Exploring the Potential Antioxidant, Anti-Inflammatory, and Anticancer Properties of Careya arborea: A Promising Underutilized Source of Natural Therapeutics
Simple Summary
Abstract
1. Introduction—Biological Profile of Careya arborea
2. Role in Traditional Medicine
3. Phytochemical Composition and Bioactive Compounds
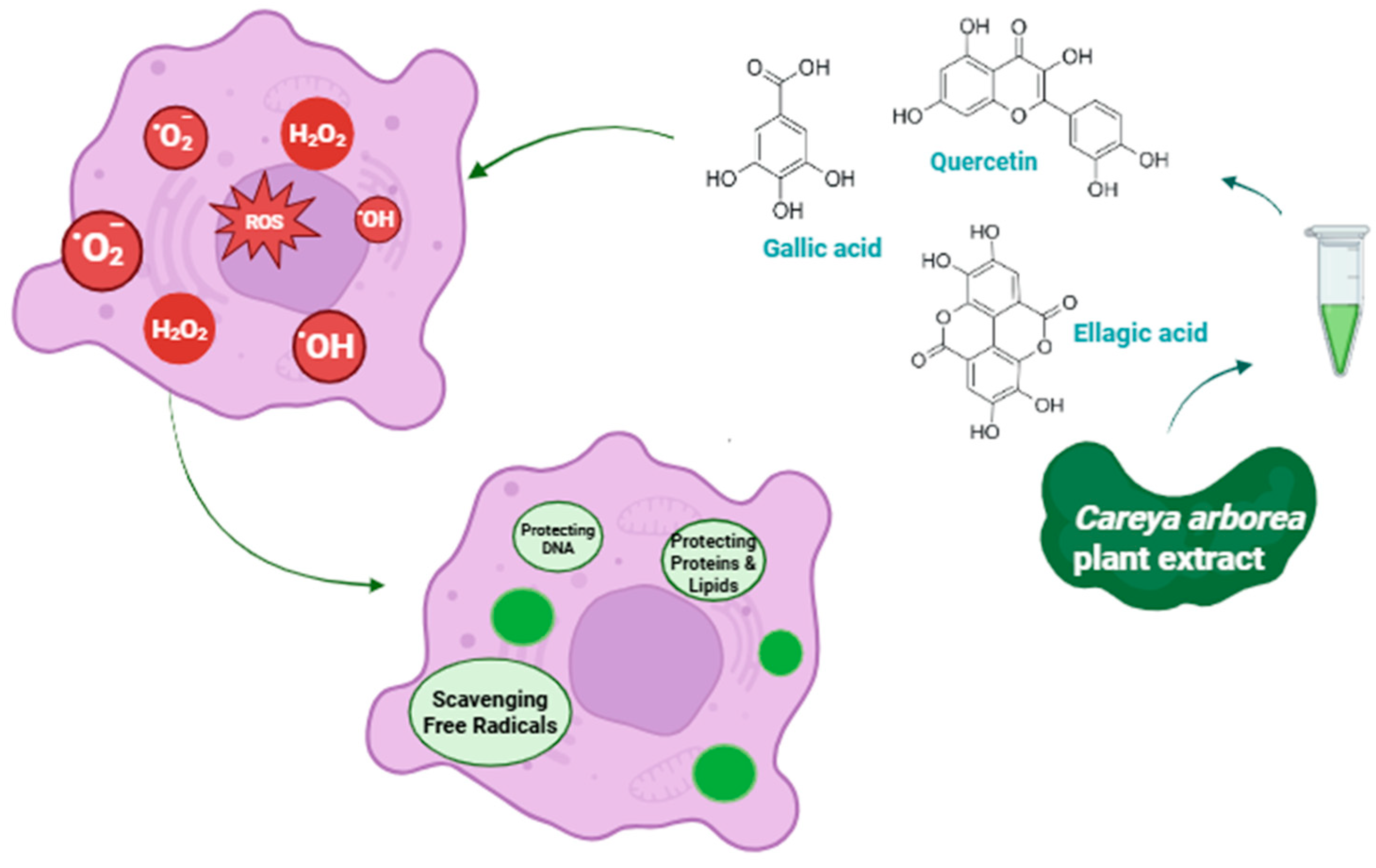
4. Antioxidant Potential of Careya arborea
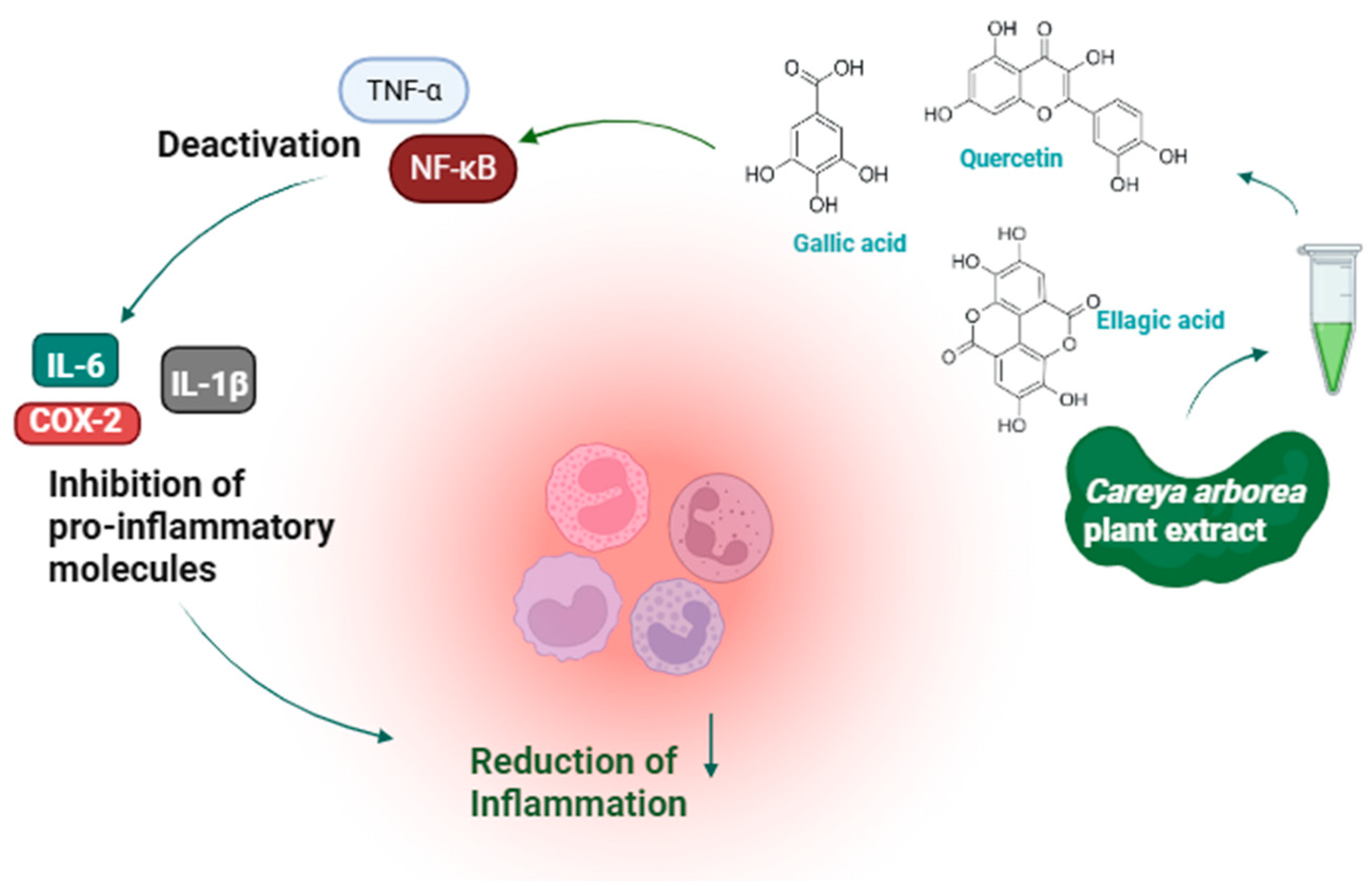
5. Anti-Inflammatory Potential of Careya arborea
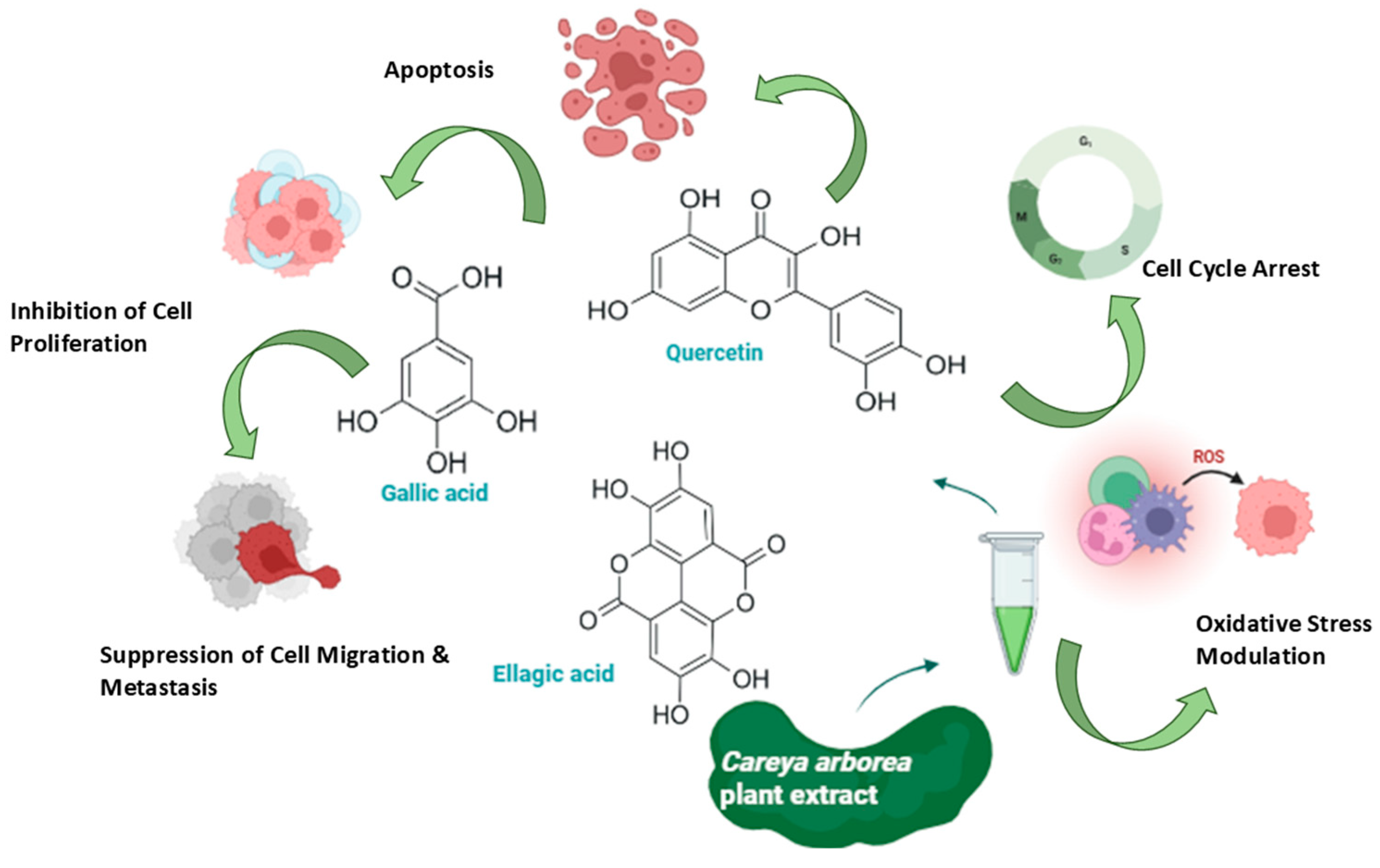
6. Anticancer Potential of Careya arborea
7. Conservation and Sustainable Harvesting of Careya arborea
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, H.D.; Xiao, P.G. Pharmaceutical resource discovery from traditional medicinal plants: Pharmacophylogeny and pharmacophylogenomics. Chin. Herb. Med. 2020, 12, 104–117. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef]
- Nadekar, L.B.; Deshmukhe, P.M.; Sonawane, A.G.; Gujarkar, M.A.; Charde, M.S.; Chakole, R.D. Careya arborea: A review. Int. J. Pharm. Pharm. Res. 2021, 21, 574–586. Available online: https://ijppr.humanjournals.com/careya-arborea-a-review/ (accessed on 10 February 2025).
- Navya, A.S.; Anitha, S. Antimicrobial activities of Careya arborea: A review. J. Pharmacogn. Phytochem. 2018, 7, 3155–3157. Available online: https://www.phytojournal.com/archives/2018.v7.i4.5436/antimicrobial-activities-of-careya-arborea-a-review (accessed on 10 February 2025).
- Ambardar, N.; Aeri, V. A better understanding of traditional uses of Careya arborea Roxb.: Phytochemical and pharmacological review. Tang [Humanit. Med.] 2013, 3, 21–27. [Google Scholar] [CrossRef]
- Kashyp, N.K.; Das, A.K.; Bhardwaj, A.K.; Roymahapatra, G.; Ghosh, A.; Hait, M. Phytochemical analysis of Careya arborea Roxb. root extracts: A qualitative analytical approach. J. Pharm. Res. 2023, 1, 959. [Google Scholar] [CrossRef]
- Abdul Khaliq, H. Pharmacognostic, physicochemical, phytochemical and pharmacological studies on Careya arborea Roxb.: A review. J. Phytopharm. 2016, 5, 27–34. Available online: www.phytopharmajournal.com (accessed on 20 March 2025). [CrossRef]
- Gupta, P.C.; Sharma, N.; Rao, C.V. Pharmacognostic studies of the leaves and stem of Careya arborea Roxb. Asian Pac. J. Trop. Biomed. 2012, 2, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, N.K.; Hait, M.; Roymahapatra, G. Physicochemical investigation of Careya arborea Roxb.: A comparative study. ES Gen. 2024, 1, 1136. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucl. 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Gupta, P.; Patil, D.; Patil, A. Qualitative HPTLC phytochemical profiling of Careya arborea Roxb. bark, leaves and seeds. 3 Biotech 2019, 9, 311. [Google Scholar] [CrossRef]
- Bhandary, M.J.; Chandrashekar, K.R. Medical ethnobotany of the Siddis of Uttara Kannada District, Karnataka, India. J. Ethnopharmacol. 1995, 47, 149–158. [Google Scholar] [CrossRef]
- Girach, R.D.; Aminuddin; Siddiqui, P.A.; Khan, S.A. Traditional plant remedies among the Kondh of District Dhenkanal (Odisha). Pharm. Biol. 1994, 32, 274–283. [Google Scholar]
- Selvanayagam, Z.E.; Gnanavendhan, S.G.; Balakrishna, K.; Bhima Rao, R. Antisnake venom botanicals from ethnomedicine. J. Herbs Spices Med. Plants 1995, 2, 45–100. [Google Scholar] [CrossRef]
- Begum, R.; Sheliya, M.A.; Mir, S.R.; Singh, E.; Sharma, M. Inhibition of proinflammatory mediators by coumaroyl lupendioic acid, a new lupane-type triterpene from Careya arborea, on inflammation-induced animal model. J. Ethnopharmacol. 2017, 206, 376–392. [Google Scholar] [CrossRef]
- Samvatsar, S.; Diwanji, V.B. Plant sources for the treatment of jaundice in the tribals of Western Madhya Pradesh of India. J. Ethnopharmacol. 2000, 73, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Mahishi, P.; Srinivasa, B.H.; Shivanna, M.B. Medicinal plant wealth of local communities in some villages in Shimoga District of Karnataka, India. J. Ethnopharmacol. 2005, 98, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.D.; Thatoi, H.N. Ethnomedicinal practices of Kol tribes in Similipal Biosphere Reserve, Orissa, India. Ethnobot. Leafl. 2009, 13, 379–387. [Google Scholar]
- Parinitha, M.; Harish, G.U.; Vivek, N.C.; Mahesh, T.; Shivanna, M.B. Ethnobotanical wealth of Bhadra Wildlife Sanctuary in Karnataka. Indian J. Tradit. Knowl. 2004, 3, 37–50. [Google Scholar]
- Shiddamallayya, N.; Yasmeen, A.; Gopakumar, K. Medico-botanical survey of Kumar Parvatha, Kukke Subramanya, Mangalore, Karnataka. Indian J. Tradit. Knowl. 2010, 9, 96–99. [Google Scholar]
- Satish, B.G.N.; Vrushabendra, S.B.M.; Kamal, K.G.; Mohan, B.G. Review on Careya arborea Roxb. Int. J. Res. Ayurveda Pharm. 2010, 1, 306–315. [Google Scholar]
- Mohanta, R.K.; Rout, S.D.; Sahu, H.K. Ethnomedicinal plant resources of Similipal Biosphere Reserve, Orissa, India. Zoos’ Print J. 2006, 21, 2372–2374. [Google Scholar] [CrossRef]
- Plant, K.; Area, P.; Lanka, S. Unveiling the hidden treasures: Exploring the ethnobotanical riches of the ‘Kahata’ plant (Careya arborea). In Proceedings of the 9th International Conference of Sabaragamuwa University of Sri Lanka, Colombo, Sri Lanka, 6–8 December 2023. [Google Scholar]
- Panda, S.K.; Rout, S.D.; Mishra, N.; Panda, T. Phytotherapy and traditional knowledge of tribal communities of Mayurbhanj District, Orissa, India. J. Pharmacogn. Phyther. 2011, 3, 101–113. [Google Scholar]
- Bhakuni, D.S.; Goel, A.K.; Jain, S.; Mehrotra, B.N.; Srimal, R.C. Screening of Indian plants for biological activity: Part XIV. Indian J. Exp. Biol. 1990, 28, 619–637. [Google Scholar] [PubMed]
- Navya, A.; Anitha, S. Review on pharmacognostic and pharmacological activities of Careya arborea plant. J. Pharmacogn. Phytochem. 2019, 8, 4165–4169. [Google Scholar]
- Nazriya, N.F.; De Costa, D.M.; Azhaar, A.S. Antioxidant phenolic constituents from the fruits of Careya arborea. J. Nat. Prod. Chem. 2007, 1, 103. [Google Scholar]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Li, K.; Gong, Q.; Lu, B.; Huang, K.; Tong, Y.; Mutsvene, T.E.; Lin, M.; Xu, Z.; Lu, F.; Li, X.; et al. Anti-inflammatory and antioxidative effects of gallic acid on experimental dry eye: In vitro and in vivo studies. Eye Vis. 2023, 10, 17. [Google Scholar] [CrossRef]
- Mandal, D.; Panda, N.; Kumar, S.; Banerjee, S.; Mandal, N.B.; Sahu, N.P. A triterpenoid saponin possessing antileishmanial activity from the leaves of Careya arborea. Phytochemistry 2006, 67, 183–190. [Google Scholar] [CrossRef]
- Lozano-Mena, G.; Sánchez-González, M.; Juan, M.E.; Planas, J.M. Maslinic acid, a natural phytoalexin-type triterpene from olives—A promising nutraceutical? Molecules 2014, 19, 11538–11559. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Garofalo, V.; Calogero, A.E. Anti-dyslipidemic and anti-diabetic properties of corosolic acid: A narrative review. Endocrines 2023, 4, 616–629. [Google Scholar] [CrossRef]
- Tiwari, S.; Gehlot, S. Review on Careya arborea Roxb. J. Biol. Sci. Online Web 2014, 2, 384–387. Available online: http://jbsoweb.com/admin/php/uploads/174_pdf.pdf (accessed on 21 March 2025).
- Szmagara, A.; Krzyszczak-Turczyn, A.; Sadok, I. Fruits of Polish medicinal plants as potential sources of natural antioxidants: Ellagic acid and quercetin. Appl. Sci. 2025, 15, 6094. [Google Scholar] [CrossRef]
- Luo, H.; Cai, Y.; Peng, Z.; Liu, T.; Yang, S. Chemical composition and in vitro evaluation of cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chem. Cent. J. 2014, 8, 1. [Google Scholar] [CrossRef]
- Kiran, A.W.; Chandrakant, S.M. Pharmacognostic profiles of bark of Careya arborea Roxb. J. Pharmacogn. Phyther. 2009, 1, 64–66. [Google Scholar]
- Kashyap, N.K.; Hait, M.; Roymahapatra, G. Proximate and elemental analysis of Careya arborea Roxb. root. ES Food Agrofor. 2022, 7, 41–47. [Google Scholar] [CrossRef]
- Agidew, M.G. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull. Natl. Res. Cent. 2022, 46, 87. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Senthilkumar, R.C.N.; Badami, S.; Cherian, M.M.; Hariharapura, B. Potent in vitro cytotoxic and antioxidant activity of Careya arborea bark extracts. Phytother. Res. 2007, 21, 492–495. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Abeysiriwardhana, H.; Jayasooriya, R. Evaluation of Sri Lankan wild fruits based on free radical scavenging activity, polyphenolic content and cytotoxic activity. Proc. KDU Res. Conf. 2022, 1, 22–29. Available online: http://ir.kdu.ac.lk/handle/345/6191 (accessed on 2 April 2025).
- Kumar, R.S.; Sivakumar, T.; Sundaram, R.S.; Sivakumar, P.; Nethaji, R.; Gupta, M. Antimicrobial and antioxidant activities of Careya arborea Roxb. stem bark. Iran. J. Pharmacol. Ther. 2006, 5, 35–41. [Google Scholar]
- Sadowska-Bartosz, G.; Bartosz, I. Evaluation of the antioxidant capacity of food products: Methods, applications and limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Ramdurga, B.; Jat, R.K.; Badami, S. In vitro cytotoxic and antioxidant activities of Careya arborea root extracts. Int. J. Pharm. Investig. 2021, 11, 127–130. [Google Scholar] [CrossRef]
- Rosenberg, H.F. Inflammation. In Fundamental Immunology, 4th ed.; Paul, W.E., Ed.; Lippincott-Raven: Philadelphia, PA, USA; New York, NY, USA, 1999; pp. 1–20. [Google Scholar]
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Evaluation of anti-inflammatory and anti-proliferation tumoral cell activities of Antrodia camphorata, Cordyceps sinensis, and Cinnamomum osmophloeum bark extracts. J. Ethnopharmacol. 2007, 114, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.U.; Pund, M.M.; Gacche, R.N. Evaluation of anti-inflammatory activity of selected medicinal plants used in Indian traditional medication system in vitro and in vivo. J. Tradit. Complement. Med. 2016, 6, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.N.; Nayak, B.S.; Seth, A.K.; Jalalpure, S.S.; Patel, K.N.; Patel, M.A.; Mishra, A.D. Search for medicinal plants as a source of anti-inflammatory and anti-arthritic agents—A review. Pharmacogn. Mag. 2006, 2, 77–86. [Google Scholar]
- Begum, R.; Sharma, M.; Pillai, K.K.; Aeri, V.; Sheliya, M.A. Inhibitory effect of Careya arborea on inflammatory biomarkers in carrageenan-induced inflammation. Pharm. Biol. 2015, 53, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Sambathkumar, R.; Sivakumar, T.; Sundaram, R.S.; Sivakumar, P.; Nethaji, R.; Vijayabasker, M.; Perumal, P.; Gupta, M.; Mazumdar, U. Anti-inflammatory and analgesic effects of Careya arborea stem bark in experimental animal models. Niger. J. Nat. Prod. Med. 2006, 9, 38–43. [Google Scholar] [CrossRef]
- Limongelli, V.; Bonomi, M.; Marinelli, L.; Gervasio, F.L.; Cavalli, A.; Novellino, E.; Parrinello, M. Molecular basis of cyclooxygenase enzymes (COXs) selective inhibition. Proc. Natl. Acad. Sci. USA 2010, 107, 5411–5416. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yi, S.S.; Yoo, K.-Y.; Park, O.K.; Yan, B.; Kim, I.Y.; Na Kim, Y.; Song, W.; Moon, S.M.; Won, M.-H.; et al. Effects of treadmill exercise on cyclooxygenase-2 in the hippocampus in type 2 diabetic rats: Correlation with neuroblasts. Brain Res. 2010, 1341, 84–92. [Google Scholar] [CrossRef]
- Feng, J.; Lucchinetti, E.; Fischer, G.; Zhu, M.; Zaugg, K.; Schaub, M.C.; Zaugg, M. Cardiac remodelling hinders activation of cyclooxygenase-2, diminishing protection by delayed pharmacological preconditioning: Role of HIF1α and CREB. Cardiovasc. Res. 2008, 78, 98–107. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Nature: A vital source of leads for anticancer drug development. Curr. Opin. Pharmacol. 2009, 9, 313–331. [Google Scholar]
- Balachandran, P.; Govindarajan, R. Cancer—An Ayurvedic perspective. Pharmacol. Res. 2005, 51, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Badami, S.; Dongre, S.H.; Godavarthi, A. Antitumor activity and antioxidant status of the methanol extract of Careya arborea bark against Dalton’s lymphoma ascites-induced tumor in mice. J. Pharmacol. Sci. 2007, 103, 12–23. [Google Scholar] [CrossRef]
- Kumar, R.S.; Sivakumar, T.; Senthil, V.; Murthy, N.V.; Balasubramaniam, V.; Sabi, R.K.; Sundram, R.S.; Perumal, P.; Mazumder, U.K.; Gupta, M. Antitumor effect of Careya arborea against Ehrlich ascites carcinoma in Swiss albino mice. Orient. Pharm. Exp. Med. 2008, 8, 154–163. [Google Scholar] [CrossRef]
- Buranrat, B.; Boontha, S.; Temkitthawon, P.; Chomchalao, P. Anticancer activities of Careya arborea Roxb. on MCF-7 human breast cancer cells. Biologia 2020, 75, 2359–2366. [Google Scholar] [CrossRef]
- Musgrove, E.A.; Caldon, C.E.; Barraclough, J.; Stone, A.; Sutherland, R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 2011, 11, 558–572. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. P21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Abu-Qare, A.W.; Abou-Donia, M.B. Biomarkers of apoptosis: Release of cytochrome c, activation of caspase-3, induction of 8-hydroxy-2′-deoxyguanosine, increased 3-nitrotyrosine, and alteration of p53 gene. J. Toxicol. Environ. Health B Crit. Rev. 2001, 4, 313–332. [Google Scholar] [CrossRef]
- Wadje Shailaja, D.; Wankhede Balaji, G.; Kalambkar Mahesh, R. Identification of bioactive compounds and cytotoxic activity of Careya arborea Roxb. leaves. J. Pharmacogn. Phytochem. 2019, 8, 362–365. [Google Scholar]
- Nair, V.K.R.S.; Snima, K.S.; Kamath, R.C.; Nair, S.V.; Lakshmanan, R. Synthesis and characterization of Careya arborea nanoparticles for assessing its in vitro efficacy in pancreatic cancer cells. J. Nat. Prod. 2015, 8, 9–15. [Google Scholar]
- Jayaweera, C.L. Gaps in in-situ and ex-situ conservation of threatened medicinal plant species of Sri Lanka: Towards their effective conservation. Int. J. Biodivers. Conserv. 2024, 1, 1–20. [Google Scholar]
- Rajakaruna, R.W.M.T.N.; Yakandawala, D.M.D.; Jayasuriya, K.M.G.G. Preserving Sri Lanka’s indigenous healing heritage: An updated checklist of medicinal plants and conservation priorities. Wild 2025, 54, 65–212. [Google Scholar]
- Gunatilleke, N.; Pethiyagoda, R.; Gunatilleke, S. Biodiversity of Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2008, 36, 25–61. [Google Scholar] [CrossRef]

| Treated Pathologies | Plant Part | References |
|---|---|---|
| Tumors, bronchitis, epileptic fits, diarrhea, dysentery (bloody stools), ear pain, hemorrhoids, intestinal sores, bedsores, vata and kapha imbalances (Ayurvedic), jaundice (bathing postpartum women), coughs and colds, scorpion stings, leech repellent | Bark/Stem | [7,17] |
| Ulcers, wounds (poultice), skin diseases, rashes | Leaves | [22] |
| Skin diseases, rashes, infertility (paste with Terminalia chebula and Emblica officinalis with ghee, consumed on an empty stomach), coughs and cold (with persistent calyx and bark juice) | Flowers | [18] |
| Wound healing (poultice), coughs and colds, infertility (combined with flowers as above) | Fruits/Pulp | [8,18,22] |
| Tuberculosis, skeletal fractures, blood dysentery (when mixed with Indigofera cassioides juice) | Root | [6,25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sewwandi, P.A.; Kugaseelan, S.; Virajini, M.P.T.; Samarakoon, K.W.; Jayasooriya, P.T.; Kuruppu, A.I. Exploring the Potential Antioxidant, Anti-Inflammatory, and Anticancer Properties of Careya arborea: A Promising Underutilized Source of Natural Therapeutics. Wild 2025, 2, 44. https://doi.org/10.3390/wild2040044
Sewwandi PA, Kugaseelan S, Virajini MPT, Samarakoon KW, Jayasooriya PT, Kuruppu AI. Exploring the Potential Antioxidant, Anti-Inflammatory, and Anticancer Properties of Careya arborea: A Promising Underutilized Source of Natural Therapeutics. Wild. 2025; 2(4):44. https://doi.org/10.3390/wild2040044
Chicago/Turabian StyleSewwandi, P. Aruni, Seenuga Kugaseelan, M. P. Theja Virajini, Kalpa W. Samarakoon, Prasad T. Jayasooriya, and Anchala I. Kuruppu. 2025. "Exploring the Potential Antioxidant, Anti-Inflammatory, and Anticancer Properties of Careya arborea: A Promising Underutilized Source of Natural Therapeutics" Wild 2, no. 4: 44. https://doi.org/10.3390/wild2040044
APA StyleSewwandi, P. A., Kugaseelan, S., Virajini, M. P. T., Samarakoon, K. W., Jayasooriya, P. T., & Kuruppu, A. I. (2025). Exploring the Potential Antioxidant, Anti-Inflammatory, and Anticancer Properties of Careya arborea: A Promising Underutilized Source of Natural Therapeutics. Wild, 2(4), 44. https://doi.org/10.3390/wild2040044






