Abstract
Breast cancer is a substantial and growing public health issue in India, with epidemiological data demonstrating distinct and often severe disease characteristics in contrast to Western countries. Contrary to the global trend, Indian women frequently develop the disease at an earlier age and tend to present with more advanced stages, emphasizing important variations in disease pathophysiology. This review compiles and critically evaluates the current literature to describe the specific pathophysiology of breast cancer in the Indian population. We investigate the unique cellular and molecular landscapes, evaluate the impact of specific Indian demographic and genetic features, and highlight crucial gaps in knowledge, diagnostic tools, and therapeutic approaches. The assessment reveals a molecular landscape determined by the incidence of specific tumor subtypes; triple-negative breast cancer, for instance, is frequently diagnosed in younger women, and genetic profiling research suggests variations in its susceptibility genes and mutation patterns when compared to global populations. While this paper brings together recent advancements, it highlights the challenges of adopting global diagnostic and treatment guidelines in the Indian healthcare system. These challenges are largely due to variances and specific demographic and socioeconomic discrepancies that create substantial hurdles for timely diagnosis and patient care. We highlight significant gaps, such as the need for more complete multi-omics profiling of Indian patient cohorts, an absence of uniform and readily available screening programs, and shortcomings in healthcare infrastructure and qualified oncology experts. Furthermore, the review highlights the crucial need for therapeutic strategies tailored to the distinct genetic and demographic profiles of Indian breast cancer patients. We present significant strategies for addressing these challenges, with a focus on integrating multi-omics data and clinical characteristics to gain deeper insight into the underlying causes of the disease. Promising avenues include using artificial intelligence and advancements in technology to improve diagnostics, developing indigenous and affordable treatment options, and establishing context-specific research frameworks for the Indian population. This review also underlines the necessity for personalized strategies to improve breast cancer outcomes in India.
1. Introduction
Breast cancer (BC) continues to pose a major public health challenge globally, with an estimated 2.3 million new cases and 685,000 deaths reported worldwide in 2020. While the incidence rates are high in developed countries, the burden is growing rapidly in developing nations such as India. In fact, in India, BC has surpassed other type of cancers to become the most common among women, with concerning forecasts for future incidence and mortality [1]. A notable and significant contrast in the Indian demographic is the average age at diagnosis, which is roughly ten years younger than in Western populations. Additionally, greater percentage of patients are diagnosed with more severe or aggressive characteristics, such as triple-negative breast cancer (TNBC) [2]. Effective management of cancer involves more than just treating the physical illness; it also needs to consider the enormous psychosocial burden that patients suffer. Being diagnosed with and treated for BC can be deeply distressing, with a high incidence of psychiatric morbidities, including sadness and anxiety. This aside, unmanaged psychological distress, including depression, has shown to adversely affect a patient’s quality of life, adherence to treatment regimens, and survival rates [3].
Breast cancer is an extremely diverse disease; its underlying molecular basis and receptor types primarily determine its prognosis and response to therapy. The global perspective of BC molecular subtypes (luminal A/B, HER2+, and TNBC) often guides therapeutic regimens. Nevertheless, research indicates that the prevalence of these subtypes differs greatly by ethnicity [4]. In India, aggressive subtypes, particularly TNBC, are reported at a higher rate than in Western countries. Additionally, distinct genetic susceptibilities, including particular mutations in BRCA1 and BRCA2, along with other novel gene variants, have been discovered in the diverse Indian gene pool, potentially influencing the early onset and severe progression of the disease. The complete scope and significance of this unique cellular and molecular landscape remains under explored.
This unique Indian BC clinical picture indicates possible variations in the underlying cellular and molecular etiology. Understanding these distinct abnormalities at the genetic and epigenetic levels is critical, as global data and treatment regimens may not be completely applicable to the Indian subcontinent. Regardless of the growing burden and apparent biological disparities, there are considerable gaps in our knowledge and management of BC in India. A key problem is the lack of a comprehensive national cancer registry and large-scale etiological research to determine the causes of early onset and distinct molecular profiles [5]. Setting this aside, diagnostic strategies are often hampered by poor public awareness and limited access to structured screening programs, which results in more advanced diagnoses. In terms of treatment, although there are internationally recognized guidelines, the expensive nature of specialized therapies (such as Trastuzumab), coupled with uneven distribution across the country, reveals significant healthcare inequities, creating a gap in effective treatment for all patients [6]. Therefore, there is an urgent need for research aimed at developing and validating new biomarkers, and for innovative treatment approaches for the Indian population.
Nonetheless, the emergence of frontier technological innovations provides hope for addressing these challenges. For example, programs like the Bharat Cancer Genome Atlas are tailoring advanced techniques, including large-scale genomics and multi-omics profiling to the heterogeneous Indian population [7]. Additionally, the integration of artificial intelligence (AI) and machine learning (ML) is transforming diagnostic options, enabling enhanced image examination and forecasting models tailored to the specific patient demographics of India. These advancements, featuring affordable screening tools, signify an essential progress towards more equitable and efficient cancer treatment.
This review integrates current knowledge of the distinct pathogenesis of breast cancer among the Indian population. It provides an overview of its diverse cellular and molecular landscape in comparison to global understanding and investigates the interplay of demographic, genetic, and environmental factors influencing these discrepancies. Additionally, this paper detects and examines crucial knowledge gaps in the current understanding of the diagnostic procedures and therapeutic strategies in the Indian subcontinent. It also proposes directions for future research and policy changes to improve patient outcomes. Finally, this review will highlight the recent developments and proposes significant future prospects, with an emphasis on Indian-specific innovations in genomics, multi-omics profiling, and artificial intelligence.
2. Breast Cancer Landscape from Indian Epidemiological Context
Breast cancer, as mentioned above, is the most common malignancy in women worldwide. With an estimated 2.3 million new cases, accounting for 11.7% of all cancer cases, it has in fact overtaken lung cancer as the leading cause of cancer incidence worldwide in 2020 [8]. According to epidemiological studies, the global implication and significance of BC is projected to surpass nearly 2 million by 2030 [9]. Especially, looking at the circumstance through an Indian perspective, the prevalence has escalated substantially, by nearly 50% between 1965 and 1985 [10]. To further illustrate this, in 2016 the predicted number of incident cases in India was 118,000 (with 95% confidence interval, 107,000 to 130,000), with women accounting for 98.1% of the total, and prevalent cases accounting for 526,000 (474,000 to 574,000). To highlight the severity, from 1990 to 2016, the age-standardized frequency rate of BC in women augmented by 39.1% (95% CI, 5.1 to 85.5), with an upsurge detected in every state of the country [11]. In fact, according to the Globocan data 2020, BC reckoned for 10% (Figure 1 and Figure 2) [12].
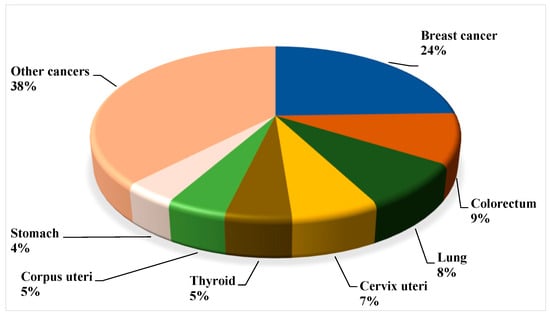
Figure 1.
Globally assessed novel cancer cases among women in 2020 (all ages), based on the data from the World Health Organization (WHO, 2020).
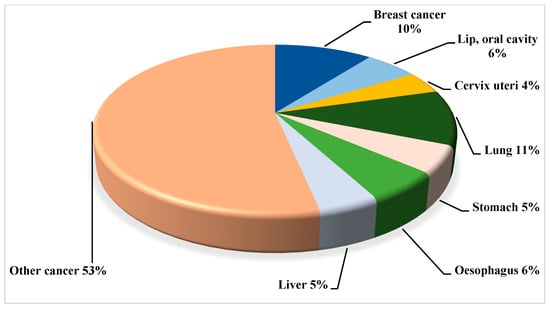
Figure 2.
Projected relative cancer burden (based on Disability Adjusted Life Years—DALYs) in India for 2030; data based on the sources such as the Global Cancer Observatory (GLOBOCAN).
In light of the facts presented, and in accordance with the existing trends, a fairly elevated proportion or incidence of the disease was seen to manifest among younger age groups among the Indian women in contrast to their Western counterparts. Furthermore, the National Cancer Registry Program investigated the statistical records from cancer depositories and registries between the years 1998 and 2013 in order to scrutinize the diversity and fluctuation in cancer prevalence. Eventually, the outcomes that ensued from the above examinations in fact revealed a compelling rise in trend of BC. In 1990, the cervix was the prominent site of cancer in India, followed by BC, in Bangalore (23.0% vs. 15.9%), Bhopal (23.2% vs. 21.4%), Chennai (28.9% vs. 17.7%), and Delhi (21.6% vs. 20.3%), respectively, whereas the breast was the prominent site of cancer in Mumbai (24.1% vs. 16.0%), as reported from the above registries. However, from 2000 to 2003 the scenario changed, with breast cancer becoming the prominent cancer site in all registries except the Barshi rural registry (16.9% vs. 36.8%). And noticeably in the case of BC, the registries in Bhopal, Chennai, and Delhi showed a considerable rising trend [13].
To further illustrate the point of conformity, patients with breast cancer have a lower survival rate in India than in Western countries, owing to their early age at the time of onset, the delayed initiation of specific and precise supervision, the late stage of the disease at the time of presentation, and ineffective/imploded treatment regimens [14].
3. Overall Global Understanding of Breast Cancer with the Indian Demographic Characteristics
3.1. Gaps in Knowledge
Compared to Western populations, Indian women have distinct BC characteristics, such as a higher incidence of premenopausal cases, a higher percentage of advanced-stage diagnoses, and a younger average age of diagnosis (40–50 years vs. 60–70 years). Moreover, there is evidence that triple-negative breast cancer (TNBC) is more common in India [15]. In addition, although BRCA1/2 mutations are known risk factors, studies indicate that Indian BC patients may have a higher incidence of these mutations than Western populations, as well as indicating a notable role for non-BRCA genes that warrants additional research.
In India, immense knowledge gaps persist regarding the precise role and function of cancer stem cells (CSCs), particularly in tumor development and progression. These gaps encompass the understanding of the basic attributes and prevalence of CSCs among Indian breast cancer patients. Epigenetic and genetic factors, distinctive to the Indian population, consequently modify CSC function, ultimately impacting therapeutic strategies that target CSCs [16]. Understanding the heterogeneity of cancer stem cells (CSCs) across various breast cancer (BC) subtypes in India and their interaction with the tumor microenvironment is undeniably crucial. This knowledge will enhance therapeutic strategies and improve treatment outcomes. Furthermore, developing these strategies necessitates a deep understanding of how this heterogeneity differs among BC subtypes in India. Therefore, more research is required to pinpoint the precise pathways and mechanisms influencing CSC behavior in these different subtypes. A collaborative, interdisciplinary approach uniting scientists, healthcare professionals, and experts in cell biology, cancer genomics, and pharmacology can bridge these knowledge gaps and illuminate the complexities of CSC biology and its role in BC in India [16].
Similarly, for precise risk assessment, timely diagnosis, and the development of precision/personalized medicine, it is essential to comprehend the unique molecular and genetic characteristics among the Indian breast cancer patients. In fact, studies are underway to determine distinct mutations (e.g., TP53 and PIK3CA) and their possible effects for personalized treatment approaches [17].
In India, socioeconomic and cultural barriers—including stigma, lack of awareness, and financial hardship—contribute to delayed breast cancer diagnosis and worse outcomes, particularly in rural regions. Therefore, to acquire a thorough understanding about the predisposing factors for BC among Indian women, studies should investigate the relationship between cultural traditions, lifestyle modifications, and genetic predispositions, which are deeply rooted in one another. This aside, with the fair amount of evidence corroborating the implication of environmental factors and lifestyle modifications, research demonstrates possible connections between BC risk and environmental pollutions in air and water, along with urbanization, dietary habits, lack of physical activity, etc., [18].
3.2. Gaps in Diagnostic and Therapeutic Approaches or Methodologies
Despite the fact that early detection of BC makes it treatable, numerous gaps in diagnostic and therapeutic approaches result in higher death rates in India than in Western nations. These disparities are especially apparent when taking into account the distinct demographics and the socioeconomic status of the Indian population as discussed below.
In terms of diagnostics, there is limited access to screening and uptake, regardless of some governmental initiatives. Breast cancer screening program participation is very low, particularly among lower socioeconomic groups and in rural regions. This could be because of a lack of knowledge and awareness, as several women are oblivious about the signs of BC and the significance of early identification [19]. Logistical challenges such as a lack of transportation, a long remote distance to screening amenities, and the necessity for a personal care assistant or attendant further hinder easy access, especially for women from rural areas [20]. This aside, Indian women typically have denser breast tissue which may make the common screening procedures, like mammography, less effective.
In addition, given all the specific and unique BC characteristics among the Indian population, there is an immense need for biomarkers that are specific to the Indian population. Therefore, additional studies and research are needed to detect genetic and epigenetic biomarkers unique to the Indian demography for timely discovery and for further individualized screening approaches [19].
Also given the severity from the genetic and molecular heterogeneity standpoint, the role of tumor microenvironment heterogeneity (TME) has a major impact on treatment resistance, metastasis, and tumor progression. Indeed, research studies focusing on the unique traits and variability of the TME among the Indian BC patients will be vital [1].
Similarly, from limited genetic testing perspective, regardless of the fact that BRCA mutations are more common in India, there is little access to genetic testing and counseling. In addition, further study is needed to determine the clinical significance of variations of unknown significance (VUS) mutations among the Indian population in order to guide treatment selection strategies [21].
However, from therapeutics standpoint, despite the recent medical advances, there is still very few high-quality, multimodal treatment centers, particularly in rural areas. The overall burden is compounded by a severe lack of medical resources, including proper infrastructure and essential equipment like radiotherapy units, and a shortage of qualified oncology specialists. Inadequate care from non-oncologists and financial restrictions lead to high rates of treatment attrition. Furthermore, the scope is complicated by limited access to expensive, novel therapies such as immunotherapy and personalized medicine [22].
On the other hand, with the inadequate studies and reports available on cancer biology from epidemiological and factors unique to India, additional research is crucial to define the frequency of molecular subtypes of BC. This, in turn, will help determine the precise genetic and environmental features that contributes to the disease. In addition, given the severity, and notwithstanding the detailed cancer surveillance initiatives, there is a lack of valid and detailed information on frequency, incidence, and mortality, which in turn impedes the implementation of successful public health interventions.
Therefore, addressing the above gaps implies the essential need to promote better public awareness, and to sensitize the public especially women about the signs and risk factors of BC, as well as the significance of early and prompt detection. Reinforcing and reducing the barriers to screening resources and services through community-centered programs, collaborations with primary healthcare personnel, and mobile screening camps may further help overcome the geographical and socioeconomic obstacles. Improving the BC management landscape requires strategic investment in healthcare personnel training, medical device accessibility, and cancer center expansion. Concurrently, enhancing policy measures—specifically through reinforced national policies, higher healthcare funding, and streamlined financial aid—is vital for addressing current shortcomings.
Ultimately, to devise successful prevention and treatment strategies, further studies and research comprising a comprehensive understanding of the multifaceted nature of BC in terms of genetics, environments, and lifestyle from Indian context, are crucial. This may, in turn, highlight the importance of investing in personalized medicine and tailored treatment approaches.
4. Genetics and Genomics: Implication of Genetic Susceptibility as a Significant Risk Factor for Breast Cancer
Essentially, a risk factor is something that affects the likelihood of developing breast cancer (BC). However, some major risk factors for BC are beyond an individual’s control, such as gender and age. For example, the disease is about 100 times more common in women than in men, and the majority of breast cancers are diagnosed in women aged 55 and older, illustrating that aging increases risk. Additionally, having a first-degree relative (mother, sister, or daughter) diagnosed with BC almost doubles a woman’s possibility of developing it [23,24].
In general, genetic mutations acquired from a parent are linked to about 5–10% of BC cases. And, in fact, an inherited mutation in the BRCA1 or BRCA2 gene is the most common cause of hereditary BC [25]. This aside, women with a BRCA1 mutation pose a 55–65% lifetime possibility of developing BC. And on the other hand, the lifetime possibility for women with a BRCA2 mutation is about 45%. Furthermore, a woman with both BRCA1 and BRCA2 gene mutation has a 70% possibility of developing BC by the age of 80. BRCA1/2 mutations significantly increase lifetime breast cancer (BC) and ovarian cancer risks, with affected individuals often diagnosed at a younger age. The risk of developing BC in the contralateral breast is also elevated. A positive family history further correlates with increased risk. Specific risks vary by gene: BRCA1 mutations are rarely associated with male breast cancer, while BRCA2 mutations carry a modest lifetime male BC risk of approximately 6.8% [25] (Figure 3 and Figure 4).
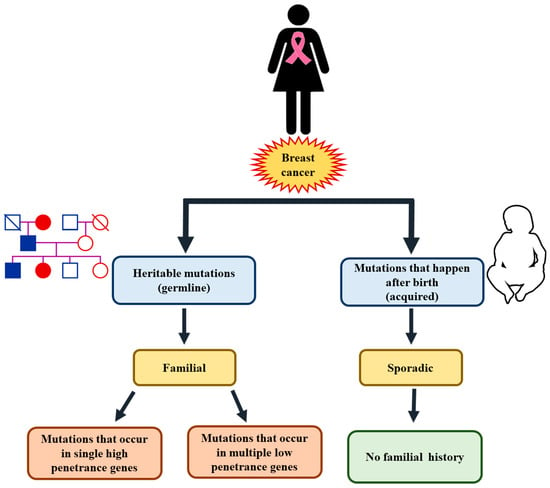
Figure 3.
Genetic susceptibility as a significant risk factor for breast cancer.
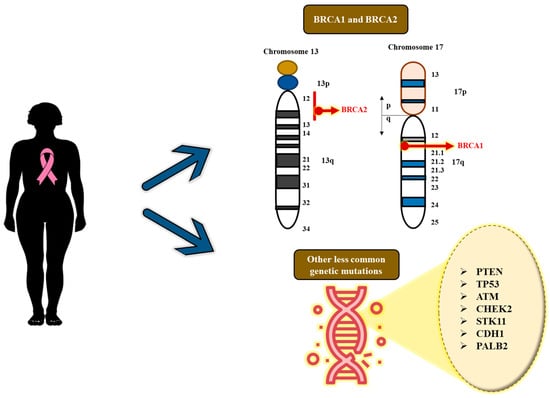
Figure 4.
Significant genetic mutations in breast cancer.
This aside, genetic mutations in numerous other genes can also lead to the development of BC, though they are less common than the BRCA mutations and the possibility of developing BC risk is relatively low [25]. A few of these mutated genes include PTEN (mutations acquired from this gene can result in Cowden’s syndrome, along with the susceptibility to non-cancerous and cancerous tumors in the breasts, in addition to growths in the thyroid, digestive tract, ovaries and uterus), TP53 (mutations of this gene results in Li-Fraumeni syndrome with an augmented susceptibility to BC, and few other cancers like leukemia, brain tumors, and sarcomas), ATM (acquiring two aberrant copies of this gene causes the disease ataxia–telangiectasia), CHEK2 (mutations in this gene can increase the liability to BC by approximately twofold), STK11 (mutations in this gene can result in Peutz–Jeghers syndrome with an increased susceptibility to several other types of cancer in addition to BC), CDH1 (these genetic mutations can result in the genetic diffuse gastric cancer with high predisposition to the invasive lobular BC), and finally PALB2 (this gene generates a protein that binds with the protein made by the BRCA2 gene, consequently resulting in mutations in this gene with a higher risk of BC) [25].
Genetic screening for mutations in genes like BRCA1, BRCA2, PTEN, and TP53 is beneficial for timely breast cancer (BC) detection and prevention in high-risk women. However, it is crucial to understand the limitations and potential drawbacks of these tests. Furthermore, the high cost of genetic testing means it is not universally applicable, and its practical use should be weighed carefully as not all women require this screening [25].
Moreover, in the context of the Indian scenario, studies indicate that Indian BC patients have a considerably higher frequency and prevalence of BRCA1 and BRCA2 mutations when compared to their Western counterparts, with one study indicating a prevalence of 29.1%. However, among the Western populations, the incidence of these mutations is normally between 5 and 10%. Additionally, although BRCA mutations are linked to early-stage BC worldwide, the association is particularly prominent in India, as studies demonstrate a substantial connection between being BRCA-positive and the occurrence of BC in younger women ranging between 20 and 45 years of age [26].
The impact of susceptibility genes like TP53, PTEN, CDH1, ATM, CHEK2, and PALB2 in India is indeed substantial for evaluating cancer risk, screening, therapeutic management, and genetic counseling. However, the thorough detailed information tailored to the Indian population is still emerging. Nevertheless, due to India’s genetic and socioeconomic variability, the incidence, expression, and the relationships between the genotype and phenotype for these genes may differ from those in the Western population [27].
However, with respect to risk assessment and penetrance, studies suggest that the range of mutations in these genes among the Indian population may show distinct disparities when compared to their Western counterparts. For example, a study in the Kashmir valley discovered a high prevalence of TP53 mutations in patients with colon cancer, indicating that it is a major contributor to this population’s elevated risk [28]. Similarly, penetrance or the risk of developing cancer because of a mutation may vary depending on the particular variant, or other genetic or environmental factors. The range of malignancies in families with Li-Fraumeni syndrome (TP53 mutation) can differ from Western cohorts, with a potentially increased occurrence of particular forms such as sarcomas [29].
This aside, numerous research studies have discovered that the mutation spectrum of cancer susceptibility genes in the Indian population is quite diverse, with notable geographical heterogeneity. In addition, although mutations in BRCA1 and BRCA2 are the most frequent, other genes are also important contributors. Germline pathogenic mutations have a higher incidence and are frequently associated with early-onset cancer.
According to studies, the comprehensive incidence of pathogenic BRCA1/2 mutations in high-risk Indian breast and ovarian cancer patients is between 20% and 30%, which is greater than that found in Western populations. In some cohorts, BRCA1 mutations are more common than BRCA2, which contrasts with many other Asian ethnicities. This aligns with India’s high prevalence of triple-negative breast cancer (TNBC), and early-onset breast cancer, which are frequently associated with BRCA1 [26,30].
This aside, numerous recurring mutations (as discussed below) have been reported, with diverse frequency across regions.
- c.68_69delAG (185delAG) in BRCA1: This mutation, which is a founder mutation in Ashkenazi Jewish communities is recurrent and seems to have originated independently among the South and West Indian populations.
- c.1961delA in BRCA1: This is one of the most common mutations discovered in current studies.
- c.5074+1G>A in BRCA1: This is most common among West Indian and other populations.
- c.5382insC in BRCA1: This is another frequently occurring pathogenic mutation.
Similarly, numerous studies among the Indian population have reported novel BRCA1/2 mutations not previously cataloged in databases like ClinVar, thus emphasizing the distinct genetic diversity of the Indian subcontinent [26].
Indeed, it will be important to address the issue of specific founder mutations for BC among the Indian population, especially in the BRCA1 and BRCA2 genes, that are associated with hereditary breast and ovarian cancer (HBOC) syndrome. India, with its diversified population and endogamous groupings, has distinct regional founder mutations that influence genetic screening and counseling procedures. Founder mutations are essentially genetic changes that occur or originate in a founding (initial) individual and are inherited by their offspring, gradually becoming increasingly prevalent in particular groups or populations that have stayed isolated owing to geographic or cultural reasons. Moreover, owing to the large and genetically diverse population of India, the distribution of BRCA1/2 mutations varies greatly by location and ethnicity. Additionally, the traditional practice of endogamy in certain communities has led to an increase in the frequency of these founder mutations and heritable conditions.
Some of the crucial insights with regard to the founder mutations in India include the following:
- Shared mutation (185delAG): The BRCA1 185delAG (as mentioned earlier) founder mutation, which is widely linked to the Ashkenazi Jewish population, can also be detected in certain areas of India, especially in the south and west. Indeed, studies indicate that this emerged or originated independently within the South Indian population, instead of originating from a common lineage with Ashkenazi Jews [31].
- Regional heterogeneity: Studies have found a wide range of BRCA1/2 variants among various Indian populations, showing that the types of mutations vary by geographical locations. This indeed highlights the importance of developing demographically specific genetic profiles [26].
- Higher frequency of mutations: Some studies in India have discovered an increased overall frequency of BRCA1/2 mutations among breast and ovarian cancer patients when compared to their Western counterparts [26,32].
Additionally, in India, population-specific founder mutations and other socioeconomic factors have a significant impact on genetic screening strategies and counseling, as discussed below:
- Targeted sequencing–next-generation sequencing (NGS): Instead of expensive, laborious, and lengthy whole gene sequencing, the testing or screening strategies can be highlighted on the most frequent founder mutations that are found in specific regional or ethnic populations. This strategy provides a faster and less expensive screening tool for high-risk people.
- Custom-designed gene panel (personalized genetic test): Genetic testing or screening can be performed with panels tailored to contain common founder mutations unique to the Indian population, along with additional pertinent genes associated with high and moderate risks.
- Overcoming barriers to access: Customized or personalized testing methods can assist in tackling the significant obstacles pertaining to the expense and availability of genetic tests in India.
5. Implication of Non-Genetic Risk Factors for Breast Cancer (Figure 5)
5.1. Familial History of Breast Cancer
Although 15% or less women with BC include a familial member who has the disease, women who have close family members who possess the disease are at an immense risk. Especially given this circumstance, having a first-degree relative like mother, sister, or daughter with BC, for instance, nearly increases the risk by twofold. And, on the other hand, having two first-degree relatives with the disease increases the risk threefold [23,24]. Intriguingly, women who have a father or brother who has BC are more likely to develop the disease themselves. Adding to this, and at an individual level, a woman who has cancer in one breast is more likely to develop cancer in the other breast or another part of the same breast [25].
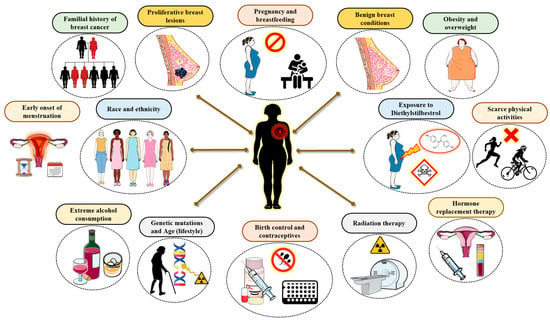
Figure 5.
Implication of non-genetic factors in breast cancer.
Familial history and genetic predisposition are more likely to found among the younger BC patients in India. Typically, the average age at which they are diagnosed is about ten years younger than that of patients in Western countries, thus highlighting the connection between genetic predispositions and the early onset of BC, and consequently assessing the importance of genetic counseling [33]. In fact, 19% of women under forty had a first- or second-degree relative who has cancer. However, the actual frequency of inherited breast cancer in India is probably miscalculated. Numerous BC disease manifestations are reported that are not linked to inherited genes or familial predisposition, indicating that familial history-based screening may overlook a large percentage of mutation carriers [26].
5.2. Proliferative Breast Lesions (PBLs)
Certain proliferating lesions with the absence of atypical hyperplasia has demonstrated to increase a woman’s susceptibility to BC [23,24]. These include fibroadenoma, sclerosing adenosis, ductal hyperplasia, papillomatosis, and radial scar. Nonetheless, certain proliferative lesions with atypical hyperplasia in the ducts or lobules of the breast tissue will boost the susceptibility to BC by 4–5 fold which comprises the atypical ductal hyperplasia (ADH) and atypical lobular hyperplasia (ALH) [34].
However, studies among the Indian population suggest that proliferative breast lesions are substantial contributing factors to BC, which increasingly aggravates young Indian women more than their Western counterparts. In fact, the diagnosis of PBL indicates the necessity for improved screening. Given this severity among the Indian population, the association between PBLs and breast cancer is especially important because of certain traits in the population such as early onset, lifestyle, and hereditary factors. In addition, owing to this higher risk, studies in India recommend that women with proliferative lesions receive more comprehensive follow-up, like clinical breast examinations and mammography screening. In addition, given the greater likelihood of advanced-stage diagnoses in India, detecting women with PBLs is a fundamental strategy for early diagnosis [1,35].
5.3. Lack of Pregnancy and Breastfeeding
To some extent, women who have never had children or who have had their first child at an age later than 30 years old have an increased risk of BC by and large. Having multiple pregnancies and/or conceiving at an early age, on the other hand, lowers the susceptibility to BC [23,24]. However, pregnancy appears to affect different types of BC in a dissimilar manner, and it in fact appears to increase the risk of triple-negative breast cancer. Breastfeeding may perhaps reduce the risk of BC to some extent, especially if it is carried on for 1.5–2 years. One possible interpretation for this conclusion is that breastfeeding decreases a woman’s aggregative number of menstrual cycles over her lifetime.
In India, a lack of pregnancy and breastfeeding, often referred to as nulliparity and never having breastfed, is a substantial and growing risk factor for BC. Although typically Indian women once had timely and numerous pregnancies and breastfed for prolonged durations, these trends are evolving, particularly among the urban, educated population. However, as India undergoes a demographic shift, the prevalence of cancer is rising. In fact, BC has exceeded cervical cancer as the most prevalent malignancy among Indian women predominantly in metropolitan areas. The upsurge in the occurrence of nulliparity and decline of breastfeeding can be attributed as important drivers of this trend, pushing the age of diagnosis to a younger demographic than in Western countries [1].
5.4. Benign Breast Conditions
Women with dense and solid mammograms have an approximately a 1.5- to 2-fold higher susceptibility to BC than women with regular breast density. However, several factors like age, the use of certain medications like menopausal hormone therapy, menopausal status, and pregnancy are found to be involved in defining breast density. This aside, a few non-proliferative lesions may have a minor effect on BC susceptibility. This may comprise fibrosis and/or simple cysts, single papilloma, duct ectasia, mild hyperplasia, adenosis, phyllodes tumor, periductal fibrosis, squamous and apocrine metaplasia, epithelial-related calcifications, and other tumors including lipoma, hamartoma, hemangioma, neurofibroma, adenomyoepithelioma, and mastitis [36].
In the Indian scenario, fibroadenomas and fibrocystic changes are the most frequent benign breast diseases mostly affecting the younger women. Moreover, although the majority of benign conditions pose little to no risk of developing cancer, certain kinds have a marginally increased risk, particularly when combined with other risk factors. Therefore, to differentiate between benign lesions and BC, it is necessary to have an early and precise diagnosis via biopsy, imaging, and clinical testing [37].
5.5. Obesity and Being Overweight
Prior to the onset of menopause, the ovaries produce the majority of the body’s estrogen, while the fat tissue produces only a small amount. Nonetheless, when a woman’s ovaries cease to produce estrogen after menopause, the majority of her estrogen is derived from the fat tissue. Therefore, harboring extra fat tissue after menopause increases estrogen levels which subsequently escalates the susceptibility to BC [23,24]. Additionally, being overweight tends to result in increased insulin levels in the blood. In turn, the elevated levels of insulin are associated with certain types of cancer, including BC. However, the relationship between weight and susceptibility to BC is complex and not yet completely interpreted.
However, given the severity among the Indian population, obesity and being overweight are intricately linked to BC risk, prognosis, and survival, which differs depending on menopausal state and body fat distribution. High body mass index (BMI) is a substantial risk factor for postmenopausal breast cancer, although central obesity is a major risk factor for both premenopausal and postmenopausal breast cancers. And, in a broader context, owing to the lifestyle modifications, the growing number of BC cases in India can be somewhat linked to trend towards sedentary lifestyle and dietary changes, leading to a rise in the average body mass index [38].
5.6. Early Onset of Menstruation
Women who begin menstruating early, especially prior to the age of 12, will end up having more menstrual cycles which consequently results in a prolonged lifetime exposure to the hormones like estrogen and progesterone, which, to some extent, increase the susceptibility to BC [23,24]. Likewise, women who reach menopause later, especially after the age of 55, will have more menstrual cycles and a longer lifetime exposure to estrogen and progesterone, respectively, which again increases their susceptibility to BC.
Early menstruation (menarche) is a major risk factor for BC in the Indian population. This link is mostly attributed to a woman’s prolonged or chronic exposure to hormones such as estrogen and progesterone, which promote breast cell proliferation and raise the risk of cancer. In addition, the landscape of BC in India, characterized by its early onset compared to in Western nations, higher rates of aggressive subtypes like triple-negative BC (TNBC), and substantial discrepancies in detection and treatment, along with lifestyle modifications, contributes its major role [1].
5.7. Race and Ethnicity
On the whole, Caucasian women are to some extent more inclined to develop BC than their African American counterparts, despite the fact that BC is more frequent among the African American women under the of age 45. In addition, they are more prone to die from BC at any age. On the other hand, women from other races, such as those with an Asian, Hispanic, or Native American background, are at a lower risk of developing and dying from BC [23,24].
More specifically, when compared to Western populations, Indian women have a higher prevalence of more aggressive, hormone receptor-negative cancers and are detected at a much younger age. The explanations for these disparities are complex, including genetic predispositions, lifestyle modifications, and socioeconomic background, which contribute to late-stage diagnosis and lower survival rates. Adding to this body of data is a unique clinical presentation with distinct biological characteristics among the Indian BC population. These include early onset, a higher incidence of more invasive tumors (such as TNBC and HER2-positive tumors), and late-stage diagnosis. Such factors make the Indian BC population’s profile more complicated than that of its Western counterparts [1].
5.8. Exposure to Environmental Risk Factors
From the 1940s to the early 1970s, a few pregnant women were prescribed to the estrogen-like drug diethylstilbestrol (DES) which was believed to reduce the incidence of miscarriage [23,24]. These women, to some extent, have an increased risk of BC, and women whose mothers used DES during pregnancy possibly will have a considerably increased risk.
On the other hand, environmental and cultural risk factors in India, together with rising urbanization, confers a substantial impact on BC trends and effects. Indeed, these factors lead to its increased prevalence, with early onset compared to in Western populations, and with diagnostic delays resulting in higher fatality rates [39]. This aside, an emerging risk factor for BC among the Indian population is exposure to pollutants like organochlorine pesticides. This is supported by a case–control study in an Indian population, which found that women with BC had considerably higher serum levels of estrogenic OCPs, including DDT metabolites (DDE) and hexachlorocyclohexane isomers (HCH), compared to healthy control subjects [18].
Similarly, exposure to fine particulate matter like PM2.5, has been linked to an increased risk of breast cancer. According to studies, these small particles can enter the bloodstream and permeate into breast tissue, potentially leading to tumor formation [40]. Likewise, another study conducted among the women from northeast India, found that chewing betel quid was a substantial and independent risk factor for the onset of BC, possibly prompting genetic modifications [41].
Other emerging environmental pollutants like phthalates, bisphenol A (BPA), and polyfluoroalkyl substances (PFASs) are being studied for their potential link to breast cancer. The possible impact of these chemicals that interfere with hormones on the progression of BC is still being investigated [42].
5.9. Scarce Physical Activities
An emerging body of evidence and documentation suggests that routine exercise and physical workouts, particularly in women after menopause, could possibly decrease the risk of breast cancer [23,24]. However, it is unclear as to how physical activity may reduce risk of BC, but it could be because of activity levels influencing body weight, inflammation, hormones, and energy balance.
In the Indian scenario, a lack of physical exercise is a key, alterable factor, leading to the rising incidence of BC, especially in younger urban women. This aside, sedentary lifestyle raises the risk of BC owing to its association with obesity, hormonal disruptions, and inflammation, all of which are proven risk factors that contribute to the illness [1].
5.10. Extreme Alcohol Consumption
Consumption of alcohol is undeniably associated with an increased susceptibility to BC, and this increased risk or susceptibility is proportional to the amount of alcohol consumed. For instance, women who consume two to three drinks per day, have a 20% higher risk of BC than women who do not consume alcohol. And, on the other hand, women who consume only one alcoholic drink per day have a minor increased susceptibility [23,24].
High levels of alcohol intake raise the likelihood of BC among Indian women, reflecting global trends. However, unique lifestyle and genetic factors in the population can lead to different risk factors. In addition, although Indian women have a traditionally lower rate of alcohol consumption than their Western counterparts do, the potential for a negative outcome is growing with the advent of urbanization and “westernized” lifestyles [1].
5.11. Genetic Mutations and Age
An extensive proportion of breast cancers is detected in women with no clear familial history. Furthermore, rather than inherited mutations, these cancers may perhaps be brought about by genetic mutations that appear as a result of the aging process and lifestyle-linked risk factors.
In the Indian population, the effects of genetic mutations and age interact significantly, ensuing in a higher prevalence of BC in younger women, with an increased proportion of aggressive subtypes, and a distinct range of genetic mutations compared to Western populations. More specifically, genetic mutations in the BRCA1 and BRCA2 genes are major contributing factors for the rise in aggressive and early-onset BC cases in India. The prevalence of these mutations, along with diverse mutation patterns, is significantly higher in India than in Western countries [26].
5.12. Birth Control and Contraceptives
Several birth control approaches contain hormones that can increase the risk of BC [23,24]. In addition, women who use oral contraceptives have a higher risk of BC to some extent than women who have never used them. However, the risk appears to return to normal after the regimen is discontinued. To ascertain this, Depo-Provera, administered as an injectable form of progesterone, has been reported to boost the risk of BC; nevertheless, there was no increased risk in women five years after they stopped receiving the shots.
On the other hand, hormonal contraceptives are linked to a minor but considerable increased risk of BC in Indian women, particularly with long-term use. However, this risk should be examined along with other risk factors prevalent in the Indian population, like being of an advanced maternal age at first childbirth and socioeconomic status. Indeed, some Indian studies have indicated a link between the usage of hormonal contraceptives, especially oral contraceptive pills (OCPs) and an increased risk of BC [43,44].
5.13. Chest Radiation Therapy
Women who have had radiation therapy to the chest for another type of cancer at a young age are more likely to develop BC [23,24]. The effect of this factor is ameliorated in case the individual had received radiation as a teen or young adult, when the breasts were still developing. On the other hand, radiation therapy after the age of 40 did not appear to increase susceptibility to BC.
For Indian BC patients, chest radiation therapy carries the same potential risks as it does to other populations, including heart and lung damage, and the development of a secondary malignancy. However, the overall impact and absolute risk of these complications are intensified by underlying factors more prevalent in India, such as increased baseline cardiovascular risk, late-stage diagnosis, restricted access to modern treatment methods, and major financial constraints [45].
5.14. Hormone Replacement Therapy After Menopause
The hormone estrogen (often in combination with progesterone) has been administered to treat menopausal symptoms and thereupon to prevent osteoporosis [23,24]. Most often, combined hormone therapy is required as the usage of estrogen alone can aggravate the risk of uterine cancer. Nevertheless, estrogen can be used alone by women who have had a hysterectomy. This aside, postmenopausal integrated hormone therapy raises the risk of BC, the likelihood of dying from BC, and the chances of detecting cancer only at a more progressive stage subsequently. This increased risk is typically prominent after 2 years of usage. Nonetheless, the increased risk from concomitant or combined HRT is reversible, and it is only relevant to current and recent users. This is because a woman’s BC risk appears to revert to that of the general population after five years of discontinuing HRT. Additionally, the use of bioidentical or “natural” estrogen and/or progesterone is not always safer or more effective and should be regarded as having the same health risks as any other type of HRT. This aside, short-term estrogen usage after menopause does not appear to significantly increase the risk of BC. Yet, long-term treatment (>15 years) has been linked to an increased risk of ovarian and BC, respectively. Therefore, it is up to a woman and her doctor to decide as to whether to use any form of HRT after deliberating the potential risks factors and benefits, and also taking into account other risk factors pertaining to heart disease, BC, and osteoporosis, which occur thereafter.
On the other hand, hormone replacement therapy following menopause offers a complicated risk–benefit scenario for Indian women, which is determined by the type of therapy, the duration it is taken, and the patient’s specific health considerations. Especially for Indian women, who reach menopause at a younger age (46 years) than Caucasian women, the necessity of HRT for symptom relief must be balanced against the long-term risks. An Indian Menopause Society study calls for customized risk assessment, highlighting that the advantages may outweigh the modest absolute risks for healthy women in the first decade after menopause [46].
6. Risk Assessment: Breast Cancer Stratification by Way of Pathogenesis, Incidence and Invasiveness
Breast cancers are classified into several types based on where they appear in the breast, for instance in the ducts, lobules, or the tissue in between. The specific cells that are affected define the type of breast cancer. Therefore, they are classified into two distinct types, viz., carcinomas and sarcomas, based on the origin of the cells involved. Principally, carcinomas are breast cancers that develop from the epithelial component of the breast, which includes the cells that outline the lobules and terminal ducts that produce milk. Sarcomas on the other hand, are a much rarer type of breast cancer that develops from the stromal components of the breast, which include myofibroblasts and blood vessel cells (Figure 6). These categories are not always necessary because a single breast tumor can be a mixture of different cell types in some cases [25].
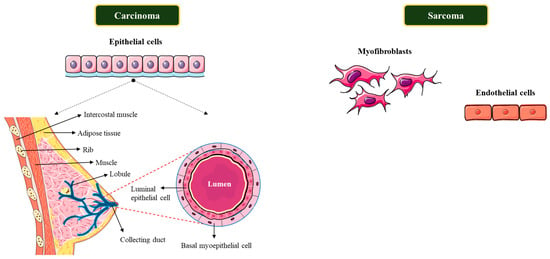
Figure 6.
Breast cancer stratification based on specific cell types.
However, the majority of breast cancers are carcinomas. Several different types of breast cancer have been described within the large group of carcinomas depending on their invasiveness corresponding to the primary tumor sites. It is indeed critical to be able to differentiate between the different subtypes since they each have diverse prognoses and treatment implications. Therefore, typically, breast cancers are classified, based on their corresponding pathological features and invasiveness, into three distinct groups: non-invasive (or in situ), invasive, and metastatic breast cancers [25].
6.1. Non-Invasive (In Situ) Breast Cancer
6.1.1. Ductal Carcinoma In Situ (Intraductal Carcinoma; DCIS)
DCIS is a non-invasive or pre-invasive BC that thrives inside from the normal pre-existing ducts, and is one of the most typical types of breast cancer [25]. And, despite the fact that DCIS is not invasive, the in situ carcinomas have a high risk of developing into invasive cancers, and therefore early and appropriate therapeutic regimen is vital in intercepting the patient from developing an invasive cancer.
6.1.2. Invasive/Infiltrating Breast Cancer
Primarily, the cancer cells in invasive breast cancers invade and propagate farther outside of the normal breast lobules and ducts, gradually moving into the adjoining breast stromal tissue [25]. And, when diagnosed with an invasive form of BC, approximately two-thirds of women are 55 or older. In addition, invasive carcinomas have the possibility to progress to other locations of the body, like the lymph nodes or other organs, and form metastases, thus classifying them as metastatic breast cancers [25]. Additionally, the invasive breast cancers are further classified into two types based on the tissue and cell types involved as follows:
6.1.3. Invasive Ductal Carcinoma (IDC)
IDC is the most common type of BC, accounting for approximately 80% of all breast cancers [25]. The IDC group of classification comprises numerous subtypes that include the tubular, medullary, mucinous, papillary, and cribriform carcinomas of the breast [25].
6.1.4. Invasive Lobular Carcinoma (ILC)
ILC is the second most common type of BC, accounting for about 10–15% of all cases [25]. While this subtype of carcinoma can affect women of any age, it is more prevalent in women over the age of 50. Additionally, the invasive lobular carcinoma occur later in life when compared to the invasive ductal carcinoma—for instance, in the early 60s in contrast to the mid-to-late 50s for IDC [25].
Overall, 90–95% of all breast cancers fall into the invasive subcategory. In addition, the invasive ductal carcinoma and the invasive lobular carcinoma have different pathological features. Lobular carcinomas develop as single cells organized individually, in single file, or in sheets, and exhibit distinct molecular and genetic abnormalities. Additionally, both the subtypes distinguish from each other in terms of prognosis and therapeutic regimen (Figure 7).
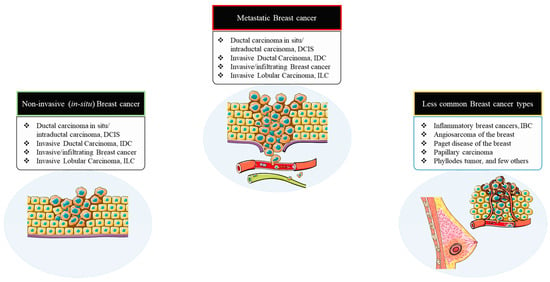
Figure 7.
Breast cancer stratification based on pathogenesis and invasiveness.
6.2. Metastatic Breast Cancer
Metastatic breast cancers are late-stage breast cancers that have spread or progressed to other organs in the body. They are also known as stage IV or advanced breast cancers [25]. Breast cancers can spread to lymph nodes in the armpit and/or distant sites like the lung, liver, bone, and brain. Even after eliminating the prime vital tumor, microscopic tumor cells or micro-metastases may still linger around and prevail in the body, thereby enabling the cancer to recur and spread. Clinically, patients may be diagnosed with metastatic disease (or de novo metastatic BC) at the start of treatment, or they may develop metastases months or years later. The prospective likelihood of BC recurrence and metastasis is not well interpreted or foreseen since it differs from person to person, owing to the tumor’s unique molecular biology and stage at the time of diagnosis. Unfortunately, given the severity, about 30% of women with early-stage BC will develop a metastatic form of the disease (Figure 7) [25].
6.3. Less Common Types of Breast Cancer
Although IDCs and ILCs account for approximately 90–95% of all BC cases, numerous rare or less common types of BC can be detected in the clinical setting [25].
6.3.1. Inflammatory Breast Cancers (IBCs)
IBC is a rare type of invasive BC that accounts for 1–5% of all breast cancers [25]. The inflammatory breast cancers differ from other group of BC in terms of their manifestations, prognosis, and therapeutic regimen. The characteristic symptoms or manifestations of IBC comprises an inflammation-like breast lump or swelling, purple or red skin discoloration, and the pitting or thickening of the breast skin. All of these are possibly triggered by cancer cells blocking the lymph vessels in the skin. However, IBC is not always associated with a breast lump and may not be detected on mammograms. This aside, IBC is more common among younger women and especially the women of African American origin, along with women who are overweight or obese. In addition, it inclines to be more aggressive than other types of BC, growing and metastasizing much more rapidly. It is invariably detected at a locally progressive stage, when the breast cancer cells have spread into the skin. And, in approximately one-third of cases, the IBCs have been detected already metastasized to distant sites of the body, thereby complicating and making treatment more difficult [25].
6.3.2. Angiosarcoma of the Breast
Angiosarcoma is a class of sarcoma that is a very unusual form of cancer that develops from the epithelial cells that line blood or lymph vessels [25]. These angiosarcomas account for 1% of all breast cancer cases and can affect either the breast tissue or the breast skin. A few cases stem as a result of previous radiation therapy in that area. Though uncommon, angiosarcomas grow and spread rapidly and should be treated appropriately.
6.3.3. Paget Disease of the Breast
This uncommon type of BC initiates in the breast ducts, extends to the nipple skin, and then gradually spreads to the areola (the dark circle around the nipple) [25]. Paget disease accounts for 3% of all BC cases and, in addition, the Paget’s cells are visually distinct from normal cells and divide rapidly. Around half of the cells express estrogen and progesterone receptors, and the majority express the HER2 protein. Predominantly, a biopsy of the tissue is usually used to diagnose cancer, and this is occasionally trailed by a mammogram, sonogram, or MRI to verify or validate the diagnosis. It is also worth noting that Paget’s disease of the breast is not medically related to other clinical settings named after Sir James Paget, such as Paget’s disease of the bone.
6.3.4. Papillary Carcinoma
This is another rare form of BC, accounting for less than 3% of all breast cancers [25]. Usually, the cells in papillary carcinoma cancer are typically arranged in finger-like projections. To illustrate further, in certain cases, the cancer cells are relatively minute in dimension and apparently form micropapillary structures. The majority of the papillary carcinomas are invasive, and are treated similarly to IDCs. Nevertheless, the invasive papillary carcinoma typically has a better prognosis than other types of invasive breast cancer. It is worth noting that papillary carcinomas can be identified while still non-invasive. Usually, a non-invasive papillary carcinoma is commonly thought to be a type of DCIS.
6.3.5. Phyllodes Tumor
This is a rare breast tumor that establishes itself in the breast’s stromal cells [25]. The majority of these tumors are benign, but approximately one-quarter are malignant. Phyllodes tumors are most common in women in their 40s, as well as in women with Li-Fraumeni syndrome, who are predisposed to this type of tumor.
6.3.6. Breast Cancers in Men, Children, and Juveniles
The male breast carcinomas can be in situ or invasive, and makes up less than 1% of all cases of breast cancer [25]. They can almost resemble as those observed in women, since the majority of cases are invasive ductal carcinomas with estrogen receptor (ER) expression. Gynecomastia (or breast enlargement) is the most common breast lesion in men, and it can affect either the unilateral or bilateral breasts. Likewise, breast lesions are rare in children and adolescents, but they do happen. These rare cases may comprise both benign and malignant lesions, such as juvenile fibro adenoma and malignant carcinoma. On the other hand, pediatric patients have more possibility to develop lymphoma or alveolar rhabdomyosarcoma that has spread or metastasized to the breast.
7. Molecular or Intrinsic Subtyping/Classification of Breast Cancer
Breast cancer entails a complex and phenotypically diverse group of diseases and consists of many biological subtypes with specific mechanisms and therapeutic responses. To elucidate further, gene expression studies have determined numerous specific subtypes of BC that vary notably in prognosis along with the therapeutic targets that exist in the cancer cell types [25]. With the advent of gene expression profiling strategies, the list of intrinsic genes that distinguish these subtypes includes several groups of genes linked with the human epidermal growth factor 2 (HER2) expression, proliferation, the estrogen receptor (ER) expression (the luminal cluster), and a distinct group of genes termed the basal cluster [25]. Accordingly, the BC subtypes are typically classified into five intrinsic or molecular subtypes established on the expression composition or scheme of specific genes using these interpretations (Figure 8).
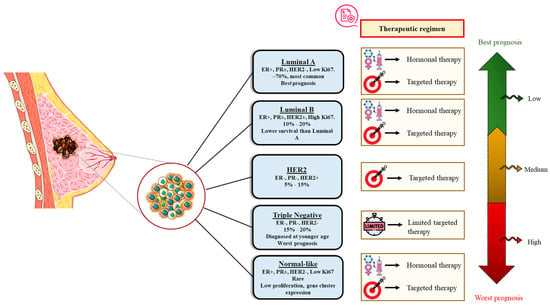
Figure 8.
Molecular or intrinsic subtyping/classification of breast cancer.
7.1. Luminal A Breast Cancer
This subtype is essentially positive for the estrogen receptor (ER) and/or progesterone receptor (PR), and negative for HER2. Luminal A cancers make up roughly 40% of all BCs and are of low grade, growing slowly, and have the best prognosis. The treatment strategy usually includes hormonal therapy [25].
Luminal A breast cancer is defined by high estrogen receptor (ER) expression, indicating its dependence on hormonal signaling. While endocrine dependence is prominent, other involved pathways can lead to resistance if disrupted. This subtype exhibits minimal Ki-67 protein levels, suggesting a slow proliferation rate and a favorable prognosis compared to other subtypes. Key genes linked to luminal epithelial cells, such as GATA3 and FOXA1, function as transcription factors for ER alpha and are significantly expressed, playing a vital role in mammary gland differentiation [47]. In addition, based on integrated genomic analysis, common mutations in the luminal A subtype include the following:
- PIK3CA: Activating mutations are commonly seen in the PI3K/AKT/mTOR pathway.
- GATA3 and MAP3K1: Mutations or alterations in these transcription factors are also frequent.
- TP53: Although not as common as in other subtypes, mutations or alterations in the tumor suppressor gene, TP53, are detected in certain luminal A tumors.
7.2. Luminal B Breast Cancer
This subtype accounts for <20% of all BCs and is ER and/or PR positive, HER2 positive or HER2 negative, and has high Ki-67 levels. Luminal B cancers grow somewhat quicker than luminal A cancers and have a slightly worse prognosis [25].
For patients diagnosed with the intrinsic luminal B subtype of breast cancer, the vital molecular mechanisms and signaling pathways are usually distinguished by an elevated proliferative rate along with increased genomic instability when compared to the luminal A subtype. In addition, despite the fact that these subtypes are estrogen receptor-positive (ER+), these tumors frequently use substitute growth pathways, resulting in a more aggressive phenotype and greater rates of endocrine therapy resistance. Similarly, these subtypes have a higher level of somatic mutations, gene copy number alterations (CNAs), and DNA methylation abnormalities than the luminal A subtype. Moreover, these extensive genetic alterations lead to an increased tumor grade with poor prognosis. Likewise, the tumor suppressor gene TP53 is more commonly altered in luminal B malignancies. In addition, deactivating this gene impairs cell cycle checkpoints and DNA repair, resulting in unregulated cell division with increased genomic instability [48]. This aside, luminal tumors frequently harbor somatic mutations in the PIK3CA gene, which encodes a PI3K subunit. However, the frequency is decreased in luminal B compared to luminal A, indicating that additional factors influence its activation. On the other hand, the deletion or lack of expression of the tumor suppressor gene PTEN, which serves as a negative regulator of the PI3K pathway, may lead to an increased pathway activity. Similarly, the Akt and mTOR proteins are activated downstream of PI3K, triggering cell growth and proliferation, while also increasing resistance to endocrine therapy [49].
7.3. HER2-Enriched Breast Cancer
This subtype accounts for 10–15% of all BCs and is defined by the lack of ER and PR expression, high HER2 and proliferation gene cluster expression, and sparse expression of the luminal and basal clusters [25]. This aside, the HER2-supplemented cancers thrive more rapidly than luminal cancers and have a poor prognosis in general. Nevertheless, they can be effectively managed with HER2-targeted therapies like Tykerb (or lapatinib), Herceptin (or trastuzumab), Kadcyla (or T-DM1 or ado-trastuzumab emtansine), and Perjeta (or pertuzumab). Additionaaly, it should be noted that the HER2-supplemented subtype does not imply clinically HER2-positive BC. Despite the fact that approximately 50% of clinical HER2-positive BCs are HER2 supplemented, the residual 50% can be of any molecular subtype but are predominantly HER2-positive luminal subtypes. Yet, approximately 30% of HER2-enriched tumors are clinically HER2-negative [25].
The main molecular mechanism underlying the HER2-enriched (HER2-E) intrinsic subtype of BC is the overexpression or amplification of the ERBB2 gene, which encodes the HER2 receptor. As a result, there is an overactivation of the HER2 signaling pathway, which triggers a number of downstream cascade of events that regulate uncontrolled cell proliferation, growth, and survival. The primary mechanism driving HER2-E BC is the occurrence of too many copies of the ERBB2 gene, which results in a surplus of the HER2 protein on the cell surface [50]. The dimerization of HER2 receptors stimulates a number of critical downstream signaling pathways including the following:
- PI3K/Akt/mTOR pathway: This signaling pathway is one of the most commonly activated downstream of HER2 and is an important regulator of cell survival, growth, metabolism, and proliferation. Mutations in the PIK3CA gene, which encodes the p110α subunit of PI3K, along with the loss of the tumor suppressor gene PTEN, are prevalent in HER2+ BC, leading to constitutive PI3K/Akt activation and treatment resistance.
- MAPK/ERK pathway: The MAPK signaling pathway is yet another important modulator of HER2 signaling, which stimulates cell proliferation. Certain metastatic HER2-positive BCs exhibit genetic modifications that trigger the MAPK pathway, including the deletion of NF1. This can lead to a “pathway switch”, rendering tumors resistant to Akt obstruction, yet more reliant on and susceptible to MEK/ERK inhibition.
- Likewise, other related signaling pathways like HIF-1, Wnt/β-catenin, and STAT signaling pathways are also known to be activated in some HER2-enriched tumors, thus contributing to the resistance of anti-HER2 therapies.
7.4. Triple-Negative/Basal-like Breast Cancer (TNBC)
Triple-negative/basal-like breast cancer are ER, PR, and HER2-negative and account for approximately 20% of all BCs [25]. TNBC is more prevalent among women with BRCA1 gene mutations, women under the age of 40, and African American women. It is a high-grade BC since it acts more destructively than other types of BC. TNBC is most commonly diagnosed with infiltrating ductal carcinoma, though an uncommon histologic subtype, medullary carcinoma, is also triple negative. Additionally, TNBC’s non-surgical treatment/management has been restricted to usual chemotherapy, in contrast to other breast cancer subtypes, which have an arsenal of targeted regimens such as ER antagonists and HER2 monoclonal antibodies, until the validation of the PARP inhibitor Olaparib for BRCA1 and BRCA2 mutation carriers, who are prone to develop TNBC [25].
Likewise, TNBC is more invasive and has a greater rate of early recurrence than other forms of BC. The prognosis is often very bad, and most patients relapse five years following surgery. Not to mention, TNBC is resistant to both targeted and endocrine therapies since ER, PR, and HER2 are negatively expressed. In addition, TNBC can only be treated with a few, often ineffective therapies, and thus there is a pressing, urgent need for novel therapies [51,52].
Additionally, targeting the mechanisms that underpin genomic instability may be required for such cancers, in which chromosomal instability is a driving factor. It is still unclear how significant many genetic dependencies and anomalies are across the various forms of breast cancer. In order to unravel the essential molecular pathways and interconnected nodes, a thorough functional screening such as CRISPR-Cas9 technology coupled with deeper analyses and data integration would be needed [53,54,55].
Furthermore, TNBC is a diverse and aggressive BC subtype, characterized by unique molecular mechanisms and signaling cascades. Certain genetic mutations and dysregulated pathways define this subtype, influencing its proliferation, metastasis, and prognosis [56]. The landscape of TNBC is intricate and can be categorized into molecular subtypes, each presenting distinct treatment-responsive deficiencies and featuring numerous significant pathways discussed below, which play a role among them.
- PI3K/Akt/mTOR pathway: The PI3K/AKT/mTOR (PAM) signaling pathway is among the most frequently modified and excessively activated in TNBC. This pathway regulates cell proliferation, survival, metabolism, and angiogenesis. Mutations in the PIK3CA gene or the deletion of the tumor suppressor PTEN are common causes of hyperactivation. This pathway may also play a role in contributing to chemoresistance and relapse [57].
- DNA damage response (DDR) pathways: A considerable proportion of TNBC tumors are distinguished by deficiencies in DNA repair processes, a feature known as “BRCAness”, due to its similarities to tumors with inherited BRCA1/2 mutations. This aside, mutations in BRCA1 are especially known to be linked to the basal-like type of TNBC.
- Wnt/β-catenin signaling pathway: The Wnt/β-catenin pathway is essential for embryonic development and adult tissue homeostasis, but it is abnormally active in certain malignancies, including TNBC. In TNBC, the Wnt/β-catenin pathway is frequently overactivated because of the high levels of Wnt receptors such Frizzled (FZD7) and the Wnt co-receptor LRP6. As a result, β-catenin is stabilized and subsequently moves into the nucleus.
7.5. Normal-like Breast Cancer
This subtype resembles to the luminal A disease. They are ER and/or PR positive, HER2 negative, and have low levels of protein Ki-67 [25]. While normal-like breast cancer has a noble prognosis, it is still somewhat worse than luminal breast cancer.
Due to the inherent cellular and molecular diversity of breast cancers (BCs), simultaneous investigation of multiple genetic alterations is essential [27]. Advances in next-generation genomics and transcriptomics have facilitated this, leading to the identification of numerous BC subtypes with distinct prognoses and therapeutic targets [58]. These intrinsic subtypes naturally divide into two main groups based on hormone receptor gene expression, reflecting the clinically understood biological differences between ER-positive and ER-negative cancers, which originate from different progenitor cells.
To sum up, the histopathological and molecular/intrinsic classification of BC are two diverse but complementary approaches used in understanding and categorizing BC. Indeed, each provides a distinctive perspective on the nature of the disease’s manifestation. While histopathology concentrates on the microscopic features of cancer cells and tissue architecture, molecular subtyping focuses on the patterns of gene expression to categorize tumors based on their biological functions. Invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), mucinous carcinoma, tubular carcinoma, medullary carcinoma, papillary carcinoma, and others are among the most prevalent histological forms. In addition, HER2-enriched, basal-like/triple-negative, luminal A, and luminal B are the most well-known molecular subtypes. In addition, molecular subtyping counts on the condition of important biomarkers like estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2), as well as proliferation markers like Ki-67.
To elaborate, histopathological classification (e.g., ILC, IDC) examines cancer cells’ microscopic organization and features, while molecular subtyping analyzes attributes like gene expression signatures to categorize tumors. Both methods have prognostic significance: specific histological subtypes like tubular carcinoma indicate favorable prognosis, while others like metaplastic cancer suggest a negative one. Similarly, the likelihood of recurrence and response to treatment vary by molecular subtype; for example, luminal A tumors generally have a better prognosis than luminal B tumors [1].
In addition, although prognostic information is provided by both the classifications, molecular subtyping might enable a more accurate evaluation of risk and possible outcomes. Similarly, while there is not always a one-to-one correlation, some histological types are more likely to be linked to particular molecular subtypes. For example, luminal A is a common presentation of invasive lobular cancer. Essentially, histopathology lays the groundwork for comprehending the visual aspects of BC, whereas molecular subtyping sheds light on the disease’s underlying biology and behavior, which in turn influences prognosis and therapeutic approaches.
8. Prevalence and Clinical Significance of Molecular Subtypes in the Indian Setting
However, in the Indian context, there is a well-established correlation between the molecular subtypes of BC, particularly those defined by immunohistochemistry (IHC), their histological attributes, and their respective clinical outcomes. In addition, although there is considerable variation across studies, recent research suggests that luminal A is the most prevalent subtype, followed by triple-negative breast cancer (TNBC) and luminal B, both of which are observed at high rates in India [59,60,61]. Luminal A, which is frequently regarded as the most prevalent subtype among Western populations, may be less common in certain Indian studies, or differences in Ki-67 evaluation may have an impact on its diagnosis. In addition, corresponding to the global trends, invasive ductal carcinoma (IDC) is the most prevalent histological form of BC among all molecular subtypes in India. Moreover, while lower histological grades (Grade I) are often linked to luminal A cancers, higher histological grades (Grade III) are frequently associated with TNBC, HER2-enriched, and luminal B subtypes.
Moreover, in terms of lymphovascular invasion (LVI), some Indian studies suggest a strong association between molecular subtypes and LVI. While the highest prevalence of LVI has been observed in various subtypes depending on the study, it is generally considered higher in aggressive subtypes, such as HER2-positive BC and TNBC compared to luminal A tumors [59,60,61]. However, some studies from India demonstrate a higher rate of lymph node positivity in TNBC, in comparison to some other regions [58]. Additionally, these studies also suggest that HER2 and TNBC-enriched subtypes might present with bigger tumors than that of luminal A.
Given the current oncological landscape of India, molecular profiling is becoming more popular, since it can help guide personalized therapy choices, especially when combined with techniques like next-generation sequencing (NGS). Although promising, a number of limitations in local data solutions and access to the gene expression assays hinder its extensive and fair implementation [21].
In terms of its positive impact on treatment decisions, it undeniably helps gain a deeper insight into tumor heterogeneity. By identifying each tumor’s distinct genetic composition, molecular profiling goes beyond conventional classification systems and enables a more specialized and prospective therapeutic regime. It advances personalized and precision medicine by diagnosing precise molecular subtypes and predicting treatment outcomes [62]. Oncotype DX and similar tests can predict recurrence risk, allowing clinicians to identify individuals who can safely evade chemotherapy, saving unnecessary toxicity exposure and expenses, thus refining prognosis and abating overtreatment [15]. Similarly, gene expression profiling (GEP) aids in the identification of appropriate chemotherapy regimens, predicting therapeutic response, and tracking disease progression [15]. Indeed, clinical studies or trials aimed at specific genetic profiles can accelerate the discovery and regulatory approval of novel therapies.
In addition, given the inadequacy of local data infrastructure and gene expression assay availability, current genomic classifiers, such as Oncotype DX and MammaPrint, are often exorbitant for most Indian patients. The fact that most genomic classifiers were established with data predominantly from Western populations raises questions about their applicability and efficacy within the heterogeneous Indian population [63]. Indeed, there is a significant scarcity of comprehensive and thorough research investigating the molecular heterogeneity of BC subtypes among the Indian population. In fact, ongoing RNA and DNA profiling studies on Indian patients are demonstrating distinct genetic and expression patterns when compared to data from Western populations [64]. In addition, due to India’s insufficient government investment in healthcare and inadequate coverage plans, a substantial amount of healthcare spending comes directly from the patients/individuals, thus making expensive examinations like GEP unaffordable to many.
Furthermore, limited access to diagnostic and treatment technologies, especially in rural areas, insufficient resources and equipment, and a scarcity of experienced specialists in molecular testing make access more challenging. Discrepancies in molecular technologies like microarray, sequencing, RNA extraction, and reverse transcription procedures can result in variations. Therefore, standardized operating procedures or guidelines across the testing centers are essential for consistency [65]. Therefore, overcoming these hurdles, and leveraging focused research, with improved governmental measures, prioritizing healthcare equity initiatives, are critical to realize the prospects of molecular profiling for breast cancer patients in India.
9. Clinical Phasing/Staging and Characteristic Survival Percentage in BC Cases
When a patient is detected with breast cancer, routine assessments are implemented to define the stage of the disease, which influences the treatment they receive. According to the American Joint Committee on Cancer (AJCC) and the International Union for Cancer Control’s (UICC) Tumor, Node, and Metastasis (TNM) breast cancer staging system, the clinical staging of breast cancer is alike across breast cancer subtypes: Stage 0, Stage I, Stage II, Stage III, and Stage IV, as detailed in Figure 9.
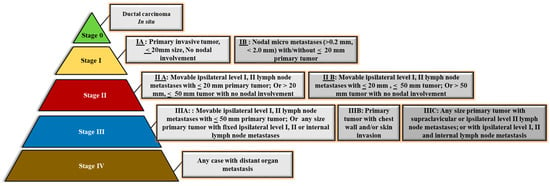
Figure 9.
Clinical phasing/staging and characteristic survival percentage in BC cases.
10. Treatment Options for Breast Cancer
Treatment options for BC are often determined by numerous factors, such as the specific type, progression of the disease, the patient’s genetic composition, and their clinical status. Treatment may consist of a single approach or be used in conjunction with various therapies, such as surgery, radiation therapy, hormone therapy, chemotherapy, immunotherapy, and targeted therapies. While some treatment strategies focus on the specific area surrounding the tumor, others are systemic, affecting the entire body with cancer-fighting agents. Table 1 below outlines and explains the mechanisms of action for various cancer therapies and their application in the Indian context, taking into account factors like population demographics, disease burden, and healthcare infrastructure.
In India, the use of breast cancer therapies is increasing, despite challenges like resource disparities, high costs, and a need for better infrastructure. These obstacles have spurred new research efforts aimed at producing more cost-effective and holistic therapeutic options, as well as gaining a deeper insight into cancer with the resident population. The most prominent challenges and the ongoing research for each of these BC treatment options in the Indian context are discussed below.
10.1. Surgical Therapy
A late-stage diagnosis presents with advanced malignancy, making surgical treatment more challenging and requiring more resources. This is further complicated by a lack of specialized oncologists along with the disproportionate distribution of cancer facilities or centers, which results in many procedures being conducted in less than ideal conditions. Moreover, in terms of research investigations, Indian guidelines are progressively promoting a multidisciplinary team (MDT) strategy for complicated surgeries, particularly for advanced tumors. Studies are also being carried out to perceive the high out-of-pocket expenditure associated with challenging cancer surgeries, including at government institutions [66].
10.2. Radiation Therapy
Indeed, there is a substantial disparity between the demand for radiation therapy and its actual application. Approximately around 50–64% of new cancer patients require radiation therapy globally, yet only 28.5% obtain it. This is due to a significant lack of radiation machines, with most treatment centers located in urban areas, resulting in challenges for rural patients who face extended wait times with limited access [67]. In the Indian context, research investigations using epidemiological data are in full swing to make a more accurate evaluation of the overall national radiotherapy requirements. Additionally, there are also initiatives to build and implement locally produced low-cost radiotherapy devices to expand access and lessen the dependence on expensive imports [68].
10.3. Hormone Therapy
About one in four individuals with hormone-sensitive breast cancer become unresponsive to these medications, resulting in a recurrence of the disease. This indeed underscores a significant obstacle in managing therapeutic effectiveness over time. In addition, most recently, a study from the Tata Memorial Centre and the National Institute of Biomedical Genomics, India, has detected critical genetic markers that contribute to hormone therapy resistance among Indian BC patients. This finding may undoubtedly pave the way for individualized or personalized treatment approaches and for remodeling the current medications to combat resistance [69].
10.4. Chemotherapy
Chemotherapy plays a fundamental role in the therapeutic regimen of BC in India, applied both before (neoadjuvant) and after (adjuvant) surgery. The standard regimen frequently includes anthracyclines and taxanes, along with additional therapies to alleviate the side effects. However, the expensive nature and harmful effects of certain advanced medications, alongside the restricted availability of newer treatments, limit the choices of treatment, especially within public healthcare [20].
10.5. Immunotherapy
Immunotherapy, which enhances the body’s immune system to combat cancer, is a recent therapeutic option available in India for specific cases. This aside, the Drug Controller General of India (DCGI) authorized atezolizumab for metastatic TNBC in combination with nab-paclitaxel in 2020, following the conclusions from the Phase III IMpassion130 trial. Indeed the DCGI made its conclusion based on the results from the Phase III IMpassion130 study, which demonstrated that the combined therapeutic regimen significantly improved progression-free survival (PFS) in patients with PD-L1-positive malignancies [70]. However, the high price of immunotherapy treatment is a significant obstacle, with each cycle costing several lakh rupees and making the treatment unaffordable for many patients. Indian organizations are actively involved in clinical trials focused on immunotherapy. To improve accessibility, personalized treatments, such as dendritic cell-based therapy (Denvax), are being pursued as more affordable alternatives to expensive, imported immunotherapies [71].
10.6. Targeted Therapies
The recent advancement in the development of Trastuzumab Deruxtecan (Enhertu), the antibody conjugate for the metastatic HER2+ BC, was introduced in India in January 2024, marking a significant breakthrough [72]. In addition, in terms of Indian generics, the availability of affordable generic drugs and biosimilars has been vital for lowering costs and increasing treatment accessibility. However, the quality of some Indian-made drugs has been subject to debate due to varying manufacturing and regulatory standards. Moreover, in terms of research context, research investigations focusing on refining targeted therapy combinations and investigating innovative drug delivery technologies are ongoing.

Table 1.
Mechanism of action for various cancer therapies.
Table 1.
Mechanism of action for various cancer therapies.
| Therapy | Mechanism of Action | References |
|---|---|---|
| Surgery | The primary mechanism of action is the physical removal of a tumor, along with the adjacent healthy tissue (often referred to as the margin). It can be effective in treating early-stage, localized cancers. For more advanced diseases, surgery can be utilized to remove as much of the tumor as possible (debulking), thereby improving the efficacy of other treatments such as chemotherapy or radiation. | [73] |
| Radiation Therapy | High-energy radiation destroys the DNA of cancer cells, stopping them from proliferating and replicating. Cancer cells are more vulnerable to DNA damage than healthy cells because they divide faster and are less effective at DNA repair. The two most common methods are external beam radiation and internal radiation (brachytherapy), both of which are localized treatments. | [74] |
| Hormone Therapy | Also known as endocrine therapy, this approach functions by inhibiting the synthesis or activity of hormones that promote the growth of hormone-sensitive malignancies, such as breast and prostate cancer. This can be accomplished with medications that inhibit hormone synthesis, the blocking of hormone receptors, or the surgical removal of hormone-producing glands. | [75] |
| Chemotherapy | Cytotoxic chemotherapy medications aim to attack and eliminate rapidly dividing cells, including cancer cells, as well as healthy cells in the bone marrow, digestive tract, and hair follicles. These drugs disrupt the process of cell division through several mechanisms like causing DNA damage, blocking essential enzymes, or interrupting the cell’s mitotic machinery. | [76] |
| Immunotherapy | This therapy activates or strengthens the body’s immune system to identify and fight cancer cells. Examples of mechanisms are as follows:
| [77] |
| Targeted Therapies | Unlike chemotherapy, targeted therapies focus on particular molecular targets that are essential for the growth, development, and survival of cancer cells, while reducing damage to normal cells. They can inhibit signals that stimulate cancer cell development, trigger cell death, deprive the tumor by preventing the formation of new blood vessels (angiogenesis inhibitors), and transport toxic compounds directly to cancer cells. | [76] |
11. Cellular Genesis of Breast Cancer
11.1. Establishment of Mammary Gland and Mammary Stem Cells
The development of the human mammary gland is a continual process that commences during embryonic life. The mammary gland is made up of a distinctly branched network of epithelial tubes that are enclosed in a complex stroma. During the process of embryonic development, an epidermal placode gives rise to the mammary epithelium. The placodes eventually descend into the underlying mesenchyme and give rise to the rudimentary ductal structure of the gland that is present at birth which is chiefly promoted by the epithelial–mesenchymal interactions [78]. The breast rudiment is shaped at birth by 10–12 nascent ductal elements that are positioned underneath the nipple–areola complex [78]. In response to steroid hormone and growth factor receptor signaling, the breast goes through dramatic alterations in size, shape, and function during puberty, pregnancy, and lactation, respectively. This aside, the mammary epithelium is extremely receptive to local and systemic signals, and exhibits profound morphological alterations in the ductal tree during puberty and pregnancy.
Transplantation and lineage tracing investigation studies have demonstrated that the mammary epithelium has a grading of stem and progenitor cells. To elucidate furthermore, lineage tracing has indeed revealed the presence of bipotent mammary stem cells (MaSCs) in situ, in addition to long-lasting unipotent cells that steer the ductal tree morphogenesis and homeostasis. A fair amount of evidence indicates the presence of a heterogeneous MaSC section that includes the fetal MaSCs, slow-cycling cells, along with the long- and short-term repopulating cells. In addition, various luminal progenitor subtypes have been described in both normal mouse and normal human mammary tissue. This in turn suggests that understanding the “cells of origin” for BC requires interpreting the normal cellular hierarchy (Figure 10) [79].
Figure 10.
Cellular genesis of breast cancer.
Mammary stem cells (MaSCs) occur as a small section of undifferentiated cells in the mammary gland that can self-renew and generate diverse types of differentiated cells through symmetric and asymmetric divisions. Basically, the progenitor cells are thought to be produced by asymmetric divisions. In addition, the durability and self-renewal properties of the stem cells in a healthy body are well-known for their role in maintenance, repair, and tissue regeneration [79,80]. Under normal conditions, the MaSCs are thought to have functional roles that involve responding to cellular requirements during the progression of reproductive life. The MaSCs are believed to operate through close communication with their distinct cellular microenvironment, also known as the mammary stem cell niche. [79,80]. Notwithstanding, the precise location of these cells, with their distinct self-renewal and differentiation attributes, is still being debated, along with their role in BC. The MaSCs are slow-operating and long-existing stem cells, or progenitor cells, that prevail as a single, role-committed population. Additionally, there is still much discussion about the cells’ ability to differentiate into one of two types of mammary epithelial cell lineages, along with the impact of cell plasticity [81].
11.2. Contribution of Mammary Stem Cells in BC Progression
The umbrella concept or the cancer stem cell (CSC) theory is one effort to elucidate MaSCs in relation to breast cancer, as these cells do have a normal role in the body [82]. Despite the fact that the CSC theory has a long history, the emphasis herein will be on the current developments and interpretations of the theory related to breast cancer (Figure 10) [79]. The main point of disagreement in this theory is regardless of whether CSCs are the cell of derivation or origin from which cancer cells progresses, a theory or hypothesis based on explanations of resemblances between tissue replenishment and tumorigenesis, or whether malignant cells acquire stem cell potential [80,83]. The discussion commendably revolves around the significance of bipotent and unipotent luminal and myoepithelial stem cells during normal cell development and carcinogenesis [79].
One of the obstacles in accruing data to back-up or disprove this theory is the inaccuracy and variability between human and mouse models of selected biomarkers, such as CD49f, EpCAM, CD10, ALDH1A1, SSEA4, CD44, and CK12/CK19 66. Understanding cellular heterogeneity, which CSC theory attempts to describe, is critical to developing more specific treatment regimens for breast cancer, where cell heterogeneity is a major barrier. The CSC theory is based on a broadly accepted cell hierarchy structure both in normal and carcinogenic cells, with stem cells at the top and cells differentiating from that point [66]. As a result of this structure, MaSCs, as opposed to mammary repopulating cells, are bi- or multipotent, which is a well-supported conclusion. Under regenerative conditions as perceived through transplantation, these mammary repopulating cells can be recognized as adopting multipotent characteristics [84].
The MaSCs are thought to prevail in a specific microenvironment known as a stem cell niche, which emphasizes the significance of mutual response interactions between MaSC-derived epithelial cells, neighboring stromal cells, and the extracellular matrix. Nevertheless, the signaling pathways and how they impart to the normal and carcinogenic roles of MaSCs remain unknown. In terms of breast CSC origin, two major cell lineages are at the heart of this debate, viz., basal cells and luminal cells [79]. Basal cells are one of the two major cell lines in the mammary gland, the other being luminal cells. In the structure of the mammary gland, basal cells border the luminal cell layer. Similarly, the lumen of the mammary ducts and alveoli is lined with luminal cells. The basal cells usually express cytokeratins 5 and 14, as well as smooth muscle actin, whereas luminal cells express cytokeratin-8 and can be positive or negative to hormone receptors [79]. Few studies have demonstrated that patient-derived breast cancer organoids can outperform mouse models for cellular lineage tracing [69]. One such project is the creation of a living breast cancer organoid biobank, which could be used as a more precise model for research on cancer cell heterogeneity and drug discovery [85,86].
12. The Role of Genetics in the Effect of Breast Cancer Drug Resistance: Requirement for Predictive Biomarkers to Address the Outstanding Questions
The treatment regimens that are extensively presented for BC are human epidermal growth factor receptor 2 (HER2)-targeted therapy, endocrine therapy, and systemic chemotherapy. To elaborate further, based on the tumor’s features or attributes, and its hormone receptor status, and the presence of HER2, progesterone receptors, and estrogen receptors, pharmacotherapy is selected accordingly so as to yield a better disease prognosis [87]. Pharmacotherapy for breast cancer frequently has variable outcomes (inter-patient heterogenicity) in different populations, which may result in therapeutic failure. This can be brought about by several forms of congenital medication resistance among individuals. Congenital drug resistance is a phenomenon that is influenced by genetic variation. The link between genetic variations and treatment resistance for breast cancer will be briefly discussed herein.
Even while the prognosis for the disease has ameliorated through the positive outcomes of chemotherapy and adjuvants, a few designated sets of populations frequently respond differently to breast cancer treatment. Such variations or differences are brought about by the intrinsic resistance to certain prescribed medications or drugs [88]. Drug resistance is one of the chief causes for the cancer treatment failure [89]. These individual differences in drug response are mostly caused by DNA mutations that can change treatments’ or drugs’ efficacy [90]. Different mechanisms like the altered drug pharmacokinetics [91], increased or decreased cell signaling [92], altered pharmacodynamic-related receptor counts [93], etc., may all contribute to resistance. In order to identify drug resistance and provide information that enables the development of personalized medicine, it is indeed extremely pertinent to investigate additional gene polymorphisms that may influence therapeutic responses in breast cancer.
Hence, this segment will specifically be focused on the effects of pharmacogenetics in breast cancer drug resistance, and will discuss the reports or studies demonstrating the relationship of breast cancer drug resistance for significant genes like ABC1, BARDI, CD24, CYP19A1, CY2C19, CY2C9, CYP2D6, and a few others.
12.1. ABCB1
The ATP-binding cassette (ABC) is a transporter for diverse drug molecules, including chemotherapy drugs. This gene transporter aids and facilitates the passage of drug molecules through cellular membranes by expelling energy. The genes ABCB, ABCG, ABCD, ABCF, ABCCI, and ABCCII make up the ABC subfamily [94]. The ABCB1 gene is found in chromosome 7 which produces a 45-kB mRNA [95]. It encrypts a drug-active transporter that helps cells secrete cytotoxic chemicals. In addition, it encodes for P-glycoprotein (P-gp), an ATP-dependent drug efflux pump which transfers or delivers numerous hydrophobic substrates like anti-cancer drugs such as anthracyclines (doxorubicin), epipodophyllotoxins (etoposide), vinca alkaloids (vincristine), dactinomycin, and paclitaxel. The P-gp is usually expressed in tissues with excretory function, like the jejunum, kidney, liver, and adrenal gland [96]. The overexpression of MDR1 will result in the augmented drug efflux activity of P-gp, resulting in multi-drug resistance (MDR).
According to a study by Vulsteke et al. (2014) [97], patients with early breast cancer treated with 5-fluorouracil (FU), eirenicon, and cyclophosphamide (FEC) had better therapeutic outcomes when they had the ABCB1 GT genotype than when they had the ABCB1 GG/GA genotype. Similarly, based on the findings from a different study, the ABCB1 2677 GG genotype polymorphisms showed resistance to paclitaxel and anthracycline therapy. This gene polymorphism may contribute to the cross-resistance between paclitaxel and anthracycline in the treatment of metastatic breast cancer. Additionally, cases of ABCB1 3435 CT genotype resistance were discovered, which led to a poorer disease control rate and shorter overall survival (OS) when anthracycline treatment was administered [98].
Similarly another study by Vishnu et al. (2013) [99] on P-gp expression as a determinant factor in predicting response to NACT (Neoaduvant chemotherapy) proved that the detection of P-gp expression status before the initiation of chemotherapy can be used as a predictive marker for NACT response and which will eventually help in evading the toxic side effects of NACT (neoadjuvant chemotherapy) among non-responders.
12.2. eNOS
Numerous intracellular and extracellular factors, such as reactive oxygen and nitrogen species (RONS), have an impact on the development of cancers, especially breast cancer. In fact, the RONS’s accumulation within the cell is thought to contribute to the development and spread of cancer by altering the gene expression profile, dysregulating signaling pathways, and thereby inducing aberrant protein modifications [100]. As a crucial prerequisite for the physiological process, nitric oxide (NO), a water-soluble signaling molecule, is widely distributed and endogenously generated throughout the body [101]. In cancer, nitric oxide renders pleiotropic effects, some of which may promote tumor development and others may inhibit it, depending on the local concentration [102]. Reports on the function of eNOS in breast cancer revealed that it is in fact manifested in the breast tumors [100]. This indeed has a positive correlation with ER expression, which in turn has negative association with tumor grade and lymph node status. Additionally, the BC cell lines [103] and advanced breast tumors have been shown to express NOS [104]. This aside, the concentration of NO in the tumor microenvironment determines whether or not NO plays a role in tumor development or regression. While a higher NO concentration has cytotoxic effects and inhibits tumor growth and apoptosis, a lower NO concentration accelerates tumor progression by boosting tumor invasion, proliferation, migration, and chemoresistance [105]. Similarly, the results from a recent meta-analysis study described the link between the eNOS 894G>T polymorphism and the likelihood of developing cancer and stroke. Indeed, the conclusion from this study reveals a number of significant details, advancing the knowledge of NO’s role in these illnesses. The results pinpoint a possible association between the eNOS polymorphisms and a higher risk of developing cancer, especially in those who have the TT or GT genotype as opposed to the GG. This correlation seems to hold true for different ethnic groups (African, Asian, and European). However, the possibility that other variables could be at play was brought to light by other studies that found no correlation. It is thus inferred from this study that the eNOS polymorphisms, especially 894G>T, have a significant risk of cancer among the overall population based on the GG vs. GT+TT genetic model. Nevertheless, in light of these conclusions, these findings should be reassessed for further prospective validation with a larger sample size [106].
12.3. BARD1
Proteins that can communicate with breast cancer genes 1 (BRCA1) and 2 (BRCA2) and thereupon cause DNA and tumor suppressor damage may be encoded by a number of genes. The BRCA1-associated RING domain 1 (BARD1) gene is one such gene [107]. In terms of structure and function, the BARD1 gene generates a protein that is comparable to the BRCA1 protein [108]. Both BRCA1 and BARD1 have the ability to form homodimer and heterodimer structures. The former can be formed by linking with the an interesting new gene (RING) finger domain in the N-terminal portion, while the latter is stabilized by bonds between the amino acid residues of 26–119 in BARD1 and 1–109 in BRCA1respectively [109]. These interactions play a significant role in determining how breast cancer tumor suppression may manifest [108]. The stability of genotype and phenotype is generally controlled by BARD1, which also participates in DNA repair and ubiquitination. However, it was demonstrated that there is a significant association, with patients who carry single nucleotide polymorphism (SNP) in BARD1 rs2070096 with minor alleles having worse relapse-free survival rates compared to patients who have received non-TCH treatment. Nonetheless, another study found no substantial association between the polymorphism of BARD1 in rs2229571 and the response of patients using docetaxel, carboplatin, and trastuzumab (TCH) treatment compared with non-TCH treatment. Therefore, this may indicate chemoresistance [88].
12.4. CD24
The cluster of differentiation 24 (CD24) gene, which encodes for sialoglycoprotein and can bring about cell proliferation and signal changes, is found at chromosome locus 6q21 [110]. It is a protein located on the cell surface that offers links to the cell membrane via glycosylphosphatidylinositol [111]. Breast cancer cases have been linked to the overexpression of the CD24 protein. The CD24 expression was typically found in luminal and HER2-positive breast cancer cells [112]. In addition, the CD24 expression has been linked to breast cancer patients’ prognoses since it can control tumor cell proliferation [87] and escalate the prospects of metastasis [113].
There are currently no strong associations between genotype differentiation and CD24 expression. According to studies, CD24 Val is involved in the response to chemotherapy and has been linked to increased sensitivity, increased autoreactive immunity, and faster disease development. In a previous study on primary breast cancer, CD24 Val showed a significant sensitivity to anthracycline- and taxane-based therapy. According to the study, CD24 Ala/Val is the only single-factor indicator of post-neoadjuvant chemotherapy (NCT) positive complete response (pCR) in breast cancer patients. This aside, the anti-tumor immune response of the host may be able to be controlled by CD24 Ala/Val to make it more autoreactive. Differences in the CD24 genotype affect how patients respond to NCT therapy, as shown by the substantial association between CD24 Val/Val and intratumoral lymphocytic [114]. However, a study by Zhou et al. [115] revealed that pCR in breast cancer patients who had received NCT therapy was not anticipated by CD24 polymorphisms in rs3838646 and rs52812045.
12.5. Cytochrome P450 (CYP450), CYP
Cytochrome P450 (CYP450) is an enzyme that acts as a xenobiotic metabolizer by engaging xenobiotics into an oxidation reaction that converts the drug into its metabolites [116]. This mechanism is vital in BC therapy because drugs like tamoxifen must be transformed further into active and effective metabolites like 4-hydroxy-tamoxifen (4-HT) and endoxifen by CYP450 3A4 and CYP2D6, resulting in an elevated binding connection for the ER [117].
In one of the studies reported by Artigalás et al. [118], it is described that the rs4646 polymorphism in the CYP19A1 gene could possibly be a prognostic determinant in the aromatase inhibitor (AI) therapy. The SNP rs4646 was concomitant with the increased time to progression (TTP) compared to the wild-type gene in metastatic BC patients treated with AI (hazard ratio (HR) = 0.51 [95% confidence interval (CI), 0.33–0.78], p = 0.002). Additionally, Liu et al. [119] discovered a statistically significant link amongst the rs4646 T alleles (G/T or T/T) and improved overall survival in women with metastatic breast cancer (HR, 2.37 [95% CI, 1.20–4.65], p = 0.001). Miron et al. [120], on the other hand, found no compelling association with OS in the same SNP. Similarly, Henry et al. [121] found no statistically significant association between 127 SNPs in CYP19A1 associated with estrogen metabolism and breast density modulation. These findings suggest that CYP19A1 genotypes may be related to OS in BC patients receiving AIs. This association, however, seems to vary significantly between patients.
This aside, in a retrospective cohort study to describe chemoresistance brought about by CYP3A4 polymorphisms, Gor et al. [122] discovered that patients with at least one CYP3A4 *1B variant allele had a substantial relation with worse disease-free survival (DFS) in comparison to those with a wild-type *1A/*1A. The causal mechanism for this chemoresistance is that the *1B polymorphism decreases Phase I enzyme activity, resulting in a suboptimal 4-hydroxy-cyclophosphamide concentration. Because of the nature of cyclophosphamide pharmacokinetics, it must be activated to 4-hydroxy-cyclophosphamide before it can be distributed into the cancer cells via Phase I CYP enzymes, one of which is 3A4. Preceding studies by Beelen et al. [123] described a substantial link between CYP2C19 variant alleles and time to treatment failure (TTF) among tamoxifen patients, with CYP2C19*2 carriers having a longer TTF and those with the CYP2C19*17 allele having a shorter TTF, but not statistically significantly. Tamoxifen metabolism is effectively influenced by CYP2C19 inhibition, with transition to active metabolites like endoxifen having been detected. Tamoxifen resistance may be linked to lower tamoxifen and trans-4-OH-tamoxifen levels stimulated by isomerization to the cis isomer, and this isomerization may also happen for endoxifen [124,125,126]. Likewise, Vulsteke et al. [97] proposed that the CYP2C9 rs1057910 polymorphism produced resistance in his study, where there was a considerably worse RFI, but the C-allele variant carrier was only observed in three subjects, thereupon signifying additional investigation.
Furthermore, Regan et al. [127] found no link between tamoxifen therapy and alterations in the CYP2D6 phenotype metabolism. Endoxifen, a tamoxifen metabolite with a greater affinity for the ER, is thought to be linked to disease control, whereas CYP2D6 polymorphism, an enzyme that can metabolize tamoxifen into its active metabolite, was thought to be linked to lower endoxifen levels, and hence poorer disease control and higher side effects. According to Regan et al. [127], the CYP2D6 metabolism phenotype did not predict tamoxifen efficacy, implying that more research into tamoxifen metabolism and its mechanism of disease control is necessary. However, Dezentjé et al. [128] spotted an incorrect interpretation in that study and repeated it while investigating whether loss of heterozygozity (LOH) could explain a Hardy–Weinberg equilibrium deviation that may exclude false genotype by LOH in tumor tissues. Nevertheless, their study did not find any link between CYP2D6 genotype differences and the efficacy of tamoxifen. Further investigations by Neven et al. [129] and Sanchez-Spitman et al. [130] backed these findings, addressing no associations between low-activity CYP2D6 genotypes and low endoxifen levels and clinical outcomes.
13. Pharmacogenetics of Breast Cancer Therapeutics in India
The concept of pharmacogenetics, the study of how genetic variants influence drug response, is surfacing as a critical element of personalized and precision medicine, particularly for BC in India. Indeed, this discipline has a great potential for improving therapeutic options and dose regimen, thereby refining efficacy and eventually reducing side effects among the Indian BC patients, as corroborated by the studies discussed in Table 2.
Identifying the significance of genetic polymorphisms in determining BC treatment response and toxicity is crucial for personalized medicine, particularly in diversified populations like India [131]. Although there is a substantial body of research conducted worldwide, studies that focus exclusively on the Indian population are emerging, bringing to light specific concerns.
In the context of clinical implications, comprehending the genetic profiles of Indian BC patients, especially with the discussed gene polymorphisms, warrants targeted screening and treatment approaches improving drug dosage, and possibly reducing side effects. The gene polymorphisms in UGT1A1 and CYP2D6 can indicate an elevated risk of adverse events from certain drugs thus streamlining for preventive and strategic management of the drug dose modifications [132]. This aside, genetic polymorphisms in ABCB1 and GSTs can facilitate the efficacy of chemotherapy and the possibility of developing drug resistance, thus aiding in therapy escalation or the selection of alternate therapies. Likewise, comprehending the frequency and the scale of BRCA1/2 mutations in the Indian population is critical for genetic counseling and for strategies in managing hereditary BC.
At present, India does not have dedicated specific and regional pharmacogenetic guidelines for BC. Nonetheless, acknowledging the need and significance of personalized and precision medicine to the distinct genetic characteristics of Indian patients, initiative attempts are underway to incorporate pharmacogenomics into oncology practice in India [21,133].
Below are some suggestions and recommendations on how this information can be incorporated into oncology practice in India:
13.1. Evidence Generation and Dissemination
- Funding research on Indian populations: Additional investigation is required to explore and examine the genetic polymorphisms unique to the Indian population and their effect on treatment and drug response in BC.
- Implementing nationwide data repositories: Developing a complete and an extensive database of pharmacogenomic variations and drug–gene interactions related to the Indian population would be tremendously beneficial.
- Utilize real-world evidence, implementing observational studies: Corroborating results from real-world research or evidence-based studies in India, demonstrating the influence of pharmacogenomics testing on BC therapeutic response, is critical for initiating therapeutic value and increasing uptake.
13.2. Practical Implementation Strategies
- Clinically actionable drug–gene interactions (DGIs): Focus on employing pharmacogenomics tests for well-recognized drug–gene interactions in BC; for example, CYP2D6 for tamoxifen.
- Establish accessible testing policies or programs: Capitalizing on commercial, affordable and effective genetic testing tools like next-generation sequencing (NGS), is critical for increasing access.
- Proof of concept programs and step-by-step implementation: Initiate proof of concept or pilot programs at all oncology centers of excellence or specialized cancer hospitals to assess the feasibility and impact of integrating pharmacogenomics testing into regular BC care.

Table 2.
Details of some key genes and their associated polymorphisms linked to Breast cancer among the Indian population along with their potential clinical implications and the drugs affected.
Table 2.
Details of some key genes and their associated polymorphisms linked to Breast cancer among the Indian population along with their potential clinical implications and the drugs affected.
| Polymorphisms | Drugs Affected | Clinical Implications Relevant to Indian Population | |
|---|---|---|---|
| CYP2D6 | Multiple variant alleles (e.g., *4 null allele, *10 reduced function allele) | Tamoxifen | Increased risk of adverse effects: A combination of specific SNPs (e.g., 2988G>A, -1584C, and 2850C>T, suggestive of *41 reduced functional allele) may predict a higher risk of fatty liver with tamoxifen therapy [134]. |
| Altered tamoxifen metabolism: Individuals with reduced function alleles (*10) may show reduced benefit from tamoxifen and poorer disease-free survival [134]. | |||
| UGT1A1 | UGT1A128, UGT1A16 | Irinotecan | Increased irinotecan toxicity: Both UGT1A128 and UGT1A16 (more common in Asians) are associated with decreased UGT1A1 activity, increasing the risk of severe neutropenia and diarrhea from irinotecan. Double heterozygosity (UGT1A1*6/*28) is also associated with increased toxicity [135]. |
| ABCB1 (MDR1) | 1236C>T, 2677G>T/A, 3435C>T | Doxorubicin, cyclophosphamide, paclitaxel, and other MDR1 substrates | Drug resistance: Polymorphisms affecting the function and expression of ABCB1 (encoding P-glycoprotein) can lead to reduced intracellular drug levels and resistance to chemotherapy agents like anthracyclines and taxanes. Some studies suggest a higher risk of non-response to FAC chemotherapy with specific ABCB1 genotypes [136]. |
| GSTM1 | Null genotype | Anthracyclines (and potentially other chemotherapy drugs metabolized by GSTM1) | Worse chemotherapy responsiveness: The null genotype has been associated with worse responsiveness to anthracycline-based chemotherapy. Some studies suggest an increased risk of breast cancer susceptibility and shorter overall survival [137]. |
| GSTT1 | Null genotype | Cyclophosphamide, doxorubicin, 5-fluorouracil (CAF regimen) | Better DFS and OS with certain chemotherapy regimens: The null genotype, in some contexts, has been associated with better disease-free survival (DFS) and overall survival (OS) when treated with CAF (cyclophosphamide, doxorubicin, and 5-fluorouracil) chemotherapy compared to other variants. It may be associated with an increased frequency of p53 mutations [136]. |
| GSTP1 | 105Val/Val genotype, GG genotype | Cyclophosphamide (CTX) | Worse DFS with CTX: The 105Val/Val genotype showed a significant association with worse DFS in patients treated with cyclophosphamide. No significant association with cyclophosphamide outcome: Another study found no significant association with the GG genotype and cyclophosphamide outcome [136]. |
| BRCA1/2 | Pathogenic mutations and variants of uncertain significance (VUS) | PARP inhibitors | Increased hereditary breast and ovarian cancer risk: Mutations in BRCA1/2 are a major cause of hereditary breast and ovarian cancer. Predictive marker for PARP inhibitors: BRCA1/2 mutations are a strong indicator for PARP inhibitor efficacy [138]. |
| HER2 | Amplification | Trastuzumab, Pertuzumab, Lapatinib, Neratinib (HER2 inhibitors and targeted agents) | Predictive marker for HER2-targeted therapies: HER2 amplification leads to overexpression, which makes tumors responsive to anti-HER2 therapies like trastuzumab and pertuzumab. Resistance mechanisms can develop over time [139,140]. |
| Other genes | |||
| TP53 | Polymorphisms | Increased breast cancer risk [141,142]. | |
| PTEN | Germline mutations | Increased breast cancer risk [27]. | |
| MTHFR | C677T, A1298C | Association with increased homocysteine levels. A link with breast cancer risk is not clearly established [143,144]. | |
| NBN, RAD51, XRCC3 | Polymorphisms | Radiotherapy | Potential biomarkers for radiotherapy toxicity (e.g., cardiac toxicity with RAD51 and skin adverse events with XRCC3) in HER2-positive breast cancer [145]. |
13.3. Policy and Screening Guidelines
- Establish and formulate guidelines specific for India: Developing national guidelines for pharmacogenomic testing and interpretation in BC is crucial for standardizing the reliability and interpretation of results for therapeutic regimens.
- Review compliance with legal and ethical guidelines: Acknowledging concerns about patient confidentiality, sharing data resources, and possible discernment based on genetic information are vital for cultivating transparency and accountability, and eventually maintaining integrity.
- Developing a standardized curriculum for precision medicine in pharmacotherapy: Incorporating pharmacogenomics into medical training programs for both undergraduate and postgraduate students will prepare the next generation of oncologists to use this discipline proficiently.
By concentrating on these aspects, India can advance pharmacogenomics in BC treatment, leading to more accurate, efficient, and safer therapies for patients.
14. The Role of Tumor Microenvironment in Breast Cancer
The basic principle of the tumor microenvironment (TME) is that a tumor is an intricate, dynamic ecosystem with both malignant and non-malignant components, rather than an isolated mass of cancer cells. This environment plays an active role in and promotes cancer initiation, growth, progression, metastasis, and resistance to therapy. The non-cancerous parts of the TME are not inactive spectators, but are “hijacked” and reprogrammed by cancer cells to enhance their existence and proliferation [146].
As discussed below, the TME is made up of several key cellular and non-cellular components that interact via complex signaling networks.
14.1. Cellular Components
- Cancer cells: These primary cancerous cells comprise the tumor.
- Cancer-associated fibroblasts: These essentially reprogrammed fibroblasts are key producers of extracellular matrix (ECM) and numerous signaling molecules. They stimulate tumor development, angiogenesis, and metastasis.
- Immune cells: A varied group of immune cells, comprising the following:
- Tumor-associated macrophages (TAMs): Tumor-associated macrophages (TAMs) are usually adopted to an M2-like phenotype, which usually suppresses anti-tumor responses, stimulating angiogenesis, and facilitating metastasis.
- Regulatory T-cells (Tregs): These are a subclass of T-cells that inhibit the activity of other immune cells, resulting in an immunosuppressive environment.
- Myeloid-derived suppressor cells (MDSCs): These underdeveloped myeloid cells inhibit anti-tumor immune responses and promote tumor development.
- Endothelial cells: These cells form the inner layer of blood vessels and are engaged and stimulated by tumors to generate new, often defective, blood vessels that provide oxygen and nutrients to the tumor (angiogenesis).
- Pericytes: These are cells that encase the blood vessels and communicate with endothelial cells, prompting the development and durability of the tumor’s vasculature.
14.2. Non-Cellular Components
- Extracellular matrix (ECM): The ECM is a complex network of proteins and large molecules (such as collagen, fibronectin, and proteoglycans) that offers structural and biochemical assistance. The ECM in tumors frequently becomes rigid and undergoes modifications, thus facilitating the spread of cancer cells and impeding the effectiveness of therapeutic delivery [147].
- Signaling molecules: Several TME cells release a network of cytokines, chemokines, and growth factors to regulate their interactions. However, depending on the circumstances, these signals might stimulate or prevent tumor growth.
- Extracellular vesicles (EVs): These are minute vesicles, such as exosomes, that are secreted by cells to transport substances such as proteins and RNA. The tumor-derived EVs have the potential to reprogram other cells in the TME, promoting tumor development and prime distant locations for metastasis [148].
Indeed, the interplay and interaction within the tumor microenvironment (TME) are critical factors in cancer development and resistance to treatment regimen.
In terms of reciprocal communication, cancer cells are not passive in their environment; they actively regulate it by secreting cytokines and growth factors (signaling molecules) that in turn attract and alter the behavior of nearby healthy cells. As a result, the reprogrammed or modified stromal and immune cells produce signals that in turn regulate cancer cell proliferation and survival [149].
In the context of angiogenesis, because of the reduced oxygen (hypoxia) levels within the tumor, the TME produces pro-angiogenic substances like vascular endothelial growth factor (VEGF). This results in the development of a defective vascular network that delivers oxygen and nutrients to the tumor while simultaneously functioning as pathway for metastasis [150]. However, it is noteworthy that in therapeutic resistance, the TME can potentially obstruct a treatment’s efficacy. Additionally, immunosuppressive cells can deactivate the body’s inherent defenses against cancer, thus reducing the effectiveness of immunotherapies [151].
The importance of TME for Indian BC patients is significant because of unique clinical and epidemiological features, including a higher prevalence of aggressive subtypes in younger, premenopausal patients compared to Western populations. Furthermore, the unique genomic profiles observed in these may be driven by or connected to distinct TME architectures, which could play a role in both cancer progression and therapy response [152].
15. Recent Developments and Key Prospective Directions for Breast Cancer Research in India
Recent advancements in Indian breast cancer research are determined by the disease’s increasing prevalence and the significant number of late-stage detections. Key research objectives should therefore focus on improving diagnostic tools, including the use of artificial intelligence (AI) and local technology (indigenous), and creating affordable, scalable screening methods for neglected rural communities. Additionally, the research focus should prioritize India-specific challenges, such as a younger average age of diagnosis, a higher prevalence of aggressive cancer subtypes, late detection, dense breast tissue, and socioeconomic hurdles affecting care. Furthermore, key directions for the future may have to focus on utilizing advanced technologies such as AI, nanotechnology, and genomics to address the specific issues of the Indian population as discussed below.
15.1. Generating Comprehensive Genomic Data Specific for Indian Population (India-Specific Genome Atlas)
Breast cancer in India frequently affects younger individuals and is often more severe than in Western populations, resulting in lower survival rates. In addition, since Indian populations are inadequately represented in global databases, large-scale genomic studies are needed to generate comprehensive data, examine the distinct genomic landscape, and create customized predictive models for India, along with nationwide policies to address systemic challenges. These studies would focus on identifying and generating Indian-specific genetic resources, classify population-specific mutations, comprehending the younger average age of onset, and better understanding the higher prevalence of aggressive subtypes like TNBC, and eventually developing customized treatments and diagnostics strategies [15].
- Genomic and multi-omics profiling:
Large-scale genomic studies in India are aimed at producing comprehensive, high-quality multi-omics data that cover the entire spectrum of breast cancer. The Indian Cancer Genome Atlas (ICGA), a pan-India consortium spearheaded by the Council of Scientific and Industrial Research (CSIR) along with other governmental bodies has initiated a non-profit foundation for creating nationwide datasets, and genetic profiles of the cancers prevalent in India. In September 2024, it launched its initial multi-omics data platform specifically for BC patients in India, and is in fact the first multi-omics data portal for cancer in India [153]. On the other hand, the Bharat Cancer Genome Atlas (BCGA), another initiative launched by organizations like IIT Madras in conjunction with Karkinos Healthcare, is also aimed at creating a complete genetic database for cancers predominant in India. In fact, the BCGA was launched in early 2025, and its first public release is aimed at breast cancer [7]. The ultimate goal of these initiatives is to provide a multi-omics database that will manage and provide a specific genetic landscape of Indian cancer patients while also reducing current inequities in worldwide cancer research.
India’s heterogeneous population necessitates sequencing initiatives across different zones and socioeconomic classes to construct genetic classifiers that are clinically relevant throughout the country. Moreover, through the initiatives like ICGA and BCGA, researchers are examining genomic, transcriptomic, epigenetic, and proteomic data from Indian cancer patients. The initial cohort of 1000 BC patients is anticipated to create an extensive dataset that will be ethically collected and standardized. In addition, through these resources it will be possible to identify genetic traits and mutations unique to the Indian population, which may vary from those found in Western populations. This in turn may pave way to identify distinct mutation patterns (for example, genes like TP53 and ZFHX3), and detect specific or unique gene fusions and drivers of metastasis, and eventually the incidence of diverse molecular subtypes of BC in India, which may differ from those found in Western populations.
Gathering representative genomic data from the Indian population may help in developing more accurate, India-specific predictive tools. This will enable the use of specific SNPs to improve polygenic scores, which will provide insight into the unique mutational landscapes and pathways that can lead to more personalized medicine for patients. Eventually, the integration of genomic, clinical, and pathological data improves the overall understanding of the relationship between genetic profiles and patient outcomes.
15.2. Addressing High Breast Density Among Indian Breast Cancer Population
The recent developments and advances in BC research in India have indeed focused on using artificial intelligence (AI) and genomics to tackle the rising incidence, by advancing early diagnosis, addressing the distinct biological and socioeconomic issues specific to the Indian subcontinent. Indeed the recent research areas include harnessing technology for early detection, leveraging India’s unique genetic diversity for genomic investigations, enhancing treatment accessibility, and establishing therapeutic protocols tailored to specific needs. Moreover, this widens the scope of key directions, which aids in developing more accessible screening tools, implementing personalized medicine, and adopting public health policies to lessen inequities in care between urban and rural areas [153].
Undeniably, new research directions for BC in India combine deep learning (DL) with digital tomosynthesis (DBT) and genomic profiling. These efforts are aimed at overcoming healthcare and population-specific obstacles to increase screening accuracy and early detection. By integrating these advanced technologies, researchers hope to address the limitations of traditional approaches such as mammography, especially given the severity of higher breast density, which are particularly common among younger Indian women [154]. Nonetheless, effectively establishing such a system would necessitate considerable funding and validation to overcome significant resource and logistical obstacles within the Indian healthcare context.
- Using Artificial Intelligence (AI), deep learning (DL), and genomic profiling to refine tomosynthesis:
Indian researchers are indeed integrating these technologies to enhance breast cancer detection. Deep learning facilitates the analysis of massive amounts of image and genetic data, DBT provides more comprehensive 3D imaging than traditional mammography, and genomic profiling provides a better understanding of the disease’s biology.
Indian women generally exhibit higher breast density, which complicates the identification of tumors using the standard mammography since both dense tissue and tumors show up as white on the imaging. However, the deep learning algorithms can evaluate the more intricate, 3D visuals obtained from DBT to distinguish between dense tissue and possible anomalies, considerably increasing accuracy. This aside, deep learning and generative adversarial networks (GANs) can produce synthetic images to train detection algorithms, possibly refining the presentation in women with dense breast tissue, by generating bigger and more diversified training datasets [155].
On the other hand, genomic profiling enhances precision by examining the biological features of the tumor, which can subsequently be paired or integrated with imaging data. It can indeed help in forecasting a patient’s specific risk of developing BC as well as the chance of recurrence. When combined with tomosynthesis imaging, it offers a more comprehensive and precise evaluation of risk [156]. Similarly, genomic testing detects specific gene mutations and molecular features of tumors, thereby guiding towards personalized medicine. Indeed, genomic profiling can help identify biomarkers, track disease progression, and the onset of drug resistance by examining circulating tumor DNA via a “liquid biopsy” [157]. With the facts presented, AI models may integrate a wide range of data types, including tomosynthesis imaging and genomic data. This integrated strategy, known as radiogenomics, links the visual landscapes of a tumor to its molecular features, offering a deeper insight into the disease.
15.3. Adapting Screening Technologies for Early Breast Cancer Detection and Screening for Indian Needs
Breast cancer research and policies in India are prioritizing several key directions to address late diagnosis and enhance treatment availability, including the use of technology, reinforcing health systems, and culturally tailoring interventions. Therefore, to acclimatize BC screening for India, novel technologies must identify and overcome constraints like high costs, inadequate facilities, a lack of public awareness, and higher breast density among Indian women. Moreover, in place of depending only on exorbitant, foreign technology, India is slowly exploring low-cost, portable, and AI-powered BC screening approaches as discussed below, for rural and resource-limited settings.
- Technological innovation and research adapted for India:
Various startups and organizations in India are working on artificial intelligence(AI) technologies and solutions for BC screening, including NiramAI, an AI-powered diagnostic. It employs thermal imaging and AI (Thermalytix) to detect temperature fluctuations produced by tumors. The method is non-invasive, radiation-free, portable, and involves little training to use, making it ideal for rural areas and places with limited resources [158]. Likewise, Indian researchers are also working on non-invasive biomarkers, like blood-based diagnostics (immunoassays) and serum biomarkers for early cancer diagnosis, which could enhance sensitivity over mammography in younger Indian women [155].
This aside, AI-powered technologies such as “MammoAssist” are being investigated to improve diagnostic accuracy and lessen the burden on radiologists. Additionally, studies are exploring AI’s application in personalized therapies and treatment strategies [159]. Similarly, the internet is being used to offer easily accessible and trustworthy details about cancer in various languages. Telemedicine and digital health serve as an important study topic for delivering remote consultations and follow-up care, particularly in rural areas in India [160].
It is noteworthy that Indian researchers have effectively created novel and sophisticated drug delivery systems that improve therapeutic efficacy for breast cancer. To benchmark this, in February 2025, researchers at IIT Madras created and secured a patent for a drug delivery system using nanoparticles, which encapsulates anti-cancer drugs and specifically targets cancer cells, thus reducing adverse effects [161]. Correspondingly, in January 2025, researchers from the Bose Institute Kolkata and the IIT, Guwahati, created an injectable hydrogel for targeted therapy. This hydrogel delivers anti-cancer medications in a regulated manner to tumor areas [162].
In addition, in terms of utilizing local technology or indigenous treatment options, India is working on developing its own innovative remedies to boost accessibility. For example, In September 2025, a study reported that diselenide 7, a newly synthesized organoselenium compound, shows potential in addressing aggressive TNBC in animal models by lowering in invasiveness and metastasis [163].
15.4. Integrating Socio-Cultural Factors for Improving Program Implementation and Access for BC Research in India
In India, breast cancer screening uptake is chiefly influenced by the socioeconomic status and deeply rooted cultural beliefs. Therefore, BC research in India should focus on incorporating socio-cultural dynamics to improve the implementation and accessibility of breast cancer programs by overcoming challenges like stigma, lack of knowledge (ignorance), and patriarchal norms. Consequently, the key directions should include developing culturally tailored outreach strategies, incorporating socio-cultural aspects that enhance the capabilities of community health workers, addressing financial and systemic barriers, and mitigating economic disparities to effectively address the diverse Indian landscape. In addition, by establishing context-specific initiatives as discussed below, researchers can enhance screening rates, promote early diagnosis, and thereby lower the disproportionately high mortality among Indian women. Studies are underway evaluating the efficiency of introducing early detection strategies or programs into India’s healthcare system to enhance outreach and reduce late-stage diagnosis.
- Community-based solutions; a culturally adapted approach:
Successful BC screening in India would necessitate a nationwide plan that should combine various technologies and leverage collaborations between public and private sectors. Therefore, to improve coverage and lower the incidence of late-stage diagnosis, studies are evaluating the efficacy of implementing early detection programs into India’s public health system [155]. Predominantly, the primary research should aim to develop and test awareness programs that are sensitive to traditions, societal expectations and education levels, leveraging community channels.
Indeed, through community-based education, public health initiatives can reduce stigma and raise understanding of screening benefits and risk factors. This fosters women’s confidence in their healthcare choices. Studies are exploring the efficiency of disseminating knowledge through local resources. These resources include community leaders (such as religious frontrunners, political figures), media networks (including local talk shows and podcasts), and social media. This aside, in terms of community-based screening models, studies are underway to determine the practicality of deploying frontline health workers, such as Accredited Social Health Activists (ASHAs), to provide breast health education and clinical breast examinations in the community. These employees are trustworthy and can assist in breaking down cultural boundaries [164]. This in turn would enable women, especially those with a familial history or other high-risk factors, to benefit from accessible and affordable genetic testing or screening for mutations like BRCA, which would allow for targeted and early surveillance. Further studies are required to build successful community engagement models through local healthcare personnel and self-help groups. Investigations in urban slums across India have already demonstrated that engaging these groups is crucial to the long-term viability of new screening procedures for breast cancer [165].
Another key direction would be to incorporate BC screening into existing programs like the National Program for the Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases, and Stroke (NPCDCS), which currently targets women aged 30 years and older. Undeniably, additional investigation is required to determine why increasing socioeconomic status and women’s empowerment characteristics may not consistently correlate with increased screening uptake in certain regions. This includes reviewing the implementation of the present and future screening programs. Similarly, studies are also underway to determine how mobile screening camps can deliver services directly to the communities, especially in urban and rural areas with limited infrastructure [166].
16. Conclusions
The intricate landscape of breast cancer in India, as discussed in this comprehensive review, highlights specific challenges rooted in the country’s diversified socioeconomic and genetic factors. This review presents integrated information on the molecular, cellular, and developmental biology of BC, with a particular emphasis on India’s distinct epidemiological and clinical landscape. The findings from this review highlight a serious public health concern, with a considerable incidence of aggressive, triple-negative breast cancers and a younger median age of initiation than Western nations. The pathophysiological and molecular subtypes of BC are outlined, highlighting disease heterogeneity and the significance of personalized therapies. The mechanisms that drive BC progression are explained, demonstrating current studies that interpret complex signaling pathways. It also identifies specific knowledge gaps as well as diagnostic and therapeutic procedures or methodologies, indicating an urgent need for multi-faceted and comprehensive interventions.
Indeed, the global data on BC provides a basis, but it is evident that simply applying Western models to the Indian setting is inadequate to tackle the high mortality rates and the current inequities in treatment regimens. Therefore, the implications of this review are clear: the effective BC control in India demands culturally sensitive awareness campaigns, the development of effective national screening programs using suitable technologies (like ultrasound for younger women), and an increased investment in genetic counseling and treatment infrastructure.
In addition, to bridge the knowledge and diagnostic gaps, significant future strategies should emphasize generating India-specific molecular data and leveraging technological innovation. Specific strategies should focus on the development of the proposed “India-specific Genome Atlas” to uncover population-specific biomarkers, and should utilize AI-driven solutions to address issues like high breast density in screening and the need for early detection tools.
Finally, a comprehensive and integrated approach that combines research, education, early diagnosis, and accessible, high-quality healthcare services is not only necessary, but is also morally required, in order to reduce the growing burden of BC and increase survival rates across India.
Author Contributions
Conceptualization, writing, reviewing, and editing: A.D. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roy, P. Breast cancer in young Indian women: Factors, challenges in screening, and upcoming diagnostics. J. Cancer Res. Clin. Oncol. 2023, 149, 14409–14427. [Google Scholar] [CrossRef]
- Kulkarni, A.; Kelkar, D.A.; Parikh, N.; Shashidhara, L.S.; Koppiker, C.B.; Kulkarni, M. Meta-Analysis of Prevalence of Triple-Negative Breast Cancer and Its Clinical Features at Incidence in Indian Patients With Breast Cancer. JCO Glob. Oncol. 2020, 6, 1052–1062. [Google Scholar] [CrossRef]
- Sivasankar Perumal, A.D. Breast cancer and mental health: Understanding the link to depressioninanindianscenario. Glob. J. Res. Anal. 2025, 14, 1–4. [Google Scholar]
- Samad, M.A.; Ahmad, I.; Khan, M.R.; Suhail, M.; Zughaibi, T.A.; Al-Abbasi, F.A.; Alhosaini, K.A.; Khan, M.S.; Kumer, A.; Tabrez, S. Breast Cancer: Molecular Pathogenesis and Targeted Therapy. MedComm 2025, 6, e70404. [Google Scholar] [CrossRef]
- Srivastava, T.P.; Goel, I.; Gogia, A.; Parshad, R.; Monga, S.; Talukdar, J.; Rai, A.; Dhar, R.; Karmakar, S. Comprehensive Overview of Breast Cancer in India. JCO Glob. Oncol. 2025, 11, e2500083. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, R.K.; Gupta, S.; Prinja, S. Cost Effectiveness of Trastuzumab for Management of Breast Cancer in India. JCO Glob. Oncol. 2020, 6, 205–216. [Google Scholar] [CrossRef]
- Mahalingam, S.; Scaria, V.; Sivasubbu, S. The Bharat Cancer Genome Atlas: Charting India’s Unique Cancer Landscape for Precision Oncology. Technol. Cancer Res. Treat. 2025, 24, 15330338251381404. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- DeSantis, C.; Siegel, R.; Bandi, P.; Jemal, A. Breast cancer statistics, 2011. CA Cancer J. Clin. 2011, 61, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Szabo, C.I.; Chopin, S.; Barjhoux, L.; Sinilnikova, O.; Lenoir, G.; Goldgar, D.E.; Bhatanager, D. BRCA1 and BRCA2 in Indian breast cancer patients. Hum. Mutat. 2002, 20, 473–474. [Google Scholar] [CrossRef]
- India State-Level Disease Burden Initiative Cancer Collaborators. The burden of cancers and their variations across the states of India: The Global Burden of Disease Study 1990–2016. Lancet Oncol. 2018, 19, 1289–1306. [Google Scholar] [CrossRef]
- Kulothungan, V.; Sathishkumar, K.; Leburu, S.; Ramamoorthy, T.; Stephen, S.; Basavarajappa, D.; Tomy, N.; Mohan, R.; Menon, G.R.; Mathur, P. Burden of cancers in India—Estimates of cancer crude incidence, YLLs, YLDs and DALYs for 2021 and 2025 based on National Cancer Registry Program. BMC Cancer 2022, 22, 527. [Google Scholar] [CrossRef]
- Takiar, R.; Srivastav, A. Time trend in breast and cervix cancer of women in India—(1990–2003). Asian Pac. J. Cancer Prev. 2008, 9, 777–780. [Google Scholar]
- Maurya, A.P.; Brahrnachari, S. Current Status of Breast Cancer Management in India. Indian J. Surg. 2021, 83 (Suppl. S2), S316–S321. [Google Scholar] [CrossRef]
- Ranganathan, S.; Dee, E.C.; Debnath, N.; Patel, T.A.; Jain, B.; Murthy, V. Access and barriers to genomic classifiers for breast cancer and prostate cancer in India. Int. J. Cancer 2024, 154, 1335–1339. [Google Scholar] [CrossRef]
- Talukdar, J.; Srivastava, T.P.; Sahoo, O.S. Cancer stem cells: Signaling pathways and therapeutic targeting. MedComm—Oncology 2023, 2, e62. [Google Scholar] [CrossRef]
- Weerarathna, I.N.; Luharia, A.; Uke, A.; Mishra, G. Challenges and Innovations in Breast Cancer Screening in India: A Review of Epidemiological Trends and Diagnostic Strategies. Int. J. Breast Cancer 2024, 2024, 6845966. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Swain, S.K.; Banerjee, B.D.; Sharma, T.; Krishnalata, T. Organochlorine pesticide exposure as a risk factor for breast cancer in young Indian women: A case-control study. South. Asian J. Cancer 2019, 8, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.N.; Alex, A.; M.S., N.; Verma, V.; Shaji, A.; Pavithran, K.; Vijaykumar, D.K.; John, D. Economic burden of breast cancer in India, 2000–2021 and forecast to 2030. Sci. Rep. 2025, 15, 1323. [Google Scholar] [CrossRef]
- Wadasadawala, T.; Mohanty, S.K.; Sen, S.; Kanala, T.S.; Maiti, S.; Puchali, N.; Gupta, S.; Sarin, R.; Parmar, V. Out-of-pocket payment and financial risk protection for breast cancer treatment: A prospective study from India. Lancet Reg. Health Southeast Asia 2024, 24, 100346. [Google Scholar] [CrossRef]
- Pemmasani, S.K.; Raman, R.; Mohapatra, R.; Vidyasagar, M.; Acharya, A. A Review on the Challenges in Indian Genomics Research for Variant Identification and Interpretation. Front. Genet. 2020, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kapoor, R. Challenges and Opportunities in Oncology in India: Is There a Paradigm Shift? Indian J. Surg. 2025, 87, 976–977. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Ozsoy, A.; Barça, N.; Dolek, B.A.; Aktaş, H.; Elverici, E.; Araz, L.; Ozkaraoğlu, O. The Relationship Between Breast Cancer and Risk Factors: A Single-Center Study. Eur. J. Breast Health 2017, 13, 145–149. [Google Scholar] [CrossRef]
- Allison, K.H. Molecular pathology of breast cancer: What a pathologist needs to know. Am. J. Clin. Pathol. 2012, 138, 770–780. [Google Scholar] [CrossRef]
- Chikkala, R.; Bhayal, D.; Rani, N.; Modali, R.; Bhatia, K.; Dubey, B. Mutational landscape of BRCA gene mutations in Indian breast cancer patients: Retrospective insights from a diagnostic lab. Egypt. J. Med. Hum. Genet. 2024, 25, 101. [Google Scholar] [CrossRef]
- Pal, M.; Das, D.; Pandey, M. Understanding genetic variations associated with familial breast cancer. World J. Surg. Oncol. 2024, 22, 271. [Google Scholar] [CrossRef]
- Rehman, S.; Sameer, A.; Zahoor, L.; Abdullah, S.; Shah, Z.; Afroze, D.; Hussain, I.; Shaffi, S.M.; Syeed, N.; Rizvi, M.A.; et al. Distinct pattern of mutations of conserved regions of TP53 in colorectal cancer patients in the Kashmir population: An emerging high-risk area. Ecancermedicalscience 2009, 3, 129. [Google Scholar] [CrossRef] [PubMed]
- Lalli, E.; Zambetti, G.P.; Ribeiro, R.C.; Figueiredo, B.C. Cancer associated to low-penetrance TP53 variants: Insights from p.R337H. Trends Cancer 2025, 11, 499–502. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]
- John, A.O.; Singh, A.; Yadav, P.; Joel, A.; Thumaty, D.B.; Ninan, K.F.; Georgy, J.T.; Cherian, A.J.; Thomas, S.; Thomas, A.; et al. The BRCA mutation spectrum among breast and ovarian cancers in India: Highlighting the need to screen BRCA1 185delAG among South Indians. Eur. J. Hum. Genet. 2024, 32, 1319–1326. [Google Scholar] [CrossRef]
- Patra, A.; Ali, S.S.; Devi, N.M.; Qadeer, A.S.; Kamalakannan, S.; Nag, S.; Kulkarni, S.S.; Rajappa, S.; Hariharan, N.; Pant, H.B.; et al. Prevalence of BRCA mutation in breast and ovarian cancer among women in India: A systematic review and meta-analysis protocol. PLoS ONE 2024, 19, e0306612. [Google Scholar] [CrossRef]
- Goyal, J.; Yadav, A.; Malhotra, H. Association of Positive Family History and Clinicopathological Features in Breast Cancer in Young Indian Females—A Pilot Study. J. Radiat. Cancer Res. 2025, 16, 23–26. [Google Scholar] [CrossRef]
- Dupont, W.D.; Parl, F.F.; Hartmann, W.H.; Brinton, L.A.; Winfield, A.C.; Worrell, J.A.; Schuyler, P.A.; Plummer, W.D. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer 1993, 71, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Liebermann, E.; Patwardhan, V.; Usmanova, G.; Aktar, N.; Agrawal, S.; Bhamare, P.; McCarthy, M.; Ginsburg, O.; Kumar, S. Barriers to Follow-Up of an Abnormal Clinical Breast Examination in Uttar Pradesh, India: A Qualitative Study. JCO Glob. Oncol. 2024, 10, e2400001. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.C.; Sellers, T.A.; Frost, M.H.; Lingle, W.L.; Degnim, A.C.; Ghosh, K.; Vierkant, R.A.; Maloney, S.D.; Pankratz, V.S.; Hillman, D.W.; et al. Benign breast disease and the risk of breast cancer. N. Engl. J. Med. 2005, 353, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Babu, A.; Singh, S.; Lakhera, K.K.; Patel, P.; Gurjar, B.; Kumar, A.; Gora, B.S.; Sharma, R.G. Patterns and Prevalence of Benign Breast Diseases: An Epidemiological Chart Review Analysis of 5965 Patients from North Western India. Indian J. Surg. Oncol. 2025, 16, 1264–1269. [Google Scholar] [CrossRef]
- Sagiraju, H.K.R.; Gogia, A.; Goel, A.; Batra, A.; Sharma, D.; Saini, S.K.; Mishra, A.; Deo, S.; Prasad, C.P.; Tanwar, P.; et al. Effect of obesity on the survival outcomes among Indian women with breast cancer. J. Clin. Oncol. 2025, 43, e12558. [Google Scholar] [CrossRef]
- Sen, S.; Khan, P.K.; Wadasadawala, T.; Mohanty, S.K. Socio-economic and regional variation in breast and cervical cancer screening among Indian women of reproductive age: A study from National Family Health Survey, 2019–2021. BMC Cancer 2022, 22, 1279. [Google Scholar] [CrossRef]
- Chatterjee, D.; McDuffie, E.E.; Smith, S.J.; Bindle, L.; van Donkelaar, A.; Hammer, M.S.; Venkataraman, C.; Brauer, M.; Martin, R.V. Source Contributions to Fine Particulate Matter and Attributable Mortality in India and the Surrounding Region. Environ. Sci. Technol. 2023, 57, 10263–10275. [Google Scholar] [CrossRef]
- Rajbongshi, N.; Mahanta, L.B.; Nath, D.C. Evaluation of Female Breast Cancer Risk Among the Betel Quid Chewer: A Bio-Statistical Assessment in Assam, India. Nepal. J. Epidemiol. 2015, 5, 494–498. [Google Scholar] [CrossRef]
- Das, A.M.; Gogia, A.; Garg, M.; Elaiyaraja, A.; Arambam, P.; Mathur, S.; Babu-Rajendran, R.; Deo, S.; Kumar, L.; Das, B.C.; et al. Urinary concentration of endocrine-disrupting phthalates and breast cancer risk in Indian women: A case-control study with a focus on mutations in phthalate-responsive genes. Cancer Epidemiol. 2022, 79, 102188. [Google Scholar]
- Maurya, A.P.; Brahmachari, S. Association of hormonal and reproductive risk factors with breast cancer in Indian women: A systematic review of case-control studies. Indian J Cancer 2023, 60, 4–11. [Google Scholar] [CrossRef]
- Vishwakarma, G.; Ndetan, H.; Das, D.N.; Gupta, G.; Suryavanshi, M.; Mehta, A.; Singh, K.P. Reproductive factors and breast cancer risk: A meta-analysis of case-control studies in Indian women. South Asian J. Cancer 2019, 8, 80–84. [Google Scholar] [CrossRef]
- Parker, S.A.; Weygand, J.; Bernat, B.G.; Jackson, A.M.; Mawlawi, O.; Barreto, I.; Hao, Y.; Khan, R.; Yorke, A.A.; Swanson, W.; et al. Assessing Radiology and Radiation Therapy Needs for Cancer Care in Low-and-Middle-Income Countries: Insight From a Global Survey of Departmental and Institutional Leaders. Adv. Radiat. Oncol. 2024, 9, 101615. [Google Scholar] [CrossRef]
- Meeta, M.; Tanvir, T. Menopause Hormone Therapy in the Changing Trends of Breast Cancer in India. J. Midlife Health 2020, 11, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Clusan, L.; Ferriere, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef]
- Rodriguez-Casanova, A.; Costa-Fraga, N.; Castro-Carballeira, C.; González-Conde, M.; Abuin, C.; Bao-Caamano, A.; García-Caballero, T.; Brozos-Vazquez, E.; Rodriguez-López, C.; Cebey, V.; et al. A genome-wide cell-free DNA methylation analysis identifies an episignature associated with metastatic luminal B breast cancer. Front. Cell Dev. Biol. 2022, 10, 1016955. [Google Scholar] [CrossRef] [PubMed]
- Ulaganathan, K.; Puranam, K.; Mukta, S.; Hanumanth, S.R. Expression profiling of luminal B breast tumor in Indian women. J. Cancer Res. Clin. Oncol. 2023, 149, 13645–13664. [Google Scholar] [CrossRef]
- Prat, A.; Pascual, T.; De Angelis, C.; Gutierrez, C.; Llombart-Cussac, A.; Wang, T.; Cortés, J.; Rexer, B.; Paré, L.; Forero, A.; et al. HER2-Enriched Subtype and ERBB2 Expression in HER2-Positive Breast Cancer Treated with Dual HER2 Blockade. J. Natl. Cancer Inst. 2020, 112, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Prat, A.; Solovieff, N.; André, F.; O’SHaughnessy, J.; Cameron, D.A.; Janni, W.; Sonke, G.S.; Yap, Y.-S.; Yardley, D.A.; Partridge, A.H.; et al. Intrinsic Subtype and Overall Survival of Patients with Advanced HR+/HER2- Breast Cancer Treated with Ribociclib and ET: Correlative Analysis of MONALEESA-2, -3, -7. Clin. Cancer Res. 2024, 30, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zou, L.; Huang, S.; Miao, H.; Liu, K.; Geng, Y.; Liu, Y.; Wu, W. The anticancer activity of bile acids in drug discovery and development. Front. Pharmacol. 2024, 15, 1362382. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Luo, M.; Huang, J.; Zhang, K.; Zheng, S.; Zhang, S.; Zhou, J. Progression from ductal carcinoma in situ to invasive breast cancer: Molecular features and clinical significance. Signal Transduct. Target. Ther. 2024, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S. Molecular targets and therapies associated with poor prognosis of triple-negative breast cancer (Review). Int. J. Oncol. 2025, 66, 52. [Google Scholar] [CrossRef]
- Hassan, A.; Aubel, C. The PI3K/Akt/mTOR Signaling Pathway in Triple-Negative Breast Cancer: A Resistance Pathway and a Prime Target for Targeted Therapies. Cancers 2025, 17, 2232. [Google Scholar] [CrossRef]
- Pereira, C.; Martis, M.; D’Souza, R.; Tauro, L.F. Correlation of Clinicopathological Features of Breast Cancer with Molecular Subtypes Taking Ki-67 into Consideration: Single Institution Experience Over 5 Years. Curr. Health Sci. J. 2021, 47, 348–352. [Google Scholar] [CrossRef]
- Jonnada, P.K.; Sushma, C.; Karyampudi, M.; Dharanikota, A. Prevalence of Molecular Subtypes of Breast Cancer in India: A Systematic Review and Meta-analysis. Indian J. Surg Oncol. 2021, 12 (Suppl. S1), 152–163. [Google Scholar] [CrossRef]
- Siddiqui, B.; Ahmed, S.; Sinha, D.; Sharma, A.V.L. Molecular Classification of Breast Carcinoma in a Tertiary Hospital of India: The Recent Trends. Indian. J. Surg. Oncol. 2023, 14, 176–180. [Google Scholar] [CrossRef]
- Jain, S.; Narang, V.; Jain, K.; Paul, D.; Singh, J.; Sohi, A.S.; Sood, S.; Aggarwal, R.; Sood, N.; Brar, G.S. Prevalence of Molecular Subtypes in Operated Cases of Breast Cancer and Its Clinicopathological Correlation: A Single Institute Study from a Tertiary Cancer Centre in North India. Indian. J. Surg. Oncol. 2021, 12, 538–544. [Google Scholar] [CrossRef]
- Rathnasamy, N.; Mullapally, S.; Sirohi, B. Precision oncology in Low and Middle income countries: A word of caution. Int. J. Cancer Care Deliv. 2021, 1, 27–31. [Google Scholar] [CrossRef]
- Suresh AVSS, M.; Dadireddy, P.K. Cost-effectiveness analysis of gene expression profiling for breast cancer treatment decisions in India. Int. J. Community Med. Public Health 2025, 12, 2100–2102. [Google Scholar] [CrossRef]
- Gulwe, A.B.; Dawkhar, S.S. Role of SNP in the Breast Cancer Development of Indian Women. Int. J. Res. Innov. Appl. Sci. 2025, 10, 1160–1173. [Google Scholar] [CrossRef]
- Nirgude, S.; Desai, S.; Khanchandani, V.; Nagarajan, V.; Thumsi, J.; Choudhary, B. Integration of exome-seq and mRNA-seq using DawnRank, identified genes involved in innate immunity as drivers of breast cancer in the Indian cohort. PeerJ 2023, 11, e16033. [Google Scholar] [CrossRef] [PubMed]
- Sengar, M.; Pramesh, C.S.; Mehndiratta, A.; Shah, S.; Munshi, A.; Vijaykumar, D.K.; Puri, A.; Mathew, B.; Arora, R.S.; T, P.K.; et al. Ensuring quality in contextualised cancer management guidelines for resource-constraint settings: Using a systematic approach. BMJ Glob. Health 2022, 7, e009584. [Google Scholar] [CrossRef]
- Zhu, H.; Chua, M.L.K.; Chitapanarux, I.; Kaidar-Person, O.; Mwaba, C.; Alghamdi, M.; Mignola, A.R.; Amrogowicz, N.; Yazici, G.; Bourhaleb, Z.; et al. Global radiotherapy demands and corresponding radiotherapy-professional workforce requirements in 2022 and predicted to 2050: A population-based study. Lancet Glob. Health 2024, 12, e1945–e1953. [Google Scholar] [CrossRef]
- Sankarapillai, J.; Ramamoorthy, T.; Sarveswaran, G.; Venugopal, G.; Mathur, P. Epidemiological analysis of radiation therapy utilization and its implications for cancer care in India-insights from National Cancer Registry Programme. BMC Cancer 2025, 25, 1062. [Google Scholar] [CrossRef]
- Ghosh, A.; Chaubal, R.; Das, C.; Parab, P.; Das, S.; Maitra, A.; Majumder, P.P.; Gupta, S.; Biswas, N.K. Genomic hallmarks of endocrine therapy resistance in ER/PR+HER2- breast tumours. Commun. Biol. 2025, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Syed, Y.Y. Atezolizumab (in Combination with Nab-Paclitaxel): A Review in Advanced Triple-Negative Breast Cancer. Drugs 2020, 80, 601–607. [Google Scholar] [CrossRef]
- Laureano, R.S.; Sprooten, J.; Vanmeerbeerk, I.; Borras, D.M.; Govaerts, J.; Naulaerts, S.; Berneman, Z.N.; Beuselinck, B.; Bol, K.F.; Borst, J.; et al. Trial watch: Dendritic cell (DC)-based immunotherapy for cancer. Oncoimmunology 2022, 11, 2096363. [Google Scholar] [CrossRef]
- Pandey, P.; Chaudhary, R.; Tripathi, D.; Lavudi, K.; Dua, K.; Weinfeld, M.; Lavasanifar, A.; Rajinikanth, P.S. Personalized treatment approach for HER2-positive metastatic breast cancer. Med. Oncol. 2024, 41, 252. [Google Scholar] [CrossRef] [PubMed]
- Deepa, K.V.; Gadgil, A.; Lofgren, J.; Mehare, S.; Bhandarkar, P.; Roy, N. Is quality of life after mastectomy comparable to that after breast conservation surgery? A 5-year follow up study from Mumbai, India. Qual. Life Res. 2020, 29, 683–692. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Asaithamby, A. Repurposing DNA repair factors to eradicate tumor cells upon radiotherapy. Transl. Cancer Res. 2017, 6 (Suppl. S5), S822–S839. [Google Scholar] [CrossRef]
- Khan, A.; Sisodiya, S.; Aftab, M.; Tanwar, P.; Hussain, S.; Gupta, V. Mechanisms and Therapeutic Strategies for Endocrine Resistance in Breast Cancer: A Comprehensive Review and Meta-Analysis. Cancers 2025, 17, 1653. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; Dhanjal, J.K.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar]
- Rai, A.; Deshpande, S.G.; Vaidya, A.; Shinde, R.K. Advancements in Immunotherapy for Breast Cancer: Mechanisms, Efficacy, and Future Directions. Cureus 2024, 16, e68351. [Google Scholar] [CrossRef] [PubMed]
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef]
- Lloyd-Lewis, B.; Harris, O.B.; Watson, C.J.; Davis, F.M. Mammary Stem Cells: Premise, Properties, and Perspectives. Trends Cell Biol. 2017, 27, 556–567. [Google Scholar] [CrossRef]
- Soteriou, D.; Fuchs, Y. A matter of life and death: Stem cell survival in tissue regeneration and tumour formation. Nat. Rev. Cancer 2018, 18, 187–201. [Google Scholar] [CrossRef]
- Mascré, G.; Dekoninck, S.; Drogat, B.; Youssef, K.K.; Brohée, S.; Sotiropoulou, P.A.; Simons, B.D.; Blanpain, C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012, 489, 257–262. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.D.; Burness, M.L.; Wicha, M.S. Therapeutic Implications of Cellular Heterogeneity and Plasticity in Breast Cancer. Cell Stem Cell 2015, 17, 260–271. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, R.; Bowman, A.N.; Nusse, R. Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 2012, 11, 387–400. [Google Scholar] [CrossRef]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef]
- Pauli, C.; Hopkins, B.D.; Prandi, D.; Shaw, R.; Fedrizzi, T.; Sboner, A.; Sailer, V.; Augello, M.; Puca, L.; Rosati, R.; et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017, 7, 462–477. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.S.; Clark, A.S.; Tchou, J.; Zhang, P.; Freedman, G.M. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. S1), 9S–16S. [Google Scholar] [CrossRef]
- Coté, D.; Eustace, A.; Toomey, S.; Cremona, M.; Milewska, M.; Furney, S.; Carr, A.; Fay, J.; Kay, E.; Kennedy, S.; et al. Germline single nucleotide polymorphisms in ERBB3 and BARD1 genes result in a worse relapse free survival response for HER2-positive breast cancer patients treated with adjuvant based docetaxel, carboplatin and trastuzumab (TCH). PLoS ONE 2018, 13, e0200996. [Google Scholar] [CrossRef]
- Polgar, O.; Bates, S.E. ABC transporters in the balance: Is there a role in multidrug resistance? Biochem. Soc. Trans. 2005, 33 Pt 1, 241–245. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Gandhi, N.; Das, G.M. Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications. Cells 2019, 8, 89. [Google Scholar] [CrossRef]
- Raghav, D.; Sharma, V. An In Silico Evaluation of Deleterious Nonsynonymous Single Nucleotide Polymorphisms in the ErbB3 Oncogene. Biores. Open Access 2013, 2, 206–211. [Google Scholar] [CrossRef]
- Thussbas, C.; Nahrig, J.; Streit, S.; Bange, J.; Kriner, M.; Kates, R.; Ulm, K.; Kiechle, M.; Hoefler, H.; Ullrich, A.; et al. FGFR4 Arg388 allele is associated with resistance to adjuvant therapy in primary breast cancer. J. Clin. Oncol. 2006, 24, 3747–3755. [Google Scholar] [CrossRef]
- Mackenzie, P.I.; Owens, I.S.; Burchell, B.; Bock, K.W.; Bairoch, A.; Belanger, A.; Gigleux, S.F.; Green, M.; Hum, D.W.; Iyanagi, T.; et al. The UDP glycosyltransferase gene superfamily: Recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 1997, 7, 255–269. [Google Scholar] [CrossRef]
- Fojo, A.; Lebo, R.; Shimizu, N.; Chin, J.E.; Roninson, I.B.; Merlino, G.T.; Gottesman, M.M.; Pastan, I. Localization of multidrug resistance-associated DNA sequences to human chromosome 7. Somat. Cell Mol. Genet. 1986, 12, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Tashbaeva, R.E.; Hwang, D.N.; Song, G.S.; Choi, N.H.; Lee, J.H.; Lyoo, Y.S.; Lee, S.J.; Jung, D.I.; Kim, H.Y.; Sur, J.H. Cellular characterization of multidrug resistance P-glycoprotein, alpha fetoprotein, and neovascular endothelium-associated antigens in canine hepatocellular carcinoma and cirrhotic liver. Vet. Pathol. 2007, 44, 600–606. [Google Scholar] [CrossRef]
- Vulsteke, C.; Pfeil, A.M.; Schwenkglenks, M.; Pettengell, R.; Szucs, T.D.; Lambrechts, D.; Peeters, M.; van Dam, P.; Dieudonné, A.S.; Hatse, S.; et al. Impact of genetic variability and treatment-related factors on outcome in early breast cancer patients receiving (neo-) adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide, and docetaxel. Breast Cancer Res. Treat. 2014, 147, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Rha, S.Y.; Jeung, H.-.-C.; Im, C.-.-K.; Ahn, J.B.; Kwon, W.S.; Yoo, N.C.; Roh, J.K.; Chung, H.C. Association of the ABCB1 gene polymorphisms 2677G>T/A and 3435C>T with clinical outcomes of paclitaxel monotherapy in metastatic breast cancer patients. Ann. Oncol. 2009, 20, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Vishnukumar, S.; Umamaheswaran, G.; Anichavezhi, D.; Indumathy, S.; Adithan, C.; Srinivasan, K.; Kadambari, D. P-glycoprotein expression as a predictor of response to neoadjuvant chemotherapy in breast cancer. Indian J. Cancer 2013, 50, 195–199. [Google Scholar] [CrossRef]
- Mishra, D.; Patel, V.; Banerjee, D. Nitric Oxide and S-Nitrosylation in Cancers: Emphasis on Breast Cancer. Breast Cancer 2020, 14, 1178223419882688. [Google Scholar] [CrossRef]
- Devendran, A.; Nampoothiri, S.; Shewade, D.G.; Chatterjee, S.; Jayaraman, B.; Chandrasekharan, A. Allele, Genotype and Haplotype Structures of Functional Polymorphic Variants in Endothelial Nitric Oxide Synthase (eNOS), Angiotensinogen (ACE) and Aldosterone Synthase (CYP11B2) Genes in Healthy Pregnant Women of Indian Ethnicity. J. Reprod. Infertil. 2015, 16, 180–192. [Google Scholar]
- Coutinho, L.L.; Femino, E.L.; Gonzalez, A.L.; Moffat, R.L.; Heinz, W.F.; Cheng, R.Y.S.; Lockett, S.J.; Rangel, M.C.; Ridnour, L.A.; Wink, D.A. NOS2 and COX-2 Co-Expression Promotes Cancer Progression: A Potential Target for Developing Agents to Prevent or Treat Highly Aggressive Breast Cancer. Int. J. Mol. Sci. 2024, 25, 6103. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef]
- Loibl, S.; von Minckwitz, G.; Weber, S.; Sinn, H.; Schini-Kerth, V.B.; Lobysheva, I.; Nepveu, F.; Wolf, G.; Strebhardt, K.; Kaufmann, M. Expression of endothelial and inducible nitric oxide synthase in benign and malignant lesions of the breast and measurement of nitric oxide using electron paramagnetic resonance spectroscopy. Cancer 2002, 95, 1191–1198. [Google Scholar] [CrossRef]
- Liang, F.; Wang, M.; Li, J.; Guo, J. The evolution of S-nitrosylation detection methodology and the role of protein S-nitrosylation in various cancers. Cancer Cell Int. 2024, 24, 408. [Google Scholar] [CrossRef] [PubMed]
- Tualeka, A.R.; Jalaludin, J.; Gasana, J.; Sopian, N.A.; Chao, H.R.; Yusmaidie, M.; Perumal, V.; Zurimi, S.; Rahmawati, P.; Ahsan, A.; et al. Association between eNOS rs1799983 (894G>T) Polymorphism with Cancer and Stroke Risk: A meta-analysis. F1000Research 2024, 12, 1467. [Google Scholar] [CrossRef]
- Suszynska, M.; Kluzniak, W.; Wokolorczyk, D.; Jakubowska, A.; Huzarski, T.; Gronwald, J.; Debniak, T.; Szwiec, M.; Ratajska, M.; Klonowska, K.; et al. BARD1 is A Low/Moderate Breast Cancer Risk Gene: Evidence Based on An Association Study of the Central European p.Q564X Recurrent Mutation. Cancers 2019, 11, 740. [Google Scholar] [CrossRef]
- Hashizume, R.; Fukuda, M.; Maeda, I.; Nishikawa, H.; Oyake, D.; Yabuki, Y.; Ogata, H.; Ohta, T. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 2001, 276, 14537–14540. [Google Scholar] [CrossRef]
- Meza, J.E.; Brzovic, P.S.; King, M.C.; Klevit, R.E. Mapping the functional domains of BRCA1. Interaction of the ring finger domains of BRCA1 and BARD1. J. Biol. Chem. 1999, 274, 5659–5665. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine N-CHs. CD24 Molecule [Homo Sapiens (Human)]. 2023. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=100133941 (accessed on 22 November 2025).
- Schabath, H.; Runz, S.; Joumaa, S.; Altevogt, P. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J. Cell Sci. 2006, 119 Pt 2, 314–325. [Google Scholar] [CrossRef]
- Kwon, M.J.; Han, J.; Seo, J.H.; Song, K.; Jeong, H.M.; Choi, J.-S.; Kim, Y.J.; Lee, S.-H.; Choi, Y.-L.; Shin, Y.K. CD24 Overexpression Is Associated with Poor Prognosis in Luminal A and Triple-Negative Breast Cancer. PLoS ONE 2015, 10, e0139112. [Google Scholar] [CrossRef]
- Baumann, P.; Cremers, N.; Kroese, F.; Orend, G.; Chiquet-Ehrismann, R.; Uede, T.; Yagita, H.; Sleeman, J.P. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005, 65, 10783–10793. [Google Scholar] [CrossRef]
- Marmé, F.; Werft, W.; Walter, A.; Keller, S.; Wang, X.; Benner, A.; Burwinkel, B.; Sinn, P.; Hug, S.; Sohn, C.; et al. CD24 Ala57Val polymorphism predicts pathologic complete response to sequential anthracycline- and taxane-based neoadjuvant chemotherapy for primary breast cancer. Breast Cancer Res. Treat. 2012, 132, 819–831. [Google Scholar] [CrossRef]
- Zhou, X. CD24 polymorphisms cannot predict pathologic complete response to anthracycline- and taxane-based neoadjuvant chemotherapy in breast cancer. Clin. Breast Cancer 2014, 14, e33–e40. [Google Scholar] [CrossRef]
- Rao, U.S.C.; Devendran, A.; Satyamoorthy, K.; Shewade, D.G.; Krishnamoorthy, R.; Chandrasekaran, A. Functional characterization of promoter region polymorphisms of human CYP2C19 gene. Mol. Biol. Rep. 2011, 38, 4171–4179. [Google Scholar]
- Crewe, H.K.; Ellis, S.W.; Lennard, M.S.; Tucker, G.T. Variable contribution of cytochromes P450 2D6, 2C9 and 3A4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochem. Pharmacol. 1997, 53, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Artigalas, O.; Vanni, T.; Hutz, M.H.; Ashton-Prolla, P.; Schwartz, I.V. Influence of CYP19A1 polymorphisms on the treatment of breast cancer with aromatase inhibitors: A systematic review and meta-analysis. BMC Med. 2015, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bai, Y.X.; Zhou, J.H.; Sun, X.W.; Sui, H.; Zhang, W.J.; Yuan, H.H.; Xie, R.; Wei, X.L.; Zhang, T.T.; et al. A polymorphism at the 3′-UTR region of the aromatase gene is associated with the efficacy of the aromatase inhibitor, anastrozole, in metastatic breast carcinoma. Int. J. Mol. Sci. 2013, 14, 18973–18988. [Google Scholar] [CrossRef]
- Miron, L.; Negura, L.; Peptanariu, D.; Marinca, M. Research on aromatase gene (CYP19A1) polymorphisms as a predictor of endocrine therapy effectiveness in breast cancer. Rev. Med. Chir. Soc. Med. Nat. Iasi 2012, 116, 997–1004. [Google Scholar]
- Henry, N.L.; Chan, H.P.; Dantzer, J.; Goswami, C.P.; Li, L.; Skaar, T.C.; Rae, J.M.; Desta, Z.; Khouri, N.; Pinsky, R.; et al. Aromatase inhibitor-induced modulation of breast density: Clinical and genetic effects. Br. J. Cancer 2013, 109, 2331–2339. [Google Scholar] [CrossRef]
- Gor, P.P.; Su, H.I.; Gray, R.J.; A Gimotty, P.; Horn, M.; Aplenc, R.; Vaughan, W.P.; Tallman, M.S.; Rebbeck, T.R.; DeMichele, A. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: A retrospective cohort study. Breast Cancer Res. 2010, 12, R26. [Google Scholar] [CrossRef]
- Beelen, K.; Opdam, M.; Severson, T.M.; Koornstra, R.H.T.; Vincent, A.D.; Hauptmann, M.; van Schaik, R.H.N.; Berns, E.M.J.J.; Vermorken, J.B.; van Diest, P.J.; et al. CYP2C19 2 predicts substantial tamoxifen benefit in postmenopausal breast cancer patients randomized between adjuvant tamoxifen and no systemic treatment. Breast Cancer Res. Treat. 2013, 139, 649–655. [Google Scholar] [CrossRef]
- Johnston, S.; Dowsett, M.; Smith, I.; Sacks, N.; Haynes, B.; Jarman, M.; Ebbs, S. Acquired tamoxifen resistance in human breast cancer and reduced intra-tumoral drug concentration. Lancet 1993, 342, 1521–1522. [Google Scholar] [CrossRef]
- Satyanarayana, C.R.; Devendran, A.; Sundaram, R.; Gopal, S.D.; Rajagopal, K.; Chandrasekaran, A. Genetic variations and haplotypes of the 5′ regulatory region of CYP2C19 in South Indian population. Drug Metab. Pharmacokinet. 2009, 24, 185–193. [Google Scholar] [CrossRef]
- Anichavezhi, D.; Chakradhara Rao, U.S.; Shewade, D.G.; Krishnamoorthy, R.; Adithan, C. Distribution of CYP2C19*17 allele and genotypes in an Indian population. J. Clin. Pharm. Ther. 2012, 37, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Regan, M.M.; Leyland-Jones, B.; Bouzyk, M.; Pagani, O.; Tang, W.; Kammler, R.; Dell’Orto, P.; Biasi, M.O.; Thürlimann, B.; Lyng, M.B.; et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The breast international group 1-98 trial. J. Natl. Cancer Inst. 2012, 104, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Dezentjé, V.O.; Van Schaik, R.H.N.; Vletter-Bogaartz, J.M.; Van Der Straaten, T.; Wessels, J.A.M.; Kranenbarg, E.M.-K.; Berns, E.M.; Seynaeve, C.; Putter, H.; Van De Velde, C.J.H.; et al. CYP2D6 genotype in relation to tamoxifen efficacy in a Dutch cohort of the tamoxifen exemestane adjuvant multinational (TEAM) trial. Breast Cancer Res. Treat. 2013, 140, 363–373. [Google Scholar] [CrossRef]
- Neven, P.; Jongen, L.; Lintermans, A.; Van Asten, K.; Blomme, C.; Lambrechts, D.; Poppe, A.; Wildiers, H.; Dieudonné, A.-S.; Brouckaert, O.; et al. Tamoxifen Metabolism and Efficacy in Breast Cancer: A Prospective Multicenter Trial. Clin. Cancer Res. 2018, 24, 2312–2318. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.; Dezentjé, V.; Swen, J.; Moes, D.J.A.r.; Böhringer, S.; Batman, E.; Van Druten, E.; Smorenburg, C.; Van Bochove, A.; Zeillemaker, A.; et al. Tamoxifen Pharmacogenetics and Metabolism: Results From the Prospective CYPTAM Study. J. Clin. Oncol. 2019, 37, 636–646. [Google Scholar] [CrossRef]
- Kadri, M.S.N.; Patel, K.M.; Bhargava, P.A.; Shah, F.D.; Badgujar, N.V.; Tarapara, B.V.; Patel, P.S.; Shaikh, M.I.; Shah, K.; Patel, A.; et al. Mutational Landscape for Indian Hereditary Breast and Ovarian Cancer Cohort Suggests Need for Identifying Population Specific Genes and Biomarkers for Screening. Front. Oncol. 2020, 10, 568786. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.R.; Joghee, S. Enhancing breast cancer treatment through pharmacogenomics: A narrative review. Clin. Chim. Acta 2024, 562, 119893. [Google Scholar] [CrossRef]
- Jadeja, N.; Rajakumar, N.; Reddy, N.; Ali, N.; Lichten, L. Reflections on my international genetic counseling rotations: Contrasts in practice between India and the United States. Genet. Med. Open 2024, 2 (Suppl. S2), 101871. [Google Scholar] [CrossRef]
- Damodaran, S.E.; Pradhan, S.C.; Umamaheswaran, G.; Kadambari, D.; Reddy, K.S.; Adithan, C. Genetic polymorphisms of CYP2D6 increase the risk for recurrence of breast cancer in patients receiving tamoxifen as an adjuvant therapy. Cancer Chemother. Pharmacol. 2012, 70, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Sharma, S.; Behera, D.; Singh, N. UGT1A1 Gene Polymorphisms in Patients with Small Cell Lung Cancer Treated with Irinotecan-Platinum Doublet Chemotherapy and Their Association with Gastrointestinal Toxicity and Overall Survival. Oncologist 2021, 26, 701–713. [Google Scholar] [CrossRef]
- Gudur, R.A.; Bhosale, S.J.; Gudur, A.K.; Kale, S.R.; More, A.L.; Datkhile, K.D. Influence of (C1236T and C3435T) Polymorphisms of ABCB1 Gene on Chemotherapy Treatment Outcome and Toxicity in Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2024, 25, 1567–1577. [Google Scholar] [CrossRef]
- Behlam, I.; Sudershan, A.; Bharti, S.; Saini, A.K.; Manhas, S.; Kumar, P. Association of GSTT1 & GSTM1 Null Mutations with Breast Cancer Risk in North Indian Population: A Case-Control Study. Afr. J. Biomed. Res. 2024, 27, 4016–4022. [Google Scholar]
- Bhaskaran, S.P.; Huang, T.; Rajendran, B.K.; Guo, M.; Luo, J.; Qin, Z.; Zhao, B.; Chian, J.; Li, S.; Wang, S.M. Ethnic-specific BRCA1/2 variation within Asia population: Evidence from over 78 000 cancer and 40 000 non-cancer cases of Indian, Chinese, Korean and Japanese populations. J. Med. Genet. 2021, 58, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, J.; Gutierrez, C.; Sethunath, V.; Mehravaran, S.; Giuliano, M.; Shea, M.J.; Mitchell, T.; Wang, T.; Nanda, S.; Pereira, R.; et al. Neratinib plus trastuzumab is superior to pertuzumab plus trastuzumab in HER2-positive breast cancer xenograft models. NPJ Breast Cancer 2021, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.P.; Avaronnan, M.; Devi, N.; Praveen Kumar Shenoy, V.P. Her2 positive metastatic breast cancer treated with low dose lapatinib in a resource-constrained setting in South India: A retrospective audit. Ecancermedicalscience 2024, 18, 1758. [Google Scholar] [CrossRef]
- Ghosh, M.; Saha, S.; Bettke, J.; Nagar, R.; Parrales, A.; Iwakuma, T.; van der Velden, A.W.; Martinez, L.A. Mutant p53 suppresses innate immune signaling to promote tumorigenesis. Cancer Cell 2021, 39, 494–508.e5. [Google Scholar] [CrossRef]
- Akhter, N.; Dar, S.A.; Chattopadhyay, S.; Haque, S.; Anwer, R.; Wahid, M.; Jawed, A.; Lohani, M.; Mandal, R.K.; Shukla, N.K.; et al. Impact of p53 arg72pro SNP on Breast Cancer Risk in North Indian Population. Curr. Genom. 2018, 19, 395–410. [Google Scholar] [CrossRef]
- Waseem, M.; Hussain, S.R.; Kumar, S.; Serajuddin, M.; Mahdi, F.; Sonkar, S.K.; Bansal, C.; Ahmad, M.K. Association of MTHFR (C677T) Gene Polymorphism with Breast Cancer in North India. Biomark. Cancer 2016, 8, 111–117. [Google Scholar] [CrossRef]
- Kuras, S.; Pence, M.E.; Arac, E.; Yildiz, A.; Erdogan, B.; Pence, S. Investigation of the effects of MTHFR gene variations and homocysteine levels in hypertensive patients. North. Clin. Istanb. 2025, 12, 55–61. [Google Scholar] [CrossRef]
- Rajagopal, T.; Seshachalam, A.; Rathnam, K.K.; Talluri, S.; Venkatabalasubramanian, S.; Dunna, N.R. Homologous recombination DNA repair gene RAD51, XRCC2 & XRCC3 polymorphisms and breast cancer risk in South Indian women. PLoS ONE 2022, 17, e0259761. [Google Scholar]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, S.; Rao, M.; Cheng, B. Tumor-derived extracellular vesicles: Key drivers of immunomodulation in breast cancer. Front. Immunol. 2025, 16, 1548535. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I. Hypoxia-driven angiogenesis in breast cancer mechanisms and therapeutic targets: A narrative review. Ann. Med. Surg. 2025, 87, 4246–4254. [Google Scholar] [CrossRef]
- Gill, G.S.; Kharb, S.; Goyal, G.; Das, P.; Kurdia, K.C.; Dhar, R.; Karmakar, S. Immune checkpoint inhibitors and immunosuppressive tumor microenvironment: Current challenges and strategies to overcome resistance. Immunopharmacol. Immunotoxicol. 2025, 47, 485–507. [Google Scholar] [CrossRef]
- McDonald, J.A.; Rao, R.; Gibbons, M.; Janardhanan, R.; Jaswal, S.; Mehrotra, R.; Pandey, M.; Radhakrishnan, V.; Ramakant, P.; Verma, N.; et al. Symposium report: Breast cancer in India-trends, environmental exposures and clinical implications. Cancer Causes Control 2021, 32, 567–575. [Google Scholar] [CrossRef]
- ICGA. Teams—Indian Cancer Genome Atlas: Mapping India’s Cancer Genome SD, Ankita Singh. The Indian Cancer Genome Atlas: A Multi-Omics Resource for Advancing Cancer Research. bioRxiv 2025. [Google Scholar]
- Bhattacharya, S.; Varshney, S.; Heidler, P.; Tripathi, S.K. Expanding the horizon for breast cancer screening in India through artificial intelligent technologies -A mini-review. Front. Digit. Health 2022, 4, 1082884. [Google Scholar] [CrossRef]
- Mangayarkarasi, V.; Durairaj, E.; Ramanathan, V. Enhancing Cancer Screening and Early Diagnosis in India: Overcoming Challenges and Leveraging Emerging Technologies. Cureus 2025, 17, e78808. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, D.; Lockhat, Z.; Brzozowski, L.; Saini, K.S.; Dlamini, Z.; Hull, R. The Convergence of Radiology and Genomics: Advancing Breast Cancer Diagnosis with Radiogenomics. Cancers 2024, 16, 1076. [Google Scholar] [CrossRef]
- Adapa, K.; Gupta, A.; Singh, S.; Kaur, H.; Trikha, A.; Sharma, A.; Rahul, K. A real world evaluation of an innovative artificial intelligence tool for population-level breast cancer screening. npj Digit. Med. 2025, 8, 2. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Saleem, S.M.; Singh, A.; Singh, S.; Tripathi, S. Empowering precision medicine: Regenerative AI in breast cancer. Front. Oncol. 2024, 14, 1465720. [Google Scholar] [CrossRef]
- Das, S.K.; Dasgupta, R.K.; Roy, S.D.; Shil, D. AI in Indian healthcare: From roadmap to reality. Intell. Pharm. 2024, 2, 329–334. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.S.; Sasmal, A.; Ariraman, S.B.M.; Raj, T.; Selvaraj, V.; Arockiarajan, A.; Sudhakar, S. Cobalt-nickel metal-organic frameworks (CNMs) as drug delivery agents for triple-negative breast cancer. Nanoscale Adv. 2025, 7, 5361–5376. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Das, T.; Kushwaha, R.; Misra, A.K.; Jana, K.; Das, D. Targeted and precise drug delivery using a glutathione-responsive ultra-short peptide-based injectable hydrogel as a breast cancer cure. Mater. Horiz. 2025, 12, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Press Information Bureau (PIB), G.o.I. New Compound Synthesised Can Combat Aggressive Breast Cancer: Press Information Bureau (PIB), Government of India; 2025 [Cited Government of India]. Available online: https://www.pib.gov.in/PressReleasePage.aspx?PRID=2162654 (accessed on 22 November 2025).
- Sangwan, R.K.; Huda, R.K.; Panigrahi, A.; Toteja, G.S.; Sharma, A.K.; Thakor, M.; Kumar, P. Strengthening breast cancer screening program through health education of women and capacity building of primary healthcare providers. Front. Public Health 2023, 11, 1276853. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Yadav, K. Breast cancer in India: Present scenario and the challenges ahead. World J. Clin. Oncol. 2022, 13, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Jamwal, N.; Gupta, R. The Chiraiya project: A retrospective analysis of breast cancer detection gaps addressed via mobile mammography in Jammu Province, India. BMC Public Health 2024, 24, 2087. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).