Abstract
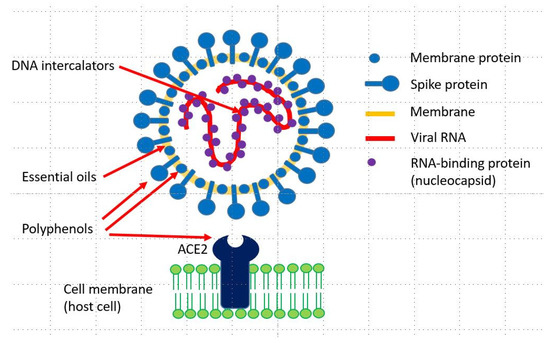
Many plants produce secondary metabolites (PSMs) with antiviral activities. Among the antiviral PSMs, lipophilic terpenoids in essential oils can disturb the lipid envelope of viruses. Phenols and polyphenols (flavonoids, rosmarinic acid and tannins) attack viral proteins present in the viral membrane or inside the virus particle. Both phenolics and essential oils are active against free viral particles but not—or to a lesser degree—after a virus has entered a host cell. Another group of PSMs is directed against DNA or RNA. These are DNA intercalators such as sanguinarine, berberine, emetine and other isoquinoline alkaloids, ß-carboline, and quinoline alkaloids such as quinine, cinchonine, dictamine and skimmianine. The DNA intercalators stabilize double-stranded nucleic acids and inhibit the replication, transcription, and translation of genetic material. These alkaloids can inhibit viral development and viral replication in cells, as shown for SARS-CoV-1 and other viruses. Since chloroquine (which is also a DNA intercalator and a chemical derivative of the alkaloid quinine) is apparently clinically helpful against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, it is assumed that intercalating alkaloids, or the medicinal plants producing them, may be interesting candidates for the development of new antiviral drugs for the treatment of coronavirus disease 2019 (COVID-19).
5. Conclusions
Antiviral secondary metabolites can target viral proteins (polyphenols), the lipid envelope (essential oils and other lipophilic PSMs) and viral nucleic acids (intercalating alkaloids). DNA-intercalating drugs inhibit DNA and RNA polymerases and protein biosynthesis, and consequently, viral replication. Whereas essential oils and polyphenols are active against the free virus, the intercalators can also inhibit the viral replication inside the host cell. The intercalating alkaloids sanguinarine, chelidonine, chelerythrine, berberine, coptisine, jatrorrhizine, palmatine, tetrandrine, cepharanthine, quinine, cinchonine, harmine and emetine (Table 3 and Table 4) represent interesting candidates for direct clinical studies or as lead compounds for the synthesis of synthetic antiviral drugs. Alternatively, extracts from medicinal plants [13] which produce these alkaloids, such as Bocconia frutescens, Chelidonium majus, Cinchona sp., Eschscholzia californica, Berberis sp., Coptis chinensis, Jateorhiza palmata, Hydrastis canadensis, Macleaya cordata, Phellodendron amurense, Psychotria ipecacuanha, Sanguinaria canadensis, Stephania tetrandra and others summarized in [34] may be more easily available than the isolated alkaloids. They might be useful as adjunctive therapeutics in the treatment of viral infections such as SARS-CoV-2 but need to be investigated in more detail.
Acknowledgments
The author thanks his former co-workers B. Latz-Brüning, T. Schmeller, B. Wetterauer, and Tamer Mahmoud who had analyzed PSMs with DNA-intercalating activities.
Conflicts of Interest
The author is the Editor-in-Chief of Diversity.
References
- Wink, M. Evolution of the Angiosperms and co-Evolution of Secondary Metabolites, Especially of Alkaloids. In Co-Evolution of Secondary Metabolites; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Heidelberg, Germany, 2020; pp. 1–24. [Google Scholar] [CrossRef]
- Harborne, J.B. Introduction to Ecological Biochemistry, 4th ed.; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genetics 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Wink, M. Biochemistry of Plant Secondary Metabolism; Annual Plant Reviews; Wiley-Blackwell: Oxford, GB, USA, 2010; Volume 40. [Google Scholar]
- Reichling, J. Plant—Microbe Interactions and Secondary Metabolites with Antibacterial, Antifungal and Antiviral Properties. In Functions and Biotechnology of Plant Secondary Metabolites; Annual Plant Reviews 39; Wink, M., Ed.; Wiley-Blackwell: Oxford, GB, USA, 2010; pp. 214–347. [Google Scholar]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral natural products and herbal medicines. J. Trad. Complement. Med. 2014, 4, 124–135. [Google Scholar] [CrossRef]
- Mukhtar, M.; Arshad, M.; Ahmad, M.; Pomerantz, R.J.; Wigdahl, B.; Parveen, Z. Antiviral potentials of medicinal plants. Virus Res. 2008, 131, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Peng, T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antivir. Res. 2013, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Tahir, I.M.; Shah, S.M.A.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytother. Res. 2018, 32, 811–822. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Sana, U.; Khan, R.U.; Alagawany, M.; et al. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens—Current knowledge and future prospects. Curr. Drug. Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World, 2nd ed.; CABI: Wallingford, UK, 2017. [Google Scholar]
- Tok, T.T.; Tatar, G. Structures and functions of coronavirus proteins: Molecular modeling of viral nucleoprotein. Int. J. Virol. Infect. Dis. 2017, 2, 1–7. [Google Scholar]
- Yang, Y.; Sahidul Islam, M.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese Medicine in the treatment of patients infected with 2019-New Coronavirus (SARS-CoV-2): A review and perspective. Int. J. Biol. Sci. 2020, 16, 1708–1717. [Google Scholar] [CrossRef]
- Alberts, B. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2015. [Google Scholar]
- Herrmann, F.; Romero, M.R.; Blazquez, A.G.; Kahl, S.; Kaufmann, D.; Marin, J.J.G.; Efferth, T.; Wink, M. Cytotoxicity and antiviral screening of 82 plants from Chinese and European phytomedicine. Diversity 2015, 3, 547–580. [Google Scholar] [CrossRef]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Molecular modes of action of cytotoxic alkaloids- From DNA intercalation, spindle poisoning, topoisomerase inhibition to apoptosis and multiple drug resistance. Alkaloids 2007, 64, 1–48. [Google Scholar] [PubMed]
- Wink, M.; Schmeller, T.; Latz-Brüning, B. Modes of action of allelochemical alkaloids: Interaction with neuroreceptors, DNA and other molecular targets. J. Chem. Ecol. 1998, 24, 1881–1937. [Google Scholar] [CrossRef]
- Wink, M.; Schimmer, O. Molecular Modes of Action of Defensive Secondary Metabolites. In Functions and Biotechnology of Plant Secondary Metabolites; Annual Plant Reviews 39; Wink, M., Ed.; Wiley-Blackwell: Oxford, UK, 2010; pp. 21–161. [Google Scholar]
- Schmeller, T.; Latz-Brüning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar] [CrossRef]
- Bombardelli, E.; Fontana, G.; Morazzoni, P.; Riva, A.; Ronchi, M. Formulations with Sanguinarine, Chelerythrine or Chelidonine for the Treatment of Warts, Verrucas and Psoriatic Plaques. Canada Patent CA2718384A1, 17 September 2009. [Google Scholar]
- Croaker, A.; King, G.J.; Pyne, J.H.; Anoopkumar-Dukie, S.; Liu, L. Sanguinaria canadensis: Traditional medicine, phytochemical composition, biological activities and current uses. Int. J. Mol. Sci. 2016, 17, 1414. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, H.S.; Park, H.; Kim, Y.C.; Yun, J.G.; Park, S.; Shin, H.J.; Kim, K. In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. J. Clin. Virol. 2008, 41, 122–128. [Google Scholar] [CrossRef]
- Chin, L.W.; Cheng, Y.; Lin, S. Anti-herpes simplex virus effects of berberine from Coptidis rhizoma, a major component of a Chinese herbal medicine, Ching-Wei-San. Arch. Virol. 2010, 155, 1933–1941. [Google Scholar] [CrossRef]
- Varghese, F.S.; Kaukinen, P.; Gläsker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kümmerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antiviral Res. 2016, 126, 117–124. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.Q.; Kim, Y.J.; Wu, J.; Wang, Q.; Hao, Y. In vivo and in vitro antiviral effects of berberine on influenza virus. Chin. J. Integr. Med. 2011, 17, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.C.; Jassey, A.; Liu, C.H.; Lin, C.J.; Lin, C.C.; Wong, S.H.; Wang, J.Y.; Yen, M.H.; Lin, L.T. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine 2019, 53, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Min, J.S.; Jang, M.S.; Lee, J.Y.; Shin, Y.S.; Song, J.H. Natural bis-benzylisoquinoline alkaloids—Tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules 2019, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, S.; Scaccabarozzi, D.; Signorini, L.; Perego, F.; Ilboudo, D.P.; Ferrante, P.; Delbue, S. The use of antimalarial drugs against viral infection. Microorganisms 2020, 8, 85. [Google Scholar] [CrossRef]
- Bleasel, M.D.; Peterson, G.M. Emetine, ipecac, ipecac alkaloids and analogues as potential antiviral agents for coronaviruses. Pharmaceuticals 2020, 13, 51. [Google Scholar] [CrossRef]
- Perez, R.M. Antiviral activity of compounds isolated from plants. Pharm. Biol. 2003, 41, 107–157. [Google Scholar] [CrossRef]
- Wink, M.; Van Wyk, B.E. Mind-Altering and Poisonous Plants of the World; Briza Press: Pretoria, South Africa, 2008. [Google Scholar]
- Li, S.Y.; Chen, C.; Zhang, H.Q.; Guo, H.Y.; Wang, H. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef]
- Chen, C.N.; Lin, C.P.C.; Huang, K.K.; Chen, W.C.; Hsieh, H.P.; Liang, P.H.; Hsu, J.T.H. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3,3’-digallate (TF3). Evid.-Based Complement. Altern. Med. 2005. [Google Scholar] [CrossRef]
- Wen, C.C.; Shyur, L.F.; Jan, J.T.; Liang, P.H.; Arulselvan, P.; Wu, J.B.; Kuo, S.C.; Yang, N.S. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J. Trad. Complement. Med. 2011, 1, 41–50. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.Y. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL (pro) inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef]
- Cheng, P.W.; Ng, L.T.; Chiang, L.C.; Lin, C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin. Exp. Pharmacol. Physiol. 2006, 33, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Michaelis, M.; Hsu, H.K.; Tsai, C.C.; Yang, K.D.; Wu, Y.C.; Cinatl, J.; Doerr, H.W. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. J. Ethnopharmacol. 2008, 30, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Zou, G.; Fan, J.; Yuan, Z. Identification of palmatine as an inhibitor of West Nile virus. Arch. Virol. 2010, 155, 1325–1329. [Google Scholar] [CrossRef]
- Ho, Y.J.; Lu, J.W.; Huang, J.L.; Lai, Z.Z. Palmatine inhibits Zika virus infection by disrupting virus binding, entry, and stability. Biochem. Biophys. Res. Commun. 2019, 518, 732–738. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).