Abstract
Urolithiasis is an increasingly common clinical problem worldwide. The formation of stones is a combination of metabolic status, environmental factors, family history and many other aspects. It is important to find new ways to quickly detect and assess urolithiasis because it causes sudden, severe pain and often comes back. One way to do this is by exploring new biomarkers. Current advances in proteomic studies provide a great opportunity for breakthroughs in this field. This study focuses on protein biomarkers and their connection to kidney damage and inflammation during urolithiasis.
1. Introduction
Urolithiasis (i.e., kidney stone disease) remains a global public health problem with increasing prevalence [1,2]. This disease is spreading in developed countries and affecting both adults and children, becoming a problem for society.
Women have a higher risk of early onset lithiasis, with a tendency to have recurrences. Late stone formation occurs more frequently in those with a BMI > 30 [3].
Urolithiasis is a disease in which deposits are formed in different parts of the urinary tract: the pyelocalyceal system, the ureter or even the bladder. The pathogenesis of the disease is complex and multifactorial. An imbalance between promoter (like calcium, oxalic acid, uric acid, cystine, cell fragments) and inhibitor (like magnesium, citrates, zinc, glycosaminoglycans, uromodulin, osteopontin, osteocalcin, bicunin) concentrations, along with specific physicochemical conditions, increases the risk of lithiasis. A very important aspect is the pH of the urine, which, depending on the type of deposits, can determine their precipitation. In the case of uric acid stones, an acidic environment stimulates their precipitation. The formation of stones is also influenced by antibiotic therapy and the reduction in intestinal colonization with the bacterium Oxalobacter formigenes. Indeed, this organism uses oxalic acid as an energy source, reducing its absorption in the gastrointestinal tract [4]. Also, a sedentary lifestyle, a diet high in animal-based protein with a high intake of salt and a low intake of water and the presence of obesity can increase the risk of stone crystallization. The chance of urolithiasis is 2–16 times higher in patients with a family history of stone disease [5]. We categorize urolithiasis into different types based on deposit composition and metabolic abnormalities. The most common type, accounting for 70–80% of cases, is calcium oxalate (CaOx). Uric acid urolithiasis is caused by excessive excretion of uric acid as a metabolite of purine metabolism. Cystine is associated with cystinuria. Magnesium ammonium phosphate (struvite) accompanies urinary tract infection. Other less common types include calcium phosphate, xanthine and 2,8-dihydroxyadenine [6]. On suspicion of urolithiasis, a comprehensive metabolic diagnosis is required to identify risk factors. The most common include hypercalciuria, hypocitraturia, hyperuricosuria and hyperoxaluria [5,7]. Most cases of deposits in the urinary tract are diagnosed during a renal colic episode or incidentally during an abdominal ultrasound performed because of non-specific symptoms, such as abdominal pain. Ultrasonography is the most important imaging study for diagnosing urolithiasis. It is widely available and sensitive and does not involve radiation doses. In the case of diagnostic problems, such as difficult localization or size of the deposit, spiral computed tomography without contrast should be performed [8,9]. Metabolic assessment is important after stone expulsion and should be repeated multiple times. It includes blood tests, urine samples and a 24 h urine collection to test for crystallization promoters and inhibitors. Analysis of the composition of the expelled stone is also an extremely important aspect. We currently have three methods: infrared spectroscopy, polarization microscopy and X-ray diffraction [8,10]. Based on the collected results, mainly from the metabolic assessment, targeted prophylaxis and conservative treatment can be implemented. Up to 80% of the deposits are expelled spontaneously, mainly those with a small diameter of up to 5 mm. The treatment of urolithiasis can be divided into conservative and invasive. Medical expulsion therapy (MET) relies on analgesics (NSAIDs) and agents that facilitate expulsion: alpha-blockers (doxazosin, tamsulosin, alfuzosin), calcium channel blockers (nifedipine) and corticosteroids. When conservative therapy is unsuccessful, procedures that are as minimally invasive as possible should be implemented. These include extracorporeal shock wave lithotripsy (ESWL), lithotripsy during ureteroscopy (URSL), percutaneous nephrolithotripsy (PCNL) and retrograde intrarenal surgery (RIRS). Because of the efficacy and prevalence of the above methods, classical surgical treatment is a rare choice [8,9].
Even though there are traditional diagnostic methods available, researchers are searching for new biomarkers to understand the risk of kidney stone formation and possible complications like AKI, CKD or urosepsis.
The principal goal of this review is to gather evidence from recent literature about protein biomarkers in urolithiasis patients that might be useful for establishing the diagnosis, monitoring the disease activity and prognosing the outcome of the disease.
2. Materials and Methods
Search Protocol
This review follows the PRISMA 2020 guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [11]. We searched PubMed and Web of Science databases. All records between January 2016 and September 2023 were checked by two independent individuals with the strategy using MeSH (Medical Subject Heading) terms and keywords for the description of population and intervention with the help of the Boolean operators ”or” and “and”. We used the combination of “urolithiasis” OR “kidney stones” AND “protein markers” AND “blood” OR “urine”. The inclusion criteria were as follows: (1) human research, (2) clinical diagnosis of urolithiasis, (3) protein marker obtained in blood or urine, with the use of ELISA method, (4) cohort and case-control investigations and (5) articles published in English in peer-reviewed journal. The exclusion criteria were as follows: (1) animal research, (2) no specific diagnosis of urolithiasis, (3) other markers like DNA/RNA molecules, bacteria and metabolome, (4) presence of factors like severe diseases or treatment that might influence the obtained results, (5) no control group, or it was not precisely chosen, (6) studies not in English and (7) full text not available.
A total of 213 records in the period of the last 7 years (2016–2023) were found, but only 25 were taken into further review. The excluded studies were duplications or did not meet inclusion criteria; for instance, there was no obvious diagnosis of urolithiasis, research was performed on animals, they were “in vitro” studies, and markers were not proteins or obtained in material other than blood or urine. There were also 10 case studies and 9 review studies excluded, 3 were not available as a full-size text, and 2 were not in English (Figure 1).
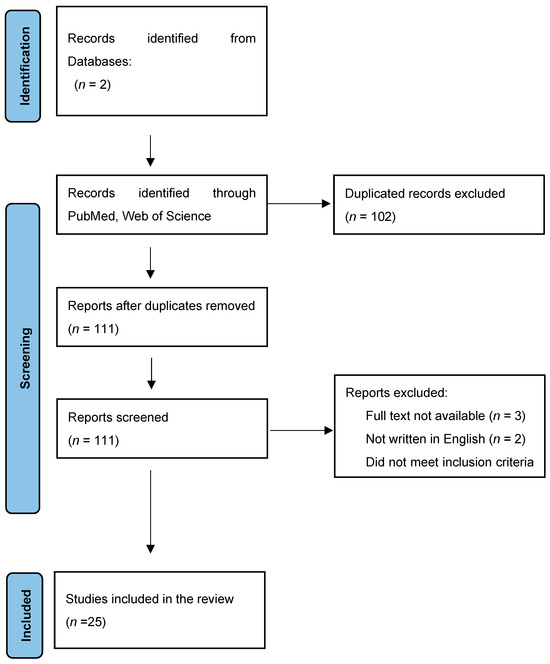
Figure 1.
Studies of the last 7 years (2016–2023) included in this review—PRISMA 2020 flow diagram. * Web of Science, PubMed/Medline.
3. Results
The review of protein biomarkers in urolithiasis patients detected a wide range of molecules in their urine and blood. Usually, they were assessed as a panel of a few proteins to get a better insight into their role in the process of stone formation as well as further progression or complications of this disorder. Detailed results of the studies from the years 2016–2023 on protein markers in urolithiasis are organized in Table 1.

Table 1.
Results of the studies from the last seven years on new protein kidney biomarkers in urolithiasis.
Cystatin C is one of the oldest and best-known proteins that may serve as a kidney function biomarker. It was not a matter of main interest in urolithiasis; however, we found two recent research studies that, among other markers, assessed cystatin C. Kovacevic L. et al. [27] performed interesting proteomic analysis of cystatin C, Neutrophil Gelatinase-associated Lipocalin (NGAL) and lysozyme C in the urine of 24 urolithiasis children and 13 healthy controls. Both cystatin C and NGAL showed significantly higher levels in affected children and nearly significantly in the case of lysozyme C. Hughes S.F. et al. [23] measured cystatin C, NGAL procalcitonin (PCT) and myeloperoxidase (MPO) in the blood of adult patients with urolithiasis to predict the risk of postoperative complications following flexible ureterorenoscopy. The blood samples were taken in time intervals before and after the procedure. Only NGAL showed a significant rise following the operation while no changes in the concentration of cystatin C, PCT or MPO were observed. There are many more recent research studies on NGAL as one of the most promising tubular protein markers in urolithiasis. Hughes S.F. et al. measured [25] NGAL and inflammatory cytokines (interleukins IL-18, IL-10, IL-8, IL-6 and TNF-alfa) in the blood of 12 lithiasis patients after shock wave lithotripsy. The samples were taken before operation and then in time intervals after the procedure. NGAL concentration was significantly increased after the procedure, similar to IL-6 and TNF-alfa. An IL-18 increase was observed but without statistical significance, whereas IL-10 and IL-8 concentrations did not change. Kandur Y. et al. [12] also measured levels of NGAL Kidney Injury Molecule—1 (KIM-1) and IL-18 in the urine of 40 children with nephrolithiasis in comparison to 23 participants with hypercalciuria and 20 healthy peers. Similarly, in this study, uNGAL/cr showed a significant rise in stone formers when compared to controls, while no differences were observed in uKIM-1/cr as well as uIL18/cr. In the study of Tasdemir M. et al. [15], urinary NGAL/cr was evaluated together with urinary KIM-1/cr and N-acetyl-beta-D-glucosaminidase (NAG)/cr in a children cohort with urolithiasis. The measurements were taken three times, in 6-month intervals. Only uNGAL/cr and uNAG/cr showed a significant rise in patients with hydronephrosis, whereas all these markers did not differ between lithiasis participants and healthy controls. In the study of Wang X. et al. [22], we also found the assessment of urinary NGAL as well as Monocyte Chemotactic Protein—1 (MCP-1), 8-isoprostane (8-IP), Liver-fatty acid binding protein (L-FABP), Heart-type fatty acid binding protein (H-FABP), clusterin and osteopontin (OPN) in 30 patients with primary hyperoxaluria and calcium oxalate (CaOx) crystals compared to healthy controls. Higher uNGAL and OPN were positively correlated with eGFR, whereas higher MCP-1 was positively correlated with CaOx supersaturation. In the study of Milisic E. et al. [30], urinary NGAL was a proposed marker of acute kidney injury in lithiasis patients who had extracorporeal shock lithotripsy (ESWL). They measured uNGAL in 62 stone formers before and 6 h, 12 h, 7 days and 3 months post-procedure. The levels were significantly rising with hours post ESWL, achieving the highest values after 12 h. UNGAL was negatively correlated with eGFR. One of the newest reviewed studies from 2023 conducted by Memmos D. et al. [35] on urinary NGAL and other biomarkers of renal injury in lithiasis participants compared the results of standard percutaneous nephrolithotomy (sPCNL) with miniaturized (mPCNL) and retrograde intrarenal surgery (RIRS). It was a randomized clinical trial, including 75 patients. No significant differences were shown in uNGAL/cr and in other markers (uKIM-1/cr or uIL-18/cr) between these subgroups. Within particular subgroups, the highest increases from baseline to 2 h post-procedure were in uNGAL/cr, uKIM-1/cr and uIL-18/cr. No changes in any of these indicators were noticed in patients who had complications, including AKI. Carbohydrate antigen 19-9 (Ca 19-9) was also assessed in patients as a predictor of renal injury due to urolithiasis. Amini E. et al. [13] in 2016 designed a study evaluating CA 19-9 in the serum and urine of 38 stone formers with hydronephrosis who underwent transurethral lithotripsy (TUL) and 24 healthy peers. The median concentration of urinary CA19-9 and serum CA19-9 was 34.0 and 15.0 kU/L in the urolithiasis group and 16.1 and 5.3 kU/L in the healthy peers, respectively (p < 0.001). Following successful TUL and hydronephrosis resolution, a significant decline was detected in serum and urinary CA19-9. The duration of ureteral obstruction was associated with higher serum and urinary CA19-9 concentrations, suggesting its predictive value for renal damage associated with urinary tract obstruction. The last reviewed study on kidney function indicators in urolithiasis was performed in China by Xiaohong F. et al. [32] on a very large population of stone formers. They included 10,281 participants divided into subgroups according to the results of a kidney ultrasound: 1—507 patients who had unilateral renal stones; 2—75 patients with bilateral renal stones; and 3—peers without urolithiasis. The participants had detailed metabolic assessments including biochemistry, eGFR (CKD was defined as eGFR < 60 mL/min/1.73 m2), albuminuria, glucose, blood pressure as well as urinary NAG/cr and urinary alfa1-MG/cr. It was shown that bilateral stone formers had the biggest proportion of CKD as well as other metabolic disturbances including hypertension and hyperglycemia. They also had higher uNAG/cr and higher alfa1-MG/cr levels in comparison to healthy peers.
Several recent studies assessed inflammatory markers in patients with urolithiasis. Venkatesan S. et al. [14] conducted cross-sectional research on 41 stone formers compared to 41 matched healthy controls assessing serum IL-6 and high-sensitivity C-reactive protein (hsCRP) together with intact parathyroid hormone (iPTH), vitamin D and 24 h urinary calcium and phosphorus excretion. Significant differences considering all evaluated parameters were observed between cases and controls. In the research of Kusumi K. et al. [21], who assessed a profile of 30 urinary cytokines in lithiasis adolescents and healthy peers, IL-13 and Macrophage Inflammatory Protein 1beta (MIP1β) were significantly increased in stone formers, whereas IL-17A was higher in controls. Cilesiz N.C. et al. [26] tried to predict spontaneous ureteral stone passage based on the concentration of procalcitonin. The patients that were able to eliminate the stone spontaneously had significantly lower levels of PCT than those who still had the stone after a 4-week follow-up. With the use of ROC curve analysis, they identified an optimal cut-off PCT value of 160 pg/mL (86.7% sensitivity, 70.8% specificity, p < 0.001; AUC 0.788 95% CI 0.658–0.917). Another research study by Taiguo Q. et al. [28] assessed the diagnostic and predictive role of IL-6 and PCT for postoperative urosepsis in 90 lithiasis patients after percutaneous nephrolithotomy. Participants with sepsis had higher IL-6 after 2 h post-surgery and higher IL-6, PCT on postoperative day one and PCT on postoperative day three when compared to patients without septic complications. IL6 was concluded to be a possible predictive marker of early diagnosis for postoperative sepsis. Ramasamy V. et al. [29] evaluated the role of the main inflammatory indicator, CRP, in predicting the outcome of MET for distal ureteric lithiasis. CRP values were higher in stone non-passers when compared to those who eliminated the calculus. Multivariate analysis showed an association between higher CRP > 1.35 mg/dL and the size of the stone >7 mm with MET failure. Wymer K. M. et al. [31] retrospectively reviewed 98 patients with urolithiasis who underwent upper urinary tract decompression to predict the risk of UTI based, within other parameters, on the serum CRP and PCT concentration. It was shown that CRP serum level >21.95 mg/dL and PCT > 0.36 µg/L had a significant predictive role. The newest study of Savin Z. et al. [36] assessed the role of white blood cells (WBC) in morphology, CRP and creatinine serum levels in renal colic patients as predictors of urinary tract obstruction because of the stone. Creatinine 0.95 mg/dL and WBC 10,000 were the most accurate thresholds for predicting the obstruction of the urinary tract.
In the group of protein markers evaluated in urolithiasis are also those that take part in the stone formation. A few of the best known are osteopontin (OPN), bikunin, uromodulin and nephrocalcin. Icer M. et al. [16] conducted a study on 88 participants (44 stone formers and 44 healthy peers) to assess urinary OPN in relation to anthropometric measurements and nutrition intake. OPN levels were lower in affected patients than in controls. Other evaluated parameters also correlated with OPN; however, it was gender-dependent. Urinary OPN excretion was decreased in patients with lithiasis in the study of Jung K. et al. [17] in contrast to increased bikunin in stone formers. These authors did not show a statistically significant increase in urinary calgranulin in affected participants. OPN and other proteins involved in the process of stone formation were subjects of interest in the research of Kovacevic L. et al. [34]. It was a proteomic analysis of several inhibitory protein profiles in children with urolithiasis. The authors confirmed that 17 proteins were significantly decreased in affected patients when compared to controls. Also, five of them (two actins, annexin A5, keratin 6B and serpin B4) were absent in the urine of stone formers. OPN urinary excretion was significantly lower in urolithiasis with hypercalciuria when compared to controls and positively correlated with urinary citrate excretion. The study of Noonin C. et al. [33], who assessed Tamm–Horsfall protein (THP) and its role in calcium oxalate stone formation, revealed that THP concentration-dependently reduced CaOx monohydrate crystal size and inhibited these crystals’ growth, aggregation and further cell adhesion. THP seemed to bind only calcium ions, not oxalate.
4. Discussion
4.1. Biomarkers of Tubular Injury
There is a strong link between urolithiasis and renal tubular injury. On the one hand, there is evidence that oxalate and CaOx crystals injure tubular epithelium.
Research using animals found that having CaOx crystals in the kidney can cause tubular cell damage and result in enzymes and debris in urine [37,38,39].
On the other hand, there are studies suggesting that tubular cell injury may stimulate crystallization. The report of Wiessner J. H. et al. revealed that both individual cells and total tubular monolayer injury exposed cell surfaces resulting in increased affinity for crystal adhesion and their further retention in the collecting duct [40].
Bigger stones, during passage down the ureter, may obstruct renal outflow, causing acute or chronic kidney injury. In patients with renal colic because of urolithiasis, elevation in creatinine serum concentration is often observed. Nevertheless, this classical kidney function indicator lacks specificity in detecting the underlying cause of renal damage and rises late in the process of acute injury. There are various biomarkers proposed to identify and monitor the process of kidney injury. A few of them may apply to urolithiasis as well.
4.2. Cystatin C
Cystatin C has definitely gained importance in diagnosing AKI and CKD. It is a low molecule (13.3 KD) removed from the bloodstream by the kidneys, and its serum levels are a more precise test of kidney function than serum creatinine levels [41]. Most studies prove that cystatin C levels are less dependent on age, gender, ethnicity, diet or muscle mass when compared to creatinine [42]. Cystatin C is a good kidney biomarker in a range of different conditions, including diabetic patients, CKD and after kidney transplantation [43].
We analyzed more recent research on cystatin C in urolithiasis but got conflicting results.
In the study of Hughes S. et al., who compared pre-ureterorenoscopy (URS) and post-URS cystatin C levels in urolithiasis patients, no significant difference was found [23]. In 2020, Kovacevic L. et al. found significant cystatin C elevation in patients with urolithiasis [27]. We should wait for new studies on larger groups of affected participants to make a reliable conclusion on cystatin C’s role in urolithiasis.
4.3. Neutrophil Gelatinase-Associated Lipocalin (NGAL)
Neutrophil gelatinase-associated lipocalin NGAL is a 25 kDa protein bound to gelatinase from neutrophils. Its expression was shown in the proximal and distant tubular cells of the kidney [44]. NGAL is upregulated during the inflammatory process because it meditates cellular proliferation and differentiation and has a bacteriostatic effect [45]. It is a marker of great interest in acute tubular damage, as the expression is upregulated 2–4 h post nephrotoxic and ischemic kidney injury [46,47,48]. Evidence from several studies points out that NGAL is useful in the detection or monitoring of kidney disorders where tubules are affected [49]. In the report of Bolgeri M. et al., not only patients with obstructive uropathy but also those who had urolithiasis without blockade in urine flow had higher sNGAL and uNGAL when compared to healthy peers [50]. Some reports give evidence that the highest uNGAL levels are observed in patients with urolithiasis combined with urinary tract infection [51], which seems to be understood as this marker rises in inflammatory conditions. In the more recent study of Tasdemir M. et al. who compared, among others, uNGAL levels in patients with nephrolithiasis, it was shown that only those who had hydronephrosis (HN) had also elevation in uNGAL [15]. This observation may propose a very careful hypothesis that in patients with nephrolithiasis without HN, markers of tubular injury are not increased because urine flow is not interrupted and tubular injury is not present in contrast with those where dilation of the renal pelvis was observed together with a rise in uNGAL/cr and uNAG/cr. The biggest limitation of this observation is the small number of patients with HD that were included. Other recent findings on NGAL in stone formers are presented in the table.
4.4. Kidney Injury Molecule 1
Kidney Injury Molecule 1 (KIM-1) is a transmembrane protein produced by proximal tubules and is present in plasma and urine after renal injury [52]. It was detected 12–24 h post-AKI, and higher urinary values were observed in patients with ischemic acute tubular necrosis than in other conditions, including CKD, diabetic nephropathy or steroid-resistant nephrotic syndrome [53]. Several studies assessed KIM-1 in nephrolithiasis, giving conflicting results. Some researchers found that uKIM-1 was increased in patients with obstructive nephropathy [54,55]. Similarly, in the study of Fahmy et al., the elevation of uKIM-1 was clear in patients who underwent retrograde intrarenal surgery and shock wave lithotripsy because of kidney stones compared to healthy controls [56]. These findings are in contrast to Urbschat A. et al. who found no difference in uKIM-1 between participants with obstructive nephropathy and controls [57]. The reviewed results of the recent research on KIM-1 as a marker of urolithiasis are gathered in Table 1.
4.5. Carbohydrate Antigen 19-9
Carbohydrate Antigen 19-9 (CA 19-9) is a 36-kD glycoprotein normally expressed in different tissues starting from the gastrointestinal tract, through the bronchi or endometrium and ending in the prostate. It is best known as a marker of pancreatic and other gastrointestinal cancers [58,59,60]. Nevertheless, some investigators revealed its higher urinary levels in urinary tract obstruction [61,62,63]. Suzuki K. and Kajbafzadeh A.M. found that CA 19-9 could be a marker for kidney injury related to urinary obstruction in their research on patients with hydronephrosis [61,62]. Amini E. et al. conducted a study on people with urolithiasis and HN before and after a procedure called transurethral lithotripsy [13]. The affected group had a significant elevation before the operation, which decreased in the following measurements.
It was also noted that the duration of ureteral obstruction was correlated with serum and urinary CA 19-9 levels, which may suggest its potential as a predictive molecule for renal damage. CA 19-9 may seem to be sensitive in the detection of urine flow blockade, but it is not specific to the urinary tract, and this significantly limits its use as a marker.
4.6. N-Acetyl-B-D-Glucosaminidase
N-acetyl-B-D-glucosaminidase (NAG) is also one marker of tubular damage; however, in vivo studies on urolithiasis and its urinary levels were not elevated in stone formers [64]. Nevertheless, in the recent study of Xiaohong F. et al., it was found that bilateral stone formers were more endangered with CKD and had, among others, an increased urine NAG/creatinine ratio (OR 1.95; 95% CI 1.21–3.16) when compared with healthy peers [32].
4.7. Myeloperoxidase
Myeloperoxidase (MPO) is involved in the generation of oxygen radicals by neutrophils in inflammatory conditions [65]. It may rise in the kidney formers; however, it was not proved in an in vivo study. Hughes S. et al. compared pre- and post-URS MPO values in stone-forming participants, and no differences were observed between these two groups [23].
4.8. Markers of Inflammation
4.8.1. Interleukins
Interleukins are cytokines involved mainly in inflammatory response. Most studied in the kidney disorders are listed below. Different tissues like macrophages, osteoblasts and smooth muscles in vessels produce IL-6, which can cause inflammation. It is also a myokine released by muscles in response to excessive contractions, and, in this role, it has mainly an anti-inflammatory effect by inhibition of TNF-alpha [65]. Its importance was shown in many diseases, including different cancers, obesity and severe COVID-19 infection [66]. In sepsis with AKI, an elevation of IL-6 was also noticed [67,68]. IL-8 is another potent cytokine that accelerates inflammation. It induces chemotaxis in target cells (neutrophils and other granulocytes) to make them migrate to the site of infection and then stimulates phagocytosis [66]. As a marker of inflammation, a urinary IL-8 increase was noticed in the course of pyelonephritis [69]. IL-18 can modulate innate and adaptive immunity, and dysregulation of its distribution can lead to autoimmune or inflammatory diseases. The primary site of IL-18 production is macrophages in various organs. It was found in the proximal tubular cells of the kidney as well. IL-18 seems to be the most involved cytokine in kidney disorders. It was even proposed to be the marker of early AKI as it increases 6–24 h post-starting factor. In the kidney, IL-18 is also associated with excessive urinary protein excretion and can be a marker of the progression of diabetic nephropathy [70,71]. Research on inflammatory cytokines in urolithiasis is not consistent. In the study of Memmos D. et al., who compared the effect of standard percutaneous nephrolithotomy (sPCNL) with miniaturized PCNL (mPCNL) and retrograde intrarenal surgery (RIRS) as a nephrolithiasis treatment and measured, among others, uIL-18/cr ratios at baseline and 2, 6, 24 and 48 h postoperatively in the above patients, no significant differences in its level were shown. Similarly, no between-group changes were observed for urinary IL-18/cr at 2 h and later time points postoperatively. Within particular groups, increases for IL-18/cr from baseline were noted at 2 h and progressively lower rises from time zero in all participants at 6, 24 and 48 h post-procedure. No significant difference in this marker level was noticed in AKI or other complications [35]. Similarly, in the study of Kandur Y. et al., who assessed urinary IL-18/cr in 40 pediatric patients diagnosed with nephrolithiasis (NL), 23 patients with hypercalciuria (HC) and 20 healthy controls, no significant differences between patient and control groups regarding urinary IL-18/cr were observed [12]. Kusumi K. et al. observed that uIL-13 was significantly increased in lithiasis participants, while IL-17A was elevated in the urine of controls [21]. More results of the most recent research on urinary interleukins as markers of urolithiasis are in Table 1. They seem to be conflicting and need further observations on larger and homogenous groups of subjects.
4.8.2. Tumor Necrosis Factor—α
Tumor necrosis factor—α (TNF-α) is both an adipokine and cytokine. As a cytokine, it is used for cell signaling. Macrophages detecting an infection release TNF to alert other immune system cells and start an inflammatory response. TNF-α regulates cell proliferation, differentiation and apoptotic death and may be used in the detection of various renal disorders [72]. Hughes S. et al. observed that TNF-α levels significantly increased in lithiasis patients undergoing SWL, peaking at 30 min post-SWL [25].
4.8.3. Monocyte Chemoattractant Protein 1
Monocyte chemoattractant protein 1 (MCP-1) is an inflammatory chemokine produced by mononuclear and intrinsic cells in the kidney to activate and recruit monocytes [73]. Its upregulation is the response to various damaging factors. Studies on several kidney disorders demonstrated its potential as a biomarker. Lupus nephritis severity correlated with urinary MCP-1 (uMCP-1) levels in pediatric patients. Similarly, MCP-1 was higher in patients with chronic kidney disease (CKD) or autosomal recessive polycystic kidney disease (ARPKD) when compared to healthy controls [74]. In urolithiasis, MCP-1 may find its place as well. In the research of Umekawa T. et al., exposure of cultured renal epithelial cells (from a rat renal proximal tubular cell line) to CaOx crystals resulted in higher expression of MCP-1 mRNA and an increased level of the protein [75]. A recent study by Wang X. et al. found that primary hyperoxaluria patients who form stones have high levels of MCP-1 in their urine, which may indicate ongoing crystallization. Other studies on MCP-1 are gathered in Table 1.
4.8.4. C-Reactive Protein, Procalcitonin
CRP was first identified by Tillet and Francis in 1930. They found that it can make streptococcus pneumoniae C-polysaccharide precipitate. CRP is synthesized in the liver as a fast response to inflammation and decreases rapidly after its resolution [76]. Similarly, procalcitonin (PCT) concentration rises as a reaction to a pro-inflammatory stimulus, especially of bacterial origin. Both CRP and PCT are commercially used in blood laboratory tests to detect severe inflammatory diseases. In several studies on urolithiasis, it was shown that elevation in CRP and PCT occurs, especially when stones cause obstruction leading to inflammation of the surrounding tissue [77]. Choosing the best treatment option for ureter obstruction depends on different factors, including the size of a stone and its location. According to The European Association of Urology, medical expulsive therapy (MET) with the use of an alpha blocker should be started in patients with renal colic and distal ureteric stones less than 5 mm, whose symptoms are controlled [78]. Classical inflammatory markers can also support the decision-making process. Observations of Cilesiz et al. show that stone formers who failed MET had higher values of serum PCT than those who spontaneously passed the stone [26]. Similar observations considering serum CRP were demonstrated earlier by Özcan C. et al. [79] as well as Aldaqadossi H. et al. [80] and Jain with colleges [81]. They gave cut-off values of CRP as a predictor of spontaneous stone passage ranging from 0.506 to 21.9 and 4.1 mg/L, respectively. Further research is needed to determine the optimal cut-off level for classical inflammatory indicators in urolithiasis resulting in ureteral obstruction, despite the certainty of their role.
4.9. Macrophage Inflammatory Protein 1beta
Macrophage Inflammatory Protein 1beta (MIP1β) is a chemokine starting recruitment of the immune cells responsible for innate and adaptive immune activity. It is involved in the process of inflammatory response during infection [82]. As another inflammatory molecule, it may be present in stone-induced kidney injury. In the study of Kusumi K. et al., urinary MIP1β levels were significantly elevated in stone-forming adolescents compared to healthy controls [21].
4.10. Other Urinary Proteins
4.10.1. Osteopontin
Osteopontin (OPN) is a phosphorylated protein that plays an essential role in bone mineralization [83]. Most probably, it is also involved in the process of inflammation, cell survival and leukocyte recruitment [84]. It has a wide tissue distribution associated with abnormal calcification including an organic matrix of the kidney stones. OPN is produced in the kidney and found in human urine. It probably acts as one of the urolithiasis inhibitors by preventing the formation of CaOx and further adhesion of crystals to renal epithelial cells [85,86]. Nevertheless, some research on animals gives exactly the opposite conclusions [87,88]. Some authors observed that OPN may increase the risk of CaOx urolithiasis by progression of renal tubular cell damage [87,88].
In the recent study by Icer et al., who compared urinary excretion of OPN between patients with urolithiasis and healthy controls, it was shown that affected participants had significantly lower levels of OPN than unaffected peers [16]. They concluded that low urinary OPN levels were correlated with a higher risk of urolithiasis. More studies on OPN are presented in Table 1.
4.10.2. Nephrocalcin
Nephrocalcin (NC) is one of the most studied molecules, taking part in the process of stone formation as an inhibitor of calcium oxalate (CaOx) crystallization. It was first described in 1978 by Nakagawa et al. as an unidentified acidic polypeptide [89]. During the next ten years, several studies showed its inhibitory effect on CaOx crystallization, and in 1987, it was isolated from the urine and named nephrocalcin [89,90,91,92,93]. This glycoprotein is in the proximal tubule and thick ascending limb of the Henle’s loop [94]. It has many polymeric forms and at least four isoforms: NC-A, NC-B, NC-C and NC-D. The risk of CaOx crystallization and nephrolithiasis depends on the proportion of the isoforms in the urine [95]. Those who are more likely to develop kidney stones excrete greater proportions of NC-C and -D than NC-A and -B [96]. Noyan et al. assessed the NC-PreA/cr ratio in the urine of 41 stone-forming children and 25 matched healthy controls. It appeared to be significantly higher in affected participants [97].
4.11. Bikunin
Another protein that slows CaOx crystallization is bikunin. It is a small chondroitin sulfate proteoglycan joined with a single glycosaminoglycan chain localized in the proximal tubule and the thin descending part of the Henle loop [98]. It is a potent inhibitor of CaOx crystal nucleation and aggregation mostly in healthy humans, whereas in the presence of urolithiasis, its preventing role is limited [99,100]. The existing literature on bikunin’s role gives conflicting results. Higher levels of bikunin were found in children with urolithiasis while in another study on adults, those affected by kidney stones had 50% lower urinary concentration when compared to healthy peers [101,102].
4.12. Calgranulins
Calgranulins, otherwise S100 proteins, are a family of calcium-binding molecules present in the cytosol. Some of them, including S100A8 (calgranulin A) and S100A9 (calgranulin B) have been classified as danger-associated molecular patterns of endogenous origin—alarmins, a group of molecules released as inflammatory signal mediators after cell death [103]. Normally, calgranulins A and B are produced mainly by neutrophils and monocytes, as well as dendritic cells, while in other cell types, they appear after the activating signal [104,105]. Momohara C. et al. found that calgranulins inhibit the crystallization, aggregation and adhesion of CaOx monocrystals to the endothelium, while Mushtaq S. et al. found that they promoted crystal aggregation [106,107].
In the more recent study of Jung K. et al. in children with urolithiasis, no significant differences were observed between the study and control groups [17].
4.13. Matrix Gla Protein
Matrix Gla protein (MGP) was identified in the bone matrix and then in other tissues, including vascular [108]. Although it is suspected to have an inhibitory effect on calcification, the recent study on stone formers does not confirm this hypothesis [24,108].
4.14. Tamm–Horsfall Protein
Tamm–Horsfall protein (THP), known as uromodulin, is one of the most extensively investigated macromolecules in nephrolithiasis. It is found in the urine of all placental invertebrates as a polymer with a molecular weight of up to several million Da [109,110]. It is involved in the pathogenesis of nephrolithiasis and tubule interstitial nephritis [111]. The studies on the particular role of THP in stone formation give conflicting answers. Some point out that it is an inhibitor of crystallization [112,113,114,115], whereas others point out its promoting role [112,113,114,115,116,117,118]. Perhaps its action depends on the concentration of this protein and other solutes [119]. In a recent study by Nonnan C. et al., THP was found to inhibit crystallization by binding calcium ions in a concentration-dependent manner [120].
4.15. Urinary Prothrombin Fragment—1
Urinary prothrombin fragment—1 (UPTF-1) is a part of thrombin. There are both in vitro and in vivo studies indicating its inhibitory effect on crystallization. Epidemiological observations confirm a bigger incidence of stone formation in people who had lower UPTF—1 [121,122].
5. Conclusions
In this review, we explored the most discussed proteins that are associated with the process of stone formation. Recent studies on proteins involved in stone formation are conflicting and inconclusive, making it difficult for clinicians to choose the best treatment.
The above might have a few explanations. First, urolithiasis, although related to idiopathic hypercalciuria in about 70–80% of cases, is not one homogenous disorder. Kidney stones may be composed of different minerals. What is more, there are various co-factors promoting their formation. The patients studied were different in age, kidney function, urinary tract defects and other health issues including medications.
What is more, the number of participants was usually low, below 100. There is one more important matter that should be taken into consideration while reviewing the existing literature and projecting future research. In the findings of Sheng X. et al., who noticed that CaOx monohydrate adhesion to the epithelium depended on the presence of protein carboxyl groups, it was pointed out that the inhibitory effect of macromolecules was related not only to their urinary levels but also the histochemical features [123]. It is important to examine not only their quantity but also quality, which was usually missed in the recent research.
Nevertheless, some proteins like NGAL, KIM-1, OPN or THP seem to be more attractive as proposed biomarkers. More reliable conclusions can be drawn in the future by conducting research on larger, similar populations with urolithiasis and using validated proteomic methods.
Funding
This research was funded by Prof. Anna Wasilewska subvention, number SUB/1/DN/22/004/1141 and APC was funded by the Medical University of Bialystok.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest, financial or otherwise.
Abbreviations
| AKI | Acute Kidney Injury |
| CKD | Chronic Kidney Disease |
| CaOx | Calcium Oxalate |
| eGFR | Estimated Glomerular Filtration Rate |
| HN | Hydronephrosis |
| hs-CRP | Highly Sensitive C-reactive protein |
| IL | Interleukin |
| KIM-1 | Kidney Injury Molecule-1 |
| L-FABP | Liver-type Fatty Acid-Binding Protein |
| MCP-1 | Monocyte Chemotactic Protein-1 |
| MET | Medical Expulsion Therapy |
| MIP1β | Macrophage Inflammatory Protein 1beta |
| MGP | Matrix Gla Protein |
| MPO | Myeloperoxidase |
| NAG | N-Acetyl-β-d-amino Glycosidase |
| NC | Nephrocalcin |
| NGAL | Neutrophil Gelatinase-Associated Lipocalin |
| OPN | Osteopontin |
| PCNL | Nephrolithotripsy |
| PTH | Parathyroid Hormone |
| RIRS | Retrograde Intrarenal Surgery |
| SSP | Spontaneous Stone Passage |
| UPTF-1 | Urinary Prothrombin Fragment-1 |
| URS | Ureterorenoscopy |
| THP | Tamm–Horsfall Protein |
| TNF | α—Tumor Necrosis Factor Alpha |
References
- Issler, N.; Dufek, S.; Kleta, R.; Bockenhauer, D.; Smeulders, N.; van‘t Hoff, W. Epidemiology of Paediatric Renal Stone Disease: A 22-Year Single Centre Experience in the UK. BMC Nephrol. 2017, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Tasian, G.E.; Ross, M.E.; Song, L.; Sas, D.J.; Keren, R.; Denburg, M.R.; Chu, D.I.; Copelovitch, L.; Saigal, C.S.; Furth, S.L. Annual Incidence of Nephrolithiasis among Children and Adults in South Carolina from 1997 to 2012. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bayne, D.; Wiener, S.; Ahn, J.; Stoller, M.; Chi, T. Stone Formation in Patients Less than 20 Years of Age Is Associated with Higher Rates of Stone Recurrence: Results from the Registry for Stones of the Kidney and Ureter (ReSKU). J. Pediatr. Urol. 2020, 16, 373.e1–373.e6. [Google Scholar] [CrossRef]
- Mehta, M.; Goldfarb, D.S.; Nazzal, L. The Role of the Microbiome in Kidney Stone Formation. Int. J. Surg. Lond. Engl. 2016, 36, 607–612. [Google Scholar] [CrossRef]
- Sharma, A.; Filler, G. Epidemiology of Pediatric Urolithiasis. Indian J. Urol. 2010, 26, 516. [Google Scholar] [CrossRef]
- Edvardsson, V. Urolithiasis in Children. In Pediatric Nephrology; Avner, E.D., Harmon, W.E., Niaudet, P., Yoshikawa, N., Emma, F., Goldstein, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–52. ISBN 978-3-642-27843-3. [Google Scholar]
- Alpay, H.; Ozen, A.; Gokce, I.; Biyikli, N. Clinical and Metabolic Features of Urolithiasis and Microlithiasis in Children. Pediatr. Nephrol. Berl. Ger. 2009, 24, 2203–2209. [Google Scholar] [CrossRef]
- Jobs, K.; Rakowska, M.; Paturej, A. Urolithiasis in The Pediatric Population—Current Opinion on Epidemiology, Patophysiology, Diagnostic Evaluation and Treatment. Dev. Period Med. 2018, 22, 201–208. [Google Scholar] [CrossRef]
- Penido, M.G.M.G.; de Sousa Tavares, M. Pediatric Primary Urolithiasis: Symptoms, Medical Management and Prevention Strategies. World J. Nephrol. 2015, 4, 444–454. [Google Scholar] [CrossRef]
- Gambaro, G.; Croppi, E.; Coe, F.; Lingeman, J.; Moe, O.; Worcester, E.; Buchholz, N.; Bushinsky, D.; Curhan, G.C.; Ferraro, P.M.; et al. Metabolic Diagnosis and Medical Prevention of Calcium Nephrolithiasis and Its Systemic Manifestations: A Consensus Statement. J. Nephrol. 2016, 29, 715–734. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kandur, Y.; Gonen, S.; Fidan, K.; Soylemezoglu, O. Evaluation of Urinary KIM-1, NGAL, and IL-18 Levels in Determining Early Renal Injury in Pediatric Cases with Hypercalciuria and/or Renal Calculi. Clin. Nephrol. 2016, 86, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Amini, E.; Pishgar, F.; Hojjat, A.; Soleimani, M.; Asgari, M.A.; Kajbafzadeh, A.-M. The Role of Serum and Urinary Carbohydrate Antigen 19-9 in Predicting Renal Injury Associated with Ureteral Stone. Ren. Fail. 2016, 38, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Chakkarai, K.; Arulvijayavani, S.; Senthilkumar, G.; Manikandan, R.; Kalyaperumal, M. Association between Vitamin D, Parathyroid Hormone and Inflammatory Markers in Urolithiasis Patients. J. Ren. Inj. Prev. 2017, 6, 240–243. [Google Scholar] [CrossRef][Green Version]
- Taşdemir, M.; Fuçucuoğlu, D.; Küçük, S.H.; Erol, M.; Yiğit, Ö.; Bilge, I. Urinary Biomarkers in the Early Detection and Follow-up of Tubular Injury in Childhood Urolithiasis. Clin. Exp. Nephrol. 2018, 22, 133–141. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M.; Sozen, S. Can Urine Osteopontin Levels, Which May Be Correlated with Nutrition Intake and Body Composition, Be Used as a New Biomarker in the Diagnosis of Nephrolithiasis? Clin. Biochem. 2018, 60, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Jobs, K.; Jung, A.; Lewicki, S.; Murawski, P.; Pączek, L.; Zdanowski, R. Assessment of Cross-Correlations between Selected Macromolecules in Urine of Children with Idiopathic Hypercalciuria. Urol. J. 2018, 15, 231–237. [Google Scholar] [CrossRef]
- Chirackal, R.S.; Jayachandran, M.; Wang, X.; Edeh, S.; Haskic, Z.; Perinpam, M.; Halling, T.M.; Mehta, R.; Rivera, M.E.; Lieske, J.C. Urinary Extracellular Vesicle-Associated MCP-1 and NGAL Derived from Specific Nephron Segments Differ between Calcium Oxalate Stone Formers and Controls. Am. J. Physiol. Renal Physiol. 2019, 317, F1475–F1482. [Google Scholar] [CrossRef]
- Shah, T.T.; Gao, C.; Peters, M.; Manning, T.; Cashman, S.; Nambiar, A.; Cumberbatch, M.; Lamb, B.; Peacock, A.; Van Son, M.J.; et al. Factors Associated with Spontaneous Stone Passage in a Contemporary Cohort of Patients Presenting with Acute Ureteric Colic: Results from the Multi-Centre Cohort Study Evaluating the Role of Inflammatory Markers In Patients Presenting with Acute Ureteric Colic (MIMIC) Study. BJU Int. 2019, 124, 504–513. [Google Scholar] [CrossRef]
- Okada, A.; Ando, R.; Taguchi, K.; Hamamoto, S.; Unno, R.; Sugino, T.; Tanaka, Y.; Mizuno, K.; Tozawa, K.; Kohri, K.; et al. Identification of New Urinary Risk Markers for Urinary Stones Using a Logistic Model and Multinomial Logit Model. Clin. Exp. Nephrol. 2019, 23, 710–716. [Google Scholar] [CrossRef]
- Kusumi, K.; Ketz, J.; Saxena, V.; Spencer, J.D.; Safadi, F.; Schwaderer, A. Adolescents with Urinary Stones Have Elevated Urine Levels of Inflammatory Mediators. Urolithiasis 2019, 47, 461–466. [Google Scholar] [CrossRef]
- Wang, X.; Bhutani, G.; Vaughan, L.E.; Enders, F.T.; Haskic, Z.; Milliner, D.; Lieske, J.C.; Assimos, D.; Baum, M.; Somers, M.; et al. Urinary Monocyte Chemoattractant Protein 1 Associated with Calcium Oxalate Crystallization in Patients with Primary Hyperoxaluria. BMC Nephrol. 2020, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.F.; Moyes, A.J.; Lamb, R.M.; Ella-Tongwiis, P.; Bell, C.; Moussa, A.; Shergill, I. The Role of Specific Biomarkers, as Predictors of Post-Operative Complications Following Flexible Ureterorenoscopy (FURS), for the Treatment of Kidney Stones: A Single-Centre Observational Clinical Pilot-Study in 37 Patients. BMC Urol. 2020, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Pottel, H.; Lieske, J.C.; Lukas, P.; Cavalier, E.; Delanaye, P.; Rule, A.D. Evaluation of Inactive Matrix-Gla-Protein (MGP) as a Biomarker for Incident and Recurrent Kidney Stones. J. Nephrol. 2020, 33, 101–107. [Google Scholar] [CrossRef]
- Hughes, S.F.; Jones, N.; Thomas-Wright, S.J.; Banwell, J.; Moyes, A.J.; Shergill, I. Shock Wave Lithotripsy, for the Treatment of Kidney Stones, Results in Changes to Routine Blood Tests and Novel Biomarkers: A Prospective Clinical Pilot-Study. Eur. J. Med. Res. 2020, 25, 18. [Google Scholar] [CrossRef]
- Cilesiz, N.C.; Ozkan, A.; Kalkanli, A.; Eroglu, A.; Gezmis, C.T.; Simsek, B.; Arslan, B. Can Serum Procalcitonin Levels Be Useful in Predicting Spontaneous Ureteral Stone Passage? BMC Urol. 2020, 20, 42. [Google Scholar] [CrossRef]
- Kovacevic, L.; Lu, H.; Kovacevic, N.; Thomas, R.; Lakshmanan, Y. Cystatin C, Neutrophil Gelatinase-Associated Lipocalin, and Lysozyme C: Urinary Biomarkers for Detection of Early Kidney Dysfunction in Children with Urolithiasis. Urology 2020, 143, 221–226. [Google Scholar] [CrossRef]
- Qi, T.; Lai, C.; Li, Y.; Chen, X.; Jin, X. The Predictive and Diagnostic Ability of IL-6 for Postoperative Urosepsis in Patients Undergoing Percutaneous Nephrolithotomy. Urolithiasis 2021, 49, 367–375. [Google Scholar] [CrossRef]
- Ramasamy, V.; Aarthy, P.; Sharma, V.; Thakur, A.P.S. Role of Inflammatory Markers and Their Trends in Predicting the Outcome of Medical Expulsive Therapy for Distal Ureteric Calculus. Urol. Ann. 2022, 14, 8–14. [Google Scholar] [CrossRef]
- Milišić, E.; Alić, J.; Zvizdić, Z.; Lepara, O.; Jonuzi, A.; Milišić, L.; Fajkić, A. Urinary Neutrophil Gelatinase-Associated Lipocalin Level as a Biomarker of Acute Kidney Injury Following Extracorporeal Shock Wave Lithotripsy. Cent. Eur. J. Urol. 2021, 74, 579–587. [Google Scholar] [CrossRef]
- Wymer, K.M.; Sharma, V.; Manka, M.; Agarwal, D.; Dodge, N.; Gettman, M.; Rivera, M. A Serum C-Reactive Protein and Procalcitonin-Based Risk Score to Predict Urinary Infection in Patients with Obstructive Urolithiasis Undergoing Decompression. J. Endourol. 2021, 35, 369–375. [Google Scholar] [CrossRef]
- Fan, X.; Ye, W.; Ma, J.; Wang, L.; Heng, W.; Zhou, Y.; Wei, S.; Xuehe, Z.; Sun, Y.; Cui, R.; et al. Metabolic Differences between Unilateral and Bilateral Renal Stones and Their Association with Markers of Kidney Injury. J. Urol. 2022, 207, 144–151. [Google Scholar] [CrossRef]
- Noonin, C.; Peerapen, P.; Yoodee, S.; Kapincharanon, C.; Kanlaya, R.; Thongboonkerd, V. Systematic Analysis of Modulating Activities of Native Human Urinary Tamm-Horsfall Protein on Calcium Oxalate Crystallization, Growth, Aggregation, Crystal-Cell Adhesion and Invasion through Extracellular Matrix. Chem. Biol. Interact. 2022, 357, 109879. [Google Scholar] [CrossRef]
- Kovacevic, L.; Kovacevic, N.; Lakshmanan, Y. Proteomic Analysis of Inhibitory Protein Profiles in the Urine of Children with Nephrolithiasis: Implication for Disease Prevention. Int. Urol. Nephrol. 2022, 54, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Memmos, D.; Sarafidis, P.; Alexandrou, M.E.; Theodorakopoulou, M.; Anastasiadis, A.; Mykoniatis, I.; Dimitriadis, G.; Dimitrios, H. The Effect of Standard Percutaneous Nephrolithotomy, Miniaturized Percutaneous Nephrolithotomy and Retrograde Intrarenal Surgery on Biomarkers of Renal Injury: A Randomized Clinical Trial. Clin. Kidney J. 2023, 16, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Savin, Z.; Mintz, I.; Lifshitz, K.; Achiam, L.; Aviram, G.; Bar-Yosef, Y.; Yossepowitch, O.; Sofer, M. The Role of Serum and Urinary Markers in Predicting Obstructing Ureteral Stones and Reducing Unjustified Non-Contrast Computerized Tomographic Scans in Emergency Departments. Emerg. Radiol. 2023, 30, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Hackett, R.L. Hyperoxaluria, Enzymuria and Nephrolithiasis. Contrib. Nephrol. 1993, 101, 190–193. [Google Scholar] [PubMed]
- Khan, S.R.; Finlayson, B.; Hackett, R.L. Histologic Study of the Early Events in Oxalate Induced Intranephronic Calculosis. Investig. Urol. 1979, 17, 199–202. [Google Scholar]
- Khan, S.R. Experimental Calcium Oxalate Nephrolithiasis and the Formation of Human Urinary Stones. Scanning Microsc. 1995, 9, 89–100. [Google Scholar]
- Wiessner, J.H.; Hasegawa, A.T.; Hung, L.Y.; Mandel, G.S.; Mandel, N.S. Mechanisms of Calcium Oxalate Crystal Attachment to Injured Renal Collecting Duct Cells. Kidney Int. 2001, 59, 637–644. [Google Scholar] [CrossRef]
- Roos, J.F.; Doust, J.; Tett, S.E.; Kirkpatrick, C.M.J. Diagnostic Accuracy of Cystatin C Compared to Serum Creatinine for the Estimation of Renal Dysfunction in Adults and Children—A Meta-Analysis. Clin. Biochem. 2007, 40, 383–391. [Google Scholar] [CrossRef]
- Onopiuk, A.; Tokarzewicz, A.; Gorodkiewicz, E. Cystatin C: A Kidney Function Biomarker. Adv. Clin. Chem. 2015, 68, 57–69. [Google Scholar] [CrossRef]
- Porto, J.; Gomes, K.; Fernandes, A.; Domingueti, C. Cystatin C: A Promising Biomarker to Evaluate Renal Function. Rev. Bras. Análises Clínicas 2016, 49, 227–234. [Google Scholar] [CrossRef]
- Alderson, H.V.; Ritchie, J.P.; Pagano, S.; Middleton, R.J.; Pruijm, M.; Vuilleumier, N.; Kalra, P.A. The Associations of Blood Kidney Injury Molecule-1 and Neutrophil Gelatinase-Associated Lipocalin with Progression from CKD to ESRD. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 2141–2149. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil Gelatinase-Associated Lipocalin—An Emerging Troponin for Kidney Injury. Nephrol. Dial. Transplant. 2008, 23, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of Renal Function Tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar] [PubMed]
- Ferguson, M.A.; Waikar, S.S. Established and Emerging Markers of Kidney Function. Clin. Chem. 2012, 58, 680–689. [Google Scholar] [CrossRef]
- Mårtensson, J.; Xu, S.; Bell, M.; Martling, C.-R.; Venge, P. Immunoassays Distinguishing between HNL/NGAL Released in Urine from Kidney Epithelial Cells and Neutrophils. Clin. Chim. Acta 2012, 413, 1661–1667. [Google Scholar] [CrossRef]
- Brunner, H.I.; Mueller, M.; Rutherford, C.; Passo, M.H.; Witte, D.; Grom, A.; Mishra, J.; Devarajan, P. Urinary Neutrophil Gelatinase-Associated Lipocalin as a Biomarker of Nephritis in Childhood-Onset Systemic Lupus Erythematosus. Arthritis Rheum. 2006, 54, 2577–2584. [Google Scholar] [CrossRef]
- Bolgeri, M.; Whiting, D.; Reche, A.; Manghat, P.; Sriprasad, S. Neutrophil Gelatinase-Associated Lipocalin (NGAL) as a Biomarker of Renal Injury in Patients with Ureteric Stones: A Pilot Study. J. Clin. Urol. 2020, 14, 205141582094756. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, M.; Wang, G.-C.; Che, J.-P.; Xu, Y.-F.; Peng, B.; Zheng, J.-H. Urinary Neutrophil Gelatinase-Associated Lipocalin, a Biomarker for Systemic Inflammatory Response Syndrome in Patients with Nephrolithiasis. J. Surg. Res. 2014, 187, 237–243. [Google Scholar] [CrossRef]
- Yin, C.; Wang, N. Kidney Injury Molecule-1 in Kidney Disease. Ren. Fail. 2016, 38, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A Novel Biomarker for Human Renal Proximal Tubule Injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Posada, D.; Dayarathna, T.; Dion, M.; Alenezi, H.; Sener, A.; Denstedt, J.D.; Pautler, S.E.; Razvi, H. KIM-1 Is a Potential Urinary Biomarker of Obstruction: Results from a Prospective Cohort Study. J. Endourol. 2017, 31, 111–118. [Google Scholar] [CrossRef]
- Xie, Y.; Xue, W.; Shao, X.; Che, X.; Xu, W.; Ni, Z.; Mou, S. Analysis of a Urinary Biomarker Panel for Obstructive Nephropathy and Clinical Outcomes. PLoS ONE 2014, 9, e112865. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.; Sener, A.; Sabbisetti, V.; Nott, L.; Lang, R.M.; Welk, B.K.; Méndez-Probst, C.E.; MacPhee, R.A.; VanEerdewijk, S.; Cadieux, P.A.; et al. Urinary Expression of Novel Tissue Markers of Kidney Injury after Ureteroscopy, Shockwave Lithotripsy, and in Normal Healthy Controls. J. Endourol. 2013, 27, 1455–1462. [Google Scholar] [CrossRef]
- Urbschat, A.; Gauer, S.; Paulus, P.; Reissig, M.; Weipert, C.; Ramos-Lopez, E.; Hofmann, R.; Hadji, P.; Geiger, H.; Obermüller, N. Serum and Urinary NGAL but Not KIM-1 Raises in Human Postrenal AKI. Eur. J. Clin. Investig. 2014, 44, 652–659. [Google Scholar] [CrossRef]
- Kilis-Pstrusinska, K.; Szajerka, U.; Zwolinska, D. Unspecific Increase of Tumor Markers in a Girl with Nephrotic Syndrome and Ovarian Teratoma. Ren. Fail. 2013, 35, 654–656. [Google Scholar] [CrossRef]
- Atkinson, B.F.; Ernst, C.S.; Herlyn, M.; Steplewski, Z.; Sears, H.F.; Koprowski, H. Gastrointestinal Cancer-Associated Antigen in Immunoperoxidase Assay. Cancer Res. 1982, 42, 4820–4823. [Google Scholar]
- Malaguarnera, G.; Giordano, M.; Paladina, I.; Rando, A.; Uccello, M.; Basile, F.; Biondi, A.; Carnazzo, S.; Alessandria, I.; Mazzarino, C. Markers of Bile Duct Tumors. World J. Gastrointest. Oncol. 2011, 3, 49–59. [Google Scholar] [CrossRef]
- Aybek, H.; Aybek, Z.; Sinik, Z.; Demir, S.; Sancak, B.; Tuncay, L. Elevation of Serum and Urinary Carbohydrate Antigen 19-9 in Benign Hydronephrosis. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2006, 13, 1380–1384. [Google Scholar] [CrossRef]
- Lopes, R.I.; Dénes, F.T.; Bartolamei, M.G.; Reis, S.; Sanches, T.R.; Leite, K.R.; Srougi, M.; Seguro, A.C. Serum and Urinary Values of CA 19-9 and TGFß1 in a Rat Model of Partial or Complete Ureteral Obstruction. Eur. J. Pediatr. Surg. 2015, 25, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Muraishi, O.; Tokue, A. The Correlation of Serum Carbohydrate Antigen 19-9 with Benign Hydronephrosis. J. Urol. 2002, 167, 16–20. [Google Scholar] [CrossRef]
- Tenstad, O.; Roald, A.B.; Grubb, A.; Aukland, K. Renal Handling of Radiolabelled Human Cystatin C in the Rat. Scand. J. Clin. Lab. Investig. 1996, 56, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Pedersen, B.K. Contraction-Induced Myokine Production and Release: Is Skeletal Muscle an Endocrine Organ? Exerc. Sport Sci. Rev. 2005, 33, 114. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Jardel, C.; Delattre, J.; Hainque, B.; Bruckert, E.; Oberlin, F. Evidence for a Link between Adipose Tissue Interleukin-6 Content and Serum C-Reactive Protein Concentrations in Obese Subjects. Circulation 1999, 99, 2221–2222. [Google Scholar] [CrossRef]
- Chawla, L.; Seneff, M.; Nelson, D.; Williams, M.; Levy, H.; Kimmel, P.; Macias, W. Elevated Plasma Concentrations of IL-6 and Elevated APACHE II Score Predict Acute Kidney Injury in Patients with Severe Sepsis. Clin. J. Am. Soc. Nephrol. CJASN 2007, 2, 22–30. [Google Scholar] [CrossRef]
- Kwon, O.; Molitoris, B.A.; Pescovitz, M.; Kelly, K.J. Urinary Actin, Interleukin-6, and Interleukin-8 May Predict Sustained ARF after Ischemic Injury in Renal Allografts. Am. J. Kidney Dis. 2003, 41, 1074–1087. [Google Scholar] [CrossRef]
- Rao, W.H.; Evans, G.S.; Finn, A. The Significance of Interleukin 8 in Urine. Arch. Dis. Child. 2001, 85, 256–262. [Google Scholar] [CrossRef]
- Liu, F.; Guo, J.; Zhang, Q.; Liu, D.; Wen, L.; Yang, Y.; Yang, L.; Liu, Z. The Expression of Tristetraprolin and Its Relationship with Urinary Proteins in Patients with Diabetic Nephropathy. PLoS ONE 2015, 10, e0141471. [Google Scholar] [CrossRef]
- Nakamura, A.; Shikata, K.; Hiramatsu, M.; Nakatou, T.; Kitamura, T.; Wada, J.; Itoshima, T.; Makino, H. Serum Interleukin-18 Levels Are Associated with Nephropathy and Atherosclerosis in Japanese Patients with Type 2 Diabetes. Diabetes Care 2005, 28, 2890–2895. [Google Scholar] [CrossRef]
- Al-Lamki, R.S.; Mayadas, T.N. TNF Receptors: Signaling Pathways and Contribution to Renal Dysfunction. Kidney Int. 2015, 87, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Tam, F.W.K. Urinary Monocyte Chemoattractant Protein-1 in Renal Disease. Clin. Chim. Acta Int. J. Clin. Chem. 2011, 412, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Rybi Szumińska, A.; Wasilewska, A.; Kamianowska, M. Protein Biomarkers in Chronic Kidney Disease in Children—What Do We Know So Far? J. Clin. Med. 2023, 12, 3934. [Google Scholar] [CrossRef]
- Umekawa, T.; Chegini, N.; Khan, S.R. Increased Expression of Monocyte Chemoattractant Protein-1 (MCP-1) by Renal Epithelial Cells in Culture on Exposure to Calcium Oxalate, Phosphate and Uric Acid Crystals. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2003, 18, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Baltz, M.L. Acute Phase Proteins with Special Reference to C-Reactive Protein and Related Proteins (Pentaxins) and Serum Amyloid A Protein. Adv. Immunol. 1983, 34, 141–212. [Google Scholar] [CrossRef]
- Crowley, A.R.; Byrne, J.C.; Vaughan, E.D.; Marion, D.N. The Effect of Acute Obstruction on Ureteral Function. J. Urol. 1990, 143, 596–599. [Google Scholar] [CrossRef]
- Türk, C.; Knoll, T.; Seitz, C.; Skolarikos, A.; Chapple, C.; McClinton, S.; European Association of Urology. Medical Expulsive Therapy for Ureterolithiasis: The EAU Recommendations in 2016. Eur. Urol. 2017, 71, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Özcan, C.; Aydoğdu, O.; Senocak, C.; Damar, E.; Eraslan, A.; Oztuna, D.; Bozkurt, O.F. Predictive Factors for Spontaneous Stone Passage and the Potential Role of Serum C-Reactive Protein in Patients with 4 to 10 Mm Distal Ureteral Stones: A Prospective Clinical Study. J. Urol. 2015, 194, 1009–1013. [Google Scholar] [CrossRef]
- Kim, B.; Park, C.; Kwon, Y.; Kim, D.; Park, C.; Kim, C. 2078 the Relationship between Natural Passage Rate of Less than 8mm Ureter Stone and C-Reactive Protein and Neutrophil Percentage. J. Urol. 2012, 187, e838. [Google Scholar] [CrossRef]
- Aldaqadossi, H.A. Stone Expulsion Rate of Small Distal Ureteric Calculi Could Be Predicted with Plasma C-Reactive Protein. Urolithiasis 2013, 41, 235–239. [Google Scholar] [CrossRef]
- Allen, S.J.; Crown, S.E.; Handel, T.M. Chemokine: Receptor Structure, Interactions, and Antagonism. Annu. Rev. Immunol. 2007, 25, 787–820. [Google Scholar] [CrossRef] [PubMed]
- Reinholt, F.P.; Hultenby, K.; Oldberg, A.; Heinegård, D. Osteopontin—A Possible Anchor of Osteoclasts to Bone. Proc. Natl. Acad. Sci. USA 1990, 87, 4473–4475. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.R.; Garvin, M.R.; Stewart, D.K.; Hinohara, T.; Simpson, J.B.; Schwartz, S.M.; Giachelli, C.M. Osteopontin Is Synthesized by Macrophage, Smooth Muscle, and Endothelial Cells in Primary and Restenotic Human Coronary Atherosclerotic Plaques. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Huang, S.-P.; Tsai, L.-Y.; Wu, W.-J.; Juo, S.-H.H.; Chou, Y.-H.; Huang, C.-H.; Wu, M.-T. The Impact of Osteopontin Promoter Polymorphisms on the Risk of Calcium Urolithiasis. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 739–743. [Google Scholar] [CrossRef]
- Yaman, F. Fetuin-a and Osteopontin in the Etiopathogenesis of Nephrolithiasis. Specialist Thesis, Pamukkale University, Denizli, Turkey, 2011. [Google Scholar]
- Hamamoto, S.; Yasui, T.; Okada, A.; Hirose, M.; Matsui, Y.; Kon, S.; Sakai, F.; Kojima, Y.; Hayashi, Y.; Tozawa, K.; et al. Crucial Role of the Cryptic Epitope SLAYGLR within Osteopontin in Renal Crystal Formation of Mice. J. Bone Miner. Res. 2011, 26, 2967–2977. [Google Scholar] [CrossRef]
- Hirose, M.; Tozawa, K.; Okada, A.; Hamamoto, S.; Higashibata, Y.; Gao, B.; Hayashi, Y.; Shimizu, H.; Kubota, Y.; Yasui, T.; et al. Role of Osteopontin in Early Phase of Renal Crystal Formation: Immunohistochemical and Microstructural Comparisons with Osteopontin Knock-out Mice. Urol. Res. 2012, 40, 121–129. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Kaiser, E.T.; Coe, F.L. Isolation and Characterization of Calcium Oxalate Crystal Growth Inhibitors from Human Urine. Biochem. Biophys. Res. Commun. 1978, 84, 1038–1044. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Margolis, H.C.; Yokoyama, S.; Kézdy, F.J.; Kaiser, E.T.; Coe, F.L. Purification and Characterization of a Calcium Oxalate Monohydrate Crystal Growth Inhibitor from Human Kidney Tissue Culture Medium. J. Biol. Chem. 1981, 256, 3936–3944. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Abram, V.; Kézdy, F.J.; Kaiser, E.T.; Coe, F.L. Purification and Characterization of the Principal Inhibitor of Calcium Oxalate Monohydrate Crystal Growth in Human Urine. J. Biol. Chem. 1983, 258, 12594–12600. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Parks, J.H.; Kézdy, F.J.; Coe, F.L. Molecular Abnormality of Urinary Glycoprotein Crystal Growth Inhibitor in Calcium Nephrolithiasis. Trans. Assoc. Am. Physicians 1985, 98, 281–289. [Google Scholar]
- Coe, F.L.; Margolis, H.C.; Deutsch, L.H.; Strauss, A.L. Urinary Macromolecular Crystal Growth Inhibitors in Calcium Nephrolithiasis. Miner. Electrolyte Metab. 1980, 3, 268–275. [Google Scholar]
- Nakagawa, Y. Immunohistochemical Localization of Nephrocalcin (NC) to Proximal Tubule and Thick Ascending Limb of Henle’s Loop (TALH) of Human and Mouse Kidney. Kidney Int. 1990, 37, 474. [Google Scholar]
- Nakagawa, Y.; Ahmed, M.; Hall, S.L.; Deganello, S.; Coe, F.L. Isolation from Human Calcium Oxalate Renal Stones of Nephrocalcin, a Glycoprotein Inhibitor of Calcium Oxalate Crystal Growth. Evidence That Nephrocalcin from Patients with Calcium Oxalate Nephrolithiasis Is Deficient in Gamma-Carboxyglutamic Acid. J. Clin. Investig. 1987, 79, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Kurutz, J.; Carvalho, M.; Nakagawa, Y. Nephrocalcin Isoforms Coat Crystal Surfaces and Differentially Affect Calcium Oxalate Monohydrate Crystal Morphology, Growth, and Aggregation. J. Cryst. Growth 2003, 255, 392–402. [Google Scholar] [CrossRef]
- Noyan, A.; Yaşar, H.; Bayazit, A.K.; Anarat, R.; Bayazit, Y.; Anarat, A. Urinary Nephrocalcin Excretion in Children with Urolithiasis. Nephron Physiol. 2003, 94, p59–p61. [Google Scholar] [CrossRef]
- Okuyama, M.; Yamaguchi, S.; Yachiku, S. Identification of Bikunin Isolated from Human Urine Inhibits Calcium Oxalate Crystal Growth and Its Localization in the Kidneys. Int. J. Urol. 2003, 10, 530–535. [Google Scholar] [CrossRef]
- Atmani, F.; Khan, S.R. Role of Urinary Bikunin in the Inhibition of Calcium Oxalate Crystallization. J. Am. Soc. Nephrol. JASN 1999, 10 (Suppl. S14), S385–S388. [Google Scholar]
- De Yoreo, J.J.; Qiu, S.R.; Hoyer, J.R. Molecular Modulation of Calcium Oxalate Crystallization. Am. J. Physiol. Renal Physiol. 2006, 291, F1123–F1131. [Google Scholar] [CrossRef]
- Bergsland, K.J.; Kelly, J.K.; Coe, B.J.; Coe, F.L. Urine Protein Markers Distinguish Stone-Forming from Non-Stone-Forming Relatives of Calcium Stone Formers. Am. J. Physiol. Renal Physiol. 2006, 291, F530–F536. [Google Scholar] [CrossRef]
- Médétognon-Benissan, J.; Tardivel, S.; Hennequin, C.; Daudon, M.; Drüeke, T.; Lacour, B. Inhibitory Effect of Bikunin on Calcium Oxalate Crystallization in Vitro and Urinary Bikunin Decrease in Renal Stone Formers. Urol. Res. 1999, 27, 69–75. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Roth, J. Mechanisms of Disease: A “DAMP” View of Inflammatory Arthritis. Nat. Clin. Pract. Rheumatol. 2007, 3, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Roth, J.; Oppenheim, J.J.; Tracey, K.J.; Vogl, T.; Feldmann, M.; Horwood, N.; Nanchahal, J. Alarmins: Awaiting a Clinical Response. J. Clin. Investig. 2012, 122, 2711–2719. [Google Scholar] [CrossRef] [PubMed]
- Edgeworth, J.; Gorman, M.; Bennett, R.; Freemont, P.; Hogg, N. Identification of P8,14 as a Highly Abundant Heterodimeric Calcium Binding Protein Complex of Myeloid Cells. J. Biol. Chem. 1991, 266, 7706–7713. [Google Scholar] [CrossRef]
- Mushtaq, S.; Siddiqui, A.A.; Naqvi, Z.A.; Rattani, A.; Talati, J.; Palmberg, C.; Shafqat, J. Identification of Myeloperoxidase, Alpha-Defensin and Calgranulin in Calcium Oxalate Renal Stones. Clin. Chim. Acta Int. J. Clin. Chem. 2007, 384, 41–47. [Google Scholar] [CrossRef]
- Momohara, C.; Tsujihata, M.; Yoshioka, I.; Tsujimura, A.; Nonomura, N.; Okuyama, A. Mechanism Underlying the Low Prevalence of Pediatric Calcium Oxalate Urolithiasis. J. Urol. 2009, 182, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous Calcification of Arteries and Cartilage in Mice Lacking Matrix GLA Protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, J.A.; Glenton, P.A.; Khan, S.R. Characterization of Tamm-Horsfall Protein in a Rat Nephrolithiasis Model. J. Urol. 2001, 166, 1492–1497. [Google Scholar] [CrossRef]
- Sikri, K.L.; Foster, C.L.; MacHugh, N.; Marshall, R.D. Localization of Tamm-Horsfall Glycoprotein in the Human Kidney Using Immuno-Fluorescence and Immuno-Electron Microscopical Techniques. J. Anat. 1981, 132, 597–605. [Google Scholar]
- Serafini-Cessi, F.; Malagolini, N.; Cavallone, D. Tamm-Horsfall Glycoprotein: Biology and Clinical Relevance. Am. J. Kidney Dis. 2003, 42, 658–676. [Google Scholar] [CrossRef]
- Ryall, R.L.; Harnett, R.M.; Hibberd, C.M.; Edyvane, K.A.; Marshall, V.R. Effects of Chondroitin Sulphate, Human Serum Albumin and Tamm-Horsfall Mucoprotein on Calcium Oxalate Crystallization in Undiluted Human Urine. Urol. Res. 1991, 19, 181–188. [Google Scholar] [CrossRef]
- Robertson, W.G.; Scurr, D.S.; Bridge, C.M. Factors Influencing the Crystallisation of Calcium Oxalate in Urine—Critique. J. Cryst. Growth 1981, 53, 182–194. [Google Scholar] [CrossRef]
- Fellström, B.; Danielson, B.G.; Ljunghall, S.; Wikström, B. Crystal Inhibition: The Effects of Polyanions on Calcium Oxalate Crystal Growth. Clin. Chim. Acta Int. J. Clin. Chem. 1986, 158, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.K.; Marshall, V.R.; Ryall, R.L. Tamm-Horsfall Mucoprotein Reduces Promotion of Calcium Oxalate Crystal Aggregation Induced by Urate in Human Urine in Vitro. Clin. Sci. Lond. Engl. 1979 1994, 87, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.A.; Sulaiman, S. Tamm-Horsfall Mucoproteins Promote Calcium Oxalate Crystal Formation in Urine: Quantitative Studies. J. Urol. 1982, 127, 177–179. [Google Scholar] [CrossRef]
- Yoshioka, T.; Koide, T.; Utsunomiya, M.; Itatani, H.; Oka, T.; Sonoda, T. Possible Role of Tamm-Horsfall Glycoprotein in Calcium Oxalate Crystallisation. Br. J. Urol. 1989, 64, 463–467. [Google Scholar] [CrossRef]
- Grover, P.K.; Ryall, R.L.; Marshall, V.R. Does Tamm-Horsfall Mucoprotein Inhibit or Promote Calcium Oxalate Crystallization in Human Urine? Clin. Chim. Acta Int. J. Clin. Chem. 1990, 190, 223–238. [Google Scholar] [CrossRef]
- Khan, S.R.; Atmani, F.; Glenton, P.; Hou, Z.; Talham, D.R.; Khurshid, M. Lipids and Membranes in the Organic Matrix of Urinary Calcific Crystals and Stones. Calcif. Tissue Int. 1996, 59, 357–365. [Google Scholar] [CrossRef]
- Khan, S.R. Role of Renal Epithelial Cells in the Initiation of Calcium Oxalate Stones. Nephron Exp. Nephrol. 2004, 98, e55–e60. [Google Scholar] [CrossRef] [PubMed]
- Doyle, I.R.; Ryall, R.L.; Marshall, V.R. Inclusion of Proteins into Calcium Oxalate Crystals Precipitated from Human Urine: A Highly Selective Phenomenon. Clin. Chem. 1991, 37, 1589–1594. [Google Scholar] [CrossRef]
- Webber, D.; Rodgers, A.L.; Sturrock, E.D. Synergism between Urinary Prothrombin Fragment 1 and Urine: A Comparison of Inhibitory Activities in Stone-Prone and Stone-Free Population Groups. Clin. Chem. Lab. Med. 2002, 40, 930–936. [Google Scholar] [CrossRef]
- Sheng, X.; Ward, M.D.; Wesson, J.A. Adhesion between Molecules and Calcium Oxalate Crystals: Critical Interactions in Kidney Stone Formation. J. Am. Chem. Soc. 2003, 125, 2854–2855. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).