultures of strains grown either on agar plates or in LB broth were taken and suspended or diluted in sterile DNAse-free distilled water, then DNA was isolated by boiling the suspensions. All PCRs were performed using DreamTaq Green Mastermix (ThermoFisher, Waltham, MA, USA) per the manufacturer’s instructions. The usual heat profile of the reactions was an initial denaturation at 94 °C for 3 min, then 30 cycles of a denaturation at 94 °C for 30 s, annelation for 30 s on the optimal temperature of the primer pair used, then extension for 1 min at 72 °C. After the last cycle, a final elongation step at 72 °C for 5 min followed. In the case of the Clermont phylotyping, the fast heat profile was used []. Results of the reactions were visualized by agarose gel electrophoresis. All the primers used in the study are listed in Table A1.
3. Results
3.1. Species Determination and Phenotypic Traits
All 30 strains isolated from the parenchymatic organs of the dead septicaemic camel calves proved to be a member of the E. coli species, according to the MALDI-TOF analysis. Six strains indicated in Table 1 showed the hmv phenotype as, in their case, it was possible to drag a mucous filament > 5 mm in length from the colonies grown on agar plates (Figure 1A), and it was considerably harder to remove them from the agar surface when compared to the colonies of the other strains (‘sticky’ phenotype). Strain TE11 showed a mucous, but not hmv colony morphology (Figure 1B), while TE10 showed a sticky but not mucous phenotype. Six strains showed hemolysis (Table 1 and Figure 1C).
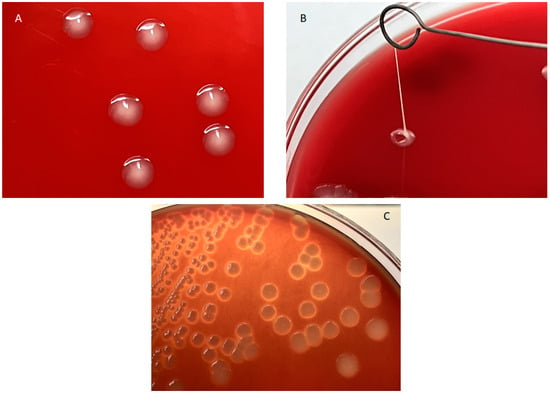
Figure 1.
Characteristic morphologies shown by the E. coli strains of camel origin investigated in the study. (A): String test of strain TE23 showing hmv phenotype. (B): Mucoid but not adherent morphotype shown by strain TE11. (C): Hemolysis shown by strain TE14.
3.2. Antimicrobial Resistance Patterns and MIC Values
To test the AMR repertoire of the strains, the MIC of the strains against the thirteen antibiotics frequently applied against E. coli was tested; the results are shown in Table 3. Notably, there were seven strains with a MIC value higher than the MIC50 for three or more antibiotics. Three strains showed a MIC against florfenicol higher than the MIC90, and two strains, TE19 and TE20, showed a MIC against colistin higher than the MIC90 value for this antibiotic in the set. In the case of gentamycin, the strains formed two groups according to susceptibility; there were eight strains with at least MIC = 16, while the rest of the strains showed a maximum MIC value of 2 against this antibiotic.
3.3. Virulence Genes
We investigated the presence of known key VGs of ExPEC, as well as those of the significant intestinal pathotypes of E. coli. The results are summarized in Table 4. An interesting finding was that strain TE16 harbored the eae gene encoding the adhesin intimin, therefore belonging to the enteropathogenic E. coli (EPEC) pathotype. TE14, TE15 and TE18 were positive for the gene encoding cytotoxic necrotizing factor 1 (cnf1) and S fimbriae (sfa).

Table 4.
Carriage of VGs, state of UPEC PAI integration sites, and phylogenetic grouping of the strains. The results of the Sp typing are shown as a four-digit code, as described in []. The intact or disrupted state of UPEC PAI integration sites is shown as: ‘i’: intact, ‘d’: disrupted.
3.4. Occupied States of PAI Integration Sites
To screen for the presence of characteristic UPEC PAIs, the intact or occupied state of their characteristic integration sites in the strains’ genomes was checked using the E. coli K-12 derivate strain MG1655 as the positive control and the prototypic UPEC 536 strain as the internal negative control, as this strain harbors a PAI in all the investigated genomic sites; therefore, it gives a negative result for all the reactions. The results are shown in Table 4. All strains had at least three of the investigated sites disrupted, showing no product for the respective reactions. The thrW and leuX sites were disrupted in all strains.
3.5. Phylogenetic Relations
The phylogenetic grouping of the strains according to the extended Clermont-scheme, which is a triplex PCR reaction with supplementary reactions and checks for the presence/absence of housekeeping marker genes, is shown in Table 4. Out of the seven possible groups, all strains belonged to either group A, B1 or D. No strain proved to be a member of groups B2, C, E or F.
The results of the Sp typing based on the prophages of the EHEC O157:H7 Sakai strain are shown in Table 4. Half of the strains carried at least one of the prophage-related marker gene originally identified in the Sakai strain, suggesting that they harbor at least one corresponding or similar prophage. The majority of the other strains carried one or two prophage regions. Strain TE16 represented type 7767, indicating the carriage of four prophage regions.
4. Discussion
In this study, we performed phenotypic and genotypic characterization of 30 E. coli strains isolated from cases of sepsis in young camel calves. As all strains were isolated from extraintestinal sites where commensal E. coli does not occur naturally, it can be reasonably supposed that the isolated strains were the causative agents of septicaemia. The source of the strains was most likely the farm environment, with the infection occurring through the peroral route.
Six of the characterized strains displayed the hmv phenotype. While being a frequent trait of hypervirulent Klebsiella pneumoniae strains [], it is rarely reported from E. coli. In all reported cases so far, the strains were ExPEC [,,,]. One study speculates that this trait could be a frequent, but overlooked, characteristic of UPEC strains []. In contrast to K. pneumoniae, where genes responsible for the hmv phenotype have been identified [], the genetic background of this trait is yet to be investigated and described in E. coli. The hmv phenotype is frequently associated with hypervirulence in K. pneumoniae, in the case of which the hmv strains seem to be more capable of causing abscesses and spreading metastatically [], suggesting that this phenotype deserves attention when characterizing and treating ExPEC infections in humans and animals.
The strains showed diverse patterns of AMR. Seven strains were potentially multidrug resistant (MDR), with one such strain and one non-MDR strain showing colistin resistance as well. Eight strains showed high resistance against gentamycin, while the rest of the strains had MIC values several dilutions lower, leading to a considerable difference between MIC50 and MIC90 for this antibiotic in the strain set. Although it is not conclusively proven [], it is very likely that food-producing animals play an important role in the dissemination of ARGs [], and our results corroborate this notion. The strains showed almost universally high MIC values for beta-lactams, which is consistent with earlier reports of ESBL-resistant E. coli being carried by camels [,], with one study suggesting the reservoir role of this animal in carrying potentially zoonotic ESBL-resistant E. coli []. The gene responsible for colistin resistance was reported from the E. coli strains of camel intestinal origin [], and this is the first time that ExPEC strains from this animal also showed resistance against this antibiotic. The worldwide emergence of colistin-resistant strains of animal origin is a worrying trend, as colistin is considered a last-line antibiotic [].
Three of the strains (TE14, TE15 and TE18) harbored the cytotoxic necrotizing factor type 1 (CNF-1) [] and S fimbriae [,] characteristic of the subset of ExPEC referred to as necrotoxigenic E. coli type 1 (NTEC1) strains. Albeit sometimes reported from diarrheal cases, they are mostly associated with extraintestinal and urinary infections in both humans and animals [,]; cnf+ strains have already been reported from diarrheic camels as well []. Only strain TE24 harbored the papC-encoding pyelonephritis associated pilus, an adhesion factor characteristic of UPEC []. There were 25 strains which were negative for all investigated VGs. Nevertheless, the site of their isolation and the pathological findings of the animals indicate the pathogenicity of the strains.
Strain TE16 carried the eae gene, encoding intimin, which is responsible for the intimate attachment of EPEC and EHEC cells to the host intestinal epithelium, and the characteristic pedestal formation by actin filaments in the affected epithelial cells (reviewed by []). The carriage of this gene classifies strain TE16 as EPEC, which is an interesting finding, as it was, similarly to other strains in the study, isolated from the parenchymatic organs of an affected animal, which died in colisepticaemia. EPEC strains are a significant intestinal pathotype affecting humans [,], which are frequently isolated from domestic animals, and as such, are considered zoonotic pathogens []. As mentioned in Section 3.5, strain TE16 also proved to be the most ‘prophage-rich’ strain with the Sp typing scheme, which could be explained by the fact that the scheme was developed for the prophages of a strain representing an intestinal pathotype. The presence of EPEC strains in the feces of both healthy and diarrheic camels was reported earlier [,], and was also isolated from camel carcasses and meat [,]. The isolation of this strain from a septic case underlines the notion that intestinal pathogenic E. coli and ExPEC cannot always be strictly differentiated [], as well as that ExPEC strains in some cases may be opportunistic pathogens []. This difficulty of differentiation was even more underlined by the results of the phylogenetic grouping of the strains. To our surprise, no strain belonged to the B2 phylogenetic group, which is otherwise overrepresented among ExPEC strains [] and includes almost all NTEC1 strains [], while our cnf1+ strains belonged to group B1.
The results of the Sp typing suggested heterogeneity in the prophage content of the strains, with ten strains containing several prophage-associated regions in multiple patterns. The investigation of characteristic UPEC PAI integration sites showed that all strains had at least three of the sites disrupted, potentially containing MGEs encoding ARGs or VGs such as cnf1 or papC. The strains showed various patterns of disruption, with very few cases of repeating patterns, further pointing to the genetic variability of the isolates.
The isolation of E. coli strains carrying the ARGs and VGs detailed above is a cause for public health concern, as the camel calves from which they originated were raised on farms involved in camel milk production, and the consumption of unpasteurized camel milk is a widespread practice in the UAE []. The high proportion of antibiotic-resistant and MDR strains could be the result of the widespread use of antibiotics [,], and suggests that the key to an effective defense against these strains lies in herd-specific autogenous vaccines or alternative agents, such as bacteriophage-based biocontrol [], while simultaneously reducing the use of antibiotics.
The whole genome sequencing (WGS) of the strains is currently under way. It could provide us with useful information on the content of the disrupted PAI-integration sites, heretofore unaccounted or unknown VGs or ARGs, as well as previously uncharacterized MGEs. Comparative genomic analysis with isolates from different sources should also provide useful insights on the zoonotic potential of these strains, as well as similar strains to be isolated from camels in the future.
5. Conclusions
This is the first report of ExPEC isolated from camel calves, showing a range of antibiotic resistance, with a diverse array of VGs and potential MGEs. The determination of WGS will provide more in-depth genomic information, especially regarding the VG and ARG repertoire, as well as MGEs including PAIs, prophages and plasmids. The unique importance of the camel as a domestic animal in the Middle East underlines the importance of exploring the pathogenic bacteria infecting them to prevent economic loss, the dissemination of antibiotic resistance and potentially zoonotic infections. Because of the genetic variability and plasticity of pathogenic E. coli, the close monitoring and frequent sampling of camel herds would be beneficial in order to explore emerging and potentially dangerous clones.
Author Contributions
Conceptualization, D.S., I.T. and L.M.; methodology, D.S., Z.S., J.M., S.V.J., J.J. (John Jeeba), A.S., J.J. (Judit Juhász), P.N., A.M.T.A., A.A.I. and L.M.; validation D.S., Z.S., I.T., J.M., S.V.J., J.J. (John Jeeba), A.S., J.J. (Judit Juhász), P.N., A.M.T.A., A.A.I. and L.M.; investigation, D.S., Z.S., J.M., S.V.J., J.J. (John Jeeba), A.S., J.J. (Judit Juhász), P.N., A.M.T.A., A.A.I. and L.M.; resources, D.S., Z.S. and L.M.; writing—original draft preparation, D.S.; writing—review and editing, Z.S., I.T., J.M., S.V.J., J.J. (John Jeeba), A.S., J.J. (Judit Juhász), P.N., A.M.T.A., A.A.I. and L.M.; visualization, D.S. and L.M.; supervision, D.S.; project administration, D.S.; funding acquisition, D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Research, Development and Innovation Office (Hungary), grant number FK 143174.
Institutional Review Board Statement
Ethical review and approval were waived for this study, because only dead animals were used.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to the research are included in the article.
Acknowledgments
We thank György Schneider (University of Pécs Medical School, Pécs, Hungary) and Ulrich Dobrindt (Institute of Hygiene, University of Münster, Münster, Germany) for directing us to the dissertation of Lubomir Grozdanov [], from where the primers for PAI-mapping were taken. We also thank Réka Sipos (HUN-REN Veterinary Medical Research Institute) for her help with the PCR reactions, and Ulrich Wernery (Central Veterinary Research Laboratory) for critical reading of the manuscript.
Conflicts of Interest
Judit Juhász, Péter Nagy, Ahmed Mohamed Taha Abdelnassir and Ahmed Abdelrhman Ismail are employees of the Farm and Veterinary Department, Emirates Industry for Camel Milk and Products, Dubai, Dubai—United Arab Emirates where the samples originate from, but these authors declare no conflicts of interest related to the study. László Makrai is the owner and CEO of Autovakcina Ltd., Budapest, Hungary, but declares no conflicts of interest to the study. All the other authors likewise declare no conflicts of interest.
Appendix A

Table A1.
List of primers for PCR reactions used in the study. Targets with more than two primers were detected with multiplex reactions. Primers taken from ref. [] encode tRNA genes. Sp1-17 refer to the prophage genes of the EHEC O157:H7 Sakai strain. Their primers were grouped into triplex reactions, as indicated (for details see ref. []). Abbreviations of pathotypes: STEC: Shiga toxigenic E. coli; EPEC: enteropathogenic E. coli; NTEC: necrotoxigenic E. coli.
Table A1.
List of primers for PCR reactions used in the study. Targets with more than two primers were detected with multiplex reactions. Primers taken from ref. [] encode tRNA genes. Sp1-17 refer to the prophage genes of the EHEC O157:H7 Sakai strain. Their primers were grouped into triplex reactions, as indicated (for details see ref. []). Abbreviations of pathotypes: STEC: Shiga toxigenic E. coli; EPEC: enteropathogenic E. coli; NTEC: necrotoxigenic E. coli.
| Target Gene/Region | Related Pathotype | Primer Name | Sequence 5′ -> 3′ | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|---|
| stx1 | STEC, EHEC | stx1-det-F1 | GTACGGGGATGCAGATAAATCGC | 52 | [] |
| stx1-det-R1 | AGCAGTCATTACATAAGAACGYCCACT | 52 | [] | ||
| stx2 | STEC, EHEC | F4 | GGCACTGTCTGAAACTGCTCCTGT | 52 | [] |
| R1 | ATTAAACTGCACTTCAGCAAATCC | 52 | [] | ||
| F4-f | CGCTGTCTGAGGCATCTCCGCT | 52 | [] | ||
| R1-e/f | TAAACTTCACCTGGGCAAAGCC | 52 | [] | ||
| eaeA | EPEC | B52 | AGGCTTCGTCACAGTTG | 52 | [] |
| B53 | CCATCGTCACCAGAGGA | 52 | [] | ||
| cdtB | various | CDT-s1 | GAAAGTAAATGGAATATAAATGTCCG | 55 | [] |
| CDT-s2 | GAAAATAAATGGAACACACATGTCCG | 55 | [] | ||
| CDT-as1 | AAATCACCAAGAATCATCCAGTTA | 55 | [] | ||
| CDT-as2 | AAATCTCCTGCAATCATCCAGTTA | 55 | [] | ||
| cnf1 | NTEC | CNF1-A | GAACTTATTAAGGATAGT | 50 | [] |
| CNF1-B | CATTATTTATAACGCTG | 50 | [] | ||
| cnf2 | NTEC | CNF1-A | AATCTAATTAAAGAGAAC | 50 | [] |
| CNF2-B | CATGCTTTGTATATCTA | 50 | [] | ||
| papC | ExPEC | pap1 | GACGGCTGTACTGCAGGGTGTGGCG | 65 | [] |
| pap2 | ATATCCTTTCTGCAGGGATGCAATA | 65 | [] | ||
| sfa | ExPEC | sfa1 | CTCCGGAGAACTGGGTGCATCTTAC | 65 | [] |
| sfa2 | CGGAGGAGTAATTACAAACCTGGCA | 65 | [] | ||
| chuA | ChuA.1 | GACGAACCAACGGTCAGGAT | 59 | [] | |
| ChuA.2 | TGCCGCCAGTACCAAAGACA | 59 | [] | ||
| yjaA | YjaA.1 | TGAAGTGTCAGGAGACGCTG | 59 | [] | |
| YjaA.2 | ATGGAGAATGCGTTCCTCAAC | 59 | [] | ||
| TspE4.C2 | TspE4C2.1 | GAGTAATGTCGGGGCATTCA | 59 | [] | |
| TspE4C2.2 | CGCGCCAACAAAGTATTACG | 59 | [] | ||
| arpA group E | ArpAgpE.f | GATTCCATCTTGTCAAAATATGCC | 59 | [] | |
| ArpAgpE.r | GAAAAGAAAAAGAATTCCCAAGAG | 59 | [] | ||
| arpA group C | trpAgpC.1 | AGTTTTATGCCCAGTGCGAG | 59 | [] | |
| trpAgpC.2 | TCTGCGCCGGTCACGCCC | 59 | [] | ||
| asnT | asnT1 | TATTCGCCCCGTTCACACG | 60 | [] | |
| asnT2 | GATACCCGCAGTTAAGCGG | 60 | [] | ||
| pheV | pheV1 | ACGAGACGAGGCGAATCAG | 60 | [] | |
| pheV2 | CCGTTGAGCGAACGGATTG | 60 | [] | ||
| serX | serX1 | CTCTCTTGCGCATTTTGATTG | 60 | [] | |
| serX2 | AGACAGGAACGACAATTTGG | 60 | [] | ||
| thrW | thrW1 | AAGGCCATTGACGCATCGC | 60 | [] | |
| thrW2 | CCATTTACCCCACTCAGCG | 60 | [] | ||
| selC | selC1 | GCGTGTATTAGGCGGAAAAAAC | 60 | [] | |
| selC2 | CCCTGAACTTCCCCACAAC | 60 | [] | ||
| leuX | leuX1 | GTGGCGTGCGACAGGTATA | 60 | [] | |
| leuX2 | GTTTCTCCGGCCCTAAGAC | 60 | [] | ||
| chuA | chu1 | GGTATTTATGGTTCAGTGATG | 60 | [] | |
| chu4 | TTTTCTCACTCAAATTGAACG | 60 | [] | ||
| waaC | waalücke 1 | CGCACTCACTGATGCCCAGCA | 60 | [] | |
| waalücke 2 | AGTCCAATCCATGCTTTACGCCAT | 60 | [] | ||
| Sp1 (reaction 1) | 31.1-f | CGCCAGCTAAATCGAACCGCAT | 68 | [] | |
| 31.1-r | CGGCTGATGATGACGACTTACTG | 68 | [] | ||
| Sp3 (reaction 1) | 84.3-f | CAGCAGATTGAAGCAGCACTCG | 68 | [] | |
| 84.3-r | GAATAAGAGCTGAGTCGTGCGG | 68 | [] | ||
| Sp4 (reaction 1) | 106.5-f | ACGATTGAGCTGACACCGGGC | 68 | [] | |
| 106.5-r | CCGGGCTTAATGTGCGGGCC | 68 | [] | ||
| Sp5 (reaction 2) | 110.2-f | CGAAGGGGCAACCGCGAAAATA | 68 | [] | |
| 110.2-r | CCCTTGTTACTTTCAGCATTCCG | 68 | [] | ||
| Sp6 (reaction 2) | 122.1-f | GGTGATGGTTTGTGGGAGAGGT | 68 | [] | |
| 122.1-r | TTGGGGGCTTAACGAATACCCC | 68 | [] | ||
| Sp8 (reaction 2) | 124.3-f | GCGTGCAGGTAATGGTAATCCG | 68 | [] | |
| 124.3-r | TTTAATGCCGTCCTGTTCCTGAGA | 68 | [] | ||
| Sp10 (reaction 3) | 145.3-f | TCCGGCATTTTCCCTGACACCA | 68 | [] | |
| 145.3-r | TATCGCGTGCCTCCTGGGTTAT | 68 | [] | ||
| Sp9 (reaction 3) | 133.1-f | GGCATCTAACGGTCTGGTGCC | 68 | [] | |
| 133.1-r | CAGCAGAAGCGAACAGCCGTCT | 68 | [] | ||
| Sp11 (reaction 3) | 164.1-f | TGACATCCACCACATCCGCAGAA | 68 | [] | |
| 164.1-r | TGTGAGGAAGAGCAGACGGAGA | 68 | [] | ||
| Sp15 (reaction 4) | 220.4-f | TACAGCGAATGCCAAATACGCTC | 68 | [] | |
| 220.4-r | TCACCCCTACAGAGAGCAAAAGAG | 68 | [] | ||
| Sp14 (reaction 4) | 204.4-f | CCAAAATACATCCACCCACCGCA | 68 | [] | |
| 204.4-r | AACGCATAGAAGAGCTGGAGGC | 68 | [] | ||
| Sp17 (reaction 4) | 276.1-f | CAGGTGGGTTGGGTAAGGTTTG | 68 | [] | |
| 276.1-r | GATGGCTGCTATGGGGATGGC | 68 | [] |
References
- Russo, T.A.; Johnson, J.R. Proposal for a New Inclusive Designation for Extraintestinal Pathogenic Isolates of Escherichia coli: ExPEC. J. Infect. Dis. 2000, 181, 1753–1754. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-Antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Bunduki, G.K.; Heinz, E.; Phiri, V.S.; Noah, P.; Feasey, N.; Musaya, J. Virulence Factors and Antimicrobial Resistance of Uropathogenic Escherichia coli (UPEC) Isolated from Urinary Tract Infections: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2021, 21, 753. [Google Scholar] [CrossRef]
- Dale, A.P.; Woodford, N. Extra-Intestinal Pathogenic Escherichia coli (ExPEC): Disease, Carriage and Clones. J. Infect. 2015, 71, 615–626. [Google Scholar] [CrossRef]
- Köhler, C.-D.; Dobrindt, U. What Defines Extraintestinal Pathogenic Escherichia coli? Int. J. Med. Microbiol. 2011, 301, 642–647. [Google Scholar] [CrossRef]
- Riley, L.W. Distinguishing Pathovars from Nonpathovars: Escherichia coli. Microbiol. Spectr. 2020, 8. [Google Scholar] [CrossRef]
- Bozcal, E. Insight into the Mobilome of Escherichia coli. In The Universe of Escherichia coli; IntechOpen: London, UK, 2019; ISBN 978-1-83881-153-2. [Google Scholar]
- Dobrindt, U.; Blum-Oehler, G.; Nagy, G.; Schneider, G.; Johann, A.; Gottschalk, G.; Hacker, J. Genetic Structure and Distribution of Four Pathogenicity Islands (PAI I536 to PAI IV536) of Uropathogenic Escherichia coli Strain 536. Infect. Immun. 2002, 70, 6365–6372. [Google Scholar] [CrossRef]
- Lloyd, A.L.; Rasko, D.A.; Mobley, H.L.T. Defining Genomic Islands and Uropathogen-Specific Genes in Uropathogenic Escherichia coli. J. Bacteriol. 2007, 189, 3532–3546. [Google Scholar] [CrossRef]
- Hochhut, B.; Wilde, C.; Balling, G.; Middendorf, B.; Dobrindt, U.; Brzuszkiewicz, E.; Gottschalk, G.; Carniel, E.; Hacker, J. Role of Pathogenicity Island-Associated Integrases in the Genome Plasticity of Uropathogenic Escherichia coli Strain 536. Mol. Microbiol. 2006, 61, 584–595. [Google Scholar] [CrossRef]
- Abbas, B.; Omer, O.H. Review of Infectious Diseases of the Camel. Vet. Bull. 2005, 75, 1N–16N. [Google Scholar]
- Bessalah, S.; Fairbrother, J.M.; Salhi, I.; Vanier, G.; Khorchani, T.; Seddik, M.M.; Hammadi, M. Antimicrobial Resistance and Molecular Characterization of Virulence Genes, Phylogenetic Groups of Escherichia coli Isolated from Diarrheic and Healthy Camel-Calves in Tunisia. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.; Tejedor-Junco, M.T.; González-Martín, M.; Corbera, J.A.; Silva, V.; Igrejas, G.; Torres, C.; Poeta, P. Escherichia coli Producing Extended-Spectrum β-Lactamases (ESBL) from Domestic Camels in the Canary Islands: A One Health Approach. Animals 2020, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Heikal, G.I.; Barhoma, R.M. Traceability of Enteropathogenic E. coli in Cattle and Camel Carcasses. Benha Vet. Med. J. 2016, 31, 50–55. [Google Scholar] [CrossRef]
- Saad, S.M.; Abou-Elroos, N.; Abou Elghar, I. Occurrence of Enterobacteriaceae in Fresh Camel Meat with Special Reference to Salmonellae. Benha Vet. Med. J. 2018, 35, 79–95. [Google Scholar] [CrossRef]
- Bottone, E.J. Hypermucoviscous Phenotype Expressed by an Isolate of Uropathogenic Escherichia coli: An Overlooked and Underappreciated Virulence Factor. Clin. Microbiol. Newsl. 2010, 32, 81–85. [Google Scholar] [CrossRef]
- Hayashi, T.; Makino, K.; Ohnishi, M.; Kurokawa, K.; Ishii, K.; Yokoyama, K.; Han, C.G.; Ohtsubo, E.; Nakayama, K.; Murata, T.; et al. Complete Genome Sequence of Enterohemorrhagic Escherichia coli O157:H7 and Genomic Comparison with a Laboratory Strain K-12. DNA Res. 2001, 8, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Dozois, C.M.; Clément, S.; Desautels, C.; Oswald, E.; Fairbrother, J.M. Expression of P, S, and F1C Adhesins by Cytotoxic Necrotizing Factor 1-Producing Escherichia coli from Septicemic and Diarrheic Pigs. FEMS Microbiol. Lett. 1997, 152, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The Complete Genome Sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Blum, G.; Ott, M.; Lischewski, A.; Ritter, A.; Imrich, H.; Tschäpe, H.; Hacker, J. Excision of Large DNA Regions Termed Pathogenicity Islands from tRNA-Specific Loci in the Chromosome of an Escherichia coli Wild-Type Pathogen. Infect. Immun. 1994, 62, 606–614. [Google Scholar] [CrossRef]
- Oswald, E.; de Rycke, J.; Lintermans, P.; van Muylem, K.; Mainil, J.; Daube, G.; Pohl, P. Virulence Factors Associated with Cytotoxic Necrotizing Factor Type Two in Bovine Diarrheic and Septicemic Strains of Escherichia coli. J. Clin. Microbiol. 1991, 29, 2522–2527. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 5th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli Phylo-Typing Method Revisited: Improvement of Specificity and Detection of New Phylo-Groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Tóth, I.; Bagyinszky, E.; Sváb, D. Multiplex Polymerase Chain Reaction Typing Scheme Based on Escherichia coli O157:H7 Sakai Prophage (Sp)-Associated Genes. Int. J. Infect. Dis. 2022, 120, 68–76. [Google Scholar] [CrossRef]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and Simple Determination of the Escherichia coli Phylogenetic Group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef]
- Shon, A.S.; Bajwa, R.P.S.; Russo, T.A. Hypervirulent (Hypermucoviscous) Klebsiella Pneumoniae. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef]
- Neumann, B.; Lippmann, N.; Wendt, S.; Karlas, T.; Lübbert, C.; Werner, G.; Pfeifer, Y.; Schuster, C.F. Recurrent Bacteremia with a Hypermucoviscous Escherichia coli Isolated from a Patient with Perihilar Cholangiocarcinoma: Insights from a Comprehensive Genome-Based Analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 28. [Google Scholar] [CrossRef]
- Harris, S.; Piotrowska, M.J.; Goldstone, R.J.; Qi, R.; Foster, G.; Dobrindt, U.; Madec, J.-Y.; Valat, C.; Rao, F.V.; Smith, D.G.E. Variant O89 O-Antigen of E. Coli Is Associated With Group 1 Capsule Loci and Multidrug Resistance. Front. Microbiol. 2018, 9, 9026. [Google Scholar] [CrossRef]
- Benham, A.; Davis, J.; Puzio, C.; Blakey, G.; Slobodov, G. Renal Abscess Yields Elusive Hypermucoviscous Phenotype of, Uropathogenic Escherichia coli: A Case Report. J. Okla. State Med. Assoc. 2013, 106, 435–438. [Google Scholar]
- Namikawa, H.; Oinuma, K.-I.; Yamada, K.; Kaneko, Y.; Kakeya, H.; Shuto, T. Differences in Severity of Bacteraemia Caused by Hypermucoviscous and Non-Hypermucoviscous Klebsiella Pneumoniae. Int. J. Antimicrob. Agents 2023, 61, 106767. [Google Scholar] [CrossRef]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fèvre, E.M.; Woolhouse, M.E.J.; van Bunnik, B.A.D. Are Food Animals Responsible for Transfer of Antimicrobial-Resistant Escherichia coli or Their Resistance Determinants to Human Populations? A Systematic Review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef]
- Zhang, S.; Abbas, M.; Rehman, M.U.; Huang, Y.; Zhou, R.; Gong, S.; Yang, H.; Chen, S.; Wang, M.; Cheng, A. Dissemination of Antibiotic Resistance Genes (ARGs) via Integrons in Escherichia coli: A Risk to Human Health. Environ. Pollut. 2020, 266, 115260. [Google Scholar] [CrossRef]
- Isler, M.; Wissmann, R.; Morach, M.; Zurfluh, K.; Stephan, R.; Nüesch-Inderbinen, M. Animal Petting Zoos as Sources of Shiga Toxin-Producing Escherichia coli, Salmonella and Extended-Spectrum β-Lactamase (ESBL)-Producing Enterobacteriaceae. Zoonoses Public Health 2021, 68, 79–87. [Google Scholar] [CrossRef]
- Fadlelmula, A.; Al-Hamam, N.A.; Al-Dughaym, A.M. A Potential Camel Reservoir for Extended-Spectrum β-Lactamase-Producing Escherichia coli Causing Human Infection in Saudi Arabia. Trop. Anim. Health Prod. 2016, 48, 427–433. [Google Scholar] [CrossRef]
- Saidani, M.; Messadi, L.; Mefteh, J.; Chaouechi, A.; Soudani, A.; Selmi, R.; Dâaloul-Jedidi, M.; Ben Chehida, F.; Mamlouk, A.; Jemli, M.H.; et al. Various Inc-Type Plasmids and Lineages of Escherichia coli and Klebsiella Pneumoniae Spreading blaCTX-M-15, blaCTX-M-1 and Mcr-1 Genes in Camels in Tunisia. J. Glob. Antimicrob. Resist. 2019, 19, 280–283. [Google Scholar] [CrossRef]
- Zhang, S.; Abbas, M.; Rehman, M.U.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhu, D.; Zhao, X.; Gao, Q.; et al. Updates on the Global Dissemination of Colistin-Resistant Escherichia coli: An Emerging Threat to Public Health. Sci. Total Environ. 2021, 799, 149280. [Google Scholar] [CrossRef]
- De Rycke, J.; González, E.A.; Blanco, J.; Oswald, E.; Blanco, M.; Boivin, R. Evidence for Two Types of Cytotoxic Necrotizing Factor in Human and Animal Clinical Isolates of Escherichia coli. J. Clin. Microbiol. 1990, 28, 694–699. [Google Scholar] [CrossRef]
- Schmoll, T.; Hoschützky, H.; Morschhäuser, J.; Lottspeich, F.; Jann, K.; Hacker, J. Analysis of Genes Coding for the Sialic Acid-Binding Adhesin and Two Other Minor Fimbrial Subunits of the S-Fimbrial Adhesin Determinant of Escherichia coli. Mol. Microbiol. 1989, 3, 1735–1744. [Google Scholar] [CrossRef]
- Dobrindt, U.; Blum-Oehler, G.; Hartsch, T.; Gottschalk, G.; Ron, E.Z.; Fünfstück, R.; Hacker, J. S-Fimbria-Encoding Determinant sfaI Is Located on Pathogenicity Island III536 of Uropathogenic Escherichia coli Strain 536. Infect. Immun. 2001, 69, 4248–4256. [Google Scholar] [CrossRef]
- Fabbri, A.; Travaglione, S.; Fiorentini, C. Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1): Toxin Biology, in Vivo Applications and Therapeutic Potential. Toxins 2010, 2, 283–296. [Google Scholar] [CrossRef]
- De Rycke, J.; Milon, A.; Oswald, E. Necrotoxic Escherichia coli (NTEC): Two Emerging Categories of Human and Animal Pathogens. Vet. Res. 1999, 30, 221–233. [Google Scholar]
- Archambaud, M.; Courcoux, P.; Labigne-Roussel, A. Detection by Molecular Hybridization of Pap, Afa, and Sea Adherence Systems in Escherichia coli Strains Associated with Urinary and Enteral Infections. Ann. De L’institut Pasteur/Microbiol. 1988, 139, 575–588. [Google Scholar] [CrossRef]
- Lai, Y.; Rosenshine, I.; Leong, J.M.; Frankel, G. Intimate Host Attachment: Enteropathogenic and Enterohaemorrhagic Escherichia coli. Cell. Microbiol. 2013, 15, 1796–1808. [Google Scholar] [CrossRef]
- Gomes, T.A.T.; Elias, W.P.; Scaletsky, I.C.A.; Guth, B.E.C.; Rodrigues, J.F.; Piazza, R.M.F.; Ferreira, L.C.S.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef]
- Yang, K.; Pagaling, E.; Yan, T. Estimating the Prevalence of Potential Enteropathogenic Escherichia coli and Intimin Gene Diversity in a Human Community by Monitoring Sanitary Sewage. Appl. Environ. Microbiol. 2014, 80, 119–127. [Google Scholar] [CrossRef]
- Hernandes, R.T.; Elias, W.P.; Vieira, M.A.M.; Gomes, T.A.T. An Overview of Atypical Enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 2009, 297, 137–149. [Google Scholar] [CrossRef]
- Narnaware, S.D.; Ranjan, R.; Jyotsana, B.; Sahoo, A. Virulence Genes and Antimicrobial Resistance Profile of Escherichia coli Isolates from Diarrhoeic Neonatal Dromedary Camels. J. Camel Pract. Res. 2022, 29, 127–132. [Google Scholar] [CrossRef]
- Le Gall, T.; Clermont, O.; Gouriou, S.; Picard, B.; Nassif, X.; Denamur, E.; Tenaillon, O. Extraintestinal Virulence Is a Coincidental By-Product of Commensalism in B2 Phylogenetic Group Escherichia coli Strains. Mol. Biol. Evol. 2007, 24, 2373–2384. [Google Scholar] [CrossRef]
- Morgan, R.N.; Saleh, S.E.; Farrag, H.A.; Aboulwafa, M.M. Prevalence and Pathologic Effects of Colibactin and Cytotoxic Necrotizing Factor-1 (Cnf 1) in Escherichia coli: Experimental and Bioinformatics Analyses. Gut Pathog. 2019, 11, 22. [Google Scholar] [CrossRef]
- Ismail, L.C.; Osaili, T.M.; Mohamad, M.N.; Zakaria, H.; Ali, A.; Tarek, A.; Ashfaq, A.; Abdouli, M.A.A.; Saleh, S.T.; Daour, R.A.; et al. Camel Milk Consumption Patterns and Perceptions in the UAE: A Cross-Sectional Study. J. Nutr. Sci. 2022, 11, e59. [Google Scholar] [CrossRef]
- Vidovic, N.; Vidovic, S. Antimicrobial Resistance and Food Animals: Influence of Livestock Environment on the Emergence and Dissemination of Antimicrobial Resistance. Antibiotics 2020, 9, 52. [Google Scholar] [CrossRef]
- Carvalho, I.; Silva, N.; Carrola, J.; Silva, V.; Currie, C.; Igrejas, G.; Poeta, P. Antibiotic Resistance. In Antibiotic Drug Resistance; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 239–259. ISBN 978-1-119-28254-9. [Google Scholar]
- Huss, P.; Raman, S. Engineered Bacteriophages as Programmable Biocontrol Agents. Curr. Opin. Biotechnol. 2020, 61, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Grozdanov, L.A. Analysis of the Genome Organization and Fitness Traits of Non-Pathogenic Escherichia coli Strain Nissle 1917 (O6:K5:H1); Universität Würzburg: Würzburg, Germany, 2004. [Google Scholar]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Piérard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S.; et al. Multicenter Evaluation of a Sequence-Based Protocol for Subtyping Shiga Toxins and Standardizing Stx Nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- China, B.; Pirson, V.; Mainil, J. Typing of Bovine Attaching and Effacing Escherichia coli by Multiplex in Vitro Amplification of Virulence-Associated Genes. Appl. Environ. Microbiol. 1996, 62, 3462–3465. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.; Herault, F.; Beutin, L.; Oswald, E. Production of Cytolethal Distending Toxins by Pathogenic Escherichia coli Strains Isolated from Human and Animal Sources: Establishment of the Existence of a New Cdt Variant (Type IV). J. Clin. Microbiol. 2003, 41, 4285–4291. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Blanco, J.E.; Blanco, J.; Alonso, M.P.; Balsalobre, C.; Mouriño, M.; Madrid, C.; Juárez, A. Polymerase Chain Reaction for Detection of Escherichia coli Strains Producing Cytotoxic Necrotizing Factor Type 1 and Type 2 (CNF1 and CNF2). J. Microbiol. Methods 1996, 26, 95–101. [Google Scholar] [CrossRef]
- Le Bouguenec, C.; Archambaud, M.; Labigne, A. Rapid and Specific Detection of the Pap, Afa, and Sfa Adhesin-Encoding Operons in Uropathogenic Escherichia coli Strains by Polymerase Chain Reaction. J. Clin. Microbiol. 1992, 30, 1189–1193. [Google Scholar] [CrossRef]
- Lescat, M.; Clermont, O.; Woerther, P.L.; Glodt, J.; Dion, S.; Skurnik, D.; Djossou, F.; Dupont, C.; Perroz, G.; Picard, B.; et al. Commensal Escherichia coli Strains in Guiana Reveal a High Genetic Diversity with Host-Dependant Population Structure. Environ. Microbiol. Rep. 2013, 5, 49–57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).