Nanoparticles for Therapeutic and Diagnostic Applications
A topical collection in Bioengineering (ISSN 2306-5354). This collection belongs to the section "Nanobiotechnology and Biofabrication".
Viewed by 95762Editor
Topical Collection Information
Dear Colleagues,
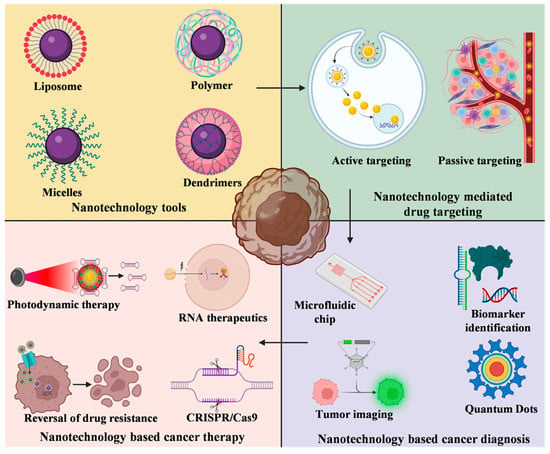
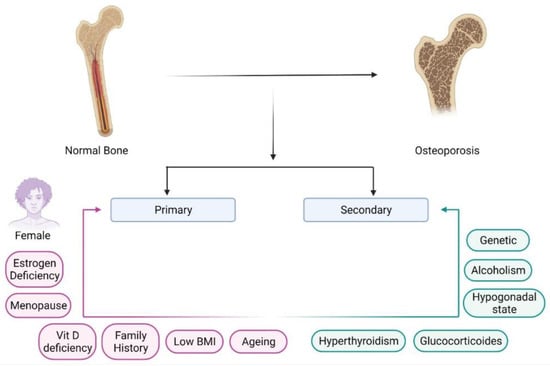
Nanoparticles have garnered intense interest as a therapeutic platform to treat a broad range of diseases, including cancer, metabolic, cardiovascular, skin, renal, inflammatory, and infectious disease. Well-designed nanoparticles can enhance the efficacy of traditional therapeutics through enhanced solubility, prolonged circulation or drug release, targeted delivery to disease sites, and reduced toxicity. Nanoparticles can be formulated for systemic, dermal, oral, and inhalation applications and optimized to overcome the delivery barriers of emerging therapeutics such as oligonucleotides, mRNA, and DNA with the potential to be safer than viral vector counterparts. Nevertheless, like any emerging technology, further advances in research are required to overcome the technological and regulatory barriers if nanoparticles are to fulfil their immense potential for human applications.
In order to offer a publication platform for researchers working in the nanotherapeutic field, the MDPI journal, Bioengineering, is dedicating a Topical Collection to Nanoparticles in Therapeutic Applications and is asking for your valuable contribution. The Issue will focus on nanoparticle development and applications in broad ranges of diseases and delivery routes. Research toward increased understanding of pharmacokinetics, pharmacodynamics, toxicity, stability, and manufacturability of nanoparticles is also highly relevant.
We look forward to receiving your contributions to this Issue of Bioengineering.
Prof. Dr. Wassana Yantasee
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Bioengineering is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.