“Malancha” [Alternanthera philoxeroides (Mart.) Griseb.]: A Potential Therapeutic Option against Viral Diseases
Abstract
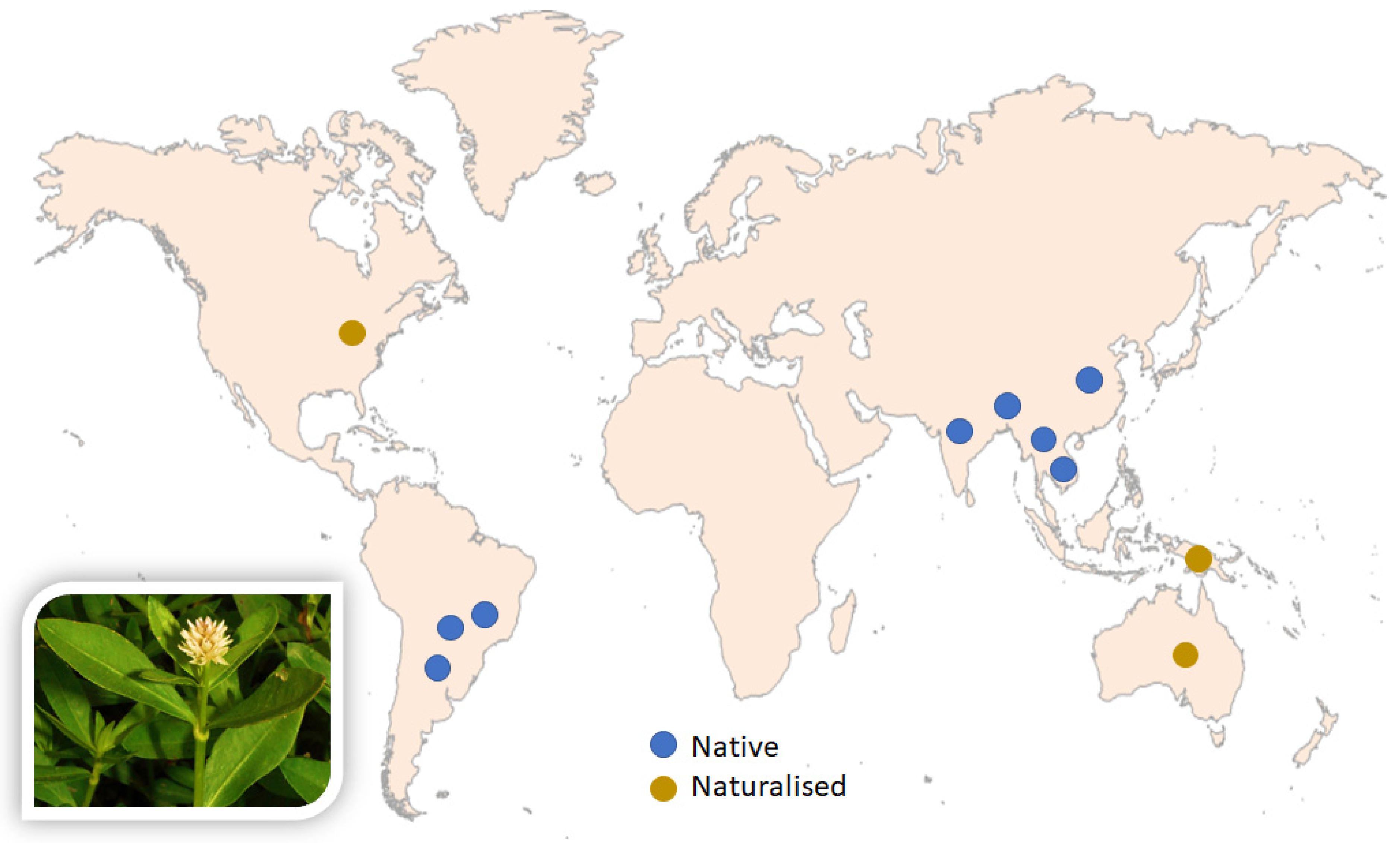
:1. Introduction
2. Phytochemical Profile and Biomolecular Interactions
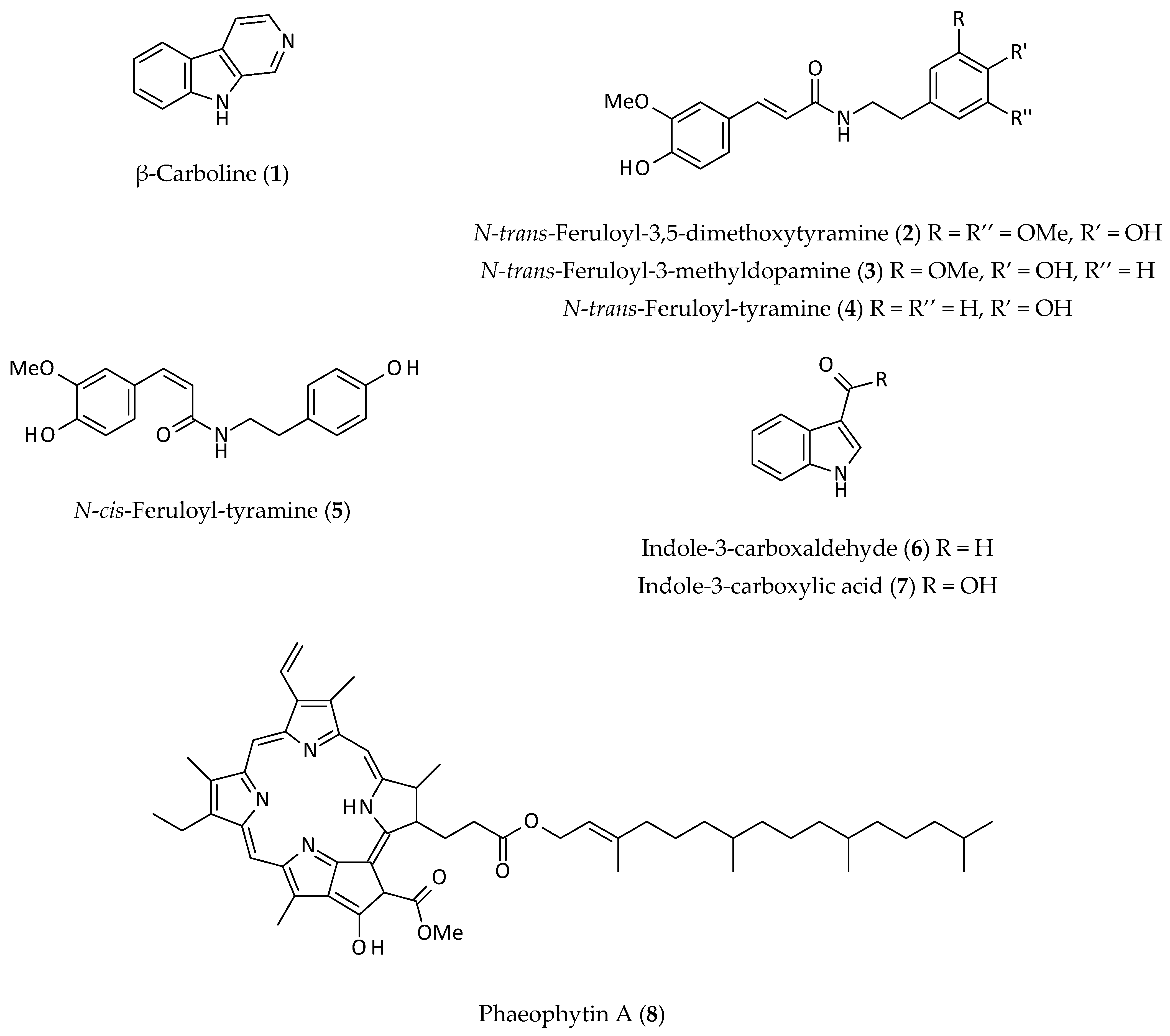
2.1. Alkaloids
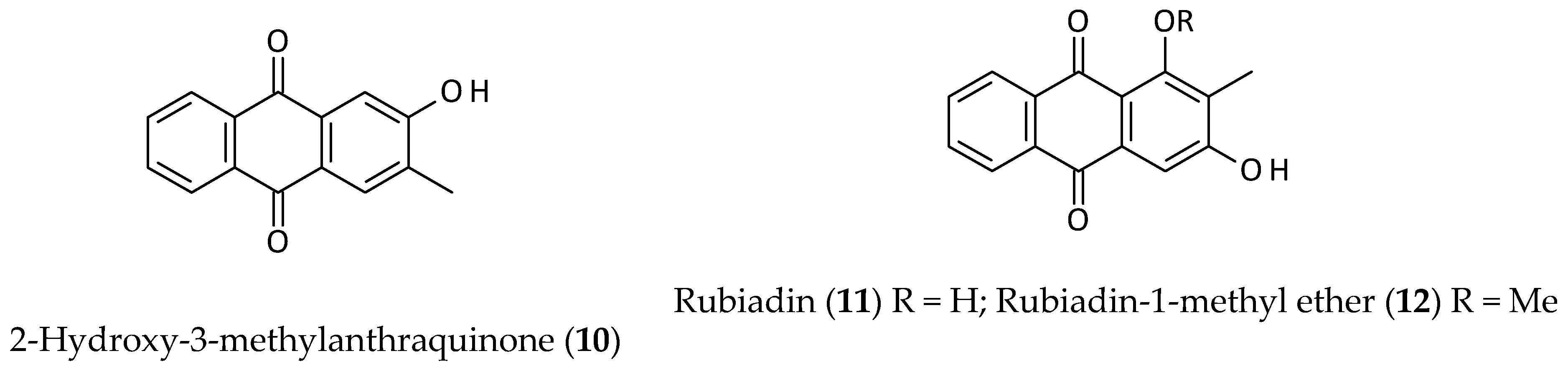
2.2. Anthraquinones
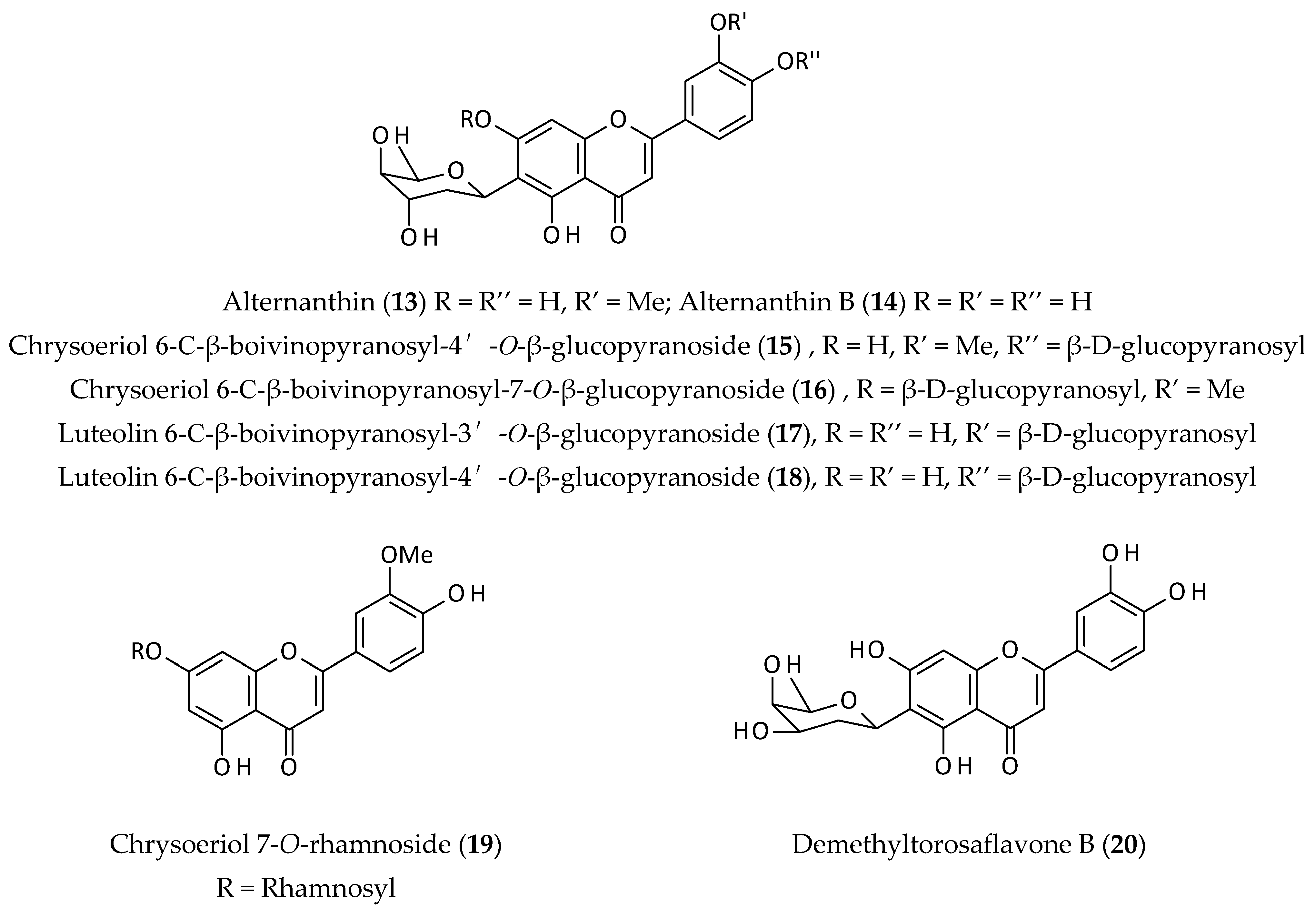
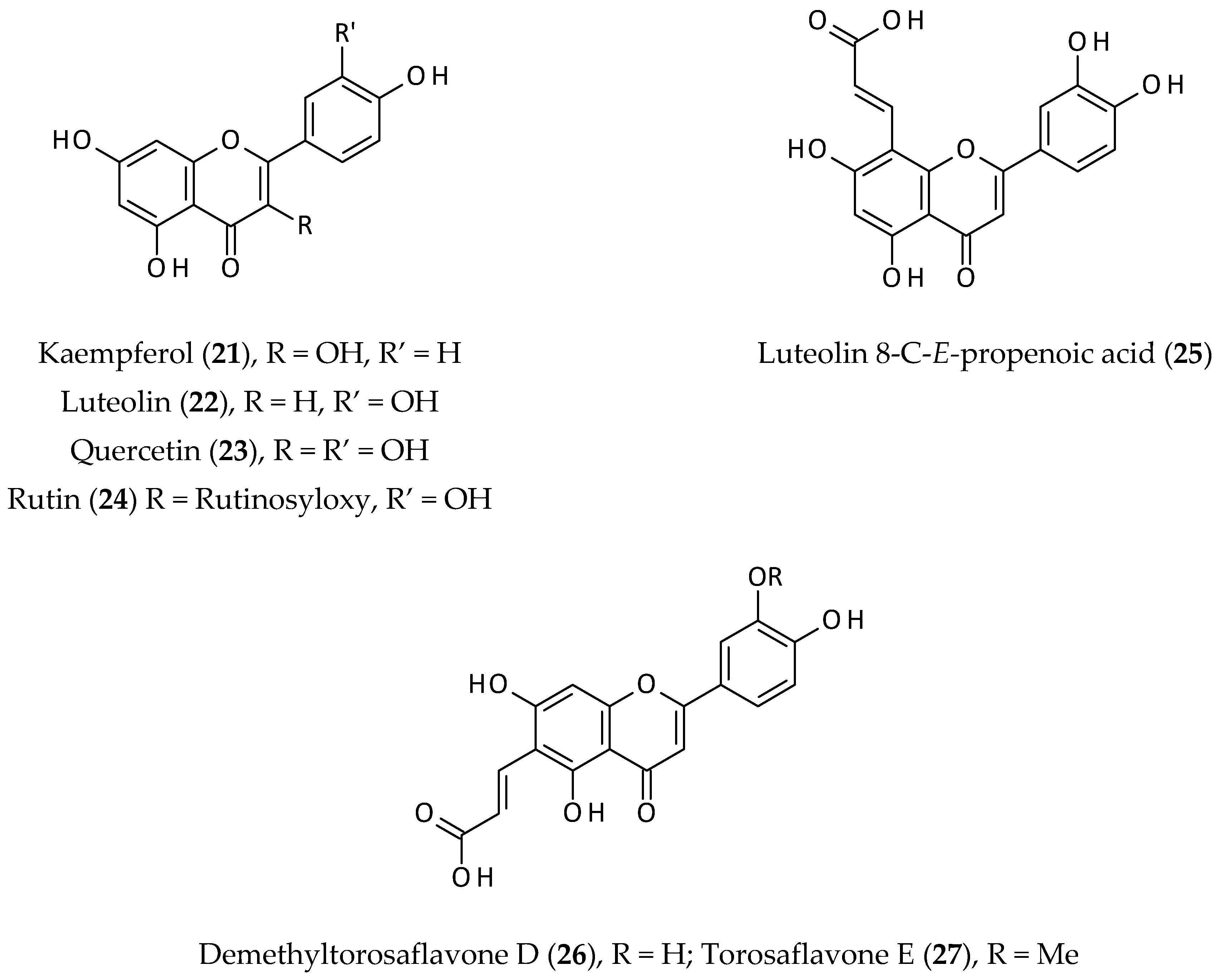
2.3. Flavonoids
2.4. Megastigmanes
2.5. Other Phenolics
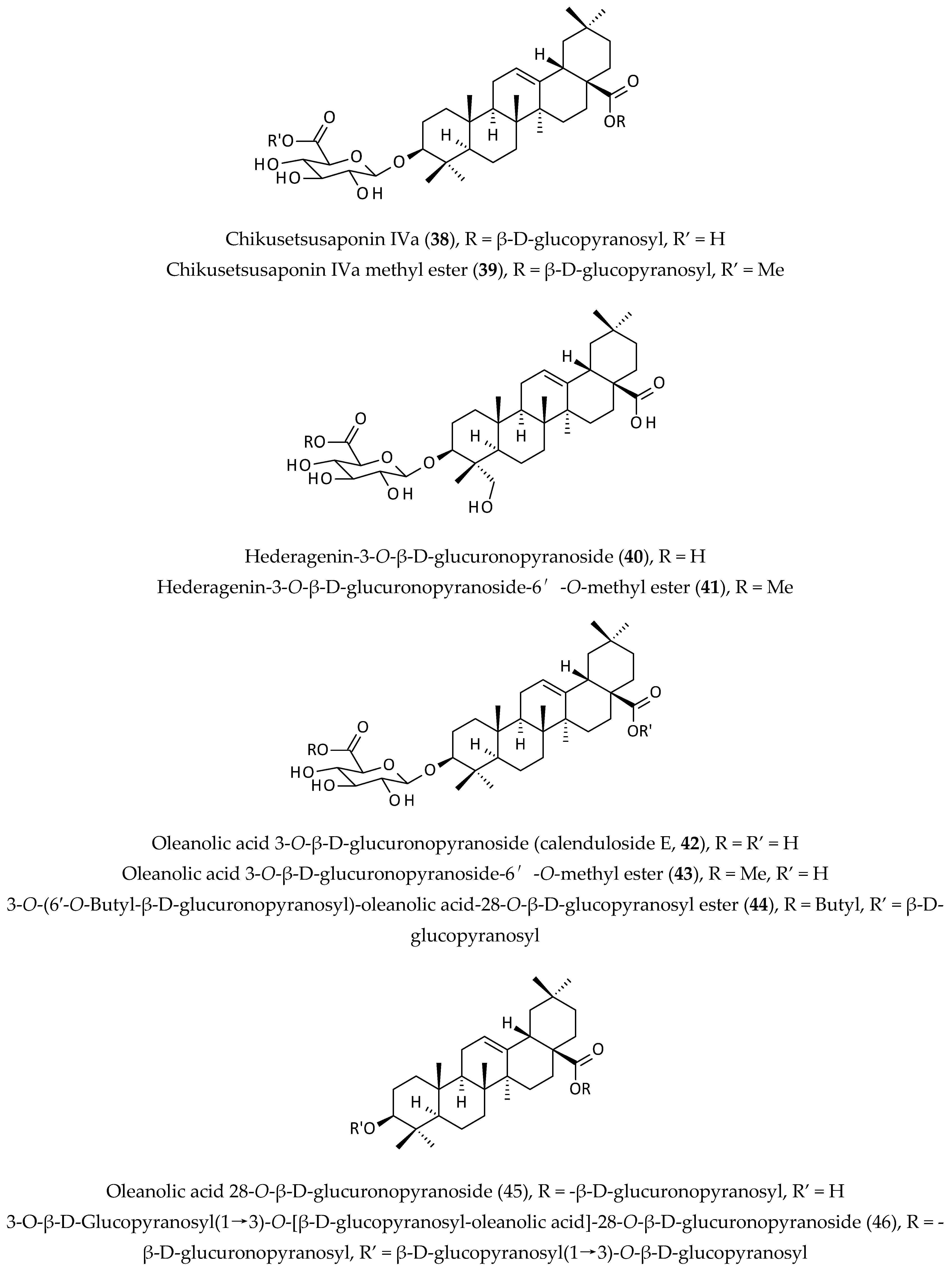
2.6. Saponins
2.7. Sterols
2.8. Terpenoids
2.9. Miscellaneous
3. Bioactivities and Therapeutic Potential as An Antiviral Agent
3.1. Antibacterial Activity
3.2. Anticancer and Antitumour Activity
3.3. Antidementia Activity
3.4. Antidepressant-Like Activity
3.5. Antihyperglycaemic Activity
3.6. Antinociceptive Activity
3.7. Antioxidant Activity
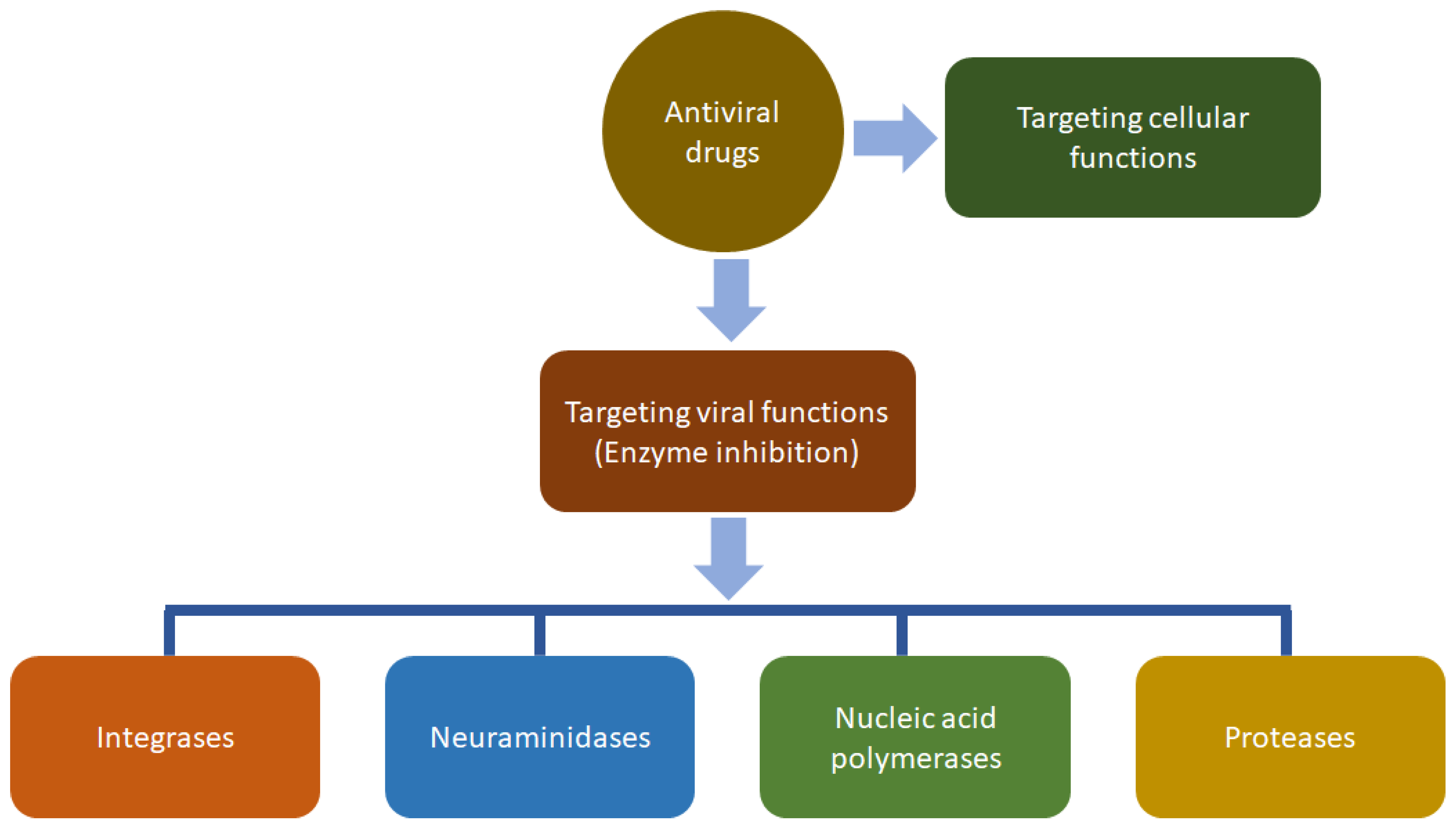
3.8. Antiviral Activity
3.9. Cardioprotective Activity
3.10. Cholinesterase Inhibitory Activity
3.11. Oestrogenic Activity
3.12. Immunomodulatory Activity
4. Toxicological Aspects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khatun, M.; Hassan, M.A.; Islam, S.N.; Rahman, M.O. Taxonomy of the leafy vegetables in Bangladesh. Bangladesh J. Plant Taxon. 2013, 20, 95–123. [Google Scholar] [CrossRef] [Green Version]
- Sunmathi, D.; Sivakumar, R. In Vitro cytotoxicity of ethanolic leaf extract of Alternanthera sessilis (L.) R. Br. Ex. DC and Alternanthera philoxeroides (Mart.) Griseb. Against human osteosarcoma cell line MG-63. Eur. J. Biomed. Pharm. Sci. 2016, 3, 416–420. [Google Scholar]
- Akbar, M.; Amin, A.; Khalil, T.; Iqbal, M.S.; Nazir, A.; Taswar, A. Antibacterial activity of Alternanthera philoxeroides (Mart.) Griseb. against bacterial phytopathogens: Erwinia carotovora, Ralstonia solanacearum and Xanthomonas axonopodis. Allelopath. J. 2021, 53, 83–92. [Google Scholar] [CrossRef]
- Zhou, B.-N.; Gabor, B.; Cordell, G.A. Alternanthin, a glycosylated flavonoid from Alternanthera philoxeroides. Phytochemistry 1988, 27, 3633–3636. [Google Scholar] [CrossRef]
- Fang, J.-B.; Jia, W.; Gao, W.-Y.; Yao, Z.; Teng, J.; Zhao, A.-H.; Duan, H.-Q. Antitumor constituents from Alternanthera Philoxeroides. J. Asian Nat. Prod. Res. 2007, 9, 511–516. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ghosh, T.; Sil, R.; Datta, A. Isolation and characterisation of methanol-soluble fraction of Alternanthera philoxeroides (Mart.) evaluation of their antioxidant, α-glucosidase inhibitory and antimicrobial activity in vitro systems. Nat. Prod. Res. 2014, 28, 2199–2202. [Google Scholar] [CrossRef]
- Kleinowski, A.M.; Ribeiro, G.A.; Milech, C.; Braga, E.J.B. Potential allelopathic and antibacterial activity from Alternanthera Philoxeroides. Hoehnea 2016, 43, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.B.; Yao, Z.; Chen, J.C.; Liu, Y.W.; Takaishi, Y.; Hongquan, Y.D. Cytotoxic triterpene saponins from Alternanthera philoxeroides. J. Asian Nat. Prod. Res. 2009, 11, 261–266. [Google Scholar] [CrossRef]
- Fang, J.B.; Liu, J.C.; Hongquan, Y.D. Constituents from Alternanthera philoxeroides and their antitumour activity. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 2009, 34, 2473–2476. [Google Scholar]
- Li, B.; Guo, Q.-L.; Tian, Y.; Liu, S.-J.; Wang, Q.; Li, C.; Dong, J.-X. New anti-HBV C-boivinopyranosyl flavones from Alternanthera philoxeroides. Molecules 2016, 21, 336. [Google Scholar] [CrossRef] [Green Version]
- Khamphukdee, C.; Monthakantirat, O.; Chulikhit, Y.; Buttachon, S.; Lee, M.; Silva, A.M.S.; Sekeroglu, N.; Kijioa, A. Chemical constituents and antidepressant-like effects in ovariectomized mice of the ethanol extract of Alternanthera philoxeroides. Molecules 2018, 23, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.-Q.; Xiong, M.-X.; Ma, Z.; Li, Q.-Y.; Liu, Y.-W. Chemical constituents of Alternanthera philoxeroides. Chin. J. Nat. Med. 2008, 6, 112–115. [Google Scholar] [CrossRef]
- Rattanathongkom, A.; Sripanidkulchai, B.O.; Kanchanapoom, T. Immunomodulatory activity of chikusetsusaponin Iva from Alternanthera philoxeroides. Isan J. Pharm. Sci. 2008, 4, 113–120. [Google Scholar]
- Fang, J.B.; Duan, H.Q.; Zhang, Y.W.; Yoshihisa, T. Chemical constituents from herb of Alternanthera philoxeroides. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 2006, 31, 1072–1075. [Google Scholar]
- Tukun, A.B.; Shaheen, N.; Banu, C.P.; Mohiduzzaman, M.; Islam, S.; Begum, M. Antioxidant capacity and total phenolic contents in hydrophilic extracts of selected Bangladeshi medicinal plants. Asian Pac. J. Trop. Med. 2014, 7, S568–S573. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.K.; Li, P.; Guo, S.H.; Wang, S.Q.; Liu, D.S. Quantitation of β-carboline and quercetin in alligator weed (Alternanthera philoxeroides (Mart.) Griseb.) by LC-MS/MS and evaluation of cardioprotective effects of the methanol extracts. Drug Discov. Ther. 2018, 12, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Pulipati, S.; Babu, P.S. In-vitro antibacterial potential of Alternanthera philoxeroides (Mart.) Griseb. against multi-drug resistant uropathogens. Int. J. Pharm. Sci. Res. 2020, 11, 3834–3840. [Google Scholar]
- Raj, A.; Sikdar, B.; Roy, A.; Mukhopadhyay, A.K.; Roy, S. Antioxidant and Antibacterial Activities of Phytochemicals in Methanolic Extracts of Five Underutilized Leafy Vegetables. Res. J. Biotechnol. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Andersen, G.; Marchinek, P.; Sulzinger, N.; Schieberle, P.; Krautwurst, D. Food sources and biomolecular targets of tyramine. Nutr. Rev. 2019, 77, 107–115. [Google Scholar] [CrossRef]
- Gillman, P.K. Monoamine oxidase inhibitors: A review concerning dietary tyramine and drug interactions. Psychotr. Comment. 2016, 1, 1–90. [Google Scholar]
- Cao, R.; Peng, W.; Wang, Z.; Xu, A. β-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.K.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of β-carboline alkaloid “harmine”. Asian J. Trop. Biomed. 2012, 2, 660–664. [Google Scholar] [CrossRef] [Green Version]
- Nahar, L.; Sarker, S.D. Chemistry for Pharmacy Students—General, Organic and Natural Product Chemistry, 2nd ed.; Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Sun, C.; Yang, J.; Cheng, H.-B.; Shen, W.-X.; Jiang, Z.-Q.; Wu, M.-J.; Li, L.; Li, W.-T.; Chen, T.-T.; Rao, X.-W.; et al. 2-Hydroxy-3-methylanthraquinone inhibits lung carcinoma cells through modulation of IL-6-induced JAK2/STAT3 pathway. Phytomedicine 2019, 61, 152848. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Henning, L.; Giannis, A.; Ortwein, J.; Kutchan, T.M.; Feng, X. Anthraquinone content in Noni (Morinda citrifolia L.). Evid.-Based Complement. Altern. Med. 2013, 208378. [Google Scholar] [CrossRef] [Green Version]
- Watroly, M.N.; Sekar, M.; Fuloria, S.; Gan, S.H.; Jeyabalan, S.; Wu, Y.S.; Subramaniyan, V.; Sathasivam, K.V.; Ravi, S.; Lum, P.T.; et al. Chemistry, biosynthesis, physicochemical and biological properties of rubiadin: A promising natural anthraquinone for new drug discovery and development. Drug Des. Dev. Ther. 2021, 15, 4527–4549. [Google Scholar] [CrossRef]
- Panigrahi, G.K.; Verma, N.; Singh, N.; Asthana, S.; Gupta, S.K.; Tripathi, A.; Das, M. Interaction of anthraquinones of Cassia occidentalis with DNA and glutathione. Toxicol. Rep. 2018, 5, 164–172. [Google Scholar] [CrossRef]
- Beckford, S.J.; Dixon, D.W. Molecular dynamics of anthraquinone DNA intercalators with polyethylene glycol side chains. J. Biomol. Struct. Dyn. 2012, 19, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Khamphukdee, C.; Chulikhit, Y.; Daodee, S.; Monthakantirat, O. Potential of Alternanthera philoxeroides on improvement of anxiety-like behavior induced by ovariectomized mice model. Indian J. Pharm. Educ. Res. 2017, 51, S494–S497. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The pharmacological activity, biochemical properties and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juca, M.M.; Filho, F.M.S.C.; de Almeida, J.C.; da Silva Mesquita, D.; de Moraes Barriga, J.R.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Loonis, M.; Dangles, O. Inhibition of the peroxidation of linoleic acid by the flavonoid quercetin within their complex with human serum albumin. Free Radic. Biol. Med. 2007, 43, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, B.; Sengupta, P.K. The interaction of quercetin with human serum albumin: A fluorescence spectroscopic study. Biochem. Biophys. Res. Commun. 2002, 299, 400–403. [Google Scholar] [CrossRef]
- Liu, S.; Guo, C.; Guo, Y.; Yu, H.; Greenway, F.; Sun, M.-Z. Comparative binding affinities of flavonoid phytochemicals with bovine serum albumin. Iran. J. Pharm. Res. 2014, 13, 1019–1028. [Google Scholar] [PubMed]
- Navarro-Retamal, C.; Caballero, J. Flavonoids as CDK1 inhibitors: Insights in their binding orientations and structure activity relationships. PLoS ONE 2016, 11, e0161111. [Google Scholar] [CrossRef]
- Attrahimovich, D.; Avni, D.; Khatib, S. Flavonoids-macromolecules interactions in human diseases with focus on Alzheimers, atherosclerosis and cancer. Antioxidants 2021, 10, 423. [Google Scholar] [CrossRef]
- Gao, M.; Tang, G.-Y. Structural basis for great protein-binding potential of flavonoids: A case study of quercetin. Nat. Prod. Commun. 2017, 12, 1817–1818. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Tian, F.; Zhang, H.-B.; Pilarinou, E.; McLaughlin, J.L. Biologically active blumenol A from the leaves of Annona glabra. Nat. Prod. Lett. 1999, 14, 77–81. [Google Scholar] [CrossRef]
- Lv, X.-J.; Li, Y.; Ma, S.-G.; Qu, J.; Liu, Y.-B.; Li, Y.-H.; Zhang, D.; Li, L.; Yu, S.-S. Bioactive megastigmane glucosides and monoterpenes from Lyonia ovalifolia. J. Asian Nat. Prod. Res. 2019, 21, 559–572. [Google Scholar] [CrossRef]
- Ha, T.K.Q.; Lee, B.W.; Nguyen, N.H.; Cho, H.M.; Venkatesan, T.; Doan, T.P.; Kim, E.; Oh, W.K. Antiviral activities of compounds isolated from Pinus densiflora (Pine Tree) against the unfluenza A virus. Biomolecules 2020, 10, 711. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chia, Y.-C.; Huang, B.-M. Phytochemicals from Polyalthia species: Potential and implication on antioxidant, anti-inflammatory, anticancer and chemopreventive activities. Molecules 2021, 26, 5369. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Q.; Zhang, M.Y.; Liu, J.J.; Hu, Z.J.; Zhu, B.L.; Liu, Y.W.; Wang, G.Z.; Wan, N.; Wu, X.L. Extraction of effective parts of Alternanthera philoxeroides (Mart.) Griseb. And its antiviral effect. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 1989, 14, 488–490, 511–512. [Google Scholar]
- Jiang, W.-L.; Luo, X.-L.; Kuang, S.-J. Effects of Alternanthera philoxeroides Griseb. against dengue virus in vitro. Di Yi Jun Yi Da Xue Xue Bao 2005, 25, 454–456. [Google Scholar] [PubMed]
- Rawel, H.M.; Meidtner, K.; Kroll, J. Binding of selected phenolic compounds to proteins. J. Agric. Food Chem. 2005, 53, 4228–4235. [Google Scholar] [CrossRef]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between phenolic acids, proteins and carbohydrates—Influence on dough and bread properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, D.; Sun, J.; Liu, X.; Jiang, L.; Guo, H.; Ren, F. Interaction of plant phenols with food macronutrients: Characterisation and nutritional-physiological consequences. Nutr. Res. Rev. 2014, 27, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Li, L.; Takamura, Y.; Yoshiike, Y.; Zhu, L.; Han, F.; Mao, X.; Ikeda, T.; Takasaki, J.-I.; Nishijo, H.; et al. Phenolic compounds prevent amyloid b-protein oligomerisation and synaptic dysfunction by site-specific binding. J. Biol. Chem. 2012, 287, 14631–14643. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.V.; Garfinkel, D.M. The bioactivity of saponins: Triterpenoid and steroidal glycosides. Drug Metab. Drug Interact. 2000, 17, 211–235. [Google Scholar] [CrossRef]
- Dogra, J.V.V.; Ojha, O.P. Saponin from Alternanthera philoxeroides (Mart.) Griseb. Comp. Physiol. Ecol. 1978, 3, 5–6. [Google Scholar]
- Rattanathongkom, A.; Lee, J.B.; Hyashi, K.; Sripanidkulchai, B.O.; Kanchanapoom, T.; Hyashi, T. Evaluation of chikusetsusaponin Iva isolated from Alternanthera philoxeroides for its potency against viral replication. Planta Med. 2009, 75, 829–835. [Google Scholar] [CrossRef]
- Guo, Q.-I.; Li, B.; Li, J.; Li, J.-J.; Xia, L.-Y.; Dong, J.-X. Triterpenoid saponins of Alternanthera philoxeroides (Mart.) Griseb. Yaoxue Xuebao Acta Pharm. Sin. 2011, 46, 428–431. [Google Scholar]
- Potter, S.M.; Jimenez-Flores, R.; Pollack, J.; Lone, T.A.; Berber-Jimenez, M.D. Protein-saponin interaction and its influence on blood lipids. J. Agric. Food Chem. 1993, 41, 1287–1291. [Google Scholar] [CrossRef]
- Verstraeten, S.L.; Lorent, J.H.; Mingeot-Leclercq, M.-P. Lipid membranes as key targets for the pharmacological actions of ginsenosides. Front. Pharmacol. 2020, 11, 576887. [Google Scholar] [CrossRef] [PubMed]
- Ilekofehinti, O.O.; Iwaloye, O.; Olawale, F.; Ariyo, E.O. Saponins in cancer treatment: Current progress and future prospects. Pathophysiology 2021, 28, 250–272. [Google Scholar] [CrossRef]
- Miettinen, T.A.; Gylling, H. Non-nutritive bioactive constituents of plants: Phytosterols. Int. J. Vitam. Nutr. Res. 2003, 73, 127–134. [Google Scholar] [CrossRef]
- De Smet, E.; Mensink, R.P.; Plat, J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Mol. Nutr. Food Res. 2012, 56, 1058–1072. [Google Scholar] [CrossRef]
- Ras, R.T.; van der Schouw, Y.T.; Trautwein, E.A.; Sioen, I.; Dalmeijer, G.W.; Zock, P.L.; Beulens, J.W. Intake of phytosterols from natural sources and risk of cardiovascular disease in the European perspective investigation into cancer and nutrition—The Netherland’s (epic-nl) population. Eur. J. Prev. Cardiol. 2015, 22, 1067–1075. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2020, 11, 599959. [Google Scholar] [CrossRef]
- Calpe-Berdiel, L.; Escola-Gil, J.C.; Blanco-Vaca, F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis 2009, 203, 18–31. [Google Scholar] [CrossRef]
- Adewusi, E.M.; Steenkamp, P.; Fouche, G.; Steenkamp, V. Isolation of cycloeucalenol from Boophone disticha and evaluation of its cytotoxicity. Nat. Prod. Commun. 2013, 8, 1213–1216. [Google Scholar] [CrossRef] [Green Version]
- Ayleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acid: An update. Evid.-Based Complement. Altern. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef] [PubMed]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic acid and its derivatives as bioactive agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [Green Version]
- Si, L.; Meng, K.; Tian, Z.; Sun, J.; Li, H.; Zhang, Z.; Soloveva, V.; Li, H.; Fu, G.; Xia, Q.; et al. Triterpenoids manipulate a broad range of virus-host fusion via wrapping the HR2 domain prevalent in viral envelop. Sci. Adv. 2018, 4, eaau8408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loza-Mejia, M.; Salazar, J.R. Sterols and triterpenoids as potential anti-inflammatories: Molecular docking studies for binding to some enzymes involved in inflammatory pathways. J. Mol. Graph. Model. 2015, 62, 18–25. [Google Scholar] [CrossRef]
- Nakhuru, K.S.; Lokho, A.; Barman, M.; Das, J.; Dwivedi, S.K. Evaluation of vitamin C of ethno-wild edible plants in Northeast India. Plant. Sci. Today 2021, 8, 473–481. [Google Scholar] [CrossRef]
- Breathnach, A.S. Pharmacological properties of azelaic acid. Clin. Drug Investig. 1995, 10, 27–33. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, R.; Yadav, P. Azelaic acid: A promising agent for dermatological applications. Curr. Drug Ther. 2020, 15, 181–193. [Google Scholar] [CrossRef]
- Subroto, E.; Putri, N.G.; Rahmani, F.R.; Nuramalia, A.F.; Musthafa, D.A. Bioavailability and bioactivity of vitamin C—A review. Int. J. Pharm. Res. 2021, 13, 128–142. [Google Scholar]
- Shawon, J.; Khan, A.M.; Rahman, A.; Hoque, M.M.; Khan, M.A.K.; Sarwar, M.G.; Halim, M.A. Molecular recognition of azelaic acid and related molecules with DNA polymerase I investigated by molecular modeling calculations. Interdiscip. Sci. 2018, 10, 525–537. [Google Scholar] [CrossRef]
- Majumder, S.; Al-Rashid, M.H.; Chowdhury, S.; Gupta, B.K.; Mandal, S.C. Physicochemical and antioxidant assay of Ayurvedic formulations of Alternanthera philoxeroides. Int. Res. J. Pharm. 2016, 7, 20–23. [Google Scholar] [CrossRef]
- Rawani, A.; Pal, S.; Chandra, G. Evaluation of antimicrobial properties of four extracts against human pathogens. Asian Pac. J. Trop. Biomed. 2011, 1, S71–S75. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogenic Xylella Fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouarab-Chibane, L.; Forquet, V.; Lanteri, P.; Clement, Y.; Leonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterisation and QSAR (quantitative- structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Ghosh, T.; Sil, R.; Datta, A. Green synthesis and characterisation of antioxidant-tagged gold nanoparticles (X-GNP) and studies on its potent antimicrobial activity. J. Exp. Nanosci. 2018, 13, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.; Liu, J.; Bilinski, K.; Xu, L.; Steiner, G.Z.; Seto, S.W.; Bensoussan, A. Herbal medicine for the treatment of vascular dementia: An overview of scientific evidence. Evid.-Based Complement. Altern. Med. 2016, 7293626. [Google Scholar] [CrossRef] [Green Version]
- Khamphukdee, C.; Monthakantirat, O.; Chulikhit, Y.; Boonyarat, C.; Daodee, S.; Aon-im, P.; Maneenet, J.; Chotritthirong, Y.; Luecha, P.; Sekeroglu, N.; et al. Antidementia effects of Alternanthera philoxeroides in ovariectomized mice supported by NMR-based metabolomic analysis. Molecules 2021, 26, 2789. [Google Scholar] [CrossRef]
- Mohammad, H.F.; Roodabeh, B.; Roja, R.; Faezeh, A.; Mohammad, A. A Systematic review of plant-derived natural compounds for anxiety disorders. Curr. Top. Med. Chem. 2016, 16, 1924–1942. [Google Scholar]
- Hernandez-Leon, A.; González-Trujano, M.E.; Fernández-GuAPti, A. The anxiolytic-like effect of rutin in rats involves GABAA receptors in the bAPolateral amygdala. Behav. Pharmacol. 2017, 28, 303–312. [Google Scholar] [CrossRef]
- Khatun, F.; Zaman, F.; Mossiab, T.; Mostafa, F.; Zaman, M.; Rehana, F.; Nasrin, D.; Jamal, F.; Nahar, N.; Rahmatullah, M. Evaluation of antinociceptive and antihyperglycemic activities in methanol extracts of whole plants of Alternanthera philoxeroides (Mart.) Griseb. (Amaranthaceae) in mice. Pak. J. Pharm. Sci. 2012, 25, 583–587. [Google Scholar]
- Noronha, A.B.; Gil, V.L.; Vicente, M.; Gonzalves, A.L. Occurrence of plant virus inhibitors om 5 species of Caryophylales 2. Alternanthera amoena, Alternanthera brasiliana, Alternanthera philoxeroides, Iresine herbstii and Talinum paniculatum. Fitopatologia Brasileira 1983, 8, 317–324. [Google Scholar]
- Niu, R. A study of the preventative and therapeutic effects of Alternanthera philoxeroides on influenza. Chin. J. Mod. Dev. Tradit. Med. 1988, 6, 29–30. [Google Scholar]
- Zhang, S.-M.; He, Y.-S.; Tabba, H.D.; Smith, K.M. Inhibitor against the human immunodeficiency virus in aqueous extracts of Alternanthera philoxeroides. Chin. Med. J. 1988, 101, 861–866. [Google Scholar] [PubMed]
- Qu, C.F.; Yang, Z.Q.; Xiang, J.M. Alternanthera philoxeroides (Mavt.) Griseb protection against fetal epidemic hemorrhagic fever virus infection in suckling mice. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 1993, 18, 304–305, 320. [Google Scholar]
- Peng, H.; Qu, C.; Yang, Z.; Liu, Y. Studies on antiviral effect of extract from Alternanthera philoxeroides Griseb. on epidemic hemorrhagic fever virus in vivo. Antivir. Res. 1997, 34, A90. [Google Scholar]
- Jiang, W.-L.; Yang, Z.-Q.; Chen, W.; Xiao, H.; Luo, X.-L. Effects of Alternanthera philoxeroides Griseb. against respiratory syncytial virus infection in mice. Nan Fang Yi Ke Da Xue Bao J. South. Med. Univ. 2007, 27, 62–64. [Google Scholar]
- Wang, W.; Chen, H.; Yang, Z. Antiviral effect of Alternanthera philoxeroides microemulsion on coxsackie virus group-3 in vitro. Wuhan Daxue Xuebao Yixue Ban 2005, 26, 700–703. [Google Scholar]
- Abd Kadir, S.L.; Yaakob, H.; Zulkiffi, R.M. Potential anti-dengue medicinal plants: A review. J. Nat. Med. 2013, 67, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Kausar, S.; Khan, F.S.; Ishaq, M.; Rehman, M.U.; Akram, M.; Riaz, M.; Rasool, G.; Khan, A.H.; Saleem, I.; Shamim, S.; et al. A review: Mechanisms of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 1–12. [Google Scholar] [CrossRef]
- Resende, F.A.; de Oliveira, A.P.S.; de Camargo, M.S.; Vilegas, W.; Varanda, E.A. Evaluation of estrogenic potential of flavonoids using a recombinant yeast strain and MCF7/BUS cell proliferation assay. PLoS ONE 2013, 8, e74881. [Google Scholar] [CrossRef]

| Bioactivities | Brief description | Types of Extract | IC50/MIC or/EC50 | References |
|---|---|---|---|---|
| Antibacterial activity | Extracts of leaves; considerable activity (zones of inhibition) against Bacillus subtilis (13.6 and 18.27 mm), Escherichia coli (14.2 and 14.8 mm), Pseudomonas aeruginosa (17.13 and 19.23 mm) and Staphylococcus aureus (13.33 and 16.2 mm) using the disc diffusion assay. | Water and CHCl3–MeOH (1:1) | MIC = 35.25–80.0 μg/mL | [73] |
| Extract of the leaves; zones of inhibition against Escherichia coli (52.14 mm) and Micrococcus luteus (34.0 mm) at a concentration of 60 μg/mL. MIC values were determined against E. coli and M. luteus. | MeOH | MIC = 11.23–16.23 μg/mL | [6] | |
| Aqueous EtOH extract (70%) of leaves and stem; no activity against Bacillus cereus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus in disc diffusion assay. | Water–EtOH (3:7) | Not determined | [7] | |
| Bioactive fraction of a MeOH extract of the aerial parts incorporated gold nanoparticles; improved activity against Acinetobacter lwoffii, Bacillus subtilis, Escherichia coli, Micrococcus luteus and Pseudomonas aeruginosa using the disc diffusion assay. | MeOH | Not determined | [76] | |
| Extract of the aerial parts; maximum antibacterial activity against MDR Staphylococcus saprophyticus, moderate against MDR Escherichia coli and Proteus vulgaris, and the least activity against MDR Proteus mirabilis. | MeOH | MIC = 12.5–25.0 μg/mL | [17] | |
| Extracts of the leaves, stem and roots; considerable activity (zone of inhibition) in the disc diffusion assay against phytopathogenic bacterial strains, Erwinia carotovora, Ralstonia solanacearum and Xanthomonas axonopodis, with the highest activity against Ralstonia solanacearum (28.1 mm, n-hexane extract of the leaves). | n-Hexane, CHCl3, EtOAc, and MeOH | Not determined | [3] | |
| Extract of the aerial parts; weak activity (zone of inhibition) in the disc diffusion assay at 12 mg/disc against Bacillus subtilis (9.2 mm), Escherichia coli (7.7 mm), Salmonella typhi (7.3 mm), Staphylococcus aureus (6.8 mm), Staphylococcus epidermidis (7.3 mm), and Vibrio cholerae (5.8 mm). | MeOH | Not determined | [18] | |
| Anticancer and antitumour activity | Alternanthin (13), alternanthin B (14), and N-trans-feruloyl-3,5-dimethoxytyramine (2), N-trans-feruloyl-3-methyldopamine (3), N-trans-feruloyl-tyramine (4) and N-cis-feruloyl-tyramine (5), isolated from an EtOH (95%) extract of the aerial parts; in vitro antitumour activity of the isolated compounds against HeLA and L929 cells. | EtOH (95%) | 13.2–72.2% inhibition at 30 μg/mL | [5] |
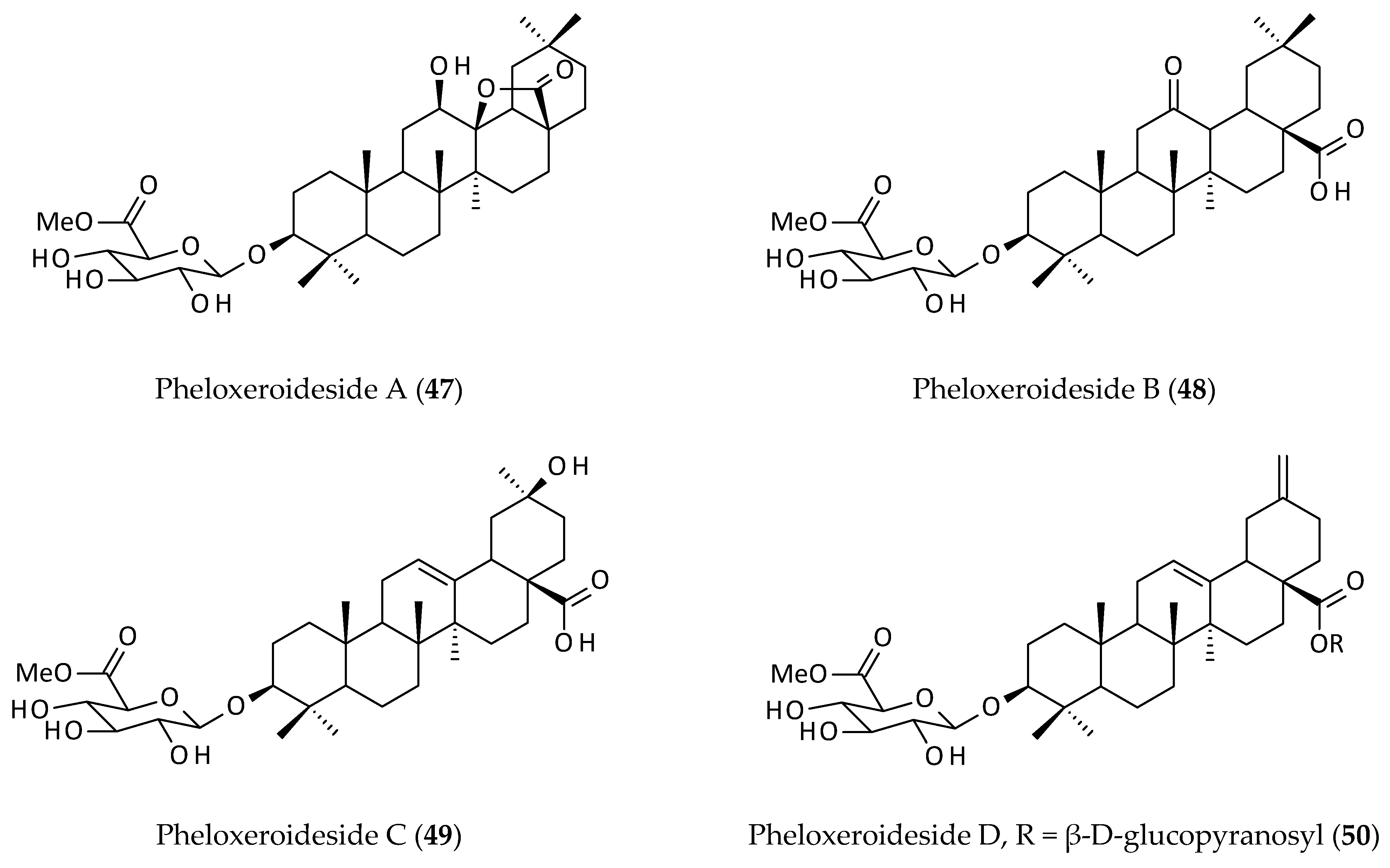
| Isolated saponins, philoxeroidesides A–D (47–50) from an EtOH (95%) extract of the aerial parts; cytotoxicity against SK–N–SH and HL60 cell lines. | EtOH (95%) | IC50 = 37.29–271.45 μg/mL | [8] | |
| Isolated compounds from an n-butyl extract of the aerial parts; in vitro antitumour activity of isolated compounds as determined by the MTT assay, with oleanolic acid 3-O-β-D-glucuronopyranoside (42) being the most active compound against HeLA and L929 cells at 30 mg/L. | n-Butane | 91.3–92.9% inhibition at 30 μg/mL | [9] | |
| Extract of the leaves; in vitro cytotoxicity against human osteosarcoma cell line MG-63. | EtOH | 67.37% inhibition at 300 μg/mL | [2] | |
| Antidementia activity | Extract of the whole plant; based on in vitro antioxidant activity, β-amyloid aggregation inhibition and cholinesterase inhibitory activity, as well as in vivo Morris water maze task, novel object recognition task, and Y-maze task. The extract as well as its flavonoids offered inhibition of β-amyloid aggregation. | EtOH | Not determined | [78] |
| Antidepressant-like activity | Extract of the aerial parts and isolated compounds; considerable in vivo antidepressant-like activity on ovariectomized mice using the tail suspension and forced swimming tests. | EtOH | Not determined | [11,29] |
| Antihyperglycaemic activity | Extract of the whole plant; in vivo antihyperglycaemic activity was evaluated through oral glucose tolerance tests in glucose-loaded mice (65.6% reduction in serum glucose level at a dose of 400 mg/kG body weight). | MeOH | Not determined | [81] |
| Extract of the leaves; α-glucosidase inhibitory property. | MeOH | IC50 = 52.41 μg/mL | [6] | |
| Antinociceptive activity | Extract of the whole plant; in vivo antinociceptive activity was evaluated by attenuation of the number of constrictions in acetic acid-induced gastric pain (44.8% reduction in constriction at a dose of 400 mg/kG body weight). | MeOH | Not determined | [81] |
| Antioxidant activity | Extract of the leaves; active in the ABTS and DPPH assays. | MeOH | ABTS IC50 = 60.76 µg/mL and DPPH IC50 = 33.94 µg/mL | [6] |
| Extract of the aerial parts; DPPH (0.14 µmol Trolox per gram equivalent). | n-Hexane-DCM (1:1) | Not determined | [15] | |
| In vitro antioxidant activity of an EtOH extract of the whole plant. | EtOH | DPPH IC50 = 222.58 µg/mL and ABTS IC50 = 384.0 µg/mL | [78] | |
| Extract of the aerial parts; TAA (3.72 mg AAE/g of extract), FRAP (14.73 mm Fe2+/mg of extract), and active in the, ABTS and SO assays. | MeOH | DPPH IC50 = 758.55 µg/mL, ABTS IC50 = 586.34 µg/mL and SO IC50 = 659.7 µg/mL | [18] | |
| Antiviral activity | Extract of the leaves; antiviral activity against tobacco mosaic virus. | EtOH | Not determined | [82] |
| Extract of the aerial parts; antiviral activity against human influenza virus. | EtOH | Not determined | [83] | |
| Aqueous extract of the aerial parts; antiviral activity against HIV. | Water | MIC = 1.8 mg/mL | [84] | |
| Various solvent extracts of several parts of this plant; antiviral activity against epidemic haemorrhagic fever virus, with petroleum ether, ether and EtOAc extracts being the active ones. | Petroleum ether, ether, and EtOAc | ED50 = 47.43 µg/mL | [43] | |
| Extract of the whole plant; in vivo antiviral activity against the epidemic haemorrhagic fever virus in suckling mice model. | EtOH | Not determined | [85] | |
| Extract of the aerial parts; in vivo antiviral activity against the epidemic haemorrhagic fever virus. | EtOH | Not determined | [86] | |
| Various solvent extracts of the aerial parts; in vitro anti-dengue virus activity, with the petroleum extract being the most active. | Petroleum ether | ED50 = 47.43 µg/mL | [44] | |
| Microemulsion of the extract; in vitro antiviral activity against coxsackie virus group B-3. | EtOH | Not determined | [88] | |
| Extract of the aerial parts; in vivo antiviral activity against respiratory syncytial virus in mice model. | EtOH | ED50 = 47.43 µg/mL | [87] | |
| Chikusetsusaponin IVa (38) and calenduloside E (42), isolated from an EtOH extract of the whole plant; Chikusetsusaponin IVa (38) exhibited antiviral activities against HSV-1, HSV-2, human cytomegalovirus, measles virus, and mumps virus, but calenduloside E (42) was inactive against all tested viruses. | EtOH | Selectivity indices (CC50/IC50) = 29, 30, 73, 25 and 25 | [13] | |
| Extract of the leaves; activity against dengue virus. | Petroleum ether | ED50 = 47.43 µg/mL | [89] | |
| Isolated C-boivinopyranosyl flavones (13–18) from an EtOH extract of the aerial parts; significant anti-HBV (hepatitis virus) activity by inhibiting the secretion of HBsAg in HepG2.215. | EtOH | IC50 = 11.39–31.54 µM | [10] | |
| Cardioprotective activity | Extract of the leaves; significant prevention of cardiomyocyte apoptosis induced by doxorubicin using H9c2 cells and determined by the MTT and Annexin V-FITC/PI staining assays. | MeOH | Not determined | [16] |
| Cholinesterase inhibitory activity | Extract of the whole plant; acetylcholinesterase and butyrylcholinesterase inhibition with IC50 values of 2.06 and 3.27 µg/mL, respectively. | EtOH | IC50 = 2.06 and 3.27 µg/mL | [78] |
| Oestrogenic activities | Extract of the aerial parts; in vitro estrogenic activity in MCF-7 breast cancer cell line. | EtOH | EC50 = 1.68 µg/mL | [11] |
| Immunomodulatory activity | Extract of the aerial parts, its n-butanol and ether fractions, and chikusetsusaponin IVa (38); the ether fraction (50 µg/mL) inhibited splenocyte proliferation, but saponin (38) (25 µg/mL) increased splenocyte proliferation. | MeOH | Not determined | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahar, L.; Nath, S.; Sarker, S.D. “Malancha” [Alternanthera philoxeroides (Mart.) Griseb.]: A Potential Therapeutic Option against Viral Diseases. Biomolecules 2022, 12, 582. https://doi.org/10.3390/biom12040582
Nahar L, Nath S, Sarker SD. “Malancha” [Alternanthera philoxeroides (Mart.) Griseb.]: A Potential Therapeutic Option against Viral Diseases. Biomolecules. 2022; 12(4):582. https://doi.org/10.3390/biom12040582
Chicago/Turabian StyleNahar, Lutfun, Sushmita Nath, and Satyajit D. Sarker. 2022. "“Malancha” [Alternanthera philoxeroides (Mart.) Griseb.]: A Potential Therapeutic Option against Viral Diseases" Biomolecules 12, no. 4: 582. https://doi.org/10.3390/biom12040582
APA StyleNahar, L., Nath, S., & Sarker, S. D. (2022). “Malancha” [Alternanthera philoxeroides (Mart.) Griseb.]: A Potential Therapeutic Option against Viral Diseases. Biomolecules, 12(4), 582. https://doi.org/10.3390/biom12040582








