Spawning-Induced pH Increase Activates Sperm Attraction and Fertilization Abilities in Eggs of the Ascidian, Phallusia philippinensis and Ciona intestinalis
Abstract
1. Introduction
2. Results
2.1. The pH in Different Ascidian Organs and Fluids Is Lower Than That of Sea Water
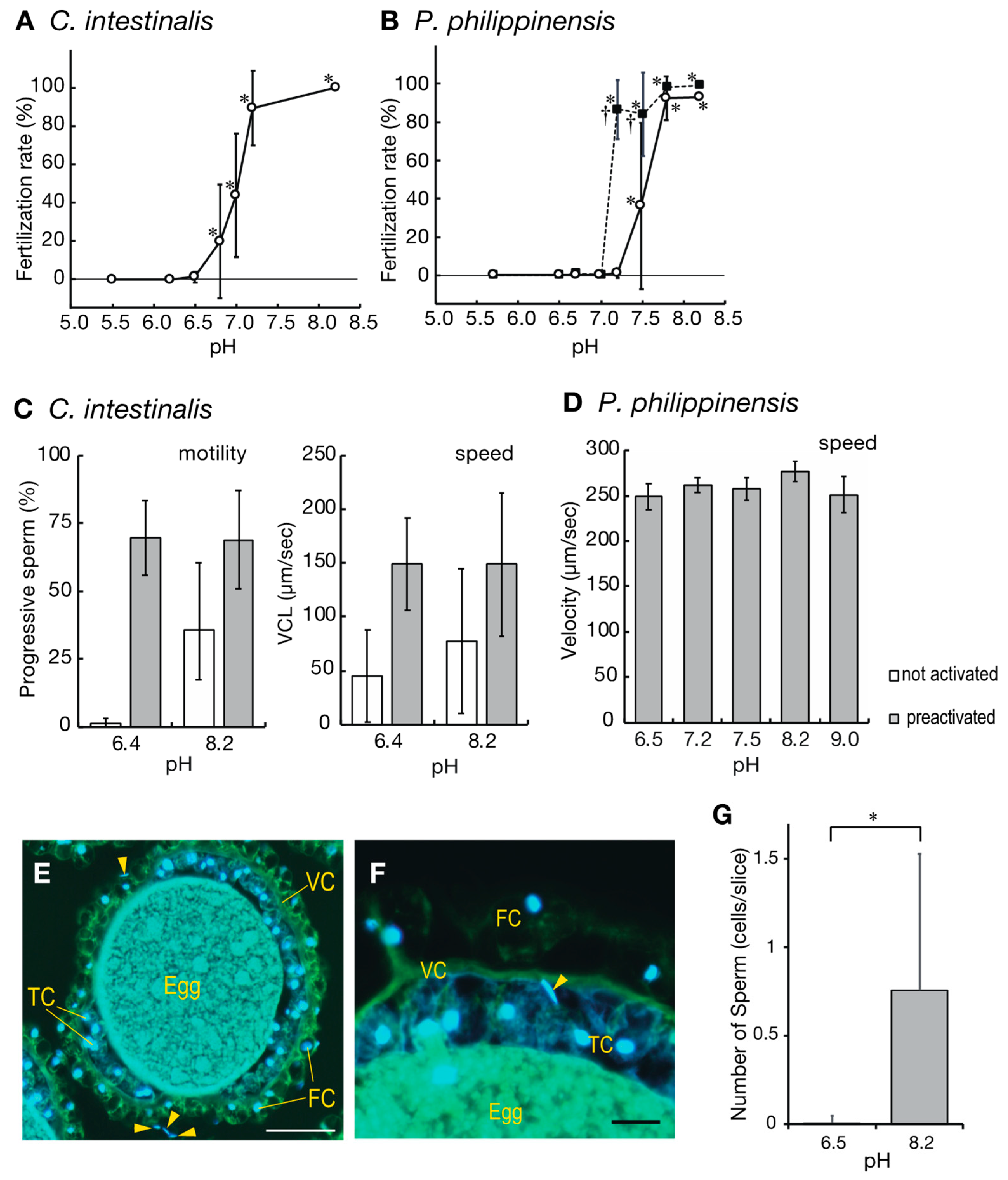
2.2. Low pH Inhibits Fertilization in Ascidians
2.3. Incubation of Eggs at pH 8.2 before Insemination Induces Their Fertility
2.4. Effect of Ambient pH on pHi of the Eggs
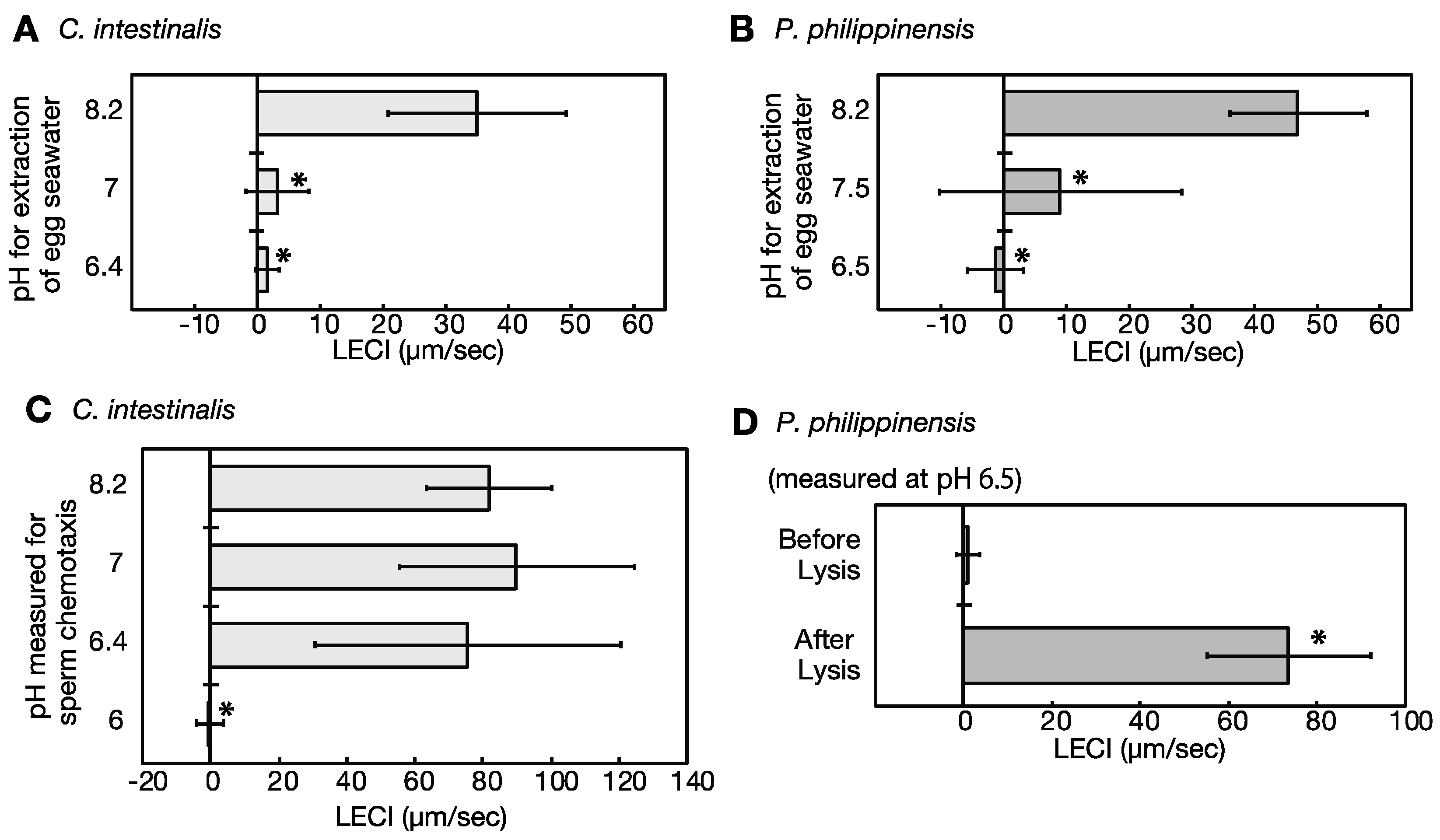
2.5. Eggs under Low pH Conditions Do Not Release Sperm Attractants
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Pre-Activation of Sperm Motility
4.3. Evaluation of Fertilization
4.4. Measurements of pH
4.5. Analysis of Sperm Motility and Chemotaxis
4.6. Evaluation of the Sperm Entry into the Perivitelline Space
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshida, M.; Yoshida, K. Modulation of Sperm Motility and Function Prior to Fertilization. In Reproductive and Developmental Strategies. Diversity and Commonality in Animals.; Kobayashi, K., Kitano, T., Iwao, Y., Kondo, M., Eds.; Springer: Tokyo, Japan, 2018; pp. 437–462. [Google Scholar]
- Wojtczak, M.; Dietrich, G.J.; Słowińska, M.; Dobosz, S.; Kuźmiński, H.; Ciereszko, A. Ovarian fluid pH enhances motility parameters of rainbow trout (Oncorhynchus mykiss) spermatozoa. Aquaculture 2007, 270, 259–264. [Google Scholar] [CrossRef]
- Nishigaki, T.; Jose, O.; Gonzalez-Cota, A.L.; Romero, F.; Trevino, C.L.; Darszon, A. Intracellular pH in sperm physiology. Biochem. Biophys. Res. Commun. 2014, 450, 1149–1158. [Google Scholar]
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction, 2nd ed.; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 1149–1158. [Google Scholar]
- Suarez, S.S. Chapter 5—Gamete and Zygote Transport. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 197–232. [Google Scholar]
- Miao, J.; Willems Hubertine, M.E.; Peters Brian, M. Exogenous Reproductive Hormones nor Candida albicans Colonization Alter the Near Neutral Mouse Vaginal pH. Infect. Immun. 2021, 89, e00550-20. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar]
- Wang, H.; McGoldrick, L.L.; Chung, J.J. Sperm ion channels and transporters in male fertility and infertility. Nat. Rev. Urol. 2021, 18, 46–66. [Google Scholar]
- Zhou, J.; Chen, L.; Li, J.; Li, H.; Hong, Z.; Xie, M.; Chen, S.; Yao, B. The Semen pH Affects Sperm Motility and Capacitation. PLoS ONE 2015, 10, e0132974. [Google Scholar] [CrossRef]
- Johnson, C.H.; Clapper, D.L.; Winkler, M.M.; Lee, H.C.; Epel, D. A volatile inhibitor immobilizes sea urchin sperm in semen by depressing the intracellular pH. Dev. Biol. 1983, 98, 493–501. [Google Scholar]
- Alavi, S.M.; Cosson, J. Sperm motility in fishes. I. Effects of temperature and pH: A review. Cell Biol. Int. 2005, 29, 101–110. [Google Scholar] [CrossRef]
- Nowosad, J.; Kucharczyk, D.; Sikora, M.; Kupren, K. Optimization of barbel (Barbus Barbus L.) fertilization and effects of ovarian fluid when there are controlled conditions for gamete activations. Anim. Reprod. Sci. 2021, 224, 106652. [Google Scholar] [CrossRef]
- Zeng, X.H.; Navarro, B.; Xia, X.M.; Clapham, D.E.; Lingle, C.J. Simultaneous knockout of Slo3 and CatSper1 abolishes all alkalization- and voltage-activated current in mouse spermatozoa. J. Gen. Physiol. 2013, 142, 305–313. [Google Scholar] [CrossRef]
- Kirichok, Y.; Navarro, B.; Clapham, D.E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006, 439, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Mannowetz, N.; Zhang, Y.; Everley, R.A.; Gygi, S.P.; Bewersdorf, J.; Lishko, P.V.; Chung, J.J. Dual Sensing of Physiologic pH and Calcium by EFCAB9 Regulates Sperm Motility. Cell 2019, 177, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Flick, M.; Bonigk, W.; Alvarez, L.; Trotschel, C.; Poetsch, A.; Muller, A.; Goodwin, N.; Pelzer, P.; Kashikar, N.D.; et al. The CatSper channel controls chemosensation in sea urchin sperm. EMBO J. 2015, 34, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.; Wei, A.; Yuan, A.; Gaut, J.; Saito, M.; Salkoff, L. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J. Biol. Chem. 1998, 273, 3509–3516. [Google Scholar] [CrossRef]
- Funahashi, H.; Ekwall, H.; Kikuchi, K.; Rodriguez-Martinez, H. Transmission electron microscopy studies of the zona reaction in pig oocytes fertilized in vivo and in vitro. Reproduction 2001, 122, 443–452. [Google Scholar] [CrossRef]
- Coy, P.; Canovas, S.; Mondejar, I.; Saavedra, M.D.; Romar, R.; Grullon, L.; Matas, C.; Aviles, M. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl. Acad. Sci. USA 2008, 105, 15809–15814. [Google Scholar] [CrossRef]
- Usui, K.; Hirohashi, N.; Chiba, K. Involvement of mitogen-activating protein kinase and intracellular pH in the duration of the metaphase I (MI) pause of starfish oocytes after spawning. Dev. Growth Differ. 2008, 50, 357–364. [Google Scholar] [CrossRef]
- Harada, K.; Oita, E.; Chiba, K. Metaphase I arrest of starfish oocytes induced via the MAP kinase pathway is released by an increase of intracellular pH. Development 2003, 130, 4581–4586. [Google Scholar] [CrossRef]
- Chiba, K. Oocyte Maturation in Starfish. Cells 2020, 9, 476. [Google Scholar] [CrossRef]
- Dehal, P.; Satou, Y.; Campbell, R.K.; Chapman, J.; Degnan, B.; De Tomaso, A.; Davidson, B.; Di Gregorio, A.; Gelpke, M.; Goodstein, D.M.; et al. The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science 2002, 298, 2157–2167. [Google Scholar]
- Yoshida, M.; Inaba, K.; Morisawa, M. Sperm chemotaxis during the process of fertilization in the ascidians Ciona savignyi and Ciona intestinalis. Dev. Biol. 1993, 157, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Hiradate, Y.; Sensui, N.; Cosson, J.; Morisawa, M. Species-specificity of sperm motility activation and chemotaxis: A study on ascidian species. Biol. Bull. 2013, 224, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, E.; Konno, A.; Inaba, K.; Oishi, T.; Murata, M.; Yoshida, M. Valosin-containing protein/p97 interacts with sperm-activating and sperm-attracting factor (SAAF) in the ascidian egg and modulates sperm-attracting activity. Dev. Growth Differ. 2008, 50, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Epel, D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature 1976, 262, 661–664. [Google Scholar] [CrossRef]
- Ueki, T.; Michibata, H. Molecular mechanism of the transport and reduction pathway of vanadium in ascidians. Coordin. Chem. Rev. 2011, 255, 2249–2257. [Google Scholar] [CrossRef]
- Dale, B.; Menezo, Y.; Cohen, J.; DiMatteo, L.; Wilding, M. Intracellular pH regulation in the human oocyte. Hum. Reprod. 1998, 13, 964–970. [Google Scholar] [CrossRef]
- Iwamatsu, T. Effects of pH on the Fertilization Response of the Medaka Egg. Dev. Growth Differ. 1984, 26, 533–544. [Google Scholar] [CrossRef]
- FitzHarris, G.; Baltz, J.M. Regulation of intracellular pH during oocyte growth and maturation in mammals. Reproduction 2009, 138, 619–627. [Google Scholar] [CrossRef]
- Erdogan, S.; FitzHarris, G.; Tartia, A.P.; Baltz, J.M. Mechanisms regulating intracellular pH are activated during growth of the mouse oocyte coincident with acquisition of meiotic competence. Dev. Biol. 2005, 286, 352–360. [Google Scholar] [CrossRef]
- Harada, K.; Fukuda, E.; Hirohashi, N.; Chiba, K. Regulation of intracellular pH by p90Rsk-dependent activation of an Na+/H+ exchanger in starfish oocytes. J. Biol. Chem. 2010, 285, 24044–24054. [Google Scholar] [CrossRef]
- Sawada, H.; Kawahigashi, M.; Yokosawa, H.; Ishii, S. Trypsin-like enzyme from eggs of the ascidian (protochordate), Halocynthia roretzi. Purification, properties, and physiological role. J. Biol. Chem. 1985, 260, 15694–15698. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Sensui, N.; Inoue, T.; Morisawa, M.; Mikoshiba, K. Role of two series of Ca2+ oscillations in activation of ascidian eggs. Dev. Biol. 1998, 203, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Sensui, N.; Yoshida, M.; Tachibana, K. Role of Mos/MEK/ERK cascade and Cdk1 in Ca2+ oscillations in fertilized ascidian eggs. Dev. Biol. 2012, 367, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Yoshida, K. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol. Hum. Reprod. 2011, 17, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, J.; Yoshida, M. Sperm Activation and Chemotaxis in Invertebrates. In Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology; Yoshida, M., Asturiano, J.F., Eds.; Springer: Singapore, 2020; pp. 31–46. [Google Scholar]
- Vandepas, L.E.; Oliveira, L.M.; Lee, S.S.; Hirose, E.; Rocha, R.M.; Swalla, B.J. Biogeography of Phallusia nigra: Is it really black and white? Biol. Bull. 2015, 228, 52–64. [Google Scholar] [CrossRef]
- Shiba, K.; Baba, S.A.; Inoue, T.; Yoshida, M. Ca2+ bursts occur around a local minimal concentration of attractant and trigger sperm chemotactic response. Proc. Natl. Acad. Sci. USA 2008, 105, 19312–19317. [Google Scholar] [CrossRef]
- Shiba, K.; Mogami, Y.; Baba, S.A. Ciliary Movement of Sea-urchin Embryos. Nat. Sci. Rep. Ochanomizu Univ. 2002, 53, 49–54. [Google Scholar]
- Yoshida, M.; Murata, M.; Inaba, K.; Morisawa, M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc. Natl. Acad. Sci. USA 2002, 99, 14831–14836. [Google Scholar] [CrossRef]
- Verstegen, J.; Iguer-Ouada, M.; Onclin, K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology 2002, 57, 149–179. [Google Scholar] [CrossRef]

| Tissue/Fluid | Ciona intestinalis | Phallusia philippinensis |
|---|---|---|
| Tissue | ||
| oviduct | 6.36 ± 0.16 (12) | 5.65 ± 0.18 (7) |
| spermiduct | 6.66 ± 0.08 (7) | 6.44 ± 0.14 (4) |
| ovary | 6.71 ± 0.07 (9) | No data |
| heart | 6.82 ± 0.09 (10) | No data |
| Fluid | ||
| blood | 6.49 ± 0.12 (31) | 6.20 ± 0.19 (14) |
| oviductal fluid | 6.18 ± 0.18 (32) | 5.69 ± 0.14 (18) |
| semen | 6.53 ± 0.13 (9) | 6.65 ± 0.18 (17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sensui, N.; Itoh, Y.; Okura, N.; Shiba, K.; Baba, S.A.; Inaba, K.; Yoshida, M. Spawning-Induced pH Increase Activates Sperm Attraction and Fertilization Abilities in Eggs of the Ascidian, Phallusia philippinensis and Ciona intestinalis. Int. J. Mol. Sci. 2023, 24, 2666. https://doi.org/10.3390/ijms24032666
Sensui N, Itoh Y, Okura N, Shiba K, Baba SA, Inaba K, Yoshida M. Spawning-Induced pH Increase Activates Sperm Attraction and Fertilization Abilities in Eggs of the Ascidian, Phallusia philippinensis and Ciona intestinalis. International Journal of Molecular Sciences. 2023; 24(3):2666. https://doi.org/10.3390/ijms24032666
Chicago/Turabian StyleSensui, Noburu, Yosinori Itoh, Nobuhiko Okura, Kogiku Shiba, Shoji A. Baba, Kazuo Inaba, and Manabu Yoshida. 2023. "Spawning-Induced pH Increase Activates Sperm Attraction and Fertilization Abilities in Eggs of the Ascidian, Phallusia philippinensis and Ciona intestinalis" International Journal of Molecular Sciences 24, no. 3: 2666. https://doi.org/10.3390/ijms24032666
APA StyleSensui, N., Itoh, Y., Okura, N., Shiba, K., Baba, S. A., Inaba, K., & Yoshida, M. (2023). Spawning-Induced pH Increase Activates Sperm Attraction and Fertilization Abilities in Eggs of the Ascidian, Phallusia philippinensis and Ciona intestinalis. International Journal of Molecular Sciences, 24(3), 2666. https://doi.org/10.3390/ijms24032666







