Evaluation of Long Bone Marrow Composition of Roe Deer (Capreolus capreolus)
Simple Summary
Abstract
1. Introduction
- To test the feasibility of using digital image analysis to quantify the color of bone marrow in a standardized and objective way;
- To analyze the relationship between marrow color (measured through RGB and decimal values) and nutritional indicators, specifically dry matter (DM) and ether extract (EE) content;
- To compare DM and EE values across different long bones, both proximal and distal, to identify which provide the most reliable and accessible information on the body condition of roe deer (Capreolus capreolus), with particular focus on whether the femur and metacarpus-metatarsus—the most commonly available bones—can provide equivalent data.
2. Materials and Methods
2.1. Data Collection
2.2. Digital Image Macroscopic Analysis
2.3. Dry Matter Analysis
2.4. Ether Extract Analysis
2.5. Statistical Analysis
3. Results
3.1. Marrow Color
3.2. DM Between Groups of Long Bones
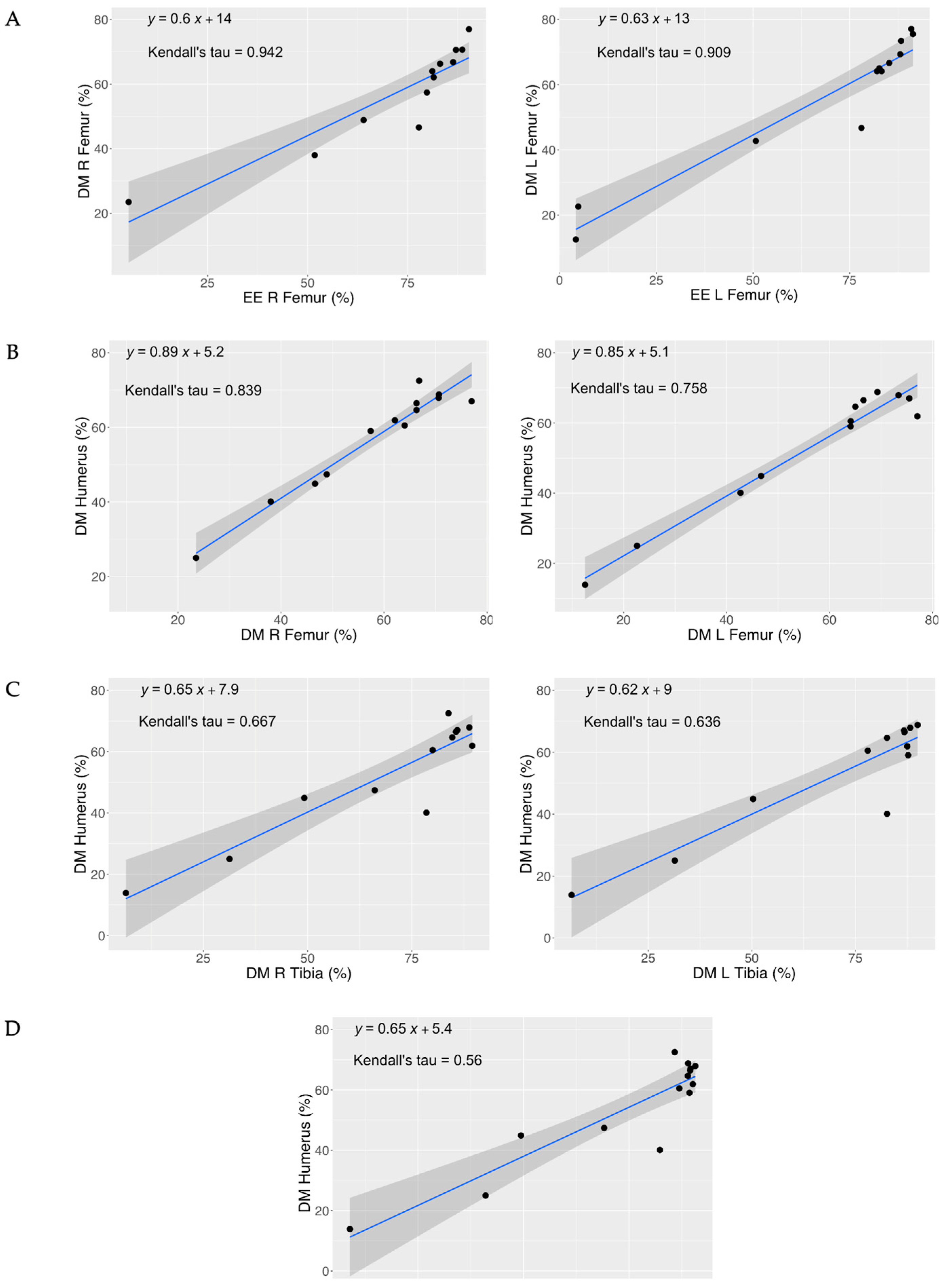
3.3. Correlation Between Marrow DM and EE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apollonio, M.; Andersen, R.; Putman, R. (Eds.) European Ungulates and Their Management in the 21st Century; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Morellet, N.; Van Moorter, B.; Cargnelutti, B.; Angibault, J.-M.; Lourtet, B.; Merlet, J.; Ladet, S.; Hewison, A.M. Landscape composition influences roe deer habitat selection at both home range and landscape scales. Landsc. Ecol. 2011, 26, 999–1010. [Google Scholar] [CrossRef]
- Jasińska, K.D.; Jackowiak, M.; Gryz, J.; Bijak, S.; Szyc, K.; Krauze-Gryz, D. Occurrence and activity of roe deer in urban forests of Warsaw. Environ. Sci. Proc. 2020, 3, 35. [Google Scholar] [CrossRef]
- Bassi, E.; Gazzola, A.; Bongi, P.; Scandura, M.; Apollonio, M. Relative impact of human harvest and wolf predation on two ungulate species in Central Italy. Ecol. Res. 2020, 35, 662–674. [Google Scholar] [CrossRef]
- Baur, S.; Peters, W.; Oettenheym, T.; Menzel, A. Weather conditions during hunting season affect the number of harvested roe deer (Capreolus capreolus). Ecol. Evol. 2021, 11, 10178–10191. [Google Scholar] [CrossRef]
- Žele Vengušt, D.; Kuhar, U.; Jerina, K.; Vengušt, G. Twenty Years of Passive Disease Surveillance of Roe Deer (Capreolus capreolus) in Slovenia. Animals 2021, 11, 407. [Google Scholar] [CrossRef]
- Ossi, F.; Hebblewhite, M.; Rocca, M.; Nicoloso, S.; Gaillard, J.M.; Cagnacci, F. Walking on the snow, feeding at the box: Drivers of winter habitat selection by roe deer (Capreolus capreolus): An empirical assessment in the Alps. In Proceedings of the IX Congresso Italiano di Teriologia, Civitella Alfedena, AQ, Italy, 7–10 May 2014; Imperio, S., Mazzaracca, S., Preatoni, D.G., Eds.; p. 17-C148. [Google Scholar]
- Hagen, R.; Ortmann, S.; Elliger, A.; Arnold, J. Advanced roe deer (Capreolus capreolus) parturition date in response to climate change. Ecosphere 2021, 12, e03819. [Google Scholar] [CrossRef]
- Ferretti, F.; Lovari, S.; Stephens, P.A. Joint effects of weather and interspecific competition on foraging behavior and survival of a mountain herbivore. Curr. Zool. 2019, 65, 165–175. [Google Scholar] [CrossRef]
- Franchini, M.; Peric, T.; Frangini, L.; Prandi, A.; Comin, A.; Rota, M.; Filacorda, S. You’re stressing me out! Effect of interspecific competition from red deer on roe deer physiological stress response. J. Zool. 2023, 320, 63–74. [Google Scholar] [CrossRef]
- Bonnot, N.; Morellet, N.; Verheyden, H.; Cargnelutti, B.; Lourtet, B.; Klein, F.; Hewison, A.J.M. Habitat use under predation risk: Hunting, roads and human dwellings influence the spatial behaviour of roe deer. Eur. J. Wildl. Res. 2013, 59, 185–193. [Google Scholar] [CrossRef]
- Tucker, M.A.; Böhning-Gaese, K.; Fagan, W.F.; Fryxell, J.M.; Van Moorter, B.; Alberts, S.C.; Ali, A.H.; Allen, A.M.; Attias, N.; Avgar, T.; et al. Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 2018, 359, 466–469. [Google Scholar] [CrossRef]
- Bonnot, N.C.; Couriot, O.; Berger, A.; Cagnacci, F.; Ciuti, S.; E De Groeve, J.; Gehr, B.; Heurich, M.; Kjellander, P.; Kröschel, M.; et al. Fear of the dark? Contrasting impacts of humans versus lynx on diel activity of roe deer across Europe. J. Anim. Ecol. 2020, 89, 132–145. [Google Scholar] [CrossRef]
- Gehr, B.; Bonnot, N.C.; Heurich, M.; Cagnacci, F.; Ciuti, S.; Hewison, A.J.M.; Gaillard, J.-M.; Ranc, N.; Premier, J.; Vogt, K.; et al. Stay home, stay safe—Site familiarity reduces predation risk in a large herbivore in two contrasting study sites. J. Anim. Ecol. 2020, 89, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Wevers, J.; Fattebert, J.; Casaer, J.; Artois, T.; Beenaerts, N. Trading fear for food in the Anthropocene: How ungulates cope with human disturbance in a multi-use, suburban ecosystem. Sci. Total. Environ. 2020, 741, 140369. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.S.; Gaynor, K.M.; Becker, J.A.; Abraham, J.O.; Mumma, M.A.; Pringle, R.M. Dynamic landscapes of fear: Understanding spatiotemporal risk. Trends Ecol. Evol. 2022, 37, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.E.; Peacor, S.D. A review of traits-mediated indirect interactions in ecological communities. Ecology 2003, 84, 1083–1100. [Google Scholar] [CrossRef]
- Bradford, A.L.; Weller, D.W.; Punt, A.E.; Ivashchenko, Y.V.; Burdin, A.M.; VanBlaricom, G.R.; Brownell, R.L., Jr. Leaner leviathans: Body condition variation in a critically endangered whale population. J. Mammal. 2012, 93, 251–266. [Google Scholar] [CrossRef]
- Schulte-Hostedde, A.I.; Millar, J.S.; Hickling, G.J. Evaluating body condition in small mammals. Can. J. Zool. 2001, 79, 1021–1029. [Google Scholar] [CrossRef]
- Risco, D.; Gonçalves, P.; Mentaberre, G.; Navarro-González, N.; Casas-Díaz, E.; Gassó, D.; Colom-Cadena, A.; Fernández-Aguilar, X.; Castillo-Contreras, R.; Velarde, R.; et al. Biometrical measurements as efficient indicators to assess wild boar body condition. Ecol. Indic. 2018, 88, 43–50. [Google Scholar] [CrossRef]
- Riney, T. Differences in proportion of fawns to hinds in red deer (Cervus elaphus) from several New Zealand environments. Nature 1956, 177, 488–489. [Google Scholar]
- Fuller, T.K.; Coy, P.L.; Peterson, W.J. Marrow fat relationships among leg bones of white-tailed deer. Wildl. Soc. Bull. 1986, 14, 73–75. [Google Scholar]
- Gerdin, J.A.; McDonough, S.P.; Reisman, R.; Scarlett, J. Circumstances, descriptive characteristics, and pathologic findings in dogs suspected of starving. Vet. Pathol. 2016, 53, 1087–1094. [Google Scholar] [CrossRef]
- Raglus, T.I.; De Groef, B.; Rochfort, S.; Rawlin, G.; McCowan, C. Bone marrow fat analysis as a diagnostic tool to document ante-mortem starvation. Vet. J. 2019, 243, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kaewthong, P.; Waiyagan, K.; Wattanachant, S. Imaging analysis by digital camera for separating broiler breast meat with low water-holding capacity. J. Poult. Sci. 2017, 54, 253–261. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Shalaby, A.A. Digital imaging devices as sensors for iron determination. Food Chem. 2019, 274, 360–367. [Google Scholar] [CrossRef]
- Turchenski, D.G.; Franco, A.J.; Turchenski, R.G.; Werner, L.C.; Weber, S.H.; Gumiel, Y.B.; Michelotto, P.V. Exploring alternatives for securing anatomical structures in capturing digital images: A comparative analysis. Anat. Histol. Embryol. 2024, 53, e12975. [Google Scholar] [CrossRef]
- Meyerholtz, K.A.; Wilson, C.R.; Everson, R.J.; Hooser, S.B. Quantitative assessment of the percent fat in domestic animal bone marrow. J. Forensic. Sci. 2011, 56, 775–777. [Google Scholar] [CrossRef]
- Borgo, C.; Dtta, R.; Rotelli, L. Valutazione e Rilievi Biometrici Della Fauna Selvatica. Ungulati, Galliformi alpi.ni e Lepre Variabile; Regione Piemonte, Istituto per le Piante da Legno e l’Ambiente. Ipla Spa: Turin, Italy, 2007. [Google Scholar]
- Hewison, A.J.; Vincent, J.P.; Joachim, J.; Angibault, J.M.; Cargnelutti, B.; Cibien, C. The effects of woodland fragmentation and human activity on roe deer distribution in agricultural landscapes. Can. J. Zool. 2001, 79, 679–689. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Harting, S.M. Basic image analysis and manipulation in ImageJ. Curr. Protoc. Mol. Biol. 2013, 102, 14–15. [Google Scholar] [CrossRef]
- Barthel, K.U. 3D-data representation with ImageJ. In Proceedings of the 1st ImageJ User & Developer Conference, Luxembourg, 18–19 May 2006; Available online: https://imagej.nih.gov/ij/plugins/color-inspector.html (accessed on 15 March 2023).
- Wu, X. Color quantization by dynamic programming and principal analysis. ACM Trans. Graph. 1992, 11, 348–372. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Bongiorno, V.; Schiavone, A.; Renna, M.; Sartore, S.; Soglia, D.; Sacchi, P.; Gariglio, M.; Castillo, A.; Mugnai, C.; Forte, C.; et al. Carcass yields and meat composition of male and female Italian slow-growing chicken breeds: Bianca di Saluzzo and Bionda Piemontese. Animals 2022, 12, 406. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Clayton, E.H.; Lamb, T.A.; Van de Ven, R.J.; Refshauge, G.; Kerr, M.; Bailes, K.; Lewandowski, P.; Ponnampalam, E. The impact of supplementing lambs with algae on growth, meat traits and oxidative status. Meat Sci. 2014, 98, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Holman, B.W.; Bailes, K.L.; Meyer, R.G.; Hopkins, D.L. Effect of modified Soxhlet (Soxtec) and Folch extraction method selection on the total lipid determination of aged beef. J. Food Sci. Technol. 2019, 56, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 17 January 2023).
- Husseman, J.S.; Murray, D.L.; Power, G.; Mack, C.M. Correlation patterns of marrow fat in Rocky Mountain elk bones. J. Wildl. Manag. 2003, 67, 742. [Google Scholar] [CrossRef]
- Tattoli, L.; Tsokos, M.; Sautter, J.; Anagnostopoulos, J.; Maselli, E.; Ingravallo, G.; Delia, M.; Solarino, B. Postmortem bone marrow analysis in forensic science: Study of 73 cases and review of the literature. Forensic Sci. Int. 2014, 234, 72–78. [Google Scholar] [CrossRef]
- Fong, D.W. Seasonal variation of marrow fat content from Newfoundland moose. J. Wildl. Manag. 1981, 45, 545. [Google Scholar] [CrossRef]
- Pewsner, M.; Origgi, F.C.; Frey, J.; Ryser-Degiorgis, M.-P. Assessing fifty years of general health surveillance of roe deer in Switzerland: A retrospective analysis of necropsy reports. PLoS ONE 2017, 12, e0170338. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Mazzamuto, M.V.; Schilling, A.-K.; Romeo, C. Wildlife disease monitoring: Methods and perspectives. Animals 2022, 12, 3032. [Google Scholar] [CrossRef]
- Nieminen, M.; Laitinen, M. Bone marrow and kidney fat as indicators of condition in reindeer. Rangifer 1986, 6, 219. [Google Scholar] [CrossRef]
- Seaman, J.P.; Kjeldsberg, C.R.; Linker, A. Gelatinous transformation of the bone marrow. Hum. Pathol. 1978, 9, 685–692. [Google Scholar] [CrossRef]
- Cowan, P.E. Weight of dried marrow as an indicator of femur fat in brushtail possums Trichosurus vulpecula. N. Z. J. Zool. 1985, 12, 349–352. [Google Scholar] [CrossRef]
- Roll, P.; Beham, A.; Beham-Schmid, C. Post-mortem histopathological investigations of the bone marrow in forensic medicine: An important issue for both the forensic and clinical pathologist. Forensic Sci. Int. 2009, 186, e17–e20. [Google Scholar] [CrossRef]
- Lamoureux, J.L.; Fitzgerald, S.D.; Church, M.K.; Agnew, D.W. The effect of environmental storage conditions on bone marrow fat determination in three species. J. Vet. Diagn. Investig. 2011, 23, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Cheatum, E.L. Bone marrow as an index of malnutrition in deer. NY State Conserv. 1949, 3, 19–22. [Google Scholar]
- Greer, K.R. A compression method indicates fat content of elk (Wapiti) femur marrows. J. Wildl. Manag. 1968, 32, 747. [Google Scholar] [CrossRef]
- Franzmann, A.W.; Arneson, P.D. Marrow fat in Alaskan moose femurs in relation to mortality factors. J. Wildl. Manag. 1976, 40, 336–339. [Google Scholar] [CrossRef]
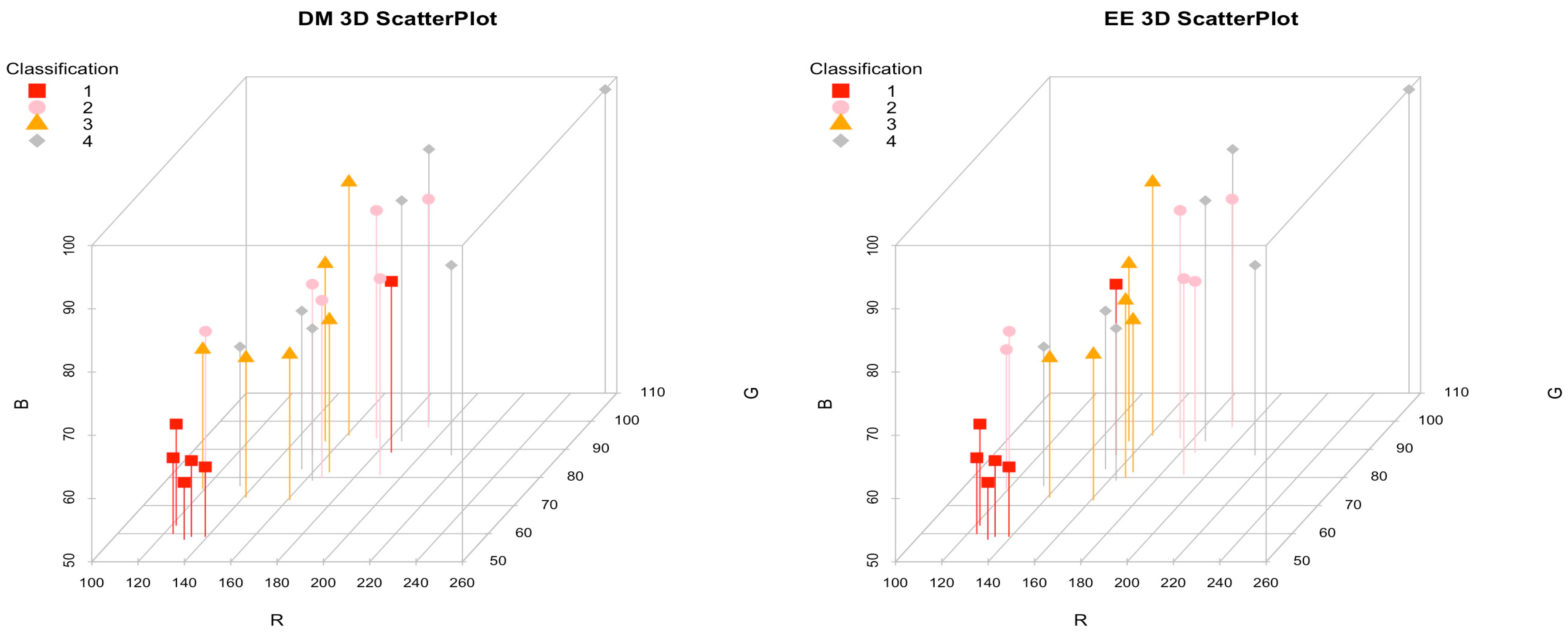
| R | G | B | Decimal * | DM | EE | |
|---|---|---|---|---|---|---|
| Min | 118 | 58 | 59 | 7,753,290 | 12.50 | 4.15 |
| 1st Q | 134 | 73 | 72 | 8,801,608 | 46.70 | 77.81 |
| Median | 161 | 81 | 75 | 10,569,801 | 64.12 | 82.68 |
| 3rd Q | 186 | 93 | 81 | 12,212,557 | 69.30 | 87.07 |
| Max | 255 | 110 | 98 | 16,739,938 | 77.06 | 91.37 |
| DM | EE | ||
|---|---|---|---|
| Decimal (RGB) | τ | 0.324 * | 0.344 * |
| p | 0.023 | 0.16 | |
| N | 25 | 25 | |
| DM | τ | - | 0.913 ** |
| p | - | 0.000 | |
| N | - | 25 | |
| DM | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fem. L | Tibia R | Tibia L | Metat. | Humer. | Radius | Metac. | |||
| DM | Fem. R | τ | 0.759 | 0.636 | 0.449 | 0.211 | 0.816 | 0.579 | 0.342 |
| p | 0.001 | 0.007 | 0.059 | 0.325 | 0 | 0.007 | 0.11 | ||
| N | 11 | 11 | 11 | 13 | 13 | 13 | 13 | ||
| Fem. L | τ | - | 0.911 | 0.595 | 0.424 | 0.758 | 0.788 | 0.364 | |
| p | - | 0 | 0.007 | 0.055 | 0.001 | 0 | 0.1 | ||
| N | - | 10 | 12 | 12 | 12 | 12 | 12 | ||
| Tibia R | τ | - | - | 0.854 | 0.545 | 0.667 | 0.939 | 0.455 | |
| p | - | - | 0.001 | 0.014 | 0.003 | 0 | 0.04 | ||
| N | - | - | 10 | 12 | 12 | 12 | 12 | ||
| Tibia L | τ | - | - | - | 0.321 | 0.657 | 0.687 | 0.443 | |
| p | - | - | - | 0.149 | 0.003 | 0.002 | 0.046 | ||
| N | - | - | - | 12 | 12 | 12 | 12 | ||
| Metat. | τ | - | - | - | - | 0.275 | 0.495 | 0.319 | |
| p | - | - | - | - | 0.171 | 0.014 | 0.112 | ||
| N | - | - | - | - | 14 | 14 | 14 | ||
| Humer. | τ | - | - | - | - | - | 0.56 | 0.385 | |
| p | - | - | - | - | - | 0.005 | 0.055 | ||
| N | - | - | - | - | - | 14 | 14 | ||
| Radius | τ | - | - | - | - | - | - | 0.341 | |
| p | - | - | - | - | - | - | 0.09 | ||
| N | - | - | - | - | - | - | 14 | ||
| EE | ||||
|---|---|---|---|---|
| Fem. R | Fem. L | |||
| DM | Fem. R | τ | 0.921 | 0.722 |
| p | 0 | 0.002 | ||
| N | 13 | 11 | ||
| Fem. L | τ | 0.745 | 0.909 | |
| p | 0.001 | 0 | ||
| N | 11 | 12 | ||
| Tibia R | τ | 0.564 | 0.867 | |
| p | 0.016 | 0 | ||
| N | 11 | 10 | ||
| Tibia L | τ | 0.477 | 0.626 | |
| p | 0.042 | 0.005 | ||
| N | 11 | 12 | ||
| Metat. | τ | 0.179 | 0.394 | |
| p | 0.393 | 0.075 | ||
| N | 13 | 12 | ||
| Humer. | τ | 0.821 | 0.727 | |
| p | 0 | 0.001 | ||
| N | 13 | 12 | ||
| Radius | τ | 0.513 | 0.818 | |
| p | 0.015 | 0 | ||
| N | 13 | 12 | ||
| Metac. | τ | 0.359 | 0.394 | |
| p | 0.088 | 0.075 | ||
| N | 13 | 12 | ||
| EE | Fem. R | τ | - | 0.709 |
| p | - | 0.002 | ||
| N | - | 11 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaydou, F.; Marucco, F.; Bianchi, C.; Rossi, L.; Schiavone, A.; Nery, J. Evaluation of Long Bone Marrow Composition of Roe Deer (Capreolus capreolus). Wild 2025, 2, 45. https://doi.org/10.3390/wild2040045
Gaydou F, Marucco F, Bianchi C, Rossi L, Schiavone A, Nery J. Evaluation of Long Bone Marrow Composition of Roe Deer (Capreolus capreolus). Wild. 2025; 2(4):45. https://doi.org/10.3390/wild2040045
Chicago/Turabian StyleGaydou, Francesca, Francesca Marucco, Chiara Bianchi, Luca Rossi, Achille Schiavone, and Joana Nery. 2025. "Evaluation of Long Bone Marrow Composition of Roe Deer (Capreolus capreolus)" Wild 2, no. 4: 45. https://doi.org/10.3390/wild2040045
APA StyleGaydou, F., Marucco, F., Bianchi, C., Rossi, L., Schiavone, A., & Nery, J. (2025). Evaluation of Long Bone Marrow Composition of Roe Deer (Capreolus capreolus). Wild, 2(4), 45. https://doi.org/10.3390/wild2040045








