Eurasian Otters’ Urban Pond Use Patterns in Southern Spain: A Case Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Period and Data Collection

2.3. Urban Pond “A” Monitoring
2.4. Urban Pond “B” and “C” Monitoring
2.5. Natural Pond Monitoring
2.6. Data Analysis
3. Results
3.1. Urban Pond “A”
3.2. Urban Ponds “B” and “C”
3.3. Natural Ponds, Model Effects, and Random Patterns
4. Discussion
4.1. Predation Patterns in Urban Pond “A”
4.2. Predation Patterns in Urban Ponds “B” and “C”
4.3. Implications of Urban Prey Depletion by Otters
4.4. Limitations of the Study
4.5. Interaction with Human Interests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Messmer, T.A. Emergence of Human-Wildlife Conflict Management: Turning Challenges into Opportunities. Int. Biodeter. 2000, 45, 97–100. [Google Scholar] [CrossRef]
- Gloor, S.; Bontadina, F.; Hegglin, D.; Deplazes, P.; Breitenmoser, U. The rise of urban fox population in Switzerland. Mamm. Biol. 2001, 66, 155–164. [Google Scholar]
- Atchison, K.A.; Rodewald, A.D. The value or urban forests to wintering birds. Nat. Area. J. 2006, 26, 280–288. [Google Scholar] [CrossRef]
- Duarte, J.; Farfán, M.A.; Fa, J.E.; Vargas, J.M. Deer population inhabiting urban areas in the south of Spain: Habitat and conflicts. Eur. J. Wildlife Res. 2015, 61, 365–377. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Carvalho, J.; Serrano, E.; Mentaberre, G.; Fernández-Aguilar, X.; Colom, C.; González-Crespo, C.; Lavín, S.; López-Olvera, J.R. Urban wild boars prefer fragmented areas with food resources near natural corridors. Sci. Total Environ. 2018, 615, 282–288. [Google Scholar] [CrossRef]
- Rodewald, A.D.; Gehrt, S.D. Wildlife population dynamics in urban landscapes. In Urban Wildlife Conservation: Theory and practice; McCleery, R.A., Moorman, C., Peterson, M.N., Eds.; Springer: New York, NY, USA, 2014; pp. 117–147. [Google Scholar]
- Linnell, J.D.C.; Cretois, B.; Nilsen, E.B.; Rolandsen, C.M.; Solberg, E.J.; Veiberg, V.; Kaczensky, P.; Van Moorter, B.; Panzacchi, M.; Rauset, G.R.; et al. The challenges and opportunities of coexisting with wild ungulates in the human-dominated landscapes of Europe’s Anthropocene. Biol. Conserv. 2020, 244, 108500. [Google Scholar] [CrossRef]
- Escobar-González, M.; López-Martín, J.M.; Valldeperes, M.; Estruch, J.; Tampach, S.; Castillo-Contreras, R.; Lavín, S.; Serrano, E.; López-Olvera, J.R. Evaluating hunting and capture methods for urban wild boar population management. Sci. Total Environ. 2024, 940, 173463. [Google Scholar] [CrossRef]
- Colomer, J.; Massei, G.; Roos, D.; Rosell, C.; Rodríguez-Teijeiro, J.D. What drives wild boar density and population growth in Mediterranean environments? Sci. Total Environ. 2024, 931, 172739. [Google Scholar] [CrossRef]
- Davison, J.; Huck, M.; Delahay, R.J.; Roper, T.J. Urban badger setts: Characteristics, patterns of use and management implications. J. Zool. 2008, 275, 190–200. [Google Scholar] [CrossRef]
- Geiger, M.; Taucher, A.L.; Gloor, S.; Hegglin, D.; Bontadina, F. In the footsteps of city foxes: Evidence for a rise of urban badger populations in Switzerland. Hystrix 2018, 29, 3–10. [Google Scholar]
- Ward, A.I.; Finney, J.K.; Beatham, S.E.; Delahay, R.J.; Robertson, P.A.; Cowan, D.P. Exclusions for resolving urban badger damage problems: Outcomes and consequences. PeerJ 2016, 4, e2579. [Google Scholar] [CrossRef]
- Tilehurst, B.A.; Baker, R.J.; Cagnacci, F.; Scott, D.M. Spatial aspects of gardens drive ranging in urban foxes (Vulpes vulpes): The resource dispersion hypothesis revisited. Animals 2020, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Padovani, R.; Shi, Z.; Harris, S. Are British urban foxes (Vulpes vulpes) “bold”? The importance of understanding human–wildlife interactions in urban areas. Ecol. Evol. 2021, 11, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Abraín, A.; Jiménez, J.; Oro, D. Pax Romana: ‘refuge abandonment’ and spread of fearless behavior in a reconciling world. Anim. Conserv. 2019, 22, 3–13. [Google Scholar] [CrossRef]
- Martínez-Abraín, A.; Jiménez, J.; Oro, D. New policies for a new wildlife: A road map for the wildlife manager of the future. Biol. Conserv. 2019, 236, 484–488. [Google Scholar] [CrossRef]
- Isaksson, C.; Rodewald, A.D.; Gil, D. Behavioural and ecological consequences of urban life in birds. Front. Ecol. Evol. 2018, 6, 50. [Google Scholar] [CrossRef]
- McCleery, R.A. Urban mammals. In Urban Ecosystem Ecology; Aitkenhead-Petersen, A., Volder, A., Eds.; Agronomy, American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2010; pp. 87–102. [Google Scholar]
- Charmantier, A.; Demeyrier, V.; Lambrechts, M.; Perret, S.; Grégoire, A. Urbanization is associated with divergence in pace-of-life in great tits. Front. Ecol. Evol. 2017, 5, 53. [Google Scholar] [CrossRef]
- Carrete, M.; Tella, J.L. Behavioural correlations associated with fear of humans differ between rural and urban burrowing owls. Front. Ecol. Evol. 2017, 5, 54. [Google Scholar] [CrossRef]
- Martínez-Abraín, A.; Jiménez, J. Anthropogenic areas as incidental substitutes for original habitats. Conserv. Biol. 2016, 30, 593–598. [Google Scholar] [CrossRef]
- Rodewald, A.D.; Arcese, P. Reproductive contributions of cardinals are consistent with a hypothesis of relaxed selection in urban landscapes. Front. Ecol. Evol. 2017, 5, 77. [Google Scholar] [CrossRef]
- Marzluff, J.M.; Rodewald, A.D. Conserving biodiversity in urbanizing areas: Non-traditional views from a bird’s perspective. Cities Environ. 2008, 1, 6–27. [Google Scholar] [CrossRef]
- Thieme, J.L.; Rodewald, A.D.; Brown, J.; Anchor, A.; Gehrt, S.D. Linking grassland and early successional bird territory density to predator activity in urban parks. Nat. Area. J. 2015, 35, 515–532. [Google Scholar] [CrossRef]
- Ruiz-Olmo, J. Nutria—Lutra lutra. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Barja, I., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2017; Available online: https://www.vertebradosibericos.org/ (accessed on 25 May 2025).
- Romero, R.; Nores, C.; García-Rovés, P.; Guitián, J.; Ruiz-Olmo, J. Distribución y uso del espacio costero de las nutrias en la fachada cántabro-atlántica. In La Nutria en España. Veinte años de Seguimiento de un Mamífero Amenazado; López-Martín, J.M., Jiménez, J., Eds.; SECEM: Malaga, Spain, 2008; pp. 397–420. [Google Scholar]
- Pedroso, N.M.; Sales-Luis, T.; Santos-Reis, M. Use of Aguieira Dam by Eurasian otters in central Portugal. Folia Zool. 2007, 56, 365–377. [Google Scholar]
- Duarte, J.; Farfán, M.A.; Vargas, J.M. The use of artificial lakes on golf courses as feeding areas by the otter (Lutra lutra) in southern Spain. IUCN Otter Spec. Group Bull. 2011, 28, 17–22. [Google Scholar]
- Ruiz-Olmo, J.; Jiménez, J.A.; Chacón, W. The importance of ponds for the otter (Lutra lutra) during drought periods in Mediterranean ecosystems: A case study in Bergantes river. Mammalia 2007, 71, 16–24. [Google Scholar] [CrossRef]
- Ruiz-Olmo, J.; Jiménez, J. Ecología de la nutria en los ambientes mediterráneos de la península Ibérica. In La Nutria en España. Veinte años de Seguimiento de un Mamífero Amenazado; López-Martín, J.M., Jiménez, J., Eds.; SECEM: Malaga, Spain, 2008; pp. 305–343. [Google Scholar]
- Basto, M.P.; Pedroso, N.M.; Mira, A.; Santos-Reis, M. Use of small and medium-sized water reservoirs by otters in a Mediterranean ecosistema. Anim. Biol. 2011, 61, 75–94. [Google Scholar] [CrossRef]
- Ruiz-Olmo, J.; López-Martín, J.L.; Palazón, S. The influence of fish abundance on the otter (Lutra lutra) populations in Iberian Mediterranean habitats. J. Zool. 2001, 254, 325–336. [Google Scholar] [CrossRef]
- Clavero, M.; Prenda, J.; Delibes, M. Trophic diversity of the otter (Lutra lutra L.) in temperate and Mediterranean freshwater habitats. J. Biogeogr. 2003, 30, 761–769. [Google Scholar] [CrossRef]
- Clavero, M.; Prenda, J.; Delibes, M. Amphibian and reptile consumption by otters (Lutra lutra L.) in a coastal area in southern Iberian Peninsula. Herpetol. J. 2005, 15, 125–131. [Google Scholar]
- Clavero, M.; Prenda, J.; Delibes, M. Does size matter? Relating consumed prey sizes and diet composition in south Iberian coastal streams. Acta Theriol. 2007, 52, 37–44. [Google Scholar] [CrossRef]
- Clavero, M.; Prenda, J.; Blanco-Garrido, F.; Delibes, M. Hydrological stability and otter trophic diversity: A scale-insensitive pattern? Can. J. Zool. 2008, 86, 1152–1158. [Google Scholar] [CrossRef]
- Ruiz-Olmo, J.; Jiménez, J. Diet diversity and breeding of top predators are determined by habitat stability and structure: A case study with the Eurasian otter (Lutra lutra L.). Eur. J. Wildlife Res. 2008, 55, 133–144. [Google Scholar] [CrossRef]
- Pedroso, N.M.; Santos-Reis, M. Summer diet of Eurasian otters in large dams of south Portugal. Hystrix 2006, 17, 117–128. [Google Scholar]
- Duarte, J.; Rubio, P. Presencia de la nutria (Lutra lutra) en campos de golf en la Costa del Sol occidental, Málaga. In Proceedings of the Resúmenes VII Jornadas SECEM, Valencia, Spain, 3–6 December 2005; p. 63. [Google Scholar]
- Duarte, J. Atropellos de nutria en el entorno de campos de golf de Málaga. Galemys 2008, 20, 105. [Google Scholar]
- Duarte, J.; Rodríguez, D.; Farfán, M.A. Eurasian otters are becoming urbanized. Front. Ecol. Environ. 2022, 20, 126. [Google Scholar] [CrossRef]
- Rodríguez, D.; Duarte, J.; Rubio, P.J.; Farfán, M.A. La nutria (Lutra lutra) en los campos de golf de la Costa del Sol: Un hábitat urbano con uso permanente. In Proceedings of the Resúmenes XV Congreso SECEM, Córdoba, Spain, 4–7 December 2021; p. 127. [Google Scholar]
- Duarte, J.; Rodríguez, D.; Rubio, P.J.; Farfán, M.A.; Bensusan, K.; Fa, J.E. La nutria (Lutra lutra) en el litoral del sur peninsular: Presencia y uso de puertos pesqueros y deportivos. In Proceedings of the Resúmenes XV Congreso SECEM, Córdoba, Spain, 4–7 December 2021; p. 49. [Google Scholar]
- Koehn, J.D. Carps (Cyprinus carpio) as a powerful invader in Australian waterways. Freshw. Biol. 2004, 49, 882–894. [Google Scholar] [CrossRef]
- Doadrio, I.; Carmona, J.A. Phylogenetic overview of the genus Squalius (Actinopterygii, Cyprinidae) in the Iberian Peninsula, with description of two new species. Cybium 2006, 30, 199–214. [Google Scholar]
- Leunda, P.M.; Elvira, B.; Ribeiro, F.; Miranda, R.; Oscoz, J.; Alves, M.J.; Collares-Pereira, M.J. International standardization of common names for Iberian endemic freshwater fishes. Limmnetica 2009, 28, 189–202. [Google Scholar] [CrossRef]
- Reuther, C.; Dolch, D.; Green, R.; Jahrl, J.; Jefferies, D.; Krekemeyer, A.; Kucerova, M.; Madsen, A.B.; Romanowski, J.; Roche, K.; et al. Surveying and Monitoring Distribution and Population Trends of the Eurasian Otter (Lutra lutra): Guidelines and evaluation of the standard method for surveys as recommended by the European section of the IUCN/SSC Otter Specialist Group. Habitat 2000, 12, 152. [Google Scholar]
- White, G.C.; Garrott, R.A. Analysis of Wildlife Radio-Tracking Data; Academic: London, UK, 1990. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; Freeman: New York, NY, USA, 1995. [Google Scholar]
- Fowler, J.; Cohen, J. Practical Statistics for Field Biology; Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Crawley, M.J. GLIM for Ecologists; Blackwell: London, UK, 1993. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Hoboken, NJ, USA, 1999. [Google Scholar]
- García, P.; Ayres, C. Depredación masiva de la nutria (Lutra lutra) sobre el galápago leproso (Mauremys leprosa). Munibe 2007, 25, 44–49. [Google Scholar]
- Lizana, M.; Pérez-Mellado, V. Depredación por la nutria (Lutra lutra) del sapo de la sierra de Gredos (Bufo bufo gredosicola). Doñana Acta Vertebr. 1990, 17, 109–112. [Google Scholar]
- Alarcos, G.; Ortiz, M.E.; Fernández, M.J.; Lizana, M. Depredación del gallipato (Pleurodeles waltl) por nutria en los Arribes del Duero, Salamanca. Bol. Asoc. Herpetol. Esp. 2006, 17, 85–88. [Google Scholar]
- Colgâlniceanu, D.; Márquez, R.; Beltrán, J.F. Impact of otter (Lutra lutra) predation on amphibians in temporary ponds in Southern Spain. Acta Herpetol. 2010, 5, 217–222. [Google Scholar]
- Ludwig, G.X.; Hokka, V.; Sulkava, R.; Ylönen, H. Otter Lutra lutra predation on farmed and free-living salmonids in boreal freshwater habitats. Wild. Biol. 2002, 8, 193–199. [Google Scholar] [CrossRef]
- The Canadian Press. Otter 6, Human 0: Battle Continues to Outs Koi Muncher from Vancouver Garden. Available online: https://ottawa.citynews.ca/2018/11/22/otter-6-humans-0-in-battle-of-wits-to-oust-koi-muncher-from-vancouver-garden/ (accessed on 4 March 2019).
- The Gobal News. Koi Tremble in Fear as Otter Makes Reappearance in Vancouver Chinese Garden. Available online: https://globalnews.ca/news/6118436/otter-returns-vancouver-garden/ (accessed on 20 March 2020).
- The Star. Otters Have Splashing Good Time in Fishponds. Available online: https://www.thestar.com.my/news/regional/2020/03/14/otters-have-splashing-good-time-in-fish-ponds (accessed on 30 March 2020).
- The Wiltshire Times. More Than 20 Fish Were Killed in Otter Raid on the Manor Fishpond at Steele Ashton. Available online: https://www.wiltshiretimes.co.uk/news/18341832.20-fish-killed-otter-raids-manor-fishpond-steeple-ashton/ (accessed on 2 April 2020).
- Shochat, E.; Lerman, S.B.; Anderies, J.M.; Warren, P.S.; Faeth, S.H.; Nilon, C.H. Invasion, competition and biodiversity loss in urban ecosystems. BioScience 2010, 60, 199–208. [Google Scholar] [CrossRef]
- Kruuk, H. Otters. Ecology, Behaviour and Conservation; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Delibes, M.; Ferreras, P.; Blázquez, M.C. Why the eurasian otter (Lutra lutra) leaves a pond? An observational test of some predictions on prey depletion. Rev. Ecol.-Terre Vie 2000, 55, 57–65. [Google Scholar] [CrossRef]
- Kruuk, H.; Wansink, D.; Moorhouse, A. Feeding patches and diving success of otters (Lutra lutra L.) in Shetland. Oikos 1990, 57, 68–72. [Google Scholar] [CrossRef]
- Mougeot, F.; Rodríguez-Ramiro, J. Commensal association of the common kingfisher wit foraging Eurasian otters. Ethology 2019, 125, 965–971. [Google Scholar] [CrossRef]
- Ruiz-Olmo, J.; Margalida, A.; Batet, A. Use of small rich patches by Eurasian otter (Lutra lutra L.) females and cubs during the pre-dispersal period. J. Zool. 2005, 265, 339–346. [Google Scholar] [CrossRef]
- Ruiz-Olmo, J.; Mañas, F.; Olmo-Vidal, J.M.; Batet, A. Breeding of Otters (Lutra lutra L.) in the Wild in the Mediterranean Area. In Otter Conference. The Return of Otters: How and Where? Conroy, J.W.H., Gutleb, A.C., Ruiz-Olmo, J., Yoxon, G.M., Eds.; Skye Island: Scotland, UK, 2003; pp. 1–11. [Google Scholar]
- Ruiz-Olmo, J.; Jiménez, J.; Margalida, A. Capture and consumption of prey of the Otter (Lutra lutra) in Mediterranean freshwater habitats of the Iberian Peninsula. Galemys 1998, 10, 209–226. [Google Scholar]
- Carrs, D.N.; Kruuk, H.; Conroy, J.W.H. Predation on adult Atlantic Salmon, Salmo salar, by otters Lutra lutra within the river Dee system, Aberdeenshire, Scotland. J. Fish Biol. 1990, 37, 935–944. [Google Scholar] [CrossRef]
- Llinares, A.; Martínez-Abraín, A.; Veiga, J. High foraging efficiency of Eurasian otters in a shallow Iberian reservoir. Wild. Biol. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Martínez-Abraín, A.; Santidrián, P.; Veiga, J. Otter diet changes in a reservoir during a severe autumn drought. J. Mammal. 2020, 101, 211–215. [Google Scholar] [CrossRef]
- Díaz-Paniagua, C. Variability in timing of larval season in an amphibian community in SW Spain. Ecography 1992, 15, 267–272. [Google Scholar] [CrossRef]
- Richter-Boix, A.; Llorente, G.A.; Montori, A. Hierarchical competition in pond-breeding anuran larvae in a Mediterranean area. Amphibia-Reptilia 2007, 28, 247–261. [Google Scholar] [CrossRef]
- Díaz-Paniagua, C. Influencia de las características del medio acuático sobre las poblaciones de larvas de anfibios en la Reserva Biológica de Doñana (Huelva, España). Doñana Acta Vertebr. 1983, 10, 41–53. [Google Scholar]
- Díaz-Paniagua, C. Reproductive period of amphibians in the Biological Reserve of Doñana (SW Spain). In Proceedings of the Third Ordinary General Meeting of the Societas Europaea Herpetologica; Rocek, Z., Ed.; Charles University: Prague, Czech Republic, 1986; pp. 429–432. [Google Scholar]
- Morales, J.J.; Ruiz-Olmo, J.; Lizana, M.; Gutiérrez, J. Diferencias en la ocupación por la nutria paleártica (Lutra lutra) de lagunas y embalses de altitud en el centro y norte de la península Ibérica. Galemys 1998, 10, 253–264. [Google Scholar]
- Jiménez, J.; López-Martín, J.M.; Ruiz-Olmo, J.; Delibes, M. ¿Por qué se está recuperando la nutria en España? In La Nutria en España. Veinte años de Seguimiento de un Mamífero Amenazado; López-Martín, J.M., Jiménez, J., Eds.; SECEM: Malaga, Spain, 2008; pp. 273–304. [Google Scholar]
- Duarte, J.; Rodríguez, D.; Rubio, P.; Farfán, M.A. Factores que determinan la presencia de la nutria (Lutra lutra) en zonas urbanas de la Costa del Sol. In Proceedings of the Resúmenes XVI Congreso SECEM, Granollers, Spain, 6–9 December 2023; p. 50. [Google Scholar]
- Fischer, J.D.; Cleeton, S.H.; Lyons, T.P.; Miller, J.R. Urbanization and the predation paradox: The role of trophic dynamics in structuring vertebrate communities. BioScience 2012, 62, 809–818. [Google Scholar] [CrossRef]



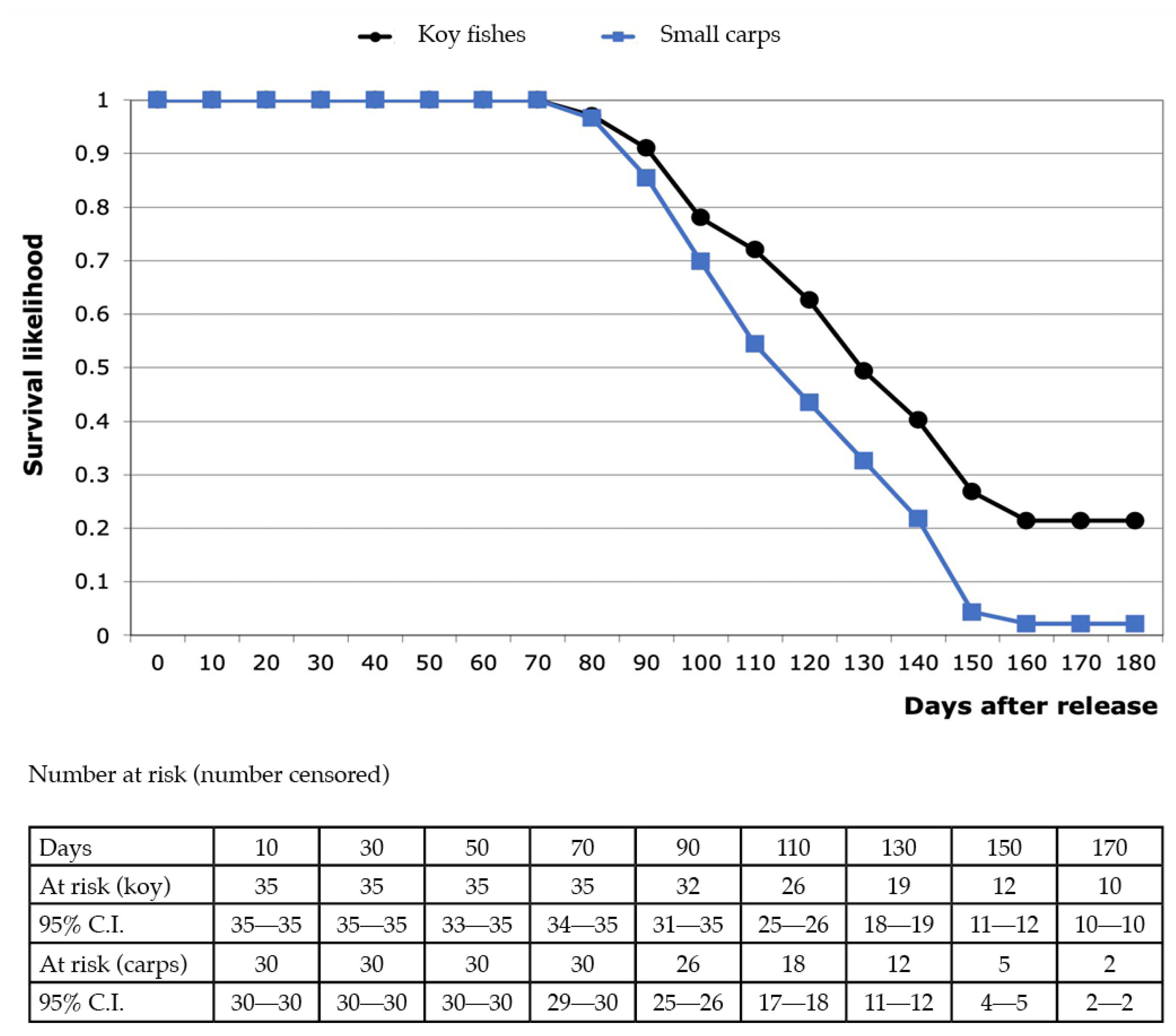


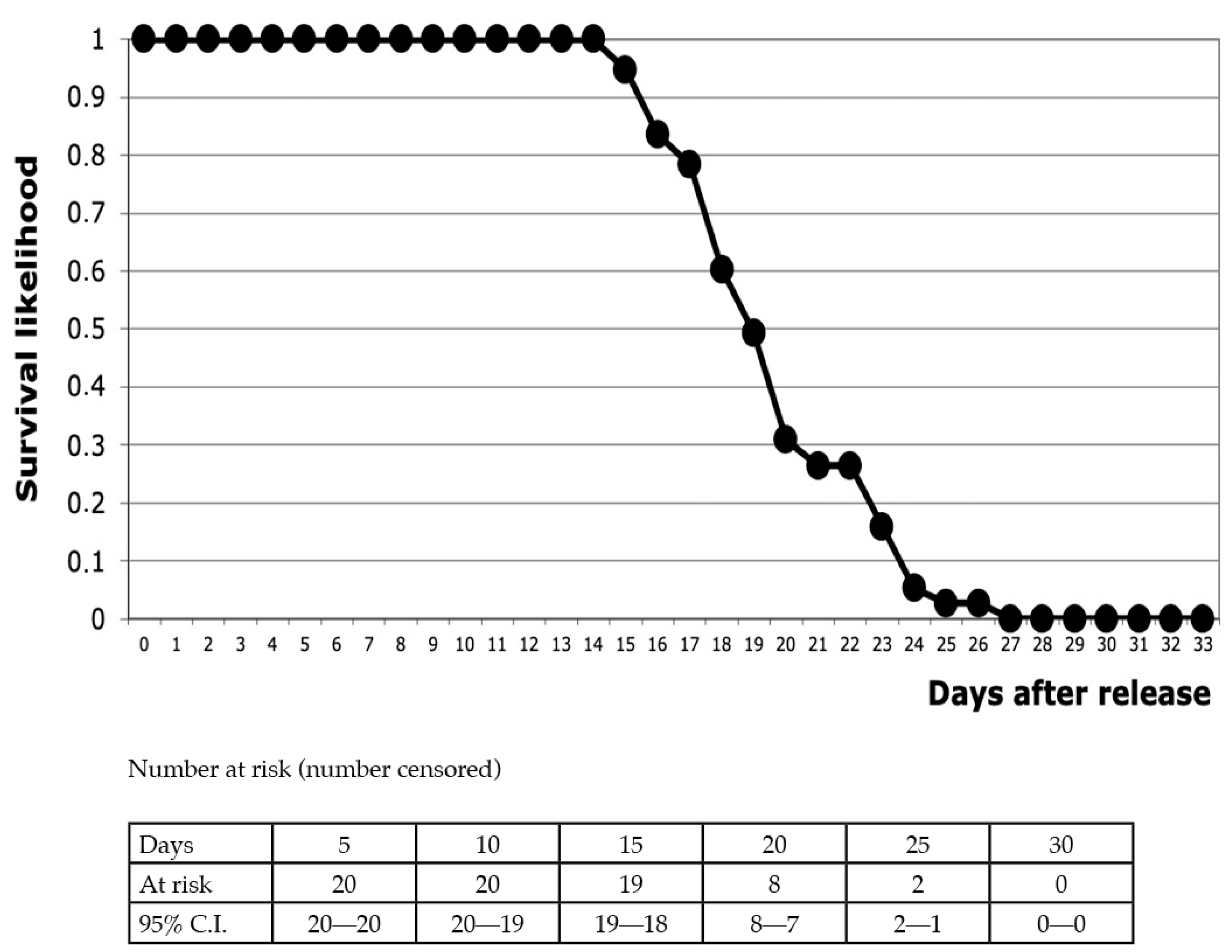
| Urban Pond A (Large) | Urban Pond A (Small) | Urban Pond B | Urban Pond C | Natural Pond Co1 | Natural Pond Co2 | |
|---|---|---|---|---|---|---|
| Location | 31°31′06″ N, 5°02′36″ W | 31°31′06″ N, 5°02′37″ W | 36°31′13″ N, 5°01′25″ W | 36°30′26″ N, 4°57′52″ W | 36°31′53″ N, 5°02′43″ W | 36°31′08″ N, 4°58′35″ W |
| Sampling period | May–Oct 2017 | Apr–Oct 2018 | Mar–May 2018 and 2019 | Jul–Aug 2018 | May–Oct 2018 | May–Oct 2019 |
| Water sheet size (m2) | 300 | 140 | 91 | 60 | 460 | 204 |
| Shore length (m) | 79 | 43 | 96 | 39 | 47 | 32 |
| Depth (m) | 0.5 | 0.5 | 0.9 | 0.3 | <1 | <1 |
| Distance to nearest river basin (m) | 48 (Guadalmina) | 67 (Guadalmina) | 1225 (Guadalmina) | 1480 (Verde) | On the riverbed | 1485 (Guadaiza) |
| Distance to nearest water stream–natural habitat (m) | 55 (Guadalmina ditch) | 75 (Guadalmina ditch) | 190 (La Leche stream) | 60 (Benavolá Stream) | On the riverbed | On the riverbed |
| Spraint abundance (spraints/meter of shore; mean ± SE) | 0.01 ± 0.001 | 0.03 ± 0.003 | Not estimated | Not estimated | 0.03 ± 0.004 | 0.03 ± 0.003 |
| Weekly otter visits (camera trapping) | Not estimated | 0.77 ± 0.22 | Not estimated | Not estimated | Not estimated | 1.25 ± 0.27 |
| Number of Cameras | Camera Sampling Effort (Trapping Days) | Sampling Effort Spraint and Prey Remains (Searching Days) | Main Prey Present | |
|---|---|---|---|---|
| Urban pond A (large) | - | - | 148 | Common carp |
| Urban pond A (small) | 3 | 157 | 157 | Brocaded carp (koi) |
| Urban pond B | - | - | 11 | Iberian water frog |
| Urban pond C | - | - | 33 | Goldfish |
| Natural pond Co1 | - | - | 160 | Andalusian barbel Malaga chub Southern straight-mouth Crayfish |
| Natural pond Co2 | 3 | 153 | 160 | Andalusian barbel Malaga chub Southern straight-mouth Crayfish |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, J.; Rodríguez, D.; Farfán, M.Á. Eurasian Otters’ Urban Pond Use Patterns in Southern Spain: A Case Study. Wild 2025, 2, 46. https://doi.org/10.3390/wild2040046
Duarte J, Rodríguez D, Farfán MÁ. Eurasian Otters’ Urban Pond Use Patterns in Southern Spain: A Case Study. Wild. 2025; 2(4):46. https://doi.org/10.3390/wild2040046
Chicago/Turabian StyleDuarte, Jesús, Diego Rodríguez, and Miguel Ángel Farfán. 2025. "Eurasian Otters’ Urban Pond Use Patterns in Southern Spain: A Case Study" Wild 2, no. 4: 46. https://doi.org/10.3390/wild2040046
APA StyleDuarte, J., Rodríguez, D., & Farfán, M. Á. (2025). Eurasian Otters’ Urban Pond Use Patterns in Southern Spain: A Case Study. Wild, 2(4), 46. https://doi.org/10.3390/wild2040046





