Abstract
Fermented foods are part of the staple diet in many different countries and populations and contain various probiotic microorganisms and non-digestible prebiotics. Fermentation is the process of breaking down sugars by bacteria and yeast species; it not only enhances food preservation but can also increase the number of beneficial gut bacteria. Regular consumption of fermented foods has been associated with a variety of health benefits (although some health risks also exist), including improved digestion, enhanced immunity, and greater weight loss, suggesting that fermented foods have the potential to help in the design of effective nutritional therapeutic approaches for obesity. In this article, we provide a comprehensive overview of the health effects of fermented foods and the corresponding mechanisms of action in obesity and obesity-related metabolic abnormalities.
1. Introduction
Obesity is a highly prevalent disease globally and increases the risk for other chronic conditions like type 2 diabetes mellitus, coronary heart disease, osteoarthritis, sleep apnea, and some types of cancer []. Anti-obesity drugs are often used to prevent and treat obesity and its comorbidities; however, various side effects and inflated healthcare costs have resulted in seeking safe and effective natural product alternatives in obesity management []. Obesity is a multi-factorial health challenge that is accompanied by immune dysfunction of white adipose tissue and intestinal microbiome dysbiosis; recently, the administration of bacteria or their metabolites from fermented foods has been found to beneficially affect adipose tissue function, inflammation, and the gut microbiome [,,,,].
Fermentation has been used for centuries to preserve foods and their texture through deactivating spoilage microorganisms [,]. Fermented foods contain enduring microorganisms that predominantly include lactic acid bacteria (LAB) and their major metabolites, i.e., lactate [,]. These foods are thought to have health-promoting effects due to a mixture of several beneficial microorganisms—not only LAB species but also acetobacter and Propionibacterium; and bioactive macromolecules—such as exopolysaccharides and bactericides [,,,]. However, the mechanisms of action are not entirely clear [,].
This paper aims to provide a comprehensive overview of available evidence from recent clinical and experimental investigations on fermented foods and obesity, focusing on the challenges faced, knowledge gaps, and future perspectives, and the potential molecular mechanisms by which fermented foods affect the gut microbiome and immune responses.
2. Fermented Foods, Gut Microbiota, and Metabolic Regulation
Fermented foods function as a relatively optimal drug delivery system as they contain a mixture of safe bioactive compounds in sufficient doses to transfer to the target site, thereby facilitating efficacy and long-term compliance [,,]. The food matrix of fermented foods can exert medicinal properties by a large number of viable microorganisms and their transformed metabolites to shield the gastrointestinal tract against pathogens, excess gastric acid, and bile salts [,]. Probiotic microorganisms in fermented foods are capable of producing short-chain fatty acids (SCFAs) via fermentation of the prebiotic non-digestible carbohydrates, which can then be taken up as a source of energy by bacteria in the colon, inhibit the overgrowth of intestinal pathogens, and regulate various metabolic pathways (e.g., cholesterol synthesis), including the secretion of appetite hormones [,,,,].
Fermented foods also contain live microorganisms or beneficial bioactive compounds that support the symbiosis between the host and the microbiome, resulting in a healthier gut environment [,]. SCFAs like acetate, propionate, and butyrate and other primary metabolites of live probiotics from fermented foods can stimulate the growth of beneficial microbial phyla in the gut, for example, by lowering intestinal luminal pH and improving conditions for intestinal commensal microflora like Bacteroides and Prevotella species [,].
The association between the gut microbiome and the risk of obesity has been known for some time [,,]. Dysbiosis refers to an imbalance of intestinal microflora that is associated with obesity [,]. Several experimental and clinical studies have demonstrated that a lower ratio of Bacteroidetes to Firmicutes may be involved in the pathogenesis of obesity; however, due to large inter-individual variation this ratio cannot be considered as a biomarker for increased obesity risk, and further research is required to identify a key bacterial community based on individual characteristics and traits that increases susceptibility to weight gain and body fat accumulation []. Although the abundance and composition of healthy gut microflora are different depending on geographical region [], decreased diversity of the microbial populations is often seen in obese subjects []. It remains to be confirmed if manipulation of the microbial community in the gut can result in a long-lasting effect on appetite regulation and body weight homeostasis.
Intestinal permeability is another factor that may mediate some of the associations between the gut microbiome and obesity. The microbiome interacts with inflammatory signal transduction pathways via the gram-negative bacteria membrane lipopolysaccharide (LPS), which binds to CD-14 and toll-like receptor-4 (TLR4) on enterocytes, resulting in the translocation of bacteria through the intestinal barrier and an inflammatory response [,,]. Relevant studies indicate that gut microbiome dysbiosis can increase intestinal permeability that can then lead to immune cell infiltration from the intestinal epithelium to the white adipose tissue and initiate low-grade systemic inflammation []. Increased abundance of the protein zonulin in feces is a marker of abnormally increased gut permeability and disturbed gut barrier function. Recent evidence demonstrates that fermented dairy products, such as kefir, can reduce intestinal permeability and gut tight junction dysfunction [,], although this does not necessarily result in beneficial changes in serum pro-inflammatory markers [].
The bioactive compounds in fermented foods can also exert beneficial effects on adipose tissue function via upregulation of the peroxisome proliferator activator receptor γ 2 (PPARγ2) []. Excess fat accumulation in white adipose tissue and reduced lipid turnover lead to increased macrophage infiltration and pro-inflammatory cytokine overproduction that predispose to metabolic dysregulation. PPARγ2 suppresses resistin expression in white adipocytes and improves insulin sensitivity []. Activation of PPARγ2 also reduces plasma free fatty acid concentrations (partly because of enhanced insulin-mediated suppression of lipolysis) and improves the plasma lipid profile. Furthermore, it suppresses pro-inflammatory mediators and increases macrophage function in visceral adipose tissue [].
Understanding the effects of fermented foods on the composition and function of the microbiome can contribute to better nutritional therapies for long-term body weight homeostasis. Since many individuals with obesity can lose weight but cannot maintain it for prolonged periods of time, finding safe and long-term dietary treatments has been of great interest []. Fermented foods produce SCFAs in the gastrointestinal tract that can regulate the intestinal microbiome, inhibit inflammatory pathways, and reduce appetite hormones [,]. Furthermore, fermented foods may contain live microorganisms or beneficial bioactive compounds that support the symbiosis between the host and the microbiome, resulting in a healthy gut environment [,].
SCFAs, including acetate, propionate, and butyrate, are the products of fermentation of dietary non-digestible carbohydrates by gut bacteria. They can inhibit lipid synthesis enzymes, reduce pathogen microorganisms, and supply energy for the intestinal epithelium []. SCFAs and other primary metabolites of live probiotics from fermented foods can stimulate the growth of beneficial microbial phyla in the gut; for example, they lower intestinal luminal pH and improve conditions for intestinal commensal microflora like Bacteroides [] and Prevotella [] species.
3. Mechanisms of Action of Fermented Foods in Obesity
There are various types of traditional fermented foods including dairy products, fruit juice, and foods of mixed composition. Although the molecular mechanisms for the observed clinical outcomes linked to consumption of distinct types of fermented food products are too broad to classify in just a few general categories, the major modes of action are summarized in the following sections and are illustrated in Table 1. To include data on the efficacy of specific fermented foods on body weight, fat mass and food intake, studies were selected according to the primary outcome considering the biological pathways relevant to metabolic regulation, immune activation, and intestinal microbiome modulation. Several molecular biomarkers have been studied in the selected clinical trials, experimental animal studies and in-vitro experiments. In most of the included studies, multiple mechanisms of action were evaluated in response to interventions with fermented food products.

Table 1.
Summary of the effects of kefir products on obesity and mechanisms of action.
3.1. Inhibition of Lipid Synthesis in the Liver and Other Metabolic Organs
Current evidence suggests some lactic acid bacteria, such as Lactobacillus plantarum, have anti-obesity effects; however, their mode of action is not adequately identified. A mixture of L. plantarum species can reduce fat synthesis and storage, the gene expression of adenosine monophosphate-activated protein kinase-α (AMPK-α), fatty acid synthetase (FAS), acetyl CoA carboxylase (ACC), and peroxisome proliferator-activated receptor-γ (PPAR-γ). At the same time, serum concentrations of total and LDL-cholesterol and triglycerides decrease and HDL-cholesterol increase []. Among over 400 strains of LABs, the Lactobacillus plantarum strain Ln4 and fermented foods containing this LAB inhibit lipid storage and adipocyte differentiation, body weight, lipid accumulation, and insulin resistance through suppression of adipokine proteins such as ANGPT-L3 (angiopoietin-like), C-reactive protein (CRP), leptin, lipocalin-2, monocyte chemoattractant protein-1 (MCP-1), and insulin-like growth factor binding proteins (IGFBPs) in white adipose tissue. They also stimulate glucose uptake by upregulation of the gene expression of hepatic lipid metabolism factors including lipoprotein lipase (LPL), insulin receptor substrate 2 (IRS2), protein kinase Bβ (Akt2), and AMPK in preadipocytes. Ln4 is a common probiotic in kimchi, a Korean fermented food, and was separated from kimchi culture that has anti-diabetic and anti-obesity effects in experimental studies []. Other types of traditional fermented foods also exhibit lipid-lowering effects. Another common fermented food in Korea is kochujang. It is fermented red pepper, soybean, and rice. Fermented kochujang can upregulate acyl-CoA synthetase (ACS), carnitine palmitoyl transferase-1 (CPT-1), and uncoupling protein-1 (UCP-1) and downregulate ACC gene expression; these changes in gene expression and subsequent protein function can result in weight reduction [].
Pumpkin (Cucurbita moschata) seeds have been used for centuries in the Native American medicinal food culture for the reduction of blood glucose, cholesterol, hypertension, and the treatment of intestinal infections and disorders []. Fermentation is an effective treatment to reduce anti-nutritional factors of fresh pumpkins. The fermented pumpkin with LAB reduces body weight, body fat content, plasma lipid profile, and liver enzymes by suppressing gene expression of lipogenic factors including PPAR-γ, CCAAT-enhancer-binding proteins (C/EBPα, C/EBPβ, C/EBPγ), and sterol regulatory element-binding transcription factor 1 (SREBP1C) []. Not only non-pathogenic LAB but also fungi exhibit beneficial effects on metabolism. For example, koji, a Japanese cultivated cooked rice with the non-pathogen fungus Aspergillus oryzae contains high levels of a growth-boosting metabolite named glucosylceramide, which enhances some beneficial gut bacteria like Blautia coccoides []. Koji glycosylceramide (KGC) increases gene expression of CYP7A1 and ABCG8, which are important in cholesterol catabolism and its excretion in the form of bile acids []. Fermentation with aspergillus strains produces metabolites like free sugars, amino acids, proteins, fibers, and polyphenolic compounds []. Okara is a Japanese soy food product that has been fermented by probiotic aspergillus strains from the koji culture []. Short-term consumption of okara can reduce body weight, fat content, improve blood and liver lipid profile, and lipid metabolism; however these changes occur without a significant effect on SCFAs in the intestine [].
Concurrent intake of antioxidants and fermentation metabolites can potentially boost metabolic effects. For instance, fermented gallate-containing green tea inhibits adipocyte differentiation without induction of apoptosis and reduces body weight without any change in calorie intake and hepatic cytotoxicity, suggesting that these effects are not dependent on appetite regulation and liver damage pathways []. Regardless of whether the fermented food contains live probiotic microorganisms or not, beneficial effects have been demonstrated in several experimental and in-vitro studies, thus implicating several metabolites from fermented food in the regulation of lipid metabolism pathways in the liver and adipose tissue that eventually lead to weight reduction.
3.2. Reduction of Appetite Hormones
Appetite control is a key factor in obesity management. Food intake and total energy expenditure are important for long-term body weight homeostasis and can be regulated by multiple physiological pathways []. Peripheral regulation of appetite consists of adipose-related factors including insulin, leptin, and adiponectin, as well as a variety of intestinal peptide hormones secreted in response to food ingestion []. Besides total calorie intake, nutrient selection can influence the regulation of hunger and satiety. Various nutrients in the food matrix produce a complex set of metabolites in the gastrointestinal tract that can affect liver, intestine, and adipose tissue metabolism and can subsequently have a profound impact on appetite-related neurotransmitters in the hypothalamus [].
Fermented dairy products slow gastric emptying, so they can influence postprandial responses like those of blood glucose, insulin, and lipids. Sangaard et al. reported a lower gastric emptying effect of fermented milk A38 due to its higher viscosity. A38 intake resulted in a greater increase but also a faster decrease in serum triglyceride concentration in all lipoprotein fractions. The same trajectory pattern (greater peak but faster decrease) was observed for cholecystokinin (CCK) and peptide YY (PYY), gastric inhibitory polypeptide (GIP), and glucagon-like peptide-1 (GLP-1). Increased GIP levels after A38 consumption can be a result of GIP response to dietary intake of fat in conjunction with slower gastric emptying. The increased postprandial CCK levels can be related to the response to the high content of whey protein. Although satiety hormones were significantly elevated, the subjective satiety sensation (self-reported on a visual analogue scale) did not change []. Likewise, a study on the satiety effects of fermented soy meal (tempeh) on appetite hormones and subjective appetite scores reported significant effects on ghrelin, insulin, and arginine, but the satiety, fullness, and hunger were not different between fermented and unfermented meals []. By contrast, a study with propionate fermented milk drink reported a significant effect on fullness, hunger, and calorie intake; however, this was a short-term effect of up to 50 min after eating [].
Another interesting study evaluated the role of fermented food as a compound delivery system. The researchers investigated whether emulsified lipid Fabuless (Olibra) can reduce dietary energy intake and improve weight management. The findings revealed a non-significant effect of Olibra, separately or with solid food, on postprandial satiety sensation. On the other hand, Olibra plus yogurt was effective in reducing appetite. The anorectic effect of Olibra was enhanced with yogurt consumption by a longer transit time from the mouth to the large intestine [].
SCFAs are the commonest metabolites of fermented foods, and they have an appetite-suppressing effect [,]. The mechanism of action of SCFAs like acetate in appetite control is not only through activation of acetyl CoA carboxylase (ACC) and peripheral inhibition of energy intake by satiety neuropeptides, but also through a central modulation of the hypothalamus and GABAergic neurons to regulate appetite []. Johansson et al. evaluated the appetite-reducing effect of fermented whole grain bread compared to unfermented whole grain, and refined products. Both fermented and unfermented whole grain products produced significant changes in fullness, hunger, and insulin postprandial responses []. In that study, the content of dietary fiber was a principal factor driving satiety, implying that the metabolites produced by fermentation were not sufficient to influence satiety. Another study from the same research team reported a significant effect of fermented sourdough rye bread on appetite. Although the mode of action is not clear, it was suggested that fermentation of sourdough can break down the viscous fibers and enhance the bolus dissolution compared to yeast-fermented rye bread []. Nevertheless, another study by the same researchers reported the opposite result, i.e., that sourdough does not have any effect on appetite and subsequent dietary intake. Despite a significant effect of sourdough on protein aggregation, the acidity was not enough in sourdough bread samples and the content of rye in different test pieces of bread was not significantly different. The lack of an effect on appetite might be related to relatively low doses of consumed rye in all groups []. Due to these controversial results, it is a bit early to draw a conclusion about the effect of fermented foods on appetite hormones and weight homeostasis. Further research is needed on the role of fermented foods on appetite regulation and weight management.
3.3. Inhibition of Proinflammatory Cytokines
Excessive accumulation of white adipose tissue demonstrates a dysregulated pattern of secretion of adipokines associated with abnormal inflammatory cytokine profile and chronic inflammation in obesity [,]. The imbalance between pro-inflammatory and anti-inflammatory cytokines in adipose tissue in obesity triggers dysregulated energy homeostasis and elevated numbers of inflammatory immune cells including M1 macrophages, neutrophils, mast cells, CD8+ T cells, and Th1 cells []. A chronic inflammatory state leads to elevated lipolysis in other tissues like muscle, liver, and pancreas, eventually followed by lipotoxicity and insulin resistance [,].
LAB strains separated from fermented dairy products have anti-inflammatory and anti-obesity effects. A mixture of LAB strains from Mozzarella di Bufala Campana (1 × 109 CFU/day) increased the number of immune-regulatory leukocytes like CD4+ T lymphocytes, CD4+ CD25+ Treg cells, and decreased pro-inflammatory leukocytes including CD8+ T lymphocytes, CD11b+ activated leukocytes, and F4/80+ macrophages during weight reduction [].
Supplementation with probiotic yogurt containing L. delbrueckii subsp. bulgaricus Streptococcus thermophilus and probiotic Bifidobacterium animalis (1 × 108 CFU/day) for 60 days elevates IgA+ cells in the intestine and reduces body weight. The IgA+ cells secrete immunoglobulin A which plays a crucial role in maintaining the efficiency of the epithelial barrier. In addition, increased levels of IL-10 could suppress inflammation in the intestinal epithelium [].
Sichuan pickles are a traditional food in China that is fermented by lactic, acetic, and ethanol pathways, thereby leading to variable characteristics and chemical features of vegetable pickles. The most abundant metabolites in sichuan are volatile organic products. The concentration of acid, ester, aldehyde, and alkenes depends on the bacterial diversity in the sichuan fermentation process []. Lactobacillus fermentum isolated from sichuan pickle can upregulate PPAR-α and downregulate PPAR-γ, suppress adipose tissue enlargement, and improve blood lipoprotein and liver damage markers []. Lactobacillus acidophilus SJLH001 (La-SJLH001), another strain isolated from Chinese fermented foods, has a potential regulatory role in glucose and cholesterol metabolism in obesity and metabolic dysfunction. It reduces serum glucose and total cholesterol concentrations and downregulates pro-inflammatory genes including CD36, Reg3γ, TLR2, and PPAR-α []. TLR2 gene expression is associated with increased innate immune response in immune disorders and type 2 diabetes as TLR2 levels are inversely associated with glucose transport [,]. The downregulation of this gene can prevent metabolic dysfunction and immune complications in obesity.
Exercise has been proposed to improve glucose tolerance and body fat-free mass; however, increases oxidative stress damage and pro-inflammatory markers in the muscle. Fermented foods in combination with exercise may exert enhanced beneficial effects on calorie intake and body fat mass as well as immune-related myokines compared to separate administration of fermented food or exercise alone [,]. Fermented soy alone reduces body fat and expression of TLR4, MyD88, and IL-6 in muscle cells compared to resistance exercise alone or control group. Fermented soy in addition to resistance exercise can compensate the adverse effects of training on inflammatory TLR-4, but the findings were not promising for all the pro-inflammatory factors (86).
There is also evidence that fermented foods may have a dose-dependent effect in weight control, glucose and lipid homeostasis, and innate immunity. For example, high-dose fermented mixed grains plus digestive enzymes reduce body weight, adiposity, leptin, keratinocyte chemo-attractant (KC), interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), and MCP-1 when compared to low doses [].
The beneficial role of fermented foods is not limited to the probiotics in these products. The modified proteins in the fermented foods can regulate immune responses through inhibition of inflammatory cytokines (MCP, IL-1β, and INF-γ) in the liver as well as improved gut microbiota and gene expression patterns of adhesion molecules [].
Fermentation of food byproducts may have both economical and health-related benefits. Citrus species are rich in ascorbic acid, citric acid, phenolics, and several bioactive compounds. Lemon (Citrus limon) peel is a byproduct that has antioxidant and anti-inflammatory characteristics []. Fermentation of lemon peel can increase the bioavailability and edibility of bioactive compounds, and can reduce body weight, adipose tissue, hepatic damage enzymes, and inflammatory cytokine gene expression in the liver and epididymal adipose tissue, leading to lower serum concentrations of inflammatory cytokines. HPLC analyses identified an array of different compounds in the fermented lemon peel like Vitexin, Cnidicin, and Byakangelicin, which have shown antioxidant properties that may be responsible for the observed beneficial effects [].
Vinegar is a product of microbial fermentation that has been used as an additive in many cuisines. Long-term administration of Nipa vinegar markedly reduces weight, lipid storage, and inflammatory markers, while enhancing gut microflora and serum adipokine levels []. Ginseng vinegar has also demonstrated anti-obesity and anti-inflammatory effects through reduction of liver and serum lipids, TNF-α, and IL-6 [].
Metabolites derived from fermented foods can trigger or modulate immune responses, enhance production of pro- and anti-inflammatory cytokines and chemokines, initiate microbial killing processes and generally affect signaling pathways related to oxidative stress and inflammation [,]. Although there is not enough evidence for every metabolite derived from fermented foods in the regulation of innate and adaptive immunity, there is a growing body of evidence suggesting an important role of these bioactive compounds in the management of obesity and obesity-related metabolic dysfunction.
3.4. Improved Glucose Metabolism
Fermented foods have a stimulatory effect on glucose metabolism via upregulation of glucose transport in the intestinal epithelium and the liver, increased glucose catabolism pathways, and inhibition of oxidative stress damage []. The beneficial effect is not only linked to the bioactive compounds alone, but also to the health-promoting effects of microbial metabolites in the intestine that can regulate intestinal microflora and upregulate glucose transporters to attenuate insulin resistance and adipogenesis []. Probiotic microbes like LAB isolated from fermented foods exhibited anti-obesity and anti-diabetic roles in both in-vitro and in-vivo experiments.
Lactobacillus plantarum Ln4 (Ln4) is a common probiotic in Korean fermented foods and some pickles and improves oral glucose tolerance and insulin tolerance via increased cellular glucose uptake and decreased protein expression of MCP-1 and IGFBP-3, as well as decreased hepatic mRNA expression of IRS2 and AMPK.
Several plants that have been used for the treatment of diabetes may exhibit stronger effects after fermentation. Although there are few studies about the anti-obesity effects of fermented medicinal plants, the current evidence is promising. Vaccinium angustifolium Ait. (Canadian lowbush blueberry) has been used as a traditional treatment for diabetes in Canada. Fermentation with a natural blueberry flora bacteria called Serratia vaccinii enhances the biological activities of antioxidant compounds in the lowbush blueberry, including phenolic compounds, by modification of phenolic chemical structure and production of gallic acid []. Administration of mature fermented blueberry juice demonstrates insulin-like and glitazone-like properties on myotubes and adipocytes by activation of PPAR-γ, AMPK, and translocation of GLUT4; however, the calcium-dependent glucose transport and insulin-related glucose metabolism pathways were not affected []. Since overeating results in abnormally increased glucose uptake and consequently, oxidative stress damage in the adipocytes by activation of protein kinase c-δ [], the regular intake of fermented medicinal plants and fruit juices may reverse cellular damage [].
The bioavailability of bioactive compounds greatly influences the effectiveness of treatment with medicinal plants, as the phenolic fraction of fermented blueberry juice with Serratia vaccinii contains catechol, chlorogenic, gallic, and protocatechuic acids that have the greatest effect on glucose-6-phosphatase levels and glucose uptake in hepatic (H4IIE, HepG2) and skeletal muscle cells (C2C12) []. Considering there are various types of fermented foods that can modulate glucose uptake and metabolism, the number of studies in this field is relatively low and there is a significant knowledge gap regarding the clinical efficacy of fermented plant products in the long-term glucose homeostasis and insulin resistance.
3.5. Modulation of Gut Microbiome
There are several studies indicating that regular consumption of fermented foods modulates intestinal microflora, but not all studies aimed to elaborate the relevant mechanisms(s) of action in subjects with overweight or obesity. Moreover, few studies have been conducted in human subjects for an adequately long period of time to analyze the abundance and diversity of microbiota and mycobiota and their association with gut-brain axis and appetite control.
Consumption of fermented foods can increase Prevotella, Bacteroides, Lactobacillus, Leuconostoc spp., and other beneficial microbial populations in the gut. Fermented foods can also affect the gene expression of enzymes that can regulate metabolic pathways of lipid synthesis and blood pressure in the host, for example, kimchi can enhance levels of Acyl-CoA synthetase long-chain family member 1 (ACSL1) through modulation of gut microbiota that inhibits triglyceride synthesis and enhances fatty acid catabolism. In addition, increased mRNA levels of aminopeptidase N (ANPEP) are inversely associated with hypertension, angiogenesis, and inflammation; this may explain the beneficial role of regular intake of kimchi on lipid profile and blood pressure [].
The microbial community of fermented foods likely determines their anti-obesity characteristics. Administration of a fermented food may exert significant changes in energy metabolism through modulation of the gut microbial population, including increased abundance of Muribaculaceae, and decreased abundance of Akkermansiaceae, Coriobacteriaceae, and Erysipelotrichaceae []. Although the modulation of gut microbiome has not been reported consistently in studies with fermented foods, the majority reveal a beneficial role in the abundance and diversity of various species in the intestine. For example, fermentation of green tea by Bacillus subtilis reduces Firmicutes/Bacteroidetes and Bacteroides/Prevotella ratios in obesity; in fact the Bacteroides/Prevotella ratio shifts towards values observed in lean subjects []. Inflammatory markers, lipid profile, and adiposity were changed after the intervention, and the probable mechanism underlying these observations is likely the modulation of intestinal microflora. Fermentation of herbal teas enhances some gut commensals like Akkermansia and Faecalibaculum, and reduces intestinal permeability and oxidative stress markers, in line with an improved microbiome profile [].
Some grain-like cereals are rich in dietary fiber, protein, and minerals and have low digestibility []. Fermentation increases the solubility of dietary fiber and its digestibility, increases the abundance of Proteobacteria, Actinobacteria, and Bacteroidetes, and reduces Firmicutes, Clostridiales, Lachnospiraceae, and Akkermansia. The abundance of various genera of the microbiome is distinct and can be associated with the biological pathways affected by different bioactive compounds. The resistant starch content is high in some types of cereals and fermentation may enhance the beneficial effects of a functional food via reduced abundance of endotoxins and pathogens in the intestine, and changes in peripheral blood metabolites [,]. In some studies, although the ratio of Firmicutes to Bacteroidetes did not change significantly, the abundance of other intestinal bacterial groups such as Akkermansia and Bacteroides and SCFAs levels were augmented []. The administration of several types of fruit juice has beneficial effects on intestinal microbiota, but the effect of fermented juice was more distinct [] and the correlation between the relative abundance of Firmicutes to Bacteroidetes with obesity markers was strong []. Not only anthropometric parameters but also several metabolic pathways such as the citrate cycle, glycerophospholipid, amino acid, and pyrimidine metabolic pathways can be improved by the modulation of the gut microbiome with fermented foods [].
The mode of anti-obesity action of fermented foods is not only related to Firmicutes/Bacteroidetes and Bacteroides/Prevotella ratios but can also be related to other bacterial phyla like Lachnospiraceae and Ruminococcaceae, which can be affected by Lactobacillus plantarum HAC01 (HAC01) and L. rhamnosus GG (LGG) intake from fermented foods like kimchi. Despite Lachnospiraceae being a member of the Firmicutes family, a higher abundance ratio has been associated with the anti-obesity effects of kimchi [].
Another example of altered bacterial phyla in the intestine is the Streptococcus genus. Intake of either fermented dairy by Lactobacillus helveticus or Greek yogurt increases abundance of Streptococcus []. Fermented protein consumption in both types increases gut microbial diversity and abundance, including the Streptococcus genus that is not dependent on the LDL receptor gene, although some genera, such as Akkermansia, Adlercreutzia, and Dubosiella, had a higher abundance in the wild-type genotype [].
Few studies investigated the effect of fermented foods on the intestinal mycobiota as well as microbiota. For example, kefir administration to C57BL/6 mice for 12 weeks markedly augmented not only LAB, but also Candida spp. and Saccharomyces spp []. Although there is some evidence for health-promoting effects of probiotic yeast species, such as saccharomyces boulardii, the molecular mechanisms are not well studied, and more research is needed to better understand the underlying biological mechanisms. In addition, it not completely clear whether the health promoting effects of fermented foods originate from the bioactive compounds, such as dairy peptides, or the microbial content of fresh fermented food. The reason behind the beneficial roles of fermented foods through the intestinal microbiome can be related to some unknown molecular mediators that lead to anti-obesity outcomes. Figure 1 and Figure 2 summarize the main effects of fermented foods on body weight and obesity through various mechanisms in cell culture and animal studies, respectively. In Figure 1, the effects of various fermented foods on triglyceride levels and glucose uptake in the cells and the expression of inflammatory and metabolic markers have been outlined in the in-vitro cell assays. In Figure 2, the main findings of fermented foods on the anthropometric and metabolic profile, antioxidant status, and the expression of immune and metabolic markers in three different animal species have been illustrated briefly.
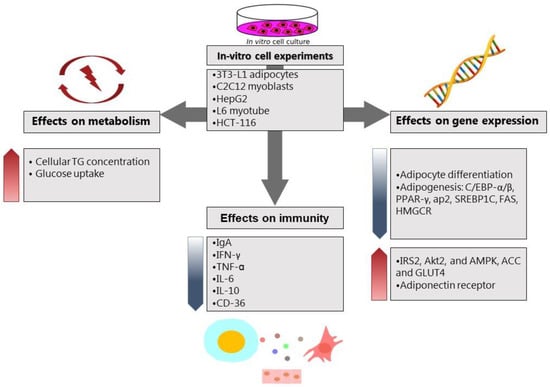
Figure 1.
The effects of various fermented foods on metabolism, immunity, and gene expression of metabolic factors in cell culture studies. Abbreviations: TG: triglyceride, IgA: immunoglobulin A, IFN-γ: interferon-γ, TNF-α: tumor necrosis factor-α, IL-6: interleukin-6, IL-10: interleukin-10, CD-36: cluster of differentiation-36, C/EBP-α/β: CCAAT/enhancer-binding protein-α/β, PPAR-γ: Peroxisome proliferator-activated receptor-γ, AP-2: adaptor protein complex-2, SREBP-1c: sterol regulatory element-binding transcription factor 1, FAS: Fas cell surface death receptor, HMGCR: HMG-CoA reductase, IRS-2: insulin receptor substrate 2, AKT-2: serine/threonine kinase, AMPK: AMP-activated protein kinase, ACC: acetyl-CoA carboxylase, GLUT4: insulin-regulated glucose transporter.
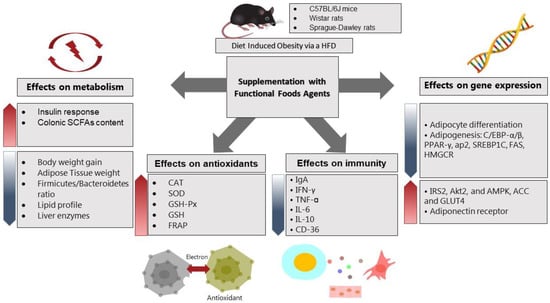
Figure 2.
The effects of various fermented foods on metabolism, antioxidant status, immunity, and gene expression of metabolic factors in three different animal models. Abbreviations: SCFAs: short chain fatty acids, CAT: catalase, SOD: superoxide dismutase, GSH-PX: glutathione peroxidase, GSH: glutathione, FRAP: fluorescence recovery after photobleaching, IgA: immunoglobulin A, IFN-γ: interferon-γ, TNF-α: tumor necrosis factor-α, IL-6: interleukin-6, IL-10: interleukin-10, CD-36: cluster of differentiation-36, C/EBP-α/β: CCAAT/enhancer-binding protein-α/β, PPAR-γ: peroxisome proliferator-activated receptor-γ, AP-2: adaptor protein complex-2, SREBP-1c: sterol regulatory element-binding transcription factor 1, FAS: Fas cell Surface death receptor, HMGCR: HMG-CoA reductase, IRS-2: insulin receptor substrate 2, AKT-2: serine/threonine kinase, AMPK: AMP-activated protein kinase, ACC: acetyl-CoA carboxylase, GLUT4: insulin-regulated glucose transporter.
4. Challenges and Risks of Fermented Foods in the Prevention and Treatment of Obesity
Several factors, including genetic susceptibility and environmental exposure to different chemical, biological, and socio-economic factors, can influence the magnitude of response to any dietary intervention. The baseline characteristics of participants have sometimes been used to distinguish between healthy and unhealthy groups, but this may not be a reliable way of classification of inter-individual variation []. The relative abundance of Bacteroidetes:Firmicutes is a common marker to compare gut microbiota in response to lifestyle intervention in individuals with obesity, but there is no fixed value for this ratio that can be used to evaluate the effect of an intervention or differences between groups. A solution to reduce inter-individual variation can be the classification of participants according to the genetic background, dietary history, antibiotic use, etc. [].
The richness and diversity of microbial phyla can change in response to ingestion of fermented foods or the combination of fermented foods with polyphenols or micronutrients. Although functional microbiology studies report significant changes after dietary interventions, it is not clear how much of this variation occurs normally and how much can be related to the effect of the administration of a specific bioactive compound or food, because the intestinal microbiome is a dynamic population, and several intrinsic and extrinsic factors play a role to maintain the balance among thousands of groups of microbial species.
Few studies have investigated the effect of fermented foods on the gut microbial eukaryote. Some concerns have been raised in this regard: the number of biological specimens, for example, feces, may be not sufficient to analyze mycobiota and targeted metagenomics assays function more efficiently in larger samples. Moreover, there is no stable and core library of fungal communities from the human gut, which makes it difficult to interpret relevant data []. Another point about mycobiota studies is that the variation of fungal communities in response to environmental changes or dietary interventions is low and requires a longer duration of intervention to observe significant changes. The effect of fungal metabolites on the richness and diversity of gut microbiome has not been studied well and the interaction between eukaryotic species with prokaryotic species requires further research [].
The interventional studies with fermented foods are highly heterogeneous and there are few randomized controlled trials with a specific fermented food. This makes it difficult to pool data in a systematic manner and draw robust conclusions. Still, the heterogeneity of available clinical trials or animal experiments can be helpful to elucidate molecular mechanisms, map the pros and cons of each type of fermented food, and design better experiments for future research in this area [].
The length of the experiments is also another factor that may be not sufficient to improve clinical outcomes, such as body weight and body fat mass in a 3-month or 6-month period. The primary outcomes in most studies include weight loss, fat mass, and food intake that depend on multiple factors and confounders that are difficult to control in clinical settings.
The amount of food consumed can also affect the findings. There are several doses of diverse types of fermented foods, drinks, or gavages that are not justified well and need more homogenous studies and systematic testing to pool data and make better sense of dose-response relationships.
Despite most of the experimental studies in animal models of obesity being methodologically sound, the reproducibility and generalizability of the findings have been considered to a limited extent. The precision, high throughput techniques and availability of biopsy tests and various specimens in animal models are undeniable []; however, most biomedical phenomena observed in homogenous animal models cannot be translated to the heterogeneous human populations or subgroups who are overweight or obese.
Another methodological concern is the sample size calculation of the experimental and clinical studies, particularly when more than two interventions are compared among more than two groups. The power of the study might be reduced due to low sample size, and multiple test errors should be corrected by the proper statistical tests such as adjusted p-value post hoc tests and false positive correction tests [].
It must also be noted that fermented foods are not completely safe. Fermentation of high-protein foods can produce biogenic amines that are hazardous for health. Fermented foods including dry-cured meat, fermented fish, and fermented legumes can produce N-nitroso compounds in the body, which have been linked to cancer []. The biogenic amines are classified into two groups: monoamines (e.g., tyramine) and diamines (e.g., histamine) []. Several studies have shown the detrimental effect of considerable amounts of biogenic amines in the body such as headache, increased blood pressure, stomach pain, and cancer []. The conservation condition of fermented foods plays a significant role in the concentration of toxic biogenic amines in the food products, for example, regular intake of canned fermented Chinese meat and soy products has been associated with gastric and esophageal cancer in low-income populations due to frequent consumption of canned fermented foods [].
Another unhealthy compound in fermented foods is their salt content. The amount of salt in some traditional fermented foods, particularly Asian soy paste, and cured meat and seafood, is too high and may contribute to carcinogenesis and other adverse health outcomes [,]. Reduction of salt content in the traditional fermented foods can be a major challenge for food safety and palatability, as salt inhibits the growth of pathogens and production of their toxic metabolites such as mycotoxins, nitrosamines, and ethyl carbamate. In addition, salt improves the texture, aroma, and taste of fermented foods as the lower salt content is associated with higher sourness and more shapeless food texture that decreases food popularity and acceptability among consumers []. Use of high throughput molecular technologies and novel treatment techniques for preservation of food products can be useful to reduce the amount of salt in fermented food processing in the food industry. Although alternative techniques, such as gamma-irradiation, microencapsulation, ultrasound, and high-pressure treatment, to prevent spoilage in the fermented foods are in early stages of development, the preliminary results seem promising [,,,].
5. Conclusions
Regular consumption of fermented foods can exert beneficial effects on body weight regulation and metabolic function through several mechanisms. Although the level of evidence for some types of fermented foods is still very low and there are considerable knowledge gaps regarding the molecular mechanisms of action of specific fermented foods, the current findings are promising. This may be especially relevant if personalized dietary interventions can be designed to include individual variation and consider multiple factors like genetic, immune, and neurological variation. Compliance to treatment is a key factor to obtain optimal results in intervention studies, and since most fermented foods are integral in the traditional cuisine in most countries, their use as a drug delivery system may increase chances of success in the long-term. Finally, analyzing omics data in addition to clinical outcomes can result in a deeper understanding of the mechanisms of action and the links among metabolism, immune responses, and eating behavior.
Author Contributions
Conceptualization, M.J., F.M.; writing—original draft preparation, M.J. and M.N., writing—review, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge Department of Nutrition, Exercise and Sports for kind administrative support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kopelman, P.G. Obesity as a Medical Problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Possible Anti-Obesity Therapeutics from Nature—A Review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Capanoglu, E.; Pateiro, M.; Mousavi Khaneghah, A.; Hano, C.; Lorenzo, J.M. Current Trends in Passiflora Genus Research: Obesity and Fermented Foods Systematic Review. Trends Food Sci. Technol. 2022, 127, 143–155. [Google Scholar] [CrossRef]
- Oh, I.; Baek, E.J.; Lee, D.H.; Choi, Y.H.; Bae, I.Y. Anti-Obesity and Anti-Inflammatory Effects of Ginseng Vinegar in High-Fat Diet Fed Mice. Food Sci. Biotechnol. 2019, 28, 1829–1836. [Google Scholar] [CrossRef]
- Pan, Y.; Tan, J.; Long, X.; Yi, R.; Zhao, X.; Park, K.-Y. Anti-Obesity Effect of Fermented Lemon Peel on High-Fat Diet-Induced Obese Mice by Modulating the Inflammatory Response. J. Food Biochem. 2022, 46, e14200. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, J.; Jin, H.; Shan, Y.; Fang, J.; Liu, F. Beneficial Impacts of Fermented Celery (Apium Graveolens L.) Juice on Obesity Prevention and Gut Microbiota Modulation in High-Fat Diet Fed Mice. Food Funct. 2021, 12, 9151–9164. [Google Scholar] [CrossRef]
- Baruah, R.; Kumar, K.; Goya, A. Functional Foods and Their Health Benefits. In High Value Fermentation Products; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 127–145. ISBN 9781119555384. [Google Scholar]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Nakamura, R.E.; Nguyen, J.G.; Michels, K.B. Does Consumption of Fermented Foods Modify the Human Gut Microbiota? J. Nutr. 2020, 150, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Baruah, R.; Ray, M.; Halami, P.M. Preventive and Therapeutic Aspects of Fermented Foods. J. Appl. Microbiol. 2022, 132, 3476–3489. [Google Scholar] [CrossRef]
- Babuchowski, A.; Laniewska-Moroz, L.; Warminska-Radyko, I. Propionibacteria in Fermented Vegetables. Lait 1999, 79, 113–124. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Beena, D.J.; Vasanthakumari, D.S.; Ismail, B. Health Benefits of Exopolysaccharides in Fermented Foods. In Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 49–62. [Google Scholar]
- Sievers, M.; Swings, J. Acetobacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; pp. 1–7. [Google Scholar]
- Masood, M.I.; Qadir, M.I.; Shirazi, J.H.; Khan, I.U. Beneficial Effects of Lactic Acid Bacteria on Human Beings. Crit. Rev. Microbiol. 2011, 37, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, A.G. Drug Delivery Systems Using Pullulan, a Biocompatible Polysaccharide Produced by Fungal Fermentation of Starch. Environ. Chem. Lett. 2019, 17, 1209–1223. [Google Scholar] [CrossRef]
- Nooshkam, M.; Babazadeh, A.; Jooyandeh, H. Lactulose: Properties, Techno-Functional Food Applications, and Food Grade Delivery System. Trends Food Sci. Technol. 2018, 80, 23–34. [Google Scholar] [CrossRef]
- Thakkar, P.; Modi, H.; Dabhi, B.; Prajapati, J. Bile Tolerance, Bile Deconjugation and Cholesterol Reducing Properties of Lactobacillus Strains Isolated from Traditional Fermented Foods. Int. J. Fermented Foods 2014, 3, 157. [Google Scholar] [CrossRef]
- Haller, D.; Colbus, H.; Gänzle, M.G.; Scherenbacher, P.; Bode, C.; Hammes, W.P. Metabolic and Functional Properties of Lactic Acid Bacteria in the Gastro-Intestinal Ecosystem: A Comparative In Vitro Study between Bacteria of Intestinal and Fermented Food Origin. Syst. Appl. Microbiol. 2001, 24, 218–226. [Google Scholar] [CrossRef]
- Adalsteinsdottir, S.A.; Magnusdottir, O.K.; Halldorsson, T.I.; Birgisdottir, B.E. Towards an Individualized Nutrition Treatment: Role of the Gastrointestinal Microbiome in the Interplay Between Diet and Obesity. Curr. Obes. Rep. 2018, 7, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Kinross, J.M.; Darzi, A.W.; Nicholson, J.K. Gut Microbiome-Host Interactions in Health and Disease. Genome Med. 2011, 3, 14. [Google Scholar] [CrossRef]
- Morgan, X.C.; Segata, N.; Huttenhower, C. Biodiversity and Functional Genomics in the Human Microbiome. Trends Genet. 2013, 29, 51–58. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S. Gut Commensal Bacteroides Acidifaciens Prevents Obesity and Improves Insulin Sensitivity in Mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef]
- Lee, Y.; Cha, Y.-S.; Park, Y.; Lee, M. PPARγ2 C1431T Polymorphism Interacts with the Antiobesogenic Effects of Kochujang, a Korean Fermented, Soybean-Based Red Pepper Paste, in Overweight/Obese Subjects: A 12-Week, Double-Blind Randomized Clinical Trial. J. Med. Food 2017, 20, 610–617. [Google Scholar] [CrossRef]
- Rajala, M.W.; Qi, Y.; Patel, H.R.; Takahashi, N.; Banerjee, R.; Pajvani, U.B.; Sinha, M.K.; Gingerich, R.L.; Scherer, P.E.; Ahima, R.S. Regulation of Resistin Expression and Circulating Levels in Obesity, Diabetes, and Fasting. Diabetes 2004, 53, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Sam, S.; Mazzone, T. Adipose Tissue Changes in Obesity and the Impact on Metabolic Function. Transl. Res. 2014, 164, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Gérard, P. Gut Microbiota and Obesity. Cell. Mol. life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Alou, M.T.; Lagier, J.-C.; Raoult, D. Diet Influence on the Gut Microbiota and Dysbiosis Related to Nutritional Disorders. Hum. Microbiome J. 2016, 1, 3–11. [Google Scholar] [CrossRef]
- Barlow, G.M.; Yu, A.; Mathur, R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr. Clin. Pract. 2015, 30, 787–797. [Google Scholar] [CrossRef]
- Loera-Rodriguez, D.; Octavio, C.; Delgado-Rizo, V.; Alvarado-Navarro, A.; Agraz-Cibrian, J.M.; Segura-Ortega, J.E.; Fafutis-Morris, M. Over-Expression of TLR4-CD14, pro-Inflammatory Cytokines, Metabolic Markers and NEFAs in Obese Non-Diabetic Mexicans. J. Inflamm. 2014, 11, 39. [Google Scholar] [CrossRef]
- Neal, M.D.; Leaphart, C.; Levy, R.; Prince, J.; Billiar, T.R.; Watkins, S.; Li, J.; Cetin, S.; Ford, H.; Schreiber, A. Enterocyte TLR4 Mediates Phagocytosis and Translocation of Bacteria across the Intestinal Barrier. J. Immunol. 2006, 176, 3070–3079. [Google Scholar] [CrossRef]
- Teixeira, T.F.S.; Collado, M.C.; Ferreira, C.L.L.F.; Bressan, J.; Maria do Carmo, G.P. Potential Mechanisms for the Emerging Link between Obesity and Increased Intestinal Permeability. Nutr. Res. 2012, 32, 637–647. [Google Scholar] [CrossRef]
- Pražnikar, Z.J.; Kenig, S.; Vardjan, T.; Bizjak, M.Č.; Petelin, A. Effects of Kefir or Milk Supplementation on Zonulin in Overweight Subjects. J. Dairy Sci. 2020, 103, 3961–3970. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, S.; Xia, Y.; Huang, J.; Ye, J.; Xuan, Z.; Li, P.; Du, B. Probiotic-Fermented Black Tartary Buckwheat Alleviates Hyperlipidemia and Gut Microbiota Dysbiosis in Rats Fed with a High-Fat Diet. Food Funct. 2021, 12, 6045–6057. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.M.; Staels, B. Peroxisome Proliferator-Activated Receptor γ and Adipose Tissue—Understanding Obesity-Related Changes in Regulation of Lipid and Glucose Metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 386–395. [Google Scholar] [CrossRef]
- Soleymani, T.; Daniel, S.; Garvey, W.T. Weight Maintenance: Challenges, Tools and Strategies for Primary Care Physicians. Obes. Rev. 2016, 17, 81–93. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. Dietary Fibers and Their Fermented Short-Chain Fatty Acids in Prevention of Human Diseases. Bioact. Carbohydr. Diet. Fibre 2019, 17, 100170. [Google Scholar] [CrossRef]
- Youn, H.-Y.; Seo, K.-H.; Kim, H.-J.; Kim, Y.-S.; Kim, H. Effect of Postbiotics Derived from Kefir Lactic Acid Bacteria-Mediated Bioconversion of Citrus Pomace Extract and Whey on High-Fat Diet-Induced Obesity and Gut Dysbiosis. Food Res. Int. 2022, 162, 111930. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, N.; Ng, L.S.; Makino, S.; Goh, L.L.; Lim, Y.J.; Ferdinandus; Sasaki, H.; Shibata, S.; Lee, C.L.K. Solid-State Fermented Okara with Aspergillus Spp. Improves Lipid Metabolism and High-Fat Diet Induced Obesity. Metabolites 2022, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Daniel, N.; Nachbar, R.T.; Tran, T.T.T.; Ouellette, A.; Varin, T.V.; Cotillard, A.; Quinquis, L.; Gagné, A.; St-Pierre, P.; Trottier, J.; et al. Gut Microbiota and Fermentation-Derived Branched Chain Hydroxy Acids Mediate Health Benefits of Yogurt Consumption in Obese Mice. Nat. Commun. 2022, 13, 1343. [Google Scholar] [CrossRef]
- Noer, E.R.; Dewi, L.; Kuo, C.H. Fermented Soybean Enhances Post-Meal Response in Appetite-Regulating Hormones among Indonesian Girls with Obesity. Obes. Res. Clin. Pract. 2021, 15, 339–344. [Google Scholar] [CrossRef]
- Kim, E.; Lee, H.G.; Han, S.; Seo, K.H.; Kim, H. Effect of Surface Layer Proteins Derived from Paraprobiotic Kefir Lactic Acid Bacteria on Inflammation and High-Fat Diet-Induced Obesity. J. Agric. Food Chem. 2021, 69, 15157–15164. [Google Scholar] [CrossRef]
- Han, Y.; Shin, Y.C.; Kim, A.H.; Kwon, E.Y.; Choi, M.S. Evaluation of the Dose-Dependent Effects of Fermented Mixed Grain Enzyme Food on Adiposity and Its Metabolic Disorders in High-Fat Diet-Induced Obese Mice. J. Med. Food 2021, 24, 873–882. [Google Scholar] [CrossRef]
- Zhong, H.; Deng, L.; Zhao, M.; Tang, J.; Liu, T.; Zhang, H.; Feng, F. Probiotic-Fermented Blueberry Juice Prevents Obesity and Hyperglycemia in High Fat Diet-Fed Mice in Association with Modulating the Gut Microbiota. Food Funct. 2020, 11, 9192–9207. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, F.; Lu, J.; Shi, J.; Guan, J.; Yan, F.; Li, B.; Huo, G. Probiotic Mixture of Lactobacillus Plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front. Microbiol. 2020, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Tan, F.; Mu, J.; Yi, R.; Zhou, X.; Zhao, X. Anti-Obesity Effects of Lactobacillus Fermentum CQPC05 Isolated from Sichuan Pickle in High-Fat Diet-Induced Obese Mice through PPAR-α Signaling Pathway. Microorganisms 2019, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, Y.; Li, Z.; Yan, H.; Li, J.; Wan, X. Mechanism Analysis of Improved Glucose Homeostasis and Cholesterol Metabolism in High-Fat-Induced Obese Mice Treated with La-SJLH001 via Transcriptomics and Culturomics. Food Funct. 2019, 10, 3556–3566. [Google Scholar] [CrossRef] [PubMed]
- Hamajima, H.; Matsunaga, H.; Fujikawa, A.; Sato, T.; Mitsutake, S.; Yanagita, T.; Nagao, K.; Nakayama, J.; Kitagaki, H. Japanese Traditional Dietary Fungus Koji Aspergillus Oryzae Functions as a Prebiotic for Blautia Coccoides through Glycosylceramide: Japanese Dietary Fungus Koji Is a New Prebiotic. Springerplus 2016, 5, 1321. [Google Scholar] [CrossRef]
- Cho, D.; Jeong, H.W.; Kim, J.K.; Kim, A.Y.; Hong, Y.D.; Lee, J.H.; Choi, J.K.; Seo, D.B. Gallocatechin Gallate-Containing Fermented Green Tea Extract Ameliorates Obesity and Hypertriglyceridemia Through the Modulation of Lipid Metabolism in Adipocytes and Myocytes. J. Med. Food 2019, 22, 779–788. [Google Scholar] [CrossRef]
- Lee, E.; Jung, S.R.; Lee, S.Y.; Lee, N.K.; Paik, H.D.; Lim, S.I. Lactobacillus Plantarum Strain Ln4 Attenuates Diet-Induced Obesity, Insulin Resistance, and Changes in Hepatic MRNA Levels Associated with Glucose and Lipid Metabolism. Nutrients 2018, 10, 643. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jang, M.-S. Anti-Obesity Effects of Laminaria Japonica Fermentation on 3T3-L1 Adipocytes Are Mediated by the Inhibition of C/EBP-α/β and PPAR-γ. Cell. Mol. Biol. (Noisy-le-grand) 2018, 64, 71–77. [Google Scholar] [CrossRef]
- Hossain, M.A.; Lee, S.J.; Park, N.H.; Birhanu, B.T.; Mechesso, A.F.; Park, J.Y.; Park, E.J.; Lee, S.P.; Youn, S.J.; Park, S.C. Enhancement of Lipid Metabolism and Hepatic Stability in Fat-Induced Obese Mice by Fermented Cucurbita Moschata Extract. Evid.-Based Complement. Altern. Med. 2018, 2018, 3908453. [Google Scholar] [CrossRef]
- Roselli, M.; Devirgiliis, C.; Zinno, P.; Guantario, B.; Finamore, A.; Rami, R.; Perozzi, G. Impact of Supplementation with a Food-Derived Microbial Community on Obesity-Associated Inflammation and Gut Microbiota Composition. Genes Nutr. 2017, 12, 25. [Google Scholar] [CrossRef]
- Joung, H.; Kim, B.; Park, H.; Lee, K.; Kim, H.H.; Sim, H.C.; Do, H.J.; Hyun, C.K.; Do, M.S. Fermented Moringa Oleifera Decreases Hepatic Adiposity and Ameliorates Glucose Intolerance in High-Fat Diet-Induced Obese Mice. J. Med. Food 2017, 20, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Beh, B.K.; Mohamad, N.E.; Yeap, S.K.; Ky, H.; Boo, S.Y.; Chua, J.Y.H.; Tan, S.W.; Ho, W.Y.; Sharifuddin, S.A.; Long, K.; et al. Anti-Obesity and Anti-Inflammatory Effects of Synthetic Acetic Acid Vinegar and Nipa Vinegar on High-Fat-Diet-Induced Obese Mice. Sci. Rep. 2017, 7, 6664. [Google Scholar] [CrossRef] [PubMed]
- Balcells, M.F.; Mariani, C.; Weill, R.; Perdigon, G.D.V.; Maldonado Galdeano, M.C. Effect of Yogurt with or without Probiotic Addition on Body Composition Changes and Immune System in an Obese Model. J. Food Sci. Nutr. 2017, 3, 1–9. [Google Scholar]
- Wang, J.H.; Bose, S.; Kim, G.C.; Hong, S.U.; Kim, J.H.; Kim, J.E.; Kim, H. Flos Lonicera Ameliorates Obesity and Associated Endotoxemia in Rats through Modulation of Gut Permeability and Intestinal Microbiota. PLoS ONE 2014, 9, e86117. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Do, H.J.; Kim, O.Y.; Chung, J.H.; Lee, J.Y.; Park, Y.S.; Hwang, K.Y.; Seong, S.I.; Shin, M.J. Fermented Soy Bean Extract Suppresses Differentiation of 3T3-L1 Preadipocytes and Facilitates Its Glucose Utilization. J. Funct. Foods 2015, 15, 516–524. [Google Scholar] [CrossRef]
- Choi, J.H.; Pichiah, P.B.T.; Kim, M.J.; Cha, Y.S. Cheonggukjang, a Soybean Paste Fermented with B. Licheniformis-67 Prevents Weight Gain and Improves Glycemic Control in High Fat Diet Induced Obese Mice. J. Clin. Biochem. Nutr. 2016, 59, 31–38. [Google Scholar] [CrossRef]
- Yoshizaki, Y.; Kawasaki, C.; Cheng, K.C.; Ushikai, M.; Amitani, H.; Asakawa, A.; Okutsu, K.; Sameshima, Y.; Takamine, K.; Inui, A. Rice Koji Reduced Body Weight Gain, Fat Accumulation, and Blood Glucose Level in High-Fat Diet-Induced Obese Mice. PeerJ 2014, 2014, e540. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Koo, B.; Seong, S.H.; Dae, Y.K.; Hee, S.S.; Cha, Y.S. Fermented Kochujang Supplement Shows Anti-Obesity Effects by Controlling Lipid Metabolism in C57BL/6J Mice Fed High Fat Diet. Food Sci. Biotechnol. 2008, 17, 336–342. [Google Scholar]
- Vuong, T.; Martineau, L.C.; Ramassamy, C.; Matar, C.; Haddad, P.S. Fermented Canadian Lowbush Blueberry Juice Stimulates Glucose Uptake and AMP-Activated Protein Kinase in Insulin-Sensitive Cultured Muscle Cells and Adipocytes. Can. J. Physiol. Pharmacol. 2007, 85, 956–965. [Google Scholar] [CrossRef]
- Sanggaard, K.M.; Holst, J.J.; Rehfeld, J.F.; Sandström, B.; Raben, A.; Tholstrup, T. Different Effects of Whole Milk and a Fermented Milk with the Same Fat and Lactose Content on Gastric Emptying and Postprandial Lipaemia, but Not on Glycaemic Response and Appetite. Br. J. Nutr. 2004, 92, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.N.; Choi, J.W.; Lim, W.C.; Kim, M.K.; Lee, I.Y.; Cho, H.Y. Kefir Inhibits 3T3-L1 Adipocyte Differentiation through down-Regulation of Adipogenic Transcription Factor Expression. J. Sci. Food Agric. 2013, 93, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chon, J.W.; Kim, H.; Seo, K.H. Modulation of Intestinal Microbiota in Mice by Kefir Administration. Food Sci. Biotechnol. 2015, 24, 1397–1403. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, Y.-T.; Hsieh, H.-H.; Chen, M.-J. Effect of Lactobacillus Mali APS1 and L. Kefiranofaciens M1 on Obesity and Glucose Homeostasis in Diet-Induced Obese Mice. J. Funct. Foods 2016, 23, 580–589. [Google Scholar] [CrossRef]
- Choi, J.W.; Kang, H.W.; Lim, W.C.; Kim, M.K.; Lee, I.Y.; Cho, H.Y. Kefir Prevented Excess Fat Accumulation in Diet-Induced Obese Mice. Biosci. Biotechnol. Biochem. 2017, 81, 958–965. [Google Scholar] [CrossRef]
- Fathi, Y.; Ghodrati, N.; Zibaeenezhad, M.J.; Faghih, S. Kefir Drink Causes a Significant yet Similar Improvement in Serum Lipid Profile, Compared with Low-Fat Milk, in a Dairy-Rich Diet in Overweight or Obese Premenopausal Women: A Randomized Controlled Trial. J. Clin. Lipidol. 2017, 11, 136–146. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kang, I.B.; Kim, H.; Song, K.Y.; Seo, K.H. Dual Function of Lactobacillus Kefiri DH5 in Preventing High-Fat-Diet-Induced Obesity: Direct Reduction of Cholesterol and Upregulation of PPAR-α in Adipose Tissue. Mol. Nutr. Food Res. 2017, 61, 1700252. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.; Jeong, D.; Kang, I.B.; Chon, J.W.; Kim, H.S.; Song, K.Y.; Seo, K.H. Kefir Alleviates Obesity and Hepatic Steatosis in High-Fat Diet-Fed Mice by Modulation of Gut Microbiota and Mycobiota: Targeted and Untargeted Community Analysis with Correlation of Biomarkers. J. Nutr. Biochem. 2017, 44, 35–43. [Google Scholar] [CrossRef]
- Lim, J.; Kale, M.; Kim, D.H.; Kim, H.S.; Chon, J.W.; Seo, K.H.; Lee, H.G.; Yokoyama, W.; Kim, H. Antiobesity Effect of Exopolysaccharides Isolated from Kefir Grains. J. Agric. Food Chem. 2017, 65, 10011–10019. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Cotter, P.D.; Willing, B.P. Traditional Kefir Reduces Weight Gain and Improves Plasma and Liver Lipid Profiles More Successfully than a Commercial Equivalent in a Mouse Model of Obesity. J. Funct. Foods 2018, 46, 29–37. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chen, H.L.; Wu, H.S.; Ho, M.H.; Chong, K.Y.; Chen, C.M. Kefir Peptides Prevent Hyperlipidemia and Obesity in High-Fat-Diet-Induced Obese Rats via Lipid Metabolism Modulation. Mol. Nutr. Food Res. 2018, 62, 1700505. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ding, G.; Li, Q.; Gong, L.; Huang, J.; Sang, Y. Tibet Kefir Milk Decreases Fat Deposition by Regulating the Gut Microbiota and Gene Expression of Lpl and Angptl4 in High Fat Diet-Fed Rats. Food Res. Int. 2019, 121, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Kim, D.H.; Yokoyama, W.H.; Kim, H. Synbiotic Effect of Whole Grape Seed Flour and Newly Isolated Kefir Lactic Acid Bacteria on Intestinal Microbiota of Diet-Induced Obese Mice. J. Agric. Food Chem. 2020, 68, 13131–13137. [Google Scholar] [CrossRef]
- Tiss, M.; Souiy, Z.; Abdeljelil, N.B.; Njima, M.; Achour, L.; Hamden, K. Fermented Soy Milk Prepared Using Kefir Grains Prevents and Ameliorates Obesity, Type 2 Diabetes, Hyperlipidemia and Liver-Kidney Toxicities in HFFD-Rats. J. Funct. Foods 2020, 67, 103869. [Google Scholar] [CrossRef]
- Gao, J.; Mao, K.; Wang, X.; Mi, S.; Fu, M.; Li, X.; Xiao, J.; Simal-Gandara, J.; Sang, Y. Tibet Kefir Milk Regulated Metabolic Changes Induced by High-Fat Diet via Amino Acids, Bile Acids, and Equol Metabolism in Human-Microbiota-Associated Rats. J. Agric. Food Chem. 2021, 69, 6720–6732. [Google Scholar] [CrossRef] [PubMed]
- Caferoglu, Z.; Sahin, G.A. The Effects of Kefir in Mixed Meals on Appetite and Food Intake: A Randomized Cross-over Trial. Rev. Nutr. 2021, 34, 1–11. [Google Scholar] [CrossRef]
- Seo, K.H.; Gyu Lee, H.; Young Eor, J.; Jin Jeon, H.; Yokoyama, W.; Kim, H. Effects of Kefir Lactic Acid Bacteria-Derived Postbiotic Components on High Fat Diet-Induced Gut Microbiota and Obesity. Food Res. Int. 2022, 157, 111445. [Google Scholar] [CrossRef] [PubMed]
- Caili, F.U.; Huan, S.; Quanhong, L.I. A Review on Pharmacological Activities and Utilization Technologies of Pumpkin. Plant Foods Hum. Nutr. 2006, 61, 70–77. [Google Scholar] [CrossRef]
- Hamajima, H.; Tanaka, M.; Miyagawa, M.; Sakamoto, M.; Nakamura, T.; Yanagita, T.; Nishimukai, M.; Mitsutake, S.; Nakayama, J.; Nagao, K.; et al. Koji Glycosylceramide Commonly Contained in Japanese Traditional Fermented Foods Alters Cholesterol Metabolism in Obese Mice. Biosci. Biotechnol. Biochem. 2019, 83, 1514–1522. [Google Scholar] [CrossRef]
- Yu, X.-H.; Qian, K.; Jiang, N.; Zheng, X.-L.; Cayabyab, F.S.; Tang, C.-K. ABCG5/ABCG8 in Cholesterol Excretion and Atherosclerosis. Clin. Chim. Acta. 2014, 428, 82–88. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S.-Q. Biovalorisation of Okara (Soybean Residue) for Food and Nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Li, B.; Qiao, M.; Lu, F. Composition, Nutrition, and Utilization of Okara (Soybean Residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Wynne, K.; Stanley, S.; McGowan, B.; Bloom, S.R. Appetite Control. J. Endocrinol. 2005, 184, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; De Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; Van Der Knaap, H.; et al. Appetite Control: Methodological Aspects of the Evaluation of Foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Ruijschop, R.M.A.J.; Boelrijk, A.E.M.; te Giffel, M.C. Satiety Effects of a Dairy Beverage Fermented with Propionic Acid Bacteria. Int. Dairy J. 2008, 18, 945–950. [Google Scholar] [CrossRef]
- Chan, Y.-K.; Strik, C.M.; Budgett, S.C.; McGill, A.-T.; Proctor, J.; Poppitt, S.D. The Emulsified Lipid Fabuless (Olibra) Does Not Decrease Food Intake but Suppresses Appetite When Consumed with Yoghurt but Not Alone or with Solid Foods: A Food Effect Study. Physiol. Behav. 2012, 105, 742–748. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The Role of Short Chain Fatty Acids in Appetite Regulation and Energy Homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Darzi, J.; Frost, G.S.; Robertson, M.D. Do SCFA Have a Role in Appetite Regulation? Proc. Nutr. Soc. 2011, 70, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Johansson, D.P.; Lee, I.; Risérus, U.; Langton, M.; Landberg, R. Effects of Unfermented and Fermented Whole Grain Rye Crisp Breads Served as Part of a Standardized Breakfast, on Appetite and Postprandial Glucose and Insulin Responses: A Randomized Cross-over Trial. PLoS ONE 2015, 10, e0122241. [Google Scholar] [CrossRef]
- Iversen, K.N.; Johansson, D.; Brunius, C.; Andlid, T.; Andersson, R.; Langton, M.; Landberg, R. Appetite and Subsequent Food Intake Were Unaffected by the Amount of Sourdough and Rye in Soft Bread-A Randomized Cross-Over Breakfast Study. Nutrients 2018, 10, 1594. [Google Scholar] [CrossRef]
- Schrager, M.A.; Metter, E.J.; Simonsick, E.; Ble, A.; Bandinelli, S.; Lauretani, F.; Ferrucci, L. Sarcopenic Obesity and Inflammation in the InCHIANTI Study. J. Appl. Physiol. 2007, 102, 919–925. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. (Lausanne) 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and Insulin Resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- de Luca, C.; Olefsky, J.M. Inflammation and Insulin Resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Qian, Y.; Tao, Y.; She, X.; Li, Y.; Chen, X.; Guo, S.; Xiang, W.; Liu, L.; Du, H. Characterization of the Microbial Communities and Their Correlations with Chemical Profiles in Assorted Vegetable Sichuan Pickles. Food Control 2020, 113, 107174. [Google Scholar] [CrossRef]
- Davis, J.E.; Braucher, D.R.; Walker-Daniels, J.; Spurlock, M.E. Absence of Tlr2 Protects against High-Fat Diet-Induced Inflammation and Results in Greater Insulin-Stimulated Glucose Transport in Cultured Adipocytes. J. Nutr. Biochem. 2011, 22, 136–141. [Google Scholar] [CrossRef]
- Perazza, L.R.; Daniel, N.; Dubois, M.-J.; Pilon, G.; Varin, T.V.; Blais, M.; Martinez Gonzales, J.L.; Bouchard, M.; Asselin, C.; Lessard, M. Distinct Effects of Milk-Derived and Fermented Dairy Protein on Gut Microbiota and Cardiometabolic Markers in Diet-Induced Obese Mice. J. Nutr. 2020, 150, 2673–2686. [Google Scholar] [CrossRef] [PubMed]
- Tinh, N.T.T.; Sitolo, G.C.; Yamamoto, Y.; Suzuki, T. Citrus Limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model. Foods 2021, 10, 240. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Triantos, C.; Mouzaki, A. The Influence of Nutritional Factors on Immunological Outcomes. Front. Immunol. 2021, 12, 665968. [Google Scholar] [CrossRef]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Prasanth, M.I.; Chaiyasut, C. A Mini Review on Antidiabetic Properties of Fermented Foods. Nutrients 2018, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Matar, C. Increase of Antioxidant Capacity of the Lowbush Blueberry (Vaccinium Angustifolium) during Fermentation by a Novel Bacterium from the Fruit Microflora. J. Sci. Food Agric. 2005, 85, 1477–1484. [Google Scholar] [CrossRef]
- Talior, I.; Yarkoni, M.; Bashan, N.; Eldar-Finkelman, H. Increased Glucose Uptake Promotes Oxidative Stress and PKC-Delta Activation in Adipocytes of Obese, Insulin-Resistant Mice. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E295–E302. [Google Scholar] [CrossRef] [PubMed]
- Nachar, A.; Eid, H.M.; Vinqvist-Tymchuk, M.; Vuong, T.; Kalt, W.; Matar, C.; Haddad, P.S. Phenolic Compounds Isolated from Fermented Blueberry Juice Decrease Hepatocellular Glucose Output and Enhance Muscle Glucose Uptake in Cultured Murine and Human Cells. BMC Complement. Altern. Med. 2017, 17, 138. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Son, H.; Chang, H.; Lee, J. Nutrients E Ff Ects of Cabbage-Apple Juice Fermented by Lactobacillus Plantarum EM on Lipid Profile Improvement and Obesity Amelioration in Rats. Nutrients 2020, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.B.; Jeong, H.W.; Cho, D.; Lee, B.J.; Lee, J.H.; Choi, J.Y.; Bae, I.H.; Lee, S.J. Fermented Green Tea Extract Alleviates Obesity and Related Complications and Alters Gut Microbiota Composition in Diet-Induced Obese Mice. J. Med. Food 2015, 18, 549–556. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Y.-L.; Zhang, X.; Wang, K.-B.; Huang, J.-A.; Liu, Z.-H.; Zhu, M.-Z. Polyphenols from Fu Brick Tea Reduce Obesity via Modulation of Gut Microbiota and Gut Microbiota-Related Intestinal Oxidative Stress and Barrier Function. J. Agric. Food Chem. 2021, 69, 14530–14543. [Google Scholar] [CrossRef]
- Wijngaard, H.; Arendt, E.K. Buckwheat. Cereal Chem. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, K.; Guo, W.; Zhang, C.; Chen, H.; Xu, T.; Lu, Y.; Wu, Q.; Li, Y.; Chen, Y. Aspergillus Niger Fermented Tartary Buckwheat Ameliorates Obesity and Gut Microbiota Dysbiosis through the NLRP3/Caspase-1 Signaling Pathway in High-Fat Diet Mice. J. Funct. Foods 2022, 95, 105171. [Google Scholar] [CrossRef]
- Tochitani, S.; Maehara, Y.; Kawase, T.; Tsukahara, T.; Shimizu, R.; Watanabe, T.; Maehara, K.; Asaoka, K.; Matsuzaki, H. Fermented Rice Bran Supplementation Ameliorates Obesity via Gut Microbiota and Metabolism Modification in Female Mice. J. Clin. Biochem. Nutr. 2022, 70, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, F.; Weng, P.; Wu, Z. The Effect of Fermented Huyou Juice on Intestinal Microbiota in a High-fat Diet-induced Obesity Mouse Model. J. Food Biochem. 2020, 44, e13480. [Google Scholar] [CrossRef]
- Guo, X.; Cao, X.; Fang, X.; Guo, A.; Li, E. Inhibitory Effects of Fermented Ougan (Citrus Reticulata Cv. Suavissima) Juice on High-Fat Diet-Induced Obesity Associated with White Adipose Tissue Browning and Gut Microbiota Modulation in Mice. Food Funct. 2021, 12, 9300–9314. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-J.; Li, M.-Z.; Gao, H.; Hu, J.-L.; Nie, Q.-X.; Chen, H.-H.; Zhang, Y.-L.; Xie, M.-Y.; Nie, S.-P. Polysaccharides from Fermented Momordica Charantia L. with Lactobacillus Plantarum NCU116 Ameliorate Metabolic Disorders and Gut Microbiota Change in Obese Rats. Food Funct. 2021, 12, 2617–2630. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ji, Y.; Jung, H.-Y.; Park, H.; Kang, J.; Choi, S.-H.; Shin, H.; Hyun, C.-K.; Kim, K.-T.; Holzapfel, W.H. Lactobacillus Plantarum HAC01 Regulates Gut Microbiota and Adipose Tissue Accumulation in a Diet-Induced Obesity Murine Model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef]
- Lin, X.; Tang, Y.; Hu, Y.; Lu, Y.; Sun, Q.; Lv, Y.; Zhang, Q.; Wu, C.; Zhu, M.; He, Q.; et al. Sodium Reduction in Traditional Fermented Foods: Challenges, Strategies, and Perspectives. J. Agric. Food Chem. 2021, 69, 8065–8080. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut Microbiome Stability and Resilience: Elucidating the Response to Perturbations in Order to Modulate Gut Health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- Huseyin, C.E.; O’Toole, P.W.; Cotter, P.D.; Scanlan, P.D. Forgotten Fungi-the Gut Mycobiome in Human Health and Disease. FEMS Microbiol. Rev. 2017, 41, 479–511. [Google Scholar] [CrossRef]
- Chin, V.K.; Yong, V.C.; Chong, P.P.; Amin Nordin, S.; Basir, R.; Abdullah, M. Mycobiome in the Gut: A Multiperspective Review. Mediat. Inflamm. 2020, 2020, 9560684. [Google Scholar] [CrossRef]
- Cote, M.P.; Lubowitz, J.H.; Rossi, M.J.; Brand, J.C. Reviews Pooling Heterogeneous, Low-Evidence, High-Bias Data Result in Incorrect Conclusions: But Heterogeneity Is an Opportunity to Explore. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2018, 34, 3126–3128. [Google Scholar] [CrossRef]
- Spanagel, R. Ten Points to Improve Reproducibility and Translation of Animal Research. Front. Behav. Neurosci. 2022, 16, 869511. [Google Scholar] [CrossRef] [PubMed]
- Vickerstaff, V.; Omar, R.Z.; Ambler, G. Methods to Adjust for Multiple Comparisons in the Analysis and Sample Size Calculation of Randomised Controlled Trials with Multiple Primary Outcomes. BMC Med. Res. Methodol. 2019, 19, 129. [Google Scholar] [CrossRef]
- WHO Cancer: Carcinogenicity of the Consumption of Red Meat and Processed Meat. Available online: https://www.who.int/news-room/questions-and-answers/item/cancer-carcinogenicity-of-the-consumption-of-red-meat-and-processed-meat (accessed on 30 November 2022).
- Silla Santos, M.H. Biogenic Amines: Their Importance in Foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Fong, F.L.Y.; El-Nezami, H.; Sze, E.T.P. Biogenic Amines—Precursors of Carcinogens in Traditional Chinese Fermented Food. NFS J. 2021, 23, 52–57. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, J.; Moon, H.; Yang, J.; Kim, M. Determination of Sodium Contents in Traditional Fermented Foods in Korea. J. Food Compos. Anal. 2017, 56, 110–114. [Google Scholar] [CrossRef]
- Ji, J.; Shankar, S.; Salmieri, S.; Lacroix, M. Combined Effects of Microencapsulated Essential Oils and γ-Irradiation on Microbiological and Physicochemical Properties of Dry Fermented Sausages during Ripening and Storage. Food Control 2022, 133, 108624. [Google Scholar] [CrossRef]
- Khurana, H.K.; Kanawjia, S.K. Recent Trends in Development of Fermented Milks. Curr. Nutr. Food Sci. 2007, 3, 91–108. [Google Scholar] [CrossRef]
- Yu, Z.; Su, Y.; Zhang, Y.; Zhu, P.; Mei, Z.; Zhou, X.; Yu, H. Potential Use of Ultrasound to Promote Fermentation, Maturation, and Properties of Fermented Foods: A Review. Food Chem. 2021, 357, 129805. [Google Scholar] [CrossRef]
- Fan, X.; Lv, X.; Meng, L.; Ai, M.; Li, C.; Teng, F.; Feng, Z. Effect of Microwave Sterilization on Maturation Time and Quality of Low-Salt Sufu. Food Sci. Nutr. 2020, 8, 584–593. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).