Leptin, Adiponectin, and Melatonin Modulate Colostrum Lymphocytes in Mothers with Obesity
Abstract
1. Introduction
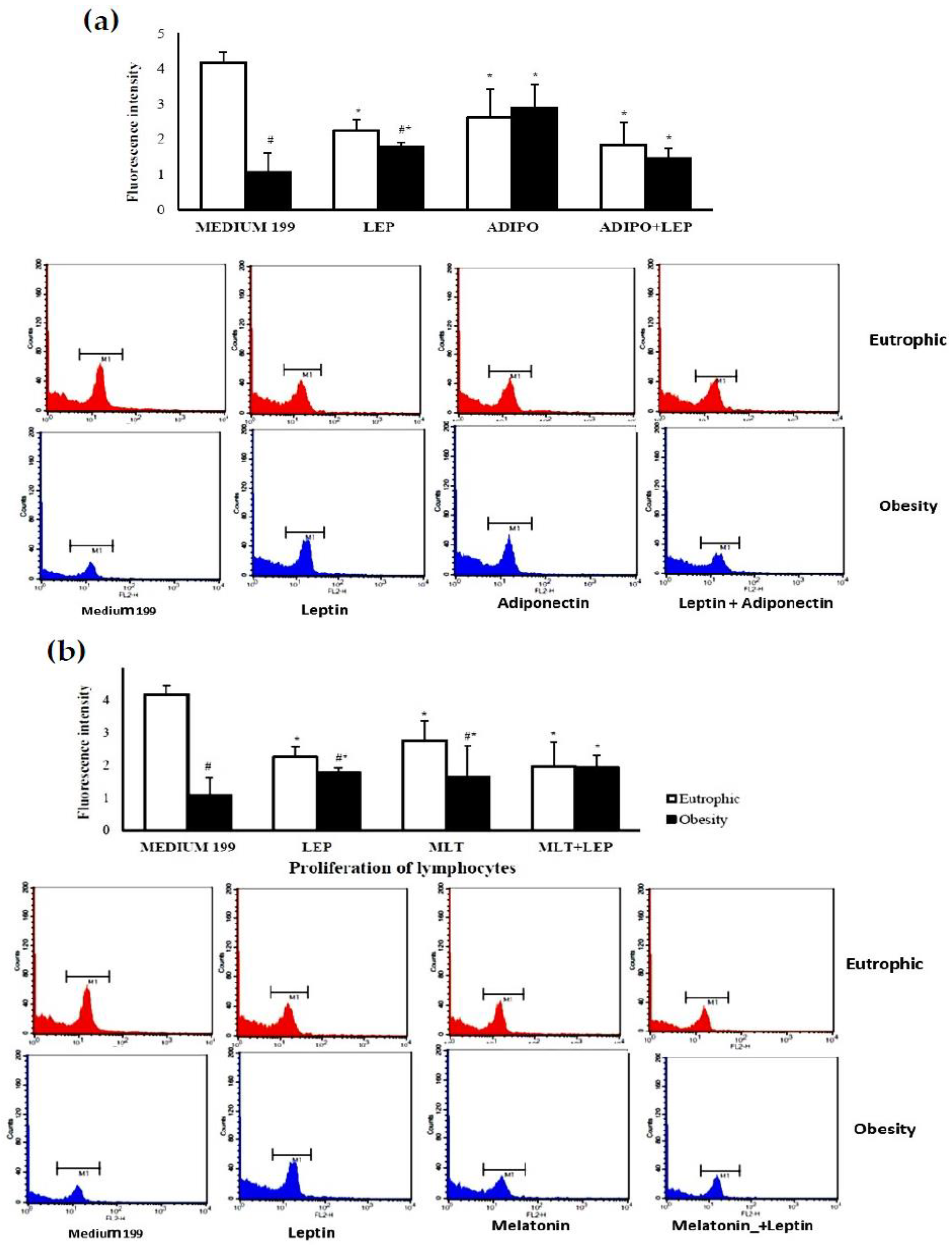
2. Results
2.1. Characterization of the Sample
2.2. Molecular Analysis of Colostrum
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Study Design and Participants
4.2. Colostrum and Cell Separation
4.3. Immunophenotyping
4.4. Stimuli
4.5. Lymphocyte Proliferation
4.6. Intracellular Calcium
4.7. Apoptosis
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Chen, J.; Lin, Y.; Xu, L.; Sang, Y.; Li, D.; Du, M. Obesity challenge drives. distinct maternal immune response changes in normal pregnant and abortion-prone mouse models. Front. Immunol. 2021, 12, 694077. [Google Scholar] [CrossRef] [PubMed]
- Rugină, C.; Mărginean, C.O.; Meliţ, L.E.; Huţanu, A.; Ghiga, D.V.; Modi, V.; Mărginean, C. Gestational obesity and subclinical inflammation: The pathway from simple assessment to complex outcome (STROBE-compliant article). Medicine 2021, 100, e26055. [Google Scholar] [CrossRef] [PubMed]
- Winer, S.; Paltser, G.; Chan, Y.; Tsui, H.; Engleman, E.; Winer, D.; Dosch, H.M. Obesity predisposes to Th17 bias. Eur. J. Immunol. 2009, 39, 2629–2635. [Google Scholar] [CrossRef]
- Fujimori, M.; França, E.L.; Fiorin, V.; Morais, T.C.; Honorio-França, A.C.; Abreu, L.C. Changes in the biochemical and immunological components of serum and colostrum of overweight and obese mothers. BMC Pregnancy Childbirth 2015, 15, 166. [Google Scholar] [CrossRef]
- Fujimori, M.; França, E.L.; Morais, T.C.; Fiorin, V.; de Abreu, L.C.; Honório-França, A.C. Cytokine and adipokine are biofactors can act in blood and colostrum of obese mothers. Biofactors 2017, 43, 243–250. [Google Scholar] [CrossRef]
- Morais, T.C.; Honorio-Franca, A.C.; Fujimori, M.; Quental, O.B.; Pessoa, R.S.; França, E.L.; Abreu, L.C. Melatonin action on the activity of phagocytes from the colostrum of obese women. Medicina 2019, 55, 625. [Google Scholar] [CrossRef]
- Morais, T.C.; de Abreu, L.C.; de Quental, O.B.; Pessoa, R.S.; Fujimori, M.; Daboin, B.E.G.; França, E.L.; Honorio-França, A.C. Obesity as an inflammatory agent can cause cellular changes in human milk due to the actions of the adipokines leptin and adiponectin. Cells 2019, 8, 519. [Google Scholar] [CrossRef]
- Farah, N.; Hogan, A.E.; O’Connor, N.; Kennelly, M.M.; O’Shea, D.; Turner, M.J. Correlation between maternal inflammatory markers and fetomaternal adiposity. Cytokine 2012, 60, 96–99. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Nehlsen-Cannarella, S.L.; Ekkens, M.; Utter, A.C.; Butterworth, D.E.; Fagoaga, O.R. Influence of obesity on immune function. J. Am. Diet. Assoc. 1999, 99, 294–299. [Google Scholar] [CrossRef]
- Elsayed, H.L.; el Gendy, Y.G.; Radwan, N.M.; Farweez, B.A.; Fouda, S. Lymphocyte subtype dysregulation in a group of children with simple obesity. Egypt J. Pediatr Allergy Immunol. 2017, 15, 63–68. [Google Scholar] [CrossRef]
- Polese, B.; Gridelet, V.; Araklioti, E.; Martens, H.; Perrier Hauterive, S.; Geenen, V. The Endocrine Milieu and CD4 T-Lymphocyte Polarization during Pregnancy. Front. Endocrinol. 2014, 5, 106. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Yuan, L.; Azevedo, S.P.; Jeong, K.; Gonzalez, A.M.; Saif, L.J. Transfer of maternal cytokines to suckling piglets: In vivo and in vitro models with implications for immunomodulation of neonatal immunity. Vet. Immunol. Immunopathol. 2007, 117, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Kugananthan, S.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human Milk Adiponectin and Leptin and Infant Body Composition over the First 12 Months of Lactation. Nutrients 2018, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Juan Castell, M.F.; Peraita-Costa, I.; Soriano, J.M.; Llopis-Morales, A.; Morales-Suarez-Varela, M. A Review of the Relationship Between the Appetite-Regulating Hormone Leptin Present in Human Milk and Infant Growth. Breastfeed. Med. 2022, 17, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Goruk, S.; Becker, A.B.; Subbarao, P.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.; Sears, M.R.; Field, C.J.; Azad, M.B. Adiponectin, leptin and insulin in breast milk: Associations with maternal characteristics and infant body composition in the first year of life. Int. J. Obes. 2018, 42, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, fat mass and immune system: Role for leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [CrossRef]
- Buonfiglio, D.; Tchio, C.; Furigo, I.; Donato, J., Jr.; Baba, K.; Cipolla-Neto, J.; Tosini, G. Removing melatonin receptor type 1 signaling leads to selective leptin resistance in the arcuate nucleus. J. Pineal Res. 2019, 67, e12580. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef]
- Corbalán-Tutau, D.; Madrid, J.A.; Nicolás, F.; Garaulet, M. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiol. Behav. 2014, 123, 231–235. [Google Scholar] [CrossRef]
- Grosshans, M.; Vollmert, C.; Vollstaedt-Klein, S.; Nolte, I.; Schwarz, E.; Wagner, X.; Leweke, M.; Mutschler, J.; Kiefer, F.; Bumb, J.M. The association of pineal gland volume and body mass in obese and normal weight individuals: A pilot study. Psychiatr. Danub. 2016, 28, 220–224. [Google Scholar]
- Cardinali, D.P.; Hardeland, R. Inflammaging, metabolic syndrome and melatonin: A call for treatment studies. Neuroendocrinology 2017, 104, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kanikowska, D.; Iwase, S.; Shimizu, Y.; Nishimura, N.; Inukai, Y.; Sato, M.; Sugenoya, J. Seasonal differences in melatonin concentrations and heart rates during sleep in obese subjects in Japan. Int. J. Biometeorol. 2013, 57, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Walecka-Kapica, E.; Błońska, A.; Winczyk, K.; Stępień, A.; Chojnacki, J. Serotonin and melatonin secretion in postmenopausal women with eating disorders. Endokrynol. Pol. 2016, 67, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.; Markus, R.P. Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes)—Melatonin in human colostrum and colostrum phagocytes. J. Pineal Res. 2006, 41, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.; Markus, R.P. Pineal melatonin and the innate immune response: The TNF-alpha increase after cesarean section suppresses nocturnal melatonin production. J. Pineal Res. 2007, 43, 365–371. [Google Scholar] [CrossRef]
- Honorio-França, A.C.; Castro, C.; Hara, P.; Ormonde, J.V.S.; Nunes, G.T.; França, E.L. Human colostrum melatonin exhibits a day-night variation and modulates the activity of colostral phagocytes. J. Appl. Biomed. 2013, 11, 153–162. [Google Scholar] [CrossRef]
- Katzer, D.; Pauli, L.; Mueller, A.; Reutter, H.; Reinsberg, J.; Fimmers, R.; Bartmann, P.; Bagci, S. Melatonin concentrations and antioxidative capacity of human breast milk according to gestational age and the time of day. J. Hum. Lact. 2016, 32, NP105–NP110. [Google Scholar] [CrossRef]
- Illnerová, H.; Buresová, M.; Presl, J. Melatonin rhythm in human milk. J. Clin. Endocrinol. Metab. 1993, 77, 838–841. [Google Scholar] [CrossRef]
- França, E.L.; Nicomedes, T.R.; Calderon, I.M.P.; Honório-França, A.C. Time-dependent alterations of soluble and cellular components in human milk. Biol. Rhythm Res. 2010, 41, 333–347. [Google Scholar] [CrossRef]
- Morceli, G.; Honorio-França, A.C.; Fagundes, D.L.; Calderon, I.M.; França, E.L. Antioxidant effect of melatonin on the functional activity of colostral phagocytes in diabetic women. PLoS ONE 2013, 8, e56915. [Google Scholar] [CrossRef]
- Ohashi, K.; Yuasa, D.; Shibata, R.; Murohara, T.; Ouchi, N. Adiponectin as a Target in Obesity-related Inflammatory State. Endocr. Metab. Immune Disord. Drug Target 2015, 15, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Aljafary, M.A.; Al-Suhaimi, E.A. Adiponectin System (Rescue Hormone): The Missing Link between Metabolic and Cardiovascular Diseases. Pharmaceutics 2022, 14, 1430. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.J.; Ferder, L.; Manucha, W.; Diez, E.R. Anti-Inflammatory Effects of Melatonin in Obesity and Hypertension. Curr. Hypertens. Rep. 2018, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Iikuni, N.; Lam, Q.L.; Lu, L.; Matarese, G.; La Cava, A. Leptin and Inflammation. Curr. Immunol. Rev. 2008, 4, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Tsuda, H.; Dickey, W.D.; Goldman, A.S. Separation of human colostral macrophages and neutrophils on gelatin and collagen-serum substrata. Cell Struct. Funct. 1984, 8, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.S. The immune system of human milk: Antimicrobial, anti-inflammatory and immunomodulating properties. Pediatr. Infect Dis. J. 1993, 12, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.S.; Chheda, S.; Keeney, S.E.; Schmalstieg, F.C. Immunology of Human Milk and Host Immunity. Fetal Neonatal Physiol. 2011, 1, 690–701. [Google Scholar] [CrossRef]
- Cabinian, A.; Sinsimer, D.; Tang, M.; Zumba, O.; Mehta, H.; Toma, A.; Sant’Angelo, D.; Laouar, Y.; Laouar, A.T. Transfer of maternal immune cells by breastfeeding: Maternal cytotoxic T lymphocytes present in breast milk localize in the Peyer’s patches of the nursed infant. PLoS ONE 2016, 11, e0156762. [Google Scholar] [CrossRef]
- Ciardelli, L.; Garofoli, F.; Stronati, M.; Mazzucchelli, I.; Avanzini, M.A.; Figar, T.; Gasparoni, A.; De Silvestri, A.; Sabatino, G.; Chirico, G. Human colostrum T lymphocytes and their effector cytokines actively aid the development of the newborn immune system. Int. J. Immunopathol. Pharmacol. 2008, 21, 781–786. [Google Scholar] [CrossRef]
- Keustermans, G.; van der Heijden, L.B.; Boer, B.; Scholman, R.; Number, R.; Pasterkamp, G.; Janse, A.J.; Schipper, H.S. Differential adipokine receptor expression on circulating leukocyte subsets in lean and obese children. PLoS ONE 2017, 12, e0187068. [Google Scholar] [CrossRef]
- Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Sánchez-Margaret, V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000, 199, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Fernández-Santos, J.M.; Martín-Lacave, I.; Calvo, J.R.; Karasek, M.; Guerrero, J.M. Human lympho-cytes-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J. Clin. Endocrinol. Metab. 2005, 90, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Sato, F.T.; Cury-Boaventura, M.F.; Guirado-Rodrigues, S.H.; Caçula, K.G.; Gonçalves Santos, C.C.; Hatanaka, E.; de Oliveira, H.H.; Santos, V.C.; Murata, G.; et al. Effect of regular circus physical exercises on lymphocytes in overweight children. PLoS ONE 2015, 10, e0120262. [Google Scholar] [CrossRef]
- Crawley, J.B.; Rawlinson, L.; Lali, F.V.; Page, T.H.; Saklatvala, J.; Foxwell, B.M. T cell proliferation in response to interleukins 2 and 7 requires p38MAP kinase activation. J. Biol. Chem. 1997, 272, 15023–15027. [Google Scholar] [CrossRef]
- Field, C.J. The Immunological components of human milk and their effect on immune development in infants. J. Nutr. 2005, 135, 1–4. [Google Scholar] [CrossRef]
- Grases-Pintó, B.; Abril-Gil, M.; Rodríguez-Lagunas, M.J.; Castell, M.; Pérez-Cano, F.J.; Franch, À. Leptin and adiponectin supple-mentation modifies mesenteric lymph node lymphocyte composition and functionality in suckling rats. Br. J. Nutr. 2018, 119, 486–495. [Google Scholar] [CrossRef]
- Fujita, Y.; Murakami, M.; Ogawa, Y.; Masuzaki, H.; Tanaka, M.; Ozaki, S.; Nakao, K.; Mimori, T. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin. Exp. Immunol. 2002, 128, 21–26. [Google Scholar] [CrossRef]
- Inserra, P.; Zhang, Z.; Ardestani, S.K.; Araghi-Niknam, M.; Liang, B.; Jiang, S.; Shaw, D.; Molitor, M.; Elliott, K.; Watson, R.R. Modulation of cytokine production by dehydroepiandrosterone (DHEA) plus melatonin (MLT) supplementation of old mice. Proc. Soc. Exp. Biol. Med. 1998, 218, 76–82. [Google Scholar] [CrossRef]
- Yoo, Y.M.; Jang, S.K.; Kim, G.H.; Park, J.Y.; Joo, S.S. Pharmacological advantages of melatonin in immunosenescence by improving activity of T lymphocytes. J. Biomed. Res. 2016, 30, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.; Melean, E.; Valero, N.; Pons, H.; Chacín-Bonilla, L.; Larreal, Y.; Bonilla, E. Effect of melatonin on lymphocyte proliferation and production of interleukin-2 (IL-2) and interleukin-1 beta (IL-1 beta) in mice splenocytes. Investig. Clin. 2003, 44, 41–50. [Google Scholar] [PubMed]
- Trellakis, S.; Rydleuskaya, A.; Fischer, C.; Canbay, A.; Tagay, S.; Scherag, A.; Bruderek, K.; Schuler, P.J.; Brandau, S. Low adiponectin, high levels of apoptosis and increased peripheral blood neutrophil activity in healthy obese subjects. Obes. Facts 2012, 5, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yu, W.; Wei, W.; Zhang, X.; Tian, Y.; Sherif, M.; Liu, X.; Dong, C.; Wu, W.; Zhang, L.; et al. Melatonin reduces intramuscular fat deposition by promoting lipolysis and increasing mitochondrial function. J. Lipid Res. 2018, 60, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Honorio-França, A.C.; Nunes, G.T.; Fagundes, D.L.G.; Marchi, P.G.F.; Silva, R.T.; França, J.L.; Botelho, A.C.F.; Albuquerque, L.C.; Varotti, F.P.; França, E.L. Intracellular calcium is a target of modulation of apoptosis in MCF-7 cells in the presence of IgA adsorbed to polyethylene glycol. Onco. Targets Ther. 2016, 9, 617–626. [Google Scholar] [CrossRef]
- Saini, N.; Lakshminarayanan, S.; Kundu, P.; Sarin, A. Notch1 modulation of cellular calcium regulates mitochondrial metabolism and anti-apoptotic activity in T-regulatory cells. Front. Immunol. 2022, 13, 832159. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Margalet, V.; Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Najib, S.; Gonzalez-Yanes, C. Role of leptin as an immunomodulator of blood mononuclear cells: Mechanisms of action. Clin. Exp. Immunol. 2003, 133, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Morshedi, A.; Zarkesh-Esfahani, S.H.; Behjati, M. Effect of leptin on neutrophils phagocytosis and lymphocytes apoptosis chal-lenge by listeria monocytogenes and Escherichia coli. Jundishapur J. Microbiol. 2013, 6, e6389. [Google Scholar] [CrossRef]
- Espino, J.; Bejarano, I.; Paredes, S.D.; Barriga, C.; Rodríguez, A.B.; Pariente, J.A. Protective effect of melatonin against human leukocyte apoptosis induced by intracellular calcium overload: Relation with its antioxidant actions. J. Pineal Res. 2011, 51, 195–206. [Google Scholar] [CrossRef]
- Delgado, J.; Terrón, M.P.; García-Martinez, V.; López-Sanchez, C.; Barriga, C.; Pariente, J.Á.; Rodríguez, A.B. Oral melatonin administration and programmed cell death of neutrophils, lymphocytes, and other cell types from rats injected with HL-60 cells. J. Appl. Biomed. 2011, 9, 197–207. [Google Scholar] [CrossRef]
- Institute of Medicine (U.S.) and National Research Council (U.S.) Committee to Reexamine IOM Pregnancy Weight Guidelines. In Weight Gain during Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Wan, C.P.; Sigh, R.V.; Lau, B.H. A simple fluorometric assay for the determination of cell numbers. J. Immunol. Methods 1994, 173, 265–272. [Google Scholar] [CrossRef]
- Burchiel, S.W.; Edwards, B.S.; Kuckuck, F.W.; Lauer, F.T.; Prossnitz, E.R.; Ransom, J.T.; Sklar, L.A. Analysis of free intracellular calcium by flow cytometry: Multiparameter and pharmacologic applications. Methods 2000, 21, 221–230. [Google Scholar] [CrossRef]




| Variable | Group | p-Value | |
|---|---|---|---|
| Eutrophic | Obese | ||
| Maternal (n = 26 per group) | |||
| Age (years) (Mean ± SD) | 24.77 ± 5.54 | 26.17 ± 4.45 | 0.3327 |
| Pre-Gestational Maternal Weight (Mean ± SD) | 55.65 ± 6.82 | 86.87 ± 8.62 # | <0.0001 |
| Maternal height in meters (Mean ± SD) | 1.59 ± 0.06 | 1.62 ± 0.07 | 0.1361 |
| Final Gestational Weight (Kg) (Mean ± SD) | 67.28 ± 8.12 | 94.22 ± 9.57# | <0.0001 |
| Pre-gestational BMI in Kg/m2 (Mean ± SD) | 21.84 ± 1.90 | 33.23 ± 2.35# | <0.0001 |
| BMI at the end of pregnancy Kg/m2 (Mean ± SD) | 26.59 ± 2.47 | 36.51 ± 2.92# | <0.0001 |
| Gestational weight gain (Mean ± SD) | 11.84 ± 4.52 | 8.20 ± 5.49# | 0.0155 |
| Gestational age in weeks (Mean ± SD) | 38.65 ± 1.52 | 38.87 ± 1.45 | 0.6023 |
| Baby (n = 26 per group) | |||
| Baby’s sex—Female (%) | 15 (57.69%) | 16 (61.54%) | 1.38 * |
| Birth weight in grams (Mean ± SD) | 3143.85 ± 356.56 | 3316.20 ± 427.51 | 0.7852 |
| Height in centimeters (Mean ± SD) | 48.35 ± 1.84 | 48.59 ± 2.14 | 0.7041 |
| Lymphocytes (n = 5 per group) | |||
| CD3+ (Mean ± SD) | 80.01 ± 13.72 | 39.72 ± 6.99 # | 0.0019 |
| CD3CD4+ (Mean ± SD) | 52.34 ± 10.73 | 22.35 ± 3.08 # | 0.0338 |
| CD3CD8+ (Mean ± SD) | 31.86 ± 12.33 | 17.37 ± 4.16 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, G.d.A.V.; Morais, T.C.; França, E.L.; Daboin, B.E.G.; Bezerra, I.M.P.; Pessoa, R.S.; de Quental, O.B.; Honório-França, A.C.; Abreu, L.C.d. Leptin, Adiponectin, and Melatonin Modulate Colostrum Lymphocytes in Mothers with Obesity. Int. J. Mol. Sci. 2023, 24, 2662. https://doi.org/10.3390/ijms24032662
Pereira GdAV, Morais TC, França EL, Daboin BEG, Bezerra IMP, Pessoa RS, de Quental OB, Honório-França AC, Abreu LCd. Leptin, Adiponectin, and Melatonin Modulate Colostrum Lymphocytes in Mothers with Obesity. International Journal of Molecular Sciences. 2023; 24(3):2662. https://doi.org/10.3390/ijms24032662
Chicago/Turabian StylePereira, Gabrielle do Amaral Virginio, Tassiane Cristina Morais, Eduardo Luzia França, Blanca Elena Guerrero Daboin, Italla Maria Pinheiro Bezerra, Rafael Souza Pessoa, Ocilma Barros de Quental, Adenilda Cristina Honório-França, and Luiz Carlos de Abreu. 2023. "Leptin, Adiponectin, and Melatonin Modulate Colostrum Lymphocytes in Mothers with Obesity" International Journal of Molecular Sciences 24, no. 3: 2662. https://doi.org/10.3390/ijms24032662
APA StylePereira, G. d. A. V., Morais, T. C., França, E. L., Daboin, B. E. G., Bezerra, I. M. P., Pessoa, R. S., de Quental, O. B., Honório-França, A. C., & Abreu, L. C. d. (2023). Leptin, Adiponectin, and Melatonin Modulate Colostrum Lymphocytes in Mothers with Obesity. International Journal of Molecular Sciences, 24(3), 2662. https://doi.org/10.3390/ijms24032662






