Freshwater Mussel Bed Habitat in an Alluvial Sand-Bed-Material-Dominated Large River: A Core Flow Sediment Refugium?
Abstract
1. Introduction
2. Materials and Methods
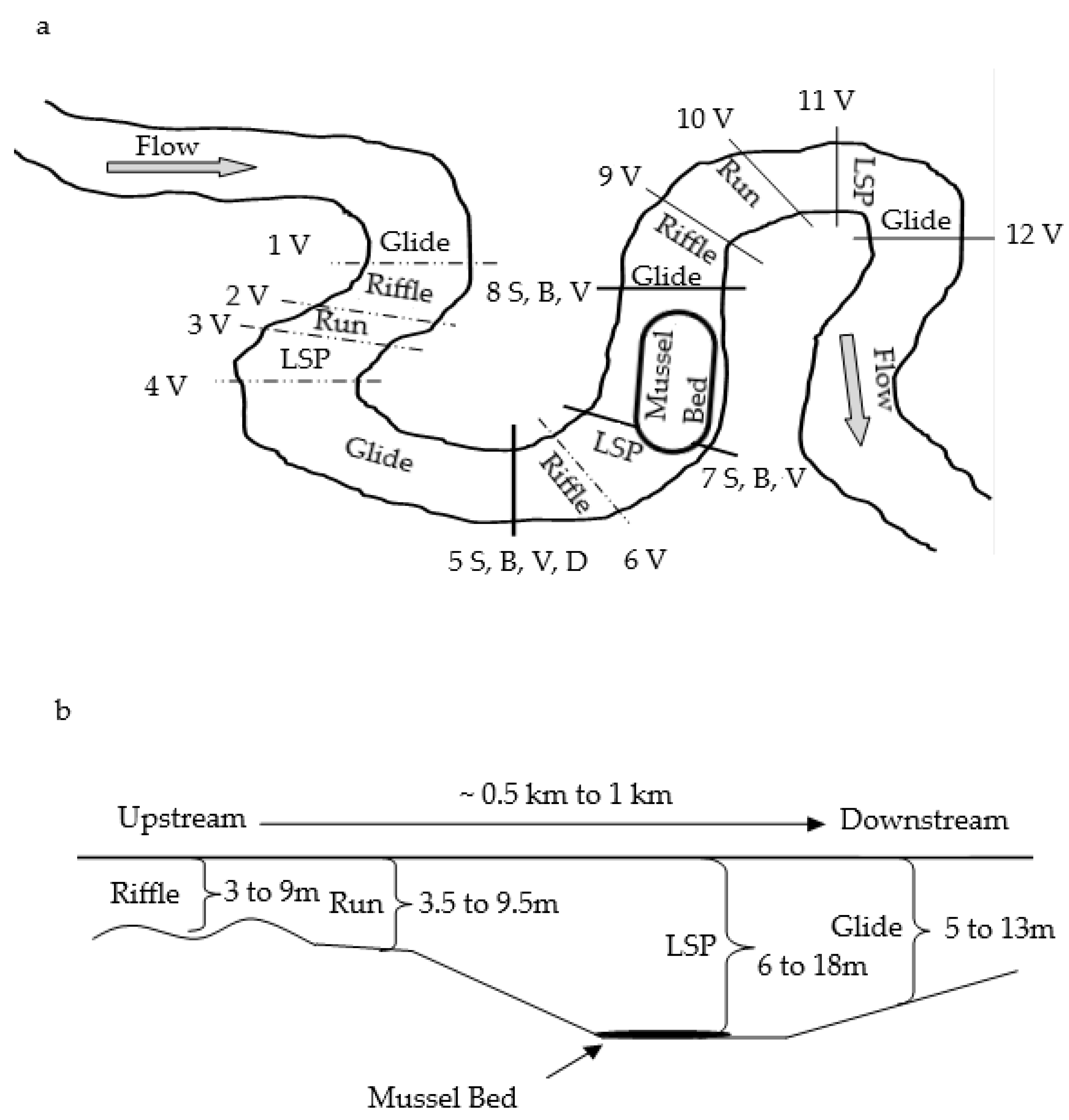
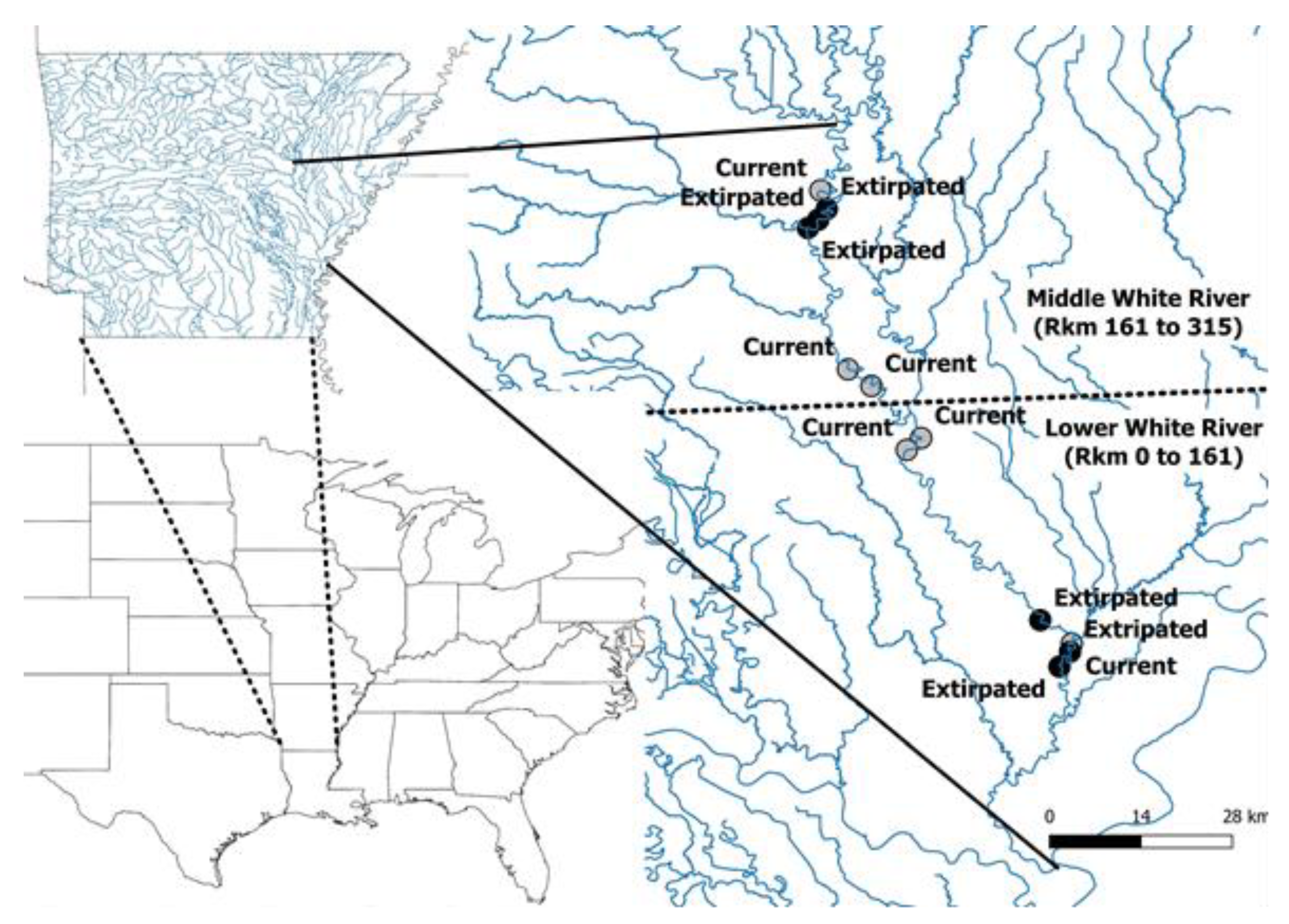
2.1. Study Area
2.2. Study Reach Identification
2.3. Sampling
2.3.1. Channel Slope
2.3.2. Discharge
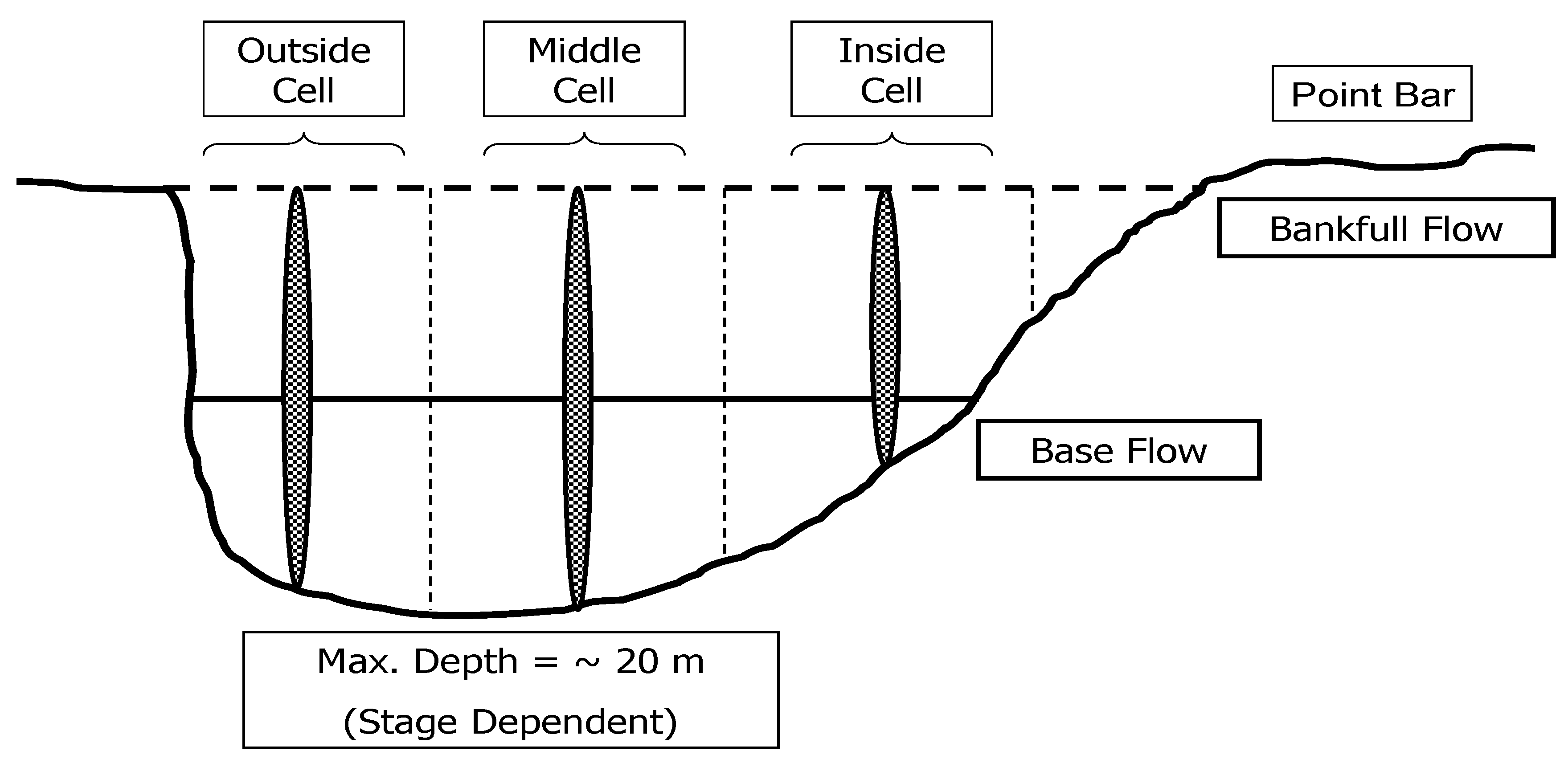
2.3.3. Velocity
2.3.4. The Froude Number
2.3.5. Shear Stress
2.3.6. Stream Power
2.3.7. Bedload
2.4. Statistical Analysis
3. Results
3.1. Channel Slope
3.2. Discharge
3.3. Mean Velocity
3.4. Bed Velocity
3.5. Froude
3.6. Shear Stress
3.7. Stream Power
3.8. Bedload Discharge
3.9. Bedload Mean Particle Size
4. Discussion
4.1. Channel Slope
4.2. Discharge
4.3. Velocity
4.4. Bed Velocity
4.5. The Froude Number
4.6. Shear Stress
4.7. Stream Power
4.8. Bedload
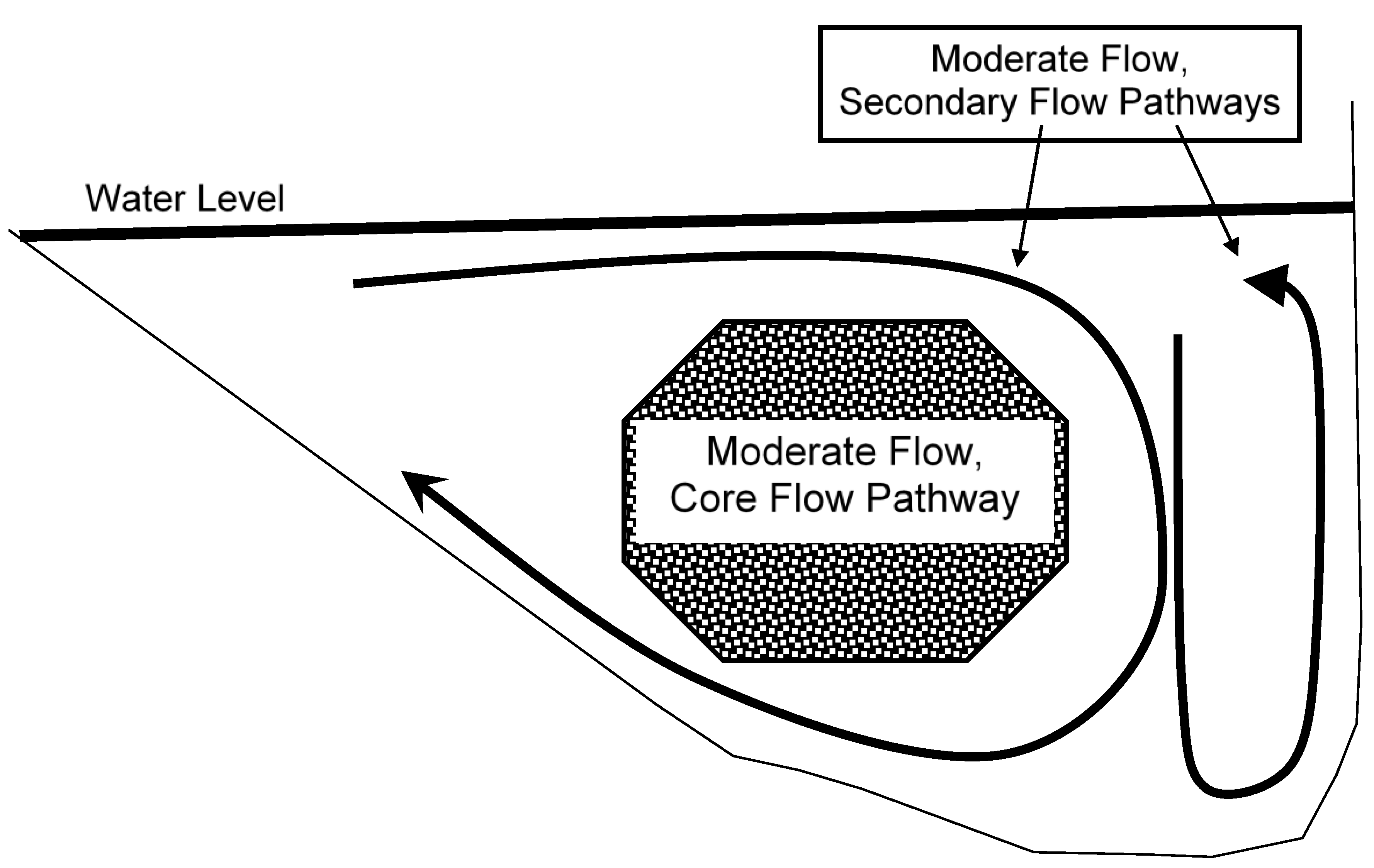
4.9. Integration—The Core Flow Concept
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Season | Region | Bed Type | Habitat | Lateral Position | ||
|---|---|---|---|---|---|---|
| Inside (±SD) | Middle (±SD) | Outside (±SD) | ||||
| Spring | Middle | Current | Riffle | 0.68 (0.15) | 0.68 (0.18) | 0.34 (0.12) |
| LSP | 0.66 (0.16) | 0.66 (0.11) | 0.38 (0.07) | |||
| Glide | 0.55 (0.19) | 0.82 (0.13) | 0.84 (0.27) | |||
| Middle | Extirpated | Riffle | 0.61 (0.10) | 0.78 (0.19) | 0.64 (0.22) | |
| LSP | 0.66 (0.18) | 0.66 (0.16) | 0.62 (0.22) | |||
| Glide | 0.45 (0.25) | 0.70 (0.20) | 0.79 (0.21) | |||
| Lower | Current | Riffle | 0.85 (0.11) | 0.84 (0.11) | 0.53 (0.31) | |
| LSP | 0.73 (0.12) | 0.83 (0.08) | 0.67 (0.11) | |||
| Glide | 0.53 (0.17) | 0.98 (0.03) | 1.01 (0.05) | |||
| Lower | Extirpated | Riffle | 0.74 (0.18) | 0.88 (0.15) | 0.69 (0.10) | |
| LSP | 0.61 (0.02) | 0.84 (0.11) | 0.72 (0.05) | |||
| Glide | 0.58 (0.06) | 0.81 (0.23) | 0.89 (0.08) | |||
| Autumn | Middle | Current | Riffle | 0.55 (0.16) | 0.66 (0.16) | 0.41 (0.13) |
| LSP | 0.55 (0.26) | 0.65 (0.13) | 0.41 (0.14) | |||
| Glide | 0.26 (0.18) | 0.50 (0.06) | 0.60 (0.09) | |||
| Middle | Extirpated | Riffle | 0.65 (0.28) | 0.68 (0.08) | 0.40 (0.07) | |
| LSP | 0.45 (0.25) | 0.59 (0.10) | 0.50 (0.27) | |||
| Glide | 0.25 (0.10) | 0.72 (0.06) | 0.71 (0.27) | |||
| Lower | Current | Riffle | 0.87 (0.15) | 0.88 (0.20) | 0.58 (0.21) | |
| LSP | 0.76 (0.26) | 0.80 (0.22) | 0.62 (0.26) | |||
| Glide | 0.63 (0.13) | 0.86 (0.20) | 0.77 (0.13) | |||
| Lower | Extirpated | Riffle | 0.96 (0.31) | 0.90 (0.25) | 0.94 (0.29) | |
| LSP | 0.71 (0.37) | 0.91 (0.27) | 0.62 (0.15) | |||
| Glide | 0.53 (0.31) | 0.71 (0.29) | 0.86 (0.21) | |||
| Winter | Middle | Current | Riffle | 1.08 (0.09) | 1.02 (0.10) | 0.74 (0.11) |
| LSP | 1.04 (0.20) | 1.01 (0.08) | 0.51 (0.07) | |||
| Glide | 0.67 (0.28) | 1.14 (0.07) | 1.20 (0.13) | |||
| Middle | Extirpated | Riffle | 1.01 (0.21) | 1.06 (0.11) | 0.83 (0.12) | |
| LSP | 0.98 (0.14) | 1.07 (0.11) | 0.68 (0.22) | |||
| Glide | 0.80 (0.10) | 1.17 (0.06) | 1.24 (0.11) | |||
| Lower | Current | Riffle | 1.10 (0.09) | 0.99 (0.08) | 0.77 (0.12) | |
| LSP | 1.04 (0.11) | 0.99 (0.05) | 0.70 (0.18) | |||
| Glide | 0.32 (0.51) | 1.02 (0.06) | 1.27 (0.22) | |||
| Lower | Extirpated | Riffle | 1.01 (0.14) | 0.97 (0.18) | 0.85 (0.22) | |
| LSP | 0.75 (0.11) | 0.92 (0.14) | 0.65 (0.17) | |||
| Glide | 0.60 (0.13) | 0.90 (0.08) | 1.00 (0.01) | |||
| Season | Region | Bed Type | Habitat | Lateral Position | ||
|---|---|---|---|---|---|---|
| Inside (±SD) | Middle (±SD) | Outside (±SD) | ||||
| Spring | Middle | Current | Riffle | 0.47 (0.10) | 0.47 (0.13) | 0.23 (0.08) |
| LSP | 0.47 (0.11) | 0.46 (0.07) | 0.26 (0.05) | |||
| Glide | 0.39 (0.13) | 0.58 (0.09) | 0.58 (0.19) | |||
| Middle | Extirpated | Riffle | 0.43 (0.07) | 0.55 (0.13) | 0.45 (0.16) | |
| LSP | 0.46 (0.12) | 0.46 (0.11) | 0.43 (0.15) | |||
| Glide | 0.31 (0.18) | 0.49 (0.14) | 0.55 (0.14) | |||
| Lower | Current | Riffle | 0.59 (0.07) | 0.59 (0.08) | 0.37 (0.22) | |
| LSP | 0.51 (0.08) | 0.58 (0.06) | 0.47 (0.07) | |||
| Glide | 0.37 (0.12) | 0.68 (0.02) | 0.71 (0.03) | |||
| Lower | Extirpated | Riffle | 0.52 (0.12) | 0.61 (0.11) | 0.48 (0.07) | |
| LSP | 0.42 (0.01) | 0.59 (0.08) | 0.50 (0.03) | |||
| Glide | 0.41 (0.05) | 0.57 (0.17) | 0.62 (0.05) | |||
| Autumn | Middle | Current | Riffle | 0.38 (0.11) | 0.46 (0.12) | 0.29 (0.09) |
| LSP | 0.38 (0.18) | 0.46 (0.09) | 0.29 (0.11) | |||
| Glide | 0.18 (0.13) | 0.34 (0.05) | 0.41 (0.07) | |||
| Middle | Extirpated | Riffle | 0.45 (0.20) | 0.47 (0.06) | 0.28 (0.05) | |
| LSP | 0.31 (0.18) | 0.41 (0.07) | 0.35 (0.19) | |||
| Glide | 0.17 (0.07) | 0.51 (0.04) | 0.50 (0.18) | |||
| Lower | Current | Riffle | 0.61 (0.10) | 0.61 (0.14) | 0.40 (0.15) | |
| LSP | 0.53 (0.18) | 0.56 (0.15) | 0.44 (0.18) | |||
| Glide | 0.44 (0.09) | 0.60 (0.14) | 0.53 (0.09) | |||
| Lower | Extirpated | Riffle | 0.67 (0.21) | 0.62 (0.18) | 0.66 (0.20) | |
| LSP | 0.50 (0.26) | 0.64 (0.19) | 0.44 (0.10) | |||
| Glide | 0.37 (0.22) | 0.50 (0.20) | 0.60 (0.25) | |||
| Winter | Middle | Current | Riffle | 0.76 (0.06) | 0.72 (0.07) | 0.52 (0.07) |
| LSP | 0.73 (0.13) | 0.72 (0.07) | 0.36 (0.05) | |||
| Glide | 0.47 (0.20) | 0.80 (0.05) | 0.84 (0.09) | |||
| Middle | Extirpated | Riffle | 0.70 (0.15) | 0.74 (0.07) | 0.58 (0.08) | |
| LSP | 0.69 (0.11) | 0.75 (0.08) | 0.47 (0.16) | |||
| Glide | 0.56 (0.07) | 0.82 (0.05) | 0.87 (0.08) | |||
| Lower | Current | Riffle | 0.77 (0.06) | 0.69 (0.05) | 0.54 (0.08) | |
| LSP | 0.73 (0.07) | 0.69 (0.03) | 0.49 (0.13) | |||
| Glide | 0.23 (0.36) | 0.71 (0.05) | 0.89 (0.15) | |||
| Lower | Extirpated | Riffle | 0.71 (0.10) | 0.68 (0.13) | 0.60 (0.16) | |
| LSP | 0.52 (0.08) | 0.64 (0.10) | 0.45 (0.12) | |||
| Glide | 0.42 (0.09) | 0.63 (0.06) | 0.70 (0.01) | |||
| Season | Region | Bed Type | Habitat | Lateral Position | ||
|---|---|---|---|---|---|---|
| Inside (±SD) | Middle (±SD) | Outside (±SD) | ||||
| Spring | Middle | Current | Riffle | 0.10 (0.02) | 0.09 (0.03) | 0.06 (0.04) |
| LSP | 0.09 (0.02) | 0.08 (0.01) | 0.05 (0.02) | |||
| Glide | 0.10 (0.06) | 0.12 (0.03) | 0.10 (0.04) | |||
| Middle | Extirpated | Riffle | 0.09 (0.03) | 0.10 (0.02) | 0.09 (0.02) | |
| LSP | 0.10 (0.01) | 0.08 (0.02) | 0.07 (0.03) | |||
| Glide | 0.08 (0.04) | 0.09 (0.03) | 0.08 (0.03) | |||
| Lower | Current | Riffle | 0.11 (0.02) | 0.11 (0.01) | 0.07 (0.04) | |
| LSP | 0.16 (0.05) | 0.11 (0.02) | 0.06 (0.01) | |||
| Glide | 0.09 (0.03) | 0.13 (0.01) | 0.12 (0.01) | |||
| Lower | Extirpated | Riffle | 0.10 (0.02) | 0.11 (0.02) | 0.08 (0.01) | |
| LSP | 0.10 (0.01) | 0.11 (0.01) | 0.08 (0.01) | |||
| Glide | 0.10 (0.02) | 0.11 (0.03) | 0.11 (0.03) | |||
| Autumn | Middle | Current | Riffle | 0.09 (0.04) | 0.11 (0.04) | 0.07 (0.03) |
| LSP | 0.12 (0.06) | 0.11 (0.03) | 0.06 (0.03) | |||
| Glide | 0.06 (0.05) | 0.08 (0.02) | 0.08 (0.02) | |||
| Middle | Extirpated | Riffle | 0.11 (0.03) | 0.12 (0.02) | 0.06 (0.02) | |
| LSP | 0.10 (0.05) | 0.10 (0.02) | 0.06 (0.03) | |||
| Glide | 0.07 (0.05) | 0.11 (0.03) | 0.09 (0.05) | |||
| Lower | Current | Riffle | 0.14 (0.04) | 0.15 (0.04) | 0.09 (0.03) | |
| LSP | 0.20 (0.06) | 0.14 (0.05) | 0.08 (0.04) | |||
| Glide | 0.13 (0.07) | 0.14 (0.04) | 0.11 (0.03) | |||
| Lower | Extirpated | Riffle | 0.17 (0.05) | 0.15 (0.04) | 0.15 (0.06) | |
| LSP | 0.16 (0.09) | 0.16 (0.04) | 0.09 (0.03) | |||
| Glide | 0.12 (0.05) | 0.12 (0.05) | 0.12 (0.03) | |||
| Winter | Middle | Current | Riffle | 0.12 (0.01) | 0.11 (0.02) | 0.08 (0.01) |
| LSP | 0.13 (0.03) | 0.11 (0.01) | 0.05 (0.01) | |||
| Glide | 0.09 (0.03) | 0.12 (0.01) | 0.11 (0.01) | |||
| Middle | Extirpated | Riffle | 0.11 (0.02) | 0.12 (0.01) | 0.09 (0.01) | |
| LSP | 0.12 (0.01) | 0.12 (0.01) | 0.06 (0.02) | |||
| Glide | 0.12 (0.01) | 0.12 (0.02) | 0.12 (0.02) | |||
| Lower | Current | Riffle | 0.12 (0.02) | 0.10 (0.01) | 0.08 (0.02) | |
| LSP | 0.15 (0.03) | 0.11 (0.00) | 0.07 (0.02) | |||
| Glide | 0.04 (0.06) | 0.11 (0.01) | 0.12 (0.02) | |||
| Lower | Extirpated | Riffle | 0.12 (0.02) | 0.11 (0.02) | 0.09 (0.02) | |
| LSP | 0.09 (0.01) | 0.10 (0.02) | 0.07 (0.02) | |||
| Glide | 0.08 (0.02) | 0.10 (0.01) | 0.11 (0.01) | |||
| Season | Region | Bed Type | Habitat | Lateral Position | ||
|---|---|---|---|---|---|---|
| Inside (±SD) | Middle (±SD) | Outside (±SD) | ||||
| Spring | Middle | Current | Riffle | 7.26 (3.93) | 5.57 (4.07) | 8.01 (7.50) |
| LSP | 6.41 (3.37) | 1.50 (2.14) | 4.06 (5.86) | |||
| Glide | 1.22 (1.21) | 3.23 (4.85) | 10.42 (10.16) | |||
| Middle | Extirpated | Riffle | 6.83 (10.42) | 12.24 (13.35) | 8.34 (7.12) | |
| LSP | 12.22 (5.89) | 5.00 (2.02) | 3.10 (3.28) | |||
| Glide | 17.90 (17.85) | 12.68 (7.89) | 12.96 (7.35) | |||
| Lower | Current | Riffle | 4.64 (4.05) | 6.83 (5.84) | 2.72 (3.74) | |
| LSP | 11.59 (7.03) | 8.81 (10.36) | 9.69 (8.76) | |||
| Glide | 10.96 (14.63) | 4.07 (3.35) | 11.07 (15.06) | |||
| Lower | Extirpated | Riffle | 4.72 (6.94) | 7.34 (6.45) | 6.78 (7.69) | |
| LSP | 8.22 (7.09) | 5.37 (6.76) | 6.59 (5.97) | |||
| Glide | 10.05 (7.43) | 6.88 (11.60) | 13.91 (3.13) | |||
| Autumn | Middle | Current | Riffle | 7.03 (1.40) | 11.67 (14.08) | 11.37 (14.44) |
| LSP | 2.80 (1.67) | 5.23 (5.63) | 10.78 (10.12) | |||
| Glide | 79.78 (117.15) | 17.19 (13.62) | 20.97 (7.29) | |||
| Middle | Extirpated | Riffle | 36.36 (33.23) | 37.57 (51.45) | 32.53 (19.32) | |
| LSP | 14.72 (8.85) | 12.92 (19.63) | 13.98 (19.90) | |||
| Glide | 46.16 (69.37) | 5.00 (7.42) | 16.15 (14.04) | |||
| Lower | Current | Riffle | 22.46 (22.26) | 8.17 (7.88) | 19.00 (3.18) | |
| LSP | 25.28 (15.29) | 30.44 (14.42) | 18.65 (16.16) | |||
| Glide | 31.95 (32.40) | 3.67 (5.08) | 19.33 (9.27) | |||
| Lower | Extirpated | Riffle | 74.33 (52.90) | 13.70 (13.95) | 8.65 (11.83) | |
| LSP | 53.80 (16.14) | 14.35 (11.65) | 22.49 (26.29) | |||
| Glide | 114.58 (112.12) | 8.79 (8.49) | 14.59 (16.39) | |||
| Winter | Middle | Current | Riffle | 13.64 (1.36) | 12.16 (4.85) | 15.52 (14.34) |
| LSP | 1.94 (3.06) | 6.32 (3.65) | 10.19 (8.81) | |||
| Glide | 7.27 (6.22) | 7.24 (4.74) | 5.95 (5.79) | |||
| Middle | Extirpated | Riffle | 25.41 (20.88) | 12.79 (10.71) | 8.16 (4.08) | |
| LSP | 9.64 (9.03) | 16.72 (13.84) | 7.43 (11.82) | |||
| Glide | 6.33 (6.13) | 4.17 (1.45) | 2.03 (1.94) | |||
| Lower | Current | Riffle | 4.08 (2.48) | 4.88 (5.30) | 5.99 (2.60) | |
| LSP | 2.08 (2.53) | 5.28 (1.43) | 2.79 (2.26) | |||
| Glide | 0.35 (0.51) | 7.72 (4.66) | 1.15 (1.49) | |||
| Lower | Extirpated | Riffle | 11.38 (1.23) | 9.68 (7.15) | 11.60 (1.67) | |
| LSP | 2.82 (2.24) | 10.58 (10.51) | 5.74 (6.35) | |||
| Glide | 5.17 (6.39) | 10.65 (2.30) | 11.12 (11.17) | |||
| Season | Region | Bed Type | Habitat | Lateral Position | ||
|---|---|---|---|---|---|---|
| Inside (±SD) | Middle (±SD) | Outside (±SD) | ||||
| Spring | Middle | Current | Riffle | 4.90 (3.02) | 4.04 (3.83) | 2.82 (3.19) |
| LSP | 3.96 (1.74) | 1.11 (1.63) | 1.78 (2.63) | |||
| Glide | 0.80 (0.78) | 2.76 (4.10) | 6.87 (4.14) | |||
| Middle | Extirpated | Riffle | 4.69 (7.30) | 8.67 (8.03) | 5.49 (5.95) | |
| LSP | 8.67 (5.43) | 3.54 (1.95) | 1.95 (1.84) | |||
| Glide | 7.86 (6.69) | 8.23 (3.65) | 9.72 (5.60) | |||
| Lower | Current | Riffle | 4.06 (3.45) | 6.18 (5.61) | 0.69 (0.66) | |
| LSP | 8.14 (3.28) | 6.71 (7.56) | 6.16 (8.05) | |||
| Glide | 5.37 (6.72) | 4.04 (3.33) | 11.45 (15.77) | |||
| Lower | Extirpated | Riffle | 2.89 (3.92) | 5.82 (4.72) | 4.43 (4.88) | |
| LSP | 4.96 (4.26) | 4.09 (4.74) | 4.90 (4.54) | |||
| Glide | 6.02 (4.92) | 4.60 (7.63) | 12.45 (3.35) | |||
| Autumn | Middle | Current | Riffle | 3.84 (1.32) | 8.28 (10.87) | 4.55 (6.17) |
| LSP | 1.48 (1.28) | 3.65 (4.42) | 3.87 (2.66) | |||
| Glide | 26.13 (39.81) | 8.73 (7.50) | 12.02 (2.24) | |||
| Middle | Extirpated | Riffle | 18.79 (14.50) | 23.72 (32.57) | 13.81 (9.18) | |
| LSP | 5.73 (4.76) | 6.83 (10.04) | 5.81 (8.44) | |||
| Glide | 10.61 (14.93) | 3.31 (4.81) | 11.26 (10.81) | |||
| Lower | Current | Riffle | 19.25 (18.67) | 6.08 (4.49) | 10.86 (4.00) | |
| LSP | 17.76 (8.02) | 26.73 (18.14) | 15.13 (17.51) | |||
| Glide | 22.77 (24.68) | 3.64 (5.14) | 14.51 (5.87) | |||
| Lower | Extirpated | Riffle | 49.50 (41.37) | 10.49 (8.63) | 7.87 (11.44) | |
| LSP | 34.23 (11.58) | 14.42 (12.06) | 13.77 (17.35) | |||
| Glide | 37.67 (32.59) | 6.33 (5.49) | 14.61 (18.89) | |||
| Winter | Middle | Current | Riffle | 14.84 (2.45) | 12.10 (3.56) | 11.93 (11.89) |
| LSP | 2.18 (3.47) | 6.20 (3.29) | 4.87 (3.75) | |||
| Glide | 3.73 (1.25) | 8.35 (5.50) | 10.42 (3.26) | |||
| Middle | Extirpated | Riffle | 23.09 (14.44) | 14.13 (12.41) | 6.46 (2.49) | |
| LSP | 9.72 (9.24) | 18.84 (16.73) | 5.67 (9.32) | |||
| Glide | 4.77 (4.11) | 4.93 (1.84) | 2.38 (2.21) | |||
| Lower | Current | Riffle | 4.51 (2.88) | 4.89 (5.59) | 4.63 (2.21) | |
| LSP | 2.28 (2.92) | 5.21 (1.39) | 2.05 (2.08) | |||
| Glide | 0.29 (0.49) | 8.02 (5.25) | 1.56 (2.07) | |||
| Lower | Extirpated | Riffle | 11.48 (1.04) | 8.99 (6.64) | 9.84 (3.09) | |
| LSP | 2.10 (1.60) | 8.90 (8.12) | 3.02 (2.75) | |||
| Glide | 3.3. (4.96) | 9.66 (2.74) | 11.10 (11.10) | |||
| Season | Region | Bed Type | Habitat | Lateral Position | ||
|---|---|---|---|---|---|---|
| Inner (±SD) | Middle (±SD) | Outer (±SD) | ||||
| Spring | Middle | Current | Riffle | 114.60 (67.47) | 848.03 (1138.40) | 28.37 (45.67 |
| LSP | 446.97 (691.48) | 85.30 (112.79) | 9.43 (15.65) | |||
| Glide | 6.03 (9.26) | 86.60 (67.74) | 120.33 (108.23) | |||
| Middle | Extirpated | Riffle | 73.27 (112.70) | 665.13 (555.18) | 115.30 (121.16) | |
| LSP | 206.07 (225.97) | 48.63 (35.68) | 9.77 (2.27) | |||
| Glide | 159.40 (272.46) | 45.30 (27.74) | 2.43 (2.56) | |||
| Lower | Current | Riffle | 636.50 (138.23) | 438.73 (726.92) | 3.33 (3.57) | |
| LSP | 537.80 (580.15) | 489.63 (376.00) | 11.70 (18.48) | |||
| Glide | 16.83 (21.40) | 385.00 (75.10) | 503.97 (716.04) | |||
| Lower | Extirpated | Riffle | 76.5 (114.60) | 181.83 (237.15) | 22.30 (26.07) | |
| LSP | 342.87 (296.95) | 776.37 (525.85) | 2.47 (2.73) | |||
| Glide | 32.83 (43.87) | 573.47 (199.21) | 493.60 (600.19) | |||
| Autumn | Middle | Current | Riffle | 358.53 (342.05) | 381.40 (583.05) | 255.10 (417.80) |
| LSP | 249.13 (205.32) | 107.10 (93.21) | 53.70 (74.37) | |||
| Glide | 0.80 (0.75) | 65.33 (39.25) | 82.13 (141.05) | |||
| Middle | Extirpated | Riffle | 366.23 (573.28) | 150.77 (166.96) | 75.10 (83.03) | |
| LSP | 496.07 (857.48) | 364.87 (375.87) | 1.77 (1.12) | |||
| Glide | 0.07 (0.12) | 652.40 (797.53) | 49.50 (82.80) | |||
| Lower | Current | Riffle | 1112.83 (1452.10) | 153.77 (107.48) | 12.77 (19.17) | |
| LSP | 4.63 (1.69) | 711.87 (706.91) | 3.57 (3.94) | |||
| Glide | 13.37 (13.12) | 1300.43 (2073.32) | 114.53 (122.80) | |||
| Lower | Extirpated | Riffle | 74.47 (49.43) | 370.27 (426.88) | 128.8 (135.14) | |
| LSP | 257.47 (11.83) | 154.1 (221.43) | 8.67 (15.01) | |||
| Glide | 7.6 (8.6) | 45.27 (42.57) | 669.2 (1004.66) | |||
| Winter | Middle | Current | Riffle | 159.50 (56.88) | 216.93 (183.47) | 53.93 (75.33) |
| LSP | 862.23 (741.18) | 595.17 (382.97) | 190.73 (283.19) | |||
| Glide | 224.63 (231.50) | 116.10 (146.32) | 76.57 (23.53) | |||
| Middle | Extirpated | Riffle | 599.73 (589.24) | 395.03 (167.88) | 154.80 (36.85) | |
| LSP | 820.70 (791.63) | 374.13 (109.73) | 2.30 (0.80) | |||
| Glide | 424.90 (289.27) | 146.33 (115.34) | 620.10 (938.07) | |||
| Lower | Current | Riffle | 201.77 (215.14) | 227.63 (212.27) | 169.53 (122.17) | |
| LSP | 2219.77 (2859.16) | 129.7 (99.30) | 18.47 (31.64) | |||
| Glide | 13.60 (17.84) | 206.47 (86.90) | 268.33 (464.77) | |||
| Lower | Extirpated | Riffle | 115.67 (73.64) | 404.00 (390.96) | 33.20 (23.90) | |
| LSP | 1218.00 (1872.08) | 338.23 (404.08) | 58.50 (58.10) | |||
| Glide | 305.47 (263.08) | 90.5 (83.15) | 302.03 (273.79) | |||
| Season | Region | Bed Type | Habitat | Lateral Position | ||
|---|---|---|---|---|---|---|
| Inner (±SD) | Middle (±SD) | Outer (±SD) | ||||
| Spring | Middle | Current | Riffle | 0.48 (0.06) | 0.20 (0.17) | 0.69 (0.31) |
| LSP | 0.51 (0.03) | 0.32 (0.02) | 0.56 (0.15) | |||
| Glide | 0.51 (0.60) | 0.35 (0.03) | 0.20 (0.35) | |||
| Middle | Extirpated | Riffle | 0.52 (0.23) | 0.38 (0.06) | 0.38 (0.07) | |
| LSP | 0.44 (0.13) | 0.35 (0.05) | 0.41 (0.10) | |||
| Glide | 0.72 (0.23) | 0.39 (0.07) | 0.33 (0.29) | |||
| Lower | Current | Riffle | 0.59 (0.09) | 0.39 (0.11) | 0.19 (0.17) | |
| LSP | 0.43 (0.09) | 0.41 (0.08) | 0.61 (0.63) | |||
| Glide | 0.53 (0.45) | 0.34 (0.01) | 0.41 (0.42) | |||
| Lower | Extirpated | Riffle | 0.60 (0.34) | 0.30 (0.03) | 0.35 (0.03) | |
| LSP | 0.32 (0.30) | 0.33 (0.00) | 0.48 (0.42) | |||
| Glide | 0.33 (0.08) | 0.43 (0.15) | 0.71 (0.20) | |||
| Autumn | Middle | Current | Riffle | 0.38 (0.06) | 0.26 (0.05) | 0.34 (0.12) |
| LSP | 0.65 (0.31) | 0.30 (0.01) | 0.53 (0.37) | |||
| Glide | 0.13 (0.14) | 0.37 (0.06) | 0.32 (0.55) | |||
| Middle | Extirpated | Riffle | 0.60 (0.24) | 0.41 (0.08) | 0.31 (0.03) | |
| LSP | 0.47 (0.48) | 0.35 (0.06) | 0.36 (0.22) | |||
| Glide | 0.25 (0.44) | 0.43 (0.03) | 0.68 (0.25) | |||
| Lower | Current | Riffle | 0.30 (0.29) | 0.31 (0.03) | 0.30 (0.10) | |
| LSP | 0.35 (0.03) | 0.38 (0.14) | 0.59 (0.51) | |||
| Glide | 0.46 (0.29) | 0.32 (0.01) | 0.44 (0.50) | |||
| Lower | Extirpated | Riffle | 0.32 (0.07) | 0.30 (0.02) | 0.30 (0.05) | |
| LSP | 0.28 (0.24) | 0.32 (0.02) | 0.23 (0.21) | |||
| Glide | 0.41 (0.17) | 0.30 (0.04) | 0.50 (0.14) | |||
| Winter | Middle | Current | Riffle | 0.38 (0.06) | 0.31 (0.02) | 0.36 (0.10) |
| LSP | 0.48 (0.13) | 0.32 (0.06) | 0.32 (0.06) | |||
| Glide | 0.39 (0.21) | 0.42 (0.10) | 0.80 (0.40) | |||
| Middle | Extirpated | Riffle | 0.45 (0.12) | 0.39 (0.08) | 0.38 (0.11) | |
| LSP | 0.49 (0.13) | 0.32 (0.04) | 0.48 (0.17) | |||
| Glide | 0.28 (0.06) | 0.35 (0.03) | 0.56 (0.17) | |||
| Lower | Current | Riffle | 0.36 (0.07) | 0.30 (0.08) | 0.38 (0.14) | |
| LSP | 0.40 (0.07) | 0.41 (0.12) | 0.40 (0.45) | |||
| Glide | 0.20 (0.17) | 0.32 (0.01) | 0.18 (0.32) | |||
| Lower | Extirpated | Riffle | 0.46 (0.04) | 0.31 (0.02) | 0.37 (0.14) | |
| LSP | 0.50 (0.12) | 0.33 (0.01) | 0.23 (0.20) | |||
| Glide | 0.35 (0.02) | 0.33 (0.02) | 0.51 (0.07) | |||
References
- Southwood, T.R.E. Tactics, Strategies and Templets. Oikos 1988, 52, 3–18. [Google Scholar] [CrossRef]
- Scarsbrook, M.R.; Townsend, C.R. Stream Community Structure in Relation to Spatial and Temporal Variation: A Habitat Templet Study of Two Contrasting New Zealand Streams. Freshw. Biol. 1993, 29, 395–410. [Google Scholar] [CrossRef]
- Townsend, C.R.; Hildrew, A.G. Species Traits in Relation to a Habitat Templet for River Systems. Freshw. Biol. 1994, 31, 265–275. [Google Scholar] [CrossRef]
- Frissell, C.A.; Liss, W.J.; Warren, C.E.; Hurley, M.D. A Hierarchical Framework for Stream Habitat Classification: Viewing Streams in a Watershed Context. Environ. Manag. 1986, 10, 199–214. [Google Scholar] [CrossRef]
- Poff, N.L. Landscape Filters and Species Traits: Towards Mechanistic Understanding and Prediction in Stream Ecology. J. N. Am. Benthol. Soc. 1997, 16, 391–409. [Google Scholar] [CrossRef]
- Neves, R.J. A State-of-the-Unionids Address. In Conservation and Management of Freshwater Mussels, Proceedings of a UMRCC Symposium, St. Louis, MO, USA, 12–14 October 1992; Cummings, K.S., Buchanan, A.C., Koch, L.M., Eds.; Upper Mississippi River Conservation Committee: Rock Island, IL, USA, 1993; pp. 1–10. [Google Scholar]
- Freshwater Mollusk Conservation Society. A National Strategy for the Conservation of Native Freshwater Mollusks. Freshw. Mollusk Biol. Conserv. 2016, 19, 1–21. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W. Fluvial Processes and Local Lithology Controlling Abundance, Structure, and Composition of Mussel Beds. Proc. Natl. Acad. Sci. USA 1982, 79, 4103–4107. [Google Scholar] [CrossRef]
- Strayer, D.L. The Effects of Surface Geology and Stream Size on Freshwater Mussel (Bivalvia, Unionidae) Distribution in Southeastern Michigan, USA. Freshw. Biol. 1983, 13, 253–264. [Google Scholar] [CrossRef]
- Neves, R.J.; Widlak, J.C. Habitat Ecology of Juvenile Freshwater Mussels (Bivalvia: Unionidae) in a Headwater Stream in Virginia. Am. Malacol. Bull. 1987, 5, 1–7. [Google Scholar]
- Bailey, R. Habitat Selection by a Freshwater Mussel: An Experimental Test. Malacologia 1989, 31, 205–210. [Google Scholar]
- Way, C.M.; Miller, A.C.; Payne, B.S. The Influence of Physical Factors on the Distribution and Abundance of Freshwater Mussels (Bivalvia: Unionidae) in the Lower Tennessee River. Nautilus 1989, 103, 96–98. [Google Scholar]
- Holland-Bartels, L.E. Physical Factors and Their Influence on the Mussel Fauna of a Main Channel Border Habitat of the Upper Mississippi River. J. N. Am. Benthol. Soc. 1990, 9, 327–335. [Google Scholar] [CrossRef]
- Strayer, D.L. Macrohabitats of Freshwater Mussels (Bivalvia: Unionacea) in Streams of the Northern Atlantic Slope. J. N. Am. Benthol. Soc. 1993, 12, 236–246. [Google Scholar] [CrossRef]
- Strayer, D.L.; Ralley, J. Microhabitat Use by an Assemblage of Stream-Dwelling Unionaceans (Bivalvia), Including Two Rare Species of Alasmidonta. J. N. Am. Benthol. Soc. 1993, 12, 247–258. [Google Scholar] [CrossRef]
- Di Maio, J.; Corkum, L.D. Relationship between the Spatial Distribution of Freshwater Mussels (Bivalvia: Unionidae) and the Hydrological Variability of Rivers. Can. J. Zool. 1995, 15, 663–671. [Google Scholar] [CrossRef]
- Di Maio, J.; Corkum, L.D. Patterns of Orientation in Unionids as a Function of Rivers with Differing Hydrological Variability. J. Molluscan Stud. 1997, 63, 531–539. [Google Scholar] [CrossRef]
- Brim-Box, J.B.; Mossa, J. Sediment, Land Use, and Freshwater Mussels: Prospects and Problems. J. N. Am. Benthol. Soc. 1999, 18, 99–117. [Google Scholar]
- Strayer, D.L. Use of Flow Refuges by Unionid Mussels in Rivers. J. N. Am. Benthol. Soc. 1999, 18, 468–476. [Google Scholar] [CrossRef]
- Hastie, L.C.; Young, M.R.; Boon, P.J. Growth Characteristics of Freshwater Pearl Mussels, Margaritifera margaritifera (L.). Freshw. Biol. 2000, 43, 243–256. [Google Scholar] [CrossRef]
- Hardison, B.S.; Layzer, J.B. Relations between Complex Hydraulics and the Localized Distribution of Mussels in Three Regulated Rivers. Regul. Rivers Res. Manag. Int. J. Devoted River Res. Manag. 2001, 17, 77–84. [Google Scholar] [CrossRef]
- Arbuckle, K.E.; Downing, J.A. The Influence of Watershed Land Use on Lake N:P in a Predominantly Agricultural Landscape. Limnol. Oceanogr. 2001, 46, 970–975. [Google Scholar] [CrossRef]
- Brim-Box, J.; Dorazio, R.M.; Liddell, W.D. Relationships between Streambed Substrate Characteristics and Freshwater Mussels (Bivalvia: Unionidae) in Coastal Plain Streams. J. N. Am. Benthol. Soc. 2002, 21, 253–260. [Google Scholar]
- Poole, K.E.; Downing, J.A. Relationship of Declining Mussel Biodiversity to Stream-Reach and Watershed Characteristics in an Agricultural Landscape. J. N. Am. Benthol. Soc. 2004, 23, 114–125. [Google Scholar] [CrossRef]
- Morales, Y.; Weber, L.; Mynett, A.; Newton, T. Effects of Substrate and Hydrodynamic Conditions on the Formation of Mussel Beds in a Large River. J. N. Am. Benthol. Soc. 2006, 25, 664–676. [Google Scholar] [CrossRef]
- Newton, T.J.; Woolnough, D.A.; Strayer, D.L. Using Landscape Ecology to Understand and Manage Freshwater Mussel Populations. J. N. Am. Benthol. Soc. 2008, 27, 424–439. [Google Scholar] [CrossRef]
- Allen, D.C.; Vaughn, C.C. Complex Hydraulic and Substrate Variables Limit Freshwater Mussel Species Richness and Abundance. J. N. Am. Benthol. Soc. 2010, 29, 383–394. [Google Scholar] [CrossRef]
- Maloney, K.O.; Lellis, W.A.; Bennett, R.M.; Waddle, T.J. Habitat Persistence for Sedentary Organisms in Managed Rivers: The Case for the Federally Endangered Dwarf Wedgemussel (Alasmidonta heterodon) in the Delaware River. Freshw. Biol. 2012, 57, 1315–1327. [Google Scholar] [CrossRef]
- Hornbach, D.J. Macrohabitat Factors Influencing the Distribution of Naiads in the St. Croix River, Minnesota and Wisconsin, USA. In Ecology and Evolution of the Freshwater Mussels Unionoida; Springer: Berlin, Germany, 2001; pp. 213–230. [Google Scholar]
- Gangloff, M.; Feminella, J. Stream Channel Geomorphology Influences Mussel Abundance in Southern Appalachian Streams, USA. Freshw. Biol. 2007, 52, 64–74. [Google Scholar] [CrossRef]
- Steuer, J.J.; Newton, T.J.; Zigler, S.J. Use of Complex Hydraulic Variables to Predict the Distribution and Density of Unionids in a Side Channel of the Upper Mississippi River. Hydrobiologia 2008, 610, 67–82. [Google Scholar] [CrossRef]
- Zigler, S.J.; Newton, T.J.; Steuer, J.J.; Bartsch, M.R.; Sauer, J.S. Importance of Physical and Hydraulic Characteristics to Unionid Mussels: A Retrospective Analysis in a Reach of Large River. Hydrobiologia 2008, 598, 343–360. [Google Scholar] [CrossRef]
- Daraio, J.A.; Weber, L.J.; Newton, T.J. Hydrodynamic Modeling of Juvenile Mussel Dispersal in a Large River: The Potential Effects of Bed Shear Stress and Other Parameters. J. N. Am. Benthol. Soc. 2010, 29, 838–851. [Google Scholar] [CrossRef]
- Daraio, J.A.; Weber, L.J.; Newton, T.J.; Nestler, J.M. A Methodological Framework for Integrating Computational Fluid Dynamics and Ecological Models Applied to Juvenile Freshwater Mussel Dispersal in the Upper Mississippi River. Ecol. Model. 2010, 221, 201–214. [Google Scholar] [CrossRef]
- Smit, R.; Kaeser, A. Defining Freshwater Mussel Mesohabitat Associations in an Alluvial, Coastal Plain River. Freshw. Sci. 2016, 35, 1276–1290. [Google Scholar] [CrossRef]
- Christian, A.D.; Harris, J.L.; Posey, W.R.; Hockmuth, J.F.; Harp, G.L. Freshwater Mussel (Bivalvia: Unionidae) Assemblages of the Lower Cache River, Arkansas. Southeast. Nat. 2005, 4, 487–512. [Google Scholar] [CrossRef]
- Bathurst, J.C.; Hey, R.D.; Thorne, C.R. Secondary Flow and Shear Stress at River Bends. J. Hydraul. Div. 1979, 105, 1277–1295. [Google Scholar]
- Bathurst, J. Environmental River Flow Hydraulics. In Applied Fluvial Geomorphology for River Engineering and Management; John Wiley & Sons, Inc.: New York, NY, USA, 1997; pp. 69–93. [Google Scholar]
- Statzner, R. Growth and Reynolds Number of Lotic Macroinvertebrates: A Problem for Adaptation of Shape to Drag. Oikos 1988, 51, 84–87. [Google Scholar] [CrossRef]
- Gordon, M.E. Mollusca of the White River, Arkansas and Missouri. Southwest. Nat. 1982, 27, 347–352. [Google Scholar] [CrossRef]
- Coker, R.E. Fresh-Water Mussels and Mussel Industries of the United States; US Government Printing Office: Washington, DC, WA, USA, 1919.
- Williams, J.D.; Warren, M.L.; Cummings, K.S.; Harris, J.L.; Neves, R.J. Conservation Status of Freshwater Mussels of the United States and Canada. Fisheries 1993, 18, 6–22. [Google Scholar] [CrossRef]
- Harris, J.L.; Posey, W.R., II; Davidson, C.L.; Farris, J.L.; Oetker, S.R.; Stoeckel, J.N.; Crump, B.G.; Barnett, M.S.; Martin, H.C.; Matthews, M.W.; et al. Unionoida (Mollusca: Margaritiferidae, Unionidae) in Arkansas, Third Status Review. J. Ark. Acad. Sci. 2009, 63, 50–84. [Google Scholar]
- Christian, A.D. Analysis of the Commercial Mussel Beds in the Cache and White Rivers in Arkansas. Master’s Thesis, Arkansas State University, Jonesboro, AR, USA, 1995. [Google Scholar]
- Christian, A.D.; Harris, J.L. Development and Assessment of a Sampling Design for Mussel Assemblages in Large Streams. Am. Midl. Nat. 2005, 153, 284–292. [Google Scholar] [CrossRef]
- Harris, J.L.; Christian, A.D. Current Status of the Freshwater Mussel Fauana of the White River, Arkansas, River Miles 10-255; Report; U.S Army Corps of Engineers: Memphis, TN, USA, 2000.
- United States Geological Survey, USGS Stream Stats. Available online: https://streamstats.usgs.gov/ss/ (accessed on 6 March 2018).
- U.S. Department of Agriculture: Forest Service. Ozark-Ouachita Highlands Assessment: Aquatic Conditions; Report 3 of 5 General Technical Report SRS-33; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, CA, USA, 1999.
- Bisson, P.A.; Nielsen, J.L.; Palmason, R.A.; Grove, L.E. A System of Naming Habitat in Small Streams, with Examples of Habitat Utilization by Salmonids during Low Streamflow. In Proceedings of the Symposium on Acquisition and Utilization of Aquatic Habitat Inventory Information, Portland, OR, USA, 28–30 October 1981; Armantrout, N.B., Ed.; Hagen Publishing Company: Los Angeles, CA, USA, 1982; pp. 62–73. [Google Scholar]
- Gordon, N.D.; McMahon, T.A.; Finlayson, B.L.; Gippel, C.; Nathan, R.J. Stream Hydrology: An Introduction for Ecologists, 2nd ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2004. [Google Scholar]
- United States Army Corps of Engineers Memphis District. White River Gauges. Available online: http://www.mvm.usace.army.mil/hydraulics/docs/white.htm (accessed on 15 January 2005).
- Rempel, L.L.; Richardson, J.S.; Healey, M.C. Macroinvertebrate Community Structure along Gradients of Hydraulic and Sedimentary Conditions in a Large Gravel-bed River. Freshw. Biol. 2000, 45, 57–73. [Google Scholar] [CrossRef]
- Das, B.M. Soil Mechanics Laboratory Manual; Engineering Press: Austin, TX, USA, 1997. [Google Scholar]
- Peck, A.J. A Reach Scale Comparison of Fluvial Geomorphological Conditions between Current and Historic Freshwater Mussel Beds in the White River, Arkansas. Master’s Thesis, Arkansas State University, Jonesboro, AR, USA, 2005. [Google Scholar]
- Lessios, H.A. Testing Electrophoretic Data for Agreement with Hardy-Weinberg Expectations. Mar. Biol. 1992, 112, 517–523. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Simon, A.; Dickerson, W.; Heins, A. Suspended-Sediment Transport Rates at the 1.5-Year Recurrence Interval for Ecoregions of the United States: Transport Conditions at the Bankfull and Effective Discharge? Geomorphology 2004, 58, 243–262. [Google Scholar] [CrossRef]
- Talling, P.J.; Sowter, M.J. Erosion, Deposition and Basin-Wide Variations in Stream Power and Bed Shear Stress. Basin Res. 1998, 10, 87–108. [Google Scholar] [CrossRef]
- Leopold, L.B.; Wolman, M.G.; Miller, J.P. Fluvial Process in Geomorphology; Freeman: San Francisco, CA, USA, 1964. [Google Scholar]
- Bridge, J.; Jarvis, J. The Dynamics of a River Bend: A Study in Flow and Sedimentary Processes. Sedimentology 1982, 29, 499–541. [Google Scholar] [CrossRef]
- Jia, Y. Minimum Froude Number and the Equilibrium of Alluvial Sand Rivers. Earth Surf. Process. Landf. 1990, 15, 199–209. [Google Scholar] [CrossRef]
- Doisy, K.E.; Rabeni, C.F. Flow Conditions, Benthic Food Resources, and Invertebrate Community Composition in a Low-Gradient Stream in Missouri. J. N. Am. Benthol. Soc. 2001, 20, 17–32. [Google Scholar] [CrossRef]
- Newson, M.; Newson, C. Geomorphology, Ecology and River Channel Habitat: Mesoscale Approaches to Basin-Scale Challenges. Prog. Phys. Geogr. 2000, 24, 195–217. [Google Scholar] [CrossRef]
- Dietrich, W.E.; Smith, J.D. Bed Load Transport in a River Meander. Water Resour. Res. 1984, 20, 1355–1380. [Google Scholar] [CrossRef]
- Gomez, B. Bedload Transport. Earth-Sci. Rev. 1991, 31, 89–132. [Google Scholar] [CrossRef]
- Dade, W. Grain Size, Sediment Transport and Alluvial Channel Pattern. Geomorphology 2000, 35, 119–126. [Google Scholar] [CrossRef]
- Kleinhans, M.G.; Brinke, W.B.T. Accuracy of Cross-Channel Sampled Sediment Transport in Large Sand-Gravel-Bed Rivers. J. Hydraul. Eng. 2001, 127, 258–269. [Google Scholar] [CrossRef]
- Church, M. Geomorphic Thresholds in Riverine Landscapes. Freshw. Biol. 2002, 47, 541–557. [Google Scholar] [CrossRef]
- Kleinhans, M.G.; van Rijn, L.C. Stochastic Prediction of Sediment Transport in Sand-Gravel Bed Rivers. J. Hydraul. Eng. 2002, 128, 412–425. [Google Scholar] [CrossRef]
- Bravo-Espinosa, M.; Osterkamp, W.; Lopes, V.L. Bedload Transport in Alluvial Channels. J. Hydraul. Eng. 2003, 129, 783–795. [Google Scholar] [CrossRef]
- Reid, I.; Bathurst, J.; Carling, P.; Walling, D.; Webb, B. Sediment Erosion, Transport, and Deposition. In Applied Fluvial Geomorphology for River Engineering and Management; Thorne, C.R., Hey, R.D., Newson, M.D., Eds.; John Wiley & Sons Ltd.: West Sussex, UK, 1997; Volume 5, pp. 95–135. [Google Scholar]
| Bed Type | Region | Study Reach | RKM | Estimated Slope (m/m) |
|---|---|---|---|---|
| Current | Middle | WR 156 | 251.1 | 0.000053 |
| Extirpated | WR 151 | 243.0 | 0.000061 | |
| Extirpated | WR 147 | 236.5 | 0.000061 | |
| Current | WR 145 | 233.4 | 0.000061 | |
| Current | WR 119 | 191.5 | 0.000063 | |
| Extirpated | WR 114 | 183.5 | 0.000063 | |
| Current | Lower | WR 096 | 154.5 | 0.000043 |
| Extirpated | WR 094 | 151.3 | 0.000043 | |
| Extirpated | WR 054 | 86.9 | 0.000050 | |
| Current | WR 048 | 77.2 | 0.000063 | |
| Extirpated | WR 046 | 74.0 | 0.000063 | |
| Current | WR 042 | 67.6 | 0.000063 |
| Region | Study Reach | Spring | Autumn | Winter |
|---|---|---|---|---|
| Middle | WR 156 | 467.60 | 239.52 | 1253.83 |
| WR 151 | 467.34 | 270.11 | 1303.89 | |
| WR 147 | 459.76 | 243.83 | 1173.46 | |
| WR 145 | 462.61 | 236.29 | 1339.48 | |
| WR 119 | 512.62 | 206.78 | 1287.95 | |
| WR 114 | 646.46 | 220.54 | 1041.18 | |
| Lower | WR 096 | 590.94 | 235.55 | 1143.26 |
| WR 094 | 580.72 | 293.82 | 1261.71 | |
| WR 054 | 616.50 | 334.09 | 732.90 | |
| WR 048 | 665.98 | 320.56 | 1006.90 | |
| WR 046 | 663.47 | 487.43 | 983.69 | |
| WR 042 | 686.61 | 230.08 | 1303.44 |
| Parameter | Significant Interaction | Nature of Interaction |
|---|---|---|
| Discharge (Q) | ||
| Season * Region | Seasonal influence dominated interaction | |
| Mean Velocity (V) | ||
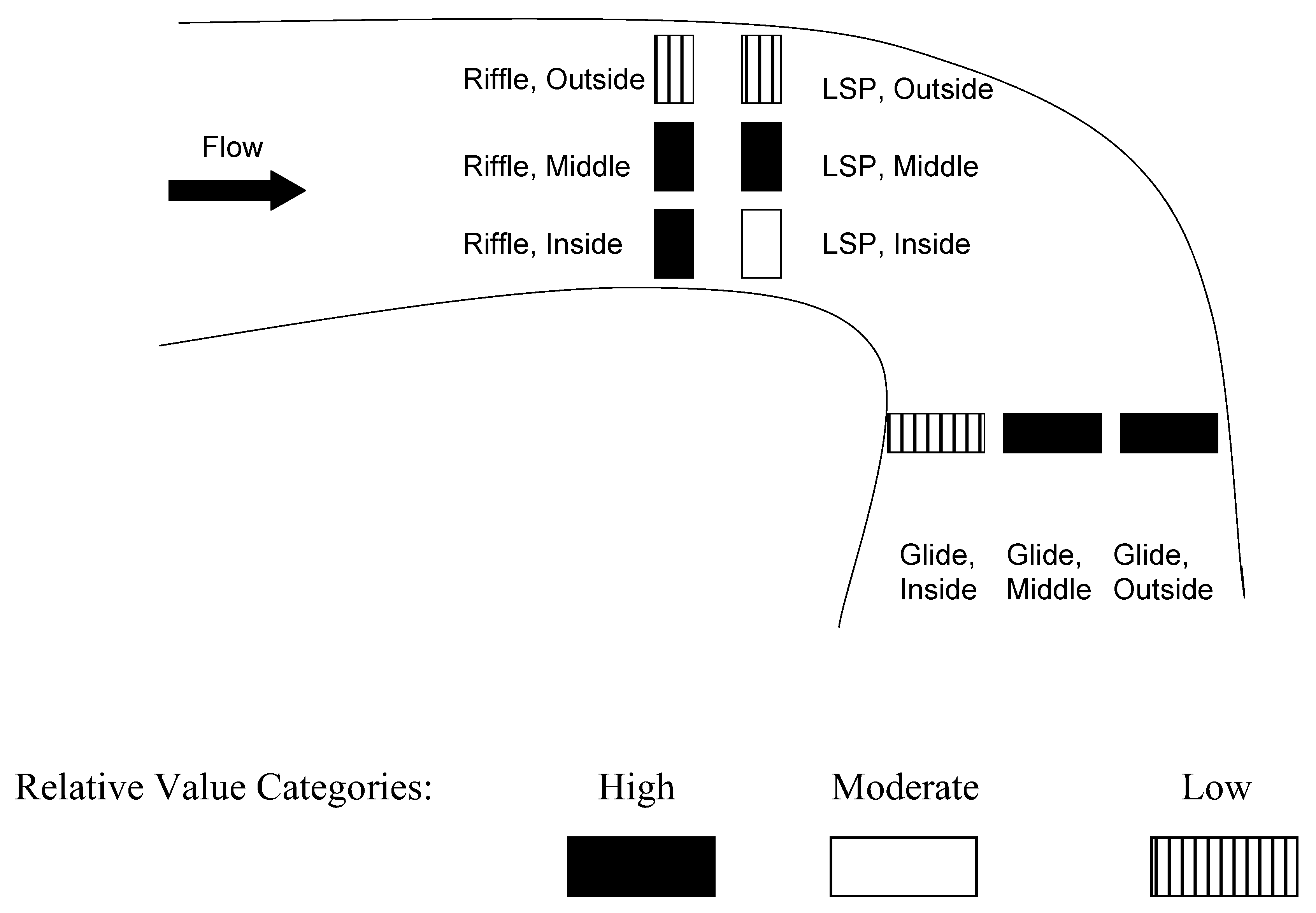
| Habitat * Lateral Position | LSP/Outside usually significantly less than other cells; except Glide/Inside and Riffle/Outside | |
| Glide/Outside usually greater than other cells | ||
| Habitat * Season | Glide/Winter significantly greater than other habitats except Riffle/Winter | |
| Lateral Position * Bed Type | Middle of Extirpated and Current sites greater than Inside/Extirpated, Inside/Current, and Outside/Current | |
| Season * Region | Strong seasonal influence, region not very influential | |
| Bed Velocity (BV) | ||
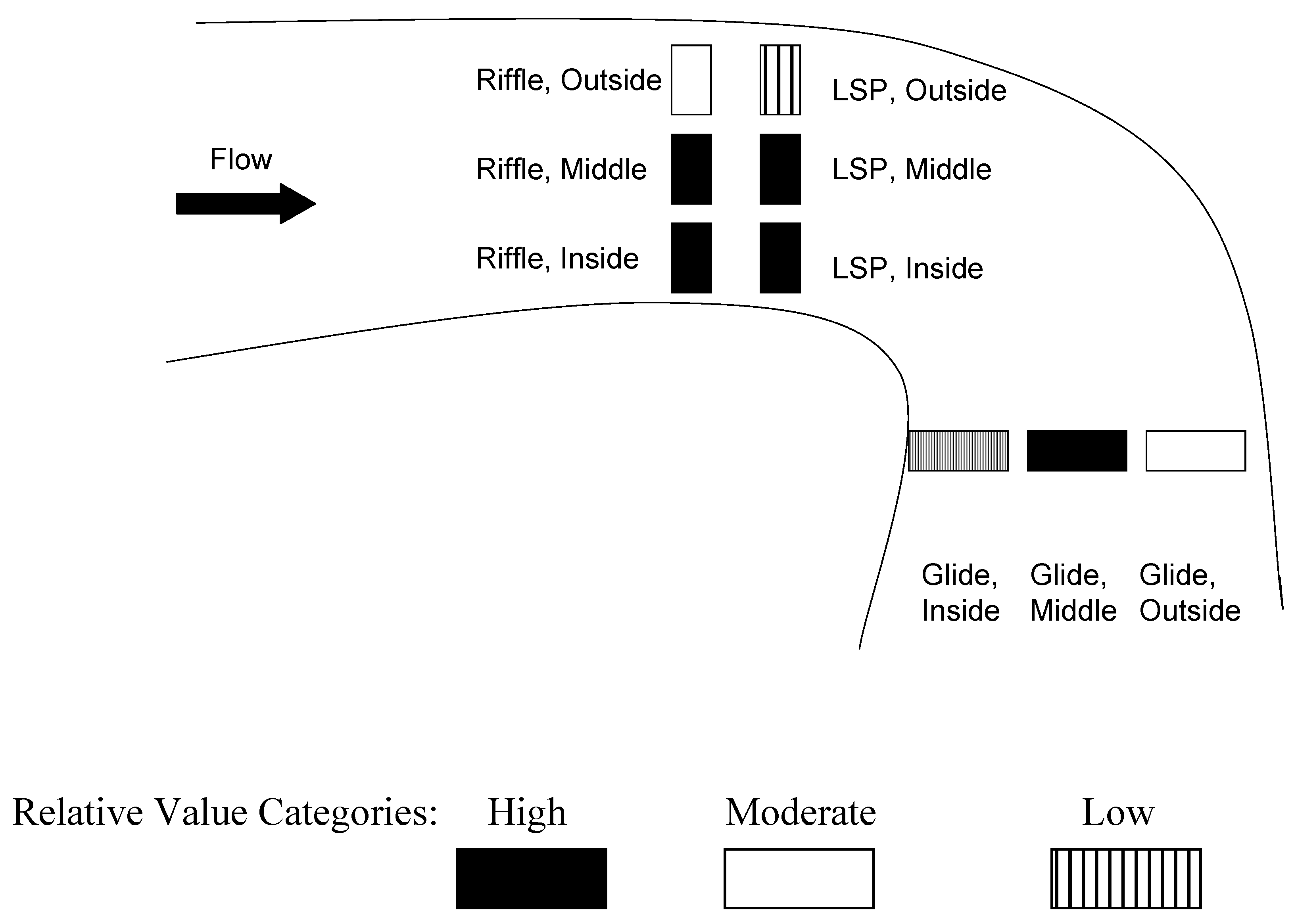
| Habitat * Lateral Position * Season | NDP | |
| Habitat * Lateral Position | LSP/Outside significantly less than most except occasionally Glide/Inside and Riffle/Outside | |
| Habitat * Season | Riffle/Winter greater than most except Glide/Winter, LSP/Winter | |
| Riffle/Winter and Glide/Winter not different, but both greater than LSP/Winter | ||
| LSP/Autumn significantly less than all others except Glide/Spring, Glide Autumn, LSP/Spring and Riffle/Autumn and Riffle/Spring | ||
| Habitat * Bed Type | NDP | |
| Froude Number (Fr) | ||
| Season * Region | Winter/Lower and Winter/Middle only similar pairs, all others significantly different | |
| Habitat * Lateral Position * Season | NDP | |
| Habitat * Lateral Position | LSP/Outside significantly less than all but Riffle/Outside | |
| Habitat * Season | Riffle/Autumn significantly greater than LSP/Spring, LSP/Winter and Riffle/Spring | |
| LSP/Autumn significantly greater than LSP/Spring and Riffle/Spring | ||
| Shear Stress (To) | Season * Region | Autumn/Lower significantly greater than all other combinations |
| Winter/Middle significantly greater than Spring/Middle | ||
| Habitat * Season | Glide/Spring and Riffle/Winter no different from any other combination | |
| Habitat * Region | NDP | |
| Stream Power (w) | ||
| Lateral Position * Season | Inside/Autumn significantly greater than others except Outside/Autumn | |
| Outside/Autumn significantly greater than Inside/Winter, Middle/Spring, Outside/Winter | ||
| Outside/Winter significantly less than Outside/Autumn | ||
| Habitat * Lateral Position | Riffle/Inside significantly greater than LSP/Outside | |
| Habitat * Season | LSP/Spring significantly less than Riffle/Autumn and Riffle/Winter | |
| Riffle/Spring less than Riffle/Autumn and Riffle/Winter | ||
| Bedload Discharge (Qbl) | ||
| Habitat * Season | Glide/Winter significantly greater than others except Riffle/Winter | |
| Season * Region | Middle/Winter significantly greater than others. | |
| Spring/Lower significantly greater than others except Middle/Winter | ||
| Autumn/Middle significantly less than others. | ||
| Autumn/Lower significantly greater than Autumn/Middle. | ||
| Autumn/Lower significantly less than Spring/Lower and Winter/Middle | ||
| Habitat * Lateral Position | NDP |
| Effect | Effect Variable | Mean Velocity (m/s) | Bed Velocity (m/s) | Froude Number (Dimensionless) | Shear Stress (N/m2) | Stream Power (W/m) | Bedload Discharge (g·s−1) |
|---|---|---|---|---|---|---|---|
| Season | |||||||
| Spring | 0.70 (±0.20) | 0.49 (±0.14) | 0.10 (±0.03) | 7.76 (±7.58) | 5.30 (±5.24) | 238.80 (±389.40) | |
| Autumn | 0.65 (±0.25) | 0.46 (±0.18) | 0.11 (±0.05) | 24.62 (±37.09) | 13.83 (±17.00) | 245.93 (±544.77) | |
| Winter | 0.91 (±0.25) | 0.64 (±0.17) | 0.10 (±0.03) | 7.94 (±7.82) | 7.38 (±7.27) | 343.19 (±677.83) | |
| Region | |||||||
| Middle | 0.71 (±0.28) | 0.49 (±0.19) | 0.09 (±0.03) | 12.85 (±22.49) | 7.85 (±9.93) | 237.31 (±391.79) | |
| Lower | 0.81 (±0.23) | 0.56 (±0.16) | 0.11 (±0.04) | 14.03 (±24.75) | 9.83 (±13.10) | 314.63 (±671.87) | |
| Habitat | |||||||
| Riffle | 0.7 (±0.24) | 0.55 (±0.17) | 0.11 (±0.03) | 13.87 (±19.02) | 9.96 (±12.85) | 260.44 (±423.57) | |
| LSP | 0.72 (±0.23) | 0.51 (±0.16) | 0.10 (±0.04) | 10.8 (±13.16) | 7.68 (±9.77) | 339.11 (±703.82) | |
| Glide | 0.76 (±0.30) | 0.54 (±0.21) | 0.10 (±0.03) | 15.6 (±33.72) | 8.89 (±12.12) | 228.37 (±483.11) | |
| Lateral Position | |||||||
| Inside | 0.70 (±0.28) | 0.49 (±0.20) | 0.11 (±0.04) | 19.48 (±36.76) | 10.91 (±15.90) | 354.36 (±729.43) | |
| Middle | 0.85 (±0.20) | 0.6 (±0.14) | 0.11 (±0.03) | 9.90 (±12.41) | 8.11 (±9.29) | 342.27 (±505.26) | |
| Outside | 0.71 (±0.27) | 0.50 (±0.19) | 0.09 (±0.03) | 10.94 (±11.03) | 7.49 (±7.98) | 131.29 (±309.20) | |
| Bed Type | |||||||
| Extirpated | 0.76 (±0.24) | 0.53 (±0.17) | 0.10 (±0.03) | 16.17 (±27.04) | 10.30 (±13.17) | 261.18 (±446.73) | |
| Current | 0.75 (±0.27) | 0.52 (±0.19) | 0.10 (±0.04) | 10.71 (±19.31) | 7.37 (±9.72) | 290.76 (±638.68) |
| Variable | Season | Region | Bed Type | Habitat | Lateral Position | Habitat × Lateral Position |
|---|---|---|---|---|---|---|
| Mean Velocity (m·s−1) | <0.0001 | <0.0001 | 0.3105 | 0.0283 | <0.0001 | <0.0001 |
| Bed Velocity (m·s−1) | <0.0001 | <0.0001 | 0.5179 | 0.0260 | <0.0001 | <0.0001 |
| Froude Number | 0.0005 | <0.0001 | 0.7262 | 0.4470 | <0.0001 | <0.0001 |
| Shear Stress (N/m2) | <0.0001 | 0.9586 | 0.0555 | 0.2171 | 0.1853 | 0.7583 |
| Stream Power (W/m) | <0.0001 | 0.2785 | 0.0438 | 0.1093 | 0.6413 | 0.0272 |
| Cell Qbl (g·s−1) | <0.0001 | 0.4139 | 0.3201 | 0.0092 | <0.0001 | <0.0001 |
| Mean Particle Size (mm) | 0.1335 | 0.1530 | 0.6668 | 0.5722 | 0.0066 | 0.1603 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christian, A.D.; Peck, A.J.; Allen, R.; Lawson, R.; Edwards, W.; Marable, G.; Seagraves, S.; Harris, J.L. Freshwater Mussel Bed Habitat in an Alluvial Sand-Bed-Material-Dominated Large River: A Core Flow Sediment Refugium? Diversity 2020, 12, 174. https://doi.org/10.3390/d12050174
Christian AD, Peck AJ, Allen R, Lawson R, Edwards W, Marable G, Seagraves S, Harris JL. Freshwater Mussel Bed Habitat in an Alluvial Sand-Bed-Material-Dominated Large River: A Core Flow Sediment Refugium? Diversity. 2020; 12(5):174. https://doi.org/10.3390/d12050174
Chicago/Turabian StyleChristian, Alan D., Andrew J. Peck, Ryan Allen, Raven Lawson, Waylon Edwards, Grace Marable, Sara Seagraves, and John L. Harris. 2020. "Freshwater Mussel Bed Habitat in an Alluvial Sand-Bed-Material-Dominated Large River: A Core Flow Sediment Refugium?" Diversity 12, no. 5: 174. https://doi.org/10.3390/d12050174
APA StyleChristian, A. D., Peck, A. J., Allen, R., Lawson, R., Edwards, W., Marable, G., Seagraves, S., & Harris, J. L. (2020). Freshwater Mussel Bed Habitat in an Alluvial Sand-Bed-Material-Dominated Large River: A Core Flow Sediment Refugium? Diversity, 12(5), 174. https://doi.org/10.3390/d12050174





