An Evaluation of the Anxiolytic Potential of Amentoflavone in Adult Zebrafish Undergoing Alcohol Withdrawal: In Vivo and In Silico Studies
Abstract
1. Introduction
2. Methodology
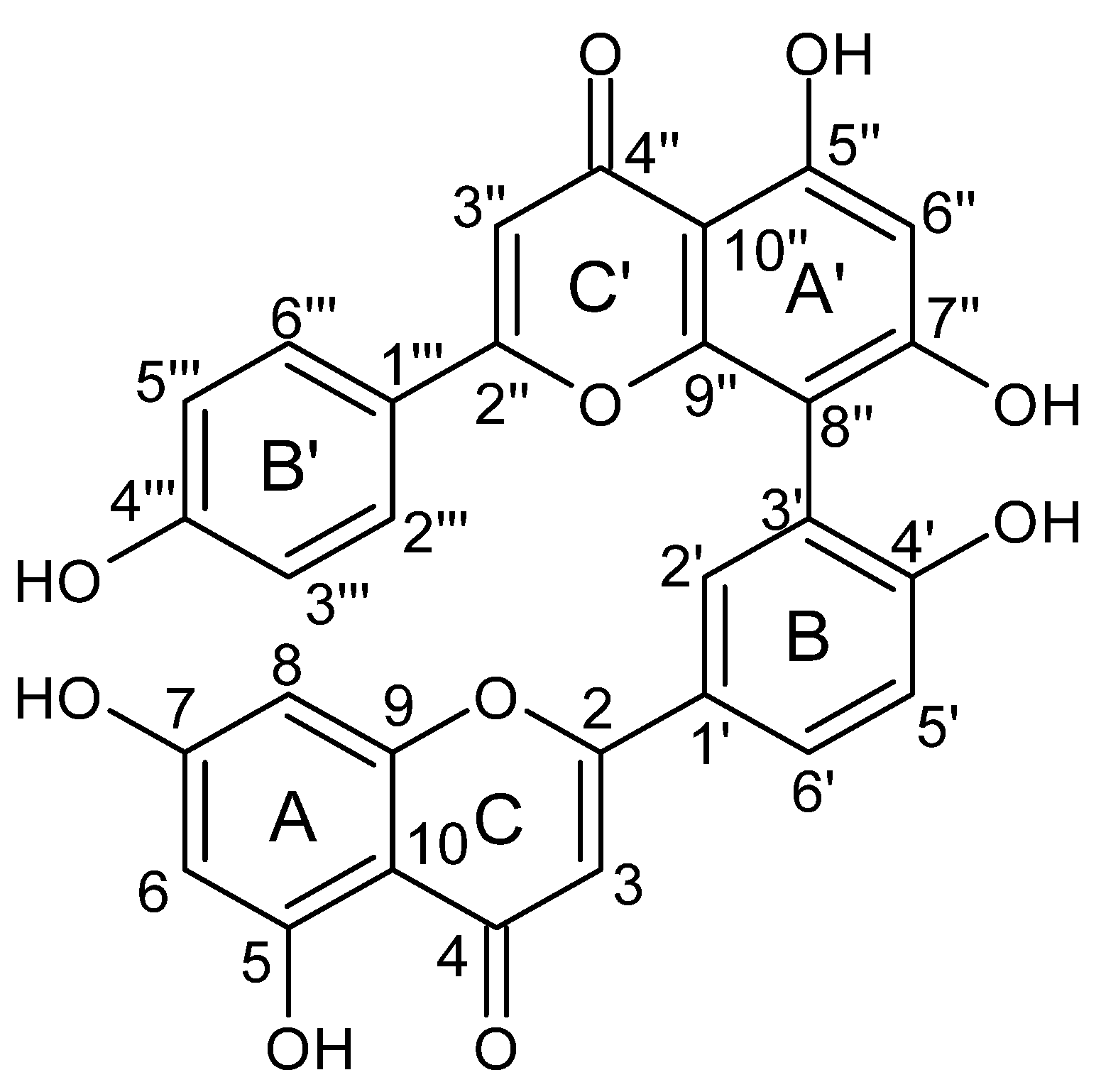
2.1. Obtaining Amentoflavone
2.2. Animals
2.3. Drugs or Pharmacological Treatments
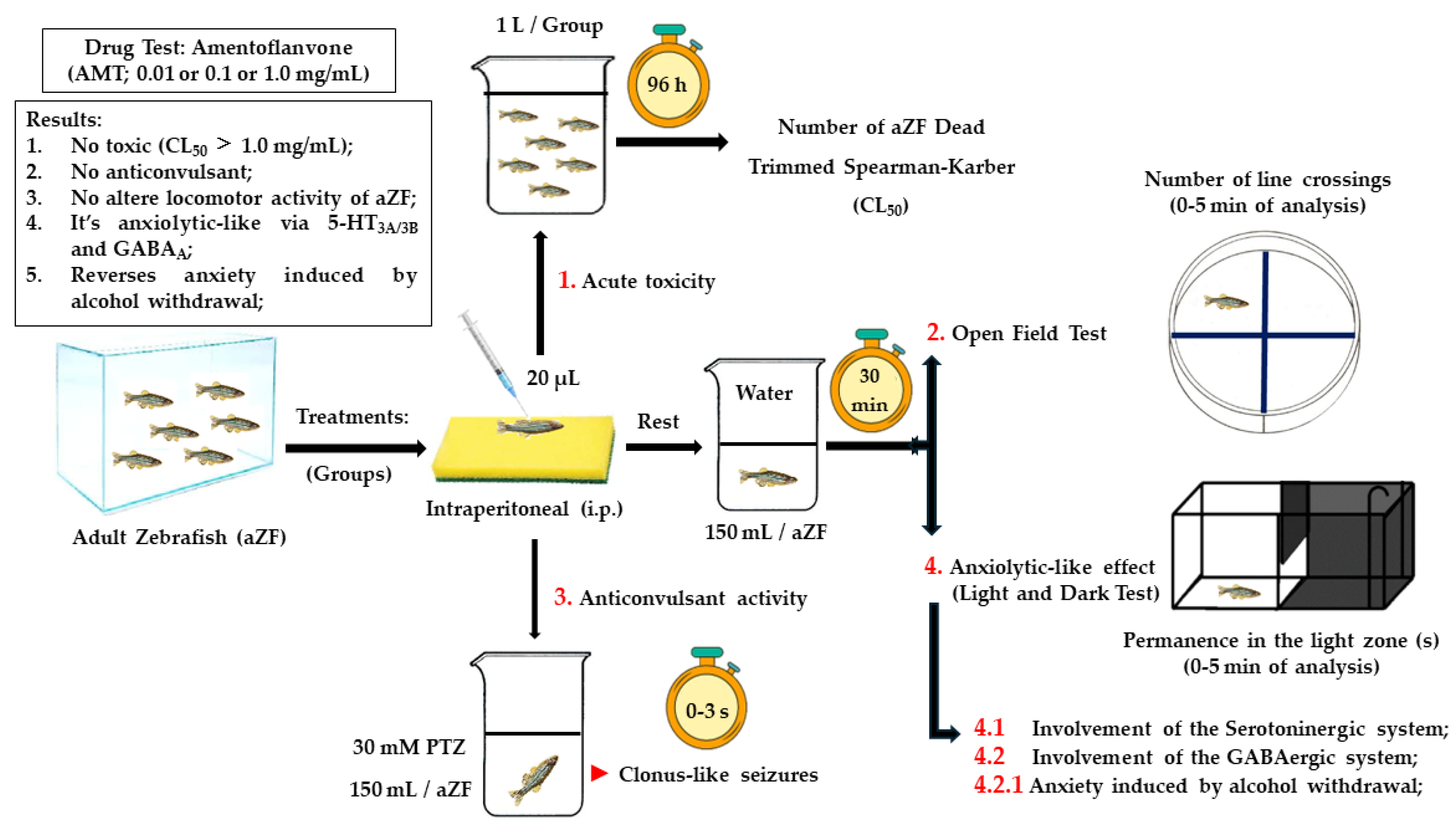
2.4. General Protocol
2.5. Anxiolytic-Like Effect
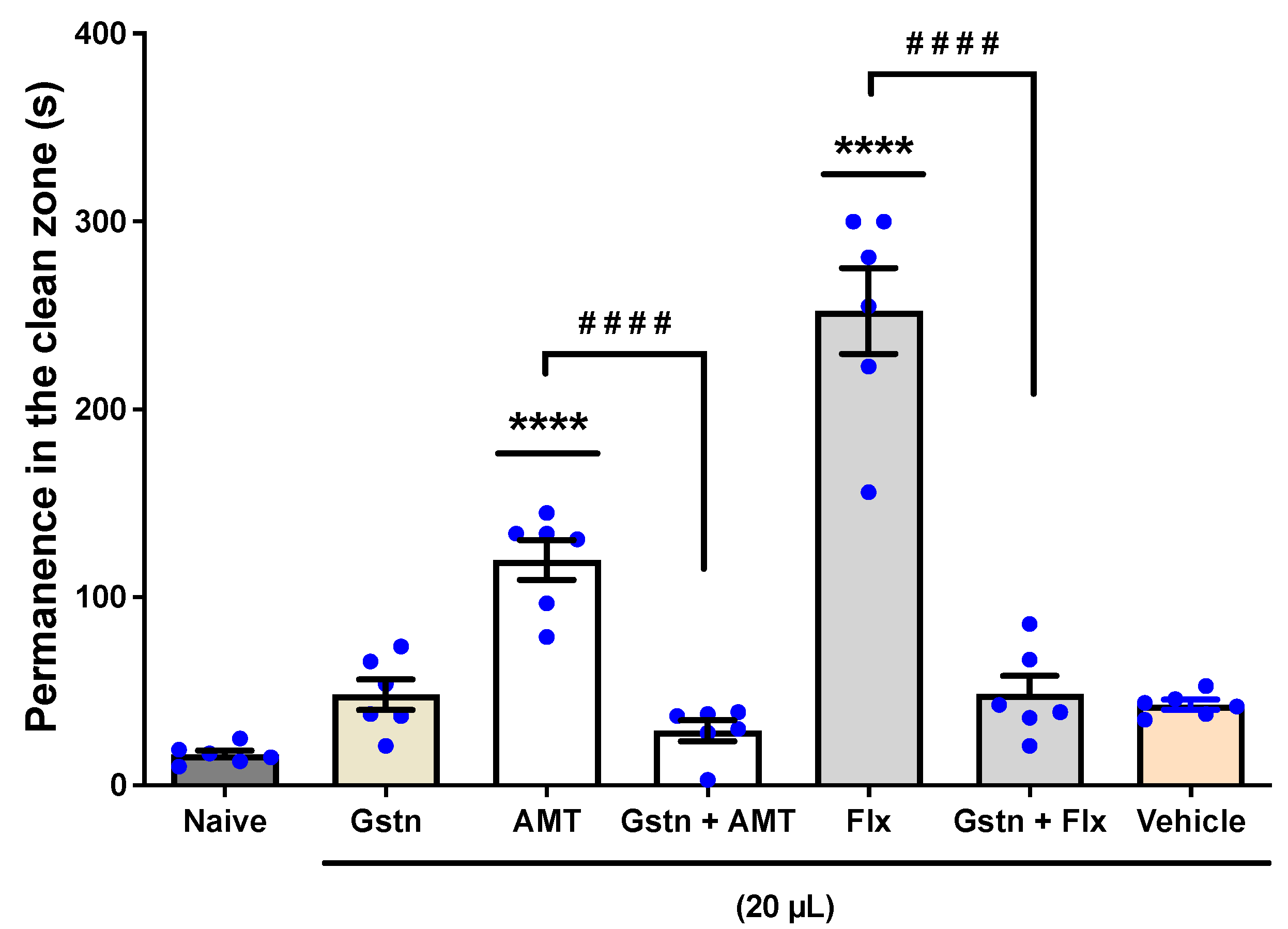
2.5.1. The Involvement of the Serotonergic System
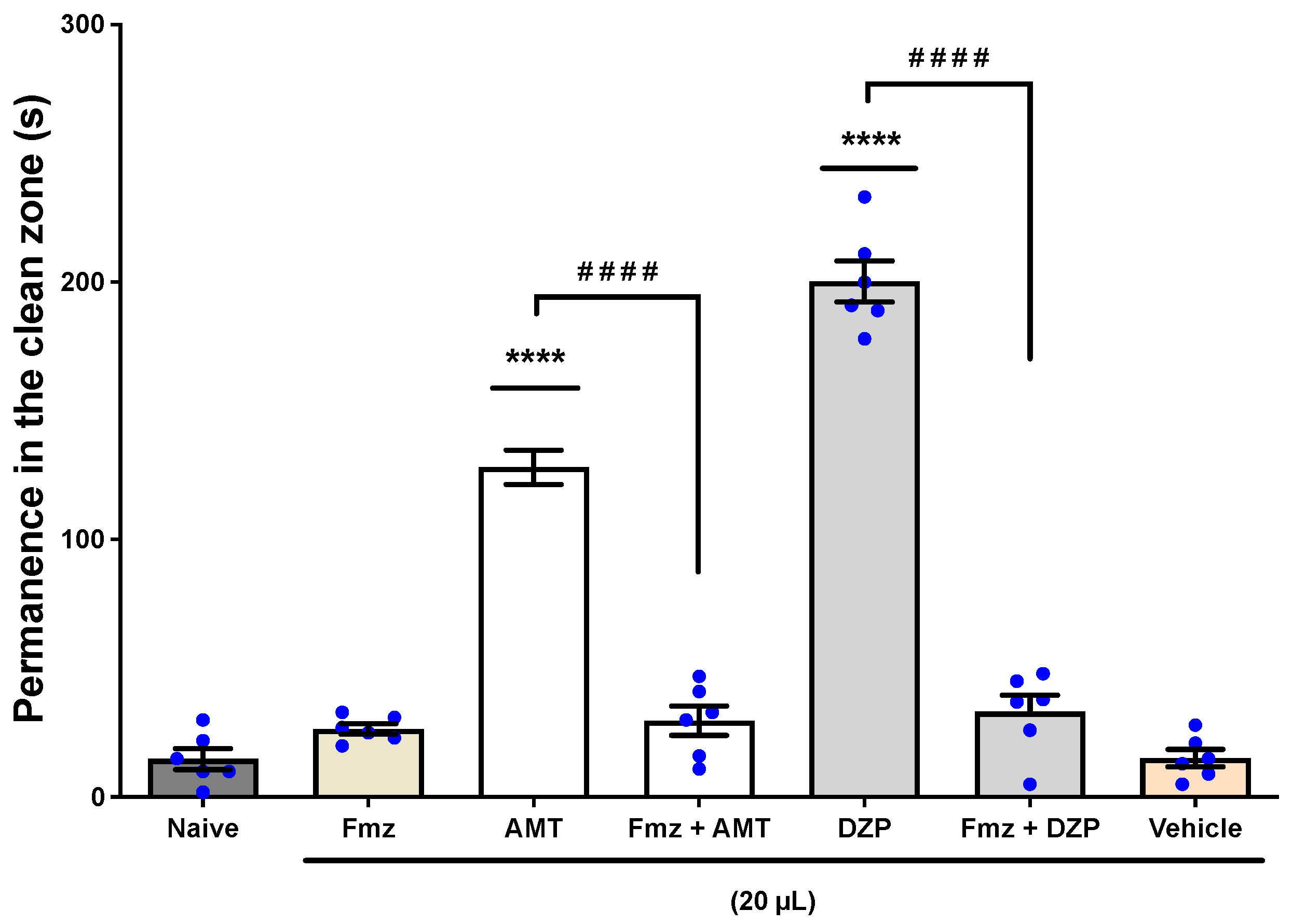
2.5.2. The Involvement of the GABAergic System
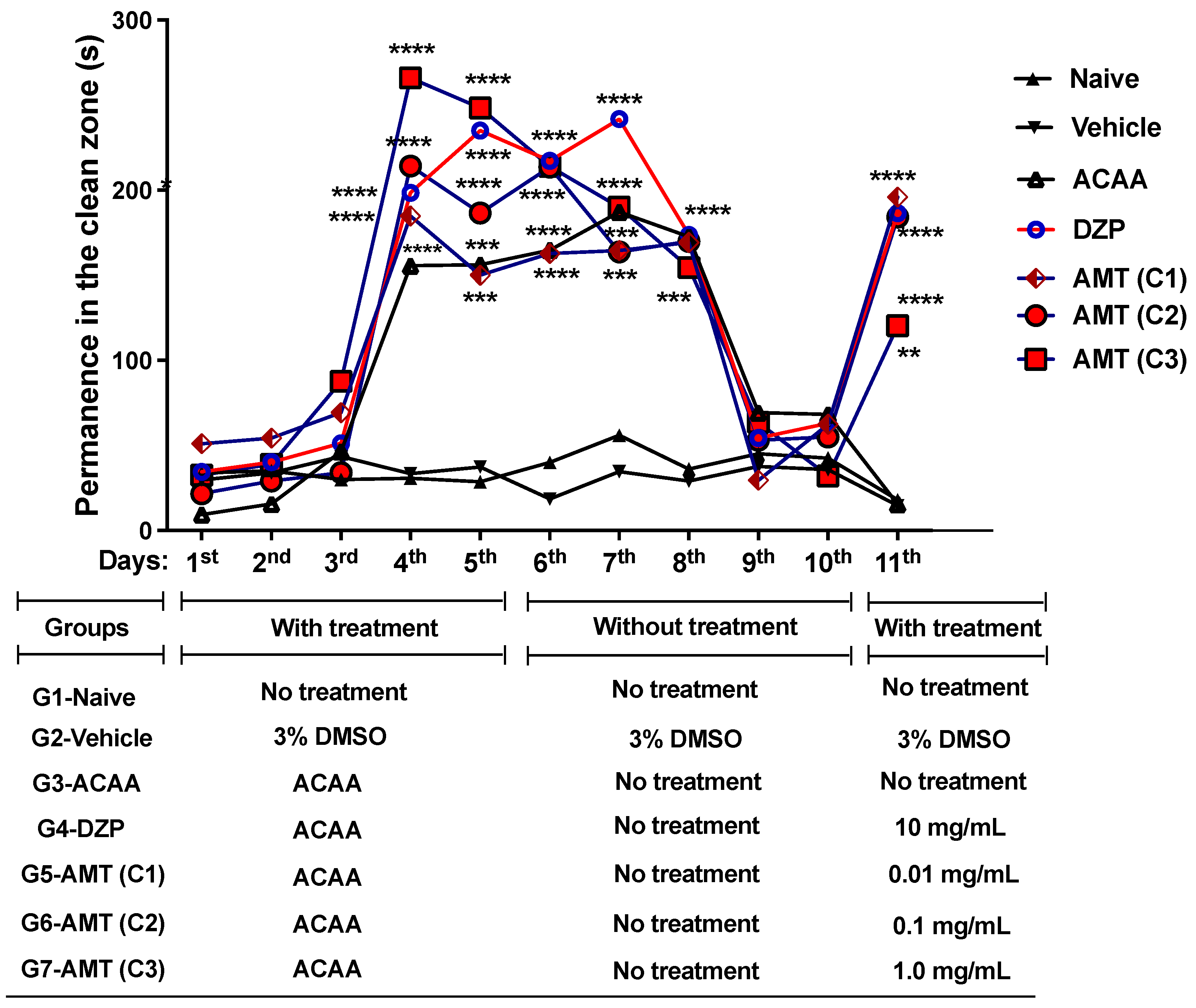
2.5.3. Anxiety Induced by Alcohol Withdrawal
- Group 1—1st to 11th day: Naive (without treatments);
- Group 2—1st to 11th day: Vehicle—3% DMSO (i.p.);
- Group 3—1st to 5th day: ACAA (p.o); 6th to 11th without ACAA treatments;
- Group 4—1st to 5th day: ACAA (p.o); 6th to 10th without ACAA treatments; 11th day: DZP (10 mg/mL; i.p.);
- Group 5—1st to 5th day: ACAA (p.o.); 6th to 10th without ACAA treatments; 11th day: AMT (0.01 mg/mL; i.p.);
- Group 6—1st to 5th day: ACAA (p.o.); 6th to 10th without ACAA treatments; 11th day: AMT (0.1 mg/mL; i.p.);
- Group 7—1st to 5th day: ACAA (p.o.); 6th to 10th without ACAA treatments; 11th day; AMT (1.0 mg/mL; i.p.).
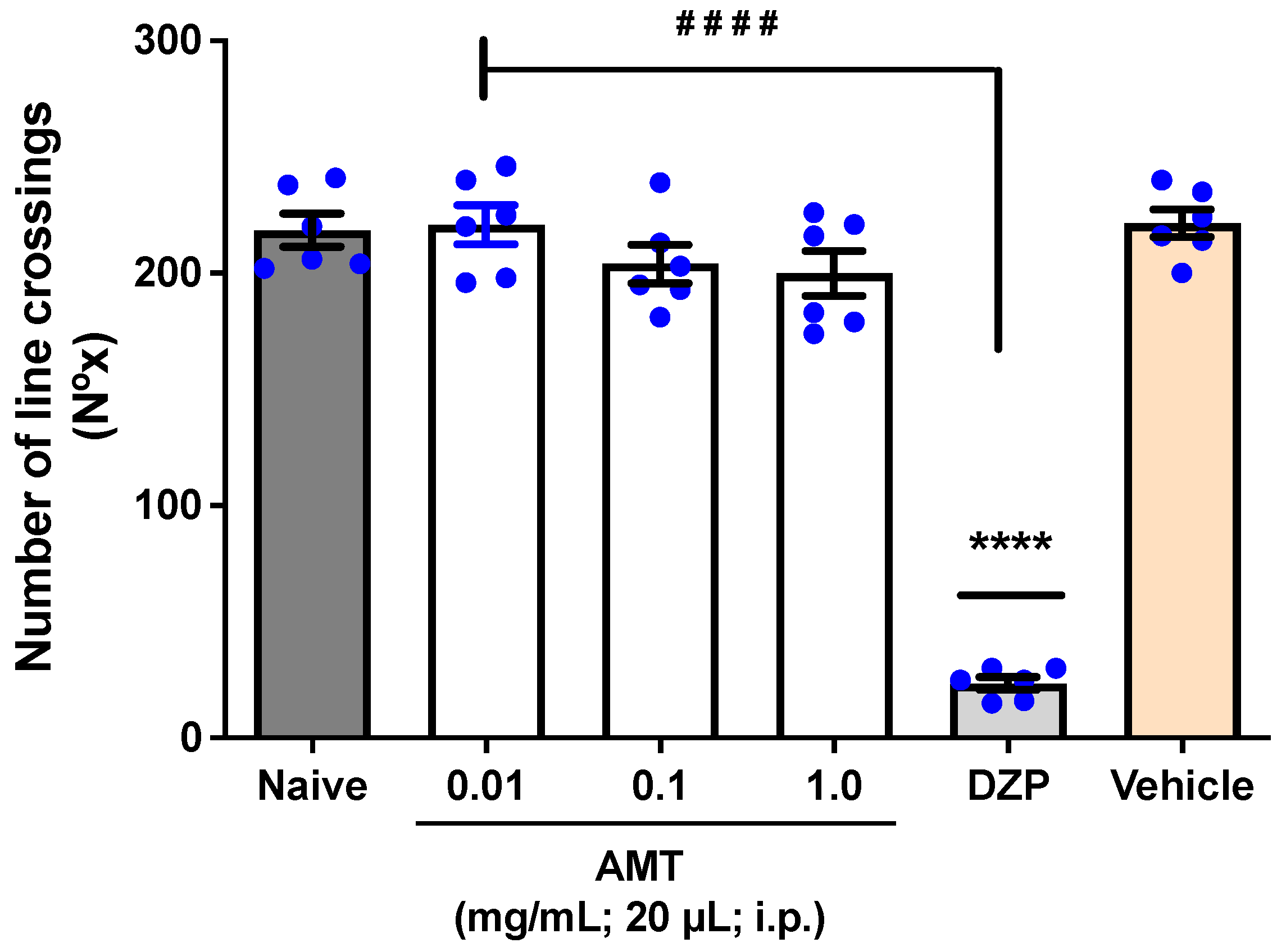
2.6. Locomotor Activity (Open Field Test)
2.7. Anticonvulsant Activity
2.8. Acute Toxicity (96 h) against Adult Zebrafish
2.9. Statistical Analysis
2.10. Molecular Docking Simulations
Preparation of Binders
3. Results
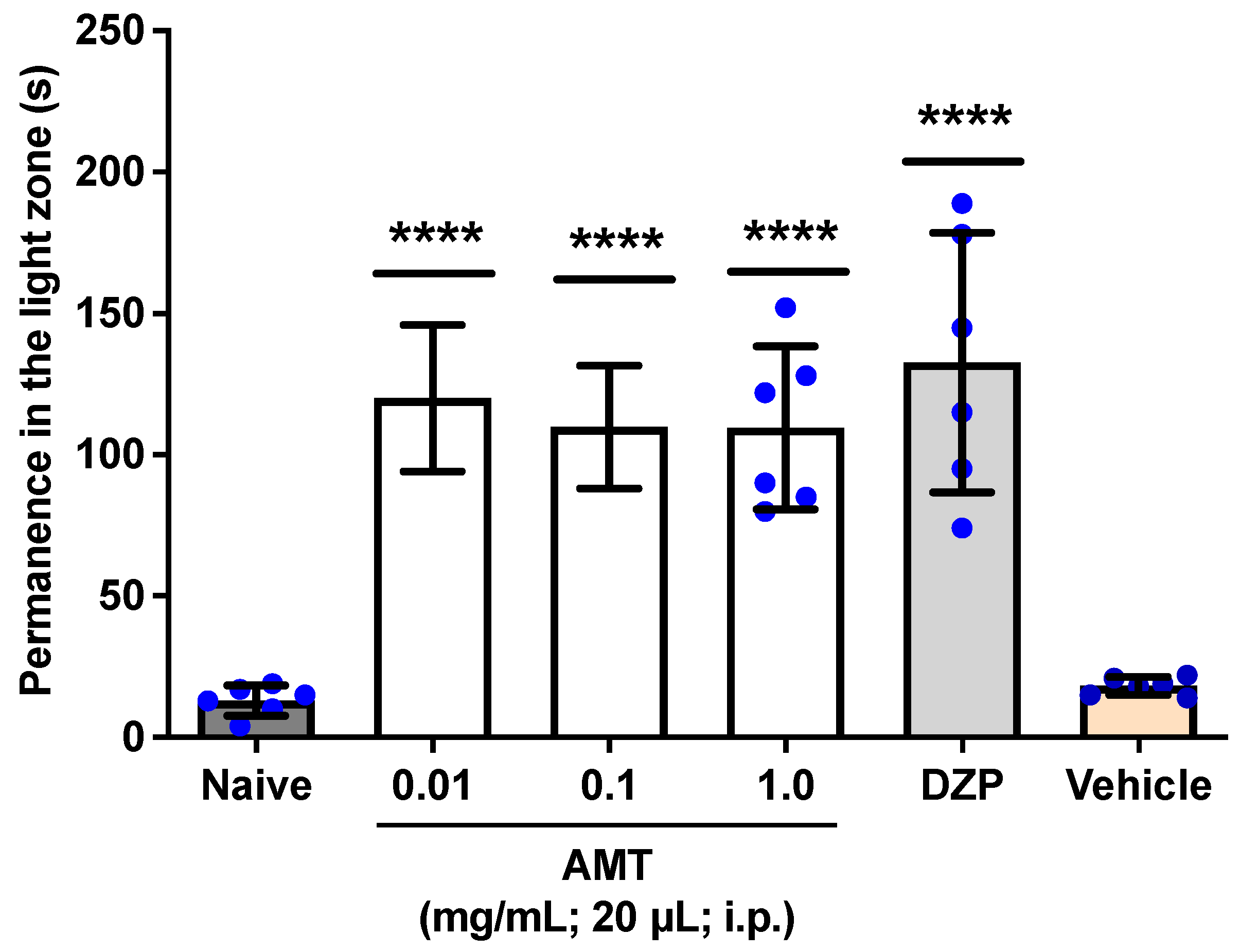
3.1. Anxiolytic-like Effect
3.1.1. The Involvement of the Serotoninergic System (5-HT2A)
3.1.2. The Involvement of the Serotoninergic System (5-HT1 and 5-HT2A/2C)
3.1.3. The Involvement of the Serotoninergic System (5-HT3A/3B)
3.1.4. The Involvement of the GABAergic System
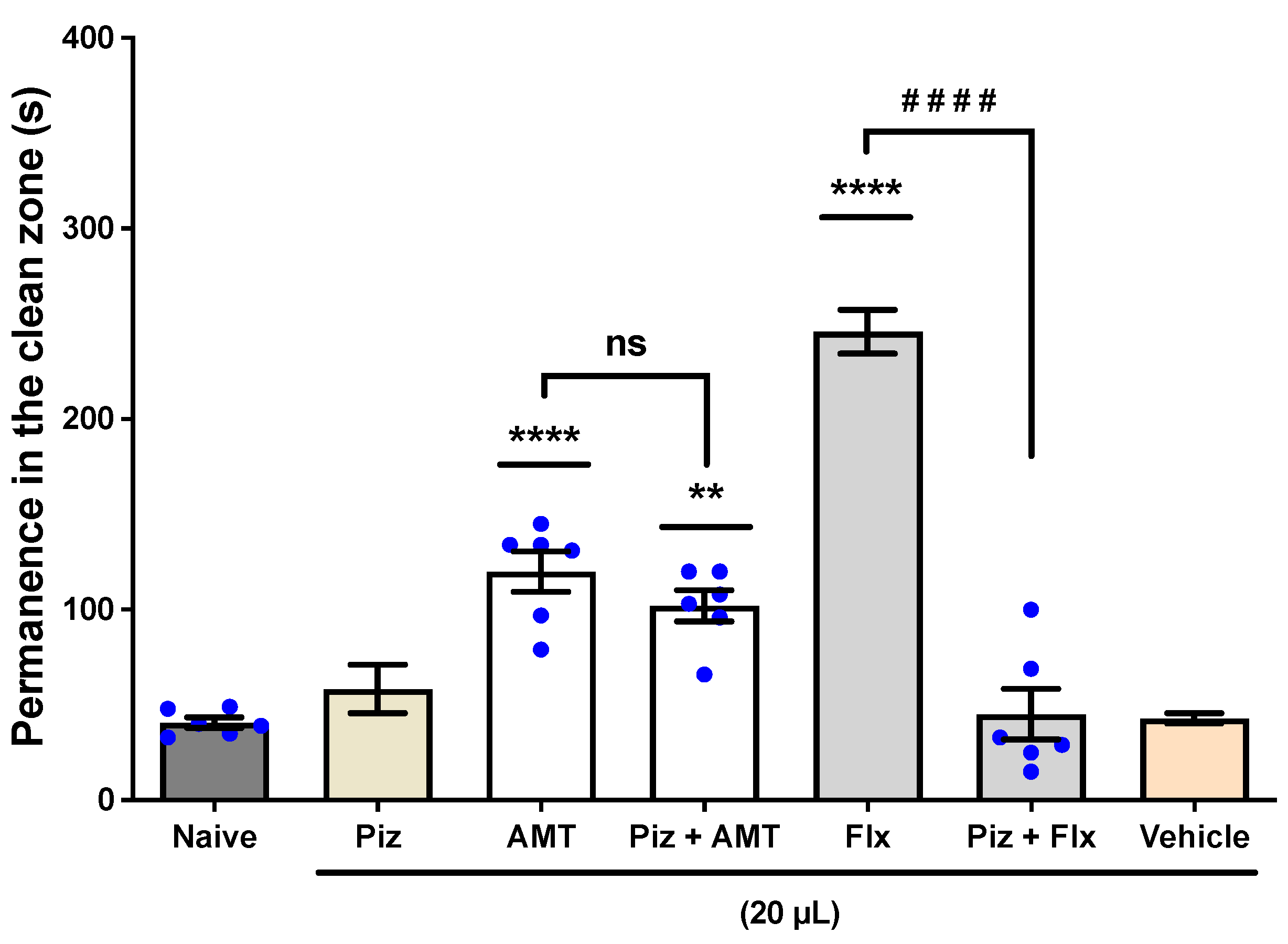
Anxiety Induced by Alcohol Withdrawal
3.2. Assessment of Locomotor Activity (Open Field Test)
3.3. Anticonvulsant Activity
3.4. Acute Toxicity against Adult Zebrafish
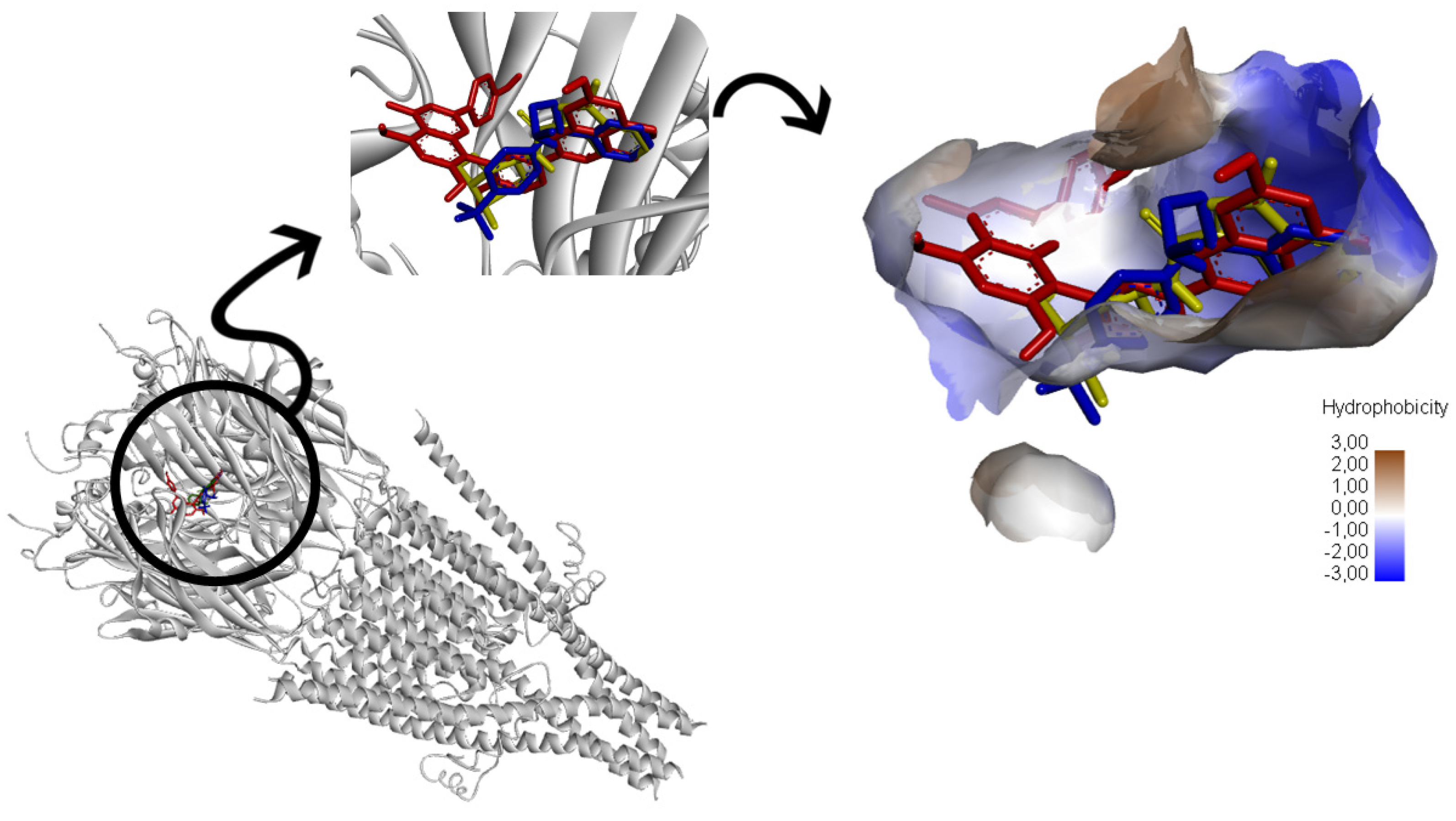
3.5. Molecular Modeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CISA Consumo de Álcool: Definições e Números No Brasil. Available online: https://ocid.es.gov.br/consumo-alcool-definicoes-numeros-Brasil-2022#:~:text=De acordo com a Vigitel,é de 25%2C6%25. (accessed on 27 July 2023).
- Queiroga, V.V.; Filgueira, E.G.K.; de Andrade Vasconcelos, A.M.; Procópio, J.V.V.; Gomes, F.W.C.; de Macêdo Gomes, C.H.F.; Gomes Filho, C.A.M.; Jacó, A.P.; de Araujo, J.M.B.G.; da Silva Nóbrega, J.C.; et al. A Pandemia Da COVID-19 e o Aumento Do Consumo de Álcool No Brasil. Res. Soc. Dev. 2021, 10, e568101118580. [Google Scholar] [CrossRef]
- Cristaldo, H.; Gandra, A.; De Acordo Com a Organização Mundial Da Saúde (OMS), o Consumo de Álcool Pode Causar Mais de 200 Doenças e Lesões. Está Associado Ao Risco de Desenvolvimento de Problemas de Saúde Como Distúrbios Mentais e Comportamentais, Incluindo Dependência Ao Álcool. Available online: https://agenciabrasil.ebc.com.br/saude/noticia/2023-02/uso-abusivo-de-alcool-entre-brasileiras-cresce-425-de-2010-2020 (accessed on 27 July 2023).
- Abrahao, K.P.; Salinas, A.G.; Lovinger, D.M. Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 2017, 96, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Villas Boas, G.R.; Paes, M.M.; Cunha, M.S.; Ponsoni, L.F.; Stefanello da Silveira, A.P.; Oesterreich, S.A. Evaluation of the Effect of Alpha-Tocopherol on Anxiety and the Neuroinflammatory Process during Alcohol Withdrawal in a Model of Forced and Chronic Self-Administration of Liquid Diet Containing Ethanol: Behavioral and Neurochemical Evidence. Alcohol 2022, 104, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Alsheikh, A.M.; Elemam, M.O.; El-bahnasawi, M. Treatment of Depression With Alcohol and Substance Dependence: A Systematic Review. Cureus 2020, 12, e11168. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in Anxiety and Depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. A Role for GABA Mechanisms in the Motivational Effects of Alcohol. Biochem. Pharmacol. 2004, 68, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Kirby, L.G.; Zeeb, F.D.; Winstanley, C.A. Contributions of Serotonin in Addiction Vulnerability. Neuropharmacology 2011, 61, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Gerlai, R. High Precision Liquid Chromatography Analysis of Dopaminergic and Serotoninergic Responses to Acute Alcohol Exposure in Zebrafish. Behav. Brain Res. 2009, 200, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Herculano, A.M.; Maximino, C. Serotonergic Modulation of Zebrafish Behavior: Towards a Paradox. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 50–66. [Google Scholar] [CrossRef]
- Widyastiwi, W.; Roseno, M. Anxiolytic Activity of Ethanolic Extract of Three Species of Indonesian Lempuyang (Zingiber Zerumbet, Zingiber Aromaticum, and Zingiber Americans). Open Access Maced. J. Med. Sci. 2022, 10, 695–701. [Google Scholar] [CrossRef]
- Castro, L.A.; Baltieri, D.A. Tratamento Farmacológico Da Dependência Do Álcool. Rev. Bras. Psiquiatr. 2004, 26, 43–46. [Google Scholar] [CrossRef]
- Ferreira, M.K.A.; da Silva, A.W.; Silva, F.C.O.; Holanda, C.L.A.; Barroso, S.M.; dos Reis Lima, J.; Neto, A.E.V.; Campos, A.R.; Bandeira, P.N.; Dos Santos, H.S.; et al. Anxiolytic-like Effect of Chalcone N-{(4′-[(E)-3-(4-Fluorophenyl)-1-(Phenyl) Prop-2-En-1-One]} Acetamide on Adult Zebrafish (Danio Rerio): Involvement of the GABAergic System. Behav. Brain Res. 2019, 374, 111871. [Google Scholar] [CrossRef] [PubMed]
- Frota, L.S.; Alves, D.R.; Marinho, M.M.; da Silva, L.P.; Almeida Neto, F.W.D.Q.; Marinho, E.S.; de Morais, S.M. Antioxidant and Anticholinesterase Activities of Amentoflavone Isolated from Ouratea Fieldingiana (Gardner) Engl. through in Vitro and Chemical-Quantum Studies. J. Biomol. Struct. Dyn. 2023, 41, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z. Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021, 12, 768708. [Google Scholar] [CrossRef]
- Cao, B.; Zeng, M.; Zhang, Q.; Zhang, B.; Cao, Y.; Wu, Y.; Feng, W.; Zheng, X. Amentoflavone Ameliorates Memory Deficits and Abnormal Autophagy in Aβ25−35-Induced Mice by MTOR Signaling. Neurochem. Res. 2021, 46, 921–934. [Google Scholar] [CrossRef]
- Vieira, J.L. Pesquisadores Da UFPB Substituem Ratos Por Peixes-Zebra Em Experimentos. Available online: https://www.ufpb.br/ufpb/contents/noticias/pesquisadores-da-ufpb-substituem-ratos-por-peixes-zebra-em-experimentos#:~:text=Pesquisadores da UFPB substituem ratos por peixes-zebra em experimentos,-O animal é&text=Um animal vertebrado ainda pouco,conhecido (accessed on 18 January 2024).
- Coulerie, P.; Nour, M.; Maciuk, A.; Eydoux, C.; Guillemot, J.-C.; Lebouvier, N.; Hnawia, E.; Leblanc, K.; Lewin, G.; Canard, B.; et al. Structure-Activity Relationship Study of Biflavonoids on the Dengue Virus Polymerase DENV-NS5 RdRp. Planta Med. 2013, 79, 1313–1318. [Google Scholar] [CrossRef]
- Lee, W.-P.; Lan, K.-L.; Liao, S.-X.; Huang, Y.-H.; Hou, M.-C.; Lan, K.-H. Inhibitory Effects of Amentoflavone and Orobol on Daclatasvir-Induced Resistance-Associated Variants of Hepatitis C Virus. Am. J. Chin. Med. 2018, 46, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, Y.A.; Garud, M.S.; Oza, M.J.; Barve, K.H.; Gaikwad, A.B. Diabetes, Diabetic Complications, and Flavonoids. In Fruits, Vegetables, and Herbs; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–104. [Google Scholar]
- MCTIC. Concea Resolução Normativa Diretrizes Da Prática Eutanásia No 37; Ministério da Ciência, Tecnologia e Inovações: Brasilia, Brazil, 2018; p. 5. [Google Scholar]
- Magalhães, F.E.A.; de Sousa, C.Á.P.B.; Santos, S.A.A.R.; Menezes, R.B.; Batista, F.L.A.; Abreu, A.O.; de Oliveira, M.V.; Moura, L.F.W.G.; Raposo, R.D.S.; Campos, A.R. Adult Zebrafish (Danio Rerio): An Alternative Behavioral Model of Formalin-Induced Nociception. Zebrafish 2017, 14, 422–429. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Pavadai, S. Anti-Inflammatory Effect of Naravelia Zeylanica DC via Suppression of Inflammatory Mediators in Carrageenan-Induced Abdominal Oedema in Zebrafish Model. Inflammopharmacology 2017, 25, 147–158. [Google Scholar] [CrossRef]
- Gebauer, D.L.; Pagnussat, N.; Piato, Â.L.; Schaefer, I.C.; Bonan, C.D.; Lara, D.R. Effects of Anxiolytics in Zebrafish: Similarities and Differences between Benzodiazepines, Buspirone and Ethanol. Pharmacol. Biochem. Behav. 2011, 99, 480–486. [Google Scholar] [CrossRef]
- Wilson, M.A.; Burghardt, P.R.; Ford, K.A.; Wilkinson, M.B.; Primeaux, S.D. Anxiolytic Effects of Diazepam and Ethanol in Two Behavioral Models: Comparison of Males and Females. Pharmacol. Biochem. Behav. 2004, 78, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Benneh, C.K.; Biney, R.P.; Mante, P.K.; Tandoh, A.; Adongo, D.W.; Woode, E. Maerua Angolensis Stem Bark Extract Reverses Anxiety and Related Behaviours in Zebrafish—Involvement of GABAergic and 5-HT Systems. J. Ethnopharmacol. 2017, 207, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Lin, O.A.; Karim, Z.A.; Vemana, H.P.; Espinosa, E.V.P.; Khasawneh, F.T. The Antidepressant 5-HT2A Receptor Antagonists Pizotifen and Cyproheptadine Inhibit Serotonin-Enhanced Platelet Function. PLoS ONE 2014, 9, e87026. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Xu, C.; Liu, Y.-X.; Ge, C.; Zhao, Z.; Zhu, Y.-B.; Bao, H.-G. Intrathecally Administered Pizotifen Alleviates Neuropathic and Inflammatory Pain in Mice by Enhancing GABAergic Inhibition. Neurosci. Lett. 2022, 775, 136545. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.S. A Review of Granisetron, 5-Hydroxytryptamine3 Receptor Antagonists, and Other Antiemetics. Am. J. Ther. 2010, 17, 476–486. [Google Scholar] [CrossRef]
- Siebel, A.M.; Menezes, F.P.; Capiotti, K.M.; Kist, L.W.; Schaefer, I.d.C.; Frantz, J.Z.; Bogo, M.R.; Da Silva, R.S.; Bonan, C.D. Role of Adenosine Signaling on Pentylenetetrazole-Induced Seizures in Zebrafish. Zebrafish 2015, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.G.G.; de Araújo, J.I.F.; Magalhães, F.E.A.; Mendes, F.R.S.; Lobo, M.D.P.; Moreira, A.C.d.O.M.; de Azevedo Moreira, R. Protein Fraction from Artocarpus Altilis Pulp Exhibits Antioxidant Properties and Reverses Anxiety Behavior in Adult Zebrafish via the Serotoninergic System. J. Funct. Foods 2020, 66, 103772. [Google Scholar] [CrossRef]
- OECD Guideline for Testing Acute Toxicity in Fishes. Test No. 203. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/1948241.pdf (accessed on 21 July 2023).
- Arellano-Aguilar, O.; Solis-Angeles, S.; Serrano-García, L.; Morales-Sierra, E.; Mendez-Serrano, A.; Montero-Montoya, R. Use of the Zebrafish Embryo Toxicity Test for Risk Assessment Purpose: Case Study. J. Fish. 2015, 9, 37–46. [Google Scholar]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software News and Updates AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Imberty, A.; Hardman, K.D.; Carver, J.P.; Perez, S. Molecular Modelling of Protein-Carbohydrate Interactions. Docking of Monosaccharides in the Binding Site of Concanavalin A. Glycobiology 1991, 1, 631–642. [Google Scholar] [CrossRef]
- Morais, S.; Cossolosso, D.; Silva, A.; de Moraes, M.; Teixeira, M.; Campello, C.; Bonilla, O.; de Paula, V.; Vila-Nova, N. Essential Oils from Croton Species: Chemical Composition, in Vitro and in Silico Antileishmanial Evaluation, Antioxidant and Cytotoxicity Activities. J. Braz. Chem. Soc. 2019, 30, 2404–2412. [Google Scholar] [CrossRef]
- Csizmadia, P. MarvinSketch and MarvinView: Molecule Applets for the World Wide Web. In The 3rd International Electronic Conference on Synthetic Organic Chemistry, 1–30 September 1999; MDPI: Basel, Switzerland, 1999; p. 1775. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Tatem, K.S.; Quinn, J.L.; Phadke, A.; Yu, Q.; Gordish-Dressman, H.; Nagaraju, K. Behavioral and Locomotor Measurements Using an Open Field Activity Monitoring System for Skeletal Muscle Diseases. J. Vis. Exp. 2014, 91, e51785. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.C.; Dewberry, L.S.; Totsch, S.K.; Yessick, L.R.; DeBerry, J.J.; Watts, S.A.; Sorge, R.E. A Novel Zebrafish-Based Model of Nociception. Physiol. Behav. 2017, 174, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.L.; Sharma, L. Bacopa Monnieri Abrogates Alcohol Abstinence-Induced Anxiety-like Behavior by Regulating Biochemical and Gabra1, Gabra4, Gabra5 Gene Expression of GABAA Receptor Signaling Pathway in Rats. Biomed. Pharmacother. 2019, 111, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Khobragade, S.; Rajaram, S.; Shingatgeri, V. Assessment of Locomotion Behavior in Adult Zebrafish after Acute Exposure to Different Pharmacological Reference Compounds. Drug Dev. Ther. 2014, 5, 127. [Google Scholar] [CrossRef]
- Maximino, C.; Marques de Brito, T.; Dias, C.A.G.D.M.; Gouveia, A., Jr.; Morato, S. Scototaxis as Anxiety-like Behavior in Fish. Nat. Protoc. 2010, 5, 209–216. [Google Scholar] [CrossRef]
- Bentué-Ferrer, D.; Bureau, M.; Patat, A.; Allain, H. Flumazenil. CNS Drug Rev. 1996, 2, 390–414. [Google Scholar] [CrossRef]
- da Silva, A.W.; Ferreira, M.K.A.; Pereira, L.R.; Rebouças, E.L.; Coutinho, M.R.; Dos, J.; Lima, R.; Guedes, M.I.F.; Bandeira, P.N.; Magalhães, F.E.A.; et al. Combretum Lanceolatum Extract Reverses Anxiety and Seizure Behavior in Adult Zebrafish through GABAergic Neurotransmission: An in Vivo and in Silico Study. J. Biomol. Struct. Dyn. 2022, 40, 9801–9814. [Google Scholar] [CrossRef]
- Andrade, J.C.; Monteiro, Á.B.; Andrade, H.H.N.; Gonzaga, T.K.S.N.; Silva, P.R.; Alves, D.N.; Castro, R.D.; Maia, M.S.; Scotti, M.T.; Sousa, D.P.; et al. Involvement of GABAA Receptors in the Anxiolytic-Like Effect of Hydroxycitronellal. Biomed. Res. Int. 2021, 2021, 9929805. [Google Scholar] [CrossRef]
- Dremencov, E.; Weizmann, Y.; Kinor, N.; Gispan-Herman, I.; Yadid, G. Modulation of Dopamine Transmission by 5HT2C and 5HT3 Receptors: A Role in the Antidepressant Response. Curr. Drug Targets 2006, 7, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Möhler, H. The GABA System in Anxiety and Depression and Its Therapeutic Potential. Neuropharmacology 2012, 62, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.D.S.; da Rocha, Y.M.; do Nascimento, G.A.; Santos, S.A.A.; Vieira, N.C.G.; Moura, L.F.W.G.; Alves, D.R.; da Silva, W.M.B.; de Morais, S.M.; de Oliveira, K.A.; et al. Potential of the Blue Calm® Food Supplement in the Treatment of Alcohol Withdrawal-Induced Anxiety in Adult Zebrafish (Danio Rerio). Neurochem. Int. 2024, 175, 105706. [Google Scholar] [CrossRef] [PubMed]
- Ishola, I.O.; Chatterjee, M.; Tota, S.; Tadigopulla, N.; Adeyemi, O.O.; Palit, G.; Shukla, R. Antidepressant and Anxiolytic Effects of Amentoflavone Isolated from Cnestis Ferruginea in Mice. Pharmacol. Biochem. Behav. 2012, 103, 322–331. [Google Scholar] [CrossRef] [PubMed]



| Sample | Adult Zebrafish Mortality | 96 h of Analysis LC50 (mg/mL)/IV | |||
|---|---|---|---|---|---|
| Vehicle | 0.01 mg/mL | 0.1 mg/mL | 1.0 mg/mL | ||
| AMT | 0 | 0 | 0 | 0 | >1.0 |
| Ligands | (kcal/mol) | RMSD (Å) |
|---|---|---|
| AMT | −9.8 | 1.207 |
| Flx | −7.7 | 1.226 |
| Gstn | −8.3 | 1.304 |
| Ligands | (kcal/mol) | RMSD (Å) |
|---|---|---|
| AMT | −9.8 | 1.177 |
| DZP | −6.3 | 1.250 |
| Fmz | −6.4 | 1.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frota, L.S.; da Silva, W.M.B.; Alves, D.R.; Santos, S.A.A.R.; do Nascimento, G.A.; Magalhães, F.E.A.; Campos, A.R.; de Morais, S.M. An Evaluation of the Anxiolytic Potential of Amentoflavone in Adult Zebrafish Undergoing Alcohol Withdrawal: In Vivo and In Silico Studies. Receptors 2024, 3, 201-219. https://doi.org/10.3390/receptors3020011
Frota LS, da Silva WMB, Alves DR, Santos SAAR, do Nascimento GA, Magalhães FEA, Campos AR, de Morais SM. An Evaluation of the Anxiolytic Potential of Amentoflavone in Adult Zebrafish Undergoing Alcohol Withdrawal: In Vivo and In Silico Studies. Receptors. 2024; 3(2):201-219. https://doi.org/10.3390/receptors3020011
Chicago/Turabian StyleFrota, Lucas Soares, Wildson Max Barbosa da Silva, Daniela Ribeiro Alves, Sacha Aubrey Alves Rodrigues Santos, Gabriela Alves do Nascimento, Francisco Ernani Alves Magalhães, Adriana Rolim Campos, and Selene Maia de Morais. 2024. "An Evaluation of the Anxiolytic Potential of Amentoflavone in Adult Zebrafish Undergoing Alcohol Withdrawal: In Vivo and In Silico Studies" Receptors 3, no. 2: 201-219. https://doi.org/10.3390/receptors3020011
APA StyleFrota, L. S., da Silva, W. M. B., Alves, D. R., Santos, S. A. A. R., do Nascimento, G. A., Magalhães, F. E. A., Campos, A. R., & de Morais, S. M. (2024). An Evaluation of the Anxiolytic Potential of Amentoflavone in Adult Zebrafish Undergoing Alcohol Withdrawal: In Vivo and In Silico Studies. Receptors, 3(2), 201-219. https://doi.org/10.3390/receptors3020011






