Abstract
Endocrine disruptors (EDCs) have been associated with the increased incidence of metabolic disorders. In this work, we conducted a systematic review of the literature in order to identify the current knowledge of the interactions between EDCs in food, the gut microbiota, and metabolic disorders in order to shed light on this complex triad. Exposure to EDCs induces a series of changes including microbial dysbiosis and the induction of xenobiotic pathways and associated genes, enzymes, and metabolites involved in EDC metabolism. The products and by-products released following the microbial metabolism of EDCs can be taken up by the host; therefore, changes in the composition of the microbiota and in the production of microbial metabolites could have a major impact on host metabolism and the development of diseases. The remediation of EDC-induced changes in the gut microbiota might represent an alternative course for the treatment and prevention of metabolic diseases.
1. Introduction
It has been widely reported that some exogenous compounds can interfere with the function of the endocrine system in the body. According to the Endocrine Society, an Endocrine Disrupting Chemical (EDC) is “an exogenous [non-natural] chemical, or mixture of chemicals, that interferes with any aspect of hormone action” [1,2]. In this respect, the main source of human exposure to EDCs is food intake. These chemicals might pass into the food chain directly when they are used as pesticides, or they might be released from food packaging containing metals, bisphenol A, or phthalates. In addition, some plant-based compounds (the so-called phytoestrogens) found in dietary supplements also exhibit endocrine disrupting potential [3].
Endocrine disruptors have been associated with the increased incidence of metabolic disorders. It has been proposed that EDCs may increase the susceptibility to these disorders by altering the adipose tissue, pancreas, liver, gastrointestinal tract, muscle, and brain homeostatic and hedonic pathways [4]. However, few studies have reported that the effects of EDCs on the gut microbiota can increase the risk of metabolic disorders such as obesity and diabetes [5,6].
Emerging evidence suggests interactions between EDCs and the microbiome, which may affect host health. A key triad between exposure to EDCs, the host genotype and phenotypic responses, and the gut microbiome has been suggested [7]. Exposure to EDCs has been shown to disrupt the microbiome, which may result in dysbiosis and the induction of pathways related to xenobiotics, microbiome-associated genes, enzymes, and the production of metabolites, which may play a crucial role in EDC biotransformation [8]. The products and by-products released following the microbial metabolism of EDCs can be taken up by the host, therefore having an impact on host health and on the development of diseases. In addition, the gut microbiota can modify the EDC profiles through different plausible mechanisms. Microbial enzymes (esterases, thiolases, azoreductases, nitroreductases, β-glucoronidases, methylases, sulfatases, lipases, and β-lyases) can be used to metabolize different types of EDC [9]. Dysbiosis and a reduced diversity of the gut microbiota may cause a reduction in the enzymatic activity, which in turn could result in a decreased metabolization of EDCs to their circulating, active forms, thereby reducing the potential EDC toxicity to the host.
In this work, we conduct a systematic review of the literature in order to identify the current knowledge regarding the interactions between the EDCs in food, thye gut microbiota and metabolic disorders, in order to shed light on this complex triad.
2. Methods
The PubMed and Web of Sciences databases were searched to identify the relevant studies. The following keywords were used: “Gut microbiota”, “Diet”, “Obesity”, “Diabetes”, “Bisphenol A”, “Bisphenol A analogs”, “Microbiota”, “Pesticides”, “Parabens”, “Polychlorinated biphenyls”, “Phytoestrogens”, “Metals”, “Cadmium”, “Arsenic”, “Lead”, “Phthalates”, “Triclosan”, and “Triclocarban”. Data published between 2006 and 2020 were considered.
The literature review was conducted in compliance with the recommendations provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Figure 1 shows the PRISMA flow diagram that maps out the number of studies identified, those included and excluded, and the reasons for exclusion ((a) papers not written in the English language, (b) no outcome of interest, and (c) not related to the subject of the study). A total of 107 studies were included for analysis [10].
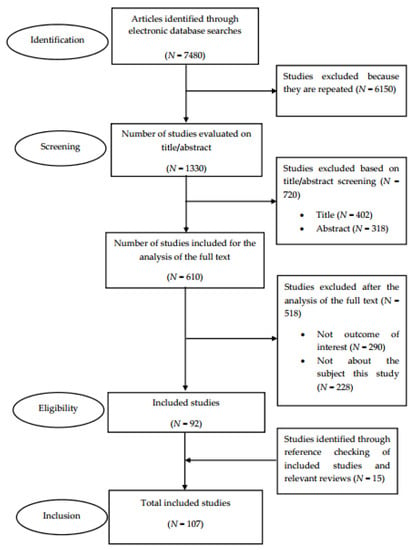
Figure 1.
A flow diagram of the literature search.
3. Results and Discussion
3.1. The Gut Microbiota in Health and Metabolic Diseases
A remarkable amount of evidence has emerged in recent years that strongly suggests that an essential role is played by the human microbiota in health and disease development via several mechanisms [11]. Variations in gut microbiota composition are considered to be physiological from the perspective of healthy gut microbiota, and these changes are related to age; sex; and external factors such as dietary habits, exercise, and antibiotic use. Indeed, dysbiosis, defined as the alteration of gut microbiota communities, is often related to health disorders.
Firmicutes and Bacteroidetes are the two primary phyla in gut microbiota, accounting for 90% of the total composition [12]. Other phyla include Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia [13]. The Firmicutes phylum is composed of more than 200 different genera such as Enterococcus, Lactobacillus, Ruminicoccus, Bacillus, and Clostridium. The phylum Bacteroidetes is dominated by the genera Prevotella and Bacteroides. The Actinobacteria phylum is comparably less abundant and mostly represented by the Bifidobacterium genus [13].
In recent years, research has demonstrated that gut microbiota could play an important role in the pathophysiology of metabolic disorders, specifically in obesity and diabetes [14]. Animal studies have shown that obesity is related to changes in the gut microbiota composition, including a reduction in species variety and alterations in the genes involved in metabolism. By contrast, data related to the human microbiota are more variable [13]. When fed with a similar diet, the comparison of the gut microbiota of genetically obese (ob/ob) mice with that of lean mice showed a greater abundance of Firmicutes and lower relative abundance of Bacteriodetes (50%) in obese mice [15]. These changes in the microbiota have also been found in different human studies [16,17]. Moreover, low relative amounts of Bifidobacterium vulgatus and high abundances of Lactobacillus spp. are detected in the microbiota of obese children [18]. Other studies have reported on the relationship between the Proteobacteria phylum and obesity, by the identification of pro-inflammatory molecules such as lipopolysaccharides and increased fat storage in the host [19]. In addition, higher abundances of Rikenellaceae and Ruminococcaceae have been revealed in leptin-resistant obese (leptin-promoting satiety) and diabetic (db/db) mice compared with in the lean members of the same litter [20]. Furthermore, other authors have demonstrated a relationship between Desulfovibrionaceae growth induction, obesity, and type 2 diabetes (T2D) [21,22].
There is also cumulative evidence from human and animal studies of an association between the development of diabetes and the existence of changes in the gut microbiota composition. Larsen et al. (2010) [23] showed that the Firmicutes phylum and Clostridia class decreased significantly in humans with T2D compared to in the healthy control group. Likewise, the β-Proteobacteria class increased in diabetics compared to in the control group and was positively associated with plasma glucose levels. Murri et al. (2013) [24] reported increased Veillonella, Bacteroidetes, and Clostridium spp.—along with a decrease in Blautia, Lactobacillus, Prevotella, and Bifidobacterium—in children with diabetes type 1 diabetes (T1D) compared to in the healthy control group.
3.2. Role of EDCs in the Microbiota
Table 1, Table 2 and Table 3 provide information concerning the effect of endocrine disruptors on gut microbiota in vitro, in animals, and in human studies.

Table 1.
Effects of endocrine disruptors on the gut microbiota in in vitro assays.

Table 2.
Effects of endocrine disruptors on the animal microbiota.

Table 3.
Effects of endocrine disruptors on the human microbiota.
3.2.1. BPA and Analogs
Bisphenol A (BPA) is an environmental chemical widely used in industry for the manufacture of polycarbonate plastics and epoxy resins, with well-known endocrine disrupting activity [25,26]. The public concern about the safety of BPA has resulted in the imposition of a ban on its use in some products and the emerging market entry of BPA analogs such as bisphenol S (BPS), bisphenol F (BPF), bisphenol AF (BPAF), and bisphenol B (BPB). However, their structural similarity to BPA has also raised concerns about their endocrine disrupting potential. Several studies have reported on the association of BPA and BPA analogs with an increased risk of developing metabolic diseases such as obesity [25,27]. However, this association is not well demonstrated, and it is challenging to find evidence for direct causality between BPA and analog exposure and metabolic diseases using epidemiological studies.
Wang et al. (2018) [28] studied the changes in metabolism and accessibility of BPA in different parts of the gastrointestinal tract using an in vitro Simulator of the Human Intestinal Microbial Ecosystem (SHIME) model. Three different BPA concentrations were investigated, which provided information regarding an extensive range of BPA daily intake values, from the human relevant exposure dose (25 μg/L) and the EPA (Environmental Protection Agency) reference dose (250 μg/L), to the 1% lowest observed adverse effect level (2500 μg/L) [28]. The toxicity of BPA, in terms of the effects on the hepatic gene expression profiles, was compared with that of the SHIME effluents, using the human hepatocellular carcinoma (HEPG2) cell line. The findings showed that BPA exposure modified the microbial composition of the colon, increasing the amount of microbes in the ascending, transverse, and descending colon. The upregulation of BPA-degrading bacteria, such as Microbacterium and Alcaligenes, was also reported.
Exposure to BPA and BPA analogs in animal models such as rodents, zebrafish, rabbits, and dogs can affect the gut microbiota and have an impact on the development of metabolic diseases. Some studies have reported that there is a sexual dysmorphic effect [6,29,30,31,32,33].
Xu et al. (2019) [33] determined the impact of BPA exposure on the development of T1D and the involvement of the host immune system and gut microbiota in a model of non-obese diabetic (NOD) mice. Adult male and female NOD mice were orally exposed to BPA at environmentally relevant doses (30 or 300 µg/kg). These doses were selected because they had previously been shown to modify the immune system and to be relevant for human exposure (30 BPA/kg body weight (bw) is within the range of human exposure levels, and 300 BPA/kg bw is also appropriate for human exposure levels based on BPA concentrations in human blood) [34]. In addition, the current EPA reference dose is 50 μg/kg/day. However, exposure to low-dose BPA also seems to have harmful effects, and as a consequence, after careful examination, the European Food Safety Authority (ESFA) has lowered the total dietary intake to 4 μg/kg bw/day.
Exposure to BPA resulted in a fast onset of T1D in female mice and slow onset in male mice. Subacute BPA exposure in female mice resulted in increased Bacteroidetes and Cyanobacteria and decreased Firmicutes, Tenericutes, and Proteobacteria. Chronic exposure to BPA in females also resulted in a proinflammatory gut microbiota, with a decrease in Bacteroidales and Lactobacillus. These results are consistent with human epidemiological studies, which show that the gut microbiota in individuals with T1D is dominated by Bacteroidetes at the phylum level and in turn with a reduction in Firmicutes in relation to control [87]. In male mice, BPA exposure results in a slow onset of T1D. Subacute exposure caused a decrease in Bacilli at the class level, which is in accordance with the findings reported in human epidemiological studies on the association between the the development of T1D and gut microbiota composition [88]. Additionally, chronic exposure to BPA in males resulted in decreased Lachnospiraceae, which has been shown to promote T1D in NOD mice models [89].
Using their NOD mouse model, Xu et al. (2019) [6] showed that BPA’s effects on the development of T1D were related to host age and gender, following various windows of exposure. Exposed juvenile NOD females (starting postnatal day (PND) 28 to PND56) and NOD offspring (starting gestation day 5 to PND28) were exposed perinatally to BPA by dosing the dams to 0 or 30 µg/kg BW. Adult NOD females were exposed to 0 or 300 µg/kg bw. Interestingly, BPA increased the risk of developing T1D in adult and juvenile females, which was related to changes in the gut microbiota, but the female offspring showed a reduced risk of developing T1D. In contrast, BPA had insignificant effects on the development of T1D in the male offspring. The changes in the gut microbiota of juvenile females associated with BPA exposure included, at the genus level, an increase of Turicibacter, Oscillospira, Ruminococcus, Jeotgalicoccus, and Lachnospiraceae, which increase the risk of T1D and inflammation, as shown in several animal models [89,90,91].
Malaisé et al. (2017) [5], in their longitudinal study, found that the perinatal exposure of C3H/HeN mice to BPA at 50 µg/kg bw/day (100 times lower the no observed adverse effect level (NOAEL), 5 mg/kg bw/day) induced dysbiosis and systemic immune imbalances at PND45. These effects were associated with a rise in glucose intolerance and a decrease in IgA and Bifidobacteria in the feces. Some strains of the Bifidobacterium genus have been shown to have anti-inflammatory properties [92].
Javurek et al. (2016) [31] explored the changes in gut microbiota related to BPA exposure in parents and their offspring in California mice (Peromyscus californicus). Female and male monogamous and biparental mice were exposed to BPA (50 mg/kg feed weight) from periconception to weaning. The dose selected has been shown to induce metabolic alterations and it is below the diet-administered maximum non toxic dose for rodents (200 mg/kg BW/day). This dose is within the presumptive NOAEL and results in concentrations similar to those reported in human serum. They demonstrated for the first time that parental exposure to concentrations of BPA environmentally relevant causes changes in the microbiota structure in non-exposed offspring. These changes were generational- and sex-dependent. In this respect, they reported that BPA (and ethinyl estradiol) exposure induced an increase in Akkermansia, Mollicutes, Prevotellaceae, Bacteroides, Erysipelotrichaceae, Methanobrevibacter, Sutterella in parents and offspring. These species have been associated to inflammatory bowel disease, obesity and metabolic disorders [93,94], autism spectrum disorders [95], colon cancer [96], and other conditions. However, Bifidobacterium was also found in higher amounts in fecal samples of female offspring. Some Bifidobacterium strains have been shown to exert health-promoting effects and is included in a number of probiotic foods and supplements [97]. DeLuca et al. (2018) [46] used a dextran sulphate sodium-induced colitis model in female C57BL/6 mice and found that BPA exposure at 50 μg/kg/day negatively affects gut physiology by reducing microbiota metabolites derived from aromatic amino acids, which might be associated with autoimmune diseases, specifically with inflammatory bowel disease. This dose was selected because it is the EPA reference dose for BPA.
Lai et al. (2016) [48] used 16S rRNA gene sequencing of cecal microbiota of CD-1 male mice to analyse the effects of dietary BPA intake on microbiota composition and physiology. Mice on high-fat high-sucrose diet were the positive controls. The findings showed that dietary BPA exposure was related to a decrease in the diversity of microbiota species. The structural changes of the gut microbiota exposed to dietary BPA were similar to those found in mice on high-fat high-sucrose diets. Additionally, the comparison between BPA and high-fat diet revealed an increase in Proteobacteria in both groups. The increased abundance of Proteobacteria has been related to different conditions such as metabolic disorders and inflammatory bowel disease [98]. Lastly, exposure to dietary BPA produced a decrease in the phylum Firmicutes, with most of the 16SRNA sequencing corresponding to the class Clostridia. Interestingly, Larsen et al. (2010) [23] demonstrated a significant reduction in Firmicutes and Clostridia in the feces of human male adults with T2D compared to the healthy group.
Dietary exposure to BPA also modified the gut microbiota in zebrafish [30,32]. In a study conducted by Liu et al. (2016) [32], the authors concluded that exposure to BPA resulted in an increase of the phylum CKC4 in both sexes probably connected to changes in the host lipid metabolism (increased triglycerides in the muscle). However, one of the limitations of this study was that the functional study of CKC4, a phylum included in the SILVA database, was very incomplete.
Chen et al. (2018) [30] exposed adult zebrafish at BPA doses of 0, 2 and 20 μg/L, titanium dioxide nanoparticles (nano-TiO2) and their binary mixtures for three months. Exposure to both compounds resulted in changes in the gut microbiota. An antagonistic interaction was observed at low BPA concentrations, but a synergistic interaction was observed at high BPA concentrations. Zebrafish growth and gut health (oxidative stress, barrier function, inflammation) were associated with sex and concentration of chemicals. Additionally, zebrafish weight was found to be positively associated with the presence of Bacteroides, closely related to the Anaerococcus, Finegoldia, and Peptoniphilus genera.
The work by Reddivari et al. (2017) [49] showed that perinatal exposure to BPA in Dutch-Belted rabbits (200 μg/kg bw/day) induced an increase in the Methanobrevibacter spp community leading to inflammation of the colon and liver, and increased gut permeability in the offspring demonstrated by increased levels of serum lipopolysaccharide. This study used rabbits as an animal model because this species has an extensive infantile period of development similar to that of humans and utilized BPA at a relatively low dose level of 200 g/kg bw/day (approximately 1/25 of the NOAEL dose). Significant positive correlations were observed between increased Methanobrevibacter spp. in the colon and systemic concentrations of lipopolysaccharide (r2 = 0.67; p = 0.023). This species can metabolize dietary substrates that lead to increased host energy intake and weight gain [93]. Lastly, perinatal exposure to BPA led to a reduction of the diversity of the microbial communities and their metabolites (short chain fatty acids) and to an increase in intestinal permeability.
The work by Koestel et al. (2017) [47] showed that the circulating BPA levels in dogs fed with canned dog food for two weeks (2.2 ± 0.15 ng/mL) were similar to the levels found in humans [99], with higher BPA concentrations related to modifications in the composition of the microbiome. These changes may lead to modifications in metabolic pathways, including the capacity to metabolize bisphenols. In this study, higher serum BPA levels were associated with decreasing relative abundances of Bacteroides spp., which may result in a reduction of BPA bacterial degradation.
We found only one study of the effects of BPA analogs on the microbiota. Catron et al. (2019) [29] exposed zebrafish during their developmental period to BPA, BPAF, BPB, BPF, and BPS at different concentrations. These concentrations were selected based on zebrafish toxicity data available through the ICSS ToxCast dashboard and previous zebrafish studies. 16S rRNA gene sequencing showed that structural microbiota disruption was highly dependent on concentration and that exposure to BPS, BPA, or BPF caused the enrichment of the microbial functions, but this did not occur with exposure to BPB or BPAF. Lastly, microbial disruption was inversely associated with host developmental toxicity and estrogenicity. The main finding of this work was that BPS, BPA, and BPF produced disruptions in microbial composition, occurring throughout the critical window of early development, at concentrations that did not cause evident developmental toxicity.
3.2.2. Pesticides
The adverse effects of agricultural pesticides derived from their endocrine disruptive activity that may affect the thyroid and the reproductive, nervous, and adipose systems were investigated [100,101,102].
In vitro studies have demonstrated that different pesticides can cause gut dysbiosis [38,39,40,41], using the chicken microphrobiome [39] and the Rumen Simulation Technique (RUSITEC) system [40,41]. Joly et al. (2013) [38] studied the impact of in vitro exposure to low doses of organophosphorus chloropyrifos in a SHIME system, and the effects in vivo in pregnant Wistar rats. Their results showed that exposure to chlorpyrifos induces the proliferation of Bacteroides spp. and Enterococcus spp. and reduces the proliferation of Bifidobacterium spp. and Lactobacillus spp.
Three studies in rodents (in ICR, C57Bl/6, and CD-1 mice and Wistar rats) showed that exposure to pesticides (carbendazim, chlorpyrifos, and organophosphorus pesticides) induces dysbiosis in the microbiota and inflammation, leading to an alteration of lipid metabolism and triggering obesity in exposed rodents [69,70,73].
Wu et al. (2018) [74] evaluated the effects of exposure to propamocarb (3, 30, and 300 mg/L for 28 days) in five week-old male Institute of Cancer Research mice. These concentrations were established based on the highest residue from the EU-Maximum Residues levels and the long-term toxicity NOAEL (20 mg/kg bw/day) [103]. The authors found that exposure induced the disruption of the transcription of hepatic genes involved in the regulation of lipid metabolism. The cecal and fecal microbiota changed at the phylum or genus levels.
Liu et al. (2017) [71] exposed adult male C57BL/6 mice, over eight weeks, to low dose p,p’-dichlorodiphenyldichloroethylene (1 mg/kg body weight/day) and β-hexachlorocyclohexane (10 mg/kg body weight/day), which are similar to the levels found in chronic exposure in humans. The authors found that exposure induced changes in the composition of the gut microbiota, particularly leading to higher abundances of Lactobacillus that are capable of deconjugating bile acids by bile salt hydrolases. These transformation reactions affect the hydrophobicity and composition of bile acids, and down-regulate the expression of genes involved in the reabsorption of bile acids in the distal ileum, but up-regulate the expression of genes involved in the hepatic synthesis of bile acids.
Tu et al. (2019) [72] evaluated the toxic effects of exposure to 2,4-dichlorophenoxyacetic acid, at an occupationally important dose, on the intestinal microbiota of specific-pathogen-free C57BL/6 male mice. Metagenomic sequencing showed a distinct gut microbial community with disturbances in the pathways of amino acid and carbohydrate metabolism and urea degradation. These findings are of particular interest, as evidence has showed that modifications of microbiome-related pathways and metabolites would produce an alteration of gut-host homeostasis, which may increase the risk of diseases [104].
The sexual dysmorphic effects of pesticide exposure have been described in animal models. Gao et al. (2017) [68] showed that exposure to a low dose of organophosphorus pesticide diazinon (4 mg/L in drinking water) modifies the gut microbiota composition and functionality in C57BL/6 mice, with more impact in male mice. At the phylum level, Bacteroidetes increased by 1.8-fold, while Firmicutes decreased by 1.8-fold, in diazinon exposed males compared to controls. As shown previously, a high Firmicutes/Bacteroidetes ratio is related to obesity, which is consistent with the observed decrease of body weight in male mice. By contrast, no effects of diazinon exposure on body weight and the Firmicutes/Bacteroidetes ratio were observed in female mice.
Finally, an epidemiological study in humans carried out by Stanaway et al. (2017) [84] found that exposure to agricultural pesticides can cause dysbiosis of the human oral microbiota. A cohort of 65 agricultural workers and 52 non-agricultural workers was studied. The results showed that workers exposed to azinfos-methyl had a decrease in common genera found in the human oral microbiome (Streptococcus, Micrococcineae, Gemella, Haemophilus, Halomonas, Actinomycineae, and Granulicatella). Although more studies are needed to confirm the results of Stanaway et al. (2017) [84], the data obtained indicate that the oral microbiome could be used as a simple biomarker for assessing pesticide exposure in epidemiological studies.
3.2.3. Polychlorinated Biphenyls
Polychlorinated biphenyls (PCBs) are well-known EDCs that were massively used until the mid-1970s as insulators for electrical equipment such as transformers, switches, capacitors, and thermostats. Despite the current ban on manufacturing, PCBs continue to be a common environmental contaminant because of their accidental and intentional release in large quantities due to their long-term stability [105,106]. The main route of exposure to PCBs is the consumption of contaminated foods such as fish and shellfish. PCBs have been related to hormone-dependent cancers, an impaired reproductive system, and cognitive and metabolic disorders (such as impaired glucose metabolism and adipocyte inflammation) [107,108].
The available evidence has shown the detrimental effects of PCB exposure on the microbiota that can trigger host metabolic disorders [36,50,51,52,53,54,55,109]. Exposure to PCBs promotes an altered microbiome, with reduced Proteobacteria [53], decreases in microbial diversity, and an increased Bacteroidetes-to-Firmicutes ratio, usually linked to intestinal and systemic inflammation [55]. In addition, changes in bile acid homeostasis that result in dysbiosis have been described in adult mice exposed to a PCB mixture [51]. Other studies have also reported the impact of exposure to PCB on amphibian [54] and zebrafish microbiomes [50]. Recently, Rude et al. (2019) [56] reported that developmental exposure to PCBs induces dysbiosis and epithelial permeability defects in the ileum and colon that result in changes to the microbial β-diversity of juvenile mice.
Petriello et al. (2018) [55] found that exposure to PCB126 (1 mmol/kg) induces disruption of the gut microbiota and host metabolism in seven week-old male Ldlr −/− mice, which are a model of cardiometabolic disease. This PCB126 dose was selected because it results in plasma concentrations similar to those found in human exposure [110]. The 16S rRNA sequencing revealed changes at the phylum and genus levels, and consistent increases in intestinal and systemic inflammation. The Firmicutes/Bacteroidetes ratio was increased after PCB126 exposure. This increase has been consistently linked to obesity, insulin resistance, inflammation, and other alterations of host metabolism [111]. Additionally, a strong PCB-dependent association was found between Bifidobacterium and circulating glucagon-like peptide-1.
Chi et al. 2019 [52] exposed adult female C57BL/6 mice to environmentally relevant low-dose PCB126 (50 μg/kg bw) once per week over six weeks and found that chronic low-dose exposure promoted dysbiosis, with changes to the microbiota’s structure and composition. In addition, PCB126 promoted dyslipidemia, hepatic damage, and a fatty liver. Lastly, metabolic indicators of these conditions seem to positively correlate with specific bacterial taxa.
The study by Chen et al. (2018) [50] showed that the exposure of zebrafish to model pollutants with different mechanisms of action and different affinities to estrogen and aryl hydrocarbon receptors (atrazine, estradiol, PCB126, and PCB153) at 1.0 μg/L for seven days resulted in changes to the microbiota and in the deterioration of intestinal and hepatic functions. The authors reported that aryl hydrocarbon and estrogen receptor signaling regulate gut microbiota physiology. Data showed that impaired Aeromonas reproduction was significantly related to oxidative damage, especially in the PCB126 groups. Previous research has linked Aeromonas spp. to intestinal inflammation and soft tissue infection [112].
3.2.4. Parabens
Parabens are widely used as preservatives and bactericides in pharmaceuticals, personal care products, and some food products [113,114,115]. Several studies have reported on the adverse health effects of parabens, including their endocrine disrupting and obesogenic activities [116,117,118,119]. However, there are few data available regarding the effects of parabens on the microbiota.
In this respect, Hu et al. (2016) [66] analyzed the effect of low-dose exposure to methylparaben, triclosan, and diethylphthalate and their mixtures, as well as the window of susceptibility, in Sprague-Dawley rats. The doses investigated result in urinary biomarker levels similar to those observed in humans [120]. The Bacteroidetes phylum was increased, while the growth of Firmicutes was decreased in all exposed rats compared to controls. However, Betaproteobacteria was increased only in the methylparaben and mixture groups, suggesting that exposure to paraben mixtures produces a distinct microbiome shift different from that resulting from individual chemicals or from simple additive effects. Surprisingly, these differences decreased in adulthood. In addition, a reduction in body weight in rats exposed during adolescence was observed [66]. This is consistent with other studies reporting a decrease in the Bacteroidetes phylum and an increase in Firmicutes, linked to weight gain [121,122]. These studies highlight i) the importance of studying the critical window of exposure such as adolescence; ii) the effect of low dose exposure, similar to a human exposure scenario; and iii) the need to evaluate the combined effect of multiple exposures. Obadia et al. (2018) [65] observed that exposure to methylparaben (0.1% and 0.3%) in fly medium reduces microbiota growth and modifies the composition and amount of the bacteria and yeasts in the intestine of the Drosophila fly.
The effects of parabens on the microbiota need further research, as their interactions are poorly understood.
3.2.5. Phytoestrogens
Phytoestrogens are compounds naturally occurring in plants that have estrogenic/antiestrogenic effects. Phytoestrogens can modulate and be metabolized by the gut microbiota [123]. Phytoestrogen activity is strongly dependent on the microbiome. Their metabolites have stronger estrogenic activity than the natural compounds themselves, and because of the variability in microbiomes, there are large differences in the effects of phytoestrogens among individuals [124,125].
Daidzen, a phytoestrogen present in soy-based foods, can be metabolized to O-desmethylangolensin (ODMA) and equol by gut microbial communities in 80–95% and 25–60% of the population, respectively. In relation to this, Frankenfeld et al. (2011, 2014) [126,127] evaluated the presence of ODMA- and equol-metabolizing phenotypes in obese, overweight, and normal-weight individuals and found that the ODMA-metabolizing phenotype, but not the equol-phenotype, was linked to obesity in adulthood.
Another study investigated the effects of S-equol on pancreatic β-cell growth and insulin secretion in male mice. The results showed that S-equol boosts β-cell function and prevents hypoglycemia in mice, suggesting that S-equol may act as a potential preventive agent against type 2 diabetes mellitus [57]. Zhou et al. (2018) [64] investigated whether genistein intake by C57BL/6 female mice can reduce the negative impact of a maternal fat-high diet on glucose and lipid metabolism in their offspring. Female mice were placed on a high-fat diet alone, a high-fat diet supplemented with genistein at low (0.25 g/kg diet) and high doses n (0.6 g/kg diet), or a genistein-free control diet, for three weeks prior to pregnancy and throughout gestation and lactation. After weaning, female offspring from the high-fat group had lower weight at birth, as well as glucose intolerance and higher insulin, triacylglycerol, and total cholesterol levels in the serum compared with the control group. Offspring from the low-dose genistein group showed an increased weight at birth, improved glucose tolerance, and decreased fasting insulin. Offspring from the high-dose genistein group showed decreased serum triacylglycerols and total cholesterol compared with the offspring from the low-dose genistein mothers. The high abundances of Bacteroides and Akkermansia in the offspring from the genistein-fed female parents might be key to the improvement of glucose metabolism. A decrease of Bacteroides has been shown in diabetes patients in comparison to in healthy controls [128]. In addition, it has been reported that Akkermansia could preserve the mucus layer thickness and is correlated with an improved metabolic profile [129]. Similarly, the increased Rikenella in the offspring from the high genistein group might be linked to the decreased triacylglycerols and total cholesterol in the serum. In addition, it has been reported that the abundance of Rikinella contributes to a lean body type phenotype [130].
Lopez et al. (2018) [59] also found, in nine-week-old male C57/BL6 mice, lower serum triglycerides, improved glucose metabolism, and lower weights in the high-fat diet and genistein group (3 mg/kg/day) compared to in mice fed the high-fat diet alone. In addition, the presence of genistein in the high-fat diet resulted in changes to the gut microbiota (increases in the Prevotella and Akkermansia genera), linked to lower circulating levels of lipopolysaccharides and the reduced expression of pro-inflammatory cytokines in the liver, compared to in mice in the high-fat diet alone group. It has been showed that the reduction of lipopolysaccharides can decrease neuroinflammation [131].
Recently, Huang et al. (2018) [58] investigated, in non-obese diabetic mice, perinatally exposed to physiological doses of genistein (20 mg/kg body weight), whether there is a sex-dependent effect on type 1 diabetes (T1D). In female offspring, perinatal exposure to genistein resulted in a higher incidence of early-onset T1D. In addition, increased Enterobacterials were found in the fecal microbiota from the PND90 female offspring, which is indicative of a pro-inflammatory response. These changes were not found in the PND30 females. However, perinatal genistein exposure in PND90 males induced changes in the gut microbiota linked to an anti-inflammatory response. The authors conclude that a strong sex-specific effect was found in the perinatal genistein exposure window and that the mechanism of T1D in non-obese diabetic females is induced by immune system modulation of the gut microbiota. These results must be taken into account, since soy milk formula consumption during infancy was related to type 1 diabetes [132].
In California mice (Peromyscus californicus), Marshall et al. (2019) [60] also determined whether perinatal exposure to genistein (250 mg/kg feed weight) promoted dysbiosis and altered gut metabolites. Female mothers were fed a diet with genistein or a genistein-free control diet. Their results showed that exposure to genistein resulted in sex-related disturbances of the gut microbiota and metabolites in the offspring. Positive associations between the gut microbiome, metabolome, and disruption of social and vocalization behaviors (audible calls above 20 kHz) were also found in the offspring of exposed dams. When male offspring from genistein-supplemented dams were compared with genistein-free offspring, calls above 20 kHz correlated with daidzein, α-tocopherol, Flexispira spp., and Odoribacter spp. The effects secondary to genistein exposure may result from disturbances to neurobehavioral programming or from changes in the microbiota linked to changes in gut metabolites. These results suggest that the gut microbiome and its metabolites can induce a disruption in the offspring’s neurobehavioral programming, known as the “microbiome gut–brain axis”.
The effect of soy intake on microbiota composition and diversity has been studied in other animal models, such as porcine models [61,63] and the Southern white rhinoceros [62]. Yeruva et al. (2016) [63] determined the influence of diet on the development of the immune system in neonates, using a porcine model. Two-day old piglets were fed soy or milk formula until day 21 and compared to a sow-fed group, and the results showed that the formula diets induced changes in the small intestine microbiome, particularly in the duodenum. Significant increases in Lactobacillaceae spp. and Clostria spp., as well as a decrease in Enterobacteriaceae spp., were found in soy-fed piglets.
There is, however, little information regarding the impact of phytoestrogens on the human microbiota. Wu et al. (2016) [82] compared the plasma metabolites in omnivores versus vegans that consume soyfoods and found significant differences in the metabolomes between the two groups, but the gut microbiota was very similar in both groups [82].
3.2.6. Metals
Metals have been considered EDCs because of their ability to bind to hormone receptors [133]. Metals are ubiquitous environmental pollutants, with the primary sources of human exposure being the inhalation of dust or direct ingestion of contaminated food and water. Metal exposure has been related to obesity, diabetes, and metabolic syndrome [134,135,136,137].
Metals can be metabolized by the colonic microbiota in humans. Van de Wiele et al. (2010) [35] reported the ability of this microbiota to methylate Arsenic (As), which suggests that the role played by microbiota metabolism should be considered when assessing the toxic effects on human health of ingested As.
Lu et al. (2014) [45] reported that the composition of the gut microbiome in C57BL/6 mice markedly alters after exposure to 10 ppm arsenic in the drinking water over 4 weeks, resulting in a decrease in four Firmicutes families. These results are in agreement with the As antiobesogenic properties described in several articles [138]. They also reported a significant association between this microbiota disruption and changes in microbiota metabolites. This suggests that exposure to As not only induces disturbances in the abundance and composition of bacterial communities but also affects their metabolomic profile, which subsequently results in the disturbance of host metabolite homeostasis. These changes in metabolite homeostasis are important risk factors involved in tissue dysfunctions, which may cause diseases such as obesity and diabetes [139].
Wu et al. (2016) [43] reported that perinatal lead (Pb) exposure (32 ppm) in the drinking water, in wild-type non-agouti (a/a) mice of the Avy strain isogenic mouse model of perinatal environmental exposure, induced changes in the adult offspring gut microbiota. These changes were sex-independent, but a strong association was found between male offspring and increased body weight. Interestingly, the quantities of the two predominant phyla (Bacteroidetes and Firmicutes) shifted inversely with Pb exposure. In addition, reduced aerobic bacteria and increased anaerobic bacteria were observed in the exposed offspring. Lastly, Pseudomonas, Enterobacter, and Desulfovibrio were found in higher abundances in exposed adult mice than in controls (p < 0.05) [43].
Ba et al. (2017) [42] demonstrated the sex-specific effects of low-dose exposure to cadmium and found that early exposure to 100 nM induced fat accumulation in adult male C57BL/6J mice. In this work, 100 nM cadmium was present in the drinking water, which is equivalent to ~ 2.5 μg/kg bw per week and corresponds to the tolerable weekly intake and the mean intake by humans [140]. They also found an increased metabolism of fatty acids and lipids as well as a decrease in the composition and diversity of the gut microbiota. At eight weeks, the gut microbiota was found to be particularly vulnerable to low-dose cadmium exposure, and exposure during this period may induce adiposity in adult mice, even if the microbiota is later restored. The role played by the gut microbiota in adiposity related to cadmium exposure was also demonstrated by microbiota transplantation and removal experiments.
Xia et al. (2018) [44] observed that short-term exposure to 10 and 30 μg/L Pb increased the volume of intestinal mucus in the adult male zebrafish. They also found decreased α-Proteobacteria and increased Firmicutes after exposure to 30 μg/L Pb for seven days. In addition, 16S rRNA sequencing demonstrated an altered gut microbiota, in terms of composition and diversity, after exposure to 30 μg/L Pb. At this dose, 52 gut microbes and 41 metabolites underwent significant changes, particularly those related to the pathways of glucose, lipid, amino acid, and nucleotide metabolism. Lastly, they also found a marked reduction in the transcription of some genes related to glycolysis and lipid metabolism after seven-day exposure to 30 μg/L Pb.
A recent study showed that the urinary concentrations of Pb in adult humans were related to changes in gut microbial composition, even at low Pb levels [81], and so an association between increased urinary Pb concentrations and increased microbiota α-diversity and richness was found. Changes in β-diversity were significantly associated with changes in urinary Pb concentrations, and Proteobacteria, including members of the Burkholderiales (a wide variety of bacterial species that perform a plethora of metabolic functions), were also associated with increased urinary Pb [81].
3.2.7. Triclosan and Triclocarban
Triclosan (TCS) and triclocarban (TCC) (TCs) are chlorinated, broad-spectrum antimicrobial endocrine disrupting chemicals found in thousands of consumer and industrial products [141], as well as in contaminated food [142,143]. These EDCs have been related to metabolic disorders such as obesity and diabetes [144,145].
It has been shown that TCS exposure induces changes in the gut microbiota of rats [66,77], mice [75], and fish [76,79]. Narrowe et al. (2015) [79] showed that even low but environmentally relevant levels of triclosan exposure (100–1000 ng/mL) can result in the disturbance of the juvenile fish gut microbiome. Seven-day exposure to triclosan in larval fathead minnows (P. promelas) resulted in significant changes, as measured by α- and β-diversity, in the gut microbiome immediately after triclosan exposure; however, the microbiome rapidly recovered following two weeks of depuration. This demonstrates the sensitivity and resilience of the gut flora to the toxic effects of environmental contaminants.
Kennedy et al. (2016) [77] found that Sprague Dawley rats with ad libitum access to commercial Harlan ground 2020X supplemented with 0.1% w/w triclocarban during gestation and lactation exhibited significant changes in the community structure of the fecal microbiota, as well as finding decreased phylogenetic diversity in exposed dams and neonatal rats. Marked differences in β-diversity were found in exposed animals compared with controls in dams at 18 days of gestation and 16 day old neonates. This dose was chosen as it has been demonstrated that the serum TCC concentration of pregnant rats after oral exposure to 0.2% w/w TCC was similar to the concentrations reported in the human serum [146].
Ma et al. (2020) [78] studied the long-term effects in adult and old rats of perinatal exposure to TCs and found that 50 mg/kg/day (the lowest toxic oral dose in rats) resulted in disturbances of the metabolism and gut microbiota that were long-lasting and persisted even after the exposure had been terminated. They also accumulated over time, inducing metabolic disorders in old rat offspring. Exposure to TCs induced an increased growth of Bacteroidetes, which has been related to lipid accumulation [42]. Additionally, a reduction in Akkermansia muciniphila, a species linked to improved metabolism in diabetic and obese mice, was observed [147].
Interestingly, probiotics have been used to modulate the microbiota and palliate intestinal metabolic disorders due to triclosan exposure in animal models [80]. In this respect, Lactobacillus plantarum ST-III has been found to increase the diversity of the gut microbiota in zebrafish, thereby reducing the toxicity of chronic exposure to triclosan. Additionally, a probiotic-rich diet reduced the risk of lipid-metabolism disorders such as increased triglyceride and total cholesterol levels. Histopathological studies demonstrated severe structural damage to the intestines, spleen, and kidney after triclosan exposure; however, this damage can be reduced by the presence of Lactobacillus.
Bever et al. (2018) [85] compared the fecal microbiome of infants fed with breast milk that had measurable levels of TCS versus infants fed with breast milk that had no detectable concentrations of TCS and found that early life exposure to exogenous contaminants induces changes to microbiome diversity. Because of the impact of a healthy infant gut microbiome on phenotypes later in life, understanding how EDCs influence the infant gut microbiome is critical to identifying and correcting problematic changes in infant gut health.
However, Ribado et al. (2017) [86] did not find that exposure to household TC-containing products induces changes to or a loss of microbial diversity, but they found increased Proteobacteria spp. in infants and mothers exposed to higher TC levels. Interestingly, increased Proteobacteria has been proposed as a potential diagnostic marker for dysbiosis and an increased risk of diabetes and colitis [148].
3.2.8. Phthalates
Phthalates are EDCs used as plasticizers in food processing and packaging, adhesives, personal care products, and cosmetics. A major source of phthalate exposure is the diet, primarily due to contamination during processing and packaging [149]. Phthalates have been considered obesogens, therefore contributing to overweightness and obesity. It has been shown that exposure to phthalates alters glucose and lipid metabolism, which increases the risk of developing insulin resistance [150,151].
A mouse model of prenatal di(2-etilhexil) ftalato (DEHP) exposure (0.2, 2, and 20 mg/kg/day) was used to study the long-term metabolic disturbances in offspring. In an ICR mouse model of prenatal DEHP exposure (0.2, 2, and 20 mg/kg/day), Fan et al. (2020) [67] showed that exposure to low-dose phthalate (0.2 mg/kg/day) in mice induced changes in glucose metabolism, energy expenditure, adipogenesis, and gut dysbiosis in a sex-dependent manner. The level of DEHP exposure was selected based on the EPA reference dose. Their findings strengthen the hypothesis that connections between the host and gut microbiota alter energy metabolism.
As mentioned above, Hu et al. (2016) [66] reported that postnatal, low-dose exposure to diethyl phthalate (DEP) in Sprague-Dawley rats from birth through adulthood induced changes in the composition of the gut microbiota, but these changes were seen only in adolescent rats. The changes include an increased relative abundance of Bacteroidetes (Prevotella) Elusimicrobia and decreased Firmicutes (Bacilli) in exposed rats versus controls. Surprisingly, these DEP-induced changes decreased in adulthood despite continuous exposure, which suggests that the effects of exposure to environmental chemicals are more severe in adolescents. They also observed a small but consistent reduction of body weight in exposed adolescent rats, which is consistent with their findings of a reduced Firmicutes/Bacteroidetes ratio.
Lei et al. (2019) [37] conducted in vivo and in vitro experiments in female C57BL/6J mice to determine the effects of low or high dose DEHP (1 or 10 mg/kg bw/day) exposure on the gut microbiota composition and metabolite profile. The authors observed an increased abundance of Lachnoclostridium and decreased Clostridium sensu stricto after DEHP exposure. The addition of DEHP to the cultured cecal microbiota enhanced the abundance of Lachnoclostridium, which is able to produce p-hydroxyphenylacetic acid, the precursor of p-cresol, a bacterial metabolite linked to neurodevelopmental disorders.
Regarding human epidemiological studies, the information is scarce. Yang et al. (2019) [83] demonstrated in a recent epidemiological study showing that DEHP exposure in newborns resulted in changes to the microbiota composition, with a decrease in Rothia sp. and Bifidobacterium longum. The presence of Rothia in human milk has been associated with a minor incidence of asthma [152], and B. longum, considered a probiotic, seems to have positive effects on infants, particularly in reducing the risk of obesity and celiac disease.
4. Conclusions
The incidence of metabolic diseases such as obesity and T2D are increasing worldwide. Exposure to EDCs related to food intake induces a series of changes including microbial dysbiosis and the induction of xenobiotic pathways and associated genes, enzymes, and metabolites involved in EDC metabolism. The products and by-products released following the microbial metabolism of EDCs can be taken up by the host and could have a major impact on host metabolism and the development of metabolic diseases. However, data regarding the effects of EDCs on the human gut microbiota are limited. The increasing EDC exposure via dietary intake requires the identification of the compounds and of the specific responses of the different species of the gut microbiome. In addition, the characterization of the common mechanisms of action of the different EDCs—such as the binding of the same hormone receptors, their possible cumulative and combined effects, and the indicative bacteria underlying the toxicity of EDCs on the gut microbiota—is also essential. In addition, the effect of exposure to low EDC levels on microbiota disruption should also be considered. The impact of other parameters such as host age and sex on the gut microbiota, described in this review, make necessary further research with broader dose ranges and analyses with more time points. This will help to determine the origin of sex-dependent effects using additional exposure windows. Lastly, the remediation of EDC-induced changes in the gut microbioma might represent an alternative for the treatment and prevention of metabolic diseases.
Author Contributions
Conceptualization, A.R.; methodology, A.R., C.M., Y.G.-O. and S.P.; writing—original draft preparation, Y.G.-O., S.P., C.M. and A.R review and editing, Y.G.-O., C.M. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the frame of GP/EFSA/ENCO/380 2018/03/G04: OBEMIRISK: Knowledge platform for assessing the risk of Bisphenols on gut microbiota and its role in obesogenic phenotype: looking for biomarkers. This research was also funded by Plan Estatal de I+D+I 2013-2016, Proyecto cofinanciado FEDER-ISCIII PI17/01758, Proyecto cofinanciado FEDER-Consejería de Salud y Familias, Junta de Andalucía PE-0250-2019 and by Fundación Mapfre MAPFRE2018.
Acknowledgments
The results presented in this article constitute part of Yolanda Gálvez-Ontiveros doctoral thesis, performed in the Nutrition and Food Sciences Doctorate Program of the University of Granada.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Saal, F.S.V. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from the Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Mantovani, A. Endocrine Disrupters and the Safety of Food Chains. Horm. Res. Paediatr. 2016, 86, 279–288. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism Disrupting Chemicals and Metabolic Disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Malaise, Y.; Menard, S.; Cartier, C.; Gaultier, E.; Lasserre, F.; Lencina, C.; Harkae, C.; Geoffre, N.; Lakhal, L.; Castan, I.; et al. Gut Dysbiosis and Impairment of Immune System Homeostasis in Perinatally-Exposed Mice to Bisphenol A Precede Obese Phenotype Development. Sci. Rep. 2017, 7, 14472. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, G.; Nagy, T.; Guo, T.L. Bisphenol A Alteration of Type 1 Diabetes in Non-Obese Diabetic (NOD) Female Mice is Dependent on Window of Exposure. Arch. Toxicol. 2019, 93, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Gut Dysbiosis in Animals due to Environmental Chemical Exposures. Front. Cell. Infect. Microbiol. 2017, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, G.; Ramprasath, T.; Gilles, M.; Swaminathan, K.; Ramasamy, S. Gut Microbiota, Endocrine-Disrupting Chemicals, and the Diabetes Epidemic. Trends Endocrinol. Metab. 2017, 28, 612–625. [Google Scholar] [CrossRef]
- Claus, S.P.; Guillou, H.; Ellero-Simatos, S. The Gut Microbiota: A Major Player in the Toxicity of Environmental Pollutants? NPJ Biofilms Microbiomes 2016, 2, 16003. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Modulation of Gut Microbiota in the Management of Metabolic Disorders: The Prospects and Challenges. Int. J. Mol. Sci. 2014, 15, 4158–4188. [Google Scholar] [CrossRef]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Orlando Mendez-Salazar, E.; Guadalupe Ortiz-Lopez, M.; de los Angeles Granados-Silvestre, M.; Palacios-Gonzalez, B.; Menjivar, M. Altered Gut Microbiota and Compositional Changes in Firmicutes and Proteobacteria in Mexican Undernourished and Obese Children. Front. Microbiol. 2018, 9, 2494. [Google Scholar] [CrossRef]
- Gallardo-Becerra, L.; Cornejo-Granados, F.; Garcia-Lopez, R.; Valdez-Lara, A.; Bikel, S.; Canizales-Quinteros, S.; Lopez-Contreras, B.E.; Mendoza-Vargas, A.; Nielsen, H.; Ochoa-Leyva, A. Metatranscriptomic Analysis to Define the Secrebiome, and 16S rRNA Profiling of the Gut Microbiome in Obesity and Metabolic Syndrome of Mexican Children. Microb. Cell Factories 2020, 19, 61. [Google Scholar] [CrossRef]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in Gut Microbiota Composition between Obese and Lean Children: A Cross-Sectional Study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Geurts, L.; Lazarevic, V.; Derrien, M.; Everard, A.; Van Roye, M.; Knauf, C.; Valet, P.; Girard, M.; Muccioli, G.G.; Francois, P.; et al. Altered Gut Microbiota and Endocannabinoid System Tone in Obese and Diabetic Leptin-Resistant Mice: Impact on Apelin Regulation in Adipose Tissue. Front. Microbiol. 2011, 2, 149. [Google Scholar] [CrossRef]
- Cani, P.D. Gut Microbiota and Obesity: Lessons from the Microbiome. Brief. Funct. Genom. 2013, 12, 381–387. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between Gut Microbiota, Host Genetics and Diet Relevant to Development of Metabolic Syndromes in Mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Abu Al-Soud, W.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Leiva, I.; Miguel Gomez-Zumaquero, J.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Isabel Queipo-Ortuno, M. Gut Microbiota in Children with Type 1 Diabetes Differs from that in Healthy Children: A Case-Control Study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Apau, J.; Acheampong, A.; Adua, E. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S can Result in Obesity in Human Body. Cogent Chem. 2018, 4, 1506601. [Google Scholar] [CrossRef]
- García, S. Estudio de la Migración de Distintos Componentes de los Materiales Plásticos a los Alimentos. Ph.D. Thesis, University of Santiago de Compostela, Santiago de Compostela, Spain, 2005. [Google Scholar]
- Verbanck, M.; Canouil, M.; Leloire, A.; Dhennin, V.; Coumoul, X.; Yengo, L.; Froguel, P.; Poulain-Godefroy, O. Low-Dose Exposure to Bisphenols A, F and S of Human Primary Adipocyte Impacts Coding and Non-Coding RNA Profiles. PLoS ONE 2017, 12, e0179583. [Google Scholar] [CrossRef]
- Wang, Y.; Rui, M.; Nie, Y.; Lu, G. Influence of Gastrointestinal Tract on Metabolism of Bisphenol A as Determined by in Vitro Simulated System. J. Hazard. Mater. 2018, 355, 111–118. [Google Scholar] [CrossRef]
- Catron, T.R.; Keely, S.P.; Brinkman, N.E.; Zurlinden, T.J.; Section, C.E.W.; Wright, J.R.; Phelps, D.; Wheaton, E.; Kvasnicka, A.; Gaballah, S.; et al. Host Developmental Toxicity of BPA and BPA Alternatives is Inversely Related to Microbiota Disruption in Zebrafish. Toxicol. Sci. 2019, 167, 468–483. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Y.; Hu, C.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Dysbiosis of Gut Microbiota by Chronic Coexposure to Titanium Dioxide Nanoparticles and Bisphenol A: Implications for Host Health in Zebrafish. Environ. Pollut. 2018, 234, 307–317. [Google Scholar] [CrossRef]
- Javurek, A.B.; Spollen, W.G.; Johnson, S.A.; Bivens, N.J.; Bromert, K.H.; Givan, S.A.; Rosenfeld, C.S. Effects of Exposure to Bisphenol A and Ethinyl Estradiol on the Gut Microbiota of Parents and their Offspring in a Rodent Model. Gut Microbes 2016, 7, 471–485. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Y.; Li, H.; Qiao, F.; Wu, J.; Du, Z.; Zhang, M. Influence of Endogenous and Exogenous Estrogenic Endocrine on Intestinal Microbiota in Zebrafish. PLoS ONE 2016, 11, e0163895. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, G.; Nagy, T.; Teng, Q.; Guo, T.L. Sex-Dependent Effects of Bisphenol A on Type 1 Diabetes Development in Non-Obese Diabetic (NOD) Mice. Arch. Toxicol. 2019, 93, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary Concentrations of Bisphenol A and 4-Nonylphenol in a Human Reference Population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiele, T.; Gallawa, C.M.; Kubachka, K.M.; Creed, J.T.; Basta, N.; Dayton, E.A.; Whitacre, S.; Du Laing, G.; Bradham, K. Arsenic Metabolism by Human Gut Microbiota upon in Vitro Digestion of Contaminated Soils. Environ. Health Perspect. 2010, 118, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.B.; Flythe, M.D.; Hennig, B. Environmental Pollutant-Mediated Disruption of Gut Microbial Metabolism of the Prebiotic Inulin. Anaerobe 2019, 55, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Menon, R.; Manteiga, S.; Alden, N.; Hunt, C.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Environmental Chemical Diethylhexyl Phthalate Alters Intestinal Microbiota Community Structure and Metabolite Profile in Mice. mSystems 2019, 4. [Google Scholar] [CrossRef]
- Joly, C.; Gay-Queheillard, J.; Leke, A.; Chardon, K.; Delanaud, S.; Bach, V.; Khorsi-Cauet, H. Impact of Chronic Exposure to Low Doses of Chlorpyrifos on the Intestinal Microbiota in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME (R)) and in the Rat. Environ. Sci. Pollut. Res. 2013, 20, 2726–2734. [Google Scholar] [CrossRef]
- Shehata, A.A.; Schroedl, W.; Aldin, A.A.; Hafez, H.M.; Krueger, M. The Effect of Glyphosate on Potential Pathogens and Beneficial Members of Poultry Microbiota in Vitro. Curr. Microbiol. 2013, 66, 350–358. [Google Scholar] [CrossRef]
- Ackermann, W.; Coenen, M.; Schroedl, W.; Shehata, A.A.; Krueger, M. The Influence of Glyphosate on the Microbiota and Production of Botulinum Neurotoxin during Ruminal Fermentation. Curr. Microbiol. 2015, 70, 374–382. [Google Scholar] [CrossRef]
- Riede, S.; Toboldt, A.; Breves, G.; Metzner, M.; Koehler, B.; Braeunig, J.; Schafft, H.; Lahrssen-Wiederholt, M.; Niemann, L. Investigations on the Possible Impact of a Glyphosate-Containing Herbicide on Ruminal Metabolism and Bacteria Invitro by Means of the ‘Rumen Simulation Technique’. J. Appl. Microbiol. 2016, 121, 644–656. [Google Scholar] [CrossRef]
- Ba, Q.; Li, M.; Chen, P.; Huang, C.; Duan, X.; Lu, L.; Li, J.; Chu, R.; Xie, D.; Song, H.; et al. Sex-Dependent Effects of Cadmium Exposure in Early Life on Gut Microbiota and Fat Accumulation in Mice. Environ. Health Perspect. 2017, 125, 437–446. [Google Scholar] [CrossRef]
- Wu, J.; Wen, X.W.; Faulk, C.; Boehnke, K.; Zhang, H.; Dolinoy, D.C.; Xi, C. Perinatal Lead Exposure Alters Gut Microbiota Composition and Results in Sex-Specific Bodyweight Increases in Adult Mice. Toxicol. Sci. 2016, 151, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Lu, L.; Jin, C.; Wang, S.; Zhou, J.; Ni, Y.; Fu, Z.; Jin, Y. Effects of Short Term Lead Exposure on Gut Microbiota and Hepatic Metabolism in Adult Zebrafish. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2018, 209, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Abo, R.P.; Schlieper, K.A.; Graffam, M.E.; Levine, S.; Wishnok, J.S.; Swenberg, J.A.; Tannenbaum, S.R.; Fox, J.G. Arsenic Exposure Perturbs the Gut Microbiome and its Metabolic Profile in Mice: An Integrated Metagenomics and Metabolomics Analysis. Environ. Health Perspect. 2014, 122, 284–291. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, J.A.A.; Allred, K.F.; Menon, R.; Riordan, R.; Weeks, B.R.; Jayaraman, A.; Allred, C.D. Bisphenol-A Alters Microbiota Metabolites Derived from Aromatic Amino Acids and Worsens Disease Activity during Colitis. Exp. Biol. Med. 2018, 243, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Koestel, Z.L.; Backus, R.C.; Tsuruta, K.; Spollen, W.G.; Johnson, S.A.; Javurek, A.B.; Ellersieck, M.R.; Wiedmeyer, C.E.; Kannan, K.; Xue, J.; et al. Bisphenol A (BPA) in the Serum of Pet Dogs Following Short-Term Consumption of Canned Dog Food and Potential Health Consequences of Exposure to BPA. Sci. Total Environ. 2017, 579, 1804–1814. [Google Scholar] [CrossRef]
- Lai, K.; Chung, Y.; Li, R.; Wan, H.; Wong, C.K. Bisphenol A Alters Gut Microbiome: Comparative Metagenomics Analysis. Environ. Pollut. 2016, 218, 923–930. [Google Scholar] [CrossRef]
- Reddivari, L.; Veeramachaneni, D.N.R.; Walters, W.A.; Lozupone, C.; Palmer, J.; Hewage, M.K.K.; Bhatnagar, R.; Amir, A.; Kennett, M.J.; Knight, R.; et al. Perinatal Bisphenol A Exposure Induces Chronic Inflammation in Rabbit Offspring via Modulation of Gut Bacteria and their Metabolites. mSystems 2017, 2, e00093-17. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, W.; Hua, J.; Hu, C.; Lai, N.L.; Jan, P.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Dysregulation of Intestinal Health by Environmental Pollutants: Involvement of the Estrogen Receptor and Aryl Hydrocarbon Receptor. Environ. Sci. Technol. 2018, 52, 2323–2330. [Google Scholar] [CrossRef]
- Cheng, S.L.; Li, X.; Lehmler, H.; Phillips, B.; Shen, D.; Cui, J.Y. Gut Microbiota Modulates Interactions between Polychlorinated Biphenyls and Bile Acid Homeostasis. Toxicol. Sci. 2018, 166, 269–287. [Google Scholar] [CrossRef]
- Chi, Y.; Lin, Y.; Lu, Y.; Huang, Q.; Ye, G.; Dong, S. Gut Microbiota Dysbiosis Correlates with a Low-Dose PCB126-Induced Dyslipidemia and Non-Alcoholic Fatty Liver Disease. Sci. Total Environ. 2019, 653, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; Eum, S.Y.; Rampersaud, E.; Daunert, S.; Abreu, M.T.; Toborek, M. Exercise Attenuates PCB-Induced Changes in the Mouse Gut Microbiome. Environ. Health Perspect. 2013, 121, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Cary, T.L.; Karasov, W.H.; Dearing, M.D. Larval Exposure to Polychlorinated Biphenyl 126 (Pcb-126) Causes Persistent Alteration of the Amphibian Gut Microbiota. Environ. Toxicol. Chem. 2015, 34, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Petriello, M.C.; Hoffman, J.B.; Vsevolozhskaya, O.; Morris, A.J.; Hennig, B. Dioxin-Like PCB 126 Increases Intestinal Inflammation and Disrupts Gut Microbiota and Metabolic Homeostasis. Environ. Pollut. 2018, 242, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Rude, K.M.; Pusceddu, M.M.; Keogh, C.E.; Sladek, J.A.; Rabasa, G.; Miller, E.N.; Sethi, S.; Keil, K.P.; Pessah, I.N.; Lein, P.J.; et al. Developmental Exposure to Polychlorinated Biphenyls (PCBs) in the Maternal Diet Causes Host-Microbe Defects in Weanling Offspring Mice. Environ. Pollut. 2019, 253, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Usami, A.; Shirai, R.; Harada, N.; Ikushiro, S.; Sakaki, T.; Nakano, Y.; Inui, H.; Yamaji, R. S -Equol Activates cAMP Signaling at the Plasma Membrane of INS-1 Pancreatic Β-Cells and Protects against Streptozotocin-Induced Hyperglycemia by Increasing Β-Cell Function in Male Mice. J. Nutr. 2017, 147, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xu, J.; Cai, D.; Chen, S.; Nagy, T.; Guo, T.L. Exacerbation of Type 1 Diabetes in Perinatally Genistein Exposed Female Non-Obese Diabetic (NOD) Mouse is Associated with Alterations of Gut Microbiota and Immune Homeostasis. Toxicol. Sci. 2018, 165, 291–301. [Google Scholar] [CrossRef]
- Lopez, P.; Sanchez, M.; Perez-Cruz, C.; Velazquez-Villegas, L.A.; Syeda, T.; Aguilar-Lopez, M.; Rocha-Viggiano, A.K.; del Carmen Silva-Lucero, M.; Torre-Villalvazo, I.; Noriega, L.G.; et al. Long-Term Genistein Consumption Modifies Gut Microbiota, Improving Glucose Metabolism, Metabolic Endotoxemia, and Cognitive Function in Mice Fed a High-Fat Diet. Mol. Nutr. Food Res. 2018, 62, 1800313. [Google Scholar] [CrossRef]
- Marshall, B.L.; Liu, Y.; Farrington, M.J.; Mao, J.; Helferich, W.G.; Schenk, A.K.; Bivens, N.J.; Sarma, S.J.; Lei, Z.; Sumner, L.W.; et al. Early Genistein Exposure of California Mice and Effects on the Gut Microbiota Brain Axis. J. Endocrinol. 2019, 242, 139–157. [Google Scholar] [CrossRef]
- Piccolo, B.D.; Mercer, K.E.; Bhattacharyya, S.; Bowlin, A.K.; Saraf, M.K.; Pack, L.; Chintapalli, S.V.; Shankar, K.; Adams, S.H.; Badger, T.M.; et al. Early Postnatal Diets Affect the Bioregional Small Intestine Microbiome and Ileal Metabolome in Neonatal Pigs. J. Nutr. 2017, 147, 1499–1509. [Google Scholar] [CrossRef]
- Williams, C.L.; Ybarra, A.R.; Meredith, A.N.; Durrant, B.S.; Tubbs, C.W. Gut Microbiota and Phytoestrogen-Associated Infertility in Southern White Rhinoceros. Mbio 2019, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Spencer, N.E.; Saraf, M.K.; Hennings, L.; Bowlin, A.K.; Cleves, M.A.; Mercer, K.; Chintapalli, S.V.; Shankar, K.; Rank, R.G.; et al. Formula Diet Alters Small Intestine Morphology, Microbial Abundance and Reduces VE-Cadherin and IL-10 Expression in Neonatal Porcine Model. BMC Gastroenterol. 2016, 16, 40. [Google Scholar]
- Zhou, L.; Xiao, X.; Zhang, Q.; Zheng, J.; Li, M.; Yu, M.; Wang, X.; Deng, M.; Zhai, X.; Li, R. Improved Glucose and Lipid Metabolism in the Early Life of Female Offspring by Maternal Dietary Genistein is Associated with Alterations in the Gut Microbiota. Front. Endocrinol. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Obadia, B.; Keebaugh, E.S.; Yamada, R.; Ludington, W.B.; Ja, W.W. Diet Influences Host-Microbiota Associations in Drosophila. Proc. Natl. Acad. Sci. USA 2018, 115, E4547–E4548. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Raikhel, V.; Gopalakrishnan, K.; Fernandez-Hernandez, H.; Lambertini, L.; Manservisi, F.; Falcioni, L.; Bua, L.; Belpoggi, F.; Teitelbaum, S.L.; et al. Effect of Postnatal Low-Dose Exposure to Environmental Chemicals on the Gut Microbiome in a Rodent Model. Microbiome 2016, 4, 26. [Google Scholar] [CrossRef]
- Fan, Y.; Qin, Y.; Chen, M.; Li, X.; Wang, R.; Huang, Z.; Xu, Q.; Yu, M.; Zhang, Y.; Han, X.; et al. Prenatal Low-Dose DEHP Exposure Induces Metabolic Adaptation and Obesity: Role of Hepatic Thiamine Metabolism. J. Hazard. Mater. 2020, 385, 121534. [Google Scholar] [CrossRef]
- Gao, B.; Bian, X.; Mahbub, R.; Lu, K. Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and its Metabolic Functions. Environ. Health Perspect. 2017, 125, 198–206. [Google Scholar] [CrossRef]
- Jin, Y.; Zeng, Z.; Wu, Y.; Zhang, S.; Fu, Z. Oral Exposure of Mice to Carbendazim Induces Hepatic Lipid Metabolism Disorder and Gut Microbiota Dysbiosis. Toxicol. Sci. 2015, 147, 116–126. [Google Scholar] [CrossRef]
- Liang, Y.; Zhan, J.; Liu, D.; Luo, M.; Han, J.; Liu, X.; Liu, C.; Cheng, Z.; Zhou, Z.; Wang, P. Organophosphorus Pesticide Chlorpyrifos Intake Promotes Obesity and Insulin Resistance through Impacting Gut and Gut Microbiota. Microbiome 2019, 7, 19. [Google Scholar] [CrossRef]
- Liu, Q.; Shao, W.; Zhang, C.; Xu, C.; Wang, Q.; Liu, H.; Sun, H.; Jiang, Z.; Gu, A. Organochloride Pesticides Modulated Gut Microbiota and Influenced Bile Acid Metabolism in Mice. Environ. Pollut. 2017, 226, 268–276. [Google Scholar] [CrossRef]
- Tu, P.; Gao, B.; Chi, L.; Lai, Y.; Bian, X.; Ru, H.; Lu, K. Subchronic Low-Dose 2,4-D Exposure Changed Plasma Acylcarnitine Levels and Induced Gut Microbiome Perturbations in Mice. Sci. Rep. 2019, 9, 4363. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, J.; Pang, G.; Ren, F.; Fang, B. Effects of Diethyl Phosphate, a Non-Specific Metabolite of Organophosphorus Pesticides, on Serum Lipid, Hormones, Inflammation, and Gut Microbiota. Molecules 2019, 24, 2003. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jin, C.; Wang, Y.; Fu, Z.; Jin, Y. Exposure to the Fungicide Propamocarb Causes Gut Microbiota Dysbiosis and Metabolic Disorder in Mice. Environ. Pollut. 2018, 237, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Tu, P.; Bian, X.; Chi, L.; Ru, H.; Lu, K. Profound Perturbation Induced by Triclosan Exposure in Mouse Gut Microbiome: A Less Resilient Microbial Community with Elevated Antibiotic and Metal Resistomes. BMC Pharmacol. Toxicol. 2017, 18, 46. [Google Scholar] [CrossRef]
- Gaulke, C.A.; Barton, C.L.; Proffitt, S.; Tanguay, R.L.; Sharpton, T.J. Triclosan Exposure is Associated with Rapid Restructuring of the Microbiome in Adult Zebrafish. PLoS ONE 2016, 11, e0154632. [Google Scholar] [CrossRef]
- Kennedy, R.C.; Fling, R.R.; Robeson, M.S.; Saxton, A.M.; Donnell, R.L.; Darcy, J.L.; Bemis, D.A.; Liu, J.; Zhao, L.; Chen, J. Temporal Development of Gut Microbiota in Triclocarban Exposed Pregnant and Neonatal Rats. Sci. Rep. 2016, 6, 33430. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Y.; Ye, H.; Zhang, J.; Ke, Y. Perinatal Triclosan Exposure in the Rat Induces Long-Term Disturbances in Metabolism and Gut Microbiota in Adulthood and Old Age. Environ. Res. 2020, 182, 109004. [Google Scholar] [CrossRef]
- Narrowe, A.B.; Albuthi-Lantz, M.; Smith, E.P.; Bower, K.J.; Roane, T.M.; Vajda, A.M.; Miller, C.S. Perturbation and Restoration of the Fathead Minnow Gut Microbiome after Low-Level Triclosan Exposure. Microbiome 2015, 3, 6. [Google Scholar] [CrossRef]
- Zang, L.; Ma, Y.; Huang, W.; Ling, Y.; Sun, L.; Wang, X.; Zeng, A.; Dahlgren, R.A.; Wang, C.; Wang, H. Dietary Lactobacillus plantarum ST-III Alleviates the Toxic Effects of Triclosan on Zebrafish (Danio rerio) via Gut Microbiota Modulation. Fish Shellfish Immunol. 2019, 84, 1157–1169. [Google Scholar] [CrossRef]
- Eggers, S.; Safdar, N.; Sethi, A.K.; Suen, G.; Peppard, P.E.; Kates, A.E.; Skarlupka, J.H.; Kanarek, M.; Malecki, K.M.C. Urinary Lead Concentration and Composition of the Adult Gut Microbiota in a Cross-Sectional Population-Based Sample. Environ. Int. 2019, 133, 105122. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative Metabolomics in Vegans and Omnivores Reveal Constraints on Diet-Dependent Gut Microbiota Metabolite Production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.S.H.; Lin, I.-H.; Chen, Y.; Lin, H.; Wu, C.; Su, Y.; Yang, Y.; Yang, S.; Suen, J. Phthalate Exposure Alters Gut Microbiota Composition and IgM Vaccine Response in Human Newborns. Food Chem. Toxicol. 2019, 132, 110700. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, I.B.; Wallace, J.C.; Shojaie, A.; Griffith, W.C.; Hong, S.; Wilder, C.S.; Green, F.H.; Tsai, J.; Knight, M.; Workman, T.; et al. Human Oral Buccal Microbiomes are Associated with Farmworker Status and Azinphos-Methyl Agricultural Pesticide Exposure. Appl. Environ. Microbiol. 2017, 83, e02149-16. [Google Scholar] [CrossRef] [PubMed]
- Bever, C.S.; Rand, A.A.; Nording, M.; Taft, D.; Kalanetra, K.M.; Mills, D.A.; Breck, M.A.; Smilowitz, J.T.; German, J.B.; Hammock, B.D. Effects of Triclosan in Breast Milk on the Infant Fecal Microbiome. Chemosphere 2018, 203, 467–473. [Google Scholar] [CrossRef]
- Ribado, J.V.; Ley, C.; Haggerty, T.D.; Tkachenko, E.; Bhatt, A.S.; Parsonnet, J. Household Triclosan and Triclocarban Effects on the Infant and Maternal Microbiome. EMBO Mol. Med. 2017, 9, 1732–1741. [Google Scholar] [CrossRef]
- Knip, M.; Siljander, H. The Role of the Intestinal Microbiota in Type 1 Diabetes Mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef]
- De Goffau, M.C.; Fuentes, S.; van den Bogert, B.; Honkanen, H.; de Vos, W.M.; Welling, G.W.; Hyoty, H.; Harmsen, H.J.M. Aberrant Gut Microbiota Composition at the Onset of Type 1 Diabetes in Young Children. Diabetologia 2014, 57, 1569–1577. [Google Scholar] [CrossRef]
- Krych, Ł.; Nielsen, D.; Hansen, A.; Hansen, C. Gut Microbial Markers are Associated with Diabetes Onset, Regulatory Imbalance, and IFN-Γ Level in NOD Mice. Gut Microbes 2015, 6, 101–109. [Google Scholar] [CrossRef]
- Chen, C.; You, L.; Huang, Q.; Fu, X.; Zhang, B.; Liu, R.; Li, C. Modulation of Gut Microbiota by Mulberry Fruit Polysaccharide Treatment of Obese Diabetic Db/Db Mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar] [CrossRef]
- Gulden, E.; Chao, C.; Tai, N.; Pearson, J.A.; Peng, J.; Majewska-Szczepanik, M.; Zhou, Z.; Wong, F.S.; Wen, L. TRIF Deficiency Protects Non-Obese Diabetic Mice from Type 1 Diabetes by Modulating the Gut Microbiota and Dendritic Cells. J. Autoimmun. 2018, 93, 57–65. [Google Scholar] [CrossRef]
- Khokhlova, E.V.; Smeianov, V.V.; Efimov, B.A.; Kafarskaia, L.I.; Pavlova, S.I.; Shkoporov, A.N. Anti-inflammatory Properties of Intestinal Bifidobacterium Strains Isolated from Healthy Infants. Microbiol. Immunol. 2012, 56, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Morton, J.M. The Human Gut Microbiome A Review of the Effect of Obesity and Surgically Induced Weight Loss. JAMA Surg. 2013, 148, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- De Theije, C.G.M.; Wopereis, H.; Ramadan, M.; van Eijndthoven, T.; Lambert, J.; Knol, J.; Garssen, J.; Kraneveld, A.D.; Oozeer, R. Altered Gut Microbiota and Activity in a Murine Model of Autism Spectrum Disorders. Brain Behav. Immun. 2014, 37, 197–206. [Google Scholar] [CrossRef]
- Baxter, N.T.; Zackular, J.P.; Chen, G.Y.; Schloss, P.D. Structure of the Gut Microbiome Following Colonization with Human Feces Determines Colonic Tumor Burden. Microbiome 2014, 2, 20. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Lorena Ruiz, S.D.; Ruas-Madiedo, P.; Sanchez, B.; Margolles, A. Bifidobacteria and their Health-Promoting Effects. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Zhang, L.; Day, A.; McKenzie, G.; Mitchell, H. Nongastric Helicobacter Species Detected in the Intestinal Tract of Children. J. Clin. Microbiol. 2006, 44, 2276–2279. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.R.; Schoenfelder, G. Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef]
- Birnbaum, L.S. When Environmental Chemicals Act Like Uncontrolled Medicine. Trends Endocrinol. Metab. 2013, 24, 321–323. [Google Scholar] [CrossRef]
- Kim, K.; Kabir, E.; Jahan, S.A. Exposure to Pesticides and the Associated Human Health Effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Zarean, M.; Poursafa, P. The Role of Environmental Disruptor Chemicals in the Development of Non Communicable Disease. Primordial Prev. Non Commun. Dis. 2019, 1121, 21–31. [Google Scholar]
- European Food Safety Authority (EFSA). Conclusion regarding the Peer Review of the Pesticide Risk Assessment of the Active Substance Propamocarb. EFSA J. 2006, 78, 1–80. [Google Scholar]
- Cho, I.; Blaser, M.J. The Human Microbiome: At the Interface of Health and Disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, R.; Martinez-Carballo, E.; Gonzalez-Barreiro, C.; Rial-Otero, R.; Simal-Gandara, J. Distribution of Polychlorinated Biphenyls in both Products and by-Products of a Mussel Shell Incinerator Facility. Environ. Sci. Pollut. Res. 2011, 18, 1139–1146. [Google Scholar] [CrossRef]
- Robson, M.; Melymuk, L.; Csiszar, S.A.; Giang, A.; Diamond, M.L.; Helm, P.A. Continuing Sources of PCBs: The Significance of Building Sealants. Environ. Int. 2010, 36, 506–513. [Google Scholar] [CrossRef]
- Baker, N.A.; Shoemaker, R.; English, V.; Larian, N.; Sunkara, M.; Morris, A.J.; Walker, M.; Yiannikouris, F.; Cassis, L.A. Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ. Health Perspect. 2015, 123, 944–950. [Google Scholar] [CrossRef]
- Mesnage, R.; Biserni, M.; Balu, S.; Frainay, C.; Poupin, N.; Jourdan, F.; Wozniak, E.; Xenakis, T.; Mein, C.A.; Antoniou, M.N. Integrated Transcriptomics and Metabolomics Reveal Signatures of Lipid Metabolism Dysregulation in HepaRG Liver Cells Exposed to PCB 126. Arch. Toxicol. 2018, 92, 2533–2547. [Google Scholar] [CrossRef]
- Iszatt, N.; Janssen, S.; Lenters, V.; Dahl, C.; Stigum, H.; Knight, R.; Mandal, S.; Peddada, S.; Gonzalez, A.; Midtvedt, T.; et al. Environmental Toxicants in Breast Milk of Norwegian Mothers and Gut Bacteria Composition and Metabolites in their Infants at 1 Month. Microbiome 2019, 7, 34. [Google Scholar] [CrossRef]
- Petriello, M.C.; Brandon, J.A.; Hoffman, J.; Wang, C.; Tripathi, H.; Abdel-Latif, A.; Ye, X.; Li, X.; Yang, L.; Lee, E.; et al. Dioxin-Like PCB 126 Increases Systemic Inflammation and Accelerates Atherosclerosis in Lean LDL Receptor-Deficient Mice. Toxicol. Sci. 2018, 162, 548–558. [Google Scholar] [CrossRef]
- Mafra, D.; Lobo, J.C.; Barros, A.F.; Koppe, L.; Vaziri, N.D.; Fouque, D. Role of Altered Intestinal Microbiota in Systemic Inflammation and Cardiovascular Disease in Chronic Kidney Disease. Future Microbiol. 2014, 9, 399–410. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The Genus Aeromonas: Taxonomy, Pathogenicity, and Infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Giulivo, M.; de Alda, M.L.; Capri, E.; Barcelo, D. Human Exposure to Endocrine Disrupting Compounds: Their Role in Reproductive Systems, Metabolic Syndrome and Breast Cancer. A Review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Monneret, C. What is an Endocrine Disruptor? C. R. Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Lin, J.; Huang, X.; Wu, Y.; Liu, J.; Wu, C. Effect of Butyl Paraben on the Development and Microbial Composition of Periphyton. Ecotoxicology 2016, 25, 342–349. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef]
- Fransway, A.F.; Fransway, P.J.; Belsito, D.V.; Yiannias, J.A. Paraben Toxicology. Dermatitis 2019, 30, 32–45. [Google Scholar] [CrossRef]
- Harley, K.G.; Berger, K.P.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C.; Calafat, A.M.; Ye, X.; Eskenazi, B. Association of Phthalates, Parabens and Phenols found in Personal Care Products with Pubertal Timing in Girls and Boys. Hum. Reprod. 2019, 34, 109–117. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Savastano, S.; Colao, A. Obesogenic Endocrine Disruptors and Obesity: Myths and Truths. Arch. Toxicol. 2017, 91, 3469–3475. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.; Li, Q.; Lambertini, L.; Belpoggi, F.; Manservisi, F.; Falcioni, L.; Bua, L.; Silva, M.J.; Ye, X.; Calafat, A.M.; et al. Paired Serum and Urine Concentrations of Biomarkers of Diethyl Phthalate, Methyl Paraben, and Triclosan in Rats. Environ. Health Perspect. 2016, 124, 39–45. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial Dysbiosis-Induced Obesity: Role of Gut Microbiota in Homoeostasis of Energy Metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Indiani, C.M.D.S.P.; Rizzardi, K.F.; Castelo, P.M.; Caldas Ferraz, L.F.; Darrieux, M.; Parisotto, T.M. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child. Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Hamm, A.K.; Manter, D.K.; Kirkwood, J.S.; Wolfe, L.M.; Cox-York, K.; Weir, T.L. The Effect of Hops (Humulus lupulus L.) Extract Supplementation on Weight Gain, Adiposity and Intestinal Function in Ovariectomized Mice. Nutrients 2019, 11, 3004. [Google Scholar] [CrossRef] [PubMed]
- Kolatorova, L.; Lapcik, O.; Starka, L. Phytoestrogens and the Intestinal Microbiome. Physiol. Res. 2018, 67, S401–S408. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Arques, J.; Medina, M.; Gaya, P.; de Las Rivas, B.; Munoz, R. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1826–1843. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L. O-Desmethylangolensin: The Importance of Equol’s Lesser Known Cousin to Human Health. Adv. Nutr. 2011, 2, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L.; Atkinson, C.; Wahala, K.; Lampe, J.W. Obesity Prevalence in Relation to Gut Microbial Environments Capable of Producing Equol or O-Desmethylangolensin from the Isoflavone Daidzein. Eur. J. Clin. Nutr. 2014, 68, 526–530. [Google Scholar] [CrossRef]
- Candela, M.; Biagi, E.; Soverini, M.; Consolandi, C.; Quercia, S.; Severgnini, M.; Peano, C.; Turroni, S.; Rampelli, S.; Pozzilli, P.; et al. Modulation of Gut Microbiota Dysbioses in Type 2 Diabetic Patients by Macrobiotic Ma-Pi 2 Diet. Br. J. Nutr. 2016, 116, 80–93. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Oki, K.; Toyama, M.; Banno, T.; Chonan, O.; Benno, Y.; Watanabe, K. Comprehensive Analysis of the Fecal Microbiota of Healthy Japanese Adults Reveals a New Bacterial Lineage Associated with a Phenotype Characterized by a High Frequency of Bowel Movements and a Lean Body Type. BMC Microbiol. 2016, 16, 284. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Jin, L.; DeCarli, C.; Phinney, B.; Sharp, F.R. Gram-Negative Bacterial Molecules Associate with Alzheimer Disease Pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef]
- Strotmeyer, E.S.; Yang, Z.; LaPorte, R.E.; Chang, Y.F.; Steenkiste, A.R.; Pietropaolo, M.; Nucci, A.M.; Shen, S.X.; Wang, L.M.; Wang, B.Y.; et al. Infant Diet and Type 1 Diabetes in China. Diabetes Res. Clin. Pract. 2004, 65, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Liu, W.; Liu, J. Potential Endocrine-Disrupting Effects of Metals via Interference with Glucocorticoid and Mineralocorticoid Receptors. Environ. Pollut. 2018, 242, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Planchart, A.; Green, A.; Hoyo, C.; Mattingly, C.J. Heavy Metal Exposure and Metabolic Syndrome: Evidence from Human and Model System Studies. Curr. Environ. Health Rep. 2018, 5, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; DiBari, J.; Bind, E.; Steffens, A.M.; Mukherjee, J.; Bartell, T.R.; Bellinger, D.C.; Hong, X.; Ji, Y.; Wang, M.; et al. In Utero Exposure to Mercury and Childhood Overweight or Obesity: Counteracting Effect of Maternal Folate Status. BMC Med. 2019, 17, 216. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Aaseth, J.; Gluhcheva, Y.G.; Ivanova, J.M.; Bjorklund, G.; Skalnaya, M.G.; Gatiatulina, E.R.; Popova, E.V.; et al. The Role of Cadmium in Obesity and Diabetes. Sci. Total Environ. 2017, 601, 741–755. [Google Scholar] [CrossRef]
- Wang, X.; Gao, D.; Zhang, G.; Zhang, X.; Li, Q.; Gao, Q.; Chen, R.; Xu, S.; Huang, L.; Zhang, Y.; et al. Exposure to Multiple Metals in Early Pregnancy and Gestational Diabetes Mellitus: A Prospective Cohort Study. Environ. Int. 2020, 135, 105370. [Google Scholar] [CrossRef]
- Paul, D.S.; Walton, F.S.; Saunders, R.J.; Styblo, M. Characterization of the Impaired Glucose Homeostasis Produced in C57BL/6 Mice by Chronic Exposure to Arsenic and High-Fat Diet. Environ. Health Perspect. 2011, 119, 1104–1109. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Statement on Tolerable Weekly Intake for Cadmium. EFSA J. 2011, 9, 1975. [Google Scholar]
- Halden, R.U.; Lindeman, A.E.; Aiello, A.E.; Andrews, D.; Arnold, W.A.; Fair, P.; Fuoco, R.E.; Geer, L.A.; Johnson, P.I.; Lohmann, R.; et al. The Florence Statement on Triclosan and Triclocarban. Environ. Health Perspect. 2017, 125, 064501. [Google Scholar] [CrossRef]
- Alvarez-Munoz, D.; Rodriguez-Mozaz, S.; Jacobs, S.; Serra-Compte, A.; Caceres, N.; Sioen, I.; Verbeke, W.; Barbosa, V.; Ferrari, F.; Fernandez-Tejedor, M.; et al. Pharmaceuticals and Endocrine Disruptors in Raw and Cooked Seafood from European Market: Concentrations and Human Exposure Levels. Environ. Int. 2018, 119, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Munoz, D.; Rambla-Alegre, M.; Carrasco, N.; Lopez de Alda, M.; Barcelo, D. Fast Analysis of Relevant Contaminants Mixture in Commercial Shellfish. Talanta 2019, 205, 119884. [Google Scholar] [CrossRef] [PubMed]
- Kalloo, G.; Calafat, A.M.; Chen, A.; Yolton, K.; Lanphear, B.P.; Braun, J.M. Early Life Triclosan Exposure and Child Adiposity at 8 Years of Age: A Prospective Cohort Study. Environ. Health 2018, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lu, C.; Wu, M.; Liang, J.; Ying, Y.; Liu, K.; Huang, X.; Zheng, S.; Du, X.; Liu, D.; et al. Association between Triclocarban and Triclosan Exposures and the Risks of Type 2 Diabetes Mellitus and Impaired Glucose Tolerance in the National Health and Nutrition Examination Survey (NHANES 2013–2014). Environ. Int. 2020, 136, 105445. [Google Scholar] [CrossRef] [PubMed]
- Schebb, N.H.; Ahn, K.C.; Dong, H.; Gee, S.J.; Hammock, B.D. Whole Blood is the Sample Matrix of Choice for Monitoring Systemic Triclocarban Levels. Chemosphere 2012, 87, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Whon, T.W.; Bae, J. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Pacyga, D.C.; Sathyanarayana, S.; Strakovsky, R.S. Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv. Nutr. 2019, 10, 803–815. [Google Scholar] [CrossRef]
- Milosevic, N.; Milanovic, M.; Sudji, J.; Zivanovic, D.B.; Stojanoski, S.; Vukovic, B.; Milic, N.; Stojanoska, M.M. Could Phthalates Exposure Contribute to the Development of Metabolic Syndrome and Liver Disease in Humans? Environ. Sci. Pollut. Res. 2020, 27, 772–784. [Google Scholar] [CrossRef]
- Martinez-Ibarra, A.; Daniel Martinez-Razo, L.; Ricardo Vazquez-Martinez, E.; Martinez-Cruz, N.; Flores-Ramirez, R.; Garcia-Gomez, E.; Lopez-Lopez, M.; Ortega-Gonzalez, C.; Camacho-Arroyo, I.; Cerbon, M. Unhealthy Levels of Phthalates and Bisphenol A in Mexican Pregnant Women with Gestational Diabetes and its Association to Altered Expression of miRNAs Involved with Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 3343. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatrics 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).