Abstract
Napier grass (Cenchrus purpureus Schumach) is an important forage crop and livestock feed. However, its yield and quality in Kenya are often limited by Napier grass headsmut and stunt disease. Napier grass genetic improvements through mutation breeding and selection could avail cultivars with increased forage. This study investigated the response of embryogenic calli to different levels of colchicine in inducing polyploidy in the two germplasms of Napier grass; South africa and Bana grass. The experiments were carried out as a factorial experiment in a completely randomized design (CRD). The colchicine concentrations used were 0, 0.05, 0.1, and 0.2%, and the exposure durations were 24, 48, and 72 h. During the shoot regeneration stage, culturing explants on an MS medium (Murashige and Skoog) supplemented with 0.2 mg L−1 Benzyl Adenine (BAP), 0.1 mg L−1 dichlorophenoxyacetic acid (2, 4-D), and 0.1 mg L−1 indole-3-butyric acid (IBA) was more suitable for shoot regeneration. Chromosome doubling was confirmed by genomic DNA and the stomata size and number. Culturing explants on an MS medium supplemented with 1 mg L−1 IBA, 1 mg L−1 2, 4-D, and 0.5 mg L−1 BAP was more suitable in inducing embryogenic calli in both genotypes. Polyploidy results revealed that a 0.1% concentration of colchicine with two days of treatment established the maximum number of octoploid plantlets induced in vitro, while a 0.2% concentration was very toxic. The stomata size and number of derived octoploid plantlets were bigger with a lower density, a shorter plant height, and a smaller stem diameter, and despite being the first to produce tillers, they were significantly higher than their progenitors. Induced mutants also had a significantly higher number of chromosomes and showed different band patterns and distances during gel electrophoresis. However, we recommend the use of flow cytometry to confirm the ploidy level. The superior mutant plantlets can be selected and recommended for characterization across representative agro-ecologies for large-scale production and used in Cenchrus purpureus breeding programs in Kenya and its environments.
1. Introduction
Kenya is one of Africa’s leading livestock producers, with smallholder farmers contributing about 80% of its beef and 50% of its dairy production, while the remainder comes from ranchers and larger dairy farms [1]. Annually, Kenya produces approximately 3.43 billion liters of milk, with an average yield of 2850 L per lactating cow, translating to 7.9 L per day [2]. Like many Eastern African countries, 80% of Kenya’s farmers practice mixed crop–livestock systems, relying primarily on rain-fed agriculture [3]. Enhancing and intensifying smallholder dairy development is a viable poverty-alleviation strategy, providing year-round income compared to seasonal crop income. Forage feed accounts for approximately 70% of the total cost of milk production [4]. However, the primary constraint in smallholder dairy farming is year-round availability and quality of forage. Key challenges to fodder production include competition for small land areas, recurrent droughts due to climate change, and low yields [4].
Napier grass (Cenchrus purpureus) is the primary livestock feed for dairy and beef cattle in Kenya, particularly in zero-grazing and semi-intensive systems [5,6]. Compared to other grasses, Napier grass has a higher leaf-to-stem ratio (3.18), better nutritional value, and can withstand repeated cutting, yielding 15 to 22 tons of dry matter per hectare [7,8,9]. As small-scale dairy farming shifts from extensive to zero-grazing systems, Napier grass constitutes up to 80% of forage [10]. Farmers with surplus fodder often sell it to others, making Napier grass a valuable income source [11]. However, the continued contribution of Napier grass to the livelihoods of small-scale farmers is threatened by low growth vigor, low biomass, and low feed value due to inferior germplasm [12]. Therefore, there is a need to enhance the genetic diversity of pasture and forage to meet growing demand, and this is a key strategy for improving food crops and enhancing plant performance [13]. Induced mutation breeding results in changes in the gene dosage, which causes chromosomal rearrangements, epigenetic remodeling, and the reunion of divergent gene regulation [14]. It is a widely adopted method in breeding programs aimed at generating genetic diversity and selecting new mutants with favorable agronomic traits like drought tolerance, tolerance to pests and diseases, increased feed-value maturity time, larger organs, and increased biomass [15]. Mutation can be induced by exposing propagules to physical (gamma irradiation, X-ray, etc.) or chemical mutagens (colchicine, oryzalin, or trifluralin) that induce polyploidy in plants [16].
Colchicine has been documented to induce a higher frequency of point mutations compared to physical mutagens like gamma rays [17]. However, the most critical factor in inducing point mutations is selecting the optimal dosage of the mutagen, which involves determining the appropriate concentration and treatment duration. Inducing polyploidy in Napier grass and selecting mutant plantlets with good agronomic traits is a promising approach to improve its productivity and resilience for use in improving livestock productivity. In light of the above background, this study aimed to investigate the response of embryogenic calli to different colchicine concentrations in inducing polyploidy for regeneration and the selection of novel Napier grass mutants.
2. Materials and Methods
2.1. Experimental Site and Plant Materials
This study was conducted at the Non-Ruminant Research Institute of Kenya Agricultural and Livestock Organization (KALRO) in collaboration with Masinde Muliro University of Science and Technology (MMUST), located in Kakamega County, Kenya. The study was carried out in three phases: callus induction, colchicine treatment and regeneration, and field evaluation. South africa Napier grass (tolerant to Napier stunt disease) was sourced from Kenya Agricultural and Livestock Organization (KALRO), Kakamega, while Bana grass (high-yielding but susceptible to Napier stunt disease) was sourced from the Food Crops Research Institute of KARLO Kitale. All these genotypes are tetraploids (2n = 4x = 28). Shoot tips from the two genotypes were collected from the greenhouse and washed thrice with running tap water and then surface-sterilized by immersing in 70% ethanol for 2 min. This was followed by immersion in 2% sodium hypochlorite solution with 2 drops of tween 20 and agitation for 15 min. The shoot tillers were rinsed three times with sterile distilled water for 6 min.
2.2. Experimental Design, Treatments, and Layout
The experiment was conducted as a factorial with three factors: four levels of colchicine (Powder from Alphatma, Moscow, Russia) concentration (C0 (0%), C1 (0.05%), C3 (0.2%), and C3 (0.2%)), three levels of exposure period (T1: 24 h, T2: 48 h, and T3: 72 h), and two Napier grass germplasms (V1 South africa Napier grass and V2 Bana grass), resulting in twenty-four treatment combinations that were replicated thrice using a factorial completely randomized design (CRD), resulting in ninety-six experimental units. However, a pre-experiment needed to be conducted to carry out somatic embryogenesis to come up with explants to be used in the next stage of the study, which was colchicine treatment and acclimatization in the greenhouse. This pre-experiment was conducted as a factorial experiment in a completely randomized design (CRD) with 3 factors: 3 growth hormones for callus induction, 3 growth hormones for shoot regeneration and rooting, and 2 germplasms of Napier grass. This resulted in eighteen treatment combinations that were replicated six times. This resulted in 108 experimental units, where the best explants that formed embryogenic calli were selected for the next stage of the study. The following callus induction medium was used: GM0 as a comparative control in hormone-free media, GM1 (MS media supplemented with 0.3 mg/L−1 BAP, 0.5 mg/L−1 2,4-D, and 0.5 mg/L−1 IBA) and GM2 (MS media supplemented with 0.5 mg/L−1 BAP, 1.0 mg/L−1 2,4-D, and 1.0 mg/L−1 IBA). For shoot regeneration, the following media were used: SRM0 as a comparative control in a hormone-free medium, SRM1 (MS media supplemented with 1 mg/L−1 BAP, 0.25 mg/L−1 2,4-D, and 0.25 mg/L−1 IBA (Protist Lab Africa Ltd., Mfangano Street, Nairobi, Kenya), and SRM2 (MS media supplemented with 2 mg/L−1 BAP, 0.5 mg/L−1 2,4-D, and 0.5 mg/L−1 IBA).
2.2.1. Embryogenic Calli Formation
Shoot tips of explants were prepared by trimming them into 1–5 mm cross-sections using a sterile scalpel. The cross-sections were then pre-soaked in a solution containing 10 mg/L citric acid and ascorbic acid to prevent browning. These sections were cultured in petri dishes on Murashige and Skoog (MS) medium, supplemented with 0, 0.5, and 1.0 mg/L dichlorophenoxyacetic acid (2,4-D), BAP (0, 0.3, and 0.5 mg/L), and 0, 0.5, and 1.0 mg/L indole-3-butyric acid (IBA) for callus induction. The pH of the induction medium was adjusted to 5.8 using 1 M HCl (Protist Lab Africa Ltd., Mfangano Street, Nairobi, Kenya), or NaOH (Protist Lab Africa Ltd., Mfangano Street, Nairobi, Kenya), before autoclaving at 121 °C for 15 min. The cultures were incubated in the dark at 27 ± 2 °C using a modified old model oven fitted with a clock timer and a fluorescent bulb, calibrated to the required standards to function as a growth chamber. Statistical analyses were performed based on a completely randomized design with three replications using the R software, version 4.2.
2.2.2. Shoot Regeneration and Rooting
In order to induce shooting, the best plant growth hormone combination for the growth of shoot tips was determined by transferring a subset of embryogenic calli into shoot regeneration media consisting of MS supplemented with various concentrations of BAP (0, 1, and 2 mg/L), 2,4-D (0, 0.25, and 0.05 mg/L), and IBA (0, 0.25, and 0.05 mg/L). The pH of all media was adjusted to 5.6–5.8 before autoclaving. The cultures were incubated in a modified old model oven fitted with a clock timer and a fluorescent bulb, which was calibrated to act as a growth chamber. This setup provided LED lighting with a 16 h light and 8 h dark photoperiod at 27 ± 2 °C, and the samples were cultured for two weeks before being treated with colchicine.
2.2.3. Treatment with Colchicine
The impact of various colchicine concentrations (0, 0.05, 0.1, and 0.2%) and the treatment duration (24, 48, and 72 h) on explant survival and polyploidy induction was examined as a factorial experiment in a completely randomized design with three replications. The explants were exposed to a treatment after 134 days of culture by immersing them in a filtered–sterilized colchicine solution for the designated times, as stated earlier, and the samples were then rinsed three times with sterile distilled water. After colchicine treatment, explants were transferred to shoot regeneration media as stated earlier and subcultured after one week in the same shooting media of MS supplemented with 1 mg/L NAA (Protist Lab Africa Ltd., Mfangano Street, Nairobi, Kenya), and 150 mg/L ascorbic acid (Protist Lab Africa Ltd., Mfangano Street, Nairobi, Kenya), for 14 days for rooting before being potted in sterilized soil and transferred into the greenhouse.
2.3. Evaluation of Induced Mutants to Determine Ploidy Level
Two months after transferring them to the greenhouse after treatment with colchicine, plants that regenerated were subjected to screening for confirmation of polyploidy by measuring stomata number and size, chromosome counting, and genomic DNA of mutant plants viz their progenitors.
2.3.1. Chromosome Number by Karyotyping
Karyotyping was conducted to determine the chromosome number by counting chromosomes from young growing leaves of putative induced mutant plants. Plantlet leaf samples of about 2 cm2 were collected from the greenhouse and fixed by immersing leaf tissues in a 1% chromic acid and 10% formaldehyde solution for 24 h at 4 °C. The segments of leaf were then softened by immersing them in sodium hydroxide (1 N) (Protist Lab Africa Ltd., Mfangano Street, Nairobi, Kenya), for 10 min at 60 °C and stained with 4%aceto-carmine (Protist Lab Africa Ltd., Mfangano Street, Nairobi, Kenya), for 10 h, and they were observed using a light microscope at ×80 and ×100. Photographs of chromosome spread were taken using AmScope MU1000 10MP USB 3.0 digital microscope camera manufactured by AmScope, Irvine City in California, USA, and chromosomes were analyzed based on their size, shape, and banding patterns before being counted at the clearest preparation.
2.3.2. Stomata Size and Number
Ploidy was assessed by examining the stomata cell number and size of the stomata, genomic DNA, and chromosome counts. For stomata measurements, 35-day-old leaves that were about 1 cm2 in size were collected from the greenhouse after 2 months from regeneration. An area of approximately 0.2 cm2 on the upper leaf epidermis was treated with a colorless nail polish (Protist Lab Africa Ltd., Mfangano Street, Nairobi, Kenya),. Once the polish dried, the layer was peeled off using adhesive tape. The tape was then affixed to a clean and clear glass slide, and the stomata density per mm2 was counted with a light microscope at ×10, ×20, and ×40. Photographs of stomata were taken using a digital camera. Stomata of length 20% or bigger than their counterpart were considered as putative polyploids [18].
2.3.3. Genomic DNA
For the genomic DNA, plant genomic DNA was extracted from leaf tissue (0.3–0.7 g) of rooted plantlets using the CTAB procedure (Murray & Thompson, 1980) [19]. The DNA was SacI-digested, separated on a 0.8% agarose gel, and transferred to a prepared agarose gel that was prepared by dissolving 0.8% agarose in electrophoresis buffer by heating, and the melted agarose was poured into a casting tray with a comp to create wells and allowed to solidify. Then, the prepared DNA samples and the DNA ladder (5 KB) were carefully loaded into the wells while using a loading dye to visualize the samples during loading. Then, the prepared gel was placed in the electrophoresis apparatus, which ran at 110 V and 150 A for a duration of 90 min. The gel products were stained and visualized on FUJI X-ray film.
2.3.4. Phenotypic Evaluations of Induced Mutants
Quantitative evaluations of phenotypes of mutant plant lines were performed in the greenhouse.
Due to high mortality as a result of the toxicity of colchicine on the explants, 30 plantlets comprising putative induced mutants and their progenitors were planted in 6 × 9 pots and transferred to the greenhouse on 20 April 2024. A completely randomized design with three replications was implemented, and morphological characteristics were collected weekly. These included the number of tillers, plant height, leaf area, and stem diameter. Stem diameter was measured at 10 cm from the base of the mature plants using a string, and then the measurements were transferred to a measuring ruler. Plant height was measured from the ground to the highest point of a mature plant using a measuring tape in (cm). Tillers were counted manually on a weekly basis and recorded.
3. Results
3.1. Response of Genotype to Tissue Culture
Analysis of variance revealed significant genotype effects for the percentage of explants with calli at 4 weeks after culture initiation, necrosis intensity, and number of embryogenic calli at p < 0.05 (Table 1). All genotypes produced the most calli 4 weeks after culture initiation (Table 1). There were no significant differences (p < 0.01) among germplasms during the 4 weeks. Consequently, no germplasm produced embryogenic calli 4 weeks after culture initiation. There were higher levels of necrosis in the South africa Napier grass germplasm, while Bana grass recorded the lowest. Ideally, the Bana grass germplasm recorded highest levels of contamination, while South africa Napier grass had the lowest.

Table 1.
Effects of different growth hormone combinations on embryogenic calli induction.
After 7 to 8 weeks of culture, embryogenic calli on all germplasms were initiated in most media, where it was evident that embryogenic calli were significantly affected by the different plant growth hormonal combinations (Table 1). The percentage of embryogenic calli after 8 weeks was 68.9% and 66.2% on germplasms V1 and V2 in media with 1.0 mg L−1 IBA, 1.0 mg L−1 2,4-D, and 0.5 mg L−1 BAP, while genotypes V1 and V2 in media with 0.5 mg L−1 IBA, 0.5 mg L−1 2,4-D, and 0.3 mg L−1 BAP produced 31.1% and 33.8% calli. Explants cultured in hormone-free media did not produce any sign of growth of calli or embryogenic calli, and they were also 100% necrotic. South africa Napier had the highest levels of necrosis after 4 weeks at 14.9% and 8 weeks at 12.2%, while Bana grass had the lowest levels of necrosis after 4 weeks at 9% and 8 weeks at 4% (Table 1).
3.2. Regeneration of Shoots and Rooting
Growth of shoots was initiated after 134 days of culture in most media (Table 2). The regeneration percentage was significantly affected by the different growth hormonal regulator combinations. Explants that were cultured in hormone-free media did not show any sign of growth and were affected by necrosis at 100%. There was 43% and 40% shooting in the MS medium supplemented with 0.05 mg L−1 IBA, 0.05 mg L−1 2,4-D, and 2 mg L−1 BAP, which was the most suitable media for shoot regeneration, while in the medium with 0.25 mg L−1 IBA, 0.25 mg L−1 2,4-D, and 1 mg L−1 BAP, the level was 20% (Table 2, Figure 1).

Table 2.
Growth hormone combination effects on shoot induction derived from shoot tillers of two genotypes of Cenchrus purpureus.

Figure 1.
Regeneration of two genotypes of Cenchrus purpureus through somatic embryogenesis. (a) Embryogenic calli in MS media supplemented with 0.5 mg/L−1 BAP, 1.0 mg/L−1 2,4-D, and 1.0 mg/L−1 IBA. (b) Sprouting embryos in different growth stages in calli treated with colchicine. (c) Shoot regeneration and root induction with MS medium supplemented with 0.05 mg L−1 IBA, 0.05 mg L−1 2,4-D, and 2 mg L−1 BAP and after being transferred into media supplemented with NAA 1 mg L−1 and 150 mg L−1 ascorbic acid. (d) Regenerated synthetic induced mutants with their progenitors after being potted and transferred into the greenhouse.
3.3. Effect of Different Colchicine Concentrations on Survival of Explants and Ploidy Induction
The explants from the two germplasms were treated with different colchicine concentrations in solid media. The percentage (%) survival rate differed depending on the colchicine concentration levels, exposure duration, and their interactions, with the callus survival ranging from 0–100% (Figure 2). Toxicity was observed at higher concentrations and with longer times of exposure to treatments, which severely affected the survival rate of the calli. Importantly, it was also observed through analysis of variance that there was a high level of significance in terms of the effects of the colchicine concentration and the time of exposure and their interaction on polyploidy induction. The frequency of induction of octoploidy in synthetic polyploidy plantlets increased with the increase in exposure time (Figure 2). A colchicine concentration of 0.05% and an exposure duration of 24 h achieved the highest survival rate (35%) but led to a low number of induced mutants of 17 (48%), presumably due to a lack of any effect of colchicine, while the treatment with 0.2% with durations of 48 and 72 h resulted in 100% mortality of the calli, and at the same time, it resulted in an altered ploidy of 5 (7%) plants that regenerated under the 24 h treatment duration (Figure 2). The results also demonstrated that a colchicine concentration of 0.1% with a 48 h duration of exposure was the most suitable for inducing polyploidy, with 12 (48%) plants (Figure 2). It was observed that the growth and induction of roots in mutant plants were severely affected by the colchicine treatment viz the control. Furthermore, induced mutant plants were shorter than their progenitors, despite being the first to produce tillers, and they also had a high number of tillers and a smaller stem diameter (Table 3, Figure 3). Leaf samples of synthetic mutant lines per germplasm were randomly selected for genomic DNA extraction and chromosome counting. It was evident that chromosome doubling indeed took place in some of the regenerated plantlets that were distinct from there progenitors (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7). The average genomic DNA size of the regenerated plants was observed to be significantly different, where single cell lines from the samples collected were characterized with the same band pattern (Figure 7).
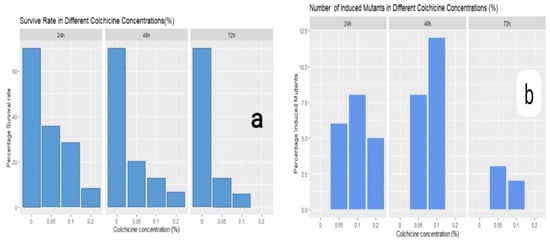
Figure 2.
Percentage explant survival rate and synthetic induced mutants after exposure to different colchicine levels and time durations. (a) Survival rate of explants after exposure to colchicine: C0 = 0, C1 = 0.05, C2 = 0.1, and C2 = 0.2%, and exposure duration: 24 hrs, 48 hrs, and 72 hrs. (b) Number of mutants induced in different colchicine concentrations.

Table 3.
Quantitative and qualitative characteristics in Cenchrus purpureus induced mutants.

Figure 3.
Tillering ability of two Cenchrus purpureus germplasms and their synthetic mutants after 4 weeks: (A1) V2 synthetic mutant, (B1) V2 progenitor, (C1) V1 synthetic mutant, and (D1) V1 progenitor; after 8 weeks: (A2) V2 synthetic mutant, (B2) V2 progenitor, (C2) V1 synthetic mutant, and (D2) V1 progenitor.
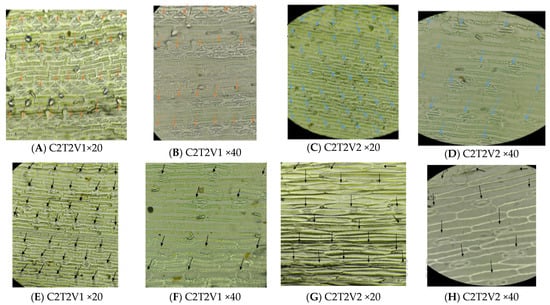
Figure 4.
Difference in stomata size and number between synthetic induced mutants and their progenitors at ×20 and ×40 magnification. Blue and orange arrows in (A–D) point to progenitor stomata, while black arrows in (E–H) point to putative induced mutants.
Figure 5.
Abnormalities shown by synthetic polyploidy after treatment with colchicine. (A–C) Albino plants and leaf chlorosis abnormalities two weeks after treatment with 0.1% colchicine concentration and 48 h duration of exposure and 0.2% colchicine concentration with 24 h and 48 h duration of exposure. (E–H) Abnormalities several weeks after establishment. (E) Chromosome incompatibility that resulting in the death of the plant. (D,F) Leaf chlorosis of new whole leaf that is dying-off. (G,H) New while leaf emerging with vigor immediately before dying-off.

Figure 6.
Chromosome number of Cenchrus purpureus visualized under a light microscope at ×100; (a) COTOV1 progenitor (2n = 4× = 28), (b) C2T2V1 synthetic induced mutant (2n = 8× = 56), (c) COTOV2 progenitor (2n = 4× = 28), and (d) C2T2V2 synthetic induced mutant (2n = 8× = 56). Measurements of some chromosomes numbers were diverse among counts, possibly due to overlaying chromosomes.

Figure 7.
Genomic DNA of induced mutants and their progenitors during gel electrophoresis. Shift in band intensity/mobility shows genomic change due to polyploidy, indicating new bands/new alleles. Well L is the ladder, wells 1, 2, 6, 7, 10, and 11 represent V1 putative mutants, while wells 3, 5, 8, and 9 represent V1 progenitors. On the other hand, wells 12, 15, 18, 19, and 21 represent V2 putative mutants, while 13, 14, 16, 17, 1, and 20 represent V2 progenitors. Progenitors have lower-molecular-weight bands (28 chromosomes) viz the mutants, making them travel farther from the well and appear slightly lower on the gel. On the other hand, putative mutants have larger-molecular-weight bands (56 chromosomes), making them travel much slower and travel a shorter distance from the well.
3.4. Phenotypic Effect on Leaf Characteristics
The stomata size and number of induced mutants were significantly different from their progenitors. Mutants exhibited larger stomata, with a stomatal length of 20 or more (>120 um) than their progenitors; however, they had a lower stomatal density (Table 3, Figure 4). Similarly, analysis of variance detected high significance in terms of tillering ability after 6 weeks of establishment in the greenhouse, where induced mutants were the first to produce tillers, but after 8 weeks, there was a high significance in terms of the number of tillers between induced mutants and their progenitors (Table 3, Figure 3). Induced plants had a slow growth rate as a result of chromosome abbreviation (deletion, duplication, inversion, and translocation) and physiological and toxic effects, which presumably reduced cell survival (Table 3, Figure 3). The mean stem diameter of the synthetic induced mutants was slightly smaller compared to their progenitors (Table 3, Figure 3).
4. Discussion
Synthetic mutant plants were produced from two Cenchrus purpureus germplasms, South africa and Bana grass, representing the first artificial report of octoploid plants from shoot tips of a tetraploid Cenchrus purpureus. For the purpose of in vitro polyploidy induction by treating shoot tillers with colchicine, it was crucial to choose an appropriate regenerating medium. Two different Cenchrus germplasms were used as explants to produce embryogenic calli through tissue culture using different media compositions with different plant growth hormones. In the present study, culturing using shoot tillers was preferred because they are less affected by contamination after various sterilization processes [20].
In the present study, MS medium supplemented with 1.0 mg L−1 IBA, 1.0 mg L−1 2,4-D, and 0.5 mg L−1 BAP was ideal and most suitable for the initiation of embryogenic calli after 8 weeks (Table 1, Figure 1). A previous study by [21,22] emphasized the use of BAP or TDZ, which played a significant role in cereal and grass species in terms of shoot regeneration. This demonstrates that low levels of 2,4-D and IBA in combination with BAP are ideal and most suitable for shoot regeneration in Cenchrus species [20,21]. Napier grass regeneration from shoot tillers is influenced by its germplasm, initiation media, colchicine concentration, and mutagen exposure period [13], which have advanced effects on the initiation of calli and, thereafter, embryogenic calli formation. In the present study, the interaction between the growth media and explants was significant, possibly as a result of gene expression and alteration, which solely depend on physiological stages [23].
A study conducted by [22], made the observation that there was the presence of green and white spots that were frequently seen on the surfaces of calli during callus induction with high regeneration ability. The use of auxin alone or in combination with cytokinins has been shown to play a vital role in organogenesis and somatic embryogenesis during critical periods of cell division in primordial cells, which result in shoot regeneration in plants [24,25]. A study conducted by [26] documented the regeneration of interspecific hybrids of elephant grass and Pearl millet via somatic embryogenesis from shoot tips of explants and reported the highest regeneration rate in MS medium supplemented with 0.5 mg/L BAP and 2 mg L−1 2,4-D using leaf whorls containing immature inflorescences. Consequently, refs. [21,25] contributed with earlier reports on the combination of BAP or TDZ in terms of shoot regeneration, but in this report, they added IAA, which was observed to increase the production of multiple shoots. Additionally, in the present study, rooting was induced by transferring explants on MS media containing 1 mg/L−1 NAA in a combination of 0.05 g/L−1 of ascorbic acid and 1 mg/L−1 IBA for 14 days before being potted and transferred to the greenhouse. Contrary to these findings, the use of NAA alone in solid or liquid media has been demonstrated to be the best in terms of inducing rooting [20,27].
The present study on in vitro induction of polyploidy demonstrates the important role of induced mutation as an efficient and cost-effective method of breeding Cenchrus purpureus. However, in vitro induction of mutation using chemical mutagenesis like colchicine has limitations, including the fact that high doses with longer exposure durations are lethal to plants, as they significantly reduced the calli survival rate and resulted in deformed plants [28]. In this study, it was evident that there was high toxicity when colchicine concentrations were increased to 2% with a longer duration of exposure in all genotypes, while a 0.1% colchicine concentration with a treatment duration of 2 days led to the maximum number of induced mutants. Other authors have reported the relationship between colchicine doses and the survival rate of explants [28,29]. Nevertheless, since the main objective of exposing plant cells to colchicine is to induce mutation, treatments that reduce survival rate can be beneficial due to the reduction in the number of progenitors and the selection of induced mutants [30]. The plant leaf whorl consists of outer and inner compartments with high numbers of primordial cells. The outer compartment results in the production of new plant cells, while the inner compartment, which houses the initial high number of primordial cells, is responsible for the production of other plant cells. Exposure of plant cells to different mutagenic doses affects plants positively and negatively by manifesting in primordial cells when they are actively dividing [31,32].
The current study showed the presence of mutants being genetically dissimilar due to phenotypic segregation. The phenotypic diversity evaluated in the induced mutants included plant height, early and late tillering, and stomata size and number. The results of the synthetically induced mutants revealed significantly bigger stomata with a lower stomatal density, findings that were also reported by [33,34,35]. Similarly, ref. [36] reported that estimation of ploidy level in closely related species can be achieved using stomatal guard cell size, as it is often significantly bigger in induced mutants than in their progenitors. The use of stomata length was equally reported in other crop grass pastures such as rye grass [34] and Miscanthus x giganteus [18]. In the present study the preliminary screening of synthetic induced mutants by comparison of stomata size and number with their corresponding counterparts was successful, as all selected plantlets were confirmed as synthetic induced mutants by chromosome counting and genomic DNA. Observations made in the chlorophyll of some induced mutants after exposure to different colchicine doses revealed plantlets with yellow and striped leaves, i.e., albino leaves. The research findings of [37] showed that the presence of chlorophyll deficiencies is a good indicator of genetic action of mutagens, where increasing mutagen doses lead to a greater frequency of chlorophyll plantlets. However, the negative effects of chlorophyll in the early stage of growth are of essence in breeding and evaluation for genomic effects and sensitivity of mutagens in crops [38].
The other main morphological changes observed in the present study were early/late tillering and reduced plant height and stem diameter in synthetic mutants of the two Pennisetum germplasms compared with their counterparts. Similar results were previously observed in Miscanthus sinensis [39], where the above physiological delays in synthetically induced mutants were apparently due to delayed development as a result of chromosome aberration. A positive correlation in terms of DNA genome size has also been observed, except that some South africa Napier grass plantlets and their progenitors could produce tillers after 6 weeks (but Bana grass had more tillers compared to South africa napier grass). However, the present study also reports that all the induced mutants and some progenitors produced tillers but with diverse numbers of tillers depending on genotype, colchicine concentration, and exposure time. A previous study [40] also reported variations in plant development, including stem diameter, tillering ability, and days taken by plants to mature as a result of mutation. It is very evident from this study that through mutagenesis, morphological traits of importance could be used to improve the overall performance in terms of yield and herbage of pasture and forage for enhanced livestock productivity. The study also suggests that in the selection of mutants with traits of importance, the effectiveness and efficiency of the mutagen is paramount. In this case, phenotype selection of induced mutants should not undeliberately result in physiological and chromosome abnormality as a result of deletion, inversion, duplication, translocation, and lethal dose. These effects on plant cells in the long run affect the cell by decreasing its survival rate and eventually eliminate the mutant.
5. Conclusions and Recommendation
In conclusion, this study reports advancements made in Napier grass following chromosome doubling and embryogenesis, where induced mutant plantlets were regenerated. Polyploidy was confirmed by chromosome counting, stomata size and number, genomic DNA, and other morphological characteristics. However, we recommend the use of flow cytometry to confirm the ploidy level. Superior mutant plantlets can be selected and recommended for characterization across representative agro-ecologies for large-scale production and can be used in Cenchrus purpureus breeding programs in Kenya and its environs.
Author Contributions
Conceptualization, M.R.W.; Methodology, M.R.W.; Software, M.R.W.; Validation, M.R.W. and E.M.M.; Formal Analysis, M.R.W.; Investigation, M.R.W.; Resources, M.R.W., E.M.M., F.N.M., L.S.W., A.I.H. and J.W.M.; Data Curation, M.R.W.; Writing—Original Draft Preparation, M.R.W.; Writing—Review and Editing, M.R.W., E.M.M., F.N.M., L.S.W. and A.I.H.; Visualization, M.R.W.; Supervision, F.N.M. and E.M.M.; Project Administration, M.R.W., F.N.M. and E.M.M.; Funding Acquisition, M.R.W., F.N.M. and E.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was primarily funded by my parents Leonard Shikuku Wafula and Annethe Barasa Shikuku and siblings Sarah Faith Shikuku and Samson Wakhisi Shikuku, with partial support from Masinde Muliro University of Science and Technology Research Fund (URF).
Data Availability Statement
All data supporting the findings of this study are contained within the article.
Acknowledgments
The authors gratefully acknowledge the first author’s parents and siblings for funding the overall research and the Director of the Kenya Agriculture and livestock Research Organization (KALRO), Kakamega, for facilitating the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Omore, A.O.; Muriuki, H.G. Improving the productivity of dairy farms in East Africa through use of strategic feed supplements. Livest. Res. Rural. Dev. 2009, 21, 198. [Google Scholar]
- Kimitei, A. Overview of the Kenya Dairy Industry (Report No. KE2024-0013); USDA Foreign Agricultural Service: Washington, DC, USA, 2004. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Overview+of+the+Kenya+Dairy%20Industry_Nairobi_Kenya_KE2024-0013.pdf (accessed on 20 November 2024).
- Muia, J.M.K.; Gitau, G.K.; Bebe, B.O.; Kahi, A.K. Effect of production system and breed on milk production and composition of smallholder dairy production in coastal Kenya. Livest. Res. Rural. Dev. 2011, 23, 116. [Google Scholar]
- Ouema, J.O.; Obare, G.A. Socioeconomic Factors Influencing Smallholder Dairy Production in Bahati Division, Nakuru District, Kenya. In Proceedings of the 8th KARI Biennial Scientific Conference, Nairobi, Kenya, 11–15 November 2002. [Google Scholar]
- Muyekho, N.F.; Mose, L.; Cheruiyot, T.D. Development and transfer of forage production technologies for smallholder dairying: Case studies of participatory evaluation of species and methods of establishment in Western Kenya. Trop. Grassl. 2003, 37, 251–256. [Google Scholar]
- Lukuyu, B.; Franzel, S.; Ongadi, P.; Duncan, A. Livestock feed resources: Current production and management practices in central and northern rift valley provinces of Kenya. Livest. Res. Rural. Dev. 2011, 23, 112. [Google Scholar]
- Muyekho, F.N.; Onginjo, E.; Lusweti, C.M.; Asaba, J.N.; Mulaa, M.; Kiiya, W. Stunting disease on Napier grass (Pennisetum purpureum): A feld evaluation of germplasm for yield and resistance/tolerance in western Kenya. In Proceedings of the 10th KARI Biennial Scientific Conference, Nairobi, Kenya, 13–17 November 2006; Esilaba, A.O., Nkonge, C., Nyongesa, D., Wandera, F.P., Mutisya, J., Nginyi, J.M., Ngigi, R., Rege, R., Kirigua, V., Eds.; KARI Headquarters: Nairobi, Kenya, 2008; Volume 2. 4p. [Google Scholar]
- Mulaa, M.; Kahi, A.; Peters, K.; Wanjohi, J. Application of Linear Programming in Feed Formulation for Smallholder Dairy Farms in Kenya. J. Agric. Sci. 2010, 2, 17–27. [Google Scholar]
- Kibiengo, M.; Wahome, R.G.; Kaguongo, W.N.; Gachene, C.K.K. Dairy farming in Kenya: Characteristics of smallholder milk production systems in western Kenya. Afr. J. Agric. Res. 2015, 10, 1607–1618. [Google Scholar] [CrossRef]
- Staal, S.; Chege, L.; Kenyanjui, M.; Kimari, A.; Lukuyu, B.; Njubi, D.; Owango, M.; Tanner, J.; Thorpe, W.; Wambugu, M. A Cross Sectional Survey of Kiambu District for the Identification of Target Groups of Smallholder Dairy Producers; KARI/ILRI Collaborative Project: Nairobi, Kenya, 1987. [Google Scholar]
- Kabirizi, J.; Muyekho, F.; Mulaa, M.; Msangi, R.; Pallangyo, B.; Kawube, G.; Zziwa, E.; Mugerwa, S.; Ajanga, S.; Lukwago, G.; et al. Napier Grass Feed Resource: Production, Constraints and Implications for Smallholder Farmers in Eastern and Central Africa; Eastern Africa Agricultural Productivity Project (EAAPP): Entebbe, Uganda, 2015; p. 13. ISBN 978-9970-9269-1-6. Available online: https://www.researchgate.net/publication/281556114_NAPIER_GRASS_FEED_RESOURCE_PRODUCTION_CONSTRAINTS_AND_IMPLICATIONS_FOR_SMALLHOLDER_FARMERS_IN_EAST_AND_CENTRAL_AFRICA (accessed on 20 November 2024).
- Jones, M.; Cuddeford, D.; Johnsen, J. Grazing behaviour of free-ranging goats in a semi-arid environment in Kenya. Small Rumin. Res. 2004, 55, 117–131. [Google Scholar]
- Ardabili, G.S.; Zakaria, R.A.; Zare, N. In Vitro Induction of Polyploidy in Sorghum bicolor L. Cytologia 2015, 80, 437–444. [Google Scholar] [CrossRef]
- Hadebe, S.T.; Modi, A.T.; Shimelis, H.A. Determination of optimum ethylmethanesulfonate conditions for chemical mutagenesis of selected vernonia (Centrapalus pauciflorus) accessions. S. Afr. J. Plant Soil 2017, 34, 311–317. [Google Scholar] [CrossRef]
- Mishra, A.K.; El-Osta, H.S.; Johnson, J.D. Factors affecting agricultural credit use in the United States: Evidence from the 2003 Agricultural Resource Management Survey. Agric. Financ. Rev. 2010, 70, 332–346. [Google Scholar]
- Zhang, X.Y.; Hu, C.G.; Yao, J.L. Tetraploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J. Plant Physiol. 2010, 167, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Van Harten, A.M. Mutation Breeding, Theory and Practical Applications; Cambridge University Press: Cambridge, UK, 1998; pp. 127–140. [Google Scholar]
- Yu, C.Y.; Kim, H.S.; Rayburn, A.L.; Widholm, J.M.; Juvik, J.A. Chromosome doubling of the bioenergy crop, Miscanthus x giganteus. Glob. Change Biol. Bioenergy 2009, 1, 404–412. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef] [PubMed]
- Umami, N.; Gondo, T.; Ishigaki, G.; Rahman, M.M.; Akashi, R. Efficient nursery production and multiple-shoot clumps formation from shoot tiller-derived shoot apices of dwarf napiergrass (Pennisetum purpureum Schumach.). Nihon Danc. Chikusan Gakkaihou 2012, 55, 121–127. (In Japanese) [Google Scholar]
- Shan, X.; Li, D.; Qu, R. Thidiazuron promotes in vitro regeneration of wheat and barley. Vitr. Cell. Dev. Biol.-Plant 2000, 36, 207–210. [Google Scholar] [CrossRef][Green Version]
- Gondo, T.; Umami, N.; Muguerza, M.; Akashi, R. Plant regeneration from embryogenic callus derived from shoot apices and production of transgenic plants by particle inflow gun in dwarf Napier grass (Pennisetum purpureum Schumach.). J. Plant Physiol. 2017, 34, 143–150. [Google Scholar] [CrossRef][Green Version]
- Vasil, I.K. Developing cell and tissue culture systems for the improvement of cereal and grass crops. J. Plant Physiol. 1987, 128, 193–218. [Google Scholar] [CrossRef]
- Gaspasr, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. Vitr. Cell. Dev. Biol.-Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Pola, S.; Saradamani, N.; Ramana, T. Enhanced shoot regeneration in tissue culture studies of Sorghum bicolor. J. Agric. Technol. 2007, 3, 275–286. [Google Scholar]
- Faleiro, F.G.; Kannan, B.; Altpeter, F. Regeneration of fertile, hexaploid, interspecific hybrids of elephant grass and pearl millet following treatment of embryogenic calli with antimitotic agents. Plant Cell Tissue Organ Cult. 2016, 124, 57–67. [Google Scholar] [CrossRef]
- Pola, S.; Saradamani, N. Somatic embryogenesis and plant regeneration in Sorghum bicolor (L.) Moench, from leaf segments. J. Cell Mol. Biol. 2006, 5, 99–107. [Google Scholar]
- Mba, C.; Afz, R.; Jankowicz-Cieslak, J.; Bado, S.; Matijevic, M.; Till, B.J. Enhancing genetic diversity through induced mutagenesis in vegetative propagated plants. In Proceedings of the International Symposium Induced Mutationsin Plants: Induced Plant Mutations in the Genomics Era, Vienna, Austria, 11–15 August 2008; International Atomic Energy Agency: Vienna, Austria, 2009. [Google Scholar]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Hafiz, I.A.; Silvestri, C. Studies on colchicine-induced chromosome doubling for enhancement of quality traits in ornamental plants. Plants 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Vainola, A.; Repo, T. Polyploidization of Rhododendron cultivars in vitro and how it affects cold hardiness. Acta Hortic. 2001, 560, 319–322. [Google Scholar] [CrossRef]
- Kiong, A.L.P.; Lai, A.G.; Hussein, S.; Harun, A.R. Physiological responses of Orthosiphon stamineus plantles to gamma irradiation. Am.-Eurasian J. Sustain. Agric. 2008, 2, 135–149. [Google Scholar]
- Dhamayanthi, K.P.M.; Gotmare, V. Induction of polyploidy in two diploid wild cotton (G. armourianum and G. aridum) species by colchicine treatment. Electron. J. Plant Breed. 2010, 1, 966–972. [Google Scholar]
- Liu, G.; Li, Z.; Bao, M. Colchicine-induced chromosome doubling in Platanus acerifolia and its effect on plant morphology. Euphytica 2007, 157, 145–154. [Google Scholar] [CrossRef]
- Quesenberry, K.H.; Dampier, J.M.; Lee, Y.Y.; Smith, R.L.; Acuña, C.A. Doubling the chromosome number of bahiagrass via tissue culture. Euphytica 2010, 175, 43–50. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Zheng, Y. Effects of polyploidization on stomatal size, density, and photosynthetic rate in Chrysanthemum lavandulifolium. Photosynthetica 2018, 56, 557–562. [Google Scholar]
- Borrino, E.M.; Powell, W. Stomatal guard cell length as an indicator of ploidy in microspore-derived plants of barley. Genome 1988, 30, 158–160. [Google Scholar] [CrossRef]
- Maluszynski, M.; Szarejko, I.; Bhatia, C.R.; Nichterlein, K.; Lagoda, P.J.L. Methodologies for generating variability. Part 4: Mutation techniques. In Plant Breeding and Farmer Participation; Ceccarelliand, S., Guimarães, E.P., Weltzien, E., Eds.; Food and Agriculture Organization of the United Nations/International Atomic Energy Agency: Vienna, Austria, 2009; pp. 159–194. Available online: https://www.iaea.org/resources/book/plant-breeding-and-farmer-participation (accessed on 20 November 2024).
- Neto, A.T.; Ando, A.; Figueira, A.; Latado, R.R.; Santos, P.C.D.; Correa, L.S.; Peres, L.E.P.; Hauagge, R.; Pulcinelli, C.E.; Ishiy, T.; et al. Genetic improvement of crops by mutation techniquesin Brazil. Plant Mutat. Rep. 2011, 2, 24–37. [Google Scholar]
- Głowacka, K.; Jeżowski, S.; Kaczmarek, Z. In vitro induction of polyploidy by colchicineTreatment of shoots and preliminary characterisation of induced polyploids in two Miscanthus species. Ind. Crops Prod. 2010, 32, 88–96. [Google Scholar] [CrossRef]
- Dhanavel, D.; Pavadai, P.; Mullainathan, L.; Mohana, D.; Raju, G.; Girija, M. Effectiveness and efficiency of chemical mutagens in cowpea (Vigna unguiculata (L.) Walp). Afr. J. Biotechnol. 2008, 7, 4116–4117. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).