A Perspective of the Epidemiology of Rabies in South Africa, 1998–2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Curation
2.2. Spatio-Temporal Analysis of Rabies Cases in South Africa
2.3. Calculating Rabies Testing Rates for South Africa
2.4. Dog Population Estimates
3. Results
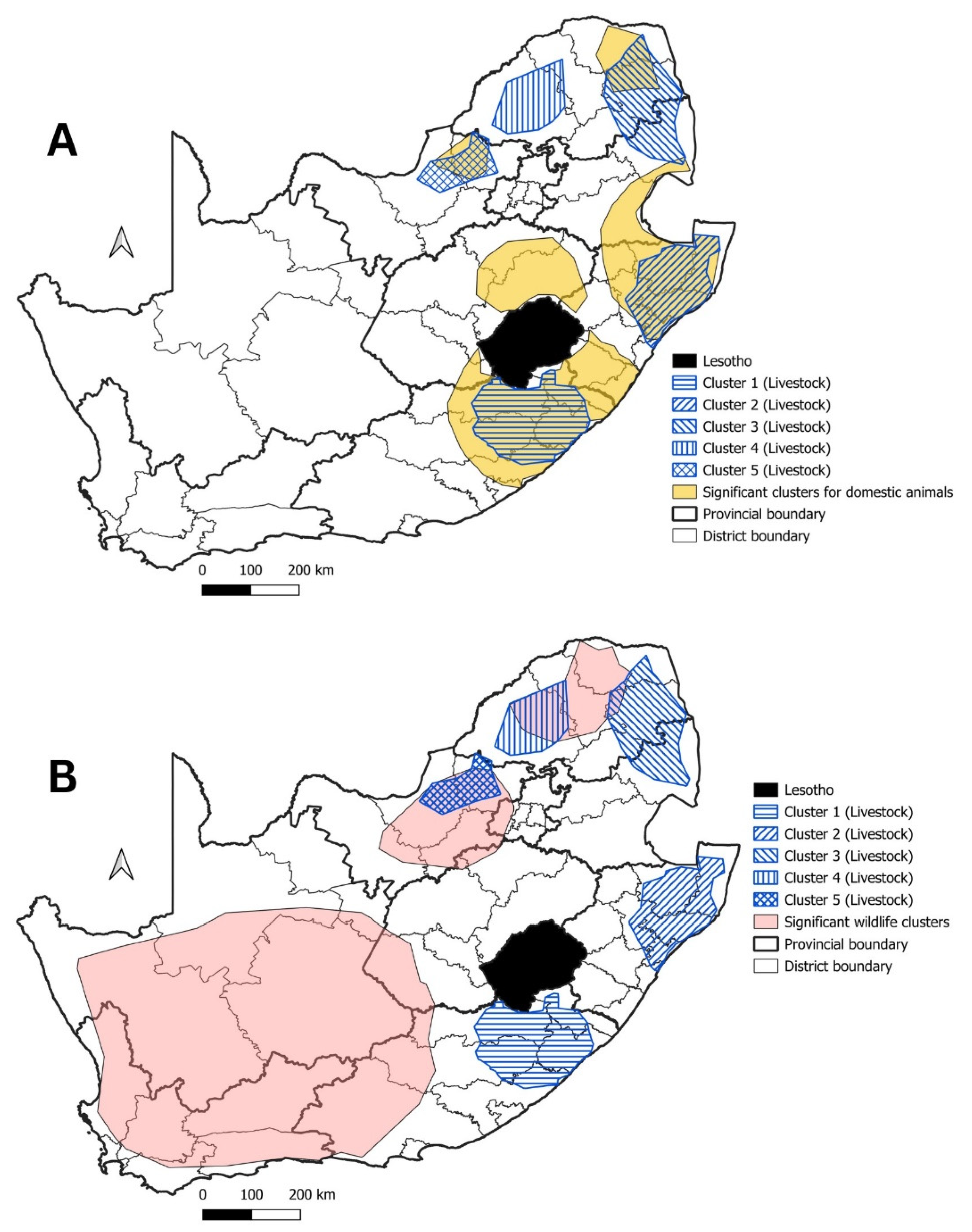
3.1. Domestic Animals
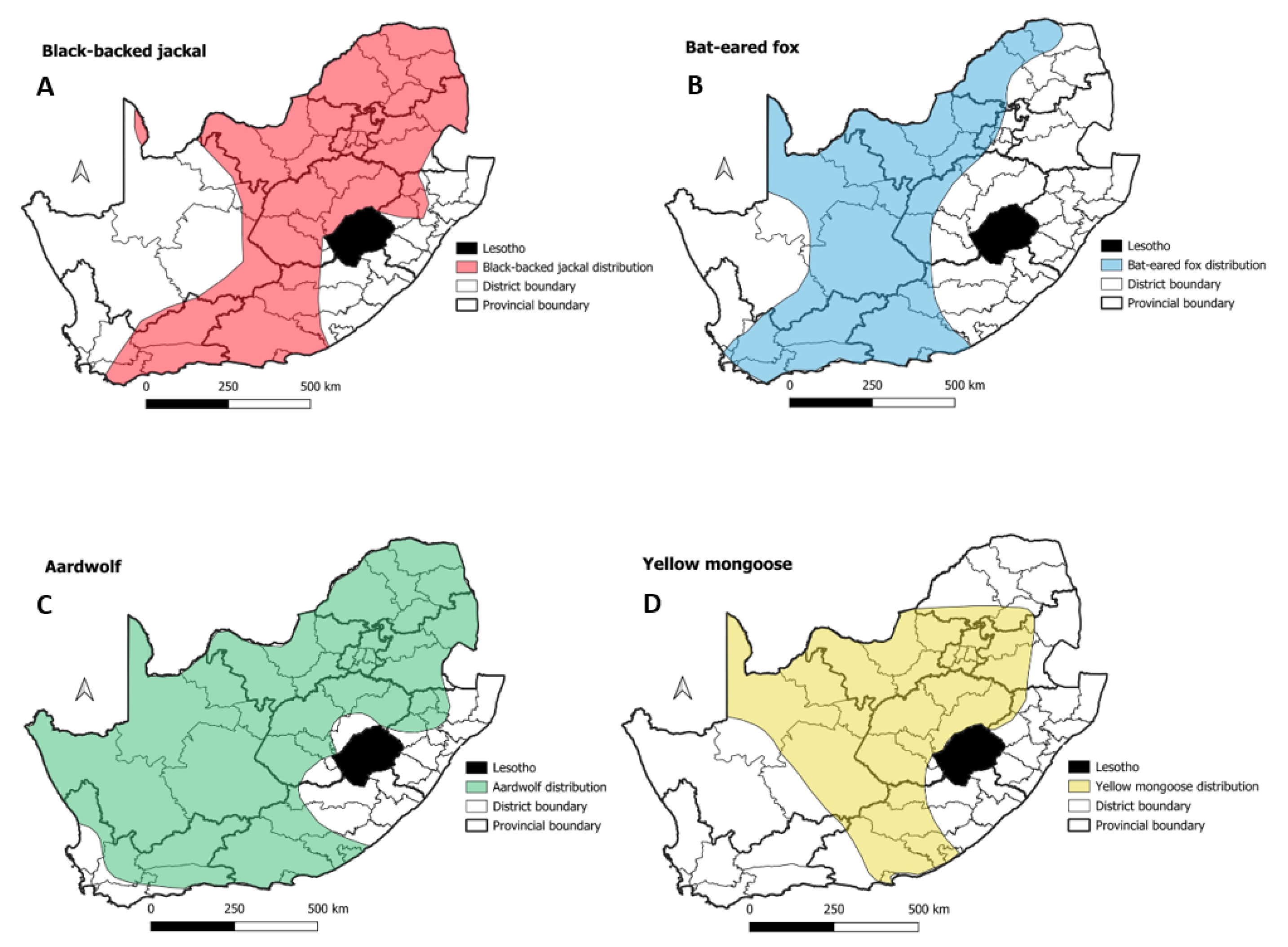
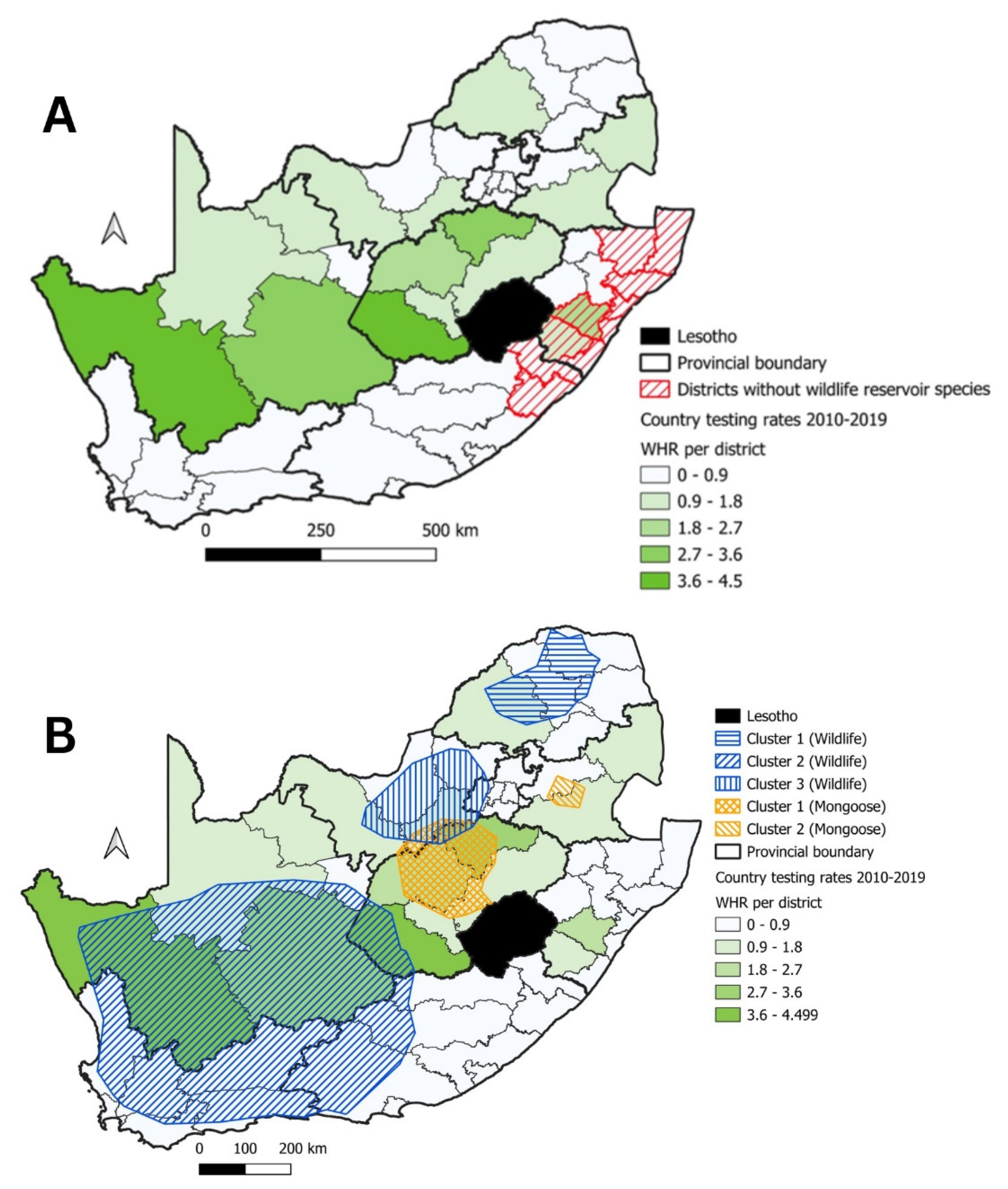
3.2. Mongooses and Other Wildlife species
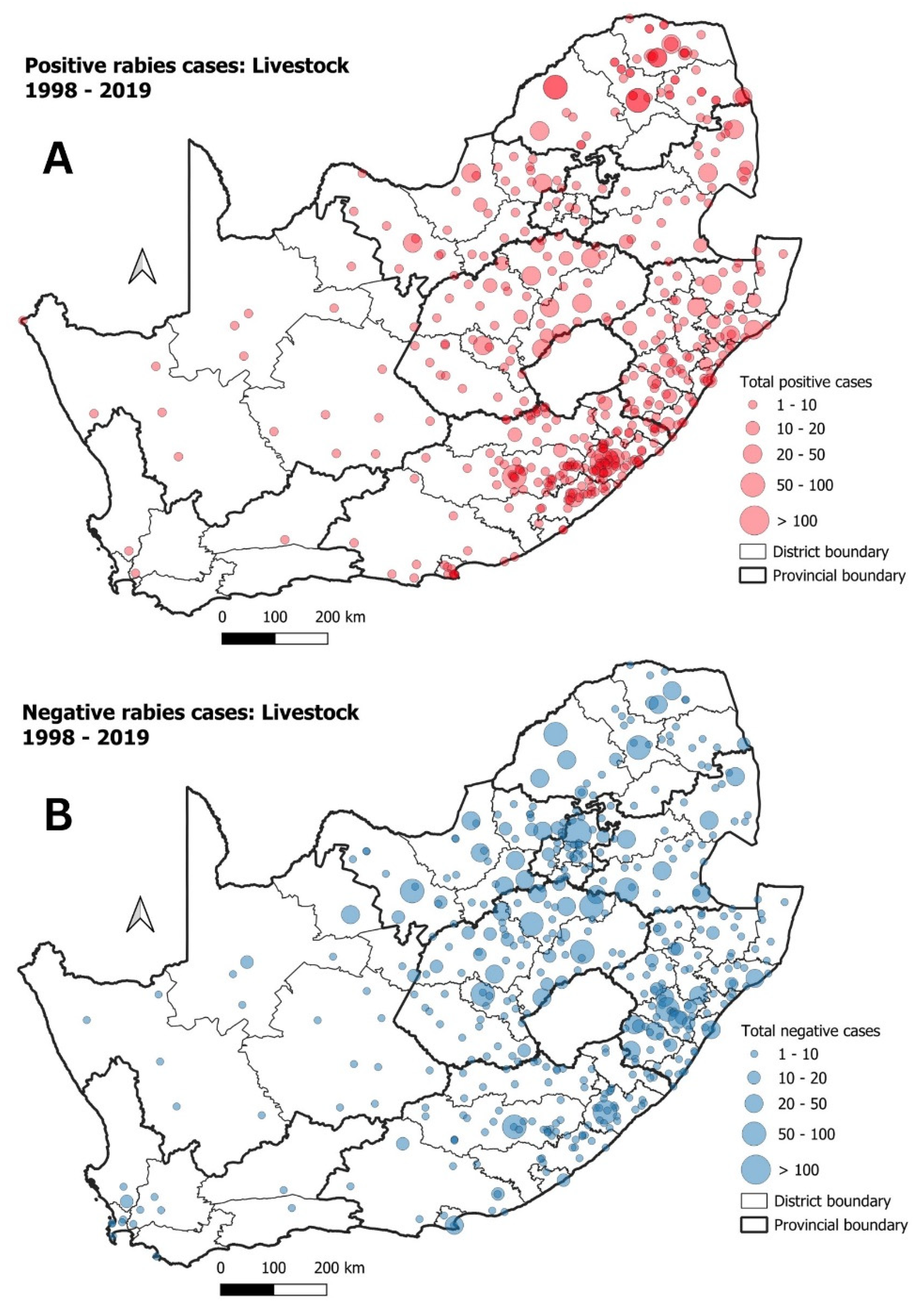
3.3. Livestock
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baer, G.M. 1—The History of Rabies, 2nd ed.; Jackson, A.C., Wunner, W.H.B.T.-R., Eds.; Academic Press: Oxford, UK, 2007; pp. 1–22. ISBN 978-0-12-369366-2. [Google Scholar]
- WHO. WHO Expert Consultation on Rabies: Third Report; WHO Technical Report Series; 1012; World Health Organization: Geneva, Switzerland, 2018; ISBN 9789241210218. [Google Scholar]
- Franka, R.; Wallace, R. Rabies Diagnosis and Surveillance in Animals in the Era of Rabies Elimination. Rev. Sci. Tech. 2018, 37, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Durr, S.; Mindekem, R.; Kaninga, Y.; Moto, D.D.; Meltzer, M.I.; Vounatsou, P.; Zinsstag, J. Effectiveness of Dog Rabies Vaccination Programmes: Comparison of Owner-Charged and Free Vaccination Campaigns. Epidemiol. Infect. 2009, 137, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag, J.; Lechenne, M.; Laager, M.; Mindekem, R.; Naïssengar, S.; Oussiguéré, A.; Bidjeh, K.; Rives, G.; Tessier, J.; Madjaninan, S.; et al. Vaccination of Dogs in an African City Interrupts Rabies Transmission and Reduces Human Exposure. Sci. Transl. Med. 2017, 9, eaaf6984. [Google Scholar] [CrossRef] [PubMed]
- Dodet, B.; Tejiokem, M.C.; Aguemon, A.-R.; Bourhy, H. Human Rabies Deaths in Africa: Breaking the Cycle of Indifference. Int. Health 2015, 7, 4–6. [Google Scholar] [CrossRef]
- Fahrion, A.S.; Taylor, L.H.; Torres, G.; Müller, T.; Dürr, S.; Knopf, L.; de Balogh, K.; Nel, L.H.; Gordoncillo, M.J.; Abela-Ridder, B. The Road to Dog Rabies Control and Elimination—What Keeps Us from Moving Faster? Front. Public Health 2017, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Nel, L.H. Discrepancies in Data Reporting for Rabies, Africa. Emerg. Infect. Dis. 2013, 19, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Townsend, S.E.; Lembo, T.; Cleaveland, S.; Meslin, F.X.; Miranda, M.E.; Putra, A.A.G.; Haydon, D.T.; Hampson, K. Surveillance Guidelines for Disease Elimination: A Case Study of Canine Rabies. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 249–261. [Google Scholar] [CrossRef]
- Swanepoel, R.; Barnard, B.J.H.; Meredith, C.D.; Bishop, G.; Bruckner, G.K.; Foggin, C.M.; Hubschle, O.J.B. Rabies in Southern Africa. Onderstepoort J. Vet. Res. 1993, 60, 325. [Google Scholar] [PubMed]
- Sabeta, C.T.; Mansfield, K.L.; McElhinney, L.M.; Fooks, A.R.; Nel, L.H. Molecular Epidemiology of Rabies in Bat-Eared Foxes (Otocyon megalotis) in South Africa. Virus Res. 2007, 129, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ngoepe, C.E.; Sabeta, C.; Nel, L. The Spread of Canine Rabies into Free State Province of South Africa: A Molecular Epidemiological Characterization. Virus Res. 2009, 142, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Mkhize, G.C.; Ngoepe, E.C.; Du Plessis, B.J.A.; Reininghaus, B.; Sabeta, C.T. Re-Emergence of Dog Rabies in Mpumalanga Province, South Africa. Vector-Borne Zoonotic Dis. 2010, 10, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Sabeta, C.T.; Weyer, J.; Geertsma, P.; Mohale, D.; Miyen, J.; Blumberg, L.H.; Leman, P.A.; Phahladira, B.; Shumba, W.; Walters, J. Emergence of Rabies in the Gauteng Province, South Africa: 2010–2011. J. S. Afr. Vet. Assoc. 2013, 84, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hergert, M.; Le Roux, K.; Nel, L.H. Characteristics of Owned Dogs in Rabies Endemic KwaZulu-Natal Province, South Africa. BMC Vet. Res. 2018, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Malan, A.J.; Coetzer, A.; Sabeta, C.T.; Nel, L.H. Epidemiological Interface of Sylvatic and Dog Rabies in the North West Province of South Africa. Trop. Med. Infect. Dis. 2022, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Ngoepe, E.; Chirima, J.G.; Mohale, D.; Mogano, K.; Suzuki, T.; Makita, K.; Sabeta, C.T. Rabies Outbreak in Black-Backed Jackals (Canis Mesomelas), South Africa, 2016. Epidemiol. Infect. 2022, 150, e137. [Google Scholar] [CrossRef] [PubMed]
- Zulu, G.C.; Sabeta, C.T.; Nel, L.H. Molecular Epidemiology of Rabies: Focus on Domestic Dogs (Canis Familiaris) and Black-Backed Jackals (Canis Mesomelas) from Northern South Africa. Virus Res. 2009, 140, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Sabeta, C.T.; Mkhize, G.C.; Ngoepe, E.C. An Evaluation of Dog Rabies Control in Limpopo Province (South Africa). Epidemiol. Infect. 2011, 139, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, N.; Markotter, W.; Nel, L.H. Evolutionary History of African Mongoose Rabies. Virus Res. 2010, 150, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Nel, L.H.; Sabeta, C.T.; von Teichman, B.; Jaftha, J.B.; Rupprecht, C.E.; Bingham, J. Mongoose Rabies in Southern Africa: A Re-Evaluation Based on Molecular Epidemiology. Virus Res. 2005, 109, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.L.; Rambaut, A.; Bourhy, H.; Holmes, E.C. The Evolutionary Dynamics of Canid and Mongoose Rabies Virus in Southern Africa. Arch. Virol. 2007, 152, 1251–1258. [Google Scholar] [CrossRef]
- Bishop, G.C.; Durrheim, D.N.; Kloeck, P.E.; Godlonton, J.D.; Bingham, J.; Speare, R.; Advisory, R. Guide for the Medical, Veterinary and Allied Professions, 2nd ed.; Department of Agriculture, Forestry, and Fisheries: Pretoria, South Africa, 2010. [Google Scholar]
- Ngoepe, C.E.; Shumba, W.; Sabeta, C. Evidence for a Host Switching in the Maintenance of Canid Rabies Variant in Two Wild Carnivore Species in the Northern Cape Province, South Africa. J. S. Afr. Vet. Assoc. 2024, 95, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, N.; Weyer, J.; Coertse, J.; Markotter, W. Evaluation of Taxonomic Characteristics of Matlo and Phala Bat Rabies-Related Lyssaviruses Identified in South Africa. Viruses 2023, 15, 2047. [Google Scholar] [CrossRef] [PubMed]
- Coertse, J.; Grobler, C.S.; Sabeta, C.T.; Seamark, E.C.J.; Kearney, T.; Paweska, J.T.; Markotter, W. Lyssaviruses in Insectivorous Bats, South Africa, 2003–2018. Emerg. Infect. Dis. 2020, 26, 3056–3060. [Google Scholar] [CrossRef] [PubMed]
- Coertse, J.; Markotter, W.; Le Roux, K.; Stewart, D.; Sabeta, C.T.; Nel, L.H. New Isolations of the Rabies-Related Mokola Virus from South Africa. BMC Vet. Res. 2017, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Coertse, J.; Geldenhuys, M.; Le Roux, K.; Markotter, W. Lagos Bat Virus, an Under-Reported Rabies-Related Lyssavirus. Viruses 2021, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Essel, V.; Weyer, J.; Kabuya, K.J. Prevention of Rabies in Humans: Published from the National Guidelines for the Prevention of Rabies in Humans, South Africa. SA Pharm. J. 2022, 89, 25–27. [Google Scholar]
- Weyer, J. Rabies in South Africa: Where Do We Stand in 2015? S. Afr. J. Infect. Dis. 2015, 30, 40–41. [Google Scholar] [CrossRef][Green Version]
- DALRRD; DoH. National Strategy for the Elimination of Canine Mediated Human Rabies in South Africa (2019–2030). Available online: https://www.dalrrd.gov.za/images/Branches/AgricProducHealthFoodSafety/animal-health/disease-control/information/dah-policy/national-strategy-for-the-elimination-of-canine-mediated-human-rabies-in-south-africa-2019.pdf (accessed on 21 March 2024).
- Mogano, K.; Suzuki, T.; Mohale, D.; Phahladira, B.; Ngoepe, E.; Kamata, Y.; Chirima, G.; Sabeta, C.; Makita, K. Spatio-Temporal Epidemiology of Animal and Human Rabies in Northern South Africa between 1998 and 2017. PLoS Neglected Trop. Dis. 2022, 16, e0010464. [Google Scholar] [CrossRef] [PubMed]
- Mohale, D.K.; Ngoepe, E.; Mparamoto, M.; Blumberg, L.; Sabeta, C.T. A Case Report on a Human Bite Contact with a Rabid Honey Badger Mellivora Capensis (Kromdraai Area, Cradle of Humankind, South Africa). Trop. Med. Infect. Dis. 2023, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, P.; Nel, L.H. Emerging Epidemic Dog Rabies in Coastal South Africa: A Molecular Epidemiological Analysis. Virus Res. 2007, 126, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Shwiff, S.A.; Hatch, B.; Anderson, A.; Nel, L.H.; Leroux, K.; Stewart, D.; de Scally, M.; Govender, P.; Rupprecht, C.E. Towards Canine Rabies Elimination in KwaZulu-Natal, South Africa: Assessment of Health Economic Data. Transbound. Emerg. Dis. 2016, 63, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Hergert, M.; Le Roux, K.; Nel, L.H. Risk Factors Associated with Nonvaccination Rabies Status of Dogs in KwaZulu-Natal, South Africa. Vet. Med. Res. Rep. 2016, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hergert, M.; Nel, L.H. Dog Bite Histories and Response to Incidents in Canine Rabies-Enzootic KwaZulu-Natal, South Africa. PLoS Negl. Trop. Dis. 2013, 7, e2059. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, K.; Kotze, J.; Perrett, K. Elimination of Dog-Mediated Human Rabies: The Burden of Human Rabies in Africa. Rev. Sci. Tech. Int. Off. Epizoot. 2018, 37, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Grewar, J. Rabies Events in the Western Cape Province 2010|Agriprobe. Available online: https://journals.co.za/doi/10.10520/EJC19017 (accessed on 26 October 2023).
- Weyer, J.; Dermaux-Msimang, V.; Grobbelaar, A.; Le Roux, C.; Moolla, N.; Paweska, J.T.; Blumberg, L.H. Epidemiology of Human Rabies in South Africa, 2008–2018. S. Afr. Med. J. 2020, 110, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Gummow, B.; Roefs, Y.A.A.; de Klerk, G. Rabies in South Africa between 1993 and 2005: What Has Been Achieved? J. S. Afr. Vet. Assoc. 2010, 81, 16–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koeppel, K.N.; van Schalkwyk, O.L.; Thompson, P.N. Patterns of Rabies Cases in South Africa between 1993 and 2019, Including the Role of Wildlife. Transbound. Emerg. Dis. 2022, 69, 836–848. [Google Scholar] [CrossRef]
- Kulldorf, M.; Information Management Services, Inc. SaTScan v8.0: Software for the Spatial and Space-Time Scan Statistics. Available online: http://www.satscan.org/ (accessed on 22 April 2022).
- Minhaj, F.S.; Bonaparte, S.C.; Boutelle, C.; Wallace, R.M. Analysis of Available Animal Testing Data to Propose Peer-Derived Quantitative Thresholds for Determining Adequate Surveillance Capacity for Rabies. Sci. Rep. 2023, 13, 3986. [Google Scholar] [CrossRef] [PubMed]
- StatsSA. Census 2022: Provinces at a Glance. 2023. Available online: https://census.statssa.gov.za/assets/documents/2022/Provinces_at_a_Glance.pdf (accessed on 28 November 2023).
- Van Sittert, S.J.; Raath, J.; Akol, G.W.; Miyen, J.M.; Mlahlwa, B.; Sabeta, C.T. Rabies in the Eastern Cape Province of South Africa-Where Are We Going Wrong? J. S. Afr. Vet. Assoc. 2010, 81, 207–215. [Google Scholar] [CrossRef]
- Conan, A.; Geerdes, J.A.C.; Akerele, O.A.; Reininghaus, B.; Simpson, G.J.G.; Knobel, D. Census and Vaccination Coverage of Owned Dog Populations in Four Resource-Limited Rural Communities, Mpumalanga Province, South Africa. J. S. Afr. Vet. Assoc. 2017, 88, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hampson, K.; Dushoff, J.; Cleaveland, S.; Haydon, D.T.; Kaare, M.; Packer, C.; Dobson, A. Transmission Dynamics and Prospects for the Elimination of Canine Rabies. PLoS Biol. 2009, 7, e53. [Google Scholar] [CrossRef] [PubMed]
- Ravensberg, M.F.; Fanoy, E.F.; Whelan, J.; Embregts, C.W.; Geurts van Kessel, C.H.; Strydom, J.H. Ongoing Rabies Outbreak in Dogs of Unprecedented Scale and Human Cases in Nelson Mandela Bay Municipality, South Africa, up to 13 February 2022. Eurosurveillance 2022, 27, 2200252. [Google Scholar] [CrossRef] [PubMed]
- Mogano, K.; Sabeta, C.T.; Suzuki, T.; Makita, K.; Chirima, G.J. Patterns of Animal Rabies Prevalence in Northern South Africa between 1998 and 2022. Trop. Med. Infect. Dis. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Kamler, J.F.; Stenkewitz, U.; Klare, U.; Jacobsen, N.F.; Macdonald, D.W. Resource Partitioning among Cape Foxes, Bat-eared Foxes, and Black-backed Jackals in South Africa. J. Wildl. Manag. 2012, 76, 1241–1253. [Google Scholar] [CrossRef]
- Kamler, J.F.; Gray, M.M.; Oh, A.; Macdonald, D.W. Genetic Structure, Spatial Organization, and Dispersal in Two Populations of Bat-eared Foxes. Ecol. Evol. 2013, 3, 2892–2902. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.L.; Pirk, C.W.W.; Bateman, P.W.; Cameron, E.Z.; Dalerum, F. Extension of the Diet of an Extreme Foraging Specialist, the Aardwolf (Proteles Cristata). Afr. Zool. 2011, 46, 194–196. [Google Scholar]
- de Vries, L.; Marneweck, D.; Dalerum, F.; Mills, M.G.L.; Yarnell, R.; Sliwa, A.; Do Linh San, E. A conservation assessment of Proteles cristata. In The Red List of mammals of South Africa, Swaziland and Lesotho; South African National Biodiversity Institute and the Endangered Wildlife Trust: Pretoria, South Africa, 2016. [Google Scholar]
- Von Teichman, B.F.; Thomson, G.R.; Meredith, C.D.; Nel, L.H. Molecular Epidemiology of Rabies Virus in South Africa: Evidence for Two Distinct Virus Groups. J. General. Virol. 1995, 76, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.L.; MacKenzie, D.I.; Nichols, J.D. Advances and Applications of Occupancy Models. Methods Ecol. Evol. 2014, 5, 1269–1279. [Google Scholar] [CrossRef]
- Pepin, B.; Choppin, J.; Ruthven, K.; Sinclair, N. Digital Curriculum Resources in Mathematics Education: Foundations for Change. ZDM 2017, 49, 645–661. [Google Scholar] [CrossRef]
- Artois, M.; Bengis, R.; Delahay, R.J.; Duchêne, M.-J.; Duff, J.P.; Ferroglio, E.; Gortazar, C.; Hutchings, M.R.; Kock, R.A.; Leighton, F.A. Wildlife Disease Surveillance and Monitoring. In Management of Disease in Wild Mammals; Springer: Tokyo, Japan, 2009; pp. 187–213. [Google Scholar] [CrossRef]
- Duncan, C.; Backus, L.; Lynn, T.; Powers, B.; Salman, M. Passive, Opportunistic Wildlife Disease Surveillance in the Rocky Mountain Region, USA. Transbound. Emerg. Dis. 2008, 55, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Lushasi, K.; Sambo, M.; Changalucha, J.; Ferguson, E.A.; Sikana, L.; Hampson, K.; Nouvellet, P.; Donnelly, C.A. Understanding the Incidence and Timing of Rabies Cases in Domestic Animals and Wildlife in South-East Tanzania in the Presence of Widespread Domestic Dog Vaccination Campaigns. Vet. Res. 2022, 53, 106. [Google Scholar] [CrossRef] [PubMed]
- Bingham, J.; Foggin, C.M. Jackal Rabies in Zimbabwe. Onderstepoort J. Vet. Res. 1993, 60, 365–366. [Google Scholar] [PubMed]

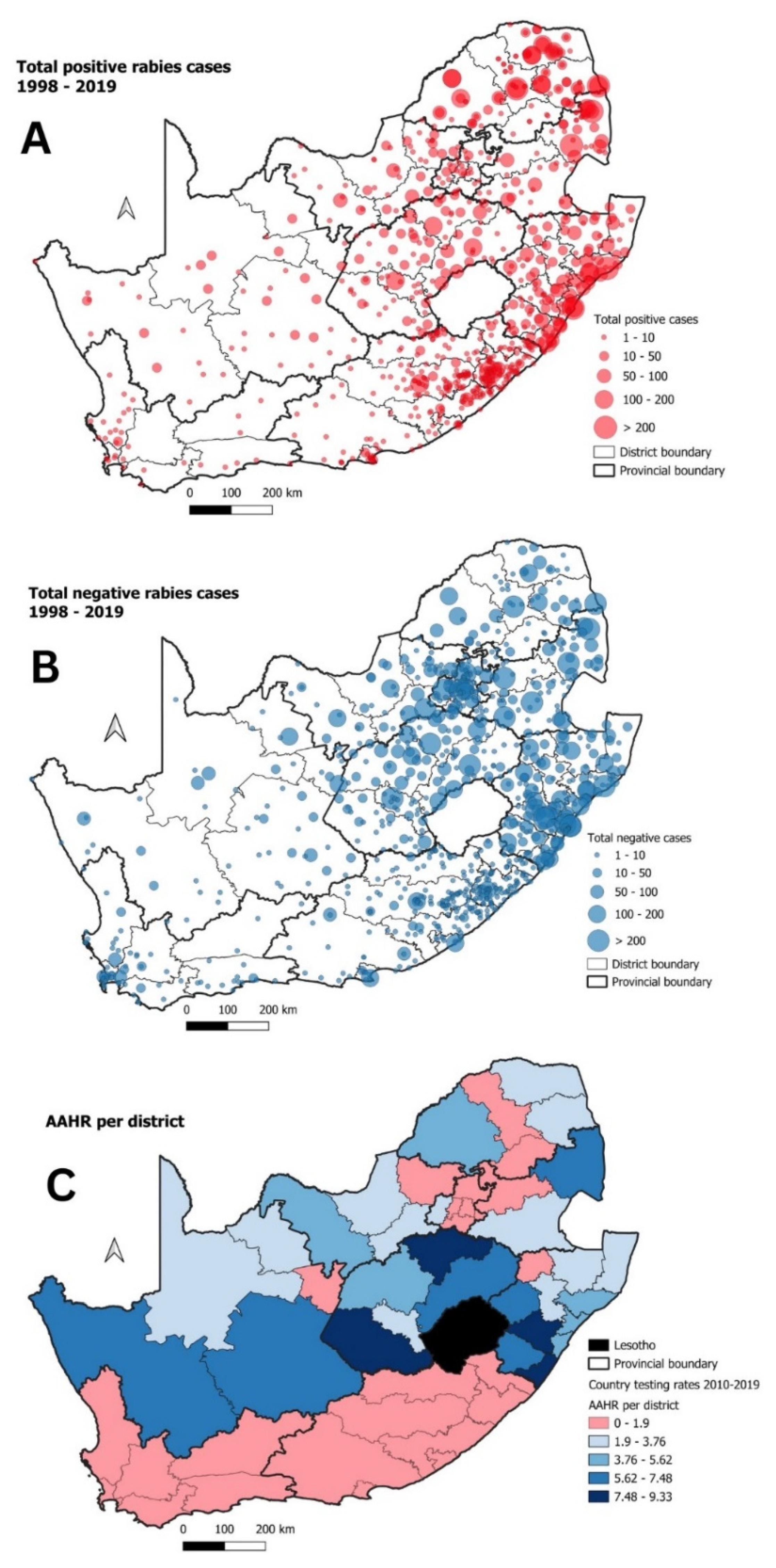
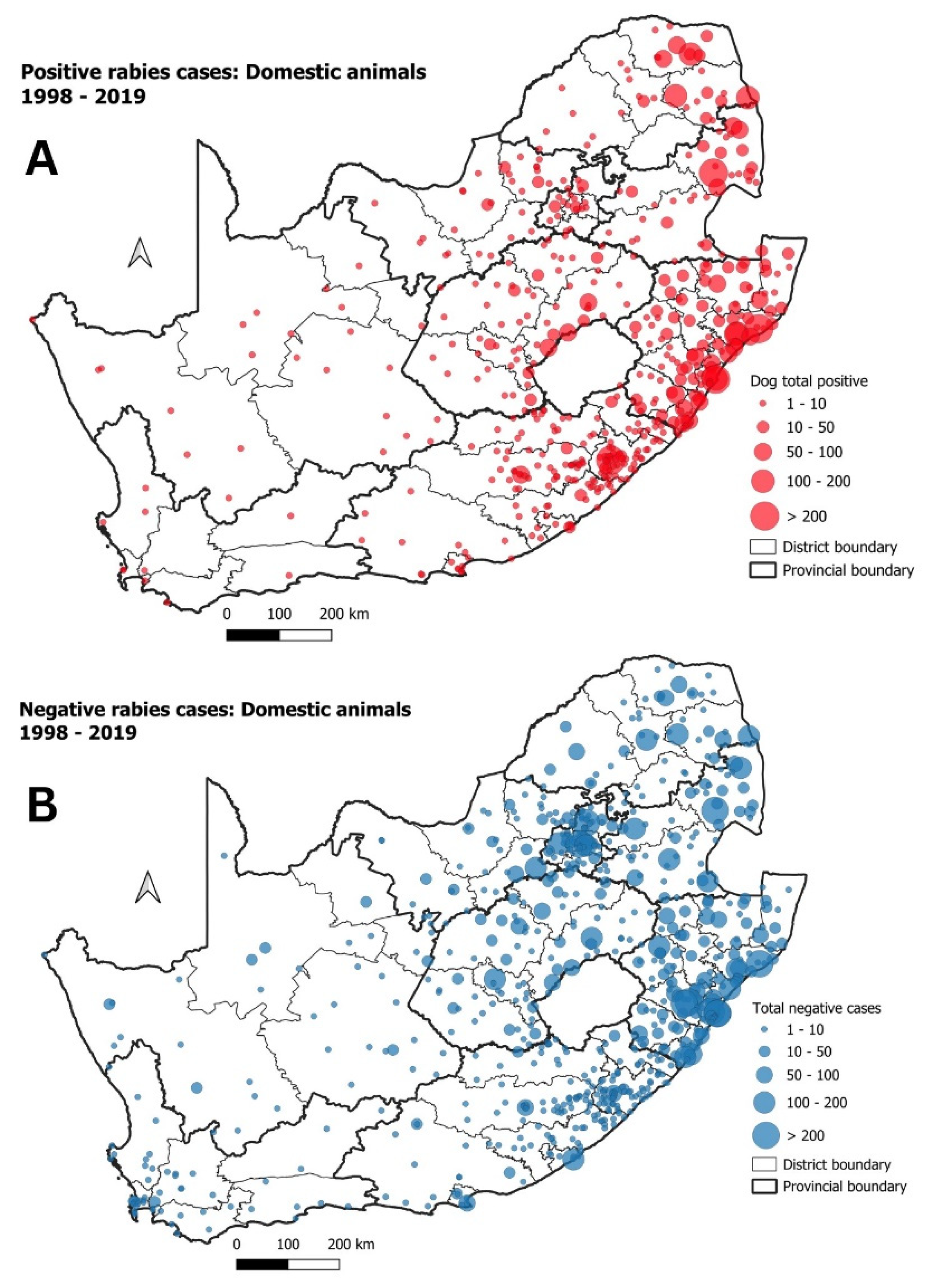
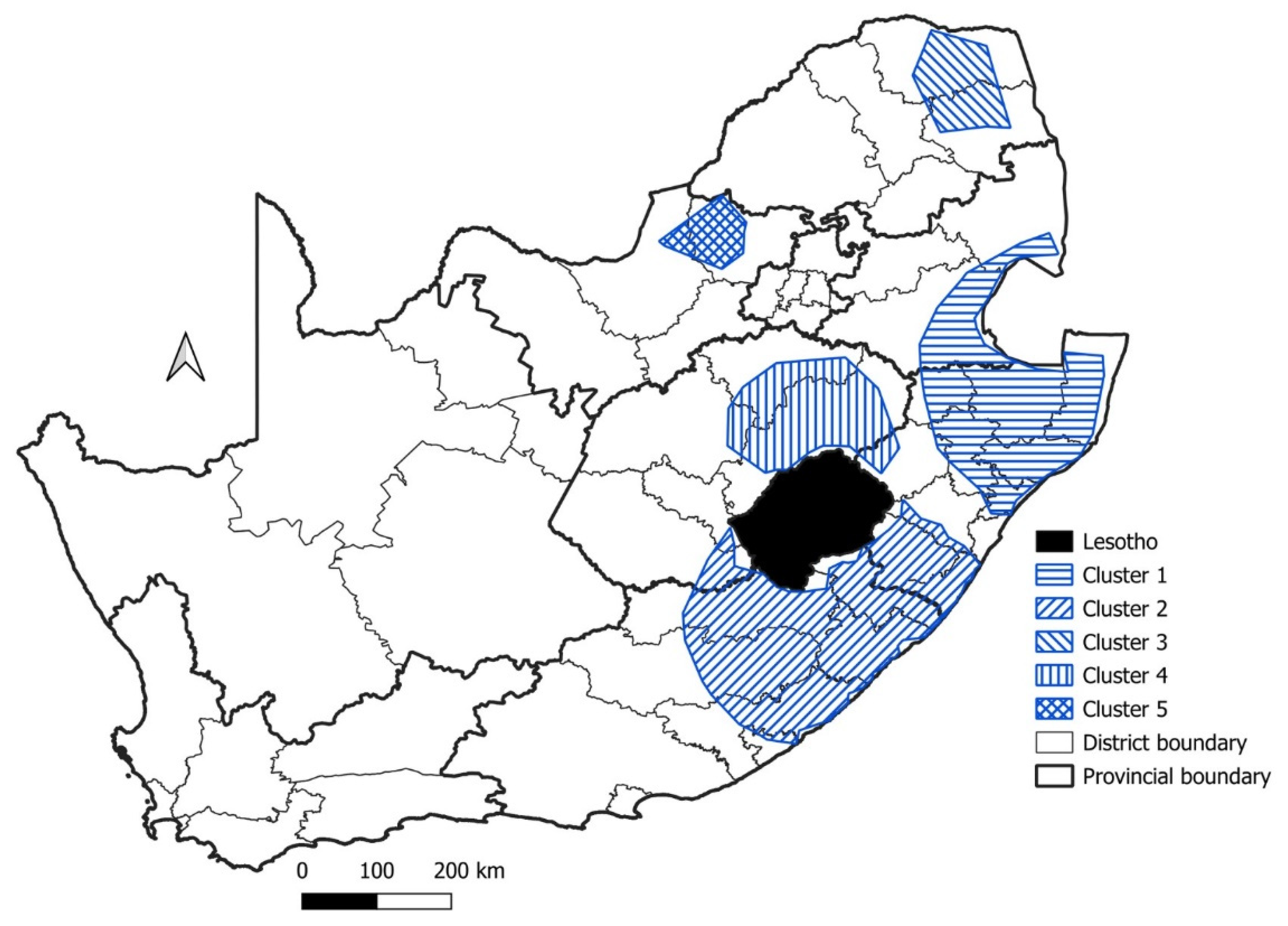
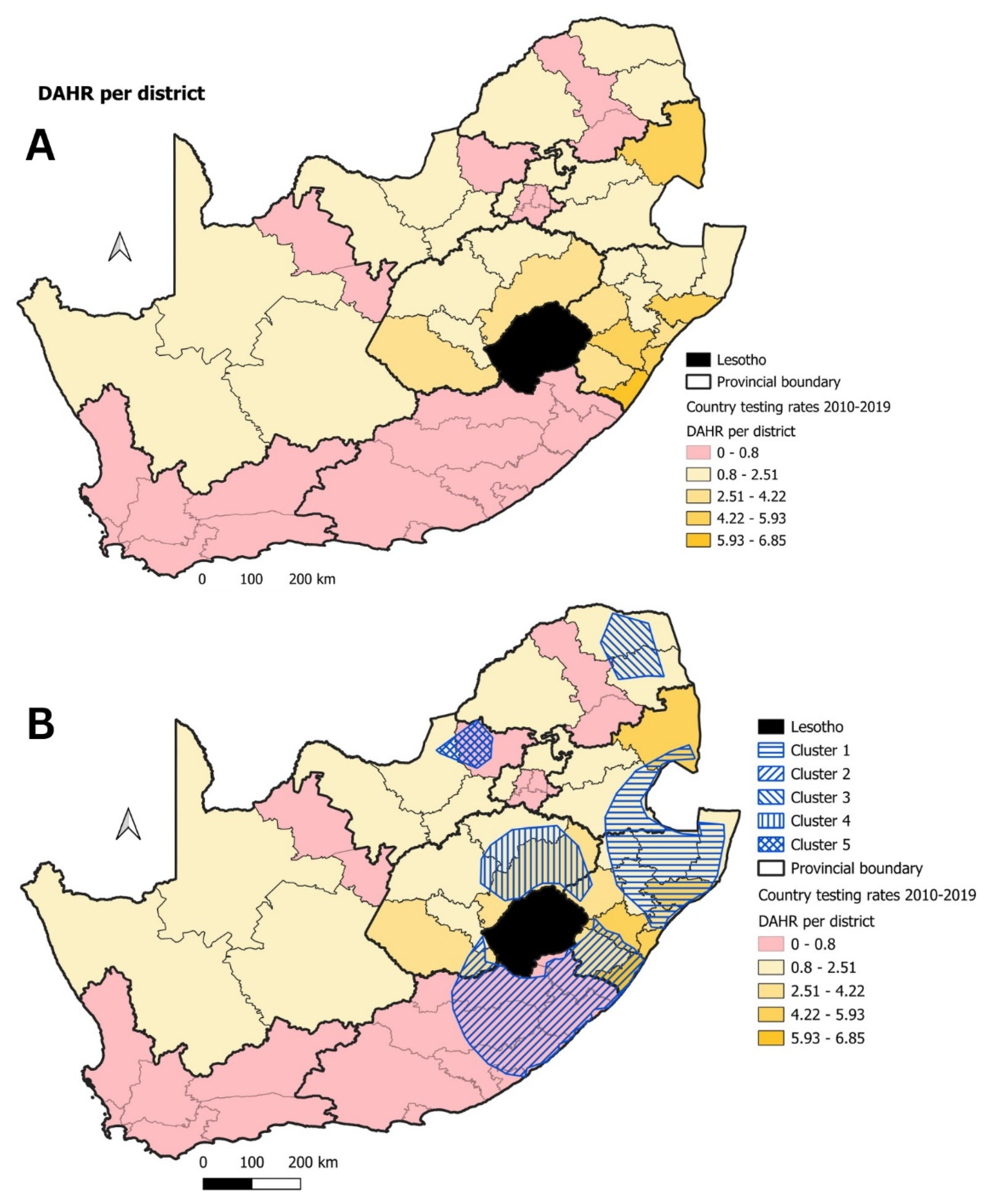
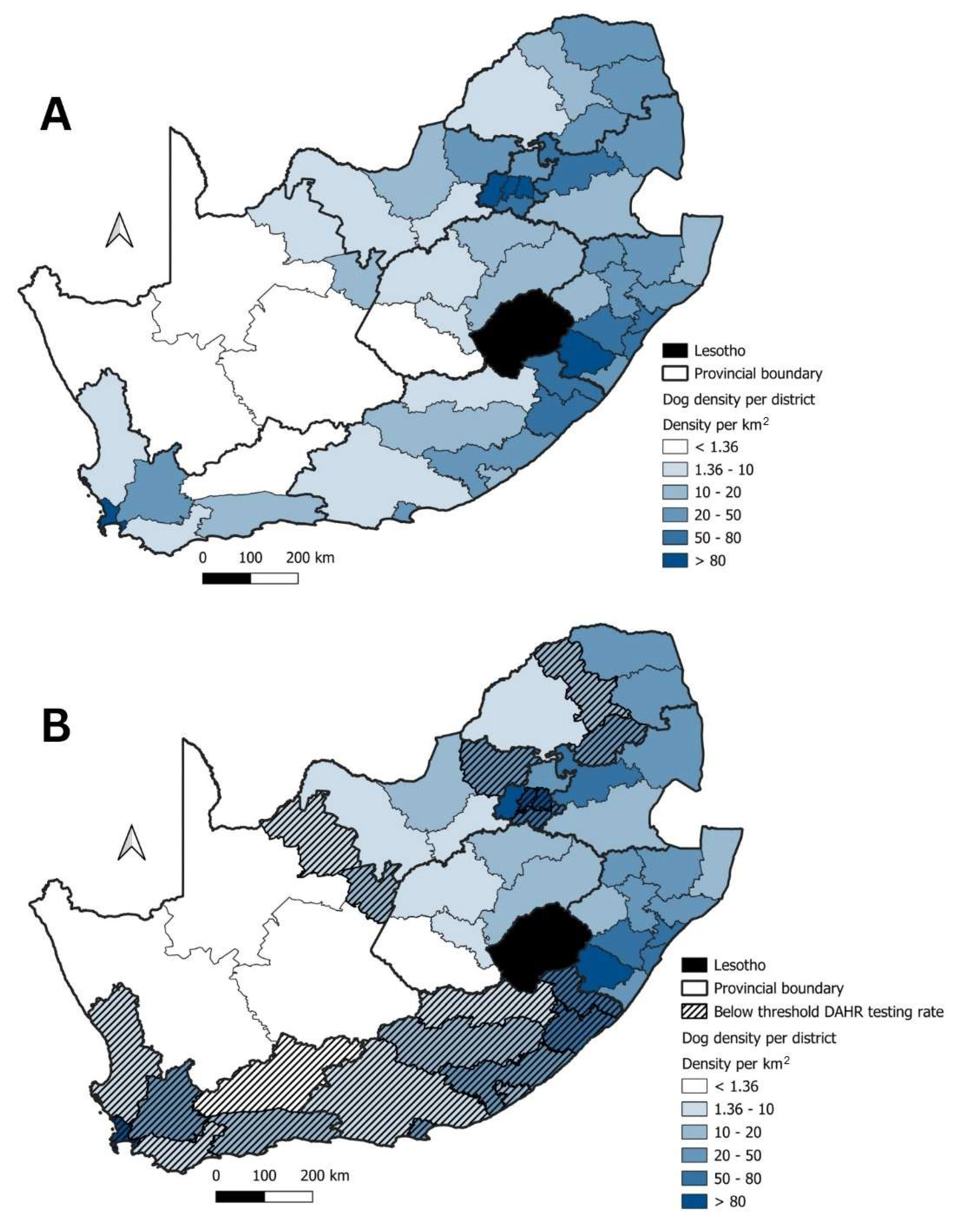
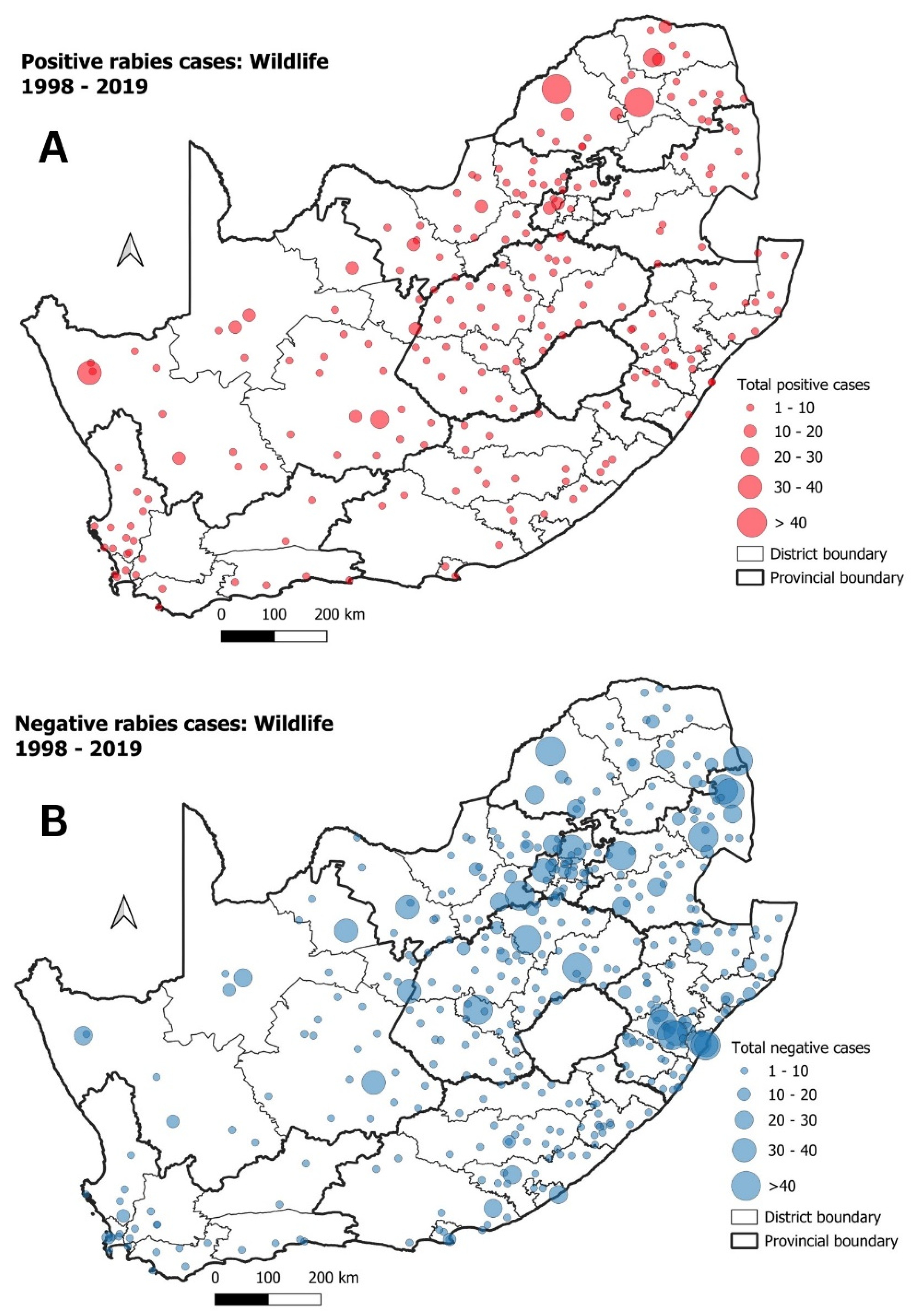
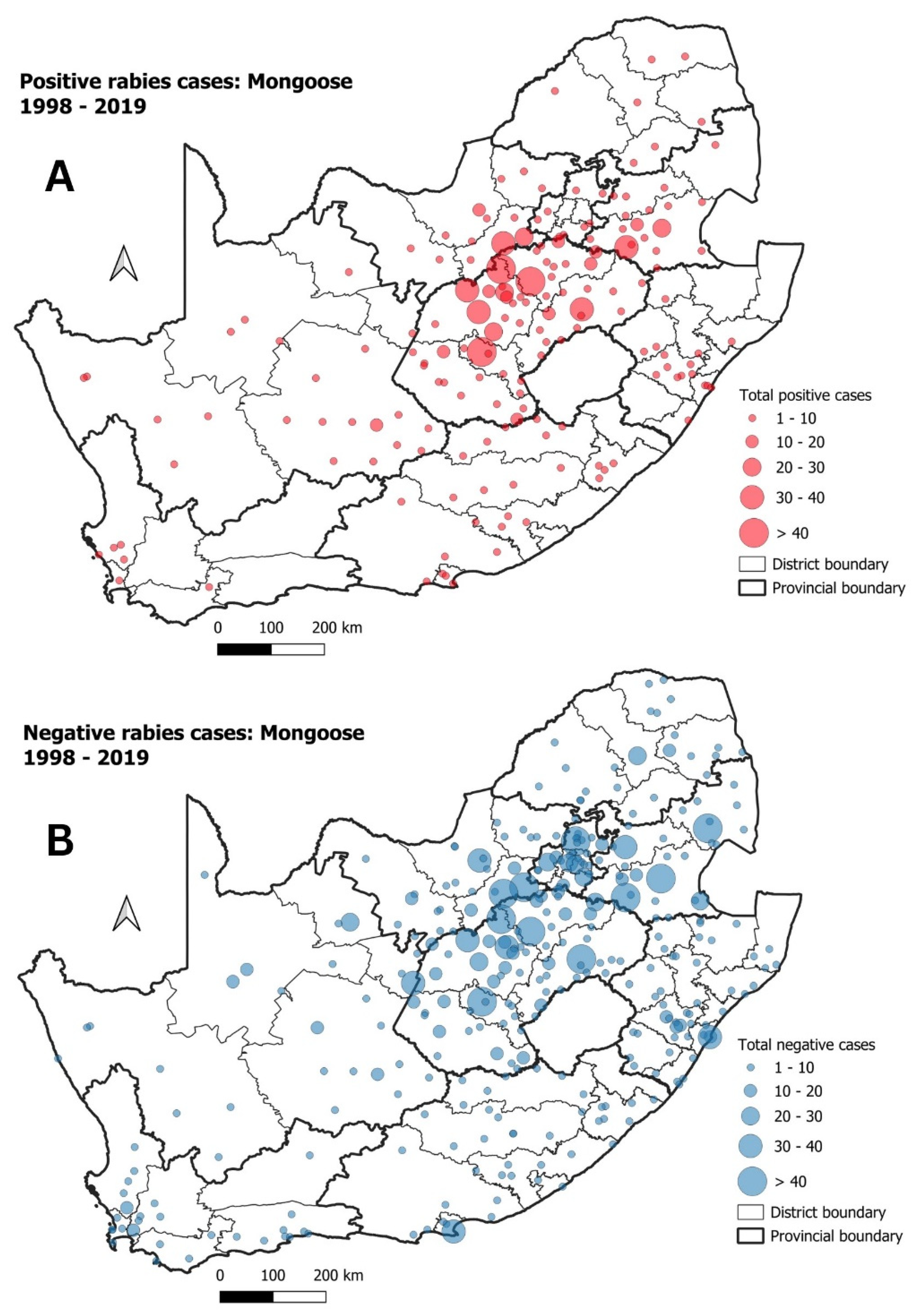
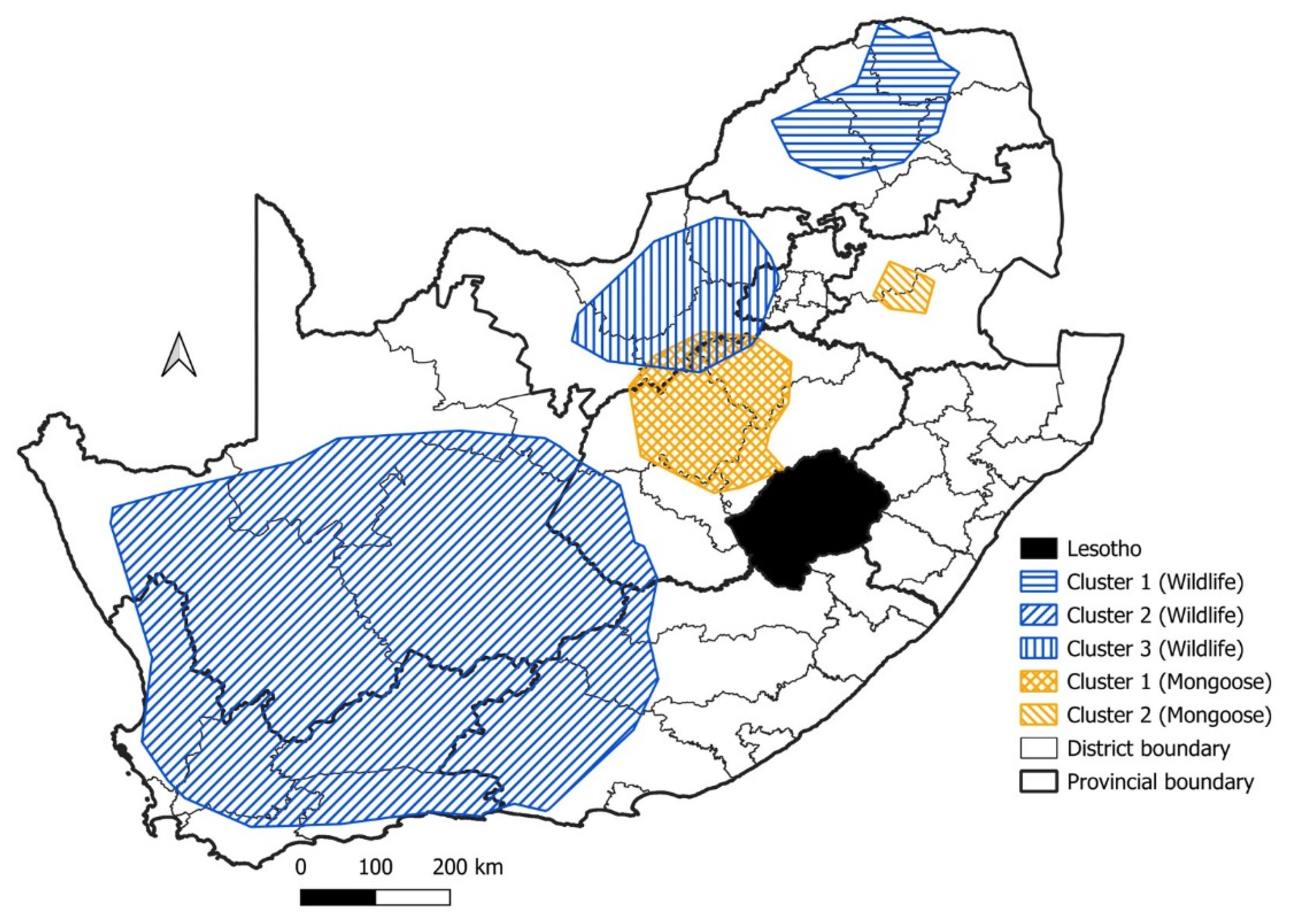

| Cluster | Start Date | End Date | p-Value | Observed Cases | Expected Cases | Relative Risk |
|---|---|---|---|---|---|---|
| 1 | 1999 | 2009 | p < 0.001 | 1455 | 835.0 | 1.94 |
| 2 | 2000 | 2010 | p < 0.001 | 935 | 488.2 | 2.06 |
| 3 | 2005 | 2015 | p < 0.001 | 447 | 231.8 | 1.99 |
| 4 | 2014 | 2016 | p < 0.05 | 35 | 15.8 | 2.22 |
| 5 | 2010 | 2012 | p < 0.05 | 96 | 58.6 | 1.65 |
| Cluster | Species | Start Date | End Date | p-Value | Observed Cases | Expected Cases | Relative Risk |
|---|---|---|---|---|---|---|---|
| 1 | Wildlife | 1998 | 2008 | p < 0.001 | 178 | 74.8 | 2.67 |
| 2 | Wildlife | 1998 | 2008 | p < 0.001 | 209 | 97.6 | 2.44 |
| 3 | Wildlife | 2016 | 2019 | p < 0.001 | 87 | 37.2 | 2.46 |
| 1 | Mongoose | 1998 | 2008 | p < 0.001 | 309 | 187.7 | 1.90 |
| 2 | Mongoose | 1998 | 2005 | p < 0.05 | 77 | 43.8 | 1.82 |
| Cluster | Start Date | End Date | Log-Likelihood Ratio (p-Value) | Observed Cases | Expected Cases | Relative Risk |
|---|---|---|---|---|---|---|
| 1 | 2002 | 2012 | 182.7 (p < 0.001) | 410 | 210.1 | 2.13 |
| 2 | 2000 | 2009 | 42.0 (p < 0.001) | 195 | 120.0 | 1.68 |
| 3 | 2005 | 2015 | 23.1 (p < 0.001) | 133 | 85.6 | 1.58 |
| 4 | 2000 | 2003 | 14.3 (p < 0.05) | 61 | 36.5 | 1.69 |
| 5 | 2014 | 2015 | 13.1 (p < 0.05) | 44 | 24.7 | 1.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malan, A.J.; Coetzer, A.; Bosch, C.; Wright, N.; Nel, L.H. A Perspective of the Epidemiology of Rabies in South Africa, 1998–2019. Trop. Med. Infect. Dis. 2024, 9, 122. https://doi.org/10.3390/tropicalmed9060122
Malan AJ, Coetzer A, Bosch C, Wright N, Nel LH. A Perspective of the Epidemiology of Rabies in South Africa, 1998–2019. Tropical Medicine and Infectious Disease. 2024; 9(6):122. https://doi.org/10.3390/tropicalmed9060122
Chicago/Turabian StyleMalan, Ayla J., Andre Coetzer, Cayla Bosch, Nicolette Wright, and Louis H. Nel. 2024. "A Perspective of the Epidemiology of Rabies in South Africa, 1998–2019" Tropical Medicine and Infectious Disease 9, no. 6: 122. https://doi.org/10.3390/tropicalmed9060122
APA StyleMalan, A. J., Coetzer, A., Bosch, C., Wright, N., & Nel, L. H. (2024). A Perspective of the Epidemiology of Rabies in South Africa, 1998–2019. Tropical Medicine and Infectious Disease, 9(6), 122. https://doi.org/10.3390/tropicalmed9060122







