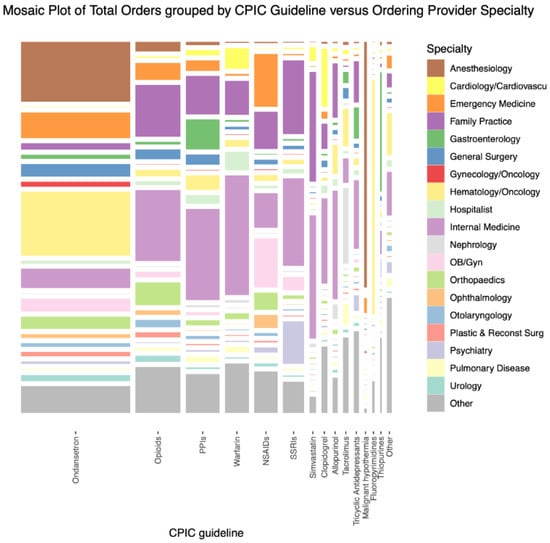
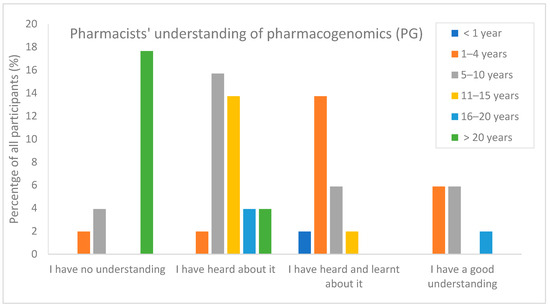
Pharmacy Services
A topical collection in Pharmacy (ISSN 2226-4787).
Submission Status: Closed (27 October 2025) | Viewed by 13961Editors
Interests: information processing and decision making related to the provision, use, and evaluation of drug products and pharmacist services
Special Issues, Collections and Topics in MDPI journals
Interests: person-centeredness; community pharmacy; medication experience; pharmacy services; implementation science; community-integrated care
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The MDPI journal Pharmacy has hosted two (2) Special Issues on Pharmacist Services. At the time of writing this invitation (November 12, 2021), 47 papers have been published in these issues (35 in Pharmacist Services I and 12 in Pharmacist Services II). These Special Issues can be found here:
https://www.mdpi.com/journal/pharmacy/special_issues/Pharmacist_Services
https://www.mdpi.com/journal/pharmacy/special_issues/Pharmacist_Services_two
As a next step, we would like to transition into a Topical Collection on 'Pharmacy Services.' Topical Collections are new to the journal and would not have a closing date for receiving papers. That way, scholars can submit at any time. It also affords us the opportunity to build upon one another's work over time through letters to the editor, commentaries, new research, replication studies, and more.
If the topical collection publishes more than 10 papers, the publisher may print a book edition. This book would be made available, in a digital format (for free) and as paperback copies (ordered via Amazon), on the MDPI platform (http://books.mdpi.com). For reference, a free download of the first book on Pharmacist Services is available at https://www.mdpi.com/books/pdfview/book/1767
Pharmacy (ISSN 2226-4787) is an international scientific open access journal on pharmacy education and practice that is published quarterly online by MDPI. The journal has already been indexed by PubMed, ESCI (Emerging Sources Citation Index), and Web of Science. Furthermore, Pharmacy is a member of the Committee on Publication Ethics (COPE), and, accordingly, submissions are peer reviewed rigorously to ensure that they conform to the highest standards in their field.
Background for the Topical Collection on 'Pharmacy Services.'
In their 2017 article, Adams and Blouin stated, "Over the past four decades, the role of the pharmacist has evolved from an individual who was primarily responsible for safely and accurately distributing a medication product to a patient, to an individual who works side-by-side with physicians, nurses, and other healthcare professionals in sophisticated, highly specialized practice settings to assure appropriate medication therapy management". (Adams ML and Blouin RA, "The Role of the Pharmacist in Health Care: Expanding and Evolving," N C Med J. 2017; 78(3):165-167).
This transformation has opened up new places for pharmacists to work that might not be licensed pharmacies (clinics, managed care, health systems, home health care, drug information, consulting, informatics, and research organizations to name a few). In light of this, what does this mean for licensed pharmacies? Should these other locations be considered "pharmacy locations" in some way? What is their role now?
Based on our research, it looks like licensed community pharmacies have been transforming from being convenient locations for obtaining products to "healthcare access points that provide and are reimbursed for patient care and public health services like medication therapy management, immunizations, and more". (Olson AW, Schommer JC, Hadsall RS. A 15 Year Ecological Comparison for the Market Dynamics of Minnesota Community Pharmacies from 2002 to 2017. Pharmacy 2018, 6, 50).
Furthermore, COVID-19 testing and vaccination services have accelerated the transformation of pharmacies into "health and personal care centers". The Scope of Practice laws in the United States (and other countries) have expanded what pharmacists and pharmacy staff can do in pharmacies. Pharmacy executives are working to transform pharmacies from "solution centers for the sick" into "hubs for the healthy". Finally, the initial findings from our 2021 National Consumer Survey (USA) show that the public is beginning to see a pharmacy as an access point not only for medication optimization, but also for wellness and prevention, patient education and support, acute and chronic care management, monitoring/lab testing, and public health.
With this in mind, we invite you to submit manuscripts in the broad area of 'Pharmacy Services'. What is changing throughout the world? What examples of innovative pharmacy practices are there? How are pharmacies adapting? What are their strengths and weaknesses? What opportunities and challenges are there? How are pharmacies meeting patients' needs? What does the future hold? What are your ideas for what is next?
Thank you for considering this invitation. When submitting your manuscript, please feel free to check with MDPI staff about the publication charges and possible discounts that would apply to you.
Prof. Dr. Jon Schommer
Dr. Anthony Olson
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pharmacy is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1800 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- pharmacy services
- service development and implementation
- service quality
- service marketing and management
- service evaluation