The Nutritional Status of Individuals Adopted Internationally as Children: A Systematic Review
Abstract
1. Introduction
1.1. Malnutrition Epidemiology
1.2. Malnutrition and Disability at Pre-Adoption Baseline
1.3. Malnutrition Post-Adoption
1.4. Research Gap
- Pre-adoption factors, e.g., early life clinical and nutritional history; underlying disability.
- Peri-adoption factors, e.g., age at adoption; length of stay in any institutional care before adoption.
- Post-adoption factors, e.g., socioeconomic and nutritional environment into which children are adopted.
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
- Anthropometric data, including weight for age, weight for height, height for age, head circumference for age and body mass index. Our main focus was on standardized values using WHO growth standards, but we also considered other growth references (e.g., CDC, NCHS growth references) and non-standard reports (e.g., unadjusted height or weight).
- Micronutrient status: either laboratory-measured values or clinical status if applicable (e.g., clinically obvious rickets suggesting vitamin D deficiency).
- Peer-reviewed studies.
- Written in English.
- Published from January 1995 to July 2020.
- They reported on individuals adopted at the age of 18 years or older.
- They focused on domestic adoption placements.
- Study reports were not peer reviewed.
- They used non-standardized anthropometric growth measurements.
2.3. Information Sources and Search Strategy
2.4. Data Extraction
2.5. Quality Assessment
2.6. Summary Measures
3. Results
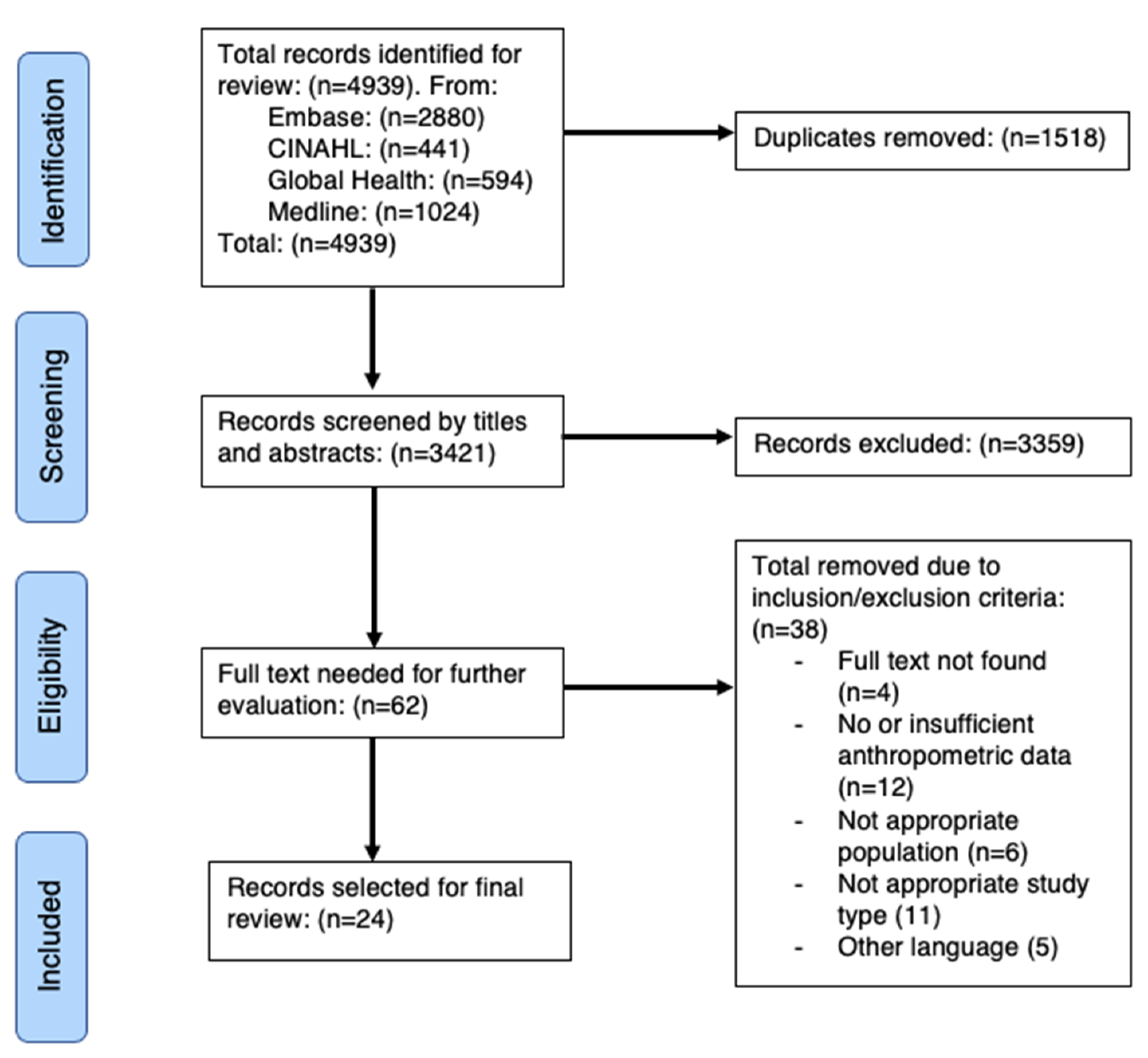
3.1. Study Selection
3.2. Study Characteristics
3.3. Anthropometric Data
3.4. Weight
3.5. Height
3.6. Head Circumference
3.7. Micronutrient Status
3.8. Age of Adoption
4. Discussion
4.1. Summary of Evidence
4.2. Pre-Adoption Factors, Country of Origin and Adoption
4.3. Micronutrient Status
4.4. Age at Adoption
4.5. Institutionalized Care and Orphanages
4.6. Sex and Nutrition Status
4.7. Disability and Nutritional Status
4.8. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| BMI | Body mass index |
| BMIZ | Body mass index Z-score |
| CAI | Children adopted internationally |
| CDC | Centers of Disease Control |
| HAZ | Height-for-age Z-score |
| HCAZ | Head circumference-for-age Z-score |
| LBW | Low birth weight |
| MDI | Mental scale index |
| OFCZ | Occipitofrontal circumference Z-score |
| PDI | Psychomotor development index |
| VDD | Vitamin D deficiency |
| WAZ | Weight-for-age Z-score |
| WHO | World Health Organization |
| WHZ | Weight-for-height Z-score |
References
- Unicef. Orphans 2020. Available online: https://www.unicef.org/media/orphans (accessed on 25 July 2020).
- Adoption.org. What International Adoption Statistics Should I Know? 2020. Available online: https://adoption.org/international-adoption-statistics-know (accessed on 25 July 2020).
- United Nations General Assembly. Guidelines for the Alternative Care of Children; UN Document A/RES/64/142; United Nations: Geneva, Switzerland, 2009. [Google Scholar]
- United Nations Human Rights Office of the High Commissioner. Convention on the Rights of the Child; United Nations Human Rights Office of the High Commissioner: New York, NY, USA, 1990. [Google Scholar]
- Mather, M. Intercountry adoption. Arch. Dis. Child. 2007, 92, 479–482. [Google Scholar] [CrossRef]
- Selman, P. The rise and fall of intercountry adoption in the 21st century. Int. Soc. Work 2009, 52, 575–594. [Google Scholar] [CrossRef]
- Smyke, A.T.; Koga, S.F.; Johnson, D.E.; Fox, N.A.; Marshall, P.J.; Nelson, C.A.; Zeanah, C.H.; BEIP Core Group. The caregiving context in institution-reared and family-reared infants and toddlers in Romania. J. Child Psychol. Psychiatry 2007, 48, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.E.; Springer, S.H. Health Care in the First Year after International Adoption. Pediatr. Clin. 2005, 52, 1331–1349. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Bothe, D.; Holsinger, E.; Kirchner, H.L.; Olness, K.; Mandalakas, A. The impact of nutritional status and longitudinal recovery of motor and cognitive milestones in internationally adopted children. Int. J. Environ. Res. Public Health 2011, 8, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Unicef. Malnutrition Prevalence Remains Alarming: Stunting Is Declining Too Slowly While Wasting Still Impacts the Lives of Far too Many Young Children 2020. Available online: https://data.unicef.org/topic/nutrition/malnutrition/ (accessed on 8 June 2020).
- Fuglestad, A.J.; Lehmann, A.E.; Kroupina, M.G.; Petryk, A.; Miller, B.S.; Iverson, S.L.; Johnson, D.E.; Georgieff, M.K. Iron deficiency in international adoptees from eastern Europe. J. Pediatr. 2008, 153, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Kertes, D.A.; Gunnar, M.R.; Madsen, N.J.; Long, J.D. Early deprivation and home basal cortisol levels: A study of internationally adopted children. Dev. Psychopathol. 2008, 20, 473–491. [Google Scholar] [CrossRef]
- Sollai, S.; Ghetti, F.; Bianchi, L.; de Martino, M.; Galli, L.; Chiappini, E. Infectious diseases prevalence, vaccination coverage, and diagnostic challenges in a population of internationally adopted children referred to a Tertiary Care Children’s Hospital from 2009 to 2015. Medicine 2017, 96, e6300. [Google Scholar] [CrossRef]
- Bortone, B.; Totaro, C.; Putignano, P.; Sollai, S.; Galli, L.; de Martino, M.; Chiappini, E. Auxo-endocrinological features in a cohort of internationally adopted children in Italy. World J. Pediatr. 2019, 15, 297–305. [Google Scholar] [CrossRef]
- DeLacey, E.; Tann, C.; Groce, N.; Kett, M.; Quiring, M.; Bergman, E.; Garcia, C.; Kerac, M. The nutritional status of children living within institutionalized care: A systematic review. PeerJ 2020, 8, e8484. [Google Scholar] [CrossRef]
- Groce, N.; Challenger, E.; Berman-Bieler, R.; Farkas, A.; Yilmaz, N.; Schultink, W.; Clark, D.; Kaplan, C.; Kerac, M. Malnutrition and disability: Unexplored opportunities for collaboration. Paediatr. Int. Child Health 2014, 34, 308–314. [Google Scholar] [CrossRef]
- Adams, M.S.; Khan, N.Z.; Begum, S.A.; Wirz, S.L.; Hesketh, T.; Pring, T.R. Feeding difficulties in children with cerebral palsy: Low-cost caregiver training in Dhaka, Bangladesh. Child Care Health Dev. 2012, 38, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Kerac, M.; McGrath, M.; Connell, N.; Compala, C.; Moore, W.; Bailey, J.; Bandsma, R.; Berkey, J.; Briend, A.; Collins, S.; et al. Severe Malnutrition: Thinking deeply, communicating simply. BMJ Glob. Health 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Marks, J.S. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults: The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Lelijveld, N.; Seal, A.; Wells, J.C.; Kirkby, J.; Opondo, C.; Chimwezi, E.; Bunn, J.; Bandsma, R.; Heyderman, R.S.; Nyirenda, M.J.; et al. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): A cohort study. Lancet Glob. Health 2016, 4, e654–e662. [Google Scholar] [CrossRef]
- Mandy, M.; Nyirenda, M. Developmental Origins of Health and Disease: The relevance to developing nations. Int. Health 2018, 10, 66–70. [Google Scholar] [CrossRef]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S.; Maternal Child Undernutrition Study Group. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J.P. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia 1992, 35, 595–601. [Google Scholar] [CrossRef]
- Adair, L.S.; Prentice, A.M. A Critical Evaluation of the Fetal Origins Hypothesis and Its Implications for Developing Countries. J. Nutr. 2004, 134, 191–193. [Google Scholar] [CrossRef]
- Ijzendoorn, M.H.V.; Bakermans-Kranenburg, M.J.; Juffer, F. Plasticity of growth in height, weight, and head circumference: Meta-analytic evidence of massive catch-up after international adoption. J. Dev. Behav. Pediatr. 2007, 28, 334–343. [Google Scholar] [CrossRef]
- Mason, P.; Narad, C. Long-term growth and puberty concerns in international adoptees. Pediatr. Clin. N. Am. 2005, 52, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health Research. International Prospective Register of Systematic Reviews: National Institute for Health Research University of York Centre for Reviews and Dissemination. 2020. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 25 July 2020).
- World Health Organization. The WHO Child Growth Standards. 2020. Available online: https://www.who.int/childgrowth/standards/en/ (accessed on 25 July 2020).
- Centers for Disease Control and Prevention. Clinical Growth Charts 2017. Available online: https://www.cdc.gov/growthcharts/clinical_charts.htm (accessed on 25 July 2020).
- Duncan, W. Hague Convention on the Protection of Children and Co-operation in Respect of Intercountry Adoption. Adopt. Fostering 1993, 17, 9–13. [Google Scholar] [CrossRef]
- National Institute for Health and Excellence. Appendix F Quality Appraisal Checklist–Quantitative Intervention Studies 2012. Available online: https://www.nice.org.uk/process/pmg4/chapter/appendix-f-quality-appraisal-checklist-quantitative-intervention-studies (accessed on 25 July 2020).
- Fuglestad, A.J.; Kroupina, M.G.; Johnson, D.E.; Georgieff, M.K. Micronutrient status and neurodevelopment in internationally adopted children. Acta Paediatr. Int. J. Paediatr. 2016, 105, e67–e76. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.L.; Eckerle, J.K.; Howard, C.R.; Andrews, B.; Polgreen, L.E. Prevalence of vitamin D deficiency in international adoptees within the first 6 months after adoption. Clin. Pediatr. 2013, 52, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Martinez Ortiz, A.; Dominguez Pinilla, N.; Wudineh, M.; Gonzalez-Granado, L.I. International adoption from Ethiopia in a 5-year period. Anales Pediatr. 2015, 82, 302–307. [Google Scholar] [CrossRef]
- Palacios, J.; Roman, M.; Camacho, C. Growth and development in internationally adopted children: Extent and timing of recovery after early adversity. Child Care Health Dev. 2011, 37, 282–288. [Google Scholar] [CrossRef]
- Pomerleau, A.; Malcuit, G.; Chicoine, J.F.; Séguin, R.; Belhumeur, C.; Germain, P.; Amyot, I.; Jéliu, G. Health status, cognitive and motor development of young children adopted from China, East Asia, and Russia across the first 6 months after adoption. Int. J. Behav. Dev. 2005, 29, 445–457. [Google Scholar] [CrossRef]
- Le Mare, L.; Audet, K. A longitudinal study of the physical growth and health of post institutionalized Romanian adoptees. Paediatr. Child Health 2006, 11, 85–91. [Google Scholar]
- Buonsenso, D.; Graffeo, R.; Scarlato, L.; Acampora, A.; Grotti, G.; Pata, D.; Colonna, A.T.; Salerno, G.; Colussi, L.; Masucci, L.; et al. Intestinal Parasitic Infections in Internationally Adopted Children: A 10-Year Retrospective Study. Pediatr. Infect. Dis. J. 2019, 38, 983–989. [Google Scholar] [CrossRef]
- Salerno, G.; Ceccarelli, M.; De Waure, C.; D’Andrea, M.; Buonsenso, D.; Faccia, V.; Pata, D.; Valentini, P. Epidemiology and risk factors of hypovitaminosis D in a cohort of internationally adopted children: A retrospective study. Italian J. Pediatr. 2018, 44, 86. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Chan, W.; Comfort, K.; Tirella, L. Health of children adopted from Guatemala: Comparison of orphanage and foster care. Pediatrics 2005, 115, e710–e717. [Google Scholar] [CrossRef] [PubMed]
- Ulijaszek, S.; Schwekendiek, D. Intercontinental differences in overweight of adopted Koreans in the United States and Europe. Econ. Hum. Biol. 2013, 11, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F.; Viviano, E. Health problems of internationally adopted children. Italian J. Pediatr. 2007, 33, 92–99. [Google Scholar]
- Chiappini, E.; Vierucci, F.; Ghetti, F.; De Martino, M.; Galli, L. Vitamin D status and predictors of hypovitaminosis D in internationally adopted children. PLoS ONE 2016, 11, e0158469. [Google Scholar] [CrossRef] [PubMed]
- Johansson-Kark, M.; Rasmussen, F.; Hjern, A. Overweight among international adoptees in Sweden: A population-based study. Acta Paediatr. Int. J. Paediatr. 2002, 91, 827–832. [Google Scholar] [CrossRef]
- Miller, L.C.; Hendrie, N.W. Health of children adopted from China. Pediatrics 2000, 105, E76. [Google Scholar] [CrossRef]
- Van Kesteren, L.; Wojciechowski, M. International adoption from Ethiopia: An overview of the health status at arrival in Belgium. Acta Clin. Belg. 2017, 72, 300–305. [Google Scholar] [CrossRef]
- Johnson, A.E.; Bruce, J.; Tarullo, A.R.; Gunnar, M.R. Growth delay as an index of allostatic load in young children: Predictions to disinhibited social approach and diurnal cortisol activity. Dev. Psychopathol. 2011, 23, 859–871. [Google Scholar] [CrossRef]
- Miller, B.S.; Spratt, E.G.; Himes, J.H.; Condon, D.; Summer, A.; Papa, C.E.; Brady, K.T. Growth failure associated with early neglect: Pilot comparison of neglected US children and international adoptees. J. Pediatr. Endocrinol. Metab. 2015, 28, 111–115. [Google Scholar] [CrossRef]
- Miller, L.C.; Tseng, B.; Tirella, L.G.; Chan, W.; Feig, E. Health of children adopted from Ethiopia. Matern. Child Health J. 2008, 12, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Tirella, L.G.; Miller, L.C. Self-Regulation in Newly Arrived International Adoptees. Phys. Occup. Ther. Pediatr. 2011, 31, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.D.; Bachrach, S.; Carpenter, T.O.; Mackenzie, W.G. Vitamin D-deficiency rickets in adopted children from the former Soviet Union: An uncommon problem with unusual clinical and biochemical features. Pediatrics 2000, 106, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Albers, L.H.; Johnson, D.E.; Hostetter, M.K.; Iverson, S.; Miller, L.C. Health of children adopted from the former Soviet Union and Eastern Europe: Comparison with preadoptive medical records. J. Am. Med. Assoc. 1997, 278, 922–924. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Soliman, A.T.; Al Dabbagh, M.M.; Habboub, A.H.; Adel, A.; Humaidy, N.A.; Abushahin, A. Linear Growth in Children with Iron Deficiency Anemia Before and After Treatment. J. Trop. Pediatr. 2009, 55, 324–327. [Google Scholar] [CrossRef]
- WHO. Worldwide Prevalence of Anaemia 1993–2005; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- WHO. Global Database on Anaemia; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Wang, M. Iron Deficiency and Other Types of Anemia in Infants and Children. Am. Fam. Physician 2016, 93, 270–278. [Google Scholar]
- Maluccio, J.A.; Hoddinott, J.; Behrman, J.R.; Martorell, R.; Quisumbing, A.R.; Stein, A.D. The Impact of Improving Nutrition during Early Childhood on Education among Guatemalan Adults. Econ. J. 2009, 119, 734–763. [Google Scholar] [CrossRef]
- The First 1000 Days of Life: The Brain’s Window of Opportunity Unicef-irc.org: Unicef, 2020. Available online: https://www.unicef-irc.org/article/958-the-first-1000-days-of-life-the-brains-window-of-opportunity.html (accessed on 25 July 2020).
- Leroy, J.L.; Ruel, M.; Habicht, J.P.; Frongillo, E.A. Linear growth deficit continues to accumulate beyond the first 1000 days in low- and middle-income countries: Global evidence from 51 national surveys. J. Nutr. 2014, 144, 1460–1466. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.; Beckett, C.; Kreppner, J.; Castle, J.; Colvert, E.; Stevens, S.; Hawkins, A.; Rutter, M. Is sub-nutrition necessary for a poor outcome following early institutional deprivation? Dev. Med. Child Neurol. 2008, 50, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Kroupina, M.G.; Eckerle, J.K.; Fuglestad, A.J.; Toemen, L.; Moberg, S.; Himes, J.H.; Miller, B.S.; Petryk, A.; Johnson, D.E. Associations between physical growth and general cognitive functioning in international adoptees from Eastern Europe at 30 months post-arrival. J. Neurodev. Disord. 2015, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Espo, M.; Kulmala, T.; Maleta, K.; Cullinan, T.; Salin, M.L.; Ashorn, P. Determinants of linear growth and predictors of severe stunting during infancy in rural Malawi. Acta Paediatr. 2002, 91, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.; Earls, F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann. N. Y. Acad. Sci. 1997, 807, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Worthman, C.M.; Stallings, J.F. Hormone measures in finger-prick blood spot samples: New field methods for reproductive endocrinology. Am. J. Phys. Anthropol. 1997, 104, 1–21. [Google Scholar] [CrossRef]
- Gunnar, M.R.; Morison, S.J.; Chisholm, K.; Schuder, M. Salivary cortisol levels in children adopted from romanian orphanages. Dev. Psychopathol. 2001, 13, 611–628. [Google Scholar] [CrossRef]
- Thurstans, S.; Opondo, C.; Seal, A.; Wells, J.; Khara, T.; Dolan, C.; Briend, A.; Myatt, M.; Garenne, M.; Sear, R.; et al. Boys are more likely to be undernourished than girls: A systematic review and meta-analysis of sex differences in undernutrition. BMJ Glob. Health 2020, 5, e004030. [Google Scholar] [CrossRef]
- Teilmann, G.; Pedersen, C.B.; Skakkebaek, N.E.; Jensen, T.K. Increased risk of precocious puberty in internationally adopted children in Denmark. Pediatrics 2006, 118, e391–e399. [Google Scholar] [CrossRef]
- Kim, E.Y. Long-term effects of gonadotropin-releasing hormone analogs in girls with central precocious puberty. Korean J. Pediatr. 2015, 58, 1–7. [Google Scholar] [CrossRef]
- Frontini, M.; Srinivasan, S.; Berenson, G. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: The Bogalusa Heart Study. Int. J. Obes. 2003, 27, 1398–1404. [Google Scholar] [CrossRef]
- Van IJzendoorn, M.H.; Palacios, J.; Sonuga-Barke, E.J.; Gunnar, M.R.; Vorria, P.; McCall, R.B.; Le Mare, L.; Bakermans-Kranenburg, M.J.; Dobrova-Krol, N.A.; Juffer, F.I. Children in Institutional Care: Delayed Development and Resilience. Monogr. Soc. Res. Child Dev. 2011, 76, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Vanderklippe, N. The Tragic tale of China’s Orphanages: 98% of Abandoned Children Have Disabilities the Global and Mail 2014. Available online: https://www.theglobeandmail.com/news/world/the-tragic-tale-of-chinas-orphanages-98-of-abandoned-children-have-disabilities/article17625887/ (accessed on 25 July 2020).
- Casey, P.H. Growth of Low Birth Weight Preterm Children. Semin. Perinatol. 2008, 32, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Christian, P. Fetal growth restriction and preterm as determinants of child growth in the first two years and potential interventions. Nestle Nutr. Inst. Workshop Ser. 2014, 78, 81–91. [Google Scholar] [PubMed]
- Onyango, A.W.; Esrey, S.A.; Kramer, M.S. Continued breastfeeding and child growth in the second year of life: A prospective cohort study in western Kenya. Lancet 1999, 354, 2041–2045. [Google Scholar] [CrossRef]
| Author, Year | Study Design | Country | Study Population | NICE Quality Assessment Score: Internal Validity/ External Validity Score | Timing of Nutritional Assessments | Sex |
|---|---|---|---|---|---|---|
| Fuglestad et al., 2016 [33] | Prospective cohort | Multi country 🡲USA | N: 58 children. Participants included children aged 8–18 months. | +/+ | Arrival and 6 months post-adoption | Females: 32 (55%) |
| Gustafson, Eckerie et al., 2013 [34] | Prospective cohort | Multi country 🡲USA | N: 160 patients. Aged 4 months to 17.8 years. | +/− | Within 6 months of adoption | Females: 83 (52%) |
| Park, Bothe et al., 2011 [9] | Prospective cohort | Multi country 🡲USA | N: 58 children. Mean age on arrival: 17.6 months. Children evaluated within 19 days. | −/− | On arrival | Females: 34 (58%) |
| Bortone, Totaro et al., 2019 [14] | Prospective cohort | Multi country 🡲Italy | N: 422 children. Median age at arrival: 6.5 years. Adopted from Europe (29.9%), Asia (26.8%), Africa (23.9%) and Latin America (19.4%). | +/− | Median 75 days after arrival | Females: 171 (40.5%) |
| Fuglestad et al., 2008 [11] | Prospective cohort | Multi country 🡲USA | N: 37 children. Children adopted from orphanages or hospitals. Low birth weight: 32%. | −/− | On arrival and 6 months post-adoption | Females: 22 (59%) |
| Martinez Ortiz, Dominguez Pinilla et al., 2015 [35] | Retrospective cohort | Ethiopia 🡲Spain | N: 251 children. Mean age of arrival: 7 months. 124 (49.4%) aged ≤ 6 months. | −/+ | Pre-adoption | |
| Palacios, Roman et al., 2011 [36] | Retrospective cohort | Multi country 🡲Spain | N: 289 children. Mean age on arrival: 34.9 months. Children from institutionalized care. Parents reported the results (approximately 38 months after arrival) of their child’s on arrival medical tests. | +/+ | On arrival and 3 years post-adoption | |
| Pomerleau et al., 2005 [37] | Prospective cohort | China, Vietnam, Taiwan, Thailand, South Korea, Cambodia, Russia and Belarus 🡲Canada | N: 123 evaluated. All children were adopted before 18 months of age. Adopted from orphanage, family-setting and some children had experience in both living situations. Assessed within one month of arrival (mean: 19.1 days). | −/− | On arrival, 3 months post-adoption and 6 months post-adoption | Females: 87 (70%) |
| Le Mare and Audet, 2006 [38] | Prospective cohort | Romania 🡲Canada | N: 36 evaluated. Lived in an orphanage for a minimum of nine months (9 to 53 months, mean = 24 months). Mean age at arrival: 23.9 months. Mean time in institution: 22.7 months. | +/− | 11 months post-adoption, 4.5 years of age and 10.5 years of age | Females: 19 (53%) |
| Buonsenso, Graffeo et al., 2019 [39] | Retrospective cohort | Multi country 🡲Italy | N: 584 evaluated, (82.19%) lived in institutions. Mean age at arrival: 5 years and 9 months. | +/+ | On arrival | |
| Salerno, Ceccarelli et al., 2018 [40] | Retrospective cohort | Multi country 🡲Italy | N: 873 children. Children were adopted from Europe and Russian federation (256, 29.8%), Latin America (231, 26.9%), Asia and Indian subcontinent (223, 26.0%), and Africa (149, 17.3%). Mean duration of institutionalization: 3 years. | +/+ | On arrival | Females: 376 (43.8%) |
| Miller, Chan et al., 2005 [41] | Retrospective cohort | Guatemala 🡲USA | N: 103 children. Mean age on arrival: 16 months. Before adoption, 25 children resided in orphanages, 56 in foster care, and 22 in mixed-care settings (time living with birth family, foster care and orphanage). | +/+ | On arrival | Females: 48 (47%) |
| Ulijaszek and Schwekendiek 2013 [42] | Retrospective cohort | Korea 🡲United States and Europe | Mean age when evaluated: 28.65 to 31.87 years old. Children adopted to America (52%) and Western Europe (44%). Adults self-reported their weight and height. | +/− | Post-adoption (mean age: males 30.46 years, females 28.65) | Females: 172 (66%) |
| Cataldo and Viviano 2007 [43] | Cross sectional | Multi country 🡲Italy | N: 36 children. Mean age at arrival: 78.5 months. Referred within 2–6 weeks of arrival. | −/− | Pre-adoption medical records and on arrival | Females: 62 (46%) |
| Chiappini, Vierucci et al., 2016 [44] | Cross sectional | Multi country 🡲Italy | Median age at arrival: 5.47 years 962 adopted from Africa (18.09%), South America (21.41%), Asia (16.32%), Europe (44.18%). | +/− | Median 72 days after arrival | Females: 381 (39.60%) |
| Johansson-Kark, Rasmussen et al., 2002 [45] | Cross sectional | Multi country 🡲Sweden | 275,026 were included in study. 2400 adults who were international adoptees were evaluated. Mean age at adoption was 1.7 years. 64% adopted before age 2. | +/+ | Post-adoption (17 years old) | Males: 275,026 (100%) |
| Miller and Hendrie 2000 [46] | Cross sectional | China 🡲USA | N: 452 children. The clinic group age at arrival: 2 months to 12 years and 4 months. Age at clinic visit: 3 months to 151 months. Children evaluated within 1.3 months. | −/+ | 1 week to 17 months of arrival | Females: 443 (98%) |
| Van Kesteren and Wojciechowski 2017 [47] | Retrospective study | Ethiopia 🡲Belgium | N: 315 children. Mean age on arrival: 3 years old. | +/+ | On arrival | Females: 151 (48%) |
| Johnson, Bruce et al., 2011 [48] | Cross sectional | Multi country 🡲USA | N: 120 children. Mean age on arrival: 6.85 years. Three groups: Post-institutionalized children, children from foster care and non-adopted children raised in the US. | +/− | On arrival | All three groups had: Male: 10 Female: 30 |
| Miller, Spratt et al., 2015 [49] | Cross sectional | Russia 🡲USA | N: 60 children. Age ranged from 3–10 years old. Three groups of children: previously institutionalized international adoptees, children with a history of neglect born in the USA, and controls. | −/+ | Post-adoption (mean age 6.1 years old) | |
| Miller, Tseng et al., 2008 [50] | Cross sectional | Ethiopia/Eritrea 🡲USA | N: 50 children. 62% were less than 4 years old. Mean age on arrival: 3 months to 15 years. Mean age at clinic visit: 51.12 months. | −/+ | On arrival | |
| Tirella and Miller 2011 [51] | Cross sectional | Multi country 🡲USA | N: 387 children. Mean age on arrival: 14.0 months. 86% of the children were evaluated within 2 months and 91% evaluated within 5 months of arrival. | −/− | On arrival | Females: 254 (66%) |
| Reeves, Bachrach et al., 2000 [52] | Case study | Soviet Union 🡲USA | Case 1: Age 2 years and 5 months, Case 2: Age 3 years and 3 months, Case 3: Age 2 years and 10 months. | −/+ | On arrival | Females: 2 (66%) |
| Albers, Johnson et al., 1997 [53] | Case study | Russia🡲USA | N: 56 adoptees from East Europe. Median age at arrival: 26 months. | +/+ | Pre-adoption medical records and on arrival | Females: 30 (54%) |
| Author, Year | Growth Reference | Weight for Age (WAZ) | Weight for Length/Height (WHZ) | Length/Height for Age (HAZ) | Body Mass Index (BMI) for Age | Head Circumference for Age (HCAZ) | Other Observations |
|---|---|---|---|---|---|---|---|
| Albers, Johnson et al., 1997 [53] | WHO growth standards | Prevalence of WAZ pre-adoption <−1: 44% Mean WAZ baseline: −1.05 (SD ± 1.06) (range −3.15 to 1.26) | Prevalence of HAZ < −1 pre-adoption: 68% Mean HAZ on arrival: −1.41 (SD ± 1.37) (range −4.52 to 1.79) | Prevalence of HCAZ < −1 pre-adoption: 43% Mean HCAZ on arrival: −1.25 (SD + 1.00) (range, −3.7 to 0.62) | Growth delay in height (68%). Growth delay in head circumference (43%). Delay in linear growth directly correlated with the amount of time living in an orphanage (p < 0.001). | ||
| Bortone, Totaro et al., 2019 [14] | WHO growth standards | Underweight prevalence baseline: 13.2%. Mean WAZ baseline: −0.4, −0.6 (above age 5) | Wasting prevalence baseline: 4.3%. Mean WHZ: −0.5, −0.9 (above age 5) | Stunting prevalence baseline: 12.9% | Stunting common in children < 5 years and in those with a disability. Disability in 72/422 (17.1%). | ||
| Cataldo and Viviano 2007 [43] | WHO growth standards | Mean WAZ baseline: −0.97 (−3.97 to 2.27) | Wasting prevalence baseline: 18.4% | Mean HAZ baseline: −1.30 (–5.98 to 2.17) Stunting prevalence baseline: 19.1% | Mean HCAZ baseline: −0.58 (−2.1 to 3.3) HCAZ <−2: 8.8% | Total Iron deficiency anemia: 74. Total rickets: 21. Total delayed bone age: 17. | |
| Fuglestad, Kroupina et al., 2016 [33] | WHO growth standards | Post-Soviet States: Mean WAZ Baseline: −0.31 (SD 1.05) 6 months follow up: 0.23 (SD 0.87) | Mean WHZ Baseline: 0.39 (SD 1.01) 6 months follow up: 0.66 (SD 1.04) | Mean WHZ Baseline: 0.39 (SD 1.01) 6 months follow up: 0.66 (SD 1.04) | Mean HCAZ Baseline: 0.05 (SD 1.31) 6 months follow up: 0.31 (SD 1.03) | Nutritional deficiencies were not eliminated at follow up. Significant growth improvements from baseline to follow up in HAZ, (p < 0.001), WAZ, (p < 0.001), WHZ, (p < 0.001), and OFCZ (p < 0.001). | |
| Ethiopia: Mean WAZ Baseline: −0.89 (SD 0.93). 6 months follow up: 0.26 (SD 0.91) | Mean WHZ Baseline: 0.17 (SD 0.84) 6 months follow up: 1.04 (SD 1.10) | Mean HAZ Baseline: −1.89 (SD 1.34) 6 months follow up: −1.09 (SD 1.23) | Mean HCAZ Baseline: 0.20 (SD 1.15) 6 months follow up: 1.23 (SD 1.14) | ||||
| Mean WAZ Baseline: −0.56 (SD 0.80) 6 months follow up: 0.02 (SD 0.96) | Mean WHZ Baseline: −0.14 (SD 0.84) 6 months follow up: 0.39 (SD 1.01) | Mean HAZ Baseline: −0.93 (SD 1.30) 6 months follow up: 0.58 (SD 0.96) | Mean HCAZ Baseline: −0.37 (SD 0.92) 6 months follow up: −0.06 (SD 1.17) | ||||
| Fuglestad, Lehmann et al., 2008 [11] | Centers for Disease Control (CDC), 2000 | Mean WAZ Baseline: −1.73 6 months follow up: 0.53 | Mean WHZ Baseline: −0.63 6 months follow up: −0.02 (WHZ) | Mean HAZ Baseline: −1.24 6 months follow up: −0.49 | Mean HCAZ Baseline: −0.67 6 months follow up: 0.11 | Mean serum ferritin concentration lower than the US population at follow up. Children with giardia lamblia at baseline had worse iron status at baseline and follow up. | |
| Johansson-Kark, Rasmussen et al., 2002 [45] | WHO growth standards | BMI range: 20.68 to 23.93 Overweight prevalence: 8.8–28.6% Overweight prevalence: 14.1% for non-adopted participants | 1.36 (1.03–1.80) increase in the odds of becoming overweight in adulthood for those who arrived in their adoptive country at a young age (0–1 year old) compared to those adopted after the age of 2. | ||||
| Martinez Ortiz, Dominguez Pinilla et al., 2015 [35] | WHO growth standards | 49% of children <3rd percentile for weight at baseline | 40% of children <3rd percentile for height at baseline | 151 (65%) had malnutrition (details not specified). Low weight for height was related to age at adoption. | |||
| Miller and Hendrie 2000 [46] | WHO growth standards | Weight: −3.77 to −2.4 (mean, SD: −1.17, 1.00) | Wasting prevalence baseline: 18% | Height: −8.64 to −2.9 (mean, SD: −1.51, 1.4), 39% stunted | Microcephaly prevalence: 28% | The amount of time living in a orphanage in months was proportional to the linear growth lag (r = 0.90; p = 0.0001) for 192 Chinese adoptees. For every 2.86 months of stay in an orphanage, children lost 1 month of height age. | |
| Palacios, Roman et al., 2011 [36] | WHO growth standards 1995 | Mean WAZ Baseline: −1.48 Follow up: 0.09 Difference: (p < 0.001) | Mean HAZ Baseline: −1.46 Follow up: −0.1 Difference: (p < 0.001) | Mean HCAZ Baseline: −0.71 Follow up: −0.46 Difference: (p < 0.001) | No significant relationship between length of institutionalization or age at arrival and growth indicators. A longer stay in orphanages was related with greater height delays (p < 0.05). Less than 7 months in orphanage, there was a negative relationship between orphanage duration and head circumference (p < 0.05). | ||
| Park, Bothe et al., 2011 [9] | WHO growth standards 2006 and CDC 2000 (for those older than 5) | Underweight prevalence baseline: 10% Mean WAZ baseline: −1.4 | Wasting prevalence baseline: 28% Mean WHZ baseline: −0.5 | Stunting prevalence baseline: 17% Mean HAZ baseline: −1.1 | Microcephaly prevalence baseline: 16% Mean HCAZ baseline: −0.8 | Growth Z-scores less than zero at baseline: HCAZ (77%), HAZ (79%), WHZ (64%) and WAZ (90%). No significant relationship between age of participants at baseline and all growth Z-scores. | |
| Salerno, Ceccarelli et al., 2018 [40] | WHO growth standards | Mean BMI baseline: 16 | No significant difference between 25(OH)D mean values for the different BMI groups (p = 0.47). | ||||
| Ulijaszek and Schwekendiek 2013 [42] | WHO growth standards | Adult BMI: USA: Males: mean BMI 25.85. Females: mean BMI 22.18. Europeans: Males: mean BMI 22.77. Females: mean BMI 21.67. USA over 25 BMI = 25.6% Europe over 25 BMI = 14.3% | Males had greater BMI than females (p < 0.001). Adoptees in Europe had lower BMI than those in the US (p < 0.001). | ||||
| Van Kesteren and Wojciechowski 2017 [47] | WHO growth standards | Wasting prevalence baseline: 8.6% | Stunting prevalence baseline: 28.9% Severe stunting prevalence baseline: 11% | Microcephaly was uncommon. Moderate microcephaly in 8 (3.3%) children. Severe microcephaly in 2 (0.8%) children. | |||
| Johnson, Bruce et al., 2011 [48] | CDC, 2000 | Mean WAZ baseline: −2.04 (post-institutionalized). Mean WAZ baseline: −0.23 (foster care). | Mean WHZ baseline: −0.94 (post-institutionalized) Mean WHZ baseline: −0.35 (foster care) | Mean HAZ baseline: −1.54 (post-institutionalized) Mean HAZ baseline: −0.03 (foster care) | For CAI linear growth delay was related with greater DSA and a more dysregulated diurnal cortisol rhythm. | ||
| Miller, Spratt et al., 2015 [49] | CDC, 2000 | Mean WAZ post-adoption: −0.59 | Mean WHZ post-adoption: −0.31 | Mean HAZ post-adoption: −0.5 | Three groups recruited: previously institutionalized CAI, US born children with history of neglect and control. Mean height growth was different (p < 0.05). Head circumference was significantly smaller (p < 0.05) in CAI. | ||
| Miller, Tseng et al., 2008 [50] | CDC, 2000 | Mean WAZ baseline: −0.59 Underweight prevalence baseline: 8% | Mean HAZ baseline: −0.64 Stunting prevalence baseline: 12% | Microcephaly prevalence baseline: 6% Mean HCAZ baseline: −0.09 | WHZ increased with age at adoption. Growth measurement Z-scores not related with age at arrival. Children from Ethiopia/Eritrea had significantly better anthropometric status at arrival than adoptees from China, Guatemala, or Russia. | ||
| Miller, Chan et al., 2005 [41] | CDC, 2000 | Mean WAZ baseline: −1.0 Underweight prevalence baseline: 20% | Mean HAZ baseline: −1.04 Stunting prevalence baseline: 16% | Microcephaly prevalence baseline: 17% Mean HCAZ baseline: −1.08 | Children who resided in orphanages had significantly lower Z-scores for all height, weight and head circumference. Children younger than 2 years at arrival, Z-scores for growth measurements related inversely with age at arrival. | ||
| Tirella and Miller 2011 [51] | CDC, 2000 | Mean WAZ baseline: −1.17 Underweight prevalence baseline: 27% | Mean HAZ baseline −0.74 Stunting prevalence baseline: 13% | Microcephaly prevalence baseline: 14% Mean HCAZ baseline: 0.8 | Children from Guatemala had greater delays in height (p = 0.007) and head circumference (p = 0.01) than those from the other countries, although these results are not significant after the Bonferroni correction. | ||
| Pomerleau et al., 2005 [37] | North American norms, 1979 (ANOVA: time, group) | Mean weight percentile baseline: 61.55 (p < 0.001) | Mean weight/height percentile baseline: 14.43 (p < 0.001) | Mean height percentile baseline: 8.44 (p < 0.001) Mean height/age percentile baseline: 7.35 (p < 0.001) | Mean head circumference percentile: 12.26 (p < 0.001) | On arrival, children from East Asia had higher percentiles for weight and height than Chinese or Russian children. Age at arrival was significantly associated with weight/height, height/age, head circumference percentile and weight percentile on arrival growth. Age at arrival was not associated with any growth indicators 6 months post-adoption. | |
| Le Mare and Audet, 2006 [38] | CDC, 2006 | Mean weight percentile (11 months post-adoption): 7.85 Mean weight percentile (4.5 years of age): 43.6 Mean weight percentile (10.5 years of age): 59.9 | Mean height percentile (4.5 years of age): 36.98 Mean height percentile (10.5 years of age): 48.8 | By phase 2 (4.5 years of age), children demonstrated almost complete weight catch up with only 3 (8.6%) children below the third percentile. By phase 3 (10.5 years of age), only 1 (2.8%) child had a weight score below the fifth percentile. |
| Author, Year | Country | Micronutrient Status (On Arrival/Pre-Adoption) | Micronutrient Status (Post-Adoption) | Clinical Signs |
|---|---|---|---|---|
| Bortone, Totaro et al., 2019 [14] | Multi country 🡲Italy | Total vitamin D deficiency: 188/416 (45.2%). | Total anemia: 40/417 (9.6%). Anemia not a risk factor for stunting (p = 0.285). | |
| Buonsenso, Graffeo et al., 2019 [39] | Multi country 🡲Italy | Total vitamin D deficiency: Moderate: 224 (38.4%) to mild: 196 (33.6%). Intestinal parasitic infections associated with vitamin D deficiency (p < 0.05). | ||
| Cataldo and Viviano 2007 [43] | Multi country 🡲Italy | Total anemia: 74 (54.4%). Total rickets: 21 (15.4%). | ||
| Chiappini, Vierucci et al., 2016 [44] | Multi country 🡲Italy | Median 25(OH)D level: 22.0 ng/mL. 73.8% of had hypovitaminosis D. Children >6 years old had an adjusted odds ratio of vitamin D deficiency and hypovitaminosis 1.87 (p < 0.01) and 2.50 (p < 0.01) times higher than children <6 years old. Age at arrival to Italy was significantly associated with both with 25-hydroxyvitamin D mean values (p < 0.01) and Vitamin D status (p < 0.01). Sex, country of origin and BMI-z-score < −2 were not associated with vitamin D status. | ||
| Fuglestad, Kroupina et al., 2016 [33] | Multi country 🡲USA | Low retinol-binding protein (33%). Zinc deficiency (29%). Vitamin D insufficiency/deficiency (21%). Iron deficiency (15%). | No significant change in micronutrient at baseline and follow up. | |
| Gustafson, Eckerie et al., 2013 [34] | Multi country 🡲USA | Total vitamin D deficiency: 7%. Total vitamin D insufficiency: 27%. | ||
| Park, Bothe et al., 2011 [9] | Multi country 🡲USA | Total anemia: 6 (11.5%). | ||
| Reeves, Bachrach et al., 2000 [52] | Soviet Union 🡲USA | Total vitamin D deficiency: 3 (100%). | Total rickets: 3 (100%). | |
| Salerno, Ceccarelli et al., 2018 [40] | Multi country 🡲Italy | A statistically significant difference was found for skin color (p = 0.011), season at first blood draw (p < 0.001), the age at the first blood draw (p < 0.001) and Vitamin D status. Time from the arrival to initial evaluation was not significantly related with 25(OH)D mean values (p = 0.388) and Vitamin D Status (p = 0.912). Female children had increased risk of severe vitamin D deficiency. | ||
| Fuglestad, Lehmann et al., 2008 [11] | Multi country 🡲USA | Total iron deficiency at baseline: 25%. Children with giardia lamblia had worse iron status at baseline and follow up. Growth rate was negatively related with change in serum ferritin concentrations between baseline and follow up (p < 0.05). | Total iron deficiency at follow up: 16%. | |
| Miller, Chan et al., 2005 [41] | Guatemala 🡲USA | Total anemia: 30%. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivey, R.; Kerac, M.; Quiring, M.; Dam, H.T.; Doig, S.; DeLacey, E. The Nutritional Status of Individuals Adopted Internationally as Children: A Systematic Review. Nutrients 2021, 13, 245. https://doi.org/10.3390/nu13010245
Ivey R, Kerac M, Quiring M, Dam HT, Doig S, DeLacey E. The Nutritional Status of Individuals Adopted Internationally as Children: A Systematic Review. Nutrients. 2021; 13(1):245. https://doi.org/10.3390/nu13010245
Chicago/Turabian StyleIvey, Richard, Marko Kerac, Michael Quiring, Hang T. Dam, Susie Doig, and Emily DeLacey. 2021. "The Nutritional Status of Individuals Adopted Internationally as Children: A Systematic Review" Nutrients 13, no. 1: 245. https://doi.org/10.3390/nu13010245
APA StyleIvey, R., Kerac, M., Quiring, M., Dam, H. T., Doig, S., & DeLacey, E. (2021). The Nutritional Status of Individuals Adopted Internationally as Children: A Systematic Review. Nutrients, 13(1), 245. https://doi.org/10.3390/nu13010245






