Immunomodulatory and Metabolic Changes after Gnetin-C Supplementation in Humans
Abstract
1. Introduction
2. Materials and Methods
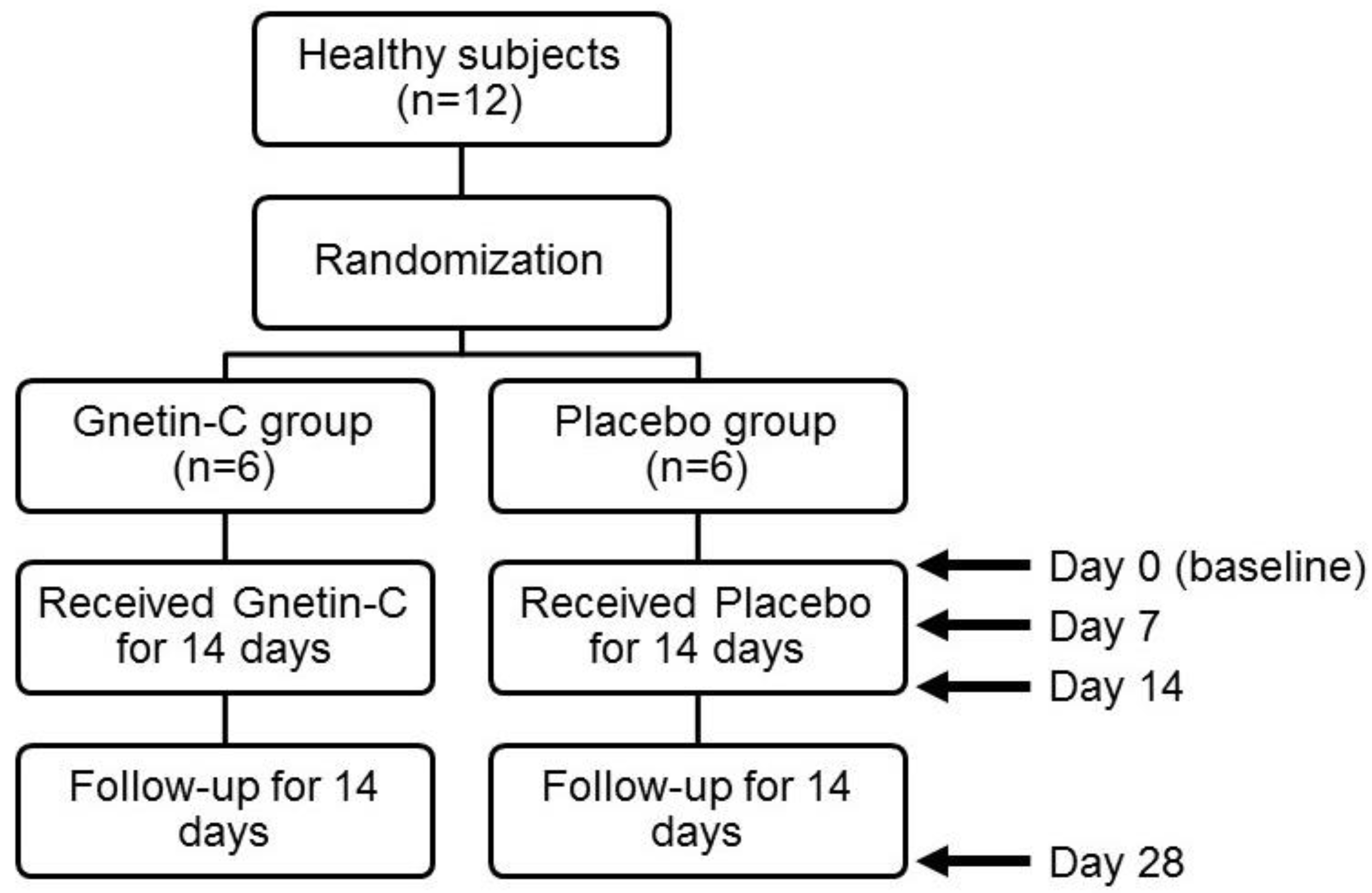
2.1. Study Design
2.2. Sample Preparation and Pharmacokinetic Evaluations
2.3. Whole Blood Cell Counts
2.4. Flow Cytometry
2.5. Cytotoxicity Assay
2.6. Serum Metabolic Profile
2.7. The Measurement of Urinary 8-Oxo-2’-Deoxyguanosine (8-OHdG)
2.8. The Measurement of Pentosidine (Advanced Glycation Endroducts, AGEs)
2.9. Statistical Analyses
3. Results
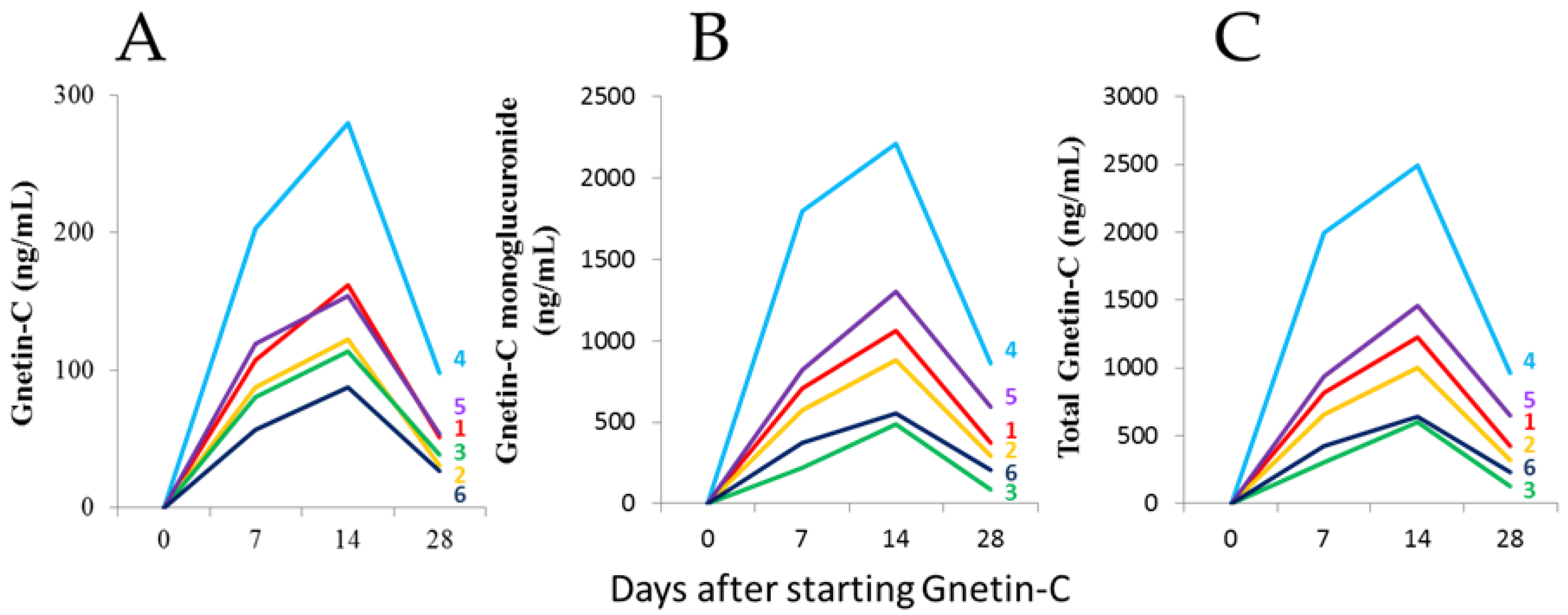
3.1. The Safety and Pharmacokinetic Evaluations
3.2. The Effects of Gnetin-C on Whole Blood Cell Counts
3.3. The Effects of Gnetin-C on Circulating Peripheral Blood Mononuclear Cells (PBMCs)
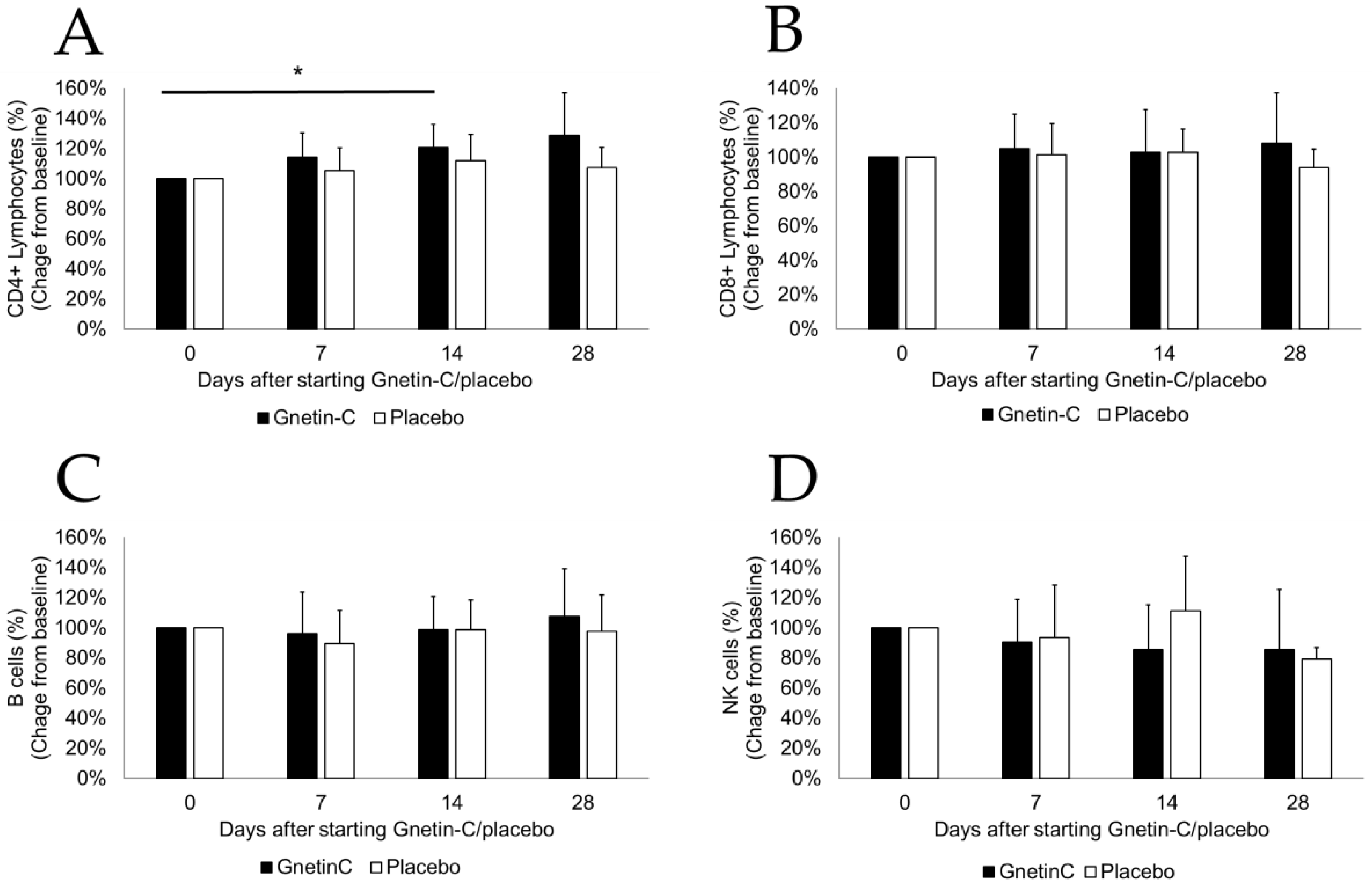
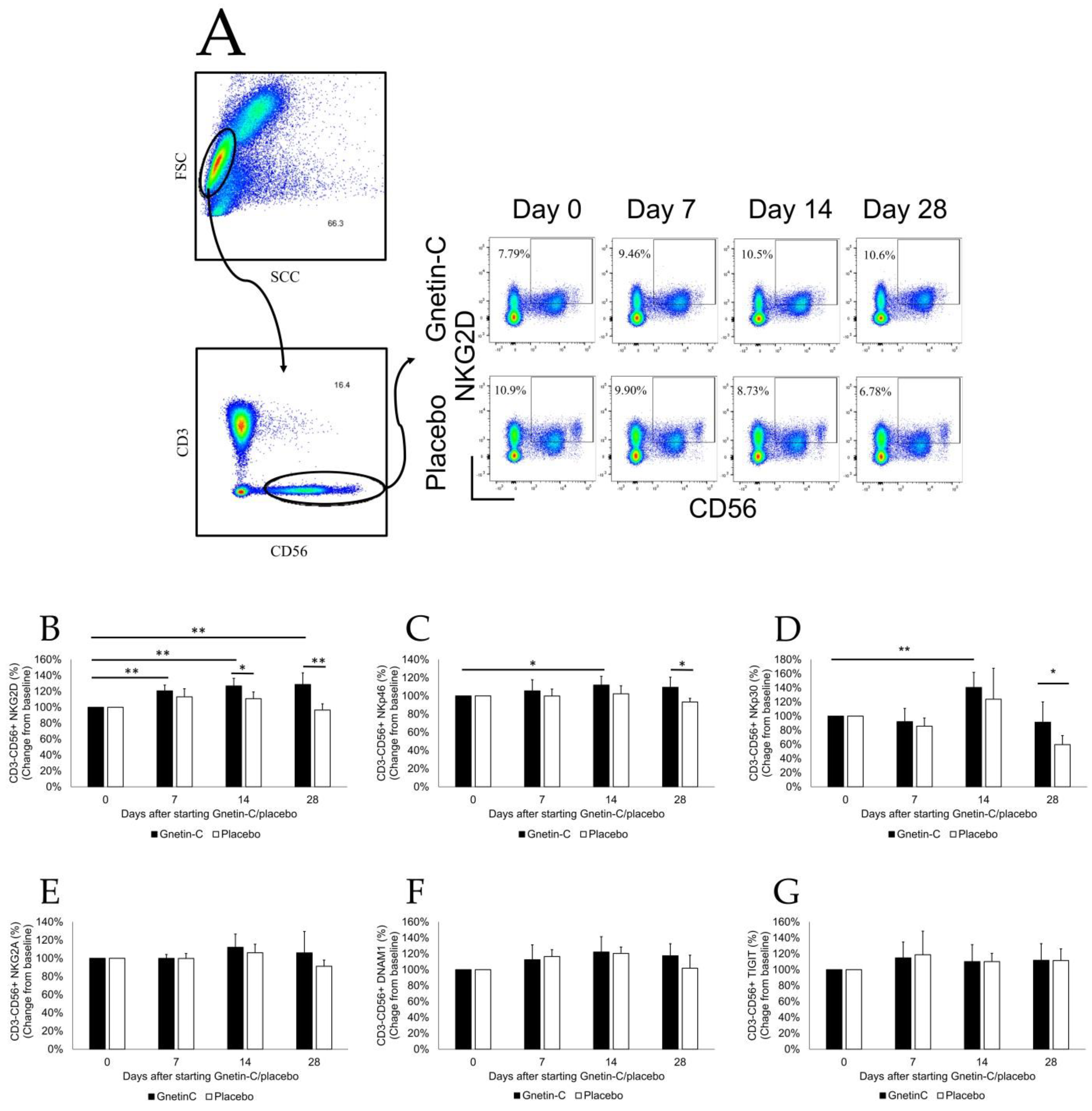
3.4. Effects of Gnetin-C on PBMC Subsets
3.5. Effect of Gnetin-C on NK Cell-Mediated Cytotoxicity
3.6. The Effects of Gnetin-C on Biochemical Tests and Lipid Profiles
3.7. The Effects of Gnetin-C on Urinary 8-OHdG and Pentosidine
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Espinoza, J.L.; Kurokawa, Y.; Takami, A. Rationale for assessing the therapeutic potential of resveratrol in hematological malignancies. Blood Rev. 2019, 33, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A.; Gollner, A.; Chiriac, M.I. Regioselective reactions for programmable resveratrol oligomer synthesis. Nature 2011, 474, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Inaoka, P.T. Gnetin-C and other resveratrol oligomers with cancer chemopreventive potential. Ann. N. Y. Acad. Sci. 2017, 1403, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R. Chemistry and Biology of Resveratrol-Derived Natural Products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Tokunaga, Y.; Sakan, F. Stilbenoids isolated from the seeds of Melinjo (Gnetum gnemon L.) and their biological activity. J. Agric. Food Chem. 2009, 57, 2544–2549. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Kunimasa, K.; Yamori, Y.; Mori, M.; Mori, H.; Nakamura, K.; Miller, G.; Manne, U.; Tiwari, A.K.; Narayanan, B. Antitumor activity of melinjo (Gnetum gnemon L.) seed extract in human and murine tumor models in vitro and in a colon-26 tumor-bearing mouse model in vivo. Cancer Med. 2015, 4, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; An, D.T.; Trung, L.Q.; Yamada, K.; Nakao, S.; Takami, A. Stilbene derivatives from melinjo extract have antioxidant and immune modulatory effects in healthy individuals. Integr. Mol. Med. 2015, 2. [Google Scholar] [CrossRef]
- Watanabe, K.; Shibuya, S.; Ozawa, Y.; Izuo, N.; Shimizu, T. Resveratrol Derivative-Rich Melinjo Seed Extract Attenuates Skin Atrophy in Sod1-Deficient Mice. Oxid. Med. Cell. Longev. 2015, 2015, 391075. [Google Scholar] [CrossRef]
- Kunimasa, K.; Ohta, T.; Tani, H.; Kato, E.; Eguchi, R.; Kaji, K.; Ikeda, K.; Mori, H.; Mori, M.; Tatefuji, T.; et al. Resveratrol derivative-rich melinjo (Gnetum gnemon L.) seed extract suppresses multiple angiogenesis-related endothelial cell functions and tumor angiogenesis. Mol. Nutr. Food Res. 2011, 55, 1730–1734. [Google Scholar] [CrossRef]
- Ikeda, E.; Ikeda, Y.; Wang, Y.; Fine, N.; Sheikh, Z.; Viniegra, A.; Barzilay, O.; Ganss, B.; Tenenbaum, H.C.; Glogauer, M. Resveratrol derivative-rich melinjo seed extract induces healing in a murine model of established periodontitis. J. Periodontol. 2018, 89, 586–595. [Google Scholar] [CrossRef]
- Kato, H.; Samizo, M.; Kawabata, R.; Takano, F.; Ohta, T. Stilbenoids from the melinjo (Gnetum gnemon L.) fruit modulate cytokine production in murine Peyer’s patch cells ex vivo. Planta Med. 2011, 77, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Hikami, S.; Iizuna, S.; Yoshimatsu, M.; Asama, T.; Ota, H.; Kimura, Y.; Tatefuji, T.; Hashimoto, K.; Higaki, K. Pharmacokinetics and safety of resveratrol derivatives in humans after oral administration of melinjo (Gnetum gnemon L.) seed extract powder. J. Agric. Food Chem. 2014, 62, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Konno, H.; Kanai, Y.; Katagiri, M.; Watanabe, T.; Mori, A.; Ikuta, T.; Tani, H.; Fukushima, S.; Tatefuji, T.; Shirasawa, T. Melinjo (Gnetum gnemon L.) Seed Extract Decreases Serum Uric Acid Levels in Nonobese Japanese Males: A Randomized Controlled Study. Evid.-Based Complement. Altern. Med. eCAM 2013, 2013, 589169. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Elbadry, M.I.; Taniwaki, M.; Harada, K.; Trung, L.Q.; Nakagawa, N.; Takami, A.; Ishiyama, K.; Yamauchi, T.; Takenaka, K.; et al. The simultaneous inhibition of the mTOR and MAPK pathways with Gnetin-C induces apoptosis in acute myeloid leukemia. Cancer Lett. 2017, 400, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, U.; Dholakia, K.; Sikorska, G.; Kumar, A.; Martinez, L.A.; Levenson, A.S. Abstract 253: Gnetin C as a candidate for targeted chemopreventive and therapeutic measures in prostate cancer. Cancer Res. 2018, 78, 253. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE), v4.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 (accessed on 15 April 2018).
- Espinoza, J.L.; Trung, L.Q.; Inaoka, P.T.; Yamada, K.; An, D.T.; Mizuno, S.; Nakao, S.; Takami, A. The Repeated Administration of Resveratrol Has Measurable Effects on Circulating T-Cell Subsets in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6781872. [Google Scholar] [CrossRef]
- Trung, L.Q.; An, D.T.T. Is Resveratrol a Cancer Immunomodulatory Molecule? Front. Pharmacol. 2018, 9, 1255. [Google Scholar] [CrossRef]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef]
- Falasca, M.; Casari, I.; Maffucci, T. Cancer chemoprevention with nuts. J. Natl. Cancer Instit. 2014, 106. [Google Scholar] [CrossRef]
- Maru, G.B.; Hudlikar, R.R.; Kumar, G.; Gandhi, K.; Mahimkar, M.B. Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: From experimental models to clinical trials. World J. Biol. Chem. 2016, 7, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Nguyen, V.H.; Ichimura, H.; Pham, T.T.; Nguyen, C.H.; Pham, T.V.; Elbadry, M.I.; Yoshioka, K.; Tanaka, J.; Trung, L.Q.; et al. A functional polymorphism in the NKG2D gene modulates NK-cell cytotoxicity and is associated with susceptibility to Human Papilloma Virus-related cancers. Sci. Rep. 2016, 6, 39231. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Takami, A.; Yoshioka, K.; Nakata, K.; Sato, T.; Kasahara, Y.; Nakao, S. Human microRNA-1245 down-regulates the NKG2D receptor in natural killer cells and impairs NKG2D-mediated functions. Haematologica 2012, 97, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Minami, M. Sensing Bacterial-Induced DNA Damaging Effects via Natural Killer Group 2 Member D Immune Receptor: From Dysbiosis to Autoimmunity and Carcinogenesis. Front. Immunol. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Bachleda, P.; Vrzal, R.; Dvorak, Z. Resveratrol enhances NK cell cytotoxicity: Possible role for aryl hydrocarbon receptor. J. Cell. Physiol. 2010, 225, 289–290. [Google Scholar] [CrossRef]
- Li, Q.; Huyan, T.; Ye, L.J.; Li, J.; Shi, J.L.; Huang, Q.S. Concentration-dependent biphasic effects of resveratrol on human natural killer cells in vitro. J. Agric. Food Chem. 2014, 62, 10928–10935. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Chen, J.K. Resveratrol enhances perforin expression and NK cell cytotoxicity through NKG2D-dependent pathways. J. Cell. Physiol. 2010, 223, 343–351. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Takami, A.; Trung, L.Q.; Nakao, S. Ataxia-telangiectasia mutated kinase-mediated upregulation of NKG2D ligands on leukemia cells by resveratrol results in enhanced natural killer cell susceptibility. Cancer Sci. 2013, 104, 657–662. [Google Scholar] [CrossRef]
- Quoc Trung, L.; Espinoza, J.L.; Takami, A.; Nakao, S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS ONE 2013, 8, e55183. [Google Scholar] [CrossRef]
- Shi, Y.W.; Wang, C.P.; Liu, L.; Liu, Y.L.; Wang, X.; Hong, Y.; Li, Z.; Kong, L.D. Antihyperuricemic and nephroprotective effects of resveratrol and its analogues in hyperuricemic mice. Mol. Nutr. Food Res. 2012, 56, 1433–1444. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, S.; Wang, Y.; Zhu, H.; Liu, Q.; Xue, Y.; Qiu, J.; Zou, H.; Zhu, X. The effect of resveratrol on the recurrent attacks of gouty arthritis. Clin. Rheumatol. 2016, 35, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; Vermeer, M.A.; Hiemstra, H.; Ras, R.T. LDL-Cholesterol Lowering of Plant Sterols and Stanols-Which Factors Influence Their Efficacy? Nutrients 2018, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.; Gotto, A.M.; LaRosa, J.C.; Maroni, J.; Szarek, M.; Grundy, S.M.; Kastelein, J.J.; Bittner, V.; Fruchart, J.C.; Treating to New Targets, I. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 2007, 357, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W. Changing Cholesterol Levels and Coronary Heart Disease Risk. Circulation 2016, 133, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.C.; Lawlor, D.A.; de Oliveira, C.; White, J.; Horta, B.L.; Barros, A.J. Role of Adiponectin in Coronary Heart Disease Risk: A Mendelian Randomization Study. Circ. Res. 2016, 119, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Woodward, L.; Akoumianakis, I.; Antoniades, C. Unravelling the adiponectin paradox: Novel roles of adiponectin in the regulation of cardiovascular disease. Br. J. Pharmacol. 2017, 174, 4007–4020. [Google Scholar] [CrossRef] [PubMed]
- Menzaghi, C.; Trischitta, V. The Adiponectin Paradox for All-Cause and Cardiovascular Mortality. Diabetes 2018, 67, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Akishita, M.; Tani, H.; Tatefuji, T.; Ogawa, S.; Iijima, K.; Eto, M.; Shirasawa, T.; Ouchi, Y. Trans-Resveratrol in Gnetum gnemon protects against oxidative-stress-induced endothelial senescence. J. Nat. Prod. 2013, 76, 1242–1247. [Google Scholar] [CrossRef] [PubMed]

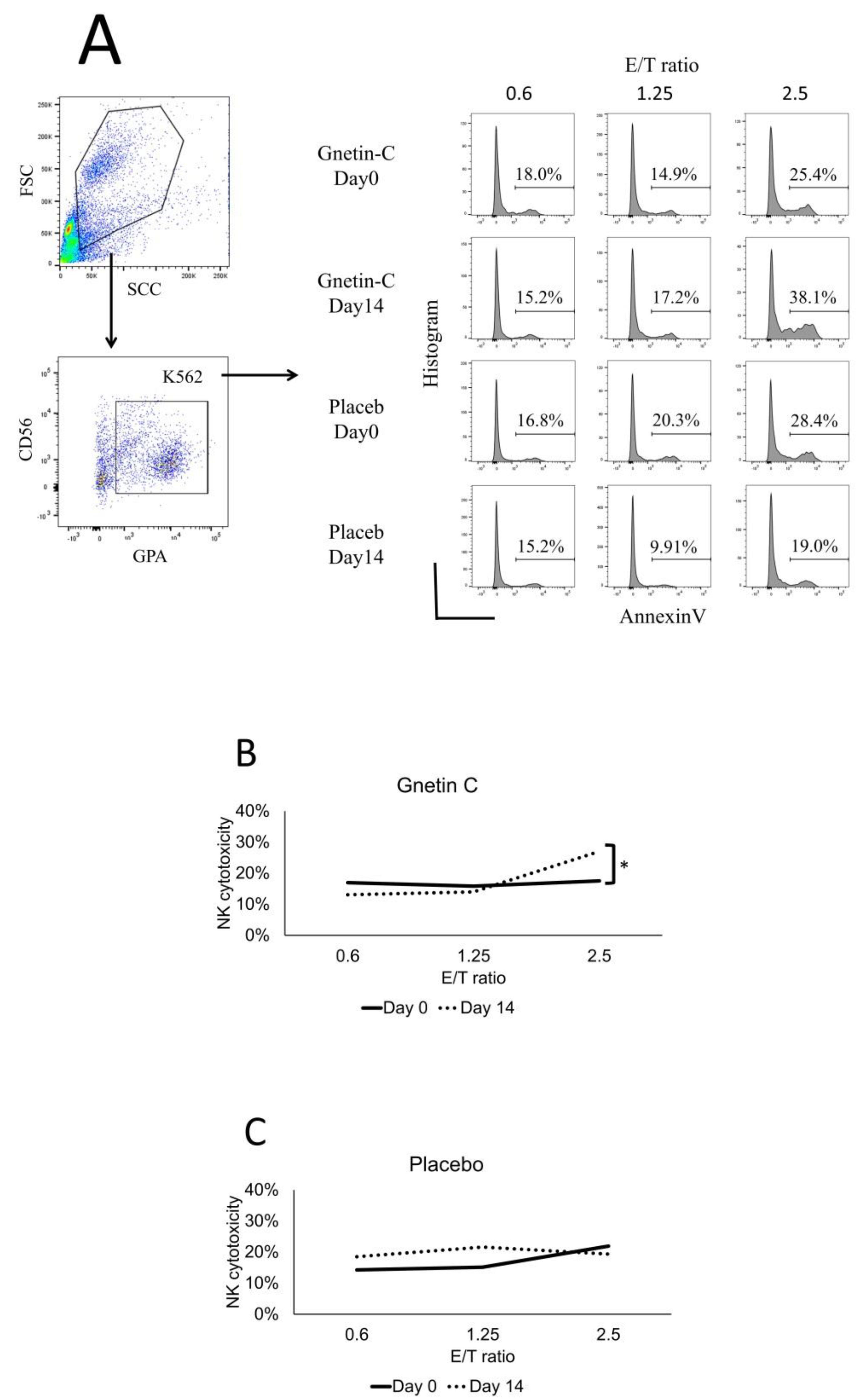

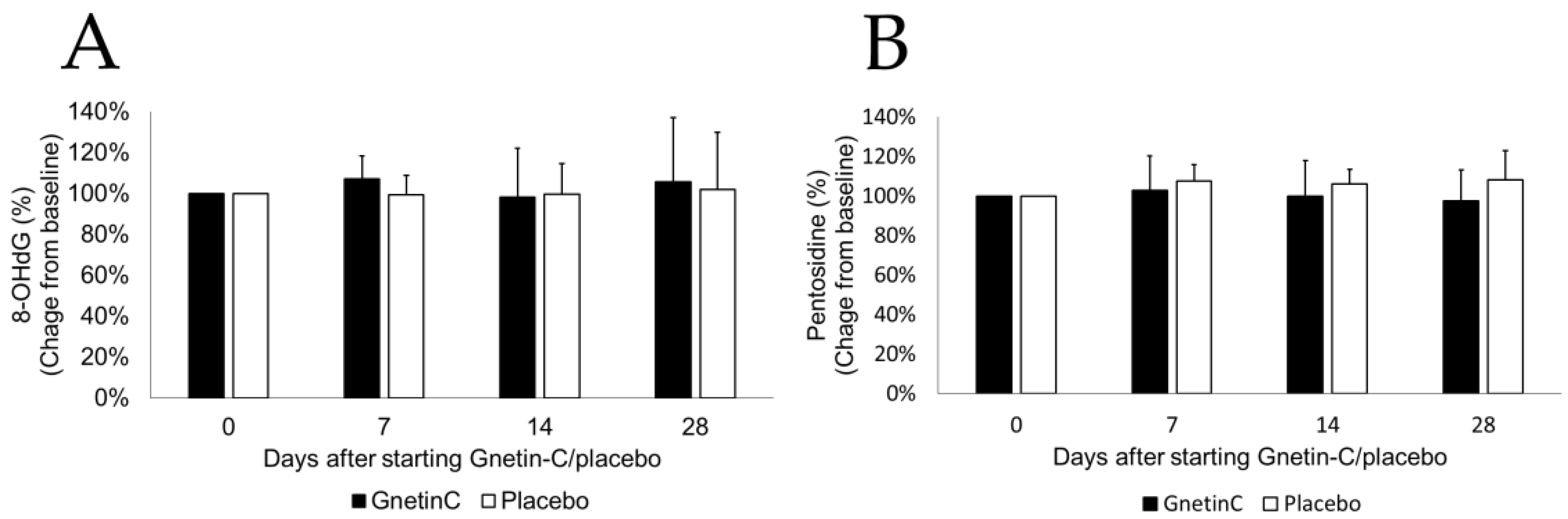
| Group | Gnetin-C | Placebo | p |
|---|---|---|---|
| n | 6 | 6 | |
| M/F | 3/3 | 3/3 | |
| Median age, years (range) | 34 (26–53) | 27.5 (24–42) | 0.26 |
| Median height, m (range) | 1.62 (1.57–1.75) | 1.65 (1.54–1.73) | 0.91 |
| Median weight, kg (range) | 55 (43–81) | 59 (42–72) | 0.90 |
| Median body mass index, kg/m2 (range) | 18.1 (14.3–26.9) | 19.6 (14.0–23.9) | 0.90 |
| Day 7 | Day 14 | Day 28 | |||||
|---|---|---|---|---|---|---|---|
| Gnetin C (n = 6) | Placebo (n = 6) | Gnetin C (n = 6) | Placebo (n = 6) | Gnetin C (n = 6) | Placebo (n = 6) | P | |
| Means ± SD | Means ± SD | Means ± SD | Means ± SD | Means ± SD | Means ± SD | ||
| Total proteins (g/dL) | 99% ± 4% | 100% ± 2% | 96% ± 4% | 99% ± 2% | 94% ± 4% | 100% ± 2% | 0.14 |
| Albumin (g/dL) | 99% ± 4% | 100% ± 2% | 97% ± 5% | 99% ± 3% | 95% ± 5% | 99% ± 2% | 0.36 |
| Total bilirubin (mg/dL) | 113% ± 27% | 91% ± 20% | 111% ± 36% | 93% ± 31% | 105% ± 30% | 104% ± 25% | 0.43 |
| Serum creatinine (mg/dL) | 102% ± 4% | 96% ± 2% | 100% ± 8% | 98% ± 4% | 102% ± 5% | 103% ± 4% | 0.63 |
| Urea nitrogen (mg/dL) | 102% ± 6% | 102% ± 18% | 96% ± 11% | 105% ± 12% | 92% ± 19% | 102% ± 8% | 0.24 |
| Aspartate transaminase (U/L) | 103% ± 18% | 96% ± 12% | 99% ± 13% | 97% ± 9% | 97% ± 12% | 95% ± 5% | 0.81 |
| Alanine aminotransferase (U/L) | 111% ± 23% | 107% ± 17% | 93% ± 22% | 101% ± 16% | 95% ± 18% | 108% ± 15% | 0.54 |
| Lactate dehydrogenase (U/L) | 96% ± 6% | 97% ± 6% | 95% ± 6% | 93% ± 4% | 95% ± 9% | 96% ± 3% | 0.76 |
| γ-glutamyltransferase (U/L) | 94% ± 13% | 94% ± 7% | 84% ± 15% | 91% ± 11% | 85% ± 20% | 91% ± 6% | 0.37 |
| Triglyceride (mg/dL) | 90% ± 13% | 92% ± 19% | 107% ± 37% | 85% ± 15% | 108% ± 30% | 106% ± 27% | 0.25 |
| C-reactive protein (mg/dL) | 87% ± 21% | 175% ± 214% | 242% ± 318% | 133% ± 57% | 132% ± 113% | 129% ± 100% | 0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagami, Y.; Suzuki, S.; Espinoza, J.L.; Vu Quang, L.; Enomoto, M.; Takasugi, S.; Nakamura, A.; Nakayama, T.; Tani, H.; Hanamura, I.; et al. Immunomodulatory and Metabolic Changes after Gnetin-C Supplementation in Humans. Nutrients 2019, 11, 1403. https://doi.org/10.3390/nu11061403
Nakagami Y, Suzuki S, Espinoza JL, Vu Quang L, Enomoto M, Takasugi S, Nakamura A, Nakayama T, Tani H, Hanamura I, et al. Immunomodulatory and Metabolic Changes after Gnetin-C Supplementation in Humans. Nutrients. 2019; 11(6):1403. https://doi.org/10.3390/nu11061403
Chicago/Turabian StyleNakagami, Yuya, Susumu Suzuki, J. Luis Espinoza, Lam Vu Quang, Megumi Enomoto, Souichi Takasugi, Ayano Nakamura, Takayuki Nakayama, Hiroya Tani, Ichiro Hanamura, and et al. 2019. "Immunomodulatory and Metabolic Changes after Gnetin-C Supplementation in Humans" Nutrients 11, no. 6: 1403. https://doi.org/10.3390/nu11061403
APA StyleNakagami, Y., Suzuki, S., Espinoza, J. L., Vu Quang, L., Enomoto, M., Takasugi, S., Nakamura, A., Nakayama, T., Tani, H., Hanamura, I., & Takami, A. (2019). Immunomodulatory and Metabolic Changes after Gnetin-C Supplementation in Humans. Nutrients, 11(6), 1403. https://doi.org/10.3390/nu11061403






