Estrogen Signals through ERβ in Breast Cancer; What We Have Learned since the Discovery of the Receptor
Abstract
1. Introduction
2. The Phenotypes of ERβ Knockout Mouse Models
3. Effects of Tissue-Specific Deletion of ERβ
4. Post-Translational Modifications of ERβ
5. ERβ and Breast Cancer Cell Proliferation
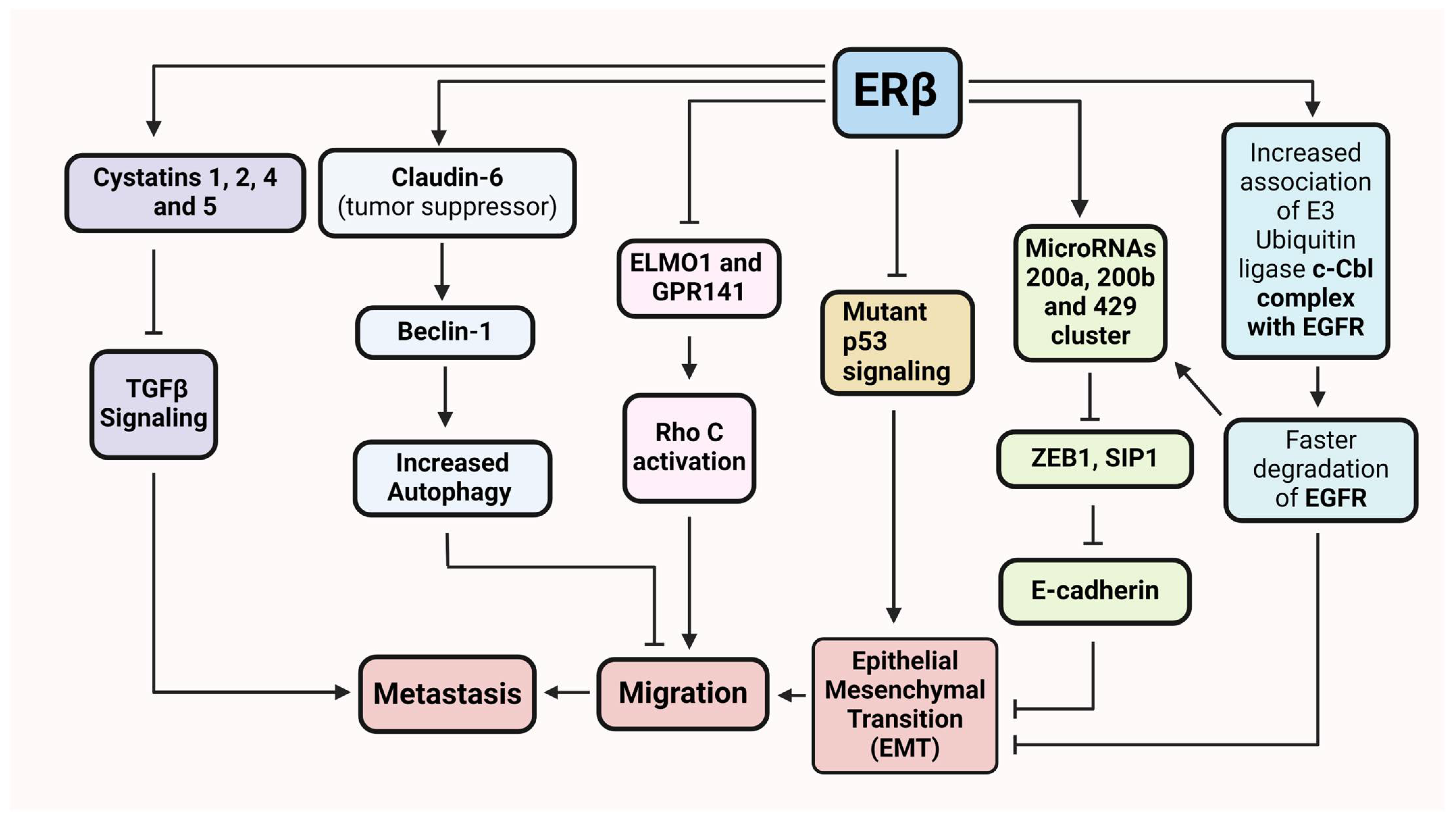
6. ERβ in Cancer Cell Invasion and Metastasis
7. ERβ in TNBC
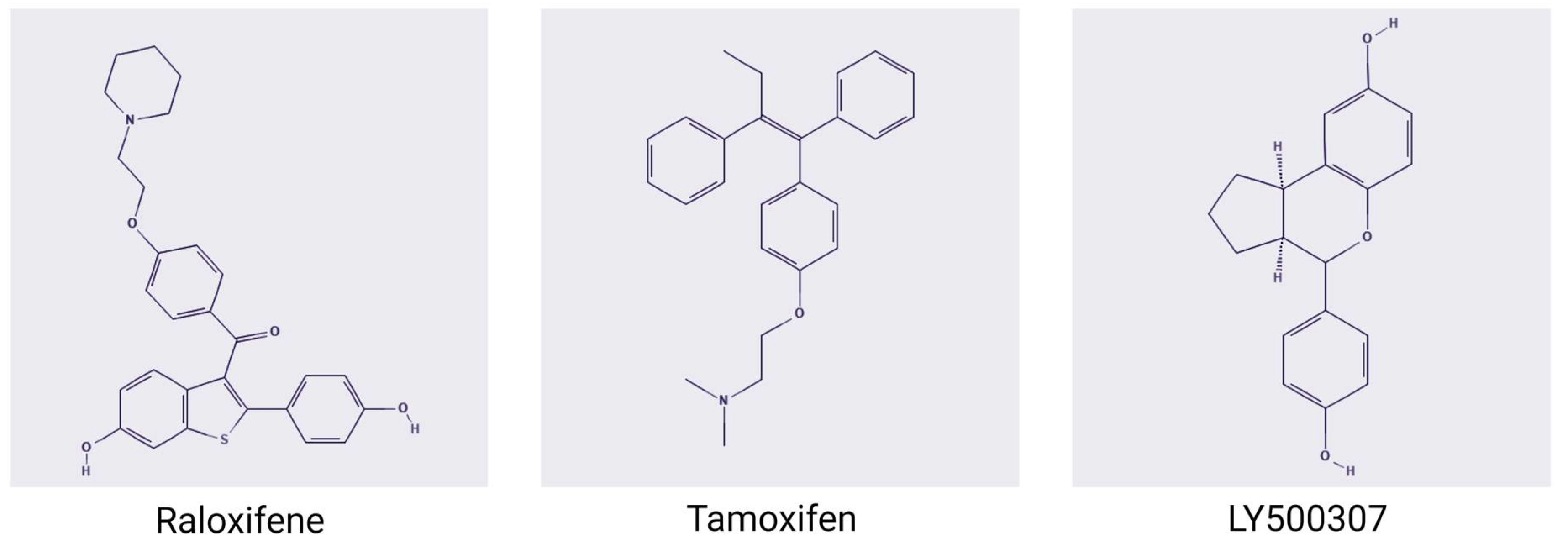
8. Synthetic Ligands and ERβ Activity
8.1. Raloxifene
8.2. Tamoxifen
8.3. LY500307
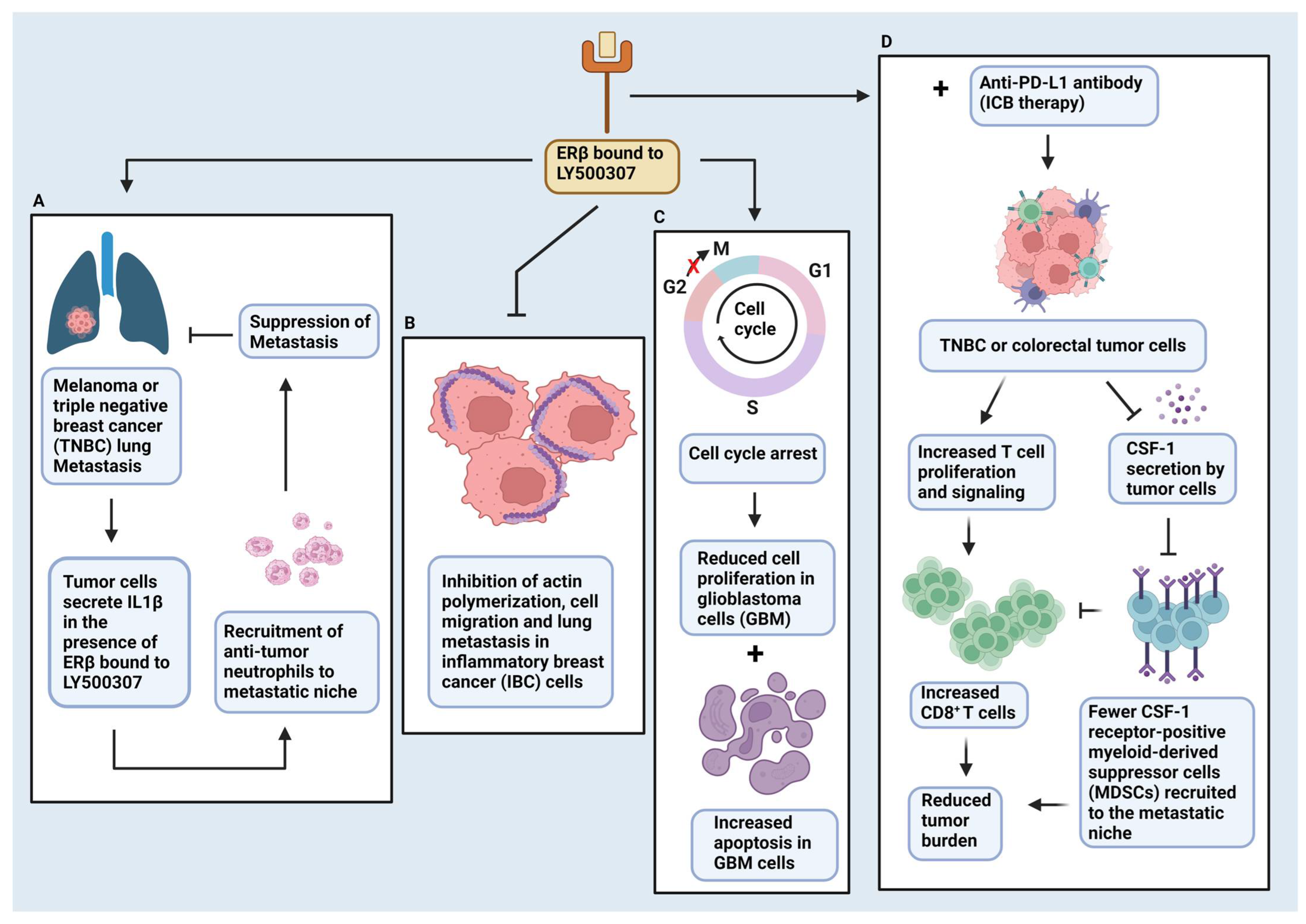
9. ERβ and the Tumor Microenvironment
10. Concluding Remarks
Funding
Conflicts of Interest
References
- Jensen, E. Studies of growth phenomena using tritium labeled steroids. In Proceedings of the 4th International Congress of Biochemistry, Vienna, Austria, 1–6 September 1958; Pergamon Press: London, UK; p. 119. [Google Scholar]
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Enmark, E.; Pelto-Huikko, M.; Grandien, K.; Lagercrantz, S.; Lagercrantz, J.; Fried, G.; Nordenskjold, M.; Gustafsson, J.A. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J. Clin. Endocrinol. Metab. 1997, 82, 4258–4265. [Google Scholar] [CrossRef] [PubMed]
- Mosselman, S.; Polman, J.; Dijkema, R. ERβ: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Shughrue, P.J.; Merchenthaler, I.; Gustafsson, J.A. The estrogen receptor beta subtype: A novel mediator of estrogen action in neuroendocrine systems. Front. Neuroendocrinol. 1998, 19, 253–286. [Google Scholar] [CrossRef]
- Taylor, A.H.; Al-Azzawi, F. Immunolocalisation of oestrogen receptor beta in human tissues. J. Mol. Endocrinol. 2000, 24, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.E.; Fuller, P. The importance of ERβ signalling in the ovary. J. Endocrinol. 2010, 205, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. Ca Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Speirs, V.; Parkes, A.T.; Kerin, M.J.; Walton, D.S.; Carleton, P.J.; Fox, J.N.; Atkin, S.L. Coexpression of estrogen receptor α and β: Poor prognostic factors in human breast cancer? Cancer Res. 1999, 59, 525–528. [Google Scholar]
- Iwao, K.; Miyoshi, Y.; Egawa, C.; Ikeda, N.; Noguchi, S. Quantitative analysis of estrogen receptor-β mRNA and its variants in human breast cancers. Int. J. Cancer 2000, 88, 733–736. [Google Scholar] [CrossRef]
- Shaw, J.A.; Udokang, K.; Mosquera, J.M.; Chauhan, H.; Jones, J.L.; Walker, R.A. Oestrogen receptors alpha and beta differ in normal human breast and breast carcinomas. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2002, 198, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-W.; Kim, K.-S.; Heo, M.-K.; Ko, S.-S.; Lee, K.S.; Hong, S.W.; Yang, W.-I.; Kim, J.-H.; Kim, G.E. treatment. Expression of estrogen receptor-β in normal mammary and tumor tissues: Is it protective in breast carcinogenesis? Breast Cancer Res. Treat. 2003, 80, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Skliris, G.P.; Munot, K.; Bell, S.M.; Carder, P.J.; Lane, S.; Horgan, K.; Lansdown, M.R.; Parkes, A.T.; Hanby, A.M.; Markham, A.F.; et al. Reduced expression of oestrogen receptor β in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2003, 201, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Rody, A.; Holtrich, U.; Solbach, C.; Kourtis, K.; Von Minckwitz, G.; Engels, K.; Kissler, S.; Gatje, R.; Karn, T.; Kaufmann, M. Methylation of estrogen receptor β promoter correlates with loss of ER-β expression in mammary carcinoma and is an early indication marker in premalignant lesions. Endocr.-Relat. Cancer 2005, 12, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.-Å.; Smithies, O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar] [CrossRef] [PubMed]
- Antal, M.C.; Krust, A.; Chambon, P.; Mark, M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERβ-null mutant. Proc. Natl. Acad. Sci. USA 2008, 105, 2433–2438. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Wu, W.-f.; Montanholi, L.; Nalvarte, I.; Antonson, P.; Gustafsson, J.-A. Ventral prostate and mammary gland phenotype in mice with complete deletion of the ERβ gene. Proc. Natl. Acad. Sci. USA 2020, 117, 4902–4909. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Krust, A.; Gansmuller, A.; Dierich, A.; Chambon, P.; Mark, M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 2000, 127, 4277–4291. [Google Scholar] [CrossRef]
- Seko, D.; Fujita, R.; Kitajima, Y.; Nakamura, K.; Imai, Y.; Ono, Y. Estrogen receptor β controls muscle growth and regeneration in young female mice. Stem Cell Rep. 2020, 15, 577–586. [Google Scholar] [CrossRef]
- Hases, L.; Indukuri, R.; Birgersson, M.; Nguyen-Vu, T.; Lozano, R.; Saxena, A.; Hartman, J.; Frasor, J.; Gustafsson, J.-Å.; Katajisto, P. Intestinal estrogen receptor beta suppresses colon inflammation and tumorigenesis in both sexes. Cancer Lett. 2020, 492, 54–62. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hugerth, L.W.; Hases, L.; Saxena, A.; Seifert, M.; Thomas, Q.; Gustafsson, J.Å.; Engstrand, L.; Williams, C. Colitis-induced colorectal cancer and intestinal epithelial estrogen receptor beta impact gut microbiota diversity. Int. J. Cancer 2019, 144, 3086–3098. [Google Scholar] [CrossRef] [PubMed]
- Hases, L.; Archer, A.; Indukuri, R.; Birgersson, M.; Savva, C.; Korach-André, M.; Williams, C. High-fat diet and estrogen impacts the colon and its transcriptome in a sex-dependent manner. Sci. Rep. 2020, 10, 16160. [Google Scholar] [CrossRef] [PubMed]
- Bado, I.; Nikolos, F.; Rajapaksa, G.; Wu, W.; Castaneda, J.; Krishnamurthy, S.; Webb, P.; Gustafsson, J.-Å.; Thomas, C. Somatic loss of estrogen receptor beta and p53 synergize to induce breast tumorigenesis. Breast Cancer Res. 2017, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Bado, I.; Nikolos, F.; Rajapaksa, G.; Gustafsson, J.A.; Thomas, C. ERbeta decreases the invasiveness of triple-negative breast cancer cells by regulating mutant p53 oncogenic function. Oncotarget 2016, 7, 13599–13611. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.-M.; Babu, C.S.; Wang, J.; Yuan, Y.; Lam, Y.-W.; Ho, S.-M.; Leung, Y.-K. Phosphorylation of human estrogen receptor-beta at serine 105 inhibits breast cancer cell migration and invasion. Mol. Cell. Endocrinol. 2012, 358, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Burke, W.; Coleman, L.; Cummings, M.; Green, C.A.; Holliday, D.L.; Horgan, K.; Maraqa, L.; Peter, M.B.; Pollock, S.; Shaaban, A.M. Phosphorylation of estrogen receptor β at serine 105 is associated with good prognosis in breast cancer. Am. J. Pathol. 2010, 177, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, K.; Lepage, J.; Sanchez, M.; Heveker, N.; Tremblay, A. Positive feedback activation of estrogen receptors by the CXCL12-CXCR4 pathway. Cancer Res. 2009, 69, 5793–5800. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.; Tremblay, G.B.; Labrie, F.; Giguère, V. Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol. Cell 1999, 3, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Hart, G.W. AlternativeO-Glycosylation/O-Phosphorylation of Serine-16 in Murine Estrogen Receptor β: POST-TRANSLATIONAL REGULATION OF TURNOVER AND TRANSACTIVATION ACTIVITY. J. Biol. Chem. 2001, 276, 10570–10575. [Google Scholar] [CrossRef]
- Cheng, X.; Cole, R.N.; Zaia, J.; Hart, G.W. Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor β. Biochemistry 2000, 39, 11609–11620. [Google Scholar] [CrossRef]
- Yuan, B.; Cheng, L.; Gupta, K.; Chiang, H.-C.; Gupta, H.B.; Sareddy, G.R.; Wang, D.; Lathrop, K.; Elledge, R.; Wang, P. Tyrosine phosphorylation regulates ERβ ubiquitination, protein turnover, and inhibition of breast cancer. Oncotarget 2016, 7, 42585. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Cheng, L.; Chiang, H.C.; Xu, X.; Han, Y.; Su, H.; Wang, L.; Zhang, B.; Lin, J.; Li, X.; et al. A phosphotyrosine switch determines the antitumor activity of ERbeta. J. Clin. Investig. 2014, 124, 3378–3390. [Google Scholar] [CrossRef] [PubMed]
- Picard, N.; Caron, V.; Bilodeau, S.; Sanchez, M.; Mascle, X.; Aubry, M.; Tremblay, A. Identification of estrogen receptor β as a SUMO-1 target reveals a novel phosphorylated sumoylation motif and regulation by glycogen synthase kinase 3β. Mol. Cell. Biol. 2012, 32, 2709–2721. [Google Scholar] [CrossRef] [PubMed]
- Roger, P.; Sahla, M.E.; Mäkelä, S.; Gustafsson, J.A.k.; Baldet, P.; Rochefort, H. Decreased expression of estrogen receptor β protein in proliferative preinvasive mammary tumors. Cancer Res. 2001, 61, 2537–2541. [Google Scholar] [PubMed]
- Järvinen, T.A.; Pelto-Huikko, M.; Holli, K.; Isola, J. Estrogen receptor β is coexpressed with ERα and PR and associated with nodal status, grade, and proliferation rate in breast cancer. Am. J. Pathol. 2000, 156, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Paruthiyil, S.; Parmar, H.; Kerekatte, V.; Cunha, G.R.; Firestone, G.L.; Leitman, D.C. Estrogen receptor β inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004, 64, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Ström, A.; Hartman, J.; Foster, J.S.; Kietz, S.; Wimalasena, J.; Gustafsson, J.-Å. Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc. Natl. Acad. Sci. USA 2004, 101, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, K.; Helguero, L.A.; Omoto, Y.; Gustafsson, J.-Å.; Haldosén, L.-A. Estrogen receptor β represses Akt signaling in breast cancer cells via downregulation of HER2/HER3 and upregulation of PTEN: Implications for tamoxifen sensitivity. Breast Cancer Res. 2011, 13, R43. [Google Scholar] [CrossRef] [PubMed]
- Cowley, S.M.; Hoare, S.; Mosselman, S.; Parker, M.G. Estrogen receptors α and β form heterodimers on DNA. J. Biol. Chem. 1997, 272, 19858–19862. [Google Scholar] [CrossRef]
- Pace, P.; Taylor, J.; Suntharalingam, S.; Coombes, R.C.; Ali, S. Human estrogen receptor β binds DNA in a manner similar to and dimerizes with estrogen receptor α. J. Biol. Chem. 1997, 272, 25832–25838. [Google Scholar] [CrossRef]
- Pettersson, K.; Grandien, K.; Kuiper, G.G.; Gustafsson, J.-A. Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Mol. Endocrinol. 1997, 11, 1486–1496. [Google Scholar] [PubMed]
- Williams, C.; Edvardsson, K.; Lewandowski, S.; Ström, A.; Gustafsson, J.-Å. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene 2008, 27, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Grober, O.; Mutarelli, M.; Giurato, G.; Ravo, M.; Cicatiello, L.; De Filippo, M.R.; Ferraro, L.; Nassa, G.; Papa, M.F.; Paris, O. Global analysis of estrogen receptor beta binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor alpha for target gene regulation. BMC Genom. 2011, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.; Lindberg, K.; Morani, A.; Inzunza, J.; Strom, A.; Gustafsson, J.-A. Estrogen receptor β inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res. 2006, 66, 11207–11213. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Katchy, A.; Williams, C. Support of a bi-faceted role of estrogen receptor beta (ERbeta) in ERalpha-positive breast cancer cells. Endocr. Relat. Cancer 2014, 21, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Lee, E.J.; Madison, L.D.; Lazennec, G. Expression of estrogen receptor β in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Lett. 2004, 566, 169–172. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Wu, W.; Strom, A.M.; Warner, M.; Gustafsson, J.-Å. Estrogen receptor β regulates AKT activity through up-regulation of INPP4B and inhibits migration of prostate cancer cell line PC-3. Proc. Natl. Acad. Sci. USA 2020, 117, 26347–26355. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Luo, Y.; Tai, R.; Zhang, N. Estrogen receptor β suppresses inflammation and the progression of prostate cancer. Mol. Med. Rep. 2019, 19, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Mal, R.; Magner, A.; David, J.; Datta, J.; Vallabhaneni, M.; Kassem, M.; Manouchehri, J.; Willingham, N.; Stover, D.; Vandeusen, J.; et al. Estrogen receptor beta (ERβ): A ligand activated tumor suppressor. Front. Oncol. 2020, 10, 587386. [Google Scholar] [CrossRef]
- Navaratnam, S.; Skliris, G.; Qing, G.; Banerji, S.; Badiani, K.; Tu, D.; Bradbury, P.A.; Leighl, N.B.; Shepherd, F.A.; Nowatzki, J.; et al. Differential role of estrogen receptor beta in early versus metastatic non-small cell lung cancer. Horm. Cancer 2012, 3, 93–100. [Google Scholar] [CrossRef]
- Omoto, Y.; Kobayashi, Y.; Nishida, K.; Tsuchiya, E.; Eguchi, H.; Nakagawa, K.; Ishikawa, Y.; Yamori, T.; Iwase, H.; Fujii, Y.; et al. Expression, function, and clinical implications of the estrogen receptor β in human lung cancers. Biochem. Biophys. Res. Commun. 2001, 285, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, C.; Li, M.; An, J.; Zhou, W.; Guo, J.; Xiao, Y. Estrogen receptor beta promotes lung cancer invasion via increasing CXCR4 expression. Cell Death Dis. 2022, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yanamala, N.; Lathrop, K.L.; Zhang, L.; Klein-Seetharaman, J.; Srinivas, H. Ligand-independent antiapoptotic function of estrogen receptor-β in lung cancer cells. Mol. Endocrinol. 2010, 24, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Mah, V.; Marquez, D.; Alavi, M.; Maresh, E.L.; Zhang, L.; Yoon, N.; Horvath, S.; Bagryanova, L.; Fishbein, M.C.; Chia, D. Expression levels of estrogen receptor beta in conjunction with aromatase predict survival in non-small cell lung cancer. Lung Cancer 2011, 74, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Hiramitsu, S.; Ishikawa, T.; Lee, W.-R.; Khan, T.; Crumbley, C.; Khwaja, N.; Zamanian, F.; Asghari, A.; Sen, M.; Zhang, Y. Estrogen receptor beta-mediated modulation of lung cancer cell proliferation by 27-hydroxycholesterol. Front. Endocrinol. 2018, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, P.A.; Stabile, L.P.; Kanterewicz, B.; Rothstein, M.E.; Gubish, C.T.; Land, S.; Shuai, Y.; Siegfried, J.M.; Nichols, M. Estrogen receptor beta (ERβ) subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J. Steroid Biochem. Mol. Biol. 2009, 116, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Stabile, L.P.; Dacic, S.; Land, S.R.; Lenzner, D.E.; Dhir, R.; Acquafondata, M.; Landreneau, R.J.; Grandis, J.R.; Siegfried, J.M. Combined analysis of estrogen receptor β-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin. Cancer Res. 2011, 17, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ding, J.; He, M.; Chen, Y.; Wang, R.; Han, Z.; Xing, E.Z.; Zhang, C.; Yeh, S. Estrogen receptor β promotes the vasculogenic mimicry (VM) and cell invasion via altering the lncRNA-MALAT1/miR-145-5p/NEDD9 signals in lung cancer. Oncogene 2019, 38, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, Y.; Dong, Y.; Liang, Y.; Qu, H.; Qi, D.; Lu, Y.; Jin, X.; Guo, Y.; Jia, Y.; et al. Estrogen receptor β inhibits breast cancer cells migration and invasion through CLDN6-mediated autophagy. J. Exp. Clin. Cancer Res. 2019, 38, 354. [Google Scholar] [CrossRef]
- Thomas, C.; Rajapaksa, G.; Nikolos, F.; Hao, R.; Katchy, A.; McCollum, C.W.; Bondesson, M.; Quinlan, P.; Thompson, A.; Krishnamurthy, S.; et al. ERβ1 represses basal-like breast cancer epithelial to mesenchymal transition by destabilizing EGFR. Breast Cancer Res. 2012, 14, R148. [Google Scholar] [CrossRef]
- Samanta, S.; Sharma, V.M.; Khan, A.; Mercurio, A.M. Regulation of IMP3 by EGFR signaling and repression by ERbeta: Implications for triple-negative breast cancer. Oncogene 2012, 31, 4689–4697. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; De Marco, P.; De Francesco, E.M.; Chimento, A.; Pezzi, V.; Maggiolini, M. Cross-talk between GPER and growth factor signaling. J. Steroid Biochem. Mol. Biol. 2013, 137, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Graeber, C.T.; Quinn, J.A.; Resnick, M.B.; Giri, D.; DeLellis, R.A.; Steinhoff, M.M.; Sabo, E. Distribution of GPR30, a seven membrane–spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin. Cancer Res. 2006, 12, 6359–6366. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Karagounis, I.V.; Srivastava, R.K.; Vrettos, N.; Nikolos, F.; Francois, N.; Huang, M.; Gong, S.; Long, Q.; Kumar, S. Estrogen receptor β-mediated inhibition of actin-based cell migration suppresses metastasis of inflammatory breast cancer. Cancer Res. 2021, 81, 2399–2414. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.M.; Bruinsma, E.S.; Nelson, A.W.; Chernukhin, I.; Carroll, J.S.; Li, Y.; Subramaniam, M.; Suman, V.J.; Negron, V.; Monroe, D.G. ERβ-mediated induction of cystatins results in suppression of TGFβ signaling and inhibition of triple-negative breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2018, 115, E9580–E9589. [Google Scholar] [CrossRef] [PubMed]
- Bouris, P.; Skandalis, S.S.; Piperigkou, Z.; Afratis, N.; Karamanou, K.; Aletras, A.J.; Moustakas, A.; Theocharis, A.D.; Karamanos, N.K. Estrogen receptor alpha mediates epithelial to mesenchymal transition, expression of specific matrix effectors and functional properties of breast cancer cells. Matrix Biol. 2015, 43, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Flamini, M.I.; Baldacci, C.; Goglia, L.; Genazzani, A.R.; Simoncini, T. Estrogen receptor-α promotes breast cancer cell motility and invasion via focal adhesion kinase and N-WASP. Mol. Endocrinol. 2010, 24, 2114–2125. [Google Scholar] [CrossRef] [PubMed]
- Giretti, M.S.; Fu, X.-D.; De Rosa, G.; Sarotto, I.; Baldacci, C.; Garibaldi, S.; Mannella, P.; Biglia, N.; Sismondi, P.; Genazzani, A.R. Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS ONE 2008, 3, e2238. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Leung, Y.-K.; Mak, P.; Hassan, S.; Ho, S.-M. Estrogen receptor (ER)-β isoforms: A key to understanding ER-β signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 13162–13167. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Chen, K.; Tang, H.; Tang, J.; Song, C.; Xie, X. ERbeta1 inversely correlates with PTEN/PI3K/AKT pathway and predicts a favorable prognosis in triple-negative breast cancer. Breast Cancer Res. Treat. 2015, 152, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Chantzi, N.I.; Tiniakos, D.G.; Palaiologou, M.; Goutas, N.; Filippidis, T.; Vassilaros, S.D.; Dhimolea, E.; Mitsiou, D.J.; Alexis, M.N. Estrogen receptor beta 2 is associated with poor prognosis in estrogen receptor alpha-negative breast carcinoma. J. Cancer Res. Clin. Oncol. 2013, 139, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.M.; Suman, V.J.; Subramaniam, M.; Wu, X.; Negron, V.; Gingery, A.; Pitel, K.S.; Shah, S.S.; Cunliffe, H.E.; McCullough, A.E.; et al. ERbeta1: Characterization, prognosis, and evaluation of treatment strategies in ERalpha-positive and -negative breast cancer. BMC Cancer 2014, 14, 749. [Google Scholar] [CrossRef] [PubMed]
- Aspros, K.G.; Carter, J.M.; Hoskin, T.L.; Suman, V.J.; Subramaniam, M.; Emch, M.J.; Ye, Z.; Sun, Z.; Sinnwell, J.P.; Thompson, K. Estrogen receptor beta repurposes EZH2 to suppress oncogenic NFκB/p65 signaling in triple negative breast cancer. NPJ Breast Cancer 2022, 8, 20. [Google Scholar] [CrossRef]
- Alexandrova, E.; Giurato, G.; Saggese, P.; Pecoraro, G.; Lamberti, J.; Ravo, M.; Rizzo, F.; Rocco, D.; Tarallo, R.; Nyman, T.A.; et al. Interaction proteomics identifies ERbeta association with chromatin repressive complexes to inhibit cholesterol biosynthesis and exert an oncosuppressive role in triple-negative breast cancer. Mol. Cell. Proteom. 2020, 19, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Dey, P.; Ziegler, Y.; Jiao, X.; Kim, S.H.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Contrasting activities of estrogen receptor beta isoforms in triple negative breast cancer. Breast Cancer Res. Treat. 2021, 185, 281–292. [Google Scholar] [CrossRef]
- Oturkar, C.C.; Gandhi, N.; Rao, P.; Eng, K.H.; Miller, A.; Singh, P.K.; Zsiros, E.; Odunsi, K.O.; Das, G.M. Estrogen Receptor-Beta2 (ERbeta2)-Mutant p53-FOXM1 Axis: A Novel Driver of Proliferation, Chemoresistance, and Disease Progression in High Grade Serous Ovarian Cancer (HGSOC). Cancers 2022, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Anestis, A.; Sarantis, P.; Theocharis, S.; Zoi, I.; Tryfonopoulos, D.; Korogiannos, A.; Koumarianou, A.; Xingi, E.; Thomaidou, D.; Kontos, M.; et al. Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Fan, P.; Wang, M.; Zhang, C.; Jiang, Y.; Huang, S.; Fang, M.; He, Z.; Wu, A.J.E.; Medicine, T. Elevated estrogen receptor β expression in triple negative breast cancer cells is associated with sensitivity to doxorubicin by inhibiting the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2020, 20, 1630–1636. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Häring, J.; Inwald, E.C.; Moehle, C.; Ortmann, O.; Treeck, O. Agonists and knockdown of estrogen receptor β differentially affect invasion of triple-negative breast cancer cells in vitro. BMC Cancer 2016, 16, 951. [Google Scholar] [CrossRef]
- Hinsche, O.; Girgert, R.; Emons, G.; Gründker, C. Estrogen receptor β selective agonists reduce invasiveness of triple-negative breast cancer cells. Int. J. Oncol. 2015, 46, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Sundberg, M.; Pristovsek, N.; Ibrahim, A.; Jonsson, P.; Katona, B.; Clausson, C.-M.; Zieba, A.; Ramström, M.; Söderberg, O. Insufficient antibody validation challenges oestrogen receptor beta research. Nat. Commun. 2017, 8, 15840. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.W.; Groen, A.J.; Miller, J.L.; Warren, A.Y.; Holmes, K.A.; Tarulli, G.A.; Tilley, W.D.; Katzenellenbogen, B.S.; Hawse, J.R.; Gnanapragasam, V.J.; et al. Comprehensive assessment of estrogen receptor beta antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity. Mol. Cell. Endocrinol. 2017, 440, 138–150. [Google Scholar] [CrossRef]
- Austin, D.; Hamilton, N.; Elshimali, Y.; Pietras, R.; Wu, Y.; Vadgama, J. Estrogen receptor-beta is a potential target for triple negative breast cancer treatment. Oncotarget 2018, 9, 33912. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.; Márquez-Garbán, D.; Mah, V.; Fernando, G.; Elshimali, Y.; Garbán, H.; Elashoff, D.; Vadgama, J.; Goodglick, L.; Pietras, R. Biologic roles of estrogen receptor-β and insulin-like growth factor-2 in triple-negative breast cancer. BioMed Res. Int. 2015, 2015, 925703. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Zakharov, M.N.; Khan, S.H.; Miki, R.; Jang, H.; Toraldo, G.; Singh, R.; Bhasin, S.; Jasuja, R. The dynamic structure of the estrogen receptor. J. Amino Acids 2011, 2011, 812540. [Google Scholar] [CrossRef] [PubMed]
- Ruff, M.; Gangloff, M.; Marie Wurtz, J.; Moras, D. Estrogen receptor transcription and transactivation Structure-function relationship in DNA-and ligand-binding domains of estrogen receptors. Breast Cancer Res. 2000, 2, 353. [Google Scholar] [CrossRef]
- Pike, A.C.; Brzozowski, A.M.; Hubbard, R.E.; Bonn, T.; Thorsell, A.-G.; Engström, O.; Ljunggren, J.; Gustafsson, J.-Å.; Carlquist, M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999, 18, 4608–4618. [Google Scholar] [CrossRef]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Strom, A.; Treuter, E.; Warner, M. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef]
- Bryant, H.U.; Glasebrook, A.L.; Yang, N.N.; Sato, M. An estrogen receptor basis for raloxifene action in bone. Proceedings of Xth International Congress on Hormonal Steroids, Quebec, Canada, 17–21 June 1998. J. Steroid Biochem. Mol. Biol. 1999, 69, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hochner-Celnikier, D. Pharmacokinetics of raloxifene and its clinical application. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 85, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Mosca, L.; Collins, P.; Geiger, M.J.; Grady, D.; Kornitzer, M.; McNabb, M.A.; Wenger, N.K. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N. Engl. J. Med. 2006, 355, 125–137. [Google Scholar] [CrossRef]
- Grady, D.; Ettinger, B.; Moscarelli, E.; Plouffe, L., Jr.; Sarkar, S.; Ciaccia, A.; Cummings, S.; Multiple Outcomes of Raloxifene Evaluation Investigators. Safety and adverse effects associated with raloxifene: Multiple outcomes of raloxifene evaluation. Obstet. Gynecol. 2004, 104, 837–844. [Google Scholar] [CrossRef]
- Delmas, P.D.; Bjarnason, N.H.; Mitlak, B.H.; Ravoux, A.-C.; Shah, A.S.; Huster, W.J.; Draper, M.; Christiansen, C. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N. Engl. J. Med. 1997, 337, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Nehoff, H.; Bahman, F.; Pritchard, T.; Taurin, S. Raloxifene nano-micelles effect on triple-negative breast cancer is mediated through estrogen receptor-β and epidermal growth factor receptor. J. Drug Target. 2019, 27, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Matsushima-Nishiwaki, R.; Yamada, N.; Hattori, Y.; Hosokawa, Y.; Tachi, J.; Hori, T.; Kozawa, O. SERMs (selective estrogen receptor modulator), acting as estrogen receptor β agonists in hepatocellular carcinoma cells, inhibit the transforming growth factor-α-induced migration via specific inhibition of AKT signaling pathway. PLoS ONE 2022, 17, e0262485. [Google Scholar] [CrossRef] [PubMed]
- Pozios, I.; Seel, N.N.; Hering, N.A.; Hartmann, L.; Liu, V.; Camaj, P.; Müller, M.H.; Lee, L.D.; Bruns, C.J.; Kreis, M.E. Raloxifene inhibits pancreatic adenocarcinoma growth by interfering with ERβ and IL-6/gp130/STAT3 signaling. Cell. Oncol. 2021, 44, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Palmer, H.; Nimick, M.; Mazumder, A.; Taurin, S.; Rana, Z.; Rosengren, R. Raloxifene Suppresses Tumor Growth and Metastasis in an Orthotopic Model of Castration-Resistant Prostate Cancer. Biomedicines 2022, 10, 853. [Google Scholar] [CrossRef]
- Love, R.R.; Mazess, R.B.; Barden, H.S.; Epstein, S.; Newcomb, P.A.; Jordan, V.C.; Carbone, P.P.; DeMets, D.L. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N. Engl. J. Med. 1992, 326, 852–856. [Google Scholar] [CrossRef]
- Kedar, R.; Bourne, T.H.; Collins, W.; Campbell, S.; Powles, T.; Ashley, S.; Cosgrove, D. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomised breast cancer prevention trial. Lancet 1994, 343, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998, 339, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef] [PubMed]
- Esslimani-Sahla, M.; Simony-Lafontaine, J.; Kramar, A.; Lavaill, R.; Mollevi, C.; Warner, M.; Gustafsson, J.-A.k.; Rochefort, H. Estrogen receptor β (ERβ) level but not its ERβcx variant helps to predict tamoxifen resistance in breast cancer. Clin. Cancer Res. 2004, 10, 5769–5776. [Google Scholar] [CrossRef]
- Hopp, T.A.; Weiss, H.L.; Parra, I.S.; Cui, Y.; Osborne, C.K.; Fuqua, S.A. Low levels of estrogen receptor β protein predict resistance to tamoxifen therapy in breast cancer. Clin. Cancer Res. 2004, 10, 7490–7499. [Google Scholar] [CrossRef] [PubMed]
- Lattrich, C.; Schüler, S.; Häring, J.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Effects of a combined treatment with tamoxifen and estrogen receptor β agonists on human breast cancer cell lines. Arch. Gynecol. Obstet. 2014, 289, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hodges-Gallagher, L.; Valentine, C.D.; Bader, S.E.; Kushner, P. Estrogen receptor beta increases the efficacy of antiestrogens by effects on apoptosis and cell cycling in breast cancer cells. Breast Cancer Res. 2008, 109, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksa, G.; Nikolos, F.; Bado, I.; Clarke, R.; Gustafsson, J.-Å.; Thomas, C. ERβ decreases breast cancer cell survival by regulating the IRE1/XBP-1 pathway. Oncogene 2015, 34, 4130–4141. [Google Scholar] [CrossRef] [PubMed]
- Razandi, M.; Pedram, A.; Jordan, V.C.; Fuqua, S.; Levin, E.R. Tamoxifen regulates cell fate through mitochondrial estrogen receptor beta in breast cancer. Oncogene 2013, 32, 3274–3285. [Google Scholar] [CrossRef]
- Langendonk, M.; de Jong, M.R.; Smit, N.; Seiler, J.; Reitsma, B.; Ammatuna, E.; Glaudemans, A.W.; van den Berg, A.; Huls, G.A.; Visser, L. Identification of the estrogen receptor beta as a possible new tamoxifen-sensitive target in diffuse large B-cell lymphoma. Blood Cancer J. 2022, 12, 36. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, S.; Mei, S.; Yang, Z.; Xu, L.; Zhou, N.; Yang, Q.; Shen, Q.; Wang, W.; Le, X.; et al. Pharmacological activation of estrogen receptor beta augments innate immunity to suppress cancer metastasis. Proc. Natl. Acad. Sci. USA 2018, 115, E3673–E3681. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhou, N.; Zhao, L.; Gimple, R.C.; Ahn, Y.H.; Zhang, P.; Wang, W.; Shao, B.; Yang, J.; Zhang, Q. Pharmacological activation of estrogen receptor beta overcomes tumor resistance to immune checkpoint blockade therapy. IScience 2020, 23, 101458. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Alejo, S.; Venkata, P.P.; Johnson, J.D.; Loeffel, I.; Pratap, U.P.; Zou, Y.; Lai, Z.; Tekmal, R.R.; Kost, E.R. Therapeutic targeting of ovarian Cancer stem cells using estrogen receptor Beta agonist. Int. J. Mol. Sci. 2022, 23, 7159. [Google Scholar] [CrossRef] [PubMed]
- Sareddy, G.R.; Li, X.; Liu, J.; Viswanadhapalli, S.; Garcia, L.; Gruslova, A.; Cavazos, D.; Garcia, M.; Strom, A.M.; Gustafsson, J.-A. Selective estrogen receptor β agonist LY500307 as a novel therapeutic agent for glioblastoma. Sci. Rep. 2016, 6, 24185. [Google Scholar] [CrossRef] [PubMed]
- Pratap, U.P.; Sareddy, G.R.; Liu, Z.; Venkata, P.P.; Liu, J.; Tang, W.; Altwegg, K.A.; Ebrahimi, B.; Li, X.; Tekmal, R.R. Histone deacetylase inhibitors enhance estrogen receptor beta expression and augment agonist-mediated tumor suppression in glioblastoma. Neuro-Oncol. Adv. 2021, 3, vdab099. [Google Scholar] [CrossRef] [PubMed]
- Pontecorvi, G.; Bellenghi, M.; Tait, S.; Tirelli, V.; Matarrese, P.; Mattia, G.; Carè, A.; Puglisi, R. Different Susceptibilities of Human Melanoma Cell Lines to G2/M Blockage and Cell Death Activation in Response to the Estrogen Receptor β agonist LY500307. J. Cancer 2022, 13, 1573. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.; Spann, M.; Myers, S.; Serviss, C.; Hu, L.; Jin, Y. Estrogen receptor beta agonist LY500307 fails to improve symptoms in men with enlarged prostate secondary to benign prostatic hypertrophy. Prostate Cancer Prostatic Dis. 2015, 18, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Breier, A. The Efficacy and Safety of a Selective Estrogen Receptor Beta Agonist (LY500307) for Negative Symptoms and Cognitive Impairment Associated With Schizophrenia—Full Text View—ClinicalTrials. gov. 2019.
- Shen, S.S.; Smith, C.L.; Hsieh, J.T.; Yu, J.; Kim, I.Y.; Jian, W.; Sonpavde, G.; Ayala, G.E.; Younes, M.; Lerner, S.P. Expression of estrogen receptors-α and-β in bladder cancer cell lines and human bladder tumor tissue. Interdiscip. Int. J. Am. Cancer Soc. 2006, 106, 2610–2616. [Google Scholar] [CrossRef]
- Kauffman, E.C.; Robinson, B.D.; Downes, M.; Marcinkiewicz, K.; Vourganti, S.; Scherr, D.S.; Gudas, L.J.; Mongan, N.P. Estrogen receptor-β expression and pharmacological targeting in bladder cancer. Oncol. Rep. 2013, 30, 131–138. [Google Scholar] [CrossRef]
- Rao, Q.; Chen, Y.; Yeh, C.-R.; Ding, J.; Li, L.; Chang, C.; Yeh, S. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget 2016, 7, 7842. [Google Scholar] [CrossRef]
- Tao, L.; Qiu, J.; Slavin, S.; Ou, Z.; Liu, Z.; Ge, J.; Zuo, L.; Guancial, E.A.; Messing, E.M.; Chang, C. Recruited T cells promote the bladder cancer metastasis via up-regulation of the estrogen receptor β/IL-1/c-MET signals. Cancer Lett. 2018, 430, 215–223. [Google Scholar] [CrossRef]
- Ali, H.R.; Chlon, L.; Pharoah, P.D.; Markowetz, F.; Caldas, C. Patterns of immune infiltration in breast cancer and their clinical implications: A gene-expression-based retrospective study. PLoS Med. 2016, 13, e1002194. [Google Scholar] [CrossRef] [PubMed]
- Onesti, C.E.; Josse, C.; Poncin, A.; Frères, P.; Poulet, C.; Bours, V.; Jerusalem, G. Predictive and prognostic role of peripheral blood eosinophil count in triple-negative and hormone receptor-negative/HER2-positive breast cancer patients undergoing neoadjuvant treatment. Oncotarget 2018, 9, 33719. [Google Scholar] [CrossRef] [PubMed]
- Ghebeh, H.; Elshenawy, M.A.; AlSayed, A.D.; Al-Tweigeri, T. Peripheral blood eosinophil count is associated with response to chemoimmunotherapy in metastatic triple-negative breast cancer. Immunotherapy 2022, 14, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Artham, S.; Chang, C.-Y.; McDonnell, D.P. Eosinophilia in cancer and its regulation by sex hormones. Trends Endocrinol. Metab. 2023, 34, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, M.J.; Carson, B.D.; Subramanian, S.; Afentoulis, M.; Vandenbark, A.A.; Ziegler, S.F.; Offner, H. Cutting edge: Estrogen drives expansion of the CD4+ CD25+ regulatory T cell compartment. J. Immunol. 2004, 173, 2227–2230. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, M.J.; Hopke, C.; Vandenbark, A.A.; Offner, H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1). Int. Immunol. 2007, 19, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, K.; Ström, A.; Jonsson, P.; Gustafsson, J.-Å.; Williams, C. Estrogen receptor β induces antiinflammatory and antitumorigenic networks in colon cancer cells. Mol. Endocrinol. 2011, 25, 969–979. [Google Scholar] [CrossRef]
- Campbell, L.; Emmerson, E.; Davies, F.; Gilliver, S.C.; Krust, A.; Chambon, P.; Ashcroft, G.S.; Hardman, M. Estrogen promotes cutaneous wound healing via estrogen receptor β independent of its antiinflammatory activities. J. Exp. Med. 2010, 207, 1825–1833. [Google Scholar] [CrossRef]
- Sun, J.; Ma, X.; Chen, Y.-X.; Rayner, K.; Hibbert, B.; McNulty, M.; Dhaliwal, B.; Simard, T.; Ramirez, D.; O’Brien, E. Attenuation of atherogenesis via the anti-inflammatory effects of the selective estrogen receptor beta modulator 8β-VE2. J. Cardiovasc. Pharmacol. 2011, 58, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Feng, W.; Miller, A.P.; Weathington, N.M.; Chen, Y.-F.; Novak, L.; Blalock, J.E.; Oparil, S.; Physiology, C. Estrogen modulates TNF-α-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-β activation. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H2607–H2612. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiu, X.; Hao, Q.; Li, D. Anti-inflammatory effects of a Chinese herbal medicine in atherosclerosis via estrogen receptor β mediating nitric oxide production and NF-κB suppression in endothelial cells. Cell Death Dis. 2013, 4, e551. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Liu, X.; Zeng, C.; Cheng, L.; Song, G.; Hou, X.; Zhu, L.; Zou, K. Estrogen receptor β activation ameliorates DSS-induced chronic colitis by inhibiting inflammation and promoting Treg differentiation. Int. Immunopharmacol. 2019, 77, 105971. [Google Scholar] [CrossRef] [PubMed]
- Pierdominici, M.; Maselli, A.; Varano, B.; Barbati, C.; Cesaro, P.; Spada, C.; Zullo, A.; Lorenzetti, R.; Rosati, M.; Rainaldi, G. Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget 2015, 6, 40443. [Google Scholar] [CrossRef]
- Yuan, B.; Clark, C.A.; Wu, B.; Yang, J.; Drerup, J.M.; Li, T.; Jin, V.X.; Hu, Y.; Curiel, T.J.; Li, R. Estrogen receptor beta signaling in CD8(+) T cells boosts T cell receptor activation and antitumor immunity through a phosphotyrosine switch. J. Immunother. Cancer 2021, 9, e001932. [Google Scholar] [CrossRef] [PubMed]
- Sapino, A.; Bosco, M.; Cassoni, P.; Castellano, I.; Arisio, R.; Cserni, G.; Dei Tos, A.P.; Fortunati, N.; Catalano, M.G.; Bussolati, G. Estrogen receptor-beta is expressed in stromal cells of fibroadenoma and phyllodes tumors of the breast. Mod. Pathol. 2006, 19, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.J.; Gherman, D.; Kim, H.J.; Omoto, Y.; Iwase, H.; Bouton, D.; Kis, L.L.; Andersson, C.T.; Warner, M.; Gustafsson, J.A. Differential expression of oestrogen receptors in human secondary lymphoid tissues. J. Pathol. 2006, 208, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Pierdominici, M.; Maselli, A.; Colasanti, T.; Giammarioli, A.M.; Delunardo, F.; Vacirca, D.; Sanchez, M.; Giovannetti, A.; Malorni, W.; Ortona, E. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol. Lett. 2010, 132, 79–85. [Google Scholar] [CrossRef]
- Bukovsky, A.; Caudle, M.R.; Cekanova, M.; Fernando, R.I.; Wimalasena, J.; Foster, J.S.; Henley, D.C.; Elder, R.F. Placental expression of estrogen receptor beta and its hormone binding variant--comparison with estrogen receptor alpha and a role for estrogen receptors in asymmetric division and differentiation of estrogen-dependent cells. Reprod. Biol. Endocrinol. 2003, 1, 36. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagandla, H.; Thomas, C. Estrogen Signals through ERβ in Breast Cancer; What We Have Learned since the Discovery of the Receptor. Receptors 2024, 3, 182-200. https://doi.org/10.3390/receptors3020010
Nagandla H, Thomas C. Estrogen Signals through ERβ in Breast Cancer; What We Have Learned since the Discovery of the Receptor. Receptors. 2024; 3(2):182-200. https://doi.org/10.3390/receptors3020010
Chicago/Turabian StyleNagandla, Harika, and Christoforos Thomas. 2024. "Estrogen Signals through ERβ in Breast Cancer; What We Have Learned since the Discovery of the Receptor" Receptors 3, no. 2: 182-200. https://doi.org/10.3390/receptors3020010
APA StyleNagandla, H., & Thomas, C. (2024). Estrogen Signals through ERβ in Breast Cancer; What We Have Learned since the Discovery of the Receptor. Receptors, 3(2), 182-200. https://doi.org/10.3390/receptors3020010





