- Article
Disparity of Prescribed Psychotropics in Alzheimer’s Disease with Neuropsychiatric Symptoms
- Samuel I. Nathaniel,
- Maggie Oliver and
- Thomas I. Nathaniel
- + 3 authors
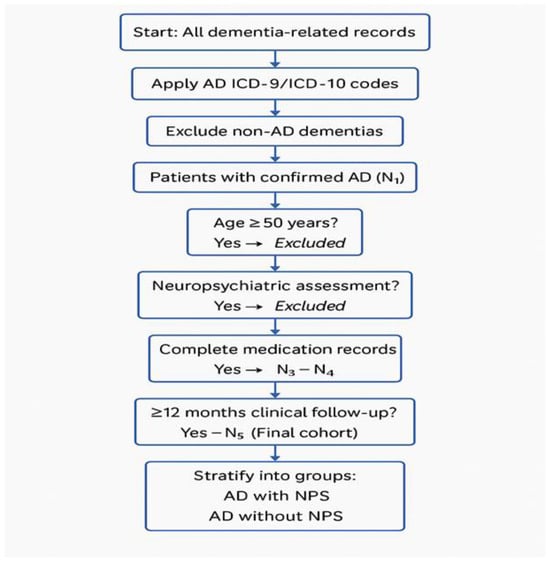
Objective: The objective of this study was to determine whether Non-Hispanic Black (NHB) or Non-Hispanic White (NHW) Alzheimer dementia patients with neuropsychiatric symptoms (ADNPS) differ regarding treatment with second-generation antipsychotics (SGAs), central acetylcholinesterase inhibitors (CAIs), and selective serotonin reuptake inhibitors (SSRIs). Methods: Pharmacologic and demographic factors associated with male and female ADNPS were examined using retrospective data collected from a registry from 2016 and 2020 in a regional AD care center. The logistic regression model was developed to generate odds ratios (OR) to determine factors that were associated with male or female ADNPS. Results: A total of 7031 AD patients were identified. Overall, 6237 patients were NHWs, and 794 were NHBs. Among the NHW AD patients, 1909 presented with behavioral disturbances or neuropsychiatric symptoms (NPS), and 168 NHB AD patients presented with NPS. In the adjusted analysis, NHW ADNPS patients were more likely to be treated with galantamine (OR = 1.538, 95% CI, 1.001–2.364, p = 0.049), memantine (OR = 1.222, 95% CI, 1.086–1.375, p < 0.001), olanzapine (OR = 2.323, 95% CI, 1.794–3.009, p < 0.001), risperidone (OR = 4.181, 95% CI, 3.539–4.939, p < 0.001), and escitalopram (OR = 1.401, 95% CI, 1.225–1.602, p < 0.001). In contrast, NHB ADNPS patients were more likely to be treated with memantine (OR = 2.601, 95% CI, 1.746–3.875, p < 0.001) and risperidone (OR = 5.526, 95% CI, 3.411–8.951, p < 0.001). Conclusions: Our findings show the use of memantine and risperidone to treat both NHB and NHW ADNPS patients. NHW ADNPS patients were more likely to be treated with galantamine, memantine, olanzapine, risperidone, and escitalopram. In contrast, NHB patients with ADNPS were more likely to be treated with memantine and risperidone.
22 December 2025