Abstract
Fructus lycii (F. lycii) is an exotic “berry-type” fruit of the plant Lycium barbarum that is characterized by a complex mixture of bioactive compounds distinguished by their high antioxidant potential. F. lycii is used in traditional Chinese home cooking and in the Chinese Pharmacopeia as an aid to vision and longevity as well as a remedy for diabetes to balance “yin” and “yang” in the body for about two centuries. Although a myriad of bioactive compounds have been isolated from F. lycii, polysaccharides, carotenoids, flavonoids, and phenolics represent the key functional components of F. lycii. F. lycii has been shown to exhibit a wide range of biological activities in experimental settings including antioxidant, anti-inflammatory, antiapoptotic, and neuroprotective effects. Despite its medicinal role dating back to the eighteenth century in the Far East and robust evidence of beneficial effects on ocular health and retinal diseases originating mainly from studies in animal models, the role of F. lycii in the clinical management of retinal diseases is yet to be established. This article comprehensively reviews the literature germane to F. lycii and retinal diseases with particular emphasis on age-related macular degeneration, diabetic retinopathy, and retinitis pigmentosa, which are commonly seen in clinical practice.
1. Introduction
Fructus lycii (F. lycii) is a “berry-type” fruit of the plant Lycium barbarum, which is a deciduous shrub belonging to the family Solanaceae. Although Lycium fruit was described in the Shennong Ben Cao Jing (ca 100AD), the oldest surviving Chinese materia medica, Carl Linnaeus, a Swedish botanist, provided the genus name ”Lycium (L)“ in the year 1753 and gave the species name ”barbarum” [1,2]. Commonly known as goji berry, F. lycii is also known as wolfberry in English, kei tze in Cantonese, and gou qi zi in Mandarin (zi meaning seed or specifically berry). Fresh F. lycii is bright orange-red in color with an ellipsoid shape measuring around 1–2 cm, has a sweet-and-tangy flavor, and is usually sun-dried as a dried fruit after harvesting in late summer or early autumn (Figure 1). Although, the berry fruits from L. barbarum and its closely related species, L. chinense, L. ruthenicum, and L. yunnanese, are interchangeable, L. barbarum is the most dominant species and produces larger berry fruits compared to other species. F. lycii is traditionally used in Chinese home cooking in tea, soups, and porridge for its pleasant flavor and in the Chinese Pharmacopeia for about two centuries as an aid to vision and longevity as well as a remedy for diabetes to balance “yin” and “yang” in the body [3,4].

Figure 1.
The fresh (left) and dried (right) berry fruits of Lycium barbarum ‘Fructus lycii’.
F. lycii is usually found in the arid and semi-arid regions of China and eastern Asia including Japan, Korea, and Vietnam. Currently, China is the world’s largest producer of F. lycii with farms in several regions, such as Qinghai, Xinjiang, Shaanxi, Gansu, Hebei, inner Mongolia, and Tibet. Approximately 90% of commercially available berry fruits are from the L. barbarum species in the autonomous regions of north-central China (Ningxia Hui) and western China (Xinjiang Uyghur), where they are grown according to Good Agricultural Practice [5]. F. lycii is fast gaining popularity as a functional food. As a result, cultivation of F. lycii has started in many regions in the west, including diverse parts of Europe and America, due to increasing demand. Besides being consumed as fresh or dried berry fruits, this ”Asian fruit” is also available in the form of processed food (cookies, chocolates, muesli, and sausages), and beverages (teas, fruit juices, wines, and beers) as well as herbal supplements that have been commercialized in western countries [6]. In recent years, several studies have been conducted to confirm and demonstrate its chemical ingredients, pharmacological properties, and clinical association with ocular health and diseases.
2. Bioactive Components
F. lycii is an excellent source of macronutrients, micronutrients, and several bioactive compounds distinguished by their high antioxidant potential (macronutrients: 46% carbohydrate, 13% protein, 1.5% fat, 16.5% dietary fiber; micronutrients: vitamins and minerals) [7]. Three bioactive compounds deserve special mention, and these are polysaccharides, carotenoids, and phenolics. The polysaccharide component (5–8% of dried fruit, hydrophilic) is the most important component and consists of six monosaccharides—glucose, galactose, arabinose, rhamnose, mannose, and xylose [8]. The carotenoids are the second most important component (0.03–0.5% of the dried weight, lipophilic) and are responsible for the characteristic bright and vivid orange to red coloration of these berries [9]. Flavonoids and phenolic acids constitute the phenolic component with quercetin-rhamno-di-hexoside and quercetin-3-O-rutinoside being the predominant flavonoids and chlorogenic acid being the most abundant phenolic acid [10]. Taurine, betaine (a natural amino acid), and heat stable vitamin C precursor represent the few other bioactive components that are found in high concentrations in these berries [11].
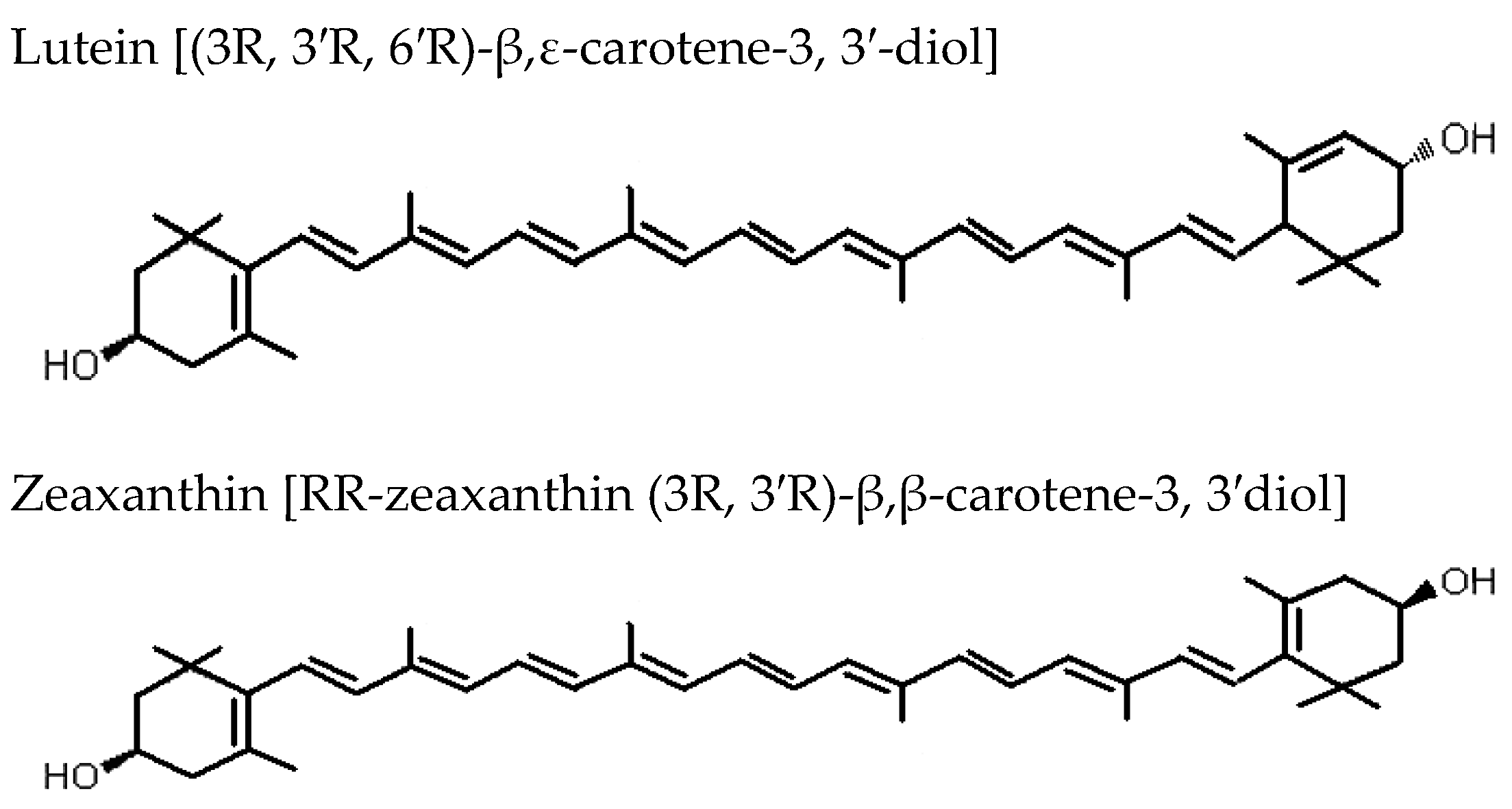
F. lycii is characterized by exceptionally high levels of the carotenoid zeaxanthin (Z) in the form of zeaxanthin dipalmitate (Figure 2) [12]. Fully ripe berry fruits contain around 60–70 times higher Z (mg/100 g fresh weight) when compared to egg yolk, another rich source of Z. This makes the berry fruits an outstanding source of Z in the human diet (F. lycii = 35.7 mg; egg yolk = 0.29 mg) [13]. The process of ripening of F. lycii is associated with two major changes: first, a change in the carotenoid profile from low levels of non-specific carotenoids to high levels of xanthophyll carotenoids, mainly esterified Z; second, a change in the carotenoid deposition state from chloroplast-specific solid state to chromoplast-specific liquid state. Ultrastructural study of F. lycii has shown that Z is deposited in a presumably J-aggregated liquid-crystalline state within the nanoscaled chromoplast tubules [14]. Although these berries constitute one of the richest sources of Z, corn, spinach, butternut squash, collard green, and tangerines represent a few other good sources of Z, which are usually consumed in the human diet.
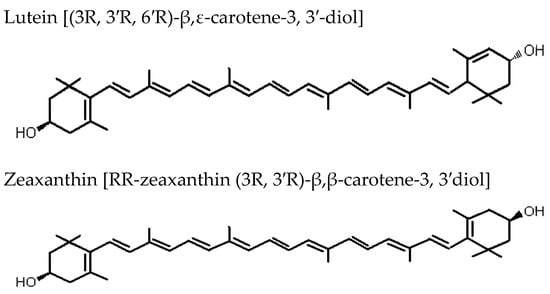
Figure 2.
Biochemical structures of the carotenoids lutein and zeaxanthin.
It is important to note that the bioactive components of F. lycii may differ geographically as well as regionally within a country. For instance, Italian F. lycii has a higher total carotenoid and Z dipalmitate content when compared to its Chinese counterpart (Carotenoids in mg/100 g: Italian = 355.4, Chinese = 224.8; Z dipalmitate: Italian = 173.50, Chinese = 33.45) [15]. A relatively higher content of micronutrients, such as vitamin K, copper, selenium, and zinc, has also been found in Italian F. lycii. Although F. lycii originating from China are overall rich in all three bioactive components with higher total antioxidant activity, there are significant differences in the carotenoid content of F. lycii originating from four different regions within China (Gansu, Ningxia, Qinghai, and Xinjiang) [16]. F. lycii from Gansu region has the highest total carotenoid content (212.5–237.1 mg/100 dw), whereas the highest content of Z dipalmitate is found in F. lycii from Qinghai region (77.57–94.03 mg/100 dw). F. lycii from Xinjiang region has the lowest total carotenoid as well as Z dipalmitate content (carotenoid = 129.3 mg/100 dw, Z = 42.60 mg/100 dw). It is believed that differences in climate, nature of soil, cultivation methods, and post-harvesting processing methods (drying and storage conditions) may result in geographic and regional differences in the bioactive components of F. lycii.
3. Bioavailability of Bioactive Components
Carotenoids are one of the well-studied components of F. lycii in terms of bioavailability. Table 1 summarizes the studies that have evaluated the bioavailability of carotenoid component of F. lycii. F. lycii contains exceptionally high levels of Z dipalmitate, an esterified form of Z formed from a combination of Z and palmitic acid. During digestion, Z esters are hydrolyzed, resulting in free Z, which is then incorporated within the chylomicron component of triacylglycerol-rich lipoproteins and transported into the blood circulation [17]. Past studies have demonstrated a good degree of bioavailability of Z from F. lycii both in animals and humans. An increase in plasma as well as retinal levels of Z (two-fold increase in macular Z levels) was demonstrated in rhesus monkeys following six weeks of supplementation with dietary F. lycii extracts [18]. Similarly, around a 2.5-fold increase in plasma levels of Z was found in healthy adults following daily consumption of F. lycii for 28 days [19,20]. These observations suggest that a modest intake of F. lycii on a daily basis markedly increases plasma levels of Z. However, a relatively smaller increase in plasma Z levels was found in elderly subjects (mean age = 67 years) when compared to younger subjects (mean age = 28 years) following intake of these berries, suggesting that there may be an age-related decline in the absorption and/or uptake of Z. Of note, these studies involving human subjects did not measure the retinal levels of Z.

Table 1.
List of studies examining the bioavailability of carotenoid zeaxanthin in Fructus lycii.
F. lycii not only represents the richest natural source of Z, but more importantly, the bioavailability of Z from this berry fruit is much higher than other dietary sources of carotenoids. The natural occurrence of the ester form of Z may account for the increased bioavailability of Z [24]. Experiments in rodents and humans showed enhanced bioavailability of esterified Z originating from F. lycii when compared to non-esterified Z [21,25], and this has been attributed to more effective micelle formation needed for lipase activity during the process of digestion. Alternatively, increased bioavailability of esterified Z from F. lycii may be due to the unique deposition state of Z. In F. lycii, Z exists in a liquid-crystalline state within tubular chromoplast (contrast solid protein-complexed Z within chloroplast in spinach) that may result in greater liberation and bioaccessibility of Z [13]. In addition, an increase in bioavailability of Z from F. lycii may also be due to the characteristic J-aggregates integral to the unique deposition state of Z. Esterification of hydroxyl group in Z dipalmitate results in development of loosely packed J-aggregates due to deterrence of hydrogen bond formation between molecules. An alternative type of aggregate for carotenoid Z is tightly packed H-aggregates that result from the formation of hydrogen bond between molecules. J-aggregated Z dipalmitate demonstrated higher bioavailability in human subjects (23% higher) as well as in-vitro bioaccessibility model (INFOGEST) when compared to H-aggregated Z [14].
The mode of consumption also affects the bioavailability of Z from F. lycii. Traditionally, F. lycii is often consumed in the form of infusion by adding hot water to the dried berries. Increase in infusion temperature and time is associated with increased activity of bioactive compounds, in particular antioxidant activity. However, F. lycii preparations of 100 °C for 1~3 h, 90 °C for 2~3 h, and 80 °C for 2.5~3 h were found to be equivalent in terms of antioxidant activity of bioactive compounds [23]. Additionally, homogenization of F. lycii in hot skimmed milk results in a formulation that has a three-fold enhanced bioavailability of Z compared with both the “classical” hot water and warm skimmed milk treatment of the berries [22]. Furthermore, higher processing temperature in presence of milk proteins further enhances Z bioavailability, probably by improving its incorporation into mixed micelles, uptake by enterocytes, and subsequent release in triacylglycerol-rich lipoproteins. Although both F. lycii and egg yolk are believed to be good dietary sources of Z, the former may represent a healthier choice, because intake of the latter is associated with incident cardiovascular diseases and all cause mortality in a dose-dependent manner [26].
Processing of F. lycii products may affect bioavailability of the bioactive components. In recent years, numerous processed products of F. lycii have become popular as ”super food” in the market, particularly in Europe and North America [27]. One such product is Himalayan Goji juice in the US that refers to the juice made from reconstituted extracts of four fruits including F. lycii, grape, apple, and pear. The process of heat treatment leads to degradation of carotenoids in F. lycii with an approximate loss of about 20–24% [28]. On the other hand, addition of citric acid can effectively control the degree of carotenoid oxidation and thereby improve the level of carotenoid, the optimal concentration being 0.2% [29]. Similarly, the process of fermentation for a period of 24 h is associated with a 17% reduction in carotenoid content and 87% decrease in sugar contents of these berries but has no effect on the polyphenol content [30]. Besides traditional extraction method using hot water, the bioactive components in F. lycii, particularly the polysaccharide component, can be extracted using novel extraction technologies, such as ultrasound assisted, enzyme assisted, microwave assisted, and supercritical fluid extraction methods. Yield of the polysaccharide component varies with the type of extraction method used with the microwave assisted method having the maximum yield of polysaccharide component (8.25% ± 0.07%) [31].
4. Fructus lycii and Retinal Diseases and Degeneration
This section comprehensively reviews the literature pertaining to F. lycii and three clinically important retinal conditions, namely age-related macular degeneration (AMD), diabetic retinopathy (DR), and retinitis pigmentosa (RP). Oxidative damage is one of the common underlying pathways for these retinal diseases. It refers to an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to scavenge these ROS with its antioxidant defense system. Under physiological conditions, ROS regulates various biological functions by acting as a second messenger in cell signaling. Excessive production of ROS leads to oxidative damage to cellular structures directly by oxidizing lipids, proteins, and nucleic acids. The excess ROS also functions as signaling molecules to activate a number of cellular stress-sensitive pathways culminating in cellular damage [32]. The retina is particularly vulnerable to chronic oxidative damage due to several reasons [33,34]. First, photoreceptors and retinal pigment epithelium (RPE) have high metabolic activity resulting in generation of ROS as a natural by-product of mitochondria; second, photoreceptors are constantly exposed to light, which in the presence of high oxygen levels results in photooxidative damage; third, there is a high content of polyunsaturated fatty acids in the photoreceptor membranous disks; fourth, lipofuscin is derived, at least in part, from oxidatively damaged photoreceptor outer segments and that in itself is a photoreactive substance. Prolonged photooxidative damage eventually triggers other pathways such as retinal apoptosis, which adds to retinal damage.
F. lycii exhibits strong antioxidant action that is believed to be attributable to the synergistic effects of its bioactive components, carotenoids, polysaccharides, and flavonoids, each of which individually are strong antioxidants. Studies in animal models and humans have consistently demonstrated that F. lycii displays scavenging activity against superoxide anion and hydroxyl radicals, the two most important ROS, as well as inhibits lipid peroxidation of low density lipoprotein cholesterol fraction as evidenced by a decrease in malondialdehyde levels [35,36,37,38]. F. lycii also enhances endogenous antioxidant defense system by increasing the levels of antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase [39,40,41]. Moreover, administration of F. lycii in-vitro significantly modulates oxidative stress by inhibiting caspase-3 activation along with ROS levels in a concentration-dependent manner [42]. At the cellular level, F. lycii can enhance macrophage nitric oxide, phagocytic capacity, and acid phosphatase as well as down-regulate gene function to prevent ROS-induced apoptosis, thus resulting in significant increase in cell viability [43,44].
4.1. F. lycii and Age-Related Macular Degeneration
AMD is a degenerative disorder of the macula characterized by progressive loss of central vision due to an atrophic and/or neovascular event and is the leading cause of blindness among the elderly individuals over 50 years of age in developed countries [45]. In the coming years, this irreversible retinal disease will become a major public health burden globally due to rapidly ageing populations, particularly in Asia, which accounts for more than 60% of the world’s population [46]. Although the exact etiopathogenesis of AMD remains unclear, it is believed to be multifactorial in origin with existing scientific evidence suggesting oxidative damage as the main underlying mechanism.
Table 2 summarizes studies that have evaluated F. lycii with respect to AMD in human RPE cell lines, animal models, and humans. Pre-treatment with F. lycii extract protected human RPE cells against acute oxidative stress injury (apoptotic cell death) induced by exposure to blue light, ultraviolet light, and hydrogen peroxide [47,48,49,50]. Experimental studies have demonstrated visible morphological and functional changes in the RPE cells, such as an increase in the number of viable cells, reduction in apoptotic cells, enhanced proliferation, and phagocytic ability with consequential decrease in lipofuscin accumulation. The protective effect of F. lycii on RPE cells is believed to be exerted by the following mechanisms: first, antioxidant property resulting in decreased levels of endogenous ROS; second, modulating action on apoptosis-related genes leading to increase in Bcl-2/Bax ratio (up-regulation of Bcl-2, an anti-apoptotic gene and down-regulation of Bax, a pro-apoptotic gene) with resultant decrease in cell apoptosis; third, attenuation of deoxyribonucleic acid (DNA) damage in a dose-dependent manner. In-vitro studies evaluating the protective effect of F. lycii on RPE cells usually involve its aqueous fraction, and although both aqueous and ethanol extracts exhibit potent antioxidant activity, the ethanol extract exhibits a stronger antioxidant effect. This is probably attributable to the synergistic activity of hydrophilic (polysaccharide) and hydrophobic (carotenoids and flavonoids) bioactive components, both possessing strong antioxidant effects. Alternatively, the stronger antioxidant effect of ethanol extract may be due to the involvement of additional signaling pathways, such as toll like receptor pathways.

Table 2.
List of studies that have evaluated F. lycii and age-related macular degeneration.
Similarly, in animal models, F. lycii effectively protected photoreceptor cells against light-induced retinal damage via its antioxidant properties [41,51]. In rats exposed to white light, there were decreased levels of oxidative stress markers, increased levels of endogenous antioxidants, and up-regulation of the antioxidative genes Nrf2 and TrxR1 following F. lycii administration. Moreover, a delay in apoptosis of photoreceptors was also observed in light-damaged retinas as suggested by decreased expression levels of PARP-14, a member of the poly-ADP-ribose polymerase family. DNA is particularly sensitive to light-induced oxidative damage, and PARP-14 plays a significant role in DNA repair. The process of apoptosis is associated with high levels of PARP due to DNA fragmentation related with apoptosis. Morphological changes, such as photoreceptor cell loss, nuclear condensation, an increased number of mitochondrial vacuoles, outer membrane disk swelling, and cristae fractures, as well as functional changes, such as loss of a- and b-wave amplitudes induced by light exposure, were also distinctively ameliorated by F. lycii in rats.
To date, there are three prospective randomized controlled clinical trials of F. lycii supplementation in human subjects with AMD. Subjects with early AMD demonstrated a delay in disease progression, as evidenced by an absence of macular hypopigmentation progression and soft drusen accumulation, following supplementation with F. lycii for a period of 90 days when compared to subjects in the control group [20]. This was accompanied by a 26% rise in plasma Z concentration and a 57% rise in antioxidant capacity in subjects on F. lycii supplementation, which is equivalent to 10 mg/day of Z. In the same cohort, F. lycii supplementation was also found to increase plasma antioxidant capacity along with enhanced immune defenses (higher immunoglobin G response, sero-conversion, and protection rates following flu vaccination) and improved yin deficiency [52]. In addition, supplementation with F. lycii for 90 days increased serum Z concentration, macular pigment optical density and visual function (best-corrected visual acuity) in patients with early AMD, without causing any detectable adverse effects [53]. The authors speculated that increase in macular pigment optical density in the central retina was the main reason for the improvement in visual function in early AMD. The main limitations of these studies are their relatively small sample size and short period of supplementation. Nevertheless, the findings are consistent with a protective effect of F. lycii in subjects with early AMD in terms of delaying disease progression and improvement in visual function. Although the underlying mechanism for the protective effect is believed to be accumulation of plasma Z and other antioxidants, no direct relationship was observed between changes in plasma Z and macular features.
The Age-Related Eye Disease Study 2 demonstrated a protective effect of lutein and Z supplementation on progression to advanced AMD when the subgroup analyses of the treatment effect were limited to those participants with the lowest dietary intake of lutein and Z [54]. F. lycii has exceptionally high level of carotenoids, particularly Z. Along with lutein, it selectively accumulates in the primate macula, around 500-fold higher concentration than in any other body tissues, where they are collectively referred to as macular pigment [55]. Although Z and lutein are structural isomers, there are a few important differences between these two carotenoids that may have functional implications: first, Z predominates in the central part of the macula, the fovea, whereas lutein predominates in the periphery, the ratio being 2.4:1 at the fovea [56]; second, Z contains two β-ring end groups, whereas lutein contains both a β-ring and a ε-ring [57]; third, Z is twice as efficacious as lutein in quenching ROS, and this is probably attributable to the extended conjugated system of Z [58]. Therefore, the potential ocular benefits of dietary consumption of F. lycii with its high Z content are self-explanatory.
4.2. F. lycii and Diabetic Retinopathy
DR is one of the most common microvascular complications of diabetes and remains a major cause of preventable blindness in the working-age population worldwide [59]. It has been estimated that more than one third of those with diabetes worldwide have some form of DR and nearly one in ten have vision threatening DR [60]. Although the molecular mechanisms underlying diabetic microvascular complications remain unclear, there is a growing body of evidence to suggest that hyperglycemia-induced oxidative stress and its downstream pathways play an important role in the pathogenesis of DR [61]. For instance, oxidative stress has been shown to induce the expression of pro- apoptotic molecules leading to apoptosis, which has been identified as one of the pivotal mechanisms for cellular damage in DR.
Table 3 summarizes studies that have evaluated F. lycii with respect to DR in animal models and human cell lines. To date, there is no study that has examined the relationship between dietary F. lycii and DR in human subjects. F. lycii administration has been shown to ameliorate retinal structural and functional changes due to diabetes in animal models [62,63,64]. Restoration of the overall retinal thickness and its individual layers, decreased structural disturbance of photoreceptors outer and inner segments, and decreased cavitation in the RPE cell layer represent just a few of the retinal structural changes observed. Similarly, structural changes altered in retinal vessels by F. lycii include reduction in basement membrane thickness, increase in vessel lumen, increase in morphologically normal capillaries, and reduction in abnormal vascular tuft and twisted capillaries suggestive of vascular proliferation. At the functional level, F. lycii administration was associated with blunting of diabetes-induced decrease in amplitude of a-wave, b-wave, and oscillatory potentials on electroretinography (ERG) in animal models of diabetes [65]. Moreover, there was also reversal of high glucose-induced Müller cell dysfunction and overexpression of glial fibrillary acid protein. Müller cells are the principal glial cells that provide nutritional and functional support to retinal neurons and exhibit the most significant changes of all the glial cells in DR [66]. The level of glial fibrillary protein is an established indicator of retinal stress and is marginally detectable in Müller cells under normal retinal conditions but markedly up-regulated in the diabetic retina [67]. Of note, b-wave is believed to reflect light-induced electrical activity in the bipolar cells with contributions by Müller cells; however, ERG observations are unable to differentiate diabetes-induced damage to bipolar cells and/or to the Müller cells.

Table 3.
List of studies that have evaluated F. lycii with respect to diabetic retinopathy.
Administration of F. lycii in diabetic rats was associated with reversal of diabetes-induced increase in vascular endothelial growth factor (VEGF) levels and suppression of pigment epithelium derived factor (PEDF) levels [65]. This resulted in reinstallation of the balance between angiogenic and anti-angiogenic factors and decreased likelihood of angiogenesis. There was also significant reduction in the levels of VEGF mRNA in the retina of experimental animals treated with F. lycii. VEGF is a pro-inflammatory peptide that is necessary for the normal maintenance of retinal and choroidal vessels, but elevated expression of VEGF induced by hypoxia is a key stimulus for aberrant growth of new vessels in proliferative DR [75]. On the other hand, PEDF is one of the protective factors that promotes anti-angiogenesis and counteracts the pro-inflammatory environment in DR. Proliferative DR is one of the main vision-threatening complications of diabetes and is characterized by pathological neovascularization of the retina [76].
F. lycii may exhibit protective effect on the outer as well as inner blood–retinal barrier (BRB) as suggested by experimental studies [69,71,74]. Diabetes-induced dysfunction of the BRB results in leakage of fluid and circulating proteins within the neural retina and this is known as diabetic macular edema. Currently, diabetic macular edema is the most prevalent vision-threatening complication of diabetes [77]. The outer BRB is formed by tight junctions between the RPE cells, a monolayer of epithelial cells that separate the vascular choroidal system from the neurosensory retina. Diabetes-induced hyperglycemia increases intracellular glucose in RPE cells, which stimulates cytosolic isoform of adenylyl cyclase, resulting in increase in intracellular cyclic AMP levels and functional impairment of the outer BRB. High glucose-induced RPE barrier impairment is ameliorated by F. lycii by reversing the activity of adenylyl cyclase and decreasing cytosolic AMP levels [74]. It has been hypothesized that this action of F. lycii is mediated via its taurine bioactive component interacting with the catechol-estrogen binding site of soluble adenylyl cyclase. F. lycii may also protect the integrity of the inner BRB, formed by tight junctions between retinal vascular endothelial cells, by targeting the ROCK signaling pathway. The ROCK pathway regulates cellular adherence, migration, proliferation, and apoptosis through the control of the actin cytoskeletal assembly and cell contraction [78,79]. In particular, this pathway regulates expression and function of intercellular adhesion molecule-1 in endothelial cells as well as directly phosphorylates occludin and other tight junctional proteins. It is believed that the retinal vascular endothelial cells are in ROCK-activated state. F. lycii administration in diabetic rats was associated with reversal of hyperglycaemia-induced up-regulation of ROCK pathway and down-regulation of phosphorylated occludin with subsequent VEGF induced hyper permeability and vascular leakage [71].
F. lycii may attenuate hyperglycemia-induced apoptosis of RPE cells through up-regulation of PPAR-γ and down-regulation of caspase-3 pathway. PPAR is prominently expressed in photoreceptor outer segments, RPE cells, and choriocapillaris in mammalian eyes, and ligand-activated PPAR-γ controls apoptosis induced by oxidative stress and thus contributes to retinal protection. Retinal expression of PPAR-γ is suppressed in experimental models of diabetes and in endothelial cells exposed to high glucose [80,81]. F. lycii extract enhanced expression of PPAR-γ-related mRNA and protein in a dose-dependent manner in human RPE cell lines exposed to high glucose [73]. At the same time, expression as well as enzymatic activity of caspase-3 was down-regulated [82,83]. Members of the caspase family are involved in the initiation and execution of apoptosis, and caspase-3, known as the executioner caspase, plays an important role in the proteolytic cascade during apoptosis. The cytoprotective effect of F. lycii on RPE cells, which is regulated via PPAR-γ-mediated caspase-3 pathway, is believed to be due to its bioactive component taurine [72]. Several lines of evidence suggest that taurine in F. lycii plays an important role in DR prevention. Taurine is a non-essential amino acid found abundantly in the retina and has an ability to cross the BRB [84]. In fact, the taurine concentration in the retina including photoreceptors and RPE cells is estimated to be around 60–80 mM, corresponding to 40–75% of the total free amino acid content deemed necessary to maintain physiological functions, such as membrane stabilization, neuromodulation, and maintaining the integrity of the retina [85,86]. Past studies have shown that plasma and tissues levels of taurine are reduced in diabetes, and there is a beneficial effect of taurine supplementation on prevention and amelioration of hyperglycemia-induced retinal changes, such as retinal glial cell apoptosis [87,88,89]. However, more conclusive studies in human subjects are warranted to understand the mechanism of action of taurine and its long-term safety and efficacy in DR.
At the cellular level, F. lycii may reduce oxidative stress to mitochondria and endoplasmic reticulum (ER), the two prime targets for diabetes-induced oxidative damage. Mitochondria, the powerhouse of the cell, are the major endogenous source of ROS but are also the targets for its damaging effects. Sustained exposure to ROS (oxidative stress) damages the mitochondria and compromises the electron transport system, which ultimately results in mitochondrial DNA damage with subsequent impairment of transcription, and propagation of a vicious cycle of ROS generation sets in [90]. Indeed, DNA damage and apoptosis of mitochondria has been associated with changes in retinal blood flow and breakdown of BRB in late stages of DR [91]. Experimental studies have demonstrated that F. lycii may enhance mitochondrial biogenesis via up-regulation of carotenoid metabolic genes in diabetic retina [68]. Diabetes-induced hyperglycemia and subsequent hypoxia initiate changes in carotenoid homeostasis through inhibition of metabolic gene expression, which in turn leads to inhibition of 5′ AMP-activated protein kinase (AMPK), decrease in mitochondrial transcription factor (TFAM), and mitochondrial dysfunction [92,93]. Administration of F. lycii in diabetic mice primarily activated AMPK alpha 2 in mitochondria and nuclei, which in turn triggered increased expression of genes related to metabolic homeostasis of carotenoid, particularly scavenger receptor B1 (SR-B1), glutathione S-transferase pi 1 (GSTP1), and beta-carotene oxygenase 2 (BCO2). In tandem, there was increased expression of proteins responsible for mitochondrial biogenesis (peroxisome proliferator-activated receptor γ coactivator-1 [PGC-1-alpha], nuclear respiratory factor 1 [NRF1], and TFAM) and decreased expression of proteins produced in response to cellular stress (hypoxia inducible factor 1-alpha [HIF-1-alpha], VEGF, and heat shock protein 60 [HSP60]). As a result, there was enhancement of mitochondrial function and subsequent neuroprotection in diabetic mice. Similarly, F. lycii may also attenuate ER stress as demonstrated by decreased expression of ER stress biomarkers in diabetic retina under experimental settings [70]. Hyperglycaemia-linked oxidative stress disrupts protein synthesis and protein folding within the ER that eventually leads to ER stress and subsequent cell apoptosis. F. lycii restored AMPK and its downstream target proteins (retinal FOX03α essential for cell survival) in diabetic retina and in human RPE cells exposed to high glucose levels [62]. Consequentially, there was increased expression of antioxidant enzymes (thioredoxin and superoxide dismutase) with resultant normalization of cellular ROS status and redox homeostasis as well as decreased ER stress. This study also documented that restoration of AMPK by F. lycii was mediated, at least partially, through its bioactive carotenoid component Z.
4.3. F. lycii and Retinitis Pigmentosa
RP is a heterogeneous group of inherited bilateral retinal pigmentary dystrophies that are characterized by progressive and sequential loss of rod and cone photoreceptors, ultimately leading to complete blindness [94]. Several mechanisms have been linked to triggering the death of photoreceptors in mouse models of RP, such as oxidative stress, ER stress, dysregulation of cyclic guanosine monophosphate (cGMP) signalling, accumulation of calcium ions, and inflammatory responses [95]. Oxidative stress may be a crucial pathway because of the following reasons: first, the retina is highly vulnerable to oxidative stress; second, antioxidant treatment improves cell survival and preserves photoreceptors function in animal models; third, antioxidant treatment may decrease inflammatory markers involved in apoptosis [96]. Although experimental therapies under investigation are aiming to repair or rescue impaired vision, an effective treatment has yet to be discovered [97,98,99]. Currently, neuroprotection using antioxidant is widely employed as a therapeutic approach to delay photoreceptor degeneration in RP patients [100].
Table 4 summarizes studies that have evaluated F. lycii with respect to RP in animal models as well as human subjects. F. lycii demonstrated a protective effect on the structure and function of retinal neural cells via its strong antioxidant property in studies involving fast degenerating photoreceptor mouse model mimicking RP [101,102]. There was restoration of photoreceptor and bipolar cell morphology, improvement in arrangement of photoreceptor cell layer, maintenance of ramified shape of microglial cells, and preservation of the outer retinal thickness in the central retina. In the retinal ganglion cells, there was an increase in response to saturated light intensities, enhanced light evoked responses, increase sensitivity, and response speed along with a decrease in spontaneous abnormal firing. There was also differential effect with ON responses better than OFF responses at early stages of photoreceptor degeneration and enhanced OFF responses than ON responses at later stages. Functionally, photopic b-wave changes in the form of decreased latency and increased amplitude as well as larger scotopic a- and b- waves were observed on ERG. These findings suggest that F. lycii may potentially improve visual processing at multiple stages of information transmission via protecting photoreceptor, bipolar cells, and ganglion cells, and perhaps, function of higher brain centers, as suggested by enhanced visual behavior.

Table 4.
List of studies that have evaluated F. lycii and retintis pigmentosa.
Furthermore, F. lycii may exhibit a long term protective effect on photoreceptors via multiple pathways in experimental settings. Following F. lycii administration, an increase in antioxidant activity was observed in animal models, as indicated by higher glutathione redox/antioxidant ratio, a ratio that is commonly used to measure oxidative stress status [102]. F. lycii also demonstrated an anti-apoptotic effect by attenuating photoreceptor cell apoptosis through modulation of PARP and caspases levels. Administration of F. lycii in animal models was associated with significant down-regulation of 9, 3, 7 pro, and cleaved caspases along with increased nuclear levels of PARP as well as cleaved PARP protein [103]. PARP is one of the substrates for caspases and activated caspase 3/7 cleaves PARP to elicit apoptosis. Additionally, F. lycii regulated photoreceptor cell apoptosis, at least partly, through down-regulation of HIF-1α and Bax protein expression in animal models [102]; the former regulates many biological processes, one of these processes being activation of transcriptional activity of p53, resulting in transcription of many pro-apoptotic proteins, such as Bax and caspase-3. In addition, F. lycii mediated expression of inflammatory mediators in animal models, partially through the nuclear factor kappa-light-chain enhancer of the activated B cells (NF-kB) signaling pathway, which is probably attributable to its polysaccharide bioactive component [102].
Similar to experimental studies in animal models, F. lycii may exhibit neuroprotective effect on the human retina and might help to minimize or delay cone degeneration in RP patients [104]. In a double-blind placebo-controlled trial with 42 RP patients, there was preservation of visual acuity, both high (90%) and low (10%) contrast, as well as average macular thickness following oral administration of F. lycii for a period of 12 months, but no significant effect was observed on visual field sensitivity or on any of the parameters of ERG. The main limitations of this study were its relatively small sample size, short duration of study period, and the active ingredients in F. lycii granules were not measured precisely. In addition, although the primary end-points (visual acuity and macular retinal thickness) reached statistical significance, these parameters may not be clinically significant. The authors proposed that F. lycii may be useful as a dietary supplement to effectively preserve photopic vision in RP patients and to help maintain quality of life; however, a large-scale longitudinal study is warranted to further investigate the long-term protective effect of F. lycii in RP patients with different genotypes.
5. Limitations of the Existing Studies
The majority of studies that have provided vital information on F. lycii in AMD, DR, and RP have been conducted in animal models or human cell lines. Experimental studies involving animal models are overall relatively inexpensive, easy to perform, and replicable; however, there are several limitations of these studies: (1) there are significant variances between humans and animals due to differences attributable to species. For instance, rodents are characterized by the absence of a macula, and rod cells are the predominant retinal photoreceptors, whereas humans have specialized macula with cone cells as the main photoreceptors [105]; (2) nearly all studies have very small sample sizes without evidence of power calculations, thereby making it difficult to draw impactful conclusions; (3) the dosages used in animal studies are usually much higher than those used clinically in humans; (4) it is difficult to measure carotenoid levels in the mouse retina, thereby hindering the evaluation of F. lycii function (due to bioactive component carotenoid) in retinal protection and diseases; (5) it remains unclear whether the histological and biochemical changes observed under experimental settings can be translated to prevent and/or delay retinal diseases in real world clinical settings.
As for DR, no single diabetic mouse model recapitulates all the features or complications of human diabetes. Generally, streptozotocin-induced type I diabetic rat models manifesting the characteristic of hyperglycemia and impaired retinal function were used for evaluating disease processes associated with DR. On the other hand, genetically programmed diabetic db/db mouse models were used for studying the development and progression of DR in type 2 diabetes. The young diabetic db/db mouse with age less than 18 weeks do not exhibit signs of DR and thus represent good animal model for studying pathogenesis of DR in type 2 diabetes. Experimental studies in AMD exposed human RPE cell lines to stimulus, such as hydrogen peroxide, blue light irradiation, ultraviolet-B light, and high intensity white light, creating acute oxidative injury, thus imitating photooxidative injury in AMD. Of note, AMD appears to involve complex interaction of genetic and environmental factors (such as chronic photooxidative damage), making it a difficult disease to mirror in animal models. Fast degenerating photoreceptors models, such as wild rd 1 and rd 10 mice, or N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague–Dawley rats, were used to mimic RP in experimental settings. Nevertheless, promising findings identified from experimental studies should warrant greater interest in this field of research.
6. Side Effects and Drug Interaction
F. lycii is considered a safe dietary supplement and a dose of 15 g per day, equivalent to 3 mg/day of Z, is deemed beneficial to ocular health [106]. In order to reap the full health benefits of F. lycii, it is important to ensure that these berry fruits are free of any external contamination that may occur during the process of production and/or post marketing. A small amount of atropine alkaloid exists as a natural content in F. lycii with a maximum detectable concentration of 19 parts per billion, which is believed to be far below the toxic levels in humans [107]. To date, none of the published reports have described any case of atropine-related side effects following F. lycii intake.
Although allergic reactions to F. lycii are extremely rare, a few cases have been reported in the literature with symptoms, such as generalized urticaria, toxic hepatitis, and rare life-threatening anaphylactic reaction [108,109]. It is believed that lipid transfer proteins may be involved in allergic sensitization of F. lycii, and therefore there is a high degree of cross-reactivity of F. lycii with peach peel and tomatoes. Caution should also be exercised in individuals with a history of any food allergy or pan allergy with nonspecific lipid transfer proteins [110,111]. In addition, there are reports describing spontaneous hemorrhages in patients on warfarin who consumed tea, juice, and wine containing F. lycii. It is believed that these hemorrhages are attributable to increased international normalized ratio, possibly due to interaction of F. lycii with warfarin, an anticoagulant medicine [112,113,114]. A history of F. lycii intake should be made mandatory in individuals who take medications with a narrow therapeutic index.
7. Future Directions and Conclusions
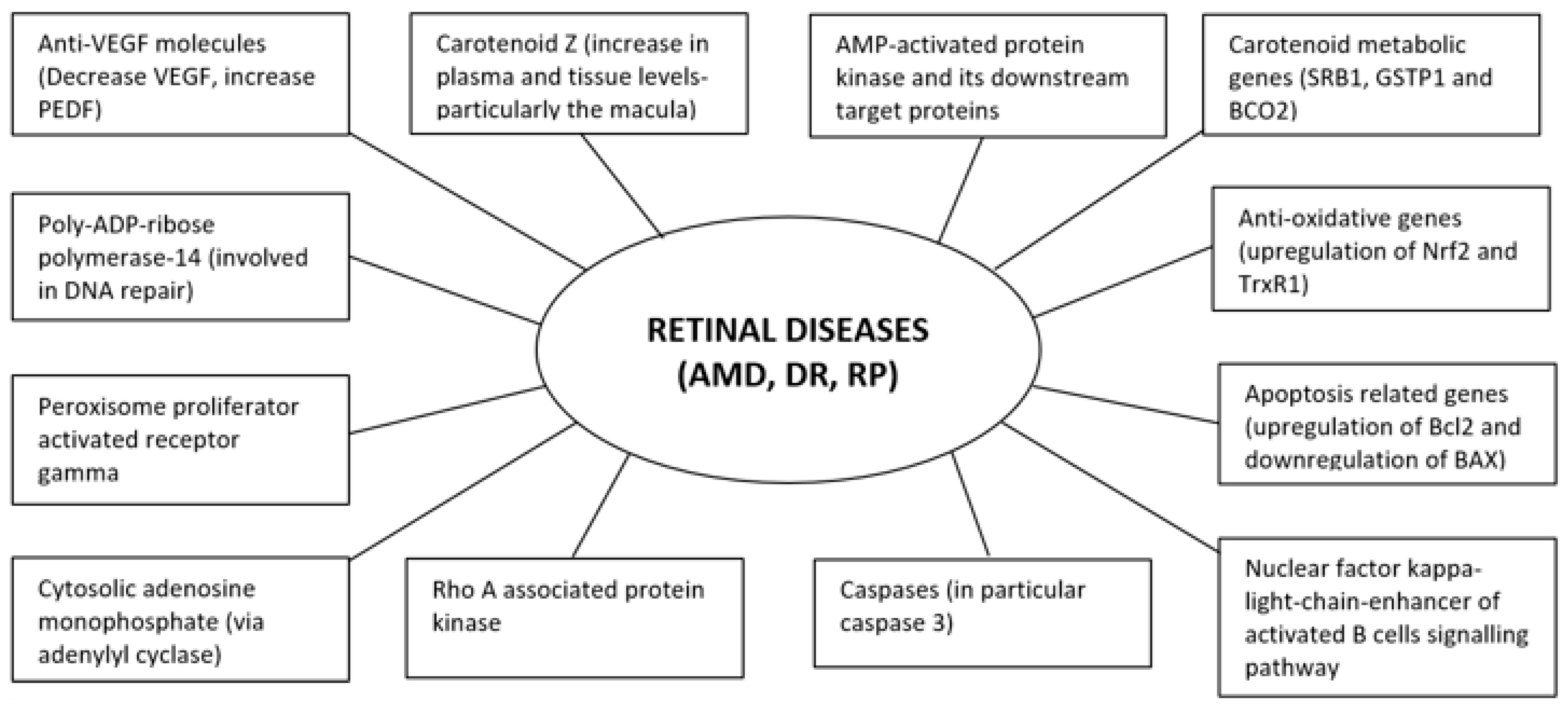
It is evident that existing literature in relation to F. lycii and retinal diseases detailed herein are mainly from experimental studies in animal models or human cell lines with a paucity of data from human subjects. Nonetheless, promising observations from these studies suggest that F. lycii has the potential to prevent and/or delay progression of retinal diseases (Figure 3) along with a favorable safety profile with few, if any, concerns regarding its adverse reactions.
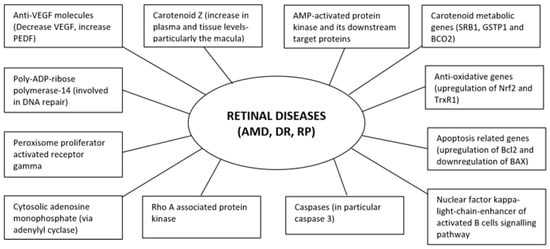
Figure 3.
Molecular targets of F. lycii in amelioration of retinal diseases.
However, there still remain a number of concerns that need to be addressed before F. lycii establishes its place in the clinical management of retinal diseases. First and foremost, observational and interventional studies, particularly long-term supplementation studies using F. lycii involving human subjects with retinal diseases, are warranted in the future to confirm the observed protective effect of F. lycii in retinal diseases. Second, there is no research grade F. lycii currently available in the market for conducting scientific studies, resulting in lack of standardization and quality control for the bioactive components in F. lycii along with adverse effects of post-marketing surveillance as another area of concern. It is thus crucial to ensure the availability of research grade F. lycii with consistent concentration of bioactive components to promote quality research in this field. Third, of all the bioactive components, carotenoids are the only bioactive component that can be precisely measured in terms of dietary intake and plasma levels as well as tissue levels, i.e., in the retina, that corresponds to the anatomical site of retinal diseases. Given that the biological effects of F. lycii are probably attributable to the additive, complementary, and/or synergistic effects from multiple bioactive components, one of the biggest challenges for future research is to segregate the specific properties of the various other bioactive components. Fourth, future studies should also examine whether the retinal benefits of F. lycii are influenced by additive or synergistic interactions with phytochemicals from other dietary sources typically consumed in the human diet. Fifth, research efforts should also be focused on studying the role of nutrigenomics (effects of nutrients on the genome, proteome, and metabolome) as well as nutrigenetics (effects of genetic variation on the interaction between diet and disease) in relation to F. lycii. Lastly, at the basic science level, studies should focus on the high order structures of bioactive components and relationship between structure and bioavailability to enhance our understanding and knowledge of F. lycii.
In summary, F. lycii offers an inexpensive and relatively safe “whole food” dietary supplement for the maintenance of retinal health as well as for prevention and/or delay in progression of retinal diseases commonly seen in clinical practice. It is important to note that these exotic berry fruits must be retailed with strict adherence to pharmacopoeia-recommended guidelines, including dosage regimes. In the near future, F. lycii supplementation may represent a model for modern medicine with successful integration between traditional Asian and Western medicine reaping the advantage of multi-functional herbal ingredients in the prevention and treatment of retinal diseases. We hope that this review article will not only increase awareness amongst the medical and scientific community regarding published work on F. lycii and retinal diseases, but will also help promote robust research work in this area to establish dietary intervention strategies aiming to promote ocular health and prevent common retinal diseases in the ageing population.
Author Contributions
K.N. and K.-G.A.E.: conceptualization; K.N., S.D., and R.S.: writing—original draft preparation; J.L.: resources; K.N. and K.-G.A.E.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Science-Translational and Applied Research (STAR19102).
Conflicts of Interest
The authors declare that they have no conflict of interest to declare.
Abbreviations
| F. lycii | Fructus lycii |
| L | Lycium |
| Z | Zeaxanthin |
| AMD | Age-related macular degeneration |
| DR | Diabetic retinopathy |
| RP | Retinitis pigmentosa |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| DNA | Deoxyribonucleic acid |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| TrxR1 | Thioredoxin reductase |
| PARP | Poly-ADP-ribose polymerase |
| ERG | Electroretinography |
| VEGF | Vascular endothelial growth factor |
| PEDF | Pigment epithelium-derived growth factor |
| mRNA | Messenger ribonucleic acid |
| BRB | Blood-retinal-barrier |
| AMPK | AMP-activated protein kinase |
| ROCK | Rho A associated protein kinase |
| PPAR | Peroxisome proliferator activated receptor |
| ER | Endoplasmic reticulum |
| TFAM | Transcription factor A, mitochondria |
| SR-B1 | Scavenger receptor class B type 1 |
| GSTP1 | Glutathione S-transferase pi 1 |
| BCO2 | β-carotene 9′,10′-oxygenase 2 |
| PCG-1 | Peroxisome proliferator-activated receptor γ coactivator-1 |
| NRF-1 | Nuclear respirator factor 1 |
| HIF | Hypoxia inducible factor |
| HSP | Heat shock protein |
| FOX03 | Forkhead O transcription factor 3 |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| cGMP | Cyclic guanosine monophosphate |
References
- Stuart, G.A.; Smith, F.P. Chinese Materia Medica; American Presbyterian Mission Press: Shanghai, China, 1991; p. 250. [Google Scholar]
- Integrated Taxonomic Information System. Lycium barbarum L. Taxonomic Serial No: 503599; 2019; p. 22. Available online: https://www.itis.gov (accessed on 29 October 2020).
- Chi, Z.S. Chemical constituents of Fructus Lycii and Folium Lycii (I)-Nutrients in Fructus lycii and Folium lycii. Zhong Yao Tong Bao 1986, 11, 41. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China Part I; Chemical Industry Press: Beijing, China, 2015. [Google Scholar]
- Cao, Y.L.; Wu, P.J. Wolfberry Germplasm Resources in China; China Forestry Publishing House: Beijing, China, 2015. [Google Scholar]
- Potterat, O. Goji (Lycium barbarum and L Chinese): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Medica 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji berries as a potential natural antioxidant medicine: An insight into their molecular mechanisms of action. Oxid. Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef] [PubMed]
- Kulczyriski, B.; Gramza-Michalowska, A. Goji berry (Lycium barbarum): Composition and health effects-a review. Pol. J. Food Nutr. Sci. 2016, 66, 67–76. [Google Scholar] [CrossRef]
- Wang, C.; Chang, S.B.; Inbaraj, S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, S. Determination of taurine in Lycium barbarum by high performance liquid chromatography with OPA-urea pre-column derivatization. Se Pu = Chin. J. Chromatogr. 1997, 15, 54–56. [Google Scholar]
- Tatania, K.; Alexander, E.; Alexander, D. Why Dietary Zeaxanthin? A Scientific Summary; Kemin Foods LC: Des Moines, IA, USA, 2012. [Google Scholar]
- Hempel, J.; Schädle, C.N.; Sprenger, J.; Heller, A.; Carle, R.; Schweiggerta, R.M. Ultrastructural deposition forms and bio accessibility of carotenoids and carotenoid esters from goji berries (Lycium barbarum L.). Food Chem. 2017, 218, 525–533. [Google Scholar] [CrossRef]
- Hempel, J.; Fischer, A.; Fischer, M.; Högel, J.; Bosy-Westphal, A.; Carle, R.; Schweiggert, R.M. Effect of aggregation forms on bioavailability of zeaxanthin in humans: A randomized cross-over study. Br. J. Nutr. 2017, 118, 698–706. [Google Scholar] [CrossRef]
- Bertoldi, D.; Cossignani, L.; Blasi, F.; Perini, M.; Barbero, A.; Pianezze, S.; Montesano, D. Characterization and geographical traceability of Italian goji berries. Food Chem. 2019, 275, 585–593. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, S.; Zhang, F.; Yan, H.; Qian, D.W.; Wang, H.Q.; Jin, L.; Duan, J.A. Comparison of Functional Components and Antioxidant Activity of Lycium barbarum L. Fruits from Different Regions in China. Molecules 2019, 24, 2228. [Google Scholar] [CrossRef] [PubMed]
- Perez-Galvez, A.; Martin, H.D.; Sies, H.; Stahl, W. Incorporation of carotenoids from paprika oleoresin into human chylomicrons. Br. J. Nutr. 2003, 89, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Leung, I.Y.F.; Tso, M.O.M.; Li, W.W.Y.; Lam, T.T. Absorption and tissue distribution of Z and L in rhesus monkeys after taking fructus lycii (Gou Qi Zi) extract. Investig. Ophthalmol. Vis. Sci. 2001, 42, 466–471. [Google Scholar]
- Cheng, C.Y.; Chung, W.Y.; Szeto, Y.T.; Benzie, I.F.F. Fasting plasma zeaxanthin response to Fructus barbarum L. (wolfberry; Kei Tze) in a food-based human supplementation trial. Br. J. Nutr. 2005, 93, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bucheli, P.; Vidal, K.; Shen, L.; Gu, Z.; Zhang, C.; Miller, L.E.; Wang, J. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom. Vis. Sci. 2011, 88, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, D.E.; Weller, P.; Wolters, M.; Hahn, A. Comparison of plasma responses in human subjects after the ingestion of 3R,3R′-zeaxanthin dipalmitate from wolfberry (Lycium barbarum) and non-esterified 3R,3R′-zeaxanthin using chiral high-performance liquid chromatography. Br. J. Nutr 2004, 91, 707–713. [Google Scholar] [CrossRef]
- Benzie, I.F.; Chung, W.Y.; Wang, J.; Richelle, M.; Bucheli, P. Enhanced bioavailability of zeaxanthin in a milk-based formulation of wolfberry (Gou Qi Zi; Fructus barbarum L.). Br. J. Nutr. 2006, 96, 154–160. [Google Scholar] [CrossRef]
- Sun, Y.; Rukeya, J.; Tao, W.; Sun, P.; Yi, Z. Bioactive compounds and antioxidant activity of wolfberry infusion. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Werklust, B.V.; Beheer, N. Carotenoid Esters for the Prevention and Treatment of Eye Diseases. Patent DE19950327, 20 April 2000. [Google Scholar]
- Leung, I.Y.F.; Ngai, J.; Lam, K.W.; Tso, M.O.M. Absorption of zeaxanthin in rats after feeding with purified zeaxanthin or a traditional Chinese medicine, Gou Qi Zi. Investig. Ophthalmol Vis. Sci. 1999, 40, S608. [Google Scholar]
- Zhong, V.W.; Horn, L.V.; Cornelis, M.C. Association of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA 2019, 32, 1081–1095. [Google Scholar] [CrossRef]
- Ulbricht, C.; Bryan, J.K.; Costa, D.; Culwell, S.; Giese, N.; Isaac, R.; Nummy, K.; Pham, T.; Rapp, C.; Rusie, E.; et al. An evidence-based systematic review of goji (Lycium spp.) by the natural standard research collaboration. J. Diet. Suppl. 2015, 12, 184–240. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, A.; Niro, S.; Alam, M.D.R.; Cinquanta, L.; Di Matteo, M.; Adiletta, G.; Panfili, G. Effect of a physical pre-treatment and drying on carotenoids of goji berries (Lycium barbarum L.). LWT - Food Sci. Technol. 2018, 92, 318–323. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, F.; Wu, M.; Yan, X.; Meng, X.; Song, Y. A rapid and effective approach for on-site assessment of total carotenoid content in wolfberry juice during processing. J. Sci. Food Agric. 2015, 95, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Dos-Reis, B.A.; Kosinka-Cagnazzo, A.; Schmitt, R. Fermentation of plant material-effect on sugar content and stability of bioactive compounds. Pol. J. Food Nutr. Sci. 2014, 64, 235–241. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, structural characterization and biological functions of Lycium barbarum polysaccharides: A review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef]
- Cutler, R.G. Oxidative stress profiling: Part I. Its potential importance in the optimization of human health. Ann. N. Y. Acad. Sci. 2005, 1055, 93–135. [Google Scholar] [CrossRef]
- Khaliq, A.; Jervis-Evans, J.; McLeod, D.; Boulton, M. Oxygen modulates the response of the retinal pigment epithelium to basic fibroblast growth factor and epidermal growth factor by receptor regulation. Investig. Ophthalmol. Vis. Sci. 1996, 37, 436–443. [Google Scholar]
- Beatty, S.; Koh, H.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Cui, B.; Liu, S.; Lin, X.; Wang, J.; Li, S.H.; Wang, Q.B.; Li, S.P. Effects of Lycium barbarum aqueous and ethanol extracts on high fat induced oxidative stress in rat liver tissue. Molecules 2011, 16, 9116–9128. [Google Scholar] [CrossRef]
- Huang, L.J.; Tian, G.Y.; Wang, Z.F.; Dong, J.B. Studies on the glycolconjugates and glycans from Lycium barbarum L in inhibiting low density lipoprotein (LDL) peroxidation. Acta Pharm Sin. 2001, 36, 108–111. [Google Scholar]
- Wu, S.; Ng, L.; Lin, C. Antioxidant activities of some common ingredients of traditional Chinese medicine, Angelica sinensis, Lycium barbarum and Poria cocos. Phytother. Res. 2004, 18, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, Y.; Liu, X. Effect of the Lycium barbarum polysaccharide on age-related oxidative stress in aged mice. J. Ehanopharmacol. 2007, 111, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, A. Evaluation of the antioxidant effects of polysaccharides extracted from Lycium barbarum. Med. Chem. Res. 2007, 15, 471–482. [Google Scholar] [CrossRef]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.P.; Ke, C.Y.; Kuo, C.C.; Lee, Y.-J. Effect of a complex lutein formula in an animal model for light-induced retinal degeneration. Chin. J. Physiol. 2016, 59, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, W.; Qi, D.; Wang, D. Lycium barbarum polysaccharide protects against LPS-induced ARDS by inhibiting apoptosis, oxidative stress and inflammation in pulmonary endothelial cells. Free Radic Res. 2018, 52, 480–490. [Google Scholar] [CrossRef]
- Gong, G.P.; Dang, T.T.; Deng, Y.N.; Han, J.; Zou, Z.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.; Wang, Z. Physiochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef]
- Liang, B.; Jin, M.; Liu, H. Water-soluble polysaccharide from dried Lycium barbarum fruits: Isolation, structural features and antioxidant activity. Carbohydr Polym 2011, 83, 1947–1951. [Google Scholar] [CrossRef]
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.H.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020and 2040: A systemic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Dong, W.H.; Du, X.J.; Guo, D.D.; Dou, R. Protective effects of Lycium barbarum polysaccharide on damaged hRPE cells induced by blue light irradiation. Int. Eye Sci. 2013, 13, 2381–2384. [Google Scholar]
- Du, X.J.; Dong, W.H.; Bi, H.S.; Xie, X.F. The anti-aging effect of Lycium barbarum polysaccharide on human retinal pigment epithelial cell. Chin. J. Exp. Ophthalmol. 2013, 31, 739–743. [Google Scholar]
- Liu, L.; Lao, W.; Ji, Q.S.; Yang, Z.H.; Yu, G.C.; Zhong, J.X. Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int. J. Ophthalmol. 2015, 8, 11–16. [Google Scholar] [PubMed]
- Hsieh, F.C.; Hung, C.T.; Cheng, K.C.; Wu, C.Y.; Chen, Y.C.; Wu, Y.J.; Liu, W.; Chiu, C.C. Protective effects of Lycium barbarum extracts on UVB-Induced damage in human retinal pigment epithelial cells accompanied by attenuating ROS and DNA Damage. Oxid. Med. Cell Longev. 2018, 2018, 4814928. [Google Scholar] [CrossRef]
- Tang, L.; Bao, S.; Du, Y.; Jiang, Z.; Wuliji, A.O.; Ren, X.; Zhang, C.; Chu, H.; Kong, L.; Ma, H. Antioxidant effects of Lycium barbarum polysaccharides on photoreceptor degeneration in the light-exposed mouse retina. Biomed. Pharmacother. 2018, 103, 829–837. [Google Scholar] [CrossRef]
- Vidal, H.; Bucheli, P.; Moulin, J.; Wang, J.; Benyacoub, J. Effect of a dietary wolfberry preparation on immune and physical status in elderly. Eur. Geriatr. Med. 2014, 5, S244–S245. [Google Scholar] [CrossRef]
- Li, S.; Liu, N.; Lin, L. Macular pigment and serum zeaxanthin levels with goji berry supplement in early age-related macular degeneration. Int. J. Ophthalmol. 2018, 11, 970–975. [Google Scholar]
- AREDS 2 Research Group. Lutein plus zeaxanthin and omega 3 fatty acids for age-related macular degeneration: The age-related eye disease study 2 (AREDS 2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Friedes, L.M.; Gomez, G.M.; Kilburn, M.D.; Menendez, E.; Vidal, I.; Wang, W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 1997, 64, 211–218. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the human macular carotenoids. Invest. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Leasher, J.L.; Bourne, R.R.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Naidoo, K.; Pesudovs, K.; Price, H.; White, R.A.; Wong, T.Y.; et al. Vision Loss Expert Group of the Global Burden of Disease Study. Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-analysis from 1990 to 2010. Diabetes Care 2016, 39, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Meta-Analysis for Eye Disease (META-EYE) Study Group. Meta-analysis for eye disease (META-EYE) study group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 207, 43603. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, Y.; Jiang, Y.; Willard, L.; Ortiz, E.; Wark, L.; Medeiros, D.; Lin, D. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp. Biol. Med. 2011, 236, 1051–1063. [Google Scholar] [CrossRef]
- Hu, C.K.; Lee, Y.J.; Colitz, C.M.; Chang, C.J.; Lin, C.T. The protective effects of Lycium barbarum and Chrysanthemum morifolum on diabetic retinopathies in rats. Vet. Ophthalmol 2012, 15 (Suppl. 2), 65–71. [Google Scholar] [CrossRef]
- Guo, J.; Xu, G.X.; Hou, Z.J.; Xu, J.B.; Huang, L.Y. Effect of Lycium barbarum polysaccharides on the retinal ultrastructure of streptozocin-induced diabetic rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 2013, 33, 1404–1407. [Google Scholar]
- Yao, Q.; Yang, Y.; Lu, X.; Zhang, Q.; Luo, M.; Li, A.; Pan, Y. Lycium barbarum polysaccharides improve retinopathy in diabetic Sprague-dawley rats. Evid Based Complement. Altern Med. 2018, 2018, 7943212. [Google Scholar] [CrossRef]
- Leith, E.; Barber, A.J.; Xu, B.; Dice, C.; Ratz, M.J.; Tanase, D.; Strother, J.M. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes 1998, 47, 815–820. [Google Scholar] [CrossRef]
- Barber, A.J.; Antonetti, A.; Gardner, T.W.; The Penn State Retina Research Group. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3561–3568. [Google Scholar]
- Yu, H.; Wark, L.; Ji, H.; Willard, L.; Jaing, Y.; Han, J.; He, H.; Ortiz, E.; Zhang, Y.; Medeiros, D.M.; et al. Dietary wolfberry up regulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Mol. Nutr. Food Res. 2013, 57, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, N.; Ma, Y. Effects of Lycium barbarum polysaccharides on retinal pathological change and expression of VEGF in retina of diabetic rats. Chin. J. Exp. Ophthalmol. 2014, 32, 334–339. [Google Scholar]
- Wang, Y.; Ding, L.; Li, Y.; Guan, C.; Guo, J. Lycium barbarum polysaccharides can reduce the oxidative damage of the retinal nerve cells in diabetic rats. Int. J. Clin. Exp. Med. 2017, 10, 5168–5174. [Google Scholar]
- Wang, J.; Yao, Y.; Liu, X.; Wang, K.; Zhou, Q.; Tang, Y. Protective effects of Lycium barbarum polysaccharides on blood-retinal barrier via ROCK1 pathway in diabetic rats. Am. J. Transl. Res. 2019, 11, 6304–6315. [Google Scholar]
- Song, M.K.; Salam, N.K.; Roufogalis, B.D.; Huang, T.H.W. Lycium barbarum (Goji Berry) extracts and its taurine component inhibit PPAR-γ-dependent gene transcription in human retinal pigment epithelial cells: Possible implications for diabetic retinopathy treatment. Biochem. Pharmacol. 2011, 82, 1209–1218. [Google Scholar] [CrossRef]
- Song, M.K.; Roufogalis, B.D.; Huang, T.H.W. Reversal of the caspase-dependent apoptotic cytotoxicity pathway by taurine from Lycium barbarum (Goji Berry) in human retinal pigment epithelial cells: Potential benefit in diabetic retinopathy. Evid. Based Complement. Altern. Med. 2012, 2012, 323784. [Google Scholar] [CrossRef]
- Pavan, B.; Capuzzo, A.; Forlani, G. High glucose-induced barrier impairment of human retinal pigment epithelium is ameliorated by treatment with Goji berry extracts through modulation of cAMP levels. Exp. Eye Res. 2014, 120, 50–54. [Google Scholar] [CrossRef]
- El Rami, H.; Barham, R.; Sun, J.K.; Silva, P.S. Evidence-Based Treatment of Diabetic Retinopathy. Semin. Ophthalmol. 2017, 32, 67–74. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, H.; Bao, S.; Wang, N.; Gillies, M.C. Diabetic macular edema: New concepts in patho-physiology and treatment. Cell Biosci. 2014, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Dörfel, M.J.; Huber, O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J. Biomed. Biotechnol. 2012, 2012, 807356. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Breslin, J.W.; Zhu, J.; Yuan, S.Y.; Wu, M.H. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 2006, 13, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Herzlich, A.A.; Tuo, J.; Chan, C.C. Peroxisome proliferator-activated receptor and age-related macular degeneration. PPAR Res. 2008, 2008, 11. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Sanders, T.; Kahook, K.; Akeel, S.; Elmarakby, A.; Al-Shabrawey, M. Suppression of retinal peroxisome proliferator-activated receptor γ in experimental diabetes and oxygen-induced retinopathy: Role of NADPH oxidase. Investig. Ophthalmol. Vis. Sci. 2009, 50, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Takatani, T.; Takahashi, Y.; Uozumi, Y.; Matsuda, T.; Ito, T.; Schaffer, S.W.; Fujio, Y.; Azuma, J. Taurine prevents the ischemia-induced apoptosis in cultured neonatal rat cardiomyocytes through Akt/caspase 9 pathway. Biochem. Biophys. Commun. 2004, 316, 484–489. [Google Scholar] [CrossRef]
- Wu, Q.D.; Wang, J.H.; Fennessy, F.; Redmond, P.H.; Bouchier-Hayes, D. Taurine prevents high glucose induced human vascular cell endothelial apoptosis. Am. J. Physiol. 1999, 227, C1229–C1238. [Google Scholar]
- Tomi, M.; Terayama, T.; Isobe, T.; Egami, F.; Morito, A.; Kurachi, M.; Ohtsuki, S.; Kang, Y.S.; Terasaki, T.; Hosoya, K. Function and regulation of taurine transport at the inner blood–retinal barrier. Microvasc. Res. 2007, 73, 100–106. [Google Scholar] [CrossRef]
- Pasantes-Morales, H.; Klethi, J.; Ledig, M.; Mandel, P. Free amino acids of chicken and rat retina. Brain Res. 1972, 41, 494–497. [Google Scholar] [CrossRef]
- Vilchis, C.; Salceda, R. Effect of diabetes on levels and uptake of putative amino acid neurotransmitters in rat retina and retinal pigment epithelium. Neurochem. Res. 1996, 21, 1167–1171. [Google Scholar] [CrossRef]
- McCarty, M.F. Exploiting complementary therapeutic strategies for the treatment of type II diabetes and prevention of its complications. Med. Hypotheses 1997, 49, 143–152. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Z.; Mi, M.; Xu, H.; Zhu, J.; Wei, N.; Chen, K.; Zhang, Q.; Zeng, K.; Wang, J.; et al. Dietary taurine supplementation ameliorates diabetic retinopathy via anti-excitotoxicity of glutamate in streptozotocin-induced Sprague-Dawley rats. Neurochem. Res. 2008, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Salam, N.K.; Roufogalis, B.D.; Huang, T.H.W. The effect of taurine in Lycium barbarum (goji berry) on diabetic retinopathy in human retinal pigment epithelial cells: Activation of PPAR-gamma. Paed Diabetes 2011, 12, 53. [Google Scholar]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochem. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mohammad, G.; dos Santos, J.M.; Zhong, Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes 2011, 60, 3023–3033. [Google Scholar] [CrossRef]
- Lobo, G.P.; Isken, A.; Hoff, S.; Babino, D.; Lintig, J.V. BCO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development 2012, 139, 2966–2967. [Google Scholar] [CrossRef] [PubMed]
- Sivitiz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Newton, F.; Megaw, R. Mechanisms of photoreceptor death in retinitis pigmentosa. Genetic 2020, 11, 1120. [Google Scholar]
- Chen, Y.; Yang, M.; Wang, Z.J. (Z)-7,40 -Dimethoxy-6-hydroxy-aurone-4-O-β-glucopyranoside mitigates retinal degeneration in Rd10 mouse model through inhibiting oxidative stress and inflammatory responses. Cutan. Ocul. Toxicol. 2019, 39, 36–42. [Google Scholar] [CrossRef]
- Bennett, J.; Tanabe, T.; Sun, D.; Zeng, Y.; Kjeldbye, H.; Gouras, P.; Maguire, A.M. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat. Med. 1996, 2, 649–654. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, R.E.; Pearson, R.A.; MacNeil, A.; Douglas, R.H.; Salt, T.E.; Akimoto, M.; Swaroop, A.; Sowden, J.C.; Ali, R.R. Retinal repair by transplantation of photoreceptor precursors. Nature 2006, 444, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Otani, A.; Dorrell, M.I.; Kinder, K.; Moreno, S.K.; Nusinowitz, S.; Banin, E.; Heckenlively, J.; Friedlander, M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J. Clin. Investig. 2004, 114, 765–774. [Google Scholar] [CrossRef]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, J.; Xiang, Z.; Xu, D.; So, K.F.; Vardi, N.; Xu, Y. Lycium barbarum Polysaccharides Protect Retina in rd1 Mice During Photoreceptor Degeneration. Investig. Opthalmol. Vis. Sci. 2018, 59, 597. [Google Scholar] [CrossRef]
- Wang, K.; Xiao, J.; Peng, B.; Xing, F.; So, K.F.; Tipoe, G.L.; Lin, B. Retinal structure and function preservation by polysaccharides of wolfberry in a mouse model of retinal degeneration. Sci. Rep. 2014, 4, 7601. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Q.; Gao, H.; Peng, X.; Wen, Y.; Dai, G. Lycium barbarum polysaccharides attenuates N-methy-N-nitrosourea-induced photoreceptor cell apoptosis in rats through regulation of poly (ADP-ribose) polymerase and caspase expression. J. Ethnopharmacol. 2016, 191, 125–134. [Google Scholar] [CrossRef]
- Chan, H.H.; Lam, H.I.; Choi, K.Y.; Li, S.Z.C.; Lakshmanan, Y.; Yu, W.Y.; Chang, R.C.C.; Lai, J.S.M.; So, K.F. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J. Ethnopharmacol. 2019, 236, 336–344. [Google Scholar] [CrossRef]
- Xue, C.; Rosen, R.; Jordan, A.; Hu, D.N. Management of ocular diseases using lutein and zeaxanthin: What have we learned from experimental animal studies? J. Ophthlamol. 2015, 2015, 523027. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Adams, M.; Wiedenmann, M.; Tittel, G.; Bauer, R. HPLC-MS trace analysis of atropine in Lycium barbarum berries. Phytochem. Anal. 2006, 17, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Monzon Ballarin, S.; Lopez-Matas, M.A.; Saenz Abad, D.; Perez-Cinto, N.; Carnes, J. Anaphylaxis associated with the ingestion of goji berries (Lycium barbarum). J. Investig. Allergol. Clin. Immunol. 2011, 21, 567–570. [Google Scholar] [PubMed]
- Arroyo-Martinez, Q.; Saenz, M.J.; Arias, F.A. Lycium barbarum: A new hepatotoxic “natural” agent? Dig. Liver Dis. 2011, 43, 749. [Google Scholar] [CrossRef] [PubMed]
- Larramendi, C.H.; Garcia-Abujeta, J.L.; Vicario, S.; Garcia-Endrino, A.; Lopez, M.A.; Garcia-Sedeno, M.D.; Carnes, J. Goji berries (Lycium barabarum) risk of allergic reactions in individuals with food allergy. J. Investig. Allergol. Clin. Immunol. 2012, 21, 345–350. [Google Scholar]
- Carnes, J.; de Larramendi, C.H.; Ferrer, A.; Huertas, A.J.; Lopez-Matas, M.A.; Pagan, J.A.; Navarro, L.A.; Garcia-Abujeta, J.L.; Vicario, S.; Pena, M. Recently introduced foods as new allergenic sources: Sensitization to Goji berries (Lycium barbarum). Food Chem. 2013, 137, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.; Hung, A.; Hui, A.C.; Chan, T.Y.K. Warfarin overdose due to possible effects of Lycium barbarum. Food Chem. Toxicol. 2008, 46, 1860–1862. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.A.; Ferro, C.L.; Bursua, A.J.; Gerber, B.S. Possible interaction between Lycium barbarum (goji) and warfarin. Pharmacotherapy 2012, 32, e50–e53. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, L.; Xie, B. Bleeding due to a probable interaction between warfarin and Gou qi zi (Lycium barbarum L.). Toxicol. Rep. 2015, 2, 1209–1212. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).