Atherogenic Index Reduction and Weight Loss in Metabolic Syndrome Patients Treated with A Novel Pectin-Enriched Formulation of Bergamot Polyphenols
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Supplement Used in The Study
2.2. Subjects
2.3. Study Design
2.4. Blood Sampling and Measurements
2.5. Statistical Analyses
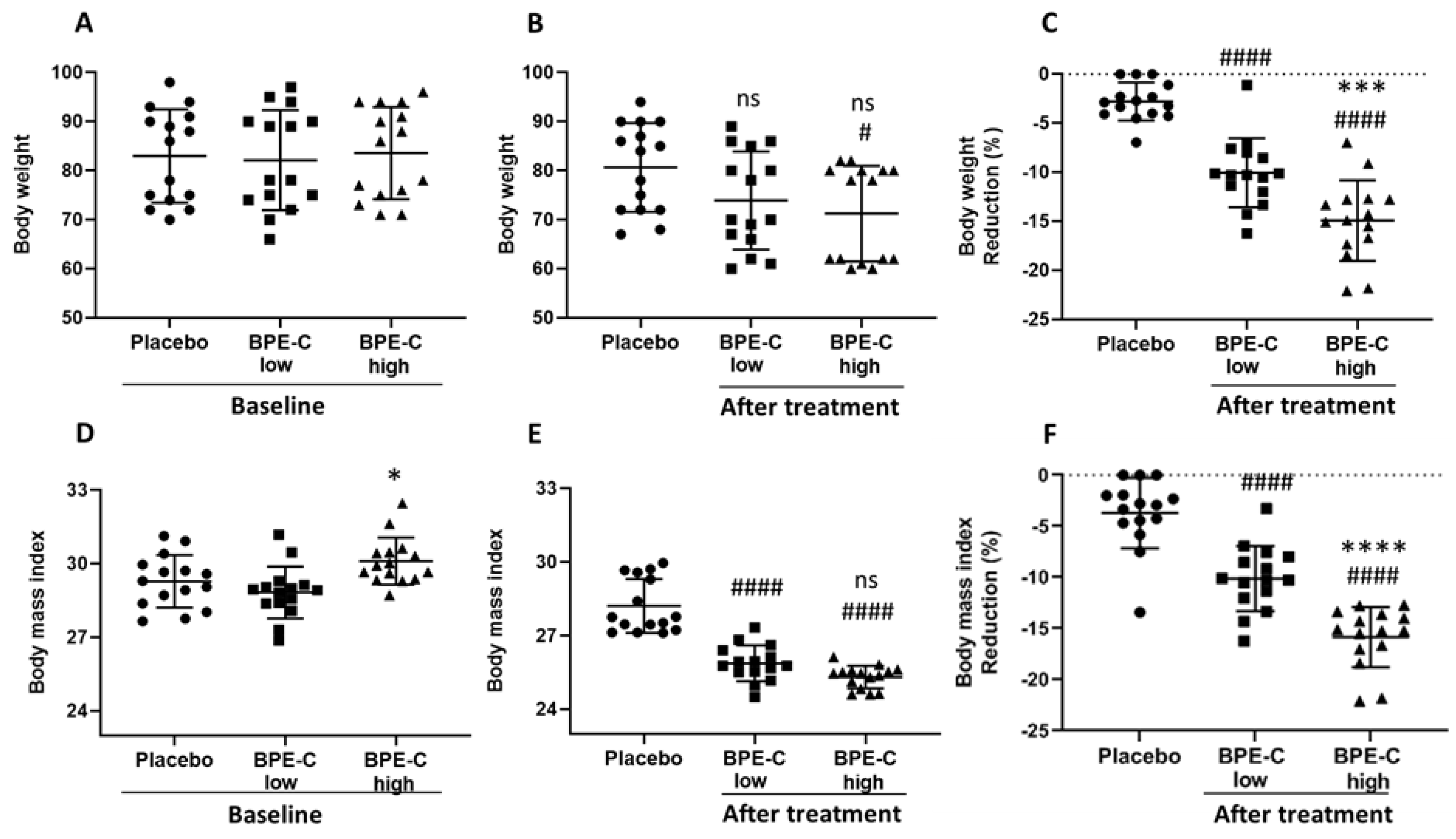
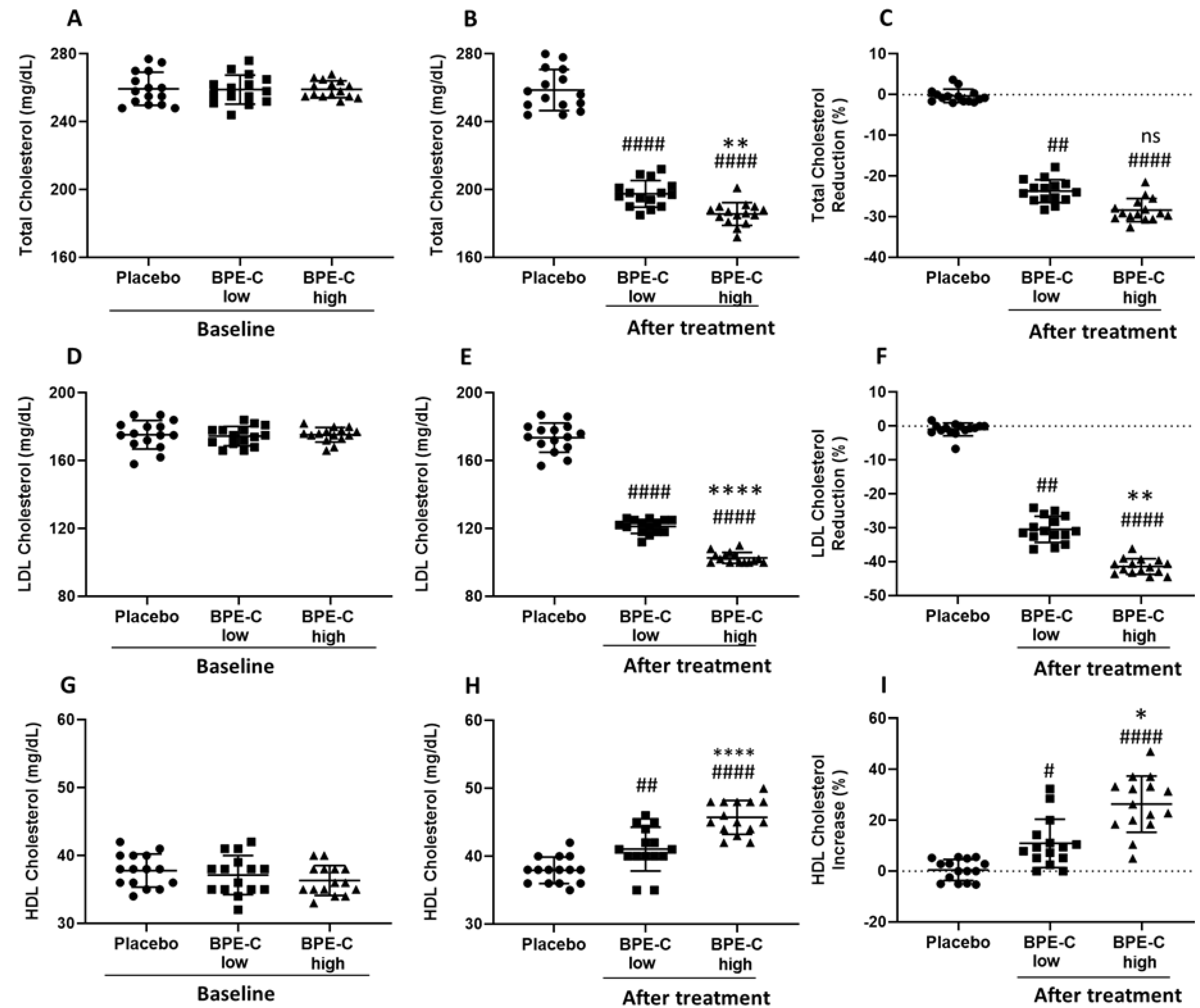
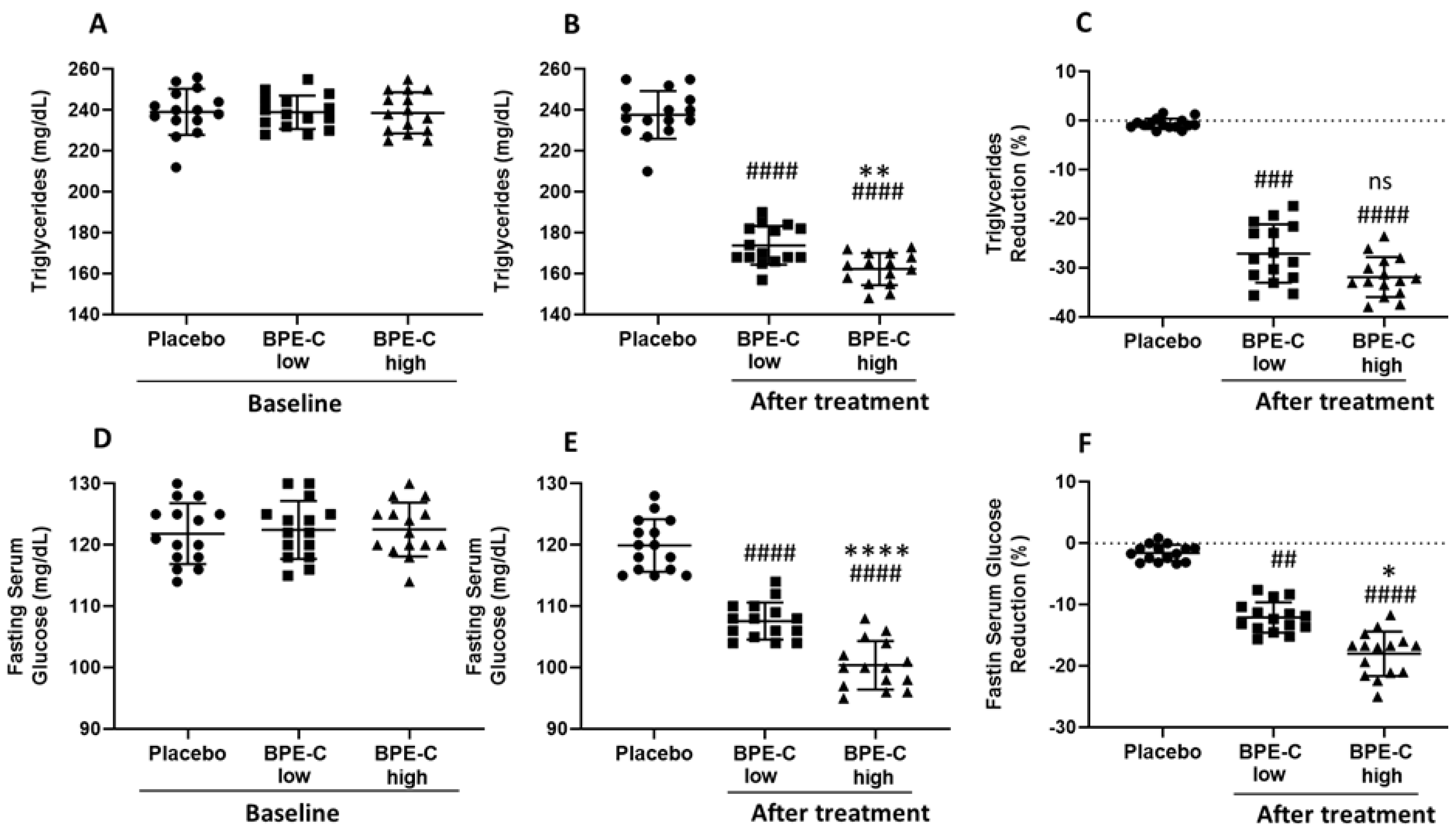
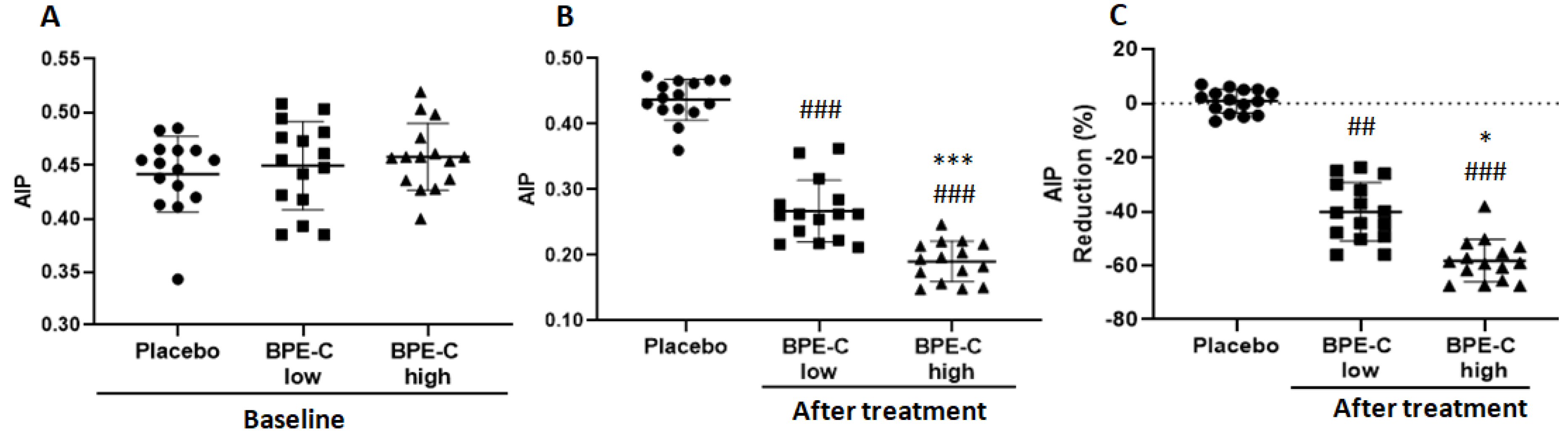
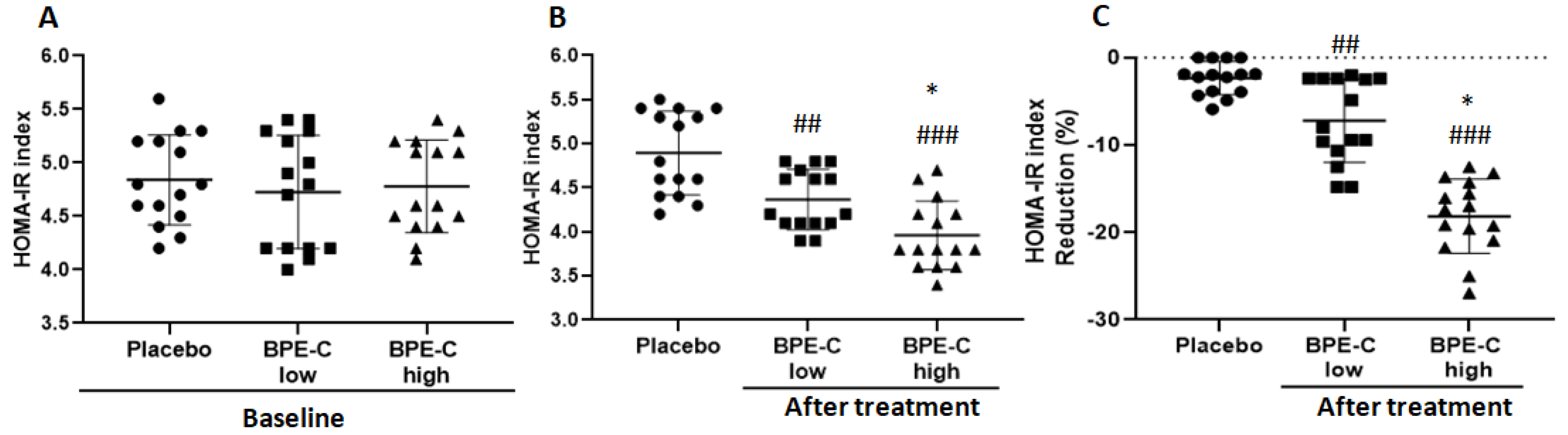
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galisteo, M.; Duarte, J.; Zarzuelo, A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 2008, 19, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Janda, E.; Mollace, V. Chapter 84—The use of bergamot-derived polyphenol fraction. In Polyphenols in Human Health and Disease; Academic Press: San Diego, CA, USA, 2014; pp. 1087–1105. [Google Scholar]
- Scuteri, A.; Laurent, S.; Cucca, F.; Cockcroft, J.; Cunha, P.G.; Manas, L.R.; Mattace Raso, F.U.; Muiesan, M.L.; Ryliskyte, L.; Rietzschel, E.; et al. Metabolic syndrome across Europe: Different clusters of risk factors. Eur. J. Prev. Cardiol. 2015, 22, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Forti, P.; Pirazzoli, G.L.; Maltoni, B.; Bianchi, G.; Magalotti, D.; Muscari, A.; Mariani, E.; Ravaglia, G.; Zoli, M. Metabolic syndrome and all-cause mortality in older men and women. Eur. J. Clin. Investig. 2012, 42, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Dobiasova, M.; Frohlich, J.; Sedova, M.; Cheung, M.C.; Brown, B.G. Cholesterol esterification and atherogenic index of plasma correlate with lipoprotein size and findings on coronary angiography. J. Lipid Res. 2011, 52, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Banach, M. Association of ideal cardiovascular health metrics with serum uric acid, inflammation and atherogenic index of plasma: A population-based survey. Atherosclerosis 2018, 284, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine 2017, 96, e8058. [Google Scholar] [CrossRef]
- Wu, T.T.; Gao, Y.; Zheng, Y.Y.; Ma, Y.T.; Xie, X. Atherogenic index of plasma (aip): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018, 17, 197. [Google Scholar] [CrossRef]
- Francisco, V.; Ruiz-Fernandez, C.; Pino, J.; Mera, A.; Angel Gonzalez-Gay, M.; Gomez, R.; Lago, F.; Mobasheri, A.; Gualillo, O. Adipokines: Linking metabolic syndrome, the immune system, and arthritic diseases. Biochem. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Ghadge, A.A.; Khaire, A.A.; Kuvalekar, A.A. Adiponectin: A potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018, 39, 151–158. [Google Scholar] [CrossRef]
- Berger, S.; Polotsky, V.Y. Leptin and leptin resistance in the pathogenesis of obstructive sleep apnea: A possible link to oxidative stress and cardiovascular complications. Oxidative Med. Cell. Longev. 2018, 2018, 5137947. [Google Scholar] [CrossRef]
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; Ter Horst, K.W.; Serlie, M.J. The pathogenesis of obesity. Metab. Clin. Exp. 2019, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Collden, G.; Tschop, M.H.; Muller, T.D. Therapeutic potential of targeting the ghrelin pathway. Int. J. Mol. Sci. 2017, 18, 798. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Dual effect of curcumin in preventing atherosclerosis: The potential role of pro-oxidant-antioxidant mechanisms. Nat. Prod. Res. 2015, 29, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Vetrani, C.; Vitale, M.; Bozzetto, L.; Della Pepa, G.; Cocozza, S.; Costabile, G.; Mangione, A.; Cipriano, P.; Annuzzi, G.; Rivellese, A.A. Association between different dietary polyphenol subclasses and the improvement in cardiometabolic risk factors: Evidence from a randomized controlled clinical trial. Acta Diabetol. 2018, 55, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Badimon, L. Effects of polyphenol intake on metabolic syndrome: Current evidences from human trials. Oxidative Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [PubMed]
- Parafati, M.; Lascala, A.; La Russa, D.; Mignogna, C.; Trimboli, F.; Morittu, V.M.; Riillo, C.; Macirella, R.; Mollace, V.; Brunelli, E.; et al. Bergamot polyphenols boost therapeutic effects of the diet on non-alcoholic steatohepatitis (nash) induced by “junk food”: Evidence for anti-inflammatory activity. Nutrients 2018, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Lascala, A.; Martino, C.; Parafati, M.; Salerno, R.; Oliverio, M.; Pellegrino, D.; Mollace, V.; Janda, E. Analysis of proautophagic activities of citrus flavonoids in liver cells reveals the superiority of a natural polyphenol mixture over pure flavones. J. Nutr. Biochem. 2018, 58, 119–130. [Google Scholar] [CrossRef]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S.; et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on ldl-cholesterol, lox-1 expression and protein kinase b phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and hypolipemic effects of a new lecithin formulation of bergamot polyphenolic fraction: A double blind, randomized, placebo- controlled study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef]
- Parafati, M.; Lascala, A.; Morittu, V.M.; Trimboli, F.; Rizzuto, A.; Brunelli, E.; Coscarelli, F.; Costa, N.; Britti, D.; Ehrlich, J.; et al. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J. Nutr. Biochem. 2015, 26, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.; Gliozzi, M.; Janda, E.; Walker, R.; Romeo, F.; Mollace, V. Effect of citrus bergamot polyphenol extract on patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2014, 109, S152–S153. [Google Scholar]
- Gliozzi, M.; Maiuolo, J.; Oppedisano, F.; Mollace, V. The effect of bergamot polyphenolic fraction in patients with non-alcoholic liver steato-hepatitis and metabolic syndrome. PharmaNutrition 2016, 4, S27–S31. [Google Scholar] [CrossRef]
- Janda, E.; Salerno, R.; Martino, C.; Lascala, A.; La Russa, D.; Oliverio, M. Qualitative and quantitative analysis of the proautophagic activity of citrus flavonoids from bergamot polyphenol fraction. Data Brief 2018, 19, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, L.; Costabile, G.; Della Pepa, G.; Ciciola, P.; Vetrani, C.; Vitale, M.; Rivellese, A.A.; Annuzzi, G. Dietary fibre as a unifying remedy for the whole spectrum of obesity-associated cardiovascular risk. Nutrients 2018, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Drew, J.E.; Reichardt, N.; Williams, L.M.; Mayer, C.D.; Walker, A.W.; Farquharson, A.J.; Kastora, S.; Farquharson, F.; Milligan, G.; Morrison, D.J.; et al. Dietary fibers inhibit obesity in mice, but host responses in the cecum and liver appear unrelated to fiber-specific changes in cecal bacterial taxonomic composition. Sci. Rep. 2018, 8, 15566. [Google Scholar] [CrossRef] [PubMed]
- Lara-Espinoza, C.; Carvajal-Millan, E.; Balandran-Quintana, R.; Lopez-Franco, Y.; Rascon-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Cai, G.; Liu, W.; Lv, S.; Wang, X.; Guo, Y.; Yan, Z.; Du, Y.; Zhou, Y. Gender-specific associations between atherogenic index of plasma and the presence and severity of acute coronary syndrome in very young adults: A hospital-based observational study. Lipids Health Dis. 2019, 18, 99. [Google Scholar] [CrossRef]
- Ni, W.; Zhou, Z.; Liu, T.; Wang, H.; Deng, J.; Liu, X.; Xing, G. Gender-and lesion number-dependent difference in “atherogenic index of plasma” in Chinese people with coronary heart disease. Sci. Rep. 2017, 7, 13207. [Google Scholar] [CrossRef]
- Gliozzi, M.; Carresi, C.; Musolino, V.; Palma, E.; Muscoli, C.; Vitale, C.; Gratteri, S.; Muscianisi, G.; Janda, E.; Muscoli, S.; et al. The effect of bergamot-derived polyphenolic fraction on ldl small dense particles and non alcoholic fatty liver disease in patients with metabolic syndrome. Adv. Biol. Chem. 2014, 4, 9. [Google Scholar] [CrossRef]
- Bruno, A.; Pandolfo, G.; Crucitti, M.; Maisano, A.; Zoccali, R.A.; Muscatello, M.R.A. Metabolic outcomes of bergamot polyphenolic fraction administration in patients treated with second-generation antipsychotics: A pilot study. J. Nutr. Biochem. 2017, 40, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Pandolfo, G.; Crucitti, M.; Cacciola, M.; Santoro, V.; Spina, E.; Zoccali, R.A.; Muscatello, M.R.A. Low-dose of bergamot-derived polyphenolic fraction (bpf) did not improve metabolic parameters in second generation antipsychotics-treated patients: Results from a 60-days open-label study. Front. Pharmacol. 2017, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- La Russa, D.; Giordano, F.; Marrone, A.; Parafati, M.; Janda, E.; Pellegrino, D. Oxidative imbalance and kidney damage in cafeteria diet-induced rat model of metabolic syndrome: Effect of bergamot polyphenolic fraction. Antioxidants 2019, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.L.; Gratz, S.W.; Peinado, D.I.; Thomson, L.M.; Garden, K.E.; Williams, P.A.; Richardson, A.J.; Ross, A.W. Effects of dietary fibre (pectin) and/or increased protein (casein or pea) on satiety, body weight, adiposity and caecal fermentation in high fat diet-induced obese rats. PLoS ONE 2016, 11, e0155871. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.L.; Thomson, L.M.; Williams, P.A.; Ross, A.W. Soluble fermentable dietary fibre (pectin) decreases caloric intake, adiposity and lipidaemia in high-fat diet-induced obese rats. PLoS ONE 2015, 10, e0140392. [Google Scholar] [CrossRef] [PubMed]
- Wanders, A.J.; van den Borne, J.J.; de Graaf, C.; Hulshof, T.; Jonathan, M.C.; Kristensen, M.; Mars, M.; Schols, H.A.; Feskens, E.J. Effects of dietary fibre on subjective appetite, energy intake and body weight: A systematic review of randomized controlled trials. Obes. Rev. 2011, 12, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, K.; Yang, H. Pectin alleviates high fat (lard) diet-induced nonalcoholic fatty liver disease in mice: Possible role of short-chain fatty acids and gut microbiota regulated by pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, S.; Luo, J.; Wu, X.; Liu, L. Effects of dietary fibers and their mixtures on short chain fatty acids and microbiota in mice guts. Food Funct. 2013, 4, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, J.; Hao, W.; Zhu, H.; Liang, N.; He, Z.; Ma, K.Y.; Chen, Z.Y. Structure-specific effects of short-chain fatty acids on plasma cholesterol concentration in male Syrian hamsters. J. Agric. Food Chem. 2017, 65, 10984–10992. [Google Scholar] [CrossRef] [PubMed]
- Gomez, B.; Gullon, B.; Remoroza, C.; Schols, H.A.; Parajo, J.C.; Alonso, J.L. Purification, characterization, and prebiotic properties of pectic oligosaccharides from orange peel wastes. J. Agric. Food Chem. 2014, 62, 9769–9782. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Curcuminoids plus piperine modulate adipokines in type 2 diabetes mellitus. Curr. Clin. Pharmacol. 2017, 12, 253–258. [Google Scholar] [CrossRef] [PubMed]
| Variables | Placebo (n = 15) | BPE-C Low (n = 15) | BPE-C High (n = 15) | p Value (Placebo vs. BPE-C low) | p Value (Placebo vs. BPE-C high) | p Value (BPE-C low vs. high) |
|---|---|---|---|---|---|---|
| Age (years) | 55.6 ± 7.4 | 59 ± 7.6 | 56.1 ± 10.9 | 0.889 | ≥0.999 | ≥0.999 |
| Weight (kg) | 83.4 ± 9.5 | 82.5 ± 10 | 84.6 ± 9.7 | 0.443 | 0.661 | ≥0.999 |
| Sex (M/F) | 8/7 | 7/8 | 9/6 | 0.715 | 0.713 | 0.464 |
| Smokers (Y/N) | 0/15 | 0/15 | 0/15 | - | - | - |
| Experimental Groups | Biomarker | |||
|---|---|---|---|---|
| Insulin (IU/L) | Leptin (ng/mL) | Ghrelin (pg/mL) | Adiponectin (mg/mL) | |
| Baseline Placebo | 20 ± 1.6 | 22.1 ± 1.1 | 619 ± 24 | 19.5 ± 1.3 |
| Baseline BPE-C low | 20 ± 1.5 | 22.6 ± 1.4 | 623 ± 19 | 19.3 ± 1.5 |
| Baseline BPE-C high | 19.6 ± 2.4 | 22 ± 2.3 | 624 ± 38 | 19.3 ± 1.4 |
| Placebo | 19.5 ± 2.6 | 22.3 ± 1.3 | 620 ± 22 | 18.6 ± 2.4 |
| BPE-C low | 16.6 ± 1.4 | 19.3 ± 2.0 | 581 ± 22 | 22.9 ± 2.5 |
| BPE-C high | 14.2 ± 1.2 | 17.3 ± 0.4 | 530 ± 21 | 23.5 ± 1.4 |
| p values | ||||
| Placebo vs. BPE-C low | 0.067 KW | 0.013KW | <0.001 | 0.003 |
| Placebo vs. BPE-C high | <0.001KW | <0.001KW | <0.001 | <0.001 |
| BPE-C high vs. BPE-C low | 0.007KW | 0.046KW | <0.001 | 0.078 |
| Change after treatment (%) | ||||
| Placebo | −2,5 ± 6.7 | −0.5 ± 4 | 0.3 ± 2 | −4.6 ± 11.6 |
| BPE-C low | −16.5 ± 6.5 | −13.9 ± 11 | −6.6 ± 2.4 | 18.1 ± 12.8 |
| BPE-C high | −26.4 ± 10.7 | −20.5 ± 8.9 | −14.9 ± 2.9 | 22.3 ± 9.6 |
| p values | ||||
| Placebo vs. BPE-C low | 0.001 | 0.002KW | <0.001 | <0.001 |
| Placebo vs. BPE-C high | <0.001 | <0.001KW | <0.001 | <0.001 |
| BPE-C high vs. BPE-C low | 0.006 | 0.490 KW | 0.007 | 0.942 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capomolla, A.S.; Janda, E.; Paone, S.; Parafati, M.; Sawicki, T.; Mollace, R.; Ragusa, S.; Mollace, V. Atherogenic Index Reduction and Weight Loss in Metabolic Syndrome Patients Treated with A Novel Pectin-Enriched Formulation of Bergamot Polyphenols. Nutrients 2019, 11, 1271. https://doi.org/10.3390/nu11061271
Capomolla AS, Janda E, Paone S, Parafati M, Sawicki T, Mollace R, Ragusa S, Mollace V. Atherogenic Index Reduction and Weight Loss in Metabolic Syndrome Patients Treated with A Novel Pectin-Enriched Formulation of Bergamot Polyphenols. Nutrients. 2019; 11(6):1271. https://doi.org/10.3390/nu11061271
Chicago/Turabian StyleCapomolla, Antonio Soccorso, Elzbieta Janda, Sara Paone, Maddalena Parafati, Tomasz Sawicki, Rocco Mollace, Salvatore Ragusa, and Vincenzo Mollace. 2019. "Atherogenic Index Reduction and Weight Loss in Metabolic Syndrome Patients Treated with A Novel Pectin-Enriched Formulation of Bergamot Polyphenols" Nutrients 11, no. 6: 1271. https://doi.org/10.3390/nu11061271
APA StyleCapomolla, A. S., Janda, E., Paone, S., Parafati, M., Sawicki, T., Mollace, R., Ragusa, S., & Mollace, V. (2019). Atherogenic Index Reduction and Weight Loss in Metabolic Syndrome Patients Treated with A Novel Pectin-Enriched Formulation of Bergamot Polyphenols. Nutrients, 11(6), 1271. https://doi.org/10.3390/nu11061271






