Navigating the Molecular and Cellular Landscape of Breast Cancer in India: From Unique Pathogenesis to the Promise of Personalized Medicine and Future Technologies
Abstract
1. Introduction
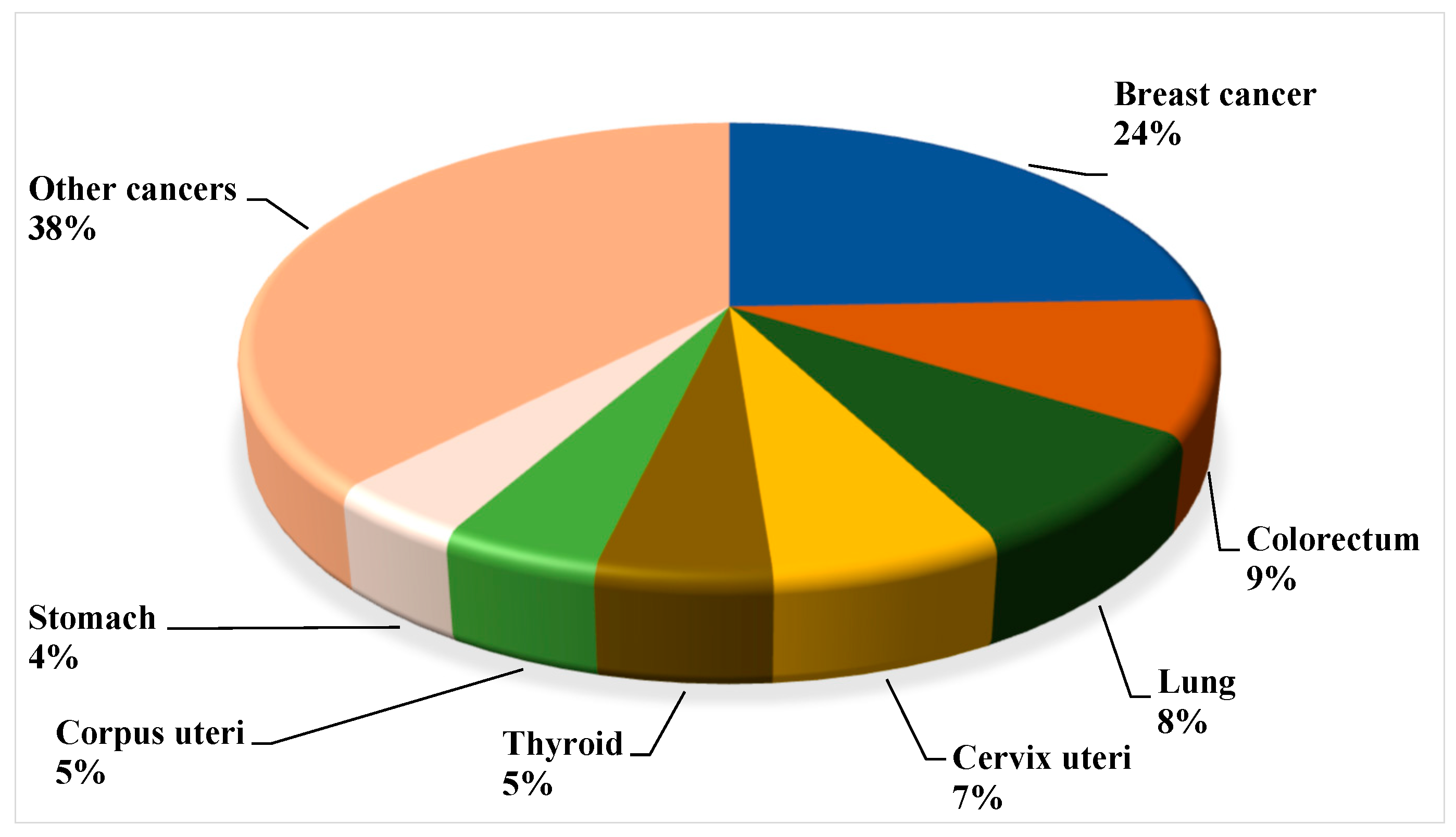
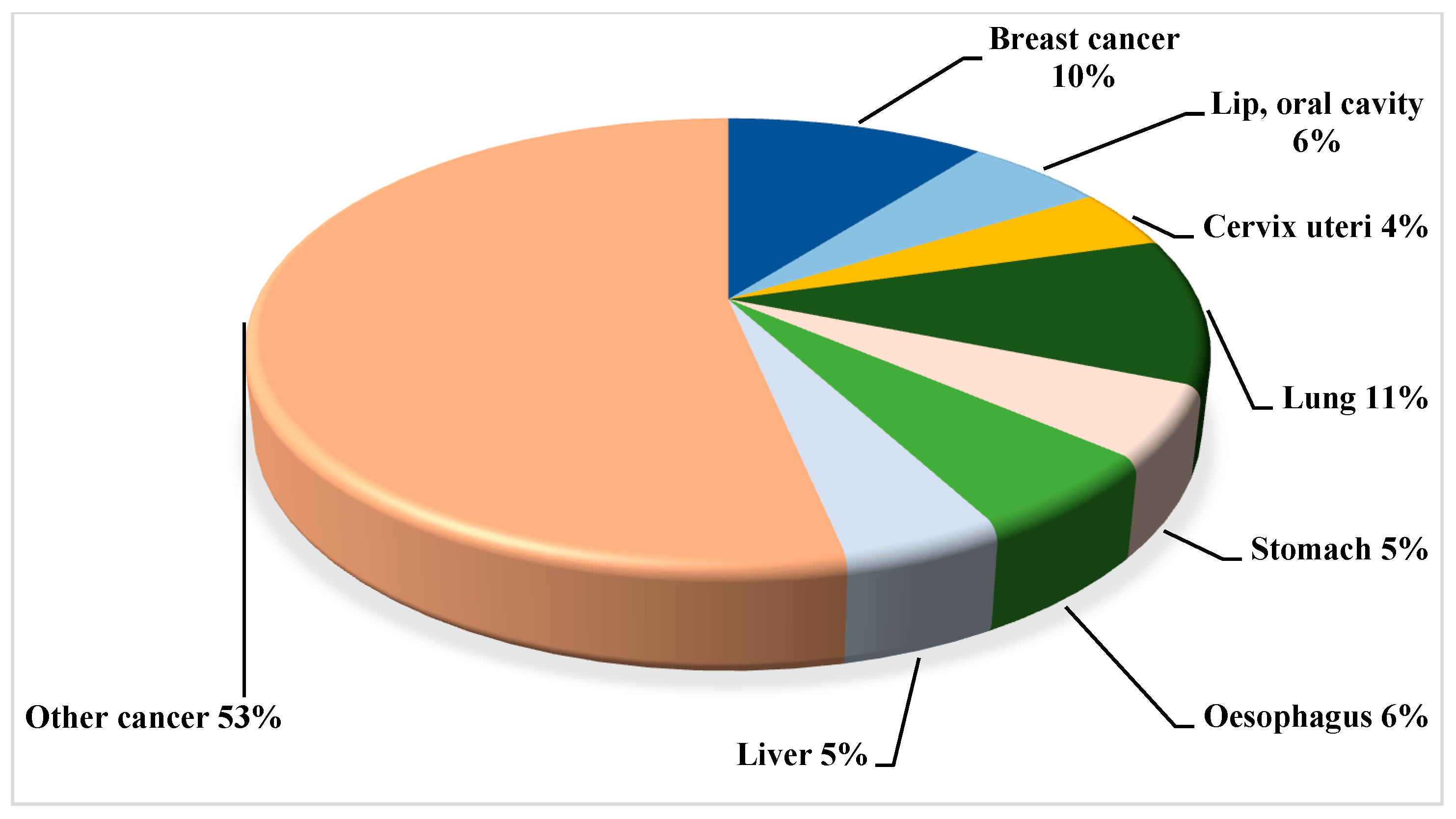
2. Breast Cancer Landscape from Indian Epidemiological Context
3. Overall Global Understanding of Breast Cancer with the Indian Demographic Characteristics
3.1. Gaps in Knowledge
3.2. Gaps in Diagnostic and Therapeutic Approaches or Methodologies
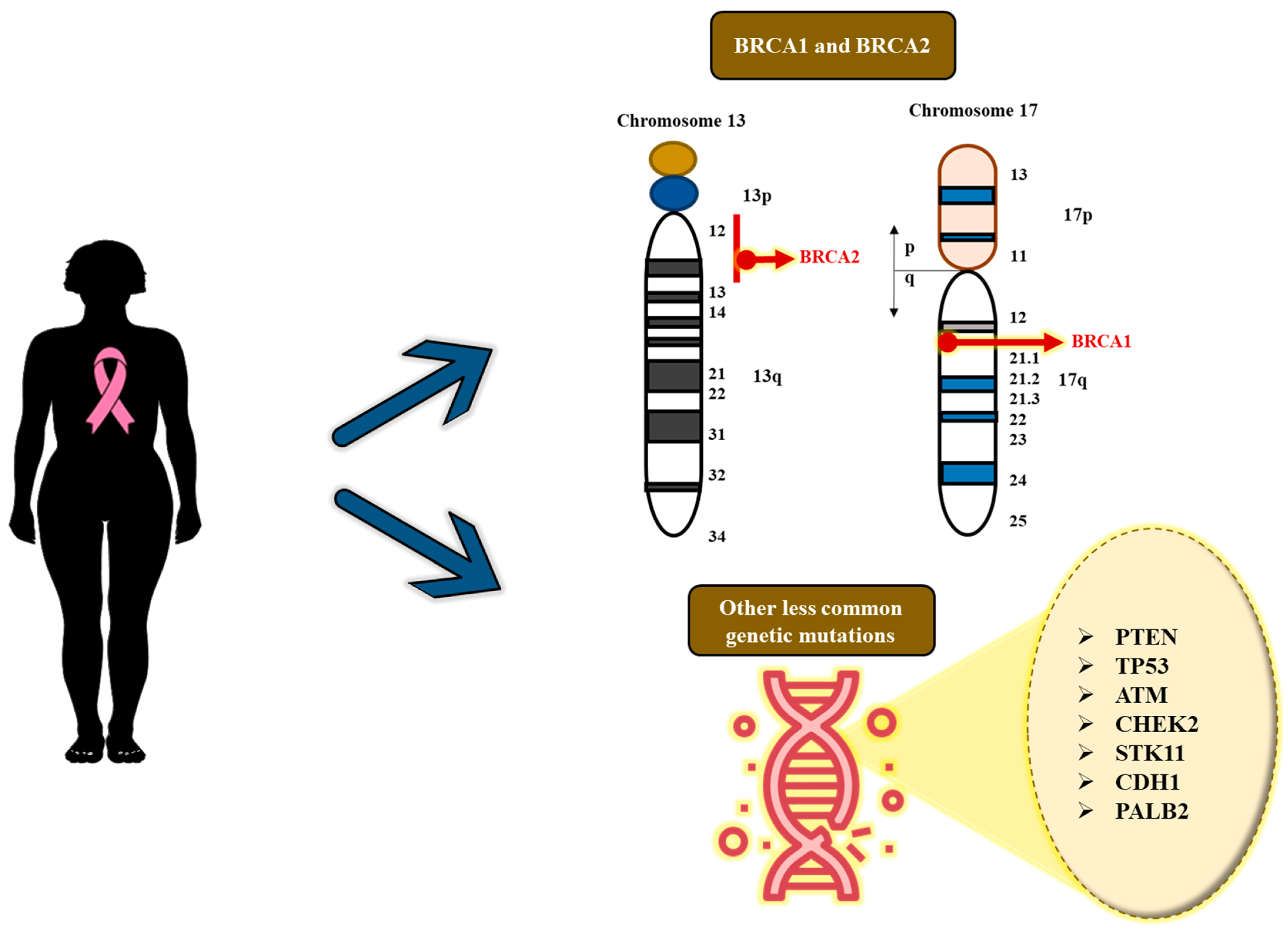
4. Genetics and Genomics: Implication of Genetic Susceptibility as a Significant Risk Factor for Breast Cancer
- c.68_69delAG (185delAG) in BRCA1: This mutation, which is a founder mutation in Ashkenazi Jewish communities is recurrent and seems to have originated independently among the South and West Indian populations.
- c.1961delA in BRCA1: This is one of the most common mutations discovered in current studies.
- c.5074+1G>A in BRCA1: This is most common among West Indian and other populations.
- c.5382insC in BRCA1: This is another frequently occurring pathogenic mutation.
- Shared mutation (185delAG): The BRCA1 185delAG (as mentioned earlier) founder mutation, which is widely linked to the Ashkenazi Jewish population, can also be detected in certain areas of India, especially in the south and west. Indeed, studies indicate that this emerged or originated independently within the South Indian population, instead of originating from a common lineage with Ashkenazi Jews [31].
- Regional heterogeneity: Studies have found a wide range of BRCA1/2 variants among various Indian populations, showing that the types of mutations vary by geographical locations. This indeed highlights the importance of developing demographically specific genetic profiles [26].
- Targeted sequencing–next-generation sequencing (NGS): Instead of expensive, laborious, and lengthy whole gene sequencing, the testing or screening strategies can be highlighted on the most frequent founder mutations that are found in specific regional or ethnic populations. This strategy provides a faster and less expensive screening tool for high-risk people.
- Custom-designed gene panel (personalized genetic test): Genetic testing or screening can be performed with panels tailored to contain common founder mutations unique to the Indian population, along with additional pertinent genes associated with high and moderate risks.
- Overcoming barriers to access: Customized or personalized testing methods can assist in tackling the significant obstacles pertaining to the expense and availability of genetic tests in India.
5. Implication of Non-Genetic Risk Factors for Breast Cancer (Figure 5)
5.1. Familial History of Breast Cancer

5.2. Proliferative Breast Lesions (PBLs)
5.3. Lack of Pregnancy and Breastfeeding
5.4. Benign Breast Conditions
5.5. Obesity and Being Overweight
5.6. Early Onset of Menstruation
5.7. Race and Ethnicity
5.8. Exposure to Environmental Risk Factors
5.9. Scarce Physical Activities
5.10. Extreme Alcohol Consumption
5.11. Genetic Mutations and Age
5.12. Birth Control and Contraceptives
5.13. Chest Radiation Therapy
5.14. Hormone Replacement Therapy After Menopause
6. Risk Assessment: Breast Cancer Stratification by Way of Pathogenesis, Incidence and Invasiveness
6.1. Non-Invasive (In Situ) Breast Cancer
6.1.1. Ductal Carcinoma In Situ (Intraductal Carcinoma; DCIS)
6.1.2. Invasive/Infiltrating Breast Cancer
6.1.3. Invasive Ductal Carcinoma (IDC)
6.1.4. Invasive Lobular Carcinoma (ILC)
6.2. Metastatic Breast Cancer
6.3. Less Common Types of Breast Cancer
6.3.1. Inflammatory Breast Cancers (IBCs)
6.3.2. Angiosarcoma of the Breast
6.3.3. Paget Disease of the Breast
6.3.4. Papillary Carcinoma
6.3.5. Phyllodes Tumor
6.3.6. Breast Cancers in Men, Children, and Juveniles
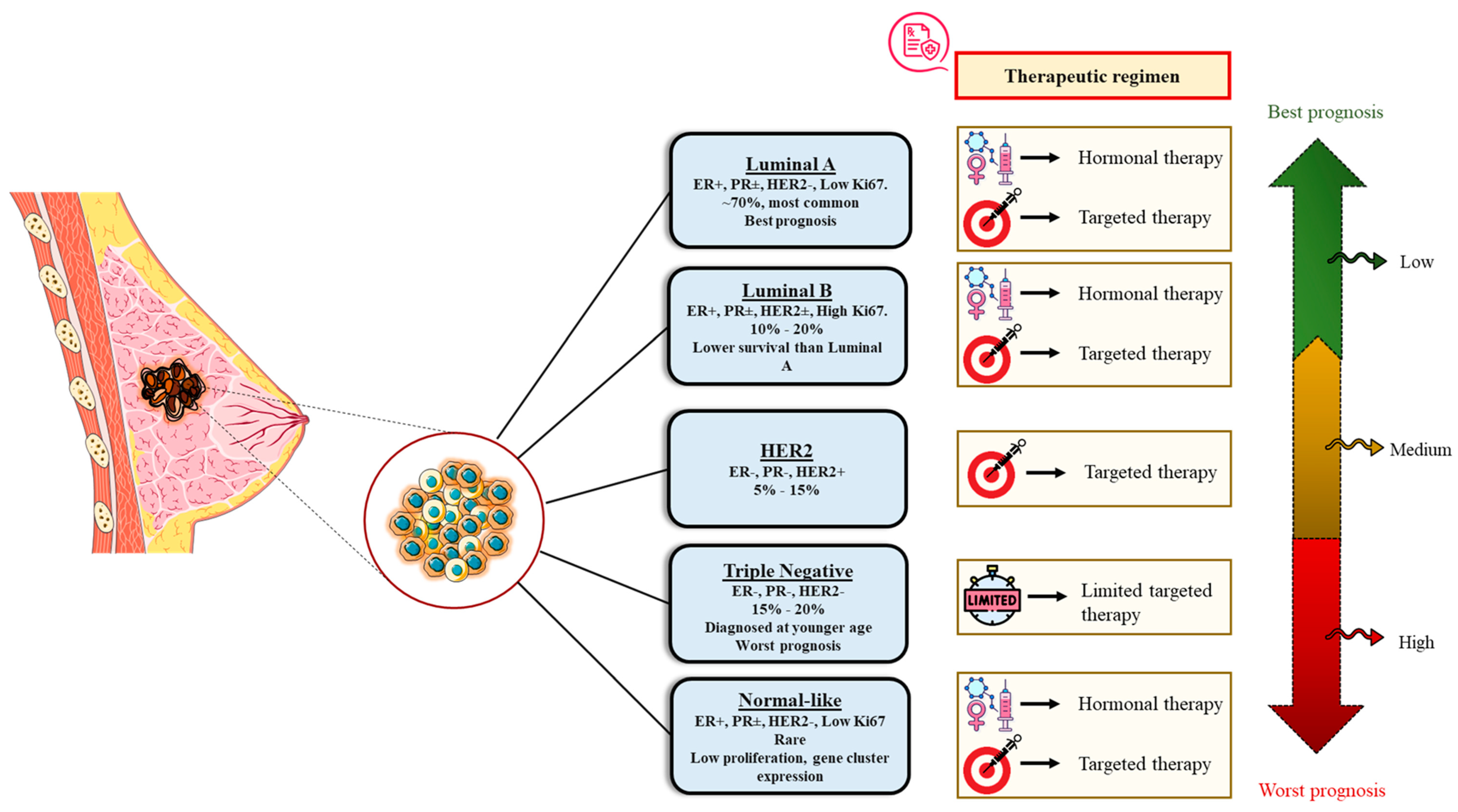
7. Molecular or Intrinsic Subtyping/Classification of Breast Cancer
7.1. Luminal A Breast Cancer
- PIK3CA: Activating mutations are commonly seen in the PI3K/AKT/mTOR pathway.
- GATA3 and MAP3K1: Mutations or alterations in these transcription factors are also frequent.
- TP53: Although not as common as in other subtypes, mutations or alterations in the tumor suppressor gene, TP53, are detected in certain luminal A tumors.
7.2. Luminal B Breast Cancer
7.3. HER2-Enriched Breast Cancer
- PI3K/Akt/mTOR pathway: This signaling pathway is one of the most commonly activated downstream of HER2 and is an important regulator of cell survival, growth, metabolism, and proliferation. Mutations in the PIK3CA gene, which encodes the p110α subunit of PI3K, along with the loss of the tumor suppressor gene PTEN, are prevalent in HER2+ BC, leading to constitutive PI3K/Akt activation and treatment resistance.
- MAPK/ERK pathway: The MAPK signaling pathway is yet another important modulator of HER2 signaling, which stimulates cell proliferation. Certain metastatic HER2-positive BCs exhibit genetic modifications that trigger the MAPK pathway, including the deletion of NF1. This can lead to a “pathway switch”, rendering tumors resistant to Akt obstruction, yet more reliant on and susceptible to MEK/ERK inhibition.
- Likewise, other related signaling pathways like HIF-1, Wnt/β-catenin, and STAT signaling pathways are also known to be activated in some HER2-enriched tumors, thus contributing to the resistance of anti-HER2 therapies.
7.4. Triple-Negative/Basal-like Breast Cancer (TNBC)
- PI3K/Akt/mTOR pathway: The PI3K/AKT/mTOR (PAM) signaling pathway is among the most frequently modified and excessively activated in TNBC. This pathway regulates cell proliferation, survival, metabolism, and angiogenesis. Mutations in the PIK3CA gene or the deletion of the tumor suppressor PTEN are common causes of hyperactivation. This pathway may also play a role in contributing to chemoresistance and relapse [57].
- DNA damage response (DDR) pathways: A considerable proportion of TNBC tumors are distinguished by deficiencies in DNA repair processes, a feature known as “BRCAness”, due to its similarities to tumors with inherited BRCA1/2 mutations. This aside, mutations in BRCA1 are especially known to be linked to the basal-like type of TNBC.
- Wnt/β-catenin signaling pathway: The Wnt/β-catenin pathway is essential for embryonic development and adult tissue homeostasis, but it is abnormally active in certain malignancies, including TNBC. In TNBC, the Wnt/β-catenin pathway is frequently overactivated because of the high levels of Wnt receptors such Frizzled (FZD7) and the Wnt co-receptor LRP6. As a result, β-catenin is stabilized and subsequently moves into the nucleus.
7.5. Normal-like Breast Cancer
8. Prevalence and Clinical Significance of Molecular Subtypes in the Indian Setting
9. Clinical Phasing/Staging and Characteristic Survival Percentage in BC Cases
10. Treatment Options for Breast Cancer
10.1. Surgical Therapy
10.2. Radiation Therapy
10.3. Hormone Therapy
10.4. Chemotherapy
10.5. Immunotherapy
10.6. Targeted Therapies
| Therapy | Mechanism of Action | References |
|---|---|---|
| Surgery | The primary mechanism of action is the physical removal of a tumor, along with the adjacent healthy tissue (often referred to as the margin). It can be effective in treating early-stage, localized cancers. For more advanced diseases, surgery can be utilized to remove as much of the tumor as possible (debulking), thereby improving the efficacy of other treatments such as chemotherapy or radiation. | [73] |
| Radiation Therapy | High-energy radiation destroys the DNA of cancer cells, stopping them from proliferating and replicating. Cancer cells are more vulnerable to DNA damage than healthy cells because they divide faster and are less effective at DNA repair. The two most common methods are external beam radiation and internal radiation (brachytherapy), both of which are localized treatments. | [74] |
| Hormone Therapy | Also known as endocrine therapy, this approach functions by inhibiting the synthesis or activity of hormones that promote the growth of hormone-sensitive malignancies, such as breast and prostate cancer. This can be accomplished with medications that inhibit hormone synthesis, the blocking of hormone receptors, or the surgical removal of hormone-producing glands. | [75] |
| Chemotherapy | Cytotoxic chemotherapy medications aim to attack and eliminate rapidly dividing cells, including cancer cells, as well as healthy cells in the bone marrow, digestive tract, and hair follicles. These drugs disrupt the process of cell division through several mechanisms like causing DNA damage, blocking essential enzymes, or interrupting the cell’s mitotic machinery. | [76] |
| Immunotherapy | This therapy activates or strengthens the body’s immune system to identify and fight cancer cells. Examples of mechanisms are as follows:
| [77] |
| Targeted Therapies | Unlike chemotherapy, targeted therapies focus on particular molecular targets that are essential for the growth, development, and survival of cancer cells, while reducing damage to normal cells. They can inhibit signals that stimulate cancer cell development, trigger cell death, deprive the tumor by preventing the formation of new blood vessels (angiogenesis inhibitors), and transport toxic compounds directly to cancer cells. | [76] |
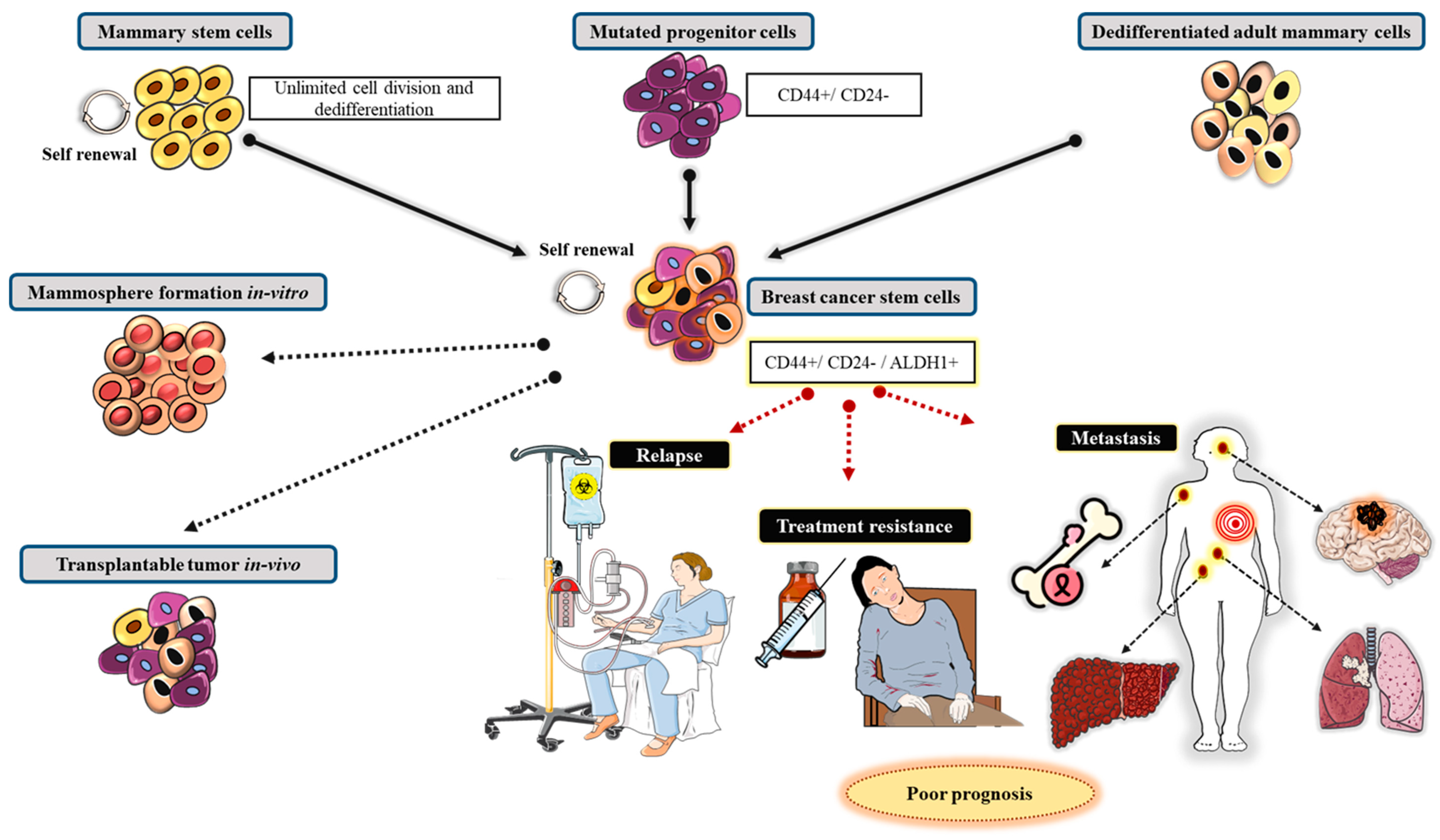
11. Cellular Genesis of Breast Cancer
11.1. Establishment of Mammary Gland and Mammary Stem Cells
11.2. Contribution of Mammary Stem Cells in BC Progression
12. The Role of Genetics in the Effect of Breast Cancer Drug Resistance: Requirement for Predictive Biomarkers to Address the Outstanding Questions
12.1. ABCB1
12.2. eNOS
12.3. BARD1
12.4. CD24
12.5. Cytochrome P450 (CYP450), CYP
13. Pharmacogenetics of Breast Cancer Therapeutics in India
13.1. Evidence Generation and Dissemination
- Funding research on Indian populations: Additional investigation is required to explore and examine the genetic polymorphisms unique to the Indian population and their effect on treatment and drug response in BC.
- Implementing nationwide data repositories: Developing a complete and an extensive database of pharmacogenomic variations and drug–gene interactions related to the Indian population would be tremendously beneficial.
- Utilize real-world evidence, implementing observational studies: Corroborating results from real-world research or evidence-based studies in India, demonstrating the influence of pharmacogenomics testing on BC therapeutic response, is critical for initiating therapeutic value and increasing uptake.
13.2. Practical Implementation Strategies
- Clinically actionable drug–gene interactions (DGIs): Focus on employing pharmacogenomics tests for well-recognized drug–gene interactions in BC; for example, CYP2D6 for tamoxifen.
- Establish accessible testing policies or programs: Capitalizing on commercial, affordable and effective genetic testing tools like next-generation sequencing (NGS), is critical for increasing access.
- Proof of concept programs and step-by-step implementation: Initiate proof of concept or pilot programs at all oncology centers of excellence or specialized cancer hospitals to assess the feasibility and impact of integrating pharmacogenomics testing into regular BC care.
| Polymorphisms | Drugs Affected | Clinical Implications Relevant to Indian Population | |
|---|---|---|---|
| CYP2D6 | Multiple variant alleles (e.g., *4 null allele, *10 reduced function allele) | Tamoxifen | Increased risk of adverse effects: A combination of specific SNPs (e.g., 2988G>A, -1584C, and 2850C>T, suggestive of *41 reduced functional allele) may predict a higher risk of fatty liver with tamoxifen therapy [134]. |
| Altered tamoxifen metabolism: Individuals with reduced function alleles (*10) may show reduced benefit from tamoxifen and poorer disease-free survival [134]. | |||
| UGT1A1 | UGT1A128, UGT1A16 | Irinotecan | Increased irinotecan toxicity: Both UGT1A128 and UGT1A16 (more common in Asians) are associated with decreased UGT1A1 activity, increasing the risk of severe neutropenia and diarrhea from irinotecan. Double heterozygosity (UGT1A1*6/*28) is also associated with increased toxicity [135]. |
| ABCB1 (MDR1) | 1236C>T, 2677G>T/A, 3435C>T | Doxorubicin, cyclophosphamide, paclitaxel, and other MDR1 substrates | Drug resistance: Polymorphisms affecting the function and expression of ABCB1 (encoding P-glycoprotein) can lead to reduced intracellular drug levels and resistance to chemotherapy agents like anthracyclines and taxanes. Some studies suggest a higher risk of non-response to FAC chemotherapy with specific ABCB1 genotypes [136]. |
| GSTM1 | Null genotype | Anthracyclines (and potentially other chemotherapy drugs metabolized by GSTM1) | Worse chemotherapy responsiveness: The null genotype has been associated with worse responsiveness to anthracycline-based chemotherapy. Some studies suggest an increased risk of breast cancer susceptibility and shorter overall survival [137]. |
| GSTT1 | Null genotype | Cyclophosphamide, doxorubicin, 5-fluorouracil (CAF regimen) | Better DFS and OS with certain chemotherapy regimens: The null genotype, in some contexts, has been associated with better disease-free survival (DFS) and overall survival (OS) when treated with CAF (cyclophosphamide, doxorubicin, and 5-fluorouracil) chemotherapy compared to other variants. It may be associated with an increased frequency of p53 mutations [136]. |
| GSTP1 | 105Val/Val genotype, GG genotype | Cyclophosphamide (CTX) | Worse DFS with CTX: The 105Val/Val genotype showed a significant association with worse DFS in patients treated with cyclophosphamide. No significant association with cyclophosphamide outcome: Another study found no significant association with the GG genotype and cyclophosphamide outcome [136]. |
| BRCA1/2 | Pathogenic mutations and variants of uncertain significance (VUS) | PARP inhibitors | Increased hereditary breast and ovarian cancer risk: Mutations in BRCA1/2 are a major cause of hereditary breast and ovarian cancer. Predictive marker for PARP inhibitors: BRCA1/2 mutations are a strong indicator for PARP inhibitor efficacy [138]. |
| HER2 | Amplification | Trastuzumab, Pertuzumab, Lapatinib, Neratinib (HER2 inhibitors and targeted agents) | Predictive marker for HER2-targeted therapies: HER2 amplification leads to overexpression, which makes tumors responsive to anti-HER2 therapies like trastuzumab and pertuzumab. Resistance mechanisms can develop over time [139,140]. |
| Other genes | |||
| TP53 | Polymorphisms | Increased breast cancer risk [141,142]. | |
| PTEN | Germline mutations | Increased breast cancer risk [27]. | |
| MTHFR | C677T, A1298C | Association with increased homocysteine levels. A link with breast cancer risk is not clearly established [143,144]. | |
| NBN, RAD51, XRCC3 | Polymorphisms | Radiotherapy | Potential biomarkers for radiotherapy toxicity (e.g., cardiac toxicity with RAD51 and skin adverse events with XRCC3) in HER2-positive breast cancer [145]. |
13.3. Policy and Screening Guidelines
- Establish and formulate guidelines specific for India: Developing national guidelines for pharmacogenomic testing and interpretation in BC is crucial for standardizing the reliability and interpretation of results for therapeutic regimens.
- Review compliance with legal and ethical guidelines: Acknowledging concerns about patient confidentiality, sharing data resources, and possible discernment based on genetic information are vital for cultivating transparency and accountability, and eventually maintaining integrity.
- Developing a standardized curriculum for precision medicine in pharmacotherapy: Incorporating pharmacogenomics into medical training programs for both undergraduate and postgraduate students will prepare the next generation of oncologists to use this discipline proficiently.
14. The Role of Tumor Microenvironment in Breast Cancer
14.1. Cellular Components
- Cancer cells: These primary cancerous cells comprise the tumor.
- Cancer-associated fibroblasts: These essentially reprogrammed fibroblasts are key producers of extracellular matrix (ECM) and numerous signaling molecules. They stimulate tumor development, angiogenesis, and metastasis.
- Immune cells: A varied group of immune cells, comprising the following:
- Tumor-associated macrophages (TAMs): Tumor-associated macrophages (TAMs) are usually adopted to an M2-like phenotype, which usually suppresses anti-tumor responses, stimulating angiogenesis, and facilitating metastasis.
- Regulatory T-cells (Tregs): These are a subclass of T-cells that inhibit the activity of other immune cells, resulting in an immunosuppressive environment.
- Myeloid-derived suppressor cells (MDSCs): These underdeveloped myeloid cells inhibit anti-tumor immune responses and promote tumor development.
- Endothelial cells: These cells form the inner layer of blood vessels and are engaged and stimulated by tumors to generate new, often defective, blood vessels that provide oxygen and nutrients to the tumor (angiogenesis).
- Pericytes: These are cells that encase the blood vessels and communicate with endothelial cells, prompting the development and durability of the tumor’s vasculature.
14.2. Non-Cellular Components
- Extracellular matrix (ECM): The ECM is a complex network of proteins and large molecules (such as collagen, fibronectin, and proteoglycans) that offers structural and biochemical assistance. The ECM in tumors frequently becomes rigid and undergoes modifications, thus facilitating the spread of cancer cells and impeding the effectiveness of therapeutic delivery [147].
- Signaling molecules: Several TME cells release a network of cytokines, chemokines, and growth factors to regulate their interactions. However, depending on the circumstances, these signals might stimulate or prevent tumor growth.
- Extracellular vesicles (EVs): These are minute vesicles, such as exosomes, that are secreted by cells to transport substances such as proteins and RNA. The tumor-derived EVs have the potential to reprogram other cells in the TME, promoting tumor development and prime distant locations for metastasis [148].
15. Recent Developments and Key Prospective Directions for Breast Cancer Research in India
15.1. Generating Comprehensive Genomic Data Specific for Indian Population (India-Specific Genome Atlas)
- Genomic and multi-omics profiling:
15.2. Addressing High Breast Density Among Indian Breast Cancer Population
- Using Artificial Intelligence (AI), deep learning (DL), and genomic profiling to refine tomosynthesis:
15.3. Adapting Screening Technologies for Early Breast Cancer Detection and Screening for Indian Needs
- Technological innovation and research adapted for India:
15.4. Integrating Socio-Cultural Factors for Improving Program Implementation and Access for BC Research in India
- Community-based solutions; a culturally adapted approach:
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, P. Breast cancer in young Indian women: Factors, challenges in screening, and upcoming diagnostics. J. Cancer Res. Clin. Oncol. 2023, 149, 14409–14427. [Google Scholar] [CrossRef]
- Kulkarni, A.; Kelkar, D.A.; Parikh, N.; Shashidhara, L.S.; Koppiker, C.B.; Kulkarni, M. Meta-Analysis of Prevalence of Triple-Negative Breast Cancer and Its Clinical Features at Incidence in Indian Patients With Breast Cancer. JCO Glob. Oncol. 2020, 6, 1052–1062. [Google Scholar] [CrossRef]
- Sivasankar Perumal, A.D. Breast cancer and mental health: Understanding the link to depressioninanindianscenario. Glob. J. Res. Anal. 2025, 14, 1–4. [Google Scholar]
- Samad, M.A.; Ahmad, I.; Khan, M.R.; Suhail, M.; Zughaibi, T.A.; Al-Abbasi, F.A.; Alhosaini, K.A.; Khan, M.S.; Kumer, A.; Tabrez, S. Breast Cancer: Molecular Pathogenesis and Targeted Therapy. MedComm 2025, 6, e70404. [Google Scholar] [CrossRef]
- Srivastava, T.P.; Goel, I.; Gogia, A.; Parshad, R.; Monga, S.; Talukdar, J.; Rai, A.; Dhar, R.; Karmakar, S. Comprehensive Overview of Breast Cancer in India. JCO Glob. Oncol. 2025, 11, e2500083. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, R.K.; Gupta, S.; Prinja, S. Cost Effectiveness of Trastuzumab for Management of Breast Cancer in India. JCO Glob. Oncol. 2020, 6, 205–216. [Google Scholar] [CrossRef]
- Mahalingam, S.; Scaria, V.; Sivasubbu, S. The Bharat Cancer Genome Atlas: Charting India’s Unique Cancer Landscape for Precision Oncology. Technol. Cancer Res. Treat. 2025, 24, 15330338251381404. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- DeSantis, C.; Siegel, R.; Bandi, P.; Jemal, A. Breast cancer statistics, 2011. CA Cancer J. Clin. 2011, 61, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Szabo, C.I.; Chopin, S.; Barjhoux, L.; Sinilnikova, O.; Lenoir, G.; Goldgar, D.E.; Bhatanager, D. BRCA1 and BRCA2 in Indian breast cancer patients. Hum. Mutat. 2002, 20, 473–474. [Google Scholar] [CrossRef]
- India State-Level Disease Burden Initiative Cancer Collaborators. The burden of cancers and their variations across the states of India: The Global Burden of Disease Study 1990–2016. Lancet Oncol. 2018, 19, 1289–1306. [Google Scholar] [CrossRef]
- Kulothungan, V.; Sathishkumar, K.; Leburu, S.; Ramamoorthy, T.; Stephen, S.; Basavarajappa, D.; Tomy, N.; Mohan, R.; Menon, G.R.; Mathur, P. Burden of cancers in India—Estimates of cancer crude incidence, YLLs, YLDs and DALYs for 2021 and 2025 based on National Cancer Registry Program. BMC Cancer 2022, 22, 527. [Google Scholar] [CrossRef]
- Takiar, R.; Srivastav, A. Time trend in breast and cervix cancer of women in India—(1990–2003). Asian Pac. J. Cancer Prev. 2008, 9, 777–780. [Google Scholar]
- Maurya, A.P.; Brahrnachari, S. Current Status of Breast Cancer Management in India. Indian J. Surg. 2021, 83 (Suppl. S2), S316–S321. [Google Scholar] [CrossRef]
- Ranganathan, S.; Dee, E.C.; Debnath, N.; Patel, T.A.; Jain, B.; Murthy, V. Access and barriers to genomic classifiers for breast cancer and prostate cancer in India. Int. J. Cancer 2024, 154, 1335–1339. [Google Scholar] [CrossRef]
- Talukdar, J.; Srivastava, T.P.; Sahoo, O.S. Cancer stem cells: Signaling pathways and therapeutic targeting. MedComm—Oncology 2023, 2, e62. [Google Scholar] [CrossRef]
- Weerarathna, I.N.; Luharia, A.; Uke, A.; Mishra, G. Challenges and Innovations in Breast Cancer Screening in India: A Review of Epidemiological Trends and Diagnostic Strategies. Int. J. Breast Cancer 2024, 2024, 6845966. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Swain, S.K.; Banerjee, B.D.; Sharma, T.; Krishnalata, T. Organochlorine pesticide exposure as a risk factor for breast cancer in young Indian women: A case-control study. South. Asian J. Cancer 2019, 8, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.N.; Alex, A.; M.S., N.; Verma, V.; Shaji, A.; Pavithran, K.; Vijaykumar, D.K.; John, D. Economic burden of breast cancer in India, 2000–2021 and forecast to 2030. Sci. Rep. 2025, 15, 1323. [Google Scholar] [CrossRef]
- Wadasadawala, T.; Mohanty, S.K.; Sen, S.; Kanala, T.S.; Maiti, S.; Puchali, N.; Gupta, S.; Sarin, R.; Parmar, V. Out-of-pocket payment and financial risk protection for breast cancer treatment: A prospective study from India. Lancet Reg. Health Southeast Asia 2024, 24, 100346. [Google Scholar] [CrossRef]
- Pemmasani, S.K.; Raman, R.; Mohapatra, R.; Vidyasagar, M.; Acharya, A. A Review on the Challenges in Indian Genomics Research for Variant Identification and Interpretation. Front. Genet. 2020, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kapoor, R. Challenges and Opportunities in Oncology in India: Is There a Paradigm Shift? Indian J. Surg. 2025, 87, 976–977. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Ozsoy, A.; Barça, N.; Dolek, B.A.; Aktaş, H.; Elverici, E.; Araz, L.; Ozkaraoğlu, O. The Relationship Between Breast Cancer and Risk Factors: A Single-Center Study. Eur. J. Breast Health 2017, 13, 145–149. [Google Scholar] [CrossRef]
- Allison, K.H. Molecular pathology of breast cancer: What a pathologist needs to know. Am. J. Clin. Pathol. 2012, 138, 770–780. [Google Scholar] [CrossRef]
- Chikkala, R.; Bhayal, D.; Rani, N.; Modali, R.; Bhatia, K.; Dubey, B. Mutational landscape of BRCA gene mutations in Indian breast cancer patients: Retrospective insights from a diagnostic lab. Egypt. J. Med. Hum. Genet. 2024, 25, 101. [Google Scholar] [CrossRef]
- Pal, M.; Das, D.; Pandey, M. Understanding genetic variations associated with familial breast cancer. World J. Surg. Oncol. 2024, 22, 271. [Google Scholar] [CrossRef]
- Rehman, S.; Sameer, A.; Zahoor, L.; Abdullah, S.; Shah, Z.; Afroze, D.; Hussain, I.; Shaffi, S.M.; Syeed, N.; Rizvi, M.A.; et al. Distinct pattern of mutations of conserved regions of TP53 in colorectal cancer patients in the Kashmir population: An emerging high-risk area. Ecancermedicalscience 2009, 3, 129. [Google Scholar] [CrossRef] [PubMed]
- Lalli, E.; Zambetti, G.P.; Ribeiro, R.C.; Figueiredo, B.C. Cancer associated to low-penetrance TP53 variants: Insights from p.R337H. Trends Cancer 2025, 11, 499–502. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]
- John, A.O.; Singh, A.; Yadav, P.; Joel, A.; Thumaty, D.B.; Ninan, K.F.; Georgy, J.T.; Cherian, A.J.; Thomas, S.; Thomas, A.; et al. The BRCA mutation spectrum among breast and ovarian cancers in India: Highlighting the need to screen BRCA1 185delAG among South Indians. Eur. J. Hum. Genet. 2024, 32, 1319–1326. [Google Scholar] [CrossRef]
- Patra, A.; Ali, S.S.; Devi, N.M.; Qadeer, A.S.; Kamalakannan, S.; Nag, S.; Kulkarni, S.S.; Rajappa, S.; Hariharan, N.; Pant, H.B.; et al. Prevalence of BRCA mutation in breast and ovarian cancer among women in India: A systematic review and meta-analysis protocol. PLoS ONE 2024, 19, e0306612. [Google Scholar] [CrossRef]
- Goyal, J.; Yadav, A.; Malhotra, H. Association of Positive Family History and Clinicopathological Features in Breast Cancer in Young Indian Females—A Pilot Study. J. Radiat. Cancer Res. 2025, 16, 23–26. [Google Scholar] [CrossRef]
- Dupont, W.D.; Parl, F.F.; Hartmann, W.H.; Brinton, L.A.; Winfield, A.C.; Worrell, J.A.; Schuyler, P.A.; Plummer, W.D. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer 1993, 71, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Liebermann, E.; Patwardhan, V.; Usmanova, G.; Aktar, N.; Agrawal, S.; Bhamare, P.; McCarthy, M.; Ginsburg, O.; Kumar, S. Barriers to Follow-Up of an Abnormal Clinical Breast Examination in Uttar Pradesh, India: A Qualitative Study. JCO Glob. Oncol. 2024, 10, e2400001. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.C.; Sellers, T.A.; Frost, M.H.; Lingle, W.L.; Degnim, A.C.; Ghosh, K.; Vierkant, R.A.; Maloney, S.D.; Pankratz, V.S.; Hillman, D.W.; et al. Benign breast disease and the risk of breast cancer. N. Engl. J. Med. 2005, 353, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Babu, A.; Singh, S.; Lakhera, K.K.; Patel, P.; Gurjar, B.; Kumar, A.; Gora, B.S.; Sharma, R.G. Patterns and Prevalence of Benign Breast Diseases: An Epidemiological Chart Review Analysis of 5965 Patients from North Western India. Indian J. Surg. Oncol. 2025, 16, 1264–1269. [Google Scholar] [CrossRef]
- Sagiraju, H.K.R.; Gogia, A.; Goel, A.; Batra, A.; Sharma, D.; Saini, S.K.; Mishra, A.; Deo, S.; Prasad, C.P.; Tanwar, P.; et al. Effect of obesity on the survival outcomes among Indian women with breast cancer. J. Clin. Oncol. 2025, 43, e12558. [Google Scholar] [CrossRef]
- Sen, S.; Khan, P.K.; Wadasadawala, T.; Mohanty, S.K. Socio-economic and regional variation in breast and cervical cancer screening among Indian women of reproductive age: A study from National Family Health Survey, 2019–2021. BMC Cancer 2022, 22, 1279. [Google Scholar] [CrossRef]
- Chatterjee, D.; McDuffie, E.E.; Smith, S.J.; Bindle, L.; van Donkelaar, A.; Hammer, M.S.; Venkataraman, C.; Brauer, M.; Martin, R.V. Source Contributions to Fine Particulate Matter and Attributable Mortality in India and the Surrounding Region. Environ. Sci. Technol. 2023, 57, 10263–10275. [Google Scholar] [CrossRef]
- Rajbongshi, N.; Mahanta, L.B.; Nath, D.C. Evaluation of Female Breast Cancer Risk Among the Betel Quid Chewer: A Bio-Statistical Assessment in Assam, India. Nepal. J. Epidemiol. 2015, 5, 494–498. [Google Scholar] [CrossRef]
- Das, A.M.; Gogia, A.; Garg, M.; Elaiyaraja, A.; Arambam, P.; Mathur, S.; Babu-Rajendran, R.; Deo, S.; Kumar, L.; Das, B.C.; et al. Urinary concentration of endocrine-disrupting phthalates and breast cancer risk in Indian women: A case-control study with a focus on mutations in phthalate-responsive genes. Cancer Epidemiol. 2022, 79, 102188. [Google Scholar]
- Maurya, A.P.; Brahmachari, S. Association of hormonal and reproductive risk factors with breast cancer in Indian women: A systematic review of case-control studies. Indian J Cancer 2023, 60, 4–11. [Google Scholar] [CrossRef]
- Vishwakarma, G.; Ndetan, H.; Das, D.N.; Gupta, G.; Suryavanshi, M.; Mehta, A.; Singh, K.P. Reproductive factors and breast cancer risk: A meta-analysis of case-control studies in Indian women. South Asian J. Cancer 2019, 8, 80–84. [Google Scholar] [CrossRef]
- Parker, S.A.; Weygand, J.; Bernat, B.G.; Jackson, A.M.; Mawlawi, O.; Barreto, I.; Hao, Y.; Khan, R.; Yorke, A.A.; Swanson, W.; et al. Assessing Radiology and Radiation Therapy Needs for Cancer Care in Low-and-Middle-Income Countries: Insight From a Global Survey of Departmental and Institutional Leaders. Adv. Radiat. Oncol. 2024, 9, 101615. [Google Scholar] [CrossRef]
- Meeta, M.; Tanvir, T. Menopause Hormone Therapy in the Changing Trends of Breast Cancer in India. J. Midlife Health 2020, 11, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Clusan, L.; Ferriere, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef]
- Rodriguez-Casanova, A.; Costa-Fraga, N.; Castro-Carballeira, C.; González-Conde, M.; Abuin, C.; Bao-Caamano, A.; García-Caballero, T.; Brozos-Vazquez, E.; Rodriguez-López, C.; Cebey, V.; et al. A genome-wide cell-free DNA methylation analysis identifies an episignature associated with metastatic luminal B breast cancer. Front. Cell Dev. Biol. 2022, 10, 1016955. [Google Scholar] [CrossRef] [PubMed]
- Ulaganathan, K.; Puranam, K.; Mukta, S.; Hanumanth, S.R. Expression profiling of luminal B breast tumor in Indian women. J. Cancer Res. Clin. Oncol. 2023, 149, 13645–13664. [Google Scholar] [CrossRef]
- Prat, A.; Pascual, T.; De Angelis, C.; Gutierrez, C.; Llombart-Cussac, A.; Wang, T.; Cortés, J.; Rexer, B.; Paré, L.; Forero, A.; et al. HER2-Enriched Subtype and ERBB2 Expression in HER2-Positive Breast Cancer Treated with Dual HER2 Blockade. J. Natl. Cancer Inst. 2020, 112, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Prat, A.; Solovieff, N.; André, F.; O’SHaughnessy, J.; Cameron, D.A.; Janni, W.; Sonke, G.S.; Yap, Y.-S.; Yardley, D.A.; Partridge, A.H.; et al. Intrinsic Subtype and Overall Survival of Patients with Advanced HR+/HER2- Breast Cancer Treated with Ribociclib and ET: Correlative Analysis of MONALEESA-2, -3, -7. Clin. Cancer Res. 2024, 30, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zou, L.; Huang, S.; Miao, H.; Liu, K.; Geng, Y.; Liu, Y.; Wu, W. The anticancer activity of bile acids in drug discovery and development. Front. Pharmacol. 2024, 15, 1362382. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Luo, M.; Huang, J.; Zhang, K.; Zheng, S.; Zhang, S.; Zhou, J. Progression from ductal carcinoma in situ to invasive breast cancer: Molecular features and clinical significance. Signal Transduct. Target. Ther. 2024, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S. Molecular targets and therapies associated with poor prognosis of triple-negative breast cancer (Review). Int. J. Oncol. 2025, 66, 52. [Google Scholar] [CrossRef]
- Hassan, A.; Aubel, C. The PI3K/Akt/mTOR Signaling Pathway in Triple-Negative Breast Cancer: A Resistance Pathway and a Prime Target for Targeted Therapies. Cancers 2025, 17, 2232. [Google Scholar] [CrossRef]
- Pereira, C.; Martis, M.; D’Souza, R.; Tauro, L.F. Correlation of Clinicopathological Features of Breast Cancer with Molecular Subtypes Taking Ki-67 into Consideration: Single Institution Experience Over 5 Years. Curr. Health Sci. J. 2021, 47, 348–352. [Google Scholar] [CrossRef]
- Jonnada, P.K.; Sushma, C.; Karyampudi, M.; Dharanikota, A. Prevalence of Molecular Subtypes of Breast Cancer in India: A Systematic Review and Meta-analysis. Indian J. Surg Oncol. 2021, 12 (Suppl. S1), 152–163. [Google Scholar] [CrossRef]
- Siddiqui, B.; Ahmed, S.; Sinha, D.; Sharma, A.V.L. Molecular Classification of Breast Carcinoma in a Tertiary Hospital of India: The Recent Trends. Indian. J. Surg. Oncol. 2023, 14, 176–180. [Google Scholar] [CrossRef]
- Jain, S.; Narang, V.; Jain, K.; Paul, D.; Singh, J.; Sohi, A.S.; Sood, S.; Aggarwal, R.; Sood, N.; Brar, G.S. Prevalence of Molecular Subtypes in Operated Cases of Breast Cancer and Its Clinicopathological Correlation: A Single Institute Study from a Tertiary Cancer Centre in North India. Indian. J. Surg. Oncol. 2021, 12, 538–544. [Google Scholar] [CrossRef]
- Rathnasamy, N.; Mullapally, S.; Sirohi, B. Precision oncology in Low and Middle income countries: A word of caution. Int. J. Cancer Care Deliv. 2021, 1, 27–31. [Google Scholar] [CrossRef]
- Suresh AVSS, M.; Dadireddy, P.K. Cost-effectiveness analysis of gene expression profiling for breast cancer treatment decisions in India. Int. J. Community Med. Public Health 2025, 12, 2100–2102. [Google Scholar] [CrossRef]
- Gulwe, A.B.; Dawkhar, S.S. Role of SNP in the Breast Cancer Development of Indian Women. Int. J. Res. Innov. Appl. Sci. 2025, 10, 1160–1173. [Google Scholar] [CrossRef]
- Nirgude, S.; Desai, S.; Khanchandani, V.; Nagarajan, V.; Thumsi, J.; Choudhary, B. Integration of exome-seq and mRNA-seq using DawnRank, identified genes involved in innate immunity as drivers of breast cancer in the Indian cohort. PeerJ 2023, 11, e16033. [Google Scholar] [CrossRef] [PubMed]
- Sengar, M.; Pramesh, C.S.; Mehndiratta, A.; Shah, S.; Munshi, A.; Vijaykumar, D.K.; Puri, A.; Mathew, B.; Arora, R.S.; T, P.K.; et al. Ensuring quality in contextualised cancer management guidelines for resource-constraint settings: Using a systematic approach. BMJ Glob. Health 2022, 7, e009584. [Google Scholar] [CrossRef]
- Zhu, H.; Chua, M.L.K.; Chitapanarux, I.; Kaidar-Person, O.; Mwaba, C.; Alghamdi, M.; Mignola, A.R.; Amrogowicz, N.; Yazici, G.; Bourhaleb, Z.; et al. Global radiotherapy demands and corresponding radiotherapy-professional workforce requirements in 2022 and predicted to 2050: A population-based study. Lancet Glob. Health 2024, 12, e1945–e1953. [Google Scholar] [CrossRef]
- Sankarapillai, J.; Ramamoorthy, T.; Sarveswaran, G.; Venugopal, G.; Mathur, P. Epidemiological analysis of radiation therapy utilization and its implications for cancer care in India-insights from National Cancer Registry Programme. BMC Cancer 2025, 25, 1062. [Google Scholar] [CrossRef]
- Ghosh, A.; Chaubal, R.; Das, C.; Parab, P.; Das, S.; Maitra, A.; Majumder, P.P.; Gupta, S.; Biswas, N.K. Genomic hallmarks of endocrine therapy resistance in ER/PR+HER2- breast tumours. Commun. Biol. 2025, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Syed, Y.Y. Atezolizumab (in Combination with Nab-Paclitaxel): A Review in Advanced Triple-Negative Breast Cancer. Drugs 2020, 80, 601–607. [Google Scholar] [CrossRef]
- Laureano, R.S.; Sprooten, J.; Vanmeerbeerk, I.; Borras, D.M.; Govaerts, J.; Naulaerts, S.; Berneman, Z.N.; Beuselinck, B.; Bol, K.F.; Borst, J.; et al. Trial watch: Dendritic cell (DC)-based immunotherapy for cancer. Oncoimmunology 2022, 11, 2096363. [Google Scholar] [CrossRef]
- Pandey, P.; Chaudhary, R.; Tripathi, D.; Lavudi, K.; Dua, K.; Weinfeld, M.; Lavasanifar, A.; Rajinikanth, P.S. Personalized treatment approach for HER2-positive metastatic breast cancer. Med. Oncol. 2024, 41, 252. [Google Scholar] [CrossRef] [PubMed]
- Deepa, K.V.; Gadgil, A.; Lofgren, J.; Mehare, S.; Bhandarkar, P.; Roy, N. Is quality of life after mastectomy comparable to that after breast conservation surgery? A 5-year follow up study from Mumbai, India. Qual. Life Res. 2020, 29, 683–692. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Asaithamby, A. Repurposing DNA repair factors to eradicate tumor cells upon radiotherapy. Transl. Cancer Res. 2017, 6 (Suppl. S5), S822–S839. [Google Scholar] [CrossRef]
- Khan, A.; Sisodiya, S.; Aftab, M.; Tanwar, P.; Hussain, S.; Gupta, V. Mechanisms and Therapeutic Strategies for Endocrine Resistance in Breast Cancer: A Comprehensive Review and Meta-Analysis. Cancers 2025, 17, 1653. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; Dhanjal, J.K.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar]
- Rai, A.; Deshpande, S.G.; Vaidya, A.; Shinde, R.K. Advancements in Immunotherapy for Breast Cancer: Mechanisms, Efficacy, and Future Directions. Cureus 2024, 16, e68351. [Google Scholar] [CrossRef] [PubMed]
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef]
- Lloyd-Lewis, B.; Harris, O.B.; Watson, C.J.; Davis, F.M. Mammary Stem Cells: Premise, Properties, and Perspectives. Trends Cell Biol. 2017, 27, 556–567. [Google Scholar] [CrossRef]
- Soteriou, D.; Fuchs, Y. A matter of life and death: Stem cell survival in tissue regeneration and tumour formation. Nat. Rev. Cancer 2018, 18, 187–201. [Google Scholar] [CrossRef]
- Mascré, G.; Dekoninck, S.; Drogat, B.; Youssef, K.K.; Brohée, S.; Sotiropoulou, P.A.; Simons, B.D.; Blanpain, C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012, 489, 257–262. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.D.; Burness, M.L.; Wicha, M.S. Therapeutic Implications of Cellular Heterogeneity and Plasticity in Breast Cancer. Cell Stem Cell 2015, 17, 260–271. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, R.; Bowman, A.N.; Nusse, R. Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 2012, 11, 387–400. [Google Scholar] [CrossRef]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef]
- Pauli, C.; Hopkins, B.D.; Prandi, D.; Shaw, R.; Fedrizzi, T.; Sboner, A.; Sailer, V.; Augello, M.; Puca, L.; Rosati, R.; et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017, 7, 462–477. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.S.; Clark, A.S.; Tchou, J.; Zhang, P.; Freedman, G.M. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. S1), 9S–16S. [Google Scholar] [CrossRef]
- Coté, D.; Eustace, A.; Toomey, S.; Cremona, M.; Milewska, M.; Furney, S.; Carr, A.; Fay, J.; Kay, E.; Kennedy, S.; et al. Germline single nucleotide polymorphisms in ERBB3 and BARD1 genes result in a worse relapse free survival response for HER2-positive breast cancer patients treated with adjuvant based docetaxel, carboplatin and trastuzumab (TCH). PLoS ONE 2018, 13, e0200996. [Google Scholar] [CrossRef]
- Polgar, O.; Bates, S.E. ABC transporters in the balance: Is there a role in multidrug resistance? Biochem. Soc. Trans. 2005, 33 Pt 1, 241–245. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Gandhi, N.; Das, G.M. Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications. Cells 2019, 8, 89. [Google Scholar] [CrossRef]
- Raghav, D.; Sharma, V. An In Silico Evaluation of Deleterious Nonsynonymous Single Nucleotide Polymorphisms in the ErbB3 Oncogene. Biores. Open Access 2013, 2, 206–211. [Google Scholar] [CrossRef]
- Thussbas, C.; Nahrig, J.; Streit, S.; Bange, J.; Kriner, M.; Kates, R.; Ulm, K.; Kiechle, M.; Hoefler, H.; Ullrich, A.; et al. FGFR4 Arg388 allele is associated with resistance to adjuvant therapy in primary breast cancer. J. Clin. Oncol. 2006, 24, 3747–3755. [Google Scholar] [CrossRef]
- Mackenzie, P.I.; Owens, I.S.; Burchell, B.; Bock, K.W.; Bairoch, A.; Belanger, A.; Gigleux, S.F.; Green, M.; Hum, D.W.; Iyanagi, T.; et al. The UDP glycosyltransferase gene superfamily: Recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 1997, 7, 255–269. [Google Scholar] [CrossRef]
- Fojo, A.; Lebo, R.; Shimizu, N.; Chin, J.E.; Roninson, I.B.; Merlino, G.T.; Gottesman, M.M.; Pastan, I. Localization of multidrug resistance-associated DNA sequences to human chromosome 7. Somat. Cell Mol. Genet. 1986, 12, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Tashbaeva, R.E.; Hwang, D.N.; Song, G.S.; Choi, N.H.; Lee, J.H.; Lyoo, Y.S.; Lee, S.J.; Jung, D.I.; Kim, H.Y.; Sur, J.H. Cellular characterization of multidrug resistance P-glycoprotein, alpha fetoprotein, and neovascular endothelium-associated antigens in canine hepatocellular carcinoma and cirrhotic liver. Vet. Pathol. 2007, 44, 600–606. [Google Scholar] [CrossRef]
- Vulsteke, C.; Pfeil, A.M.; Schwenkglenks, M.; Pettengell, R.; Szucs, T.D.; Lambrechts, D.; Peeters, M.; van Dam, P.; Dieudonné, A.S.; Hatse, S.; et al. Impact of genetic variability and treatment-related factors on outcome in early breast cancer patients receiving (neo-) adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide, and docetaxel. Breast Cancer Res. Treat. 2014, 147, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Rha, S.Y.; Jeung, H.-.-C.; Im, C.-.-K.; Ahn, J.B.; Kwon, W.S.; Yoo, N.C.; Roh, J.K.; Chung, H.C. Association of the ABCB1 gene polymorphisms 2677G>T/A and 3435C>T with clinical outcomes of paclitaxel monotherapy in metastatic breast cancer patients. Ann. Oncol. 2009, 20, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Vishnukumar, S.; Umamaheswaran, G.; Anichavezhi, D.; Indumathy, S.; Adithan, C.; Srinivasan, K.; Kadambari, D. P-glycoprotein expression as a predictor of response to neoadjuvant chemotherapy in breast cancer. Indian J. Cancer 2013, 50, 195–199. [Google Scholar] [CrossRef]
- Mishra, D.; Patel, V.; Banerjee, D. Nitric Oxide and S-Nitrosylation in Cancers: Emphasis on Breast Cancer. Breast Cancer 2020, 14, 1178223419882688. [Google Scholar] [CrossRef]
- Devendran, A.; Nampoothiri, S.; Shewade, D.G.; Chatterjee, S.; Jayaraman, B.; Chandrasekharan, A. Allele, Genotype and Haplotype Structures of Functional Polymorphic Variants in Endothelial Nitric Oxide Synthase (eNOS), Angiotensinogen (ACE) and Aldosterone Synthase (CYP11B2) Genes in Healthy Pregnant Women of Indian Ethnicity. J. Reprod. Infertil. 2015, 16, 180–192. [Google Scholar]
- Coutinho, L.L.; Femino, E.L.; Gonzalez, A.L.; Moffat, R.L.; Heinz, W.F.; Cheng, R.Y.S.; Lockett, S.J.; Rangel, M.C.; Ridnour, L.A.; Wink, D.A. NOS2 and COX-2 Co-Expression Promotes Cancer Progression: A Potential Target for Developing Agents to Prevent or Treat Highly Aggressive Breast Cancer. Int. J. Mol. Sci. 2024, 25, 6103. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef]
- Loibl, S.; von Minckwitz, G.; Weber, S.; Sinn, H.; Schini-Kerth, V.B.; Lobysheva, I.; Nepveu, F.; Wolf, G.; Strebhardt, K.; Kaufmann, M. Expression of endothelial and inducible nitric oxide synthase in benign and malignant lesions of the breast and measurement of nitric oxide using electron paramagnetic resonance spectroscopy. Cancer 2002, 95, 1191–1198. [Google Scholar] [CrossRef]
- Liang, F.; Wang, M.; Li, J.; Guo, J. The evolution of S-nitrosylation detection methodology and the role of protein S-nitrosylation in various cancers. Cancer Cell Int. 2024, 24, 408. [Google Scholar] [CrossRef] [PubMed]
- Tualeka, A.R.; Jalaludin, J.; Gasana, J.; Sopian, N.A.; Chao, H.R.; Yusmaidie, M.; Perumal, V.; Zurimi, S.; Rahmawati, P.; Ahsan, A.; et al. Association between eNOS rs1799983 (894G>T) Polymorphism with Cancer and Stroke Risk: A meta-analysis. F1000Research 2024, 12, 1467. [Google Scholar] [CrossRef]
- Suszynska, M.; Kluzniak, W.; Wokolorczyk, D.; Jakubowska, A.; Huzarski, T.; Gronwald, J.; Debniak, T.; Szwiec, M.; Ratajska, M.; Klonowska, K.; et al. BARD1 is A Low/Moderate Breast Cancer Risk Gene: Evidence Based on An Association Study of the Central European p.Q564X Recurrent Mutation. Cancers 2019, 11, 740. [Google Scholar] [CrossRef]
- Hashizume, R.; Fukuda, M.; Maeda, I.; Nishikawa, H.; Oyake, D.; Yabuki, Y.; Ogata, H.; Ohta, T. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 2001, 276, 14537–14540. [Google Scholar] [CrossRef]
- Meza, J.E.; Brzovic, P.S.; King, M.C.; Klevit, R.E. Mapping the functional domains of BRCA1. Interaction of the ring finger domains of BRCA1 and BARD1. J. Biol. Chem. 1999, 274, 5659–5665. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine N-CHs. CD24 Molecule [Homo Sapiens (Human)]. 2023. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=100133941 (accessed on 22 November 2025).
- Schabath, H.; Runz, S.; Joumaa, S.; Altevogt, P. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J. Cell Sci. 2006, 119 Pt 2, 314–325. [Google Scholar] [CrossRef]
- Kwon, M.J.; Han, J.; Seo, J.H.; Song, K.; Jeong, H.M.; Choi, J.-S.; Kim, Y.J.; Lee, S.-H.; Choi, Y.-L.; Shin, Y.K. CD24 Overexpression Is Associated with Poor Prognosis in Luminal A and Triple-Negative Breast Cancer. PLoS ONE 2015, 10, e0139112. [Google Scholar] [CrossRef]
- Baumann, P.; Cremers, N.; Kroese, F.; Orend, G.; Chiquet-Ehrismann, R.; Uede, T.; Yagita, H.; Sleeman, J.P. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005, 65, 10783–10793. [Google Scholar] [CrossRef]
- Marmé, F.; Werft, W.; Walter, A.; Keller, S.; Wang, X.; Benner, A.; Burwinkel, B.; Sinn, P.; Hug, S.; Sohn, C.; et al. CD24 Ala57Val polymorphism predicts pathologic complete response to sequential anthracycline- and taxane-based neoadjuvant chemotherapy for primary breast cancer. Breast Cancer Res. Treat. 2012, 132, 819–831. [Google Scholar] [CrossRef]
- Zhou, X. CD24 polymorphisms cannot predict pathologic complete response to anthracycline- and taxane-based neoadjuvant chemotherapy in breast cancer. Clin. Breast Cancer 2014, 14, e33–e40. [Google Scholar] [CrossRef]
- Rao, U.S.C.; Devendran, A.; Satyamoorthy, K.; Shewade, D.G.; Krishnamoorthy, R.; Chandrasekaran, A. Functional characterization of promoter region polymorphisms of human CYP2C19 gene. Mol. Biol. Rep. 2011, 38, 4171–4179. [Google Scholar]
- Crewe, H.K.; Ellis, S.W.; Lennard, M.S.; Tucker, G.T. Variable contribution of cytochromes P450 2D6, 2C9 and 3A4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochem. Pharmacol. 1997, 53, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Artigalas, O.; Vanni, T.; Hutz, M.H.; Ashton-Prolla, P.; Schwartz, I.V. Influence of CYP19A1 polymorphisms on the treatment of breast cancer with aromatase inhibitors: A systematic review and meta-analysis. BMC Med. 2015, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bai, Y.X.; Zhou, J.H.; Sun, X.W.; Sui, H.; Zhang, W.J.; Yuan, H.H.; Xie, R.; Wei, X.L.; Zhang, T.T.; et al. A polymorphism at the 3′-UTR region of the aromatase gene is associated with the efficacy of the aromatase inhibitor, anastrozole, in metastatic breast carcinoma. Int. J. Mol. Sci. 2013, 14, 18973–18988. [Google Scholar] [CrossRef]
- Miron, L.; Negura, L.; Peptanariu, D.; Marinca, M. Research on aromatase gene (CYP19A1) polymorphisms as a predictor of endocrine therapy effectiveness in breast cancer. Rev. Med. Chir. Soc. Med. Nat. Iasi 2012, 116, 997–1004. [Google Scholar]
- Henry, N.L.; Chan, H.P.; Dantzer, J.; Goswami, C.P.; Li, L.; Skaar, T.C.; Rae, J.M.; Desta, Z.; Khouri, N.; Pinsky, R.; et al. Aromatase inhibitor-induced modulation of breast density: Clinical and genetic effects. Br. J. Cancer 2013, 109, 2331–2339. [Google Scholar] [CrossRef]
- Gor, P.P.; Su, H.I.; Gray, R.J.; A Gimotty, P.; Horn, M.; Aplenc, R.; Vaughan, W.P.; Tallman, M.S.; Rebbeck, T.R.; DeMichele, A. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: A retrospective cohort study. Breast Cancer Res. 2010, 12, R26. [Google Scholar] [CrossRef]
- Beelen, K.; Opdam, M.; Severson, T.M.; Koornstra, R.H.T.; Vincent, A.D.; Hauptmann, M.; van Schaik, R.H.N.; Berns, E.M.J.J.; Vermorken, J.B.; van Diest, P.J.; et al. CYP2C19 2 predicts substantial tamoxifen benefit in postmenopausal breast cancer patients randomized between adjuvant tamoxifen and no systemic treatment. Breast Cancer Res. Treat. 2013, 139, 649–655. [Google Scholar] [CrossRef]
- Johnston, S.; Dowsett, M.; Smith, I.; Sacks, N.; Haynes, B.; Jarman, M.; Ebbs, S. Acquired tamoxifen resistance in human breast cancer and reduced intra-tumoral drug concentration. Lancet 1993, 342, 1521–1522. [Google Scholar] [CrossRef]
- Satyanarayana, C.R.; Devendran, A.; Sundaram, R.; Gopal, S.D.; Rajagopal, K.; Chandrasekaran, A. Genetic variations and haplotypes of the 5′ regulatory region of CYP2C19 in South Indian population. Drug Metab. Pharmacokinet. 2009, 24, 185–193. [Google Scholar] [CrossRef]
- Anichavezhi, D.; Chakradhara Rao, U.S.; Shewade, D.G.; Krishnamoorthy, R.; Adithan, C. Distribution of CYP2C19*17 allele and genotypes in an Indian population. J. Clin. Pharm. Ther. 2012, 37, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Regan, M.M.; Leyland-Jones, B.; Bouzyk, M.; Pagani, O.; Tang, W.; Kammler, R.; Dell’Orto, P.; Biasi, M.O.; Thürlimann, B.; Lyng, M.B.; et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The breast international group 1-98 trial. J. Natl. Cancer Inst. 2012, 104, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Dezentjé, V.O.; Van Schaik, R.H.N.; Vletter-Bogaartz, J.M.; Van Der Straaten, T.; Wessels, J.A.M.; Kranenbarg, E.M.-K.; Berns, E.M.; Seynaeve, C.; Putter, H.; Van De Velde, C.J.H.; et al. CYP2D6 genotype in relation to tamoxifen efficacy in a Dutch cohort of the tamoxifen exemestane adjuvant multinational (TEAM) trial. Breast Cancer Res. Treat. 2013, 140, 363–373. [Google Scholar] [CrossRef]
- Neven, P.; Jongen, L.; Lintermans, A.; Van Asten, K.; Blomme, C.; Lambrechts, D.; Poppe, A.; Wildiers, H.; Dieudonné, A.-S.; Brouckaert, O.; et al. Tamoxifen Metabolism and Efficacy in Breast Cancer: A Prospective Multicenter Trial. Clin. Cancer Res. 2018, 24, 2312–2318. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.; Dezentjé, V.; Swen, J.; Moes, D.J.A.r.; Böhringer, S.; Batman, E.; Van Druten, E.; Smorenburg, C.; Van Bochove, A.; Zeillemaker, A.; et al. Tamoxifen Pharmacogenetics and Metabolism: Results From the Prospective CYPTAM Study. J. Clin. Oncol. 2019, 37, 636–646. [Google Scholar] [CrossRef]
- Kadri, M.S.N.; Patel, K.M.; Bhargava, P.A.; Shah, F.D.; Badgujar, N.V.; Tarapara, B.V.; Patel, P.S.; Shaikh, M.I.; Shah, K.; Patel, A.; et al. Mutational Landscape for Indian Hereditary Breast and Ovarian Cancer Cohort Suggests Need for Identifying Population Specific Genes and Biomarkers for Screening. Front. Oncol. 2020, 10, 568786. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.R.; Joghee, S. Enhancing breast cancer treatment through pharmacogenomics: A narrative review. Clin. Chim. Acta 2024, 562, 119893. [Google Scholar] [CrossRef]
- Jadeja, N.; Rajakumar, N.; Reddy, N.; Ali, N.; Lichten, L. Reflections on my international genetic counseling rotations: Contrasts in practice between India and the United States. Genet. Med. Open 2024, 2 (Suppl. S2), 101871. [Google Scholar] [CrossRef]
- Damodaran, S.E.; Pradhan, S.C.; Umamaheswaran, G.; Kadambari, D.; Reddy, K.S.; Adithan, C. Genetic polymorphisms of CYP2D6 increase the risk for recurrence of breast cancer in patients receiving tamoxifen as an adjuvant therapy. Cancer Chemother. Pharmacol. 2012, 70, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Sharma, S.; Behera, D.; Singh, N. UGT1A1 Gene Polymorphisms in Patients with Small Cell Lung Cancer Treated with Irinotecan-Platinum Doublet Chemotherapy and Their Association with Gastrointestinal Toxicity and Overall Survival. Oncologist 2021, 26, 701–713. [Google Scholar] [CrossRef]
- Gudur, R.A.; Bhosale, S.J.; Gudur, A.K.; Kale, S.R.; More, A.L.; Datkhile, K.D. Influence of (C1236T and C3435T) Polymorphisms of ABCB1 Gene on Chemotherapy Treatment Outcome and Toxicity in Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2024, 25, 1567–1577. [Google Scholar] [CrossRef]
- Behlam, I.; Sudershan, A.; Bharti, S.; Saini, A.K.; Manhas, S.; Kumar, P. Association of GSTT1 & GSTM1 Null Mutations with Breast Cancer Risk in North Indian Population: A Case-Control Study. Afr. J. Biomed. Res. 2024, 27, 4016–4022. [Google Scholar]
- Bhaskaran, S.P.; Huang, T.; Rajendran, B.K.; Guo, M.; Luo, J.; Qin, Z.; Zhao, B.; Chian, J.; Li, S.; Wang, S.M. Ethnic-specific BRCA1/2 variation within Asia population: Evidence from over 78 000 cancer and 40 000 non-cancer cases of Indian, Chinese, Korean and Japanese populations. J. Med. Genet. 2021, 58, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, J.; Gutierrez, C.; Sethunath, V.; Mehravaran, S.; Giuliano, M.; Shea, M.J.; Mitchell, T.; Wang, T.; Nanda, S.; Pereira, R.; et al. Neratinib plus trastuzumab is superior to pertuzumab plus trastuzumab in HER2-positive breast cancer xenograft models. NPJ Breast Cancer 2021, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.P.; Avaronnan, M.; Devi, N.; Praveen Kumar Shenoy, V.P. Her2 positive metastatic breast cancer treated with low dose lapatinib in a resource-constrained setting in South India: A retrospective audit. Ecancermedicalscience 2024, 18, 1758. [Google Scholar] [CrossRef]
- Ghosh, M.; Saha, S.; Bettke, J.; Nagar, R.; Parrales, A.; Iwakuma, T.; van der Velden, A.W.; Martinez, L.A. Mutant p53 suppresses innate immune signaling to promote tumorigenesis. Cancer Cell 2021, 39, 494–508.e5. [Google Scholar] [CrossRef]
- Akhter, N.; Dar, S.A.; Chattopadhyay, S.; Haque, S.; Anwer, R.; Wahid, M.; Jawed, A.; Lohani, M.; Mandal, R.K.; Shukla, N.K.; et al. Impact of p53 arg72pro SNP on Breast Cancer Risk in North Indian Population. Curr. Genom. 2018, 19, 395–410. [Google Scholar] [CrossRef]
- Waseem, M.; Hussain, S.R.; Kumar, S.; Serajuddin, M.; Mahdi, F.; Sonkar, S.K.; Bansal, C.; Ahmad, M.K. Association of MTHFR (C677T) Gene Polymorphism with Breast Cancer in North India. Biomark. Cancer 2016, 8, 111–117. [Google Scholar] [CrossRef]
- Kuras, S.; Pence, M.E.; Arac, E.; Yildiz, A.; Erdogan, B.; Pence, S. Investigation of the effects of MTHFR gene variations and homocysteine levels in hypertensive patients. North. Clin. Istanb. 2025, 12, 55–61. [Google Scholar] [CrossRef]
- Rajagopal, T.; Seshachalam, A.; Rathnam, K.K.; Talluri, S.; Venkatabalasubramanian, S.; Dunna, N.R. Homologous recombination DNA repair gene RAD51, XRCC2 & XRCC3 polymorphisms and breast cancer risk in South Indian women. PLoS ONE 2022, 17, e0259761. [Google Scholar]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, S.; Rao, M.; Cheng, B. Tumor-derived extracellular vesicles: Key drivers of immunomodulation in breast cancer. Front. Immunol. 2025, 16, 1548535. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I. Hypoxia-driven angiogenesis in breast cancer mechanisms and therapeutic targets: A narrative review. Ann. Med. Surg. 2025, 87, 4246–4254. [Google Scholar] [CrossRef]
- Gill, G.S.; Kharb, S.; Goyal, G.; Das, P.; Kurdia, K.C.; Dhar, R.; Karmakar, S. Immune checkpoint inhibitors and immunosuppressive tumor microenvironment: Current challenges and strategies to overcome resistance. Immunopharmacol. Immunotoxicol. 2025, 47, 485–507. [Google Scholar] [CrossRef]
- McDonald, J.A.; Rao, R.; Gibbons, M.; Janardhanan, R.; Jaswal, S.; Mehrotra, R.; Pandey, M.; Radhakrishnan, V.; Ramakant, P.; Verma, N.; et al. Symposium report: Breast cancer in India-trends, environmental exposures and clinical implications. Cancer Causes Control 2021, 32, 567–575. [Google Scholar] [CrossRef]
- ICGA. Teams—Indian Cancer Genome Atlas: Mapping India’s Cancer Genome SD, Ankita Singh. The Indian Cancer Genome Atlas: A Multi-Omics Resource for Advancing Cancer Research. bioRxiv 2025. [Google Scholar]
- Bhattacharya, S.; Varshney, S.; Heidler, P.; Tripathi, S.K. Expanding the horizon for breast cancer screening in India through artificial intelligent technologies -A mini-review. Front. Digit. Health 2022, 4, 1082884. [Google Scholar] [CrossRef]
- Mangayarkarasi, V.; Durairaj, E.; Ramanathan, V. Enhancing Cancer Screening and Early Diagnosis in India: Overcoming Challenges and Leveraging Emerging Technologies. Cureus 2025, 17, e78808. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, D.; Lockhat, Z.; Brzozowski, L.; Saini, K.S.; Dlamini, Z.; Hull, R. The Convergence of Radiology and Genomics: Advancing Breast Cancer Diagnosis with Radiogenomics. Cancers 2024, 16, 1076. [Google Scholar] [CrossRef]
- Adapa, K.; Gupta, A.; Singh, S.; Kaur, H.; Trikha, A.; Sharma, A.; Rahul, K. A real world evaluation of an innovative artificial intelligence tool for population-level breast cancer screening. npj Digit. Med. 2025, 8, 2. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Saleem, S.M.; Singh, A.; Singh, S.; Tripathi, S. Empowering precision medicine: Regenerative AI in breast cancer. Front. Oncol. 2024, 14, 1465720. [Google Scholar] [CrossRef]
- Das, S.K.; Dasgupta, R.K.; Roy, S.D.; Shil, D. AI in Indian healthcare: From roadmap to reality. Intell. Pharm. 2024, 2, 329–334. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.S.; Sasmal, A.; Ariraman, S.B.M.; Raj, T.; Selvaraj, V.; Arockiarajan, A.; Sudhakar, S. Cobalt-nickel metal-organic frameworks (CNMs) as drug delivery agents for triple-negative breast cancer. Nanoscale Adv. 2025, 7, 5361–5376. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Das, T.; Kushwaha, R.; Misra, A.K.; Jana, K.; Das, D. Targeted and precise drug delivery using a glutathione-responsive ultra-short peptide-based injectable hydrogel as a breast cancer cure. Mater. Horiz. 2025, 12, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Press Information Bureau (PIB), G.o.I. New Compound Synthesised Can Combat Aggressive Breast Cancer: Press Information Bureau (PIB), Government of India; 2025 [Cited Government of India]. Available online: https://www.pib.gov.in/PressReleasePage.aspx?PRID=2162654 (accessed on 22 November 2025).
- Sangwan, R.K.; Huda, R.K.; Panigrahi, A.; Toteja, G.S.; Sharma, A.K.; Thakor, M.; Kumar, P. Strengthening breast cancer screening program through health education of women and capacity building of primary healthcare providers. Front. Public Health 2023, 11, 1276853. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Yadav, K. Breast cancer in India: Present scenario and the challenges ahead. World J. Clin. Oncol. 2022, 13, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Jamwal, N.; Gupta, R. The Chiraiya project: A retrospective analysis of breast cancer detection gaps addressed via mobile mammography in Jammu Province, India. BMC Public Health 2024, 24, 2087. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devendran, A.; Perumal, S. Navigating the Molecular and Cellular Landscape of Breast Cancer in India: From Unique Pathogenesis to the Promise of Personalized Medicine and Future Technologies. Targets 2025, 3, 38. https://doi.org/10.3390/targets3040038
Devendran A, Perumal S. Navigating the Molecular and Cellular Landscape of Breast Cancer in India: From Unique Pathogenesis to the Promise of Personalized Medicine and Future Technologies. Targets. 2025; 3(4):38. https://doi.org/10.3390/targets3040038
Chicago/Turabian StyleDevendran, Anichavezhi, and Sivasankar Perumal. 2025. "Navigating the Molecular and Cellular Landscape of Breast Cancer in India: From Unique Pathogenesis to the Promise of Personalized Medicine and Future Technologies" Targets 3, no. 4: 38. https://doi.org/10.3390/targets3040038
APA StyleDevendran, A., & Perumal, S. (2025). Navigating the Molecular and Cellular Landscape of Breast Cancer in India: From Unique Pathogenesis to the Promise of Personalized Medicine and Future Technologies. Targets, 3(4), 38. https://doi.org/10.3390/targets3040038








