Advances in Organoid Technology: Bridging the Gap between Research and Therapy
Special Issue Information
Dear Colleagues,
The upcoming Special Issue, “Advances in Organoid Technology: Bridging the Gap between Research and Therapy”, highlights the groundbreaking research and discussions presented at the “Organoids Are Us 2024” symposium. Organized by Prof. Elizabeth Vincan, Prof. Ramanuj DasGupta, and Asst. Prof. Somponnat Sampattavanich, this conference is a premier event in the field, bringing together leading experts and emerging scientists to share their latest findings and innovative approaches in organoid technology.
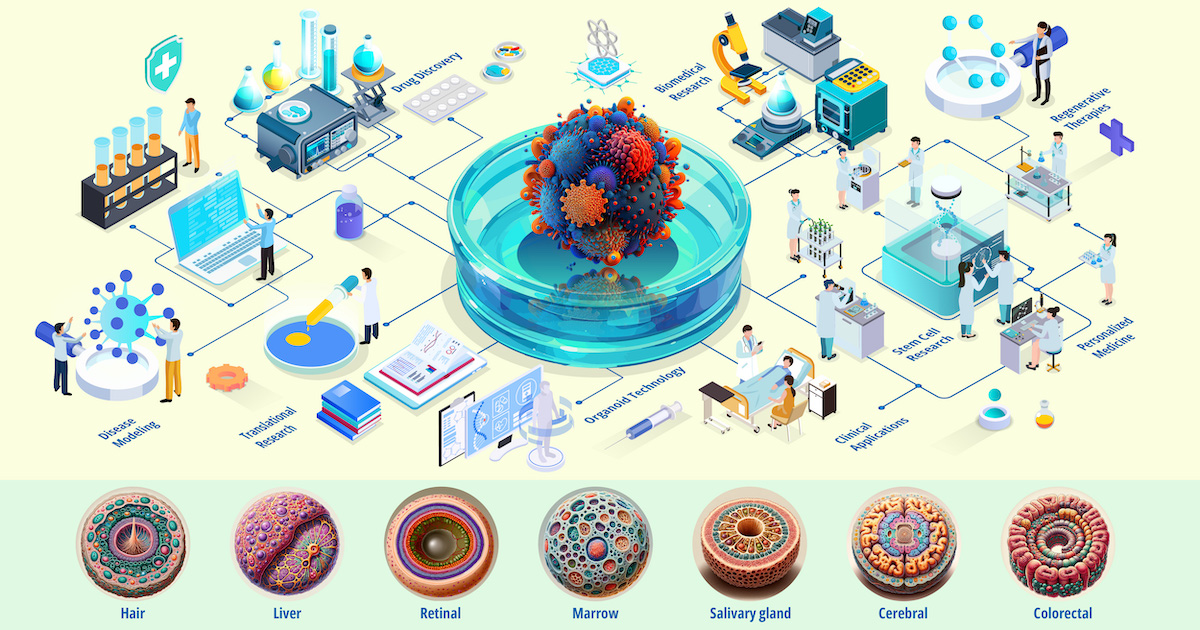
“Organoids Are Us” is renowned for fostering interdisciplinary collaboration and driving advancements in biomedical research. The symposium provides a unique platform for presenting pioneering work on organoids, which are three-dimensional structures derived from stem cells that replicate the complexity and functionality of real organs. Organoids offer unparalleled insights into human biology, disease mechanisms, and therapeutic potential, making them invaluable tools for both basic research and clinical applications.
The “Organoids Are Us 2024” symposium, now in its sixth year, marks the first time it will be held outside of Australia. Scheduled to take place from Tuesday, August 6, 2024 to Thursday, August 8, 2024, at the Ozo North Pattaya in Thailand, the symposium promises to be a landmark event. Conceived and named by Special Issue Guest Editor Professor Elizabeth Vincan, the symposium underscores the fundamental truth that “organoids” are indeed "us."
Conference participants are invited to contribute original research papers or reviews to this Special Issue of Organoids. Submissions from conference participants will be completely free of charge. We welcome previously unpublished work on all aspects and applications of organoids. This Special Issue aims to bridge the gap between laboratory discoveries and therapeutic applications, covering topics such as innovative methods for generating and manipulating organoids, their use in disease modeling, drug discovery, personalized medicine, and regenerative therapies. By featuring contributions from this esteemed symposium, the collection of work seeks to drive scientific innovation and translate organoid technology from the bench to the bedside.
Prof. Dr. Elizabeth Vincan
Dr. Ramanuj DasGupta
Dr. Somponnat Sampattavanich
Dr. Joao Ferreira
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Organoids is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- organoids in biomedical research
- stem cell research
- regenerative therapies
- disease modeling and drug discovery
- personalized medicine and translational research
Benefits of Publishing in a Special Issue
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- e-Book format: Special Issues with more than 10 articles can be published as dedicated e-books, ensuring wide and rapid dissemination.

