Simple Summary
Around 30–60% of the patients with lung cancer develop bone metastases, which are associated with decreased survival, bone pain, and skeletal-related events such as need for radiation. Patients with an epidermal growth factor mutation (EGFR), a subgroup of the patients with lung cancer, seem to develop more bone metastases than other patients with lung cancer. Due to prolonged survival of these patients, they live longer with bone metastases and/or skeletal-related events, therefore optimal management is warranted. The aim of our systematic review is to gain more insight in reporting of bone metastases, skeletal-related events, and bone-specific outcome of treatment in clinical trials enrolling patients with EGFR-mutated lung cancer. We found that data on bone metastases and bone-related outcomes are largely lacking in clinical trials. There should be more focus on reporting and preventing of skeletal-related events in these patients.
Abstract
Bone metastases, occurring in 30–60% of patients with non-small cell lung cancer (NSCLC), are associated with decreased survival, cancer-induced bone pain, and skeletal-related events (SREs). Those with an activating epidermal growth factor mutation (EGFR+) seem to be more prone to develop bone metastases. To gain more insight into bone metastases-related outcomes in EGFR+ NSCLC, we performed a systematic review on Pubmed (2006–2021). Main inclusion criteria: prospective, phase II/III trials evaluating EGFR-tyrosine kinase inhibitors, ≥10 EGFR+ patients included, data on bone metastases and/or bone-related outcomes available. Out of 663 articles, 21 (3176 EGFR+ patients) met the eligibility criteria; 4 phase III (one double blind), 17 phase II trials (three randomized) were included. In seven trials dedicated bone imaging was performed at baseline. Mean incidence of bone metastases at diagnosis was 42%; 3–33% had progression in the bone upon progression. Except for one trial, it was not specified whether the use of bone target agents was permitted, and in none of the trials, occurrence of SREs was reported. Despite the high incidence of bone metastases in EGFR+ adenocarcinoma, there is a lack of screening for, and reporting on bone metastases in clinical trials, as well as permitted bone-targeted agents and SREs.
1. Introduction
Activating epidermal growth factor mutations (EGFR) are found in approximately 10% of the Caucasian and 50% of the Asian patients with lung adenocarcinoma [1,2,3]. EGFR mutations are prognostic and are also predictive for efficacy of EGFR-tyrosine kinase inhibitors (TKI). For patients with advanced lung adenocarcinoma and an EGFR mutation, treated with epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), the five-year survival rate is 40–50% [4,5]. This is more favorable than the historical (i.e., before the introduction of immune checkpoint inhibitors) 5.8% five-year survival rate of patients without oncogenic-driven non-small-cell lung carcinoma (NSCLC) [6]. Immune checkpoint inhibitors (ICI) as monotherapy result in disappointing outcomes in patients with EGFR-mutated lung adenocarcinoma, with low responses rates and low survival and should only be considered after exhaustion of other systemic therapies [7]. ICI combined with EGFR-TKI has been evaluated in clinical studies but the combinations were either too toxic or did not provide an advantage over EGFR-TKI alone [7].
Importantly, the biological predisposition for distant metastases seems to vary between the different molecular subgroups of non-squamous NSCLC [8,9]. The largest series is a nationwide Dutch database analysis (n = 2052), including all patients with metastasized non-squamous NSCLC (ns-NSCLC) at initial diagnosis with data from molecular analysis and metastasis pattern at diagnosis of stage IV disease. A significantly higher bone metastases incidence was reported in patients with an EGFR mutation compared with other molecular subgroups (54% vs. 33% Kirsten rat sarcoma [KRAS+] vs. 30.5% anaplastic lymphoma kinase fusion [ALK+] vs. 31.5% triple negative patients, p < 0.001) [9]. However, other studies (n = 189–1063) evaluating the incidence of bone metastases in different molecular subgroups, i.e., EGFR-mutated, KRAS-mutated, ALK-rearranged or wildtype patients with non-squamous NSCLC, showed conflicting results [8,10,11,12,13].
The currently available clinical trials evaluating bone-targeted agents (BTAs) and clinical guidelines (European Society for Medical Oncology (ESMO), National Comprehensive Cancer Network (NCCN)) providing recommendations for the management of bone metastases and skeletal-related events (SREs), do not focus on specific molecular subgroups of lung adenocarcinoma [14,15,16,17,18]. The guidelines state that it is advised to treat patients with bone metastases and a favorable survival (specified as at least three months) with BTAs. However, a more personalized advise is important, as in clinical trials especially the patients with a prolonged overall survival (OS) (i.e., historically mainly patients with metastatic breast and prostate cancer) benefit most from BTAs (i.e., significant reduction of SREs) such as bisphosphonates or denosumab [19,20,21]. As the clinical behavior of EGFR-mutated lung adenocarcinoma resembles metastatic breast and prostate cancer, with a real possibility for prolonged survival, data for this subgroup is also needed. However, to the best of our knowledge, clinical trials evaluating BTAs specifically in EGFR-mutated lung adenocarcinoma do not exist.
The risk of a negative influence on quality of life (QoL) and OS, caused by SREs, could be significant in patients with EGFR-mutated lung adenocarcinoma and bone metastases [22,23]. In one retrospective case-control study (n = 189, no use of BTAs) survival post-bone metastases diagnosis was superior for patients with an EGFR mutation compared to patients with a KRAS mutation or those without an EGFR/KRAS mutation, while time to first SRE was not significantly different [12]. As a result, these patients live longer with SREs. Therefore, optimal management, treatment, and outcome of bone metastases in this specific patient population is necessary and should be further evaluated.
As large prospective series on bone metastases-related outcomes are lacking for patients with EGFR-mutated lung adenocarcinoma, we performed a systematic review to gain more insight in the reporting of bone metastases and/or SREs, and bone-specific outcomes in patients with EGFR-mutated lung adenocarcinoma included in phase II/III EGFR-TKI trials. Improved knowledge about bone-related events in EGFR-mutated tumors can lead to better advice about the use of BTA in this subgroup of patients.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
A systematic search was performed using the Pubmed database. The search period was limited to January 2006 until January 2021 (search data 8 January 2021). The start date of January 2006 was chosen, as in 2006 EGFR-TKIs were approved by the United States of America’s Food and Drug Administration (FDA), and became standard treatment for patients with lung adenocarcinoma and an EGFR mutation. Published studies were identified using a search strategy based on the patient intervention comparator outcome (PICO) method (shown in Table S1) [24]. Because the outcome variable of interest (i.e., bone metastases and SRE incidence) was an undefined endpoint in EGFR-TKI trials, we decided to exclude this outcome variable in the search strategy to prevent missing data. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 checklist for systematic reviews is shown in Table S2. Furthermore, the control intervention was not included in the search strategy to include single arm EGFR-TKI trials. Trials had to include a minimum of ten patients with non-squamous NSCLC and an EGFR mutation, as trials in the beginning of the TKI era also included patients with wildtype EGFR. Only prospective, phase II and III trials were included. All inclusion criteria are summarized in Table 1.

Table 1.
Inclusion criteria.
To minimize missing articles, in the same time period, we searched for relevant articles in the meeting libraries of the American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO) and International Association for the Study of Lung Cancer (IASLC).
2.2. Study Selection
The titles of the retrieved studies, and the abstracts of the eligible studies based on title screening, were evaluated independently by two reviewers (A.B. and S.D.). The same reviewers independently examined the full text of the remaining articles regarding the inclusion criteria. Studies were included if they met the pre-specified inclusion criteria as shown in Table 1. To complete the search, the references of all eligible articles were manually searched for additional relevant articles. In case of disagreement during study inclusion, consensus was sought.
2.3. Data Selection
Two reviewers (A.B. and S.D.) independently extracted relevant characteristics of each eligible study. When available and if applicable, the following data were extracted: year of publication; phase II or III trial, number of study arms, randomization method, blinding method, duration of study and follow-up, histological diagnosis, method of staging, number of patients and number of patients with an EGFR+ mutation, intervention (i.e., type, dose, duration, route and frequency of administration of TKIs), bone metastases (i.e., incidence, outcome, treatment), SREs (i.e., incidence, outcome, treatment), secondary and primary objectives of the trial, and OS. The Jadad scale was used to assess the methodological quality of the included trials [25]. We did not perform a formal test of heterogeneity because of the heterogeneous type of trials included in the systemic review, with three quarter of the included trials being single arm (i.e., per definition high risk of bias).
3. Results
3.1. Study Selection
The literature search identified 663 unique articles in total. About 317 articles were excluded because of non-relevant titles. About 160 of the 346 remaining articles were excluded because they did not fulfill the inclusion criteria based on the abstract. The full text of the remaining 186 articles was screened; 166 articles were excluded due to: no information about bone metastases or SREs (n = 139), unknown EGFR mutational status (n = 10), unknown if the patients with NSCLC and bone metastases were patients with an EGFR mutation or if they were wildtype patients (n = 9), insufficient patients with an EGFR mutation (n = 4), other reasons (n = 4). A manual search of the reference list of the included articles revealed one additional relevant article. No additional studies were identified by searching the meeting libraries of the ASCO, ESMO, and IASLC conferences in the period 2006–2021. Ultimately, 21 articles were included in this review. The flowchart for article selection is shown in Figure 1.
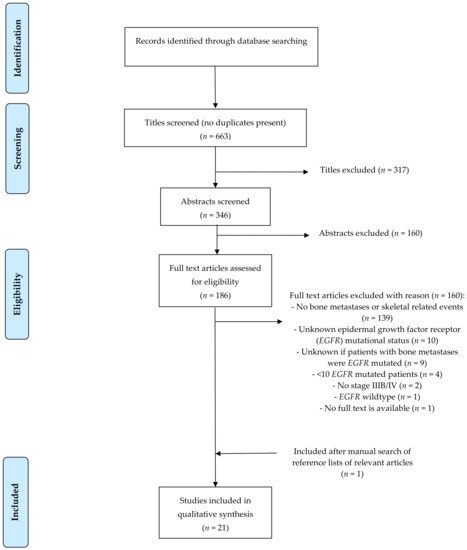
Figure 1.
Flowchart.
3.2. Description of Studies
Four phase III trials [26,27,28,29], of which one double blind randomized [29], and 17 phase II trials [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], of which three randomized [37,39,40], were included. The main characteristics of the included studies are shown in Table 2.

Table 2.
Main characteristics of the included studies.
The number of patients included in the studies ranged from 10 [38] to 556 (Flaura [29]), leading to in total 3176 patients with advanced NSCLC and an EGFR mutation included in this review. One trial also enrolled patients with advanced non-squamous NSCLC and EGFR wildtype, the results were specified per molecular subgroup [46]. The inclusion criteria were generally similar across the included trials (i.e., pathological proven locally advanced NSCLC not suitable for treatment with radical radio-chemotherapy or metastatic NSCLC). All studies, except one in which also patients with non-squamous NSCLC and EGFR wildtype were included [46], enrolled exclusively patients with an activating EGFR mutation. The other exception in inclusion criteria was systemic treatment history of the patients. The exclusion criteria concerning comorbidities were comparable among all studies. The Aura 3 was the only trial in which explicitly a statement about bone-targeted agents (BTAs) was added, it was permitted for patients to use medication (e.g., denosumab) for painful bone metastases [26]. The other trials provided no information about BTAs [26,27,28,29,30,31,32,33,34,36,37,38,39,40,41,42,43,44,45,46].
In seven trials the primary endpoint was objective response rate (ORR) on EGFR-TKI treatment [30,31,32,33,34,36] or chemotherapy combined with EGFR-TKI treatment [43]. ORR was evaluated in different patient categories: patients with NSCLC and EGFR mutation pretreated with chemotherapy or TKI (Aura 2 [32], [30], KCSG-Lu15-09 [31]), irrespective of previous chemotherapy [36], patients that were chemotherapy or TKI-naïve [33,34] and treatment naïve [43]. Progression-free survival (PFS) was the primary outcome in 11 trials [26,27,28,29,35,37,38,39,41,42,45]. PFS was evaluated in different patient categories: patients with NSCLC and an EGFR mutation who were treatment naïve in one trial [45] or treatment naïve for advanced disease in five trials (Lux-Lung 7 [40], Jo22903 and Jo25567 trial [39], Flaura, [27,29,38]). In the other trials patients with NSCLC and EGFR mutation were pretreated with EGFR-TKIs (Aura 3, [35,42,47]), were chemotherapy naïve (Aspiration study [41,46]) and chemotherapy naïve for advanced disease (Eurtac [28], Insight study [37]). The study of Yoshimura (2013) in which patients with NSCLC and an EGFR mutation who previously were treated with EGFR-TKIs and in the trial were treated with pemetrexed in combination with erlotinib or gefitinib had disease control rate as primary outcome. Two trials had co-primary endpoints: PFS and response to treatment [46] and PFS, time to treatment failure, OS in Lung-Lux 7 [40]. None of the trials had bone-metastases-related outcomes as primary or secondary endpoint.
3.3. Assessment of the Risk of Bias Within Studies
A formal test of heterogeneity was not performed due to the heterogeneous type of trials included in this systematic review: only four trials were randomized controlled trials, whereas two-third of the trials being single arm had per definition a high risk of bias. Instead, we used the Jadad scale to assess the methodological quality of the included studies [25]. The methodological quality of four of 21 studies were assessed as high (i.e., Jadad score ≥ 3) [27,28,29,40]. The other 17 studies were assessed as poor methodological quality (i.e., Jadad score ≤ 2) [26,30,31,32,33,34,35,36,37,38,39,41,42,43,44,45,46].
3.4. Results of Individual Studies
3.4.1. Imaging and Incidence of Bone Metastases at Baseline
In 12 out of 21 studies the mandated imaging at study entry was described [28,30,31,32,33,36,39,42,43,44,45,46]. Dedicated bone imaging was performed in seven out of 21 trials, by means of a bone scintigraphy [33,36,39,43,44] or a 2-deoxy-2-[fluorine-18]fluoro-D-glucose positron emission tomography-computer tomography scan (FDG-PET-CT scan) [45,46].
The incidence of bone metastases at baseline was reported in 14 studies (total 1196 patients) [27,28,31,34,35,36,37,38,40,42,43,44,45,46]. Out of these 1196 patients, 502 (42%) had bone metastases at baseline (range 14–90%). In none of these studies the bone metastasis was a stratification factor.
3.4.2. Imaging and Incidence of Bone Metastases during Follow-Up
In two out of 21 studies dedicated bone imaging during follow-up was performed: with FDG-PET-CT scan [45,46] or with a bone scintigraphy [33]. Bone scintigraphy was performed in the study of Reguart when clinically indicated [42].
In ten studies (total 2378 patients) the incidence of bone metastases as site of progressive disease (PD) was reported ([27,30,33,38,39,46], Flaura [29], Aura 2 [32], Aura 3 [26], Aspiration study [41]). Three to 26% of the patients had development or progression of bone metastases as site of PD (215 of 2378 patients). In none of the included studies data were provided whether bone progression was the only site of progression or not.
3.4.3. Skeletal Related Events
In none of the included studies information about SREs was provided. In Table 3 a summary of the reported imaging, incidence of bone metastases and SREs is shown.

Table 3.
Summary of the reported imaging, incidence of bone metastases and SREs of the included studies.
4. Discussion
Bone metastases with their risk of SRE development and resulting impact on QoL, can become a clinically relevant problem in patients with lung adenocarcinoma and an EGFR mutation because of their prolonged post-bone metastases diagnosis survival [4,12,23,48,49]. To gain more insight into bone metastases and their outcomes, we performed a systematic review focusing on screening, treatment, and reporting of bone metastases and/or SREs and bone-specific outcomes in EGFR-TKI trials.
In none of the trials, primary or secondary outcomes related to bone metastases and/or its complications were mentioned. A 42% median baseline incidence of bone metastases in patients with NSCLC and an EGFR mutation was reported, which is slightly lower compared to the 54% baseline incidence reported in the Dutch nationwide database study and other retrospective studies [9,12,50]. Of note, in only seven of the included trials specific bone imaging was performed at baseline, possibly resulting in an underestimation of the real incidence of bone metastases. Up to 26% of patients had progression in the bone upon PD. This probably is also underestimated as in only two of the trials standardized follow-up bone imaging was performed. Patients in the Aura 2 trial were permitted to use BTAs in case of painful bone metastases, but further information on actual BTA use and outcome was not provided. In all other trials, all data regarding BTA was lacking.
EGFR-TKIs have a high efficacy in patients with lung adenocarcinoma and an EGFR mutation and bone metastases [51,52]. Their efficacy in bone is mediated by blockade of receptor activator of NF-κB ligand (RANKL)-mediated osteoclast activation and by inhibiting epidermal growth factor (EGF) signaling in bone stromal cells [53]. Therefore, it could be that in this specific patient population, bone metastases do not frequently lead to SREs. Indeed, a retrospective study in patients with lung adenocarcinoma and bone metastases (n = 410) reported a preventive effect of EGFR-TKIs on the development of SREs: 23.5% of the patients with lung adenocarcinoma who were treated with EGFR-TKIs experienced SREs compared with 61.7% of patients without EGFR-TKI treatment (information about specific treatment in this group is not provided) (p < 0.001) [54]. However, even with EGFR-TKI use almost a quarter of the patients experienced SREs in this study, and in other studies, the reported frequency of SREs is even higher (37.3% to 58%) in patients with EGFR-mutated lung adenocarcinoma mainly treated with EGFR-TKIs [12,49,54,55,56,57]. A recently published retrospective study, which evaluated the type and frequency of SREs in patients with EGFR-mutated lung adenocarcinoma and bone metastases (n = 274, of which 148 treated with EGFR-TKI), showed that one-third of these patients developed their first SRE before start of EGFR-TKI treatment, the other two-third of the patients developed SREs in the first year of EGFR-TKI treatment [49]. The above summarized SRE percentages were observed in patients with EGFR-mutated lung adenocarcinoma, treated with first or second generation EGFR-TKIs. To the best of our knowledge, no data are available for the different generation EGFR-TKIs (i.e., first/second versus third) regarding efficacy specifically on bone metastases. Mouse models were set up to investigate the efficacy of osimertinib with or without bevacizumab on bone metastases of NSCLC. Treatment with osimertinib (with and without bevacizumab), showed tumor regression and bone remodeling [58]. Based on these results, it is not clear whether osimertinib is superior to earlier generation TKI in humans in the treatment of bone metastases.
In the abovementioned retrospective studies, use of BTAs varied from 0 to 65% [12,49,54,55,56,57]. Interestingly, in vitro and in vivo studies showed that bisphosphonates can act synergistically with EGFR-TKIs [54,55,59]. The in vitro study of Chang on the HCC827 NSCLC cell line expressing mutated EGFR, suggested that the combination of gefitinib and zoledronic acid caused more tumor suppression [59]. A small retrospective study of Cui et al. (n = 38) studied the efficacy of bisphosphonates in patients with EGFR-mutated lung adenocarcinoma and bone metastases, treated with EGFR-TKIs. They showed a significant additive effect of bisphosphonates on OS post-bone metastases diagnosis: post-bone metastases OS in EGFR-TKI + bisphosphonate group: 28.3 months versus 22.0 months in the EGFR-TKI only group, p = 0.0587 [55]. Another small retrospective study studied the effects of bisphosphonates in patients with EGFR-mutated lung adenocarcinoma and bone metastases (n = 62) and found comparable results (PFS and OS prolonged in the bisphosphonate + EGFR-TKI group compared with the EGFR-TKI group) [54]. As these are retrospective, small series, these data are only hypothesis generating. To the best of our knowledge, no data on denosumab combined with EGFR-TKI are available and it would be interesting to prospectively evaluate this.
Due to the increasing number of treatment options (e.g., EGFR-TKI in combination with chemotherapy, or combination of EGFR-TKI with angiogenesis inhibition), survival is further improving for patients with an EGFR mutation [47,51,52,60,61]. In the Flaura trial, the median OS with first line osimertinib was 38.6 months, in the NEJ009 trial (combination gefitinib with carboplatinum/pemetrexed versus gefitinib alone), median OS was 50.9 months [51,60]. Five-year survival rates for these trials have not been reported yet, but with a median OS of 50 months, 5-year survival rates resemble that of advanced breast or prostate cancer in which 28.1–30.2% of the patients are alive five years after the diagnosis [48,62,63]. This is important, as it is suggested in retrospective series that patients with EGFR-mutated NSCLC have a long post-bone metastatic survival of 15.5 to 28.0 months [12,49], implying that these patients live long with SREs.
Despite the similarities in the incidence and nature of EGFR-mutated lung adenocarcinoma and breast cancer bone metastases, current guidelines (e.g., ESMO, National Comprehensive Cancer Network [NCCN], and National Institute for Health and Care Excellence [NICE], ASCO) provide different recommendations for screening of bone metastases for different primary tumors [18,64,65,66,67]. The most important difference between the guidelines is the recommendation to screen all breast cancer patients, whereas for NSCLC only the Lung Cancer South East French Guidelines recommend to screen for bone metastases in NSCLC [18,64,65,66,67,68]. The French guideline also recommends to evaluate each bone metastasis for pain, neurological risk, and fracture risk to aid in defining the optimal bone metastasis management in harmony with the oncological treatment [68]. BTAs demonstrated benefit in reducing SREs and providing better pain control, in advanced breast patients diagnosed with bone metastases [19]. In the ESMO guideline on advanced breast cancer it is recommended to use BTAs in these patients (level of evidence I, grade of recommendation A [65]. Guidelines for lung cancer are less clear in their recommendations: the NCCN NSCLC guideline advises to consider BTAs in patients with NSCLC and bone metastases [16]. The ESMO guideline on bone health further specifies and recommends using BTAs in patients with a life expectancy of >3 months (i.e., almost all patients with an EGFR mutation) [18,67]. No specific recommendations for patients with EGFR-mutated lung adenocarcinoma were found in these guidelines. Probably because of the historically poor OS of NSCLC compared to advanced breast cancer, only 15–33% of patients with NSCLC and bone metastases are treated with BTAs in daily practice [69,70]. To the best of our knowledge no trials are ongoing that evaluate BTAs in patients with an EGFR mutation, although trials in patients without an oncogenic driver are ongoing [(NCT03669523 trial: denosumab in combination with nivolumab, NCT01951586 trial: denosumab in combination with chemotherapy (recently finished, results are not published)].
Drawbacks for this systematic review are: (1) The heterogeneity of the included trials with differences in populations (e.g., ethnicity) and/or follow-up which could have led to the observed differences in reported incidences of SREs; (2) the lack of primary or secondary outcomes related to bone metastases and/or related complications in studies could have led to underreporting of these outcomes.
5. Conclusions
Despite long (post-bone metastatic) OS of patients with EGFR-mutated NSCLC, and the high incidence of bone metastases in this patient population, occurrence of SRE and outcome of bone metastases is barely reported in clinical trials. Based on in vitro data found and retrospective series there might be synergistic activity of EGFR-TKI and BTA. However, prospective research is needed to validate these observations. Furthermore, the results of this systematic review stress on the importance of screening for bone metastases and reporting of clinical outcomes of treatment on bone metastases future trials for patients with EGFR-mutated NSCLC.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13133144/s1. Table S1: search strategy based on PICO method, Table S2: PRISMA 2009 Checklist.
Author Contributions
Conceptualization, A.B., L.H. and A.-M.C.D.; methodology, A.B. and L.H.; software, not applicable; validation, A.B., S.D. and L.H.; formal analysis, not applicable.; investigation, A.B., S.D. and L.H.; resources, not applicable; data curation, A.B.; writing—original draft preparation, A.B. and L.H.; writing—review and editing, S.D., G.B. and A.-M.C.D.; visualization, A.B.; supervision, L.H.; project administration, A.B.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kerner, G.S.M.A.; Schuuring, E.; Sietsma, J.; Hiltermann, T.J.N.; Pieterman, R.M.; De Leede, G.P.J.; Van Putten, J.W.G.; Liesker, J.; Renkema, T.E.J.; Van Hengel, P.; et al. Common and Rare EGFR and KRAS Mutations in a Dutch Non-Small-Cell Lung Cancer Population and Their Clinical Outcome. PLoS ONE 2013, 8, e70346. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M.; et al. Screening for Epidermal Growth Factor Receptor Mutations in Lung Cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef]
- Sequist, L.V.; Heist, R.S.; Shaw, A.T.; Fidias, P.; Rosovsky, R.; Temel, J.S.; Lennes, I.T.; Digumarthy, S.; Waltman, B.A.; Bast, E.; et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann. Oncol. 2011, 22, 2616–2624. [Google Scholar] [CrossRef]
- Yamamoto, N.; Seto, T.; Nishio, M.; Goto, K.; Okamoto, I.; Yamanaka, T.; Tanaka, M.; Takahashi, K.; Fukuoka, M.; Yamamoto, N. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first-line treatment for advanced EGFR mutation–positive non-squamous non–small-cell lung cancer (NSCLC): Survival follow-up results of JO25567. J. Clin. Oncol. 2018, 36, 9007. [Google Scholar] [CrossRef]
- Maemondo, M.; Fukuhara, T.; Saito, H.; Furuya, N.; Watanabe, K.; Sugawara, S.; Iwasawa, S.; Tsunezuka, Y.; Yamaguchi, O.; Okada, M.; et al. NEJ026: Final overall survival analysis of bevacizumab plus erlotinib treatment for NSCLC patients harboring activating EGFR-mutations. J. Clin. Oncol. 2020, 38, 9506. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 6 June 2021).
- Remon, J.; Hendriks, L.; Cabrera, C.; Reguart, N.; Besse, B. Immunotherapy for oncogenic-driven advanced non-small cell lung cancers: Is the time ripe for a change? Cancer Treat. Rev. 2018, 71, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Dormieux, A.; Mezquita, L.; Cournede, P.H.; Remon, J.; Tazdait, M.; Lacroix, L.; Rouleau, E.; Adam, J.; Bluthgen, M.V.; Facchinetti, F.; et al. Association of metastatic pattern and molecular status in stage IV non-small cell lung cancer adenocarcinoma. Eur. Radiol. 2020, 30, 5021–5028. [Google Scholar] [CrossRef]
- Kuijpers, C.; Hendriks, L.; Derks, J.; Dingemans, A.-M.; van Lindert, A.; Heuvel, M.V.D.; Damhuis, R.; Willems, S. Association of molecular status and metastatic organs at diagnosis in patients with stage IV non-squamous non-small cell lung cancer. Lung Cancer 2018, 121, 76–81. [Google Scholar] [CrossRef]
- Doebele, R.C.; Lu, X.; Sumey, C.; Bs, D.A.M.; Weickhardt, A.J.; Oton, A.B.; Bunn, P.A.; Barón, A.E.; Franklin, W.A.; Aisner, D.L.; et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012, 118, 4502–4511. [Google Scholar] [CrossRef]
- Guan, J.; Chen, M.; Xiao, N.; Li, L.; Zhang, Y.; Li, Q.; Yang, M.; Liu, L.; Chen, L. EGFR mutations are associated with higher incidence of distant metastases and smaller tumor size in patients with non-small-cell lung cancer based on PET/CT scan. Med Oncol. 2016, 33, 1–8. [Google Scholar] [CrossRef]
- Hendriks, L.; Smit, E.; Vosse, B.; Mellema, W.; Heideman, D.; Bootsma, G.; Westenend, M.; Pitz, C.; de Vries, G.; Houben, R.; et al. EGFR mutated non-small cell lung cancer patients: More prone to development of bone and brain metastases? Lung Cancer 2014, 84, 86–91. [Google Scholar] [CrossRef]
- Li, H.; Cao, J.; Zhang, X.; Song, X.; Wang, W.; Jia, S.; Li, Z.; Jia, H.; Cao, X.; Zhou, W.; et al. Correlation between status of epidermal growth factor receptor mutation and distant metastases of lung adenocarcinoma upon initial diagnosis based on 1063 patients in China. Clin. Exp. Metastasis 2017, 34, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Brouns, A.J.W.M.; Hendriks, L.E.L.; Van Der Noort, V.; Van De Borne, B.E.E.M.; Schramel, F.M.N.H.; Groen, H.J.; Biesma, B.; Smit, H.J.M.; Dingemans, A.-M.C. Efficacy of Ibandronate Loading Dose on Rapid Pain Relief in Patients With Non-Small Cell Lung Cancer and Cancer Induced Bone Pain: The NVALT-9 Trial. Front. Oncol. 2020, 10, 890. [Google Scholar] [CrossRef]
- Levasseur, N.; Clemons, M.; Hutton, B.; Shorr, R.; Jacobs, C. Bone-targeted therapy use in patients with bone metastases from lung cancer: A systematic review of randomized controlled trials. Cancer Treat. Rev. 2016, 50, 183–193. [Google Scholar] [CrossRef] [PubMed]
- NCCN. NCCN Guidelines Version 3.2020 Non-Small Cell Lung Cancer; NCCN Evidence Books: Plymouth, PA, USA, 2020. [Google Scholar]
- Peters, S.; Danson, S.; Hasan, B.; Dafni, U.; Reinmuth, N.; Majem, M.; Tournoy, K.G.; Mark, M.T.; Pless, M.; Cobo, M.; et al. A randomised open-label phase iii trial evaluating the addition of denosumab to standard first-line treatment in advanced nsclc—The etop and eortc splendour trial. J. Thorac. Oncol. 2020, 15, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- O’Carrigan, B.; Wong, M.H.; Willson, M.L.; Stockler, M.R.; Pavlakis, N.; Goodwin, A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2017, 10, CD003474. [Google Scholar] [CrossRef]
- Gartrell, B.A.; Coleman, R.; Efstathiou, E.; Fizazi, K.; Logothetis, C.J.; Smith, M.R.; Sonpavde, G.; Sartor, O.; Saad, F. Metastatic Prostate Cancer and the Bone: Significance and Therapeutic Options. Eur. Urol. 2015, 68, 850–858. [Google Scholar] [CrossRef]
- Macherey, S.; Monsef, I.; Jahn, F.; Jordan, K.; Yuen, K.K.; Heidenreich, A.; Skoetz, N. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst. Rev. 2017, 12, CD006250. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; Sandro, B.; Salvatore, I.; Alfredo, F.; Francesco, F.; Domenico, G.; Luca, M.; Nicla, L.V.; Toni, I.; Fausto, P.; et al. Natural history of non-small-cell lung cancer with bone metastases. Sci. Rep. 2015, 5, 1–9. [Google Scholar]
- Silva, G.T.; Silva, L.M.; Bergmann, A.; Thuler, L.C. Bone metastases and skeletal-related events: Incidence and prognosis according to histological subtype of lung cancer. Futur. Oncol. 2019, 15, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.d.C.; Pimenta, C.A.d.M.; Nobre, M.R.C. The pico strategy for the research question construction and evidence search. Revista Latino-Americana de Enfermagem 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.M.E.; et al. Osimertinib or platinum–pemetrexed in egfr t790m–positive lung cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Noronha, V.; Patil, V.M.; Joshi, A.; Menon, N.; Chougule, A.; Mahajan, A.; Janu, A.; Purandare, N.; Kumar, R.; More, S.; et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J. Clin. Oncol. 2020, 38, 124–136. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in UntreatedEGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Ahn, M.J.; Tsai, C.M.; Shepherd, F.A.; Bazhenova, L.; Sequist, L.V.; Hida, T.; Yang, J.C.H.; Ramalingam, S.S.; Mitsudomi, T.; Jänne, P.A.; et al. Osimertinib in patients with t790m mutation-positive, advanced non–small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer 2019, 125, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lim, S.H.; An, H.J.; Kim, K.H.; Park, K.U.; Kang, E.J.; Choi, Y.H.; Ahn, M.S.; Lee, M.H.; Sun, J.-M.; et al. Osimertinib for Patients With Non–Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J. Clin. Oncol. 2020, 38, 488–495. [Google Scholar] [CrossRef]
- Goss, G.; Tsai, C.-M.; Shepherd, A.F.; Bazhenova, L.; Lee, J.S.; Chang, G.-C.; Crino, L.; Satouchi, M.; Chu, Q.; Hida, T.; et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016, 17, 1643–1652. [Google Scholar] [CrossRef]
- Hirano, S.; Naka, G.; Takeda, Y.; Iikura, M.; Hayama, N.; Yanagisawa, A.; Amano, H.; Nakamura, M.; Nakamura, S.; Tabeta, H.; et al. A prospective, multicenter phase II trial of low-dose erlotinib as maintenance treatment after platinum doublet chemotherapy for advanced non-small cell lung cancer harboring EGFR mutation. Chin. Clin. Oncol. 2016, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Kobayashi, K.; Usui, K.; Maemondo, M.; Okinaga, S.; Mikami, I.; Ando, M.; Yamazaki, K.; Saijo, Y.; Gemma, A.; et al. First-Line Gefitinib for Patients With Advanced Non–Small-Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Mutations Without Indication for Chemotherapy. J. Clin. Oncol. 2009, 27, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Park, S.; Kim, Y.; Cho, J.H.; Park, S.E.; Lee, H.; Kim, H.K.; Kim, S.M.; Sun, J.M.; Lee, S.-H.; et al. Continuation of gefitinib beyond progression in patients with EGFR mutation-positive non-small-cell lung cancer: A phase II single-arm trial. Lung Cancer 2018, 124, 293–297. [Google Scholar] [CrossRef]
- Sunaga, N.; Tomizawa, Y.; Yanagitani, N.; Iijima, H.; Kaira, K.; Shimizu, K.; Tanaka, S.; Suga, T.; Hisada, T.; Ishizuka, T.; et al. Phase II prospective study of the efficacy of gefitinib for the treatment of stage III/IV non-small cell lung cancer with EGFR mutations, irrespective of previous chemotherapy. Lung Cancer 2007, 56, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Cheng, Y.; Zhou, J.; Lu, S.; Zhang, Y.; Zhao, J.; Kim, D.-W.; Soo, R.A.; Kim, S.-W.; Pan, H.; et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): An open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir. Med. 2020, 8, 1132–1143. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Xu, Z.; Yang, Q.; Zhu, G.; Liao, X.; Chen, X.; Zhu, B.; Duan, Y.; Sun, J. Concurrent EGFR-TKI and Thoracic Radiotherapy as First-Line Treatment for Stage IV Non-Small Cell Lung Cancer Harboring EGFR Active Mutations. Oncologist 2019, 24, 1031. [Google Scholar] [CrossRef]
- Atagi, S.; Goto, K.; Seto, T.; Yamamoto, N.; Tamura, T.; Tajima, K.; Inagaki, N. Erlotinib for Japanese patients with activating EGFR mutation-positive non-small-cell lung cancer: Combined analyses from two Phase II studies. Futur. Oncol. 2016, 12, 2117–2126. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.-H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.-H.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Park, K.; Yu, C.J.; Kim, S.W.; Lin, M.C.; Sriuranpong, V.; Tsai, C.M.; Lee, J.S.; Kang, J.H.; Chan, K.C.A.; Perez-Moreno, P.; et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer the aspiration study. JAMA Oncology 2016, 2, 305–312. [Google Scholar] [CrossRef]
- Reguart, N.; Rosell, R.; Cardenal, F.; Cardona, A.F.; Isla, D.; Palmero, R.; Moran, T.; Rolfo, C.; Pallarès, M.C.; Insa, A.; et al. Phase I/II trial of vorinostat (SAHA) and erlotinib for non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations after erlotinib progression. Lung Cancer 2014, 84, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Kudoh, S.; Mitsuoka, S.; Yoshimoto, N.; Oka, T.; Nakai, T.; Suzumira, T.; Matusura, K.; Tochino, Y.; Asai, K.; et al. Phase II study of a combination regimen of gefitinib and pemetrexed as first-line treatment in patients with advanced non-small cell lung cancer harboring a sensitive EGFR mutation. Lung Cancer 2015, 90, 65–70. [Google Scholar] [CrossRef]
- Yoshimura, N.; Okishio, K.; Mitsuoka, S.; Kimura, T.; Kawaguchi, T.; Kobayashi, M.; Hirashima, T.; Daga, H.; Takeda, K.; Hirata, K.; et al. Prospective Assessment of Continuation of Erlotinib or Gefitinib in Patients with Acquired Resistance to Erlotinib or Gefitinib Followed by the Addition of Pemetrexed. J. Thorac. Oncol. 2013, 8, 96–101. [Google Scholar] [CrossRef]
- Zwitter, M.; Rajer, M.; Stanic, K.; Vrankar, M.; Doma, A.; Cuderman, A.; Grmek, M.; Kern, I.; Kovac, V. Intercalated chemotherapy and erlotinib for non-small cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations. Cancer Biol. Ther. 2016, 17, 833–839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zwitter, M.; Stanic, K.; Rajer, M.; Kern, I.; Vrankar, M.; Edelbaher, N.; Kovac, V. Intercalated chemotherapy and erlotinib for advanced NSCLC: High proportion of complete remissions and prolonged progression-free survival among patients with EGFR activating mutations. Radiol. Oncol. 2014, 48, 361–368. [Google Scholar] [CrossRef]
- Mok, T.S.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Lee, M.; Linke, R.; Rosell, R.; Corral, J.; et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and egfr-activating mutations. J. Clin. Oncol. 2018, 36, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Morita, S.; Tashiro, N.; Imamura, F.; Inoue, A.; Seto, T.; Yamamoto, N.; Ohe, Y.; Nakagawa, K.; Fukuoka, M. Real world treatment and outcomes in EGFR mutation-positive non-small cell lung cancer: Long-term follow-up of a large patient cohort. Lung Cancer 2018, 117, 14–19. [Google Scholar] [CrossRef]
- Laganà, M.; Gurizzan, C.; Roca, E.; Cortinovis, D.; Signorelli, D.; Pagani, F.; Bettini, A.; Bonomi, L.; Rinaldi, S.; Berardi, R.; et al. High Prevalence and Early Occurrence of Skeletal Complications in EGFR Mutated NSCLC Patients With Bone Metastases. Front. Oncol. 2020, 10, 588862. [Google Scholar] [CrossRef]
- Fujimoto, D.; Ueda, H.; Shimizu, R.; Kato, R.; Otoshi, T.; Kawamura, T.; Tamai, K.; Shibata, Y.; Matsumoto, T.; Nagata, K.; et al. Features and prognostic impact of distant metastasis in patients with stage IV lung adenocarcinoma harboring EGFR mutations: Importance of bone metastasis. Clin. Exp. Metastasis 2014, 31, 543–551. [Google Scholar] [CrossRef]
- Hosomi, Y.; Morita, S.; Sugawara, S.; Kato, T.; Fukuhara, T.; Gemma, A.; Takahashi, K.; Fujita, Y.; Harada, T.; Minato, K.; et al. Gefitinib alone versus gefitinib plus chemotherapy for non–small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J. Clin. Oncol. 2020, 38, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Garon, E.B.; Seto, T.; Nishio, M.; Aix, S.P.; Paz-Ares, L.; Chiu, C.-H.; Park, K.; Novello, S.; Nadal, E.; et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1655–1669. [Google Scholar] [CrossRef]
- D’Antonio, C.; Passaro, A.; Gori, B.; Del Signore, E.; Migliorino, M.R.; Ricciardi, S.; Fulvi, A.; de Marinis, F. Bone and brain metastasis in lung cancer: Recent advances in therapeutic strategies. Ther. Adv. Med Oncol. 2014, 6, 101–114. [Google Scholar] [CrossRef]
- Huang, S.-M.; Yang, J.-J.; Chen, H.-J.; Wu, S.-P.; Bai, X.-Y.; Zhou, Q.; Tu, H.-Y.; Wu, Y.-L. Epidermal growth factor receptor is associated with the onset of skeletal related events in non-small cell lung cancer. Oncotarget 2017, 8, 81369–81376. [Google Scholar] [CrossRef][Green Version]
- Cui, X.; Li, S.; Gu, J.; Lin, Z.; Lai, B.; Huang, L.; Feng, J.; Liu, B.; Zhou, Y. Retrospective study on the efficacy of bisphosphonates in tyrosine kinase inhibitor-treated patients with non-small cell lung cancer exhibiting bone metastasis. Oncol. Lett. 2019, 18, 5437–5447. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, R.; Zhang, Z.; Jiang, T.; Ren, S.; Ma, Z.; Zhao, S.; Zhou, C.; Zhang, J. Bisphosphonates enhance antitumor effect of EGFR-TKIs in patients with advanced EGFR mutant NSCLC and bone metastases. Sci. Rep. 2017, 7, 42979. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Kudoh, S.; Mitsuoka, S.; Suzumura, T.; Umekawa, K.; Tanaka, H.; Matsuura, K.; Kimura, T.; Yoshimura, N.; Hirata, K. Skeletal-related events in advanced lung adenocarcinoma patients evaluated EGFR mutations. Osaka City Med. J. 2013, 59, 45–52. [Google Scholar]
- Higuchi, T.; Sugisawa, N.; Park, J.H.; Sun, Y.; Zhu, G.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Igarashi, K.; et al. Osimertinib regressed an EGFR-mutant lung-adenocarcinoma bone-metastasis mouse model and increased long-term survival. Transl. Oncol. 2020, 13, 100826. [Google Scholar] [CrossRef]
- Chang, J.W.-C.; Hsieh, J.-J.; Shen, Y.-C.; Yeh, K.-Y.; Wang, C.-H.; Li, Y.-Y.; Hsu, T. Bisphosphonate zoledronic acid enhances the inhibitory effects of gefitinib on EGFR-mutated non-small cell lung carcinoma cells. Cancer Lett. 2009, 278, 17–26. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Fukuhara, T.; Furuya, N.; Watanabe, K.; Sugawara, S.; Iwasawa, S.; Tsunezuka, Y.; Yamaguchi, O.; Okada, M.; Yoshimori, K.; et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019, 20, 625–635. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 6 June 2021).
- Cancer Stat Facts: Prostate Cancer. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 6 June 2021).
- Hanna, N.; Johnson, D.; Temin, S.; Baker, S.; Brahmer, J.; Ellis, P.M.; Giaccone, G.; Hesketh, P.J.; Jaiyesimi, I.; Leighl, N.B.; et al. Systemic therapy for stage iv non–small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2017, 35, 3484–3515. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th eso-esmo international consensus guidelines for advanced breast cancer (abc 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Hadji, P.; Body, J.-J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Non-Small Cell Lung Cancer. Version 3. 2020. Available online: https://www2.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf (accessed on 6 June 2021).
- Confavreux, C.B.; Pialat, J.-B.; Bellière, A.; Brevet, M.; Decroisette, C.; Tescaru, A.; Wegrzyn, J.; Barrey, C.; Mornex, F.; Souquet, P.-J.; et al. Bone metastases from lung cancer: A paradigm for multidisciplinary onco-rheumatology management. Jt. Bone Spine 2019, 86, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, M.; DeLea, T.E.; Cong, Z.; Chung, K. Utilization of intravenous bisphosphonates in patients with bone metastases secondary to breast, lung, or prostate cancer. Support. Care Cancer 2013, 22, 103–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oster, G.; Lamerato, L.; Glass, A.G.; Richert-Boe, K.E.; López, A.; Chung, K.; Richhariya, A.; Dodge, T.; Wolff, G.G.; Balakumaran, A.; et al. Use of intravenous bisphosphonates in patients with breast, lung, or prostate cancer and metastases to bone: A 15-year study in two large US health systems. Support. Care Cancer 2014, 22, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).