Oxidative Stability of the Oil from Camelina (Camelina sativa L.) Seeds: Effects of Ascorbyl Palmitate Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Oil Content of the Seeds
2.3. Analysis of Fatty Acid Composition
2.4. Determination of Conjugated Dines and Trienes Content
2.5. Determination of Total Tocopherols Content
2.6. Oxidation Procedure and Determination of the Kinetic Parameters
3. Results and Discussion
3.1. Fatty Acid Composition
3.2. Oxidative Stability
3.2.1. Current Status of Camelina Seed Oil
3.2.2. Kinetics of Lipid Autoxidation and Inhibition Effect of Antioxidants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AscPH | Ascorbyl palmitate |
| RA | Rosmarinic acid |
| IPA | Induction period |
| R | Initial rate of oxidation |
References
- Montero-Muñoz, I.; Mostaza-Colado, D.; Capuano, A.; Mauri Ablanque, P.V. Seed and Straw Characterization of Nine New Varieties of Camelina sativa (L.) Crantz. Land 2023, 12, 328. [Google Scholar] [CrossRef]
- Afzal, M.F.; Khalid, W.; Armghan Khalid, M.; Zubair, M.; Akram, S.; Kauser, S.; Noreen, S.; Jamal, A.; Kamran Khan, M.; Al-Farga, A. Recent industrials extraction of plants seeds oil used in the development of functional food products: A Review. Int. J. Food Prop. 2022, 25, 2530–2550. [Google Scholar] [CrossRef]
- Xu, T.-T.; Li, J.; Fan, Y.-W.; Zheng, T.-W.; Deng, Z.-Y. Comparison of Oxidative Stability among Edible Oils under Continuous Frying Conditions. Int. J. Food Prop. 2015, 18, 1478–1490. [Google Scholar] [CrossRef]
- Arshad, M.; Mohanty, A.K.; Van Acker, R.; Riddle, R.; Todd, J.; Khalil, H.; Misra, M. Valorization of camelina oil to biobased materials and biofuels for new industrial uses: A review. RSC Adv. 2022, 12, 27230–27245. [Google Scholar] [CrossRef]
- Berhow, M.A.; Polat, U.; Glinski, J.A.; Glensk, M.; Vaughn, S.F.; Isbell, T.; Ayala-Diaz, I.; Marek, L.; Gardner, C. Optimized analysis and quantification of glucosinolates from Camelina sativa seeds by reverse-phase liquid chromatography. Ind. Crops Prod. 2013, 43, 119–125. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Mishra, A.S. Glucosinolates in animal nutrition: A review. Anim. Feed Sci. Technol. 2007, 132, 1–27. [Google Scholar] [CrossRef]
- Alim, M.A.; Iqbal, Z.; Dutta, P.C. Studies on the characterization and distribution of fatty acids and minor components of high-erucic acid mustard oil and low-erucic acid rapeseed oil. Emir. J. Food Agric. 2012, 24, 281–287. [Google Scholar]
- Commission Regulation (EU) 2019/1870. Available online: https://eur-lex.europa.eu/eli/reg/2019/1870/oj/eng (accessed on 1 August 2025).
- Scientific Opinion on erucic acid in feed and food. EFSA J. 2016, 14, 4593.
- Sydor, M.; Kurasiak-Popowska, D.; Stuper-Szablewska, K.; Rogoziński, T. Camelina sativa. Status quo and future perspectives. Ind. Crops Prod. 2022, 187, 115531. [Google Scholar] [CrossRef]
- Murphy, E.J. Camelina (Camelina sativa). In Industrial Oil Crops; AOCS Press: Champaign, IL, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 207–230. [Google Scholar] [CrossRef]
- Wang, J.; Han, L.; Wang, D.; Sun, Y.; Huang, J.; Shahidi, F. Stability and stabilization of omega-3 oils: A review. Trends Food Sci. Technol. 2021, 118, 17–35. [Google Scholar] [CrossRef]
- Garcia-Mendoza, M.d.P.; Espinosa-Pardo, F.A.; Savoire, R.; Harscoat-Schiavo, C.; Cansell, M.; Subra-Paternault, P. Improvement of the oxidative stability of camelina oil by enrichment with phospholipid-quercetin formulations. Food Chem. 2021, 34, 128234. [Google Scholar] [CrossRef]
- Pokorny, J.; Yanishlieva, N.; Gordon, M. Antioxidants in Food. Practical Applications; Woodhead Publishing Ltd.: Cambridge, UK, 2001. [Google Scholar]
- Plut, C.; Seyrig, C.; Leclerc, C. Huile de cameline. Phytotherapie 2010, 8, 105–108. [Google Scholar] [CrossRef]
- Froehlich, A.; O’Dea, G.; Hackett, R.; O’Beirne, D.; Ni Eidhin, D.; Burke, J. Stabilization of Camelina Oil with Synthetic and Natural Antioxidants. J. Am. Oil Chem. Soc. 2012, 89, 837–847. [Google Scholar] [CrossRef]
- Pathak, R.; Mohsin, M.; Mehta, S.S. An assessment of in vitro antioxidant potential of Camelina sativa L. seed oil and estimation of tocopherol content using HPTLC method. J. Sci. Res. 2021, 13, 589–600. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Ryńska, B.; Stuper-Szablewska, K. Analysis of Distribution of Selected Bioactive Compounds in Camelina sativa from Seeds to Pomace and Oil. Agronomy 2019, 9, 168. [Google Scholar] [CrossRef]
- Chantsalnyam, B.; Otgonbayar, C.; Enkhtungalag, O.; Odonmajig, P. Physical and chemical characteristics and fatty acids composition of seeds oil isolated from Camelina sativa (L). cultivated in Mongolia. Mong. J. Chem. 2013, 14, 80–83. [Google Scholar] [CrossRef]
- Rakita, S.; Spasevski, N.; Savić, I.; Gajić, I.S.; Lazarević, J.; Dragojlović, D.; Duragić, O. Comparative Evaluation of Camelina Seed Oils Obtained by Cold-Pressing and Solvent Extraction. Foods 2024, 13, 3605. [Google Scholar] [CrossRef]
- Shukla, V.K.S.; Dutta, P.C.; Artz, W.E. Camelina oil and its unusual cholesterol content. J. Am. Oil Chem. Soc. 2002, 79, 965–969. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gürtler, R.; Husøy, T.; et al. Opinion on the re-evaluation of ascorbyl palmitate (E 304i) as a food additive in foods for infants below 16 weeks of age and the follow-up of its re-evaluation as a food additive for uses in foods for all population groups. EFSA J. 2020, 18, 6153. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.-W.; Kanner, J.; Bruce German, J. Interfacial Phenomena in the Evaluation of Antioxidants: Bulk Oils vs. Emulsions. J. Agric. Food Chem. 1994, 42, 1054–1059. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Kalu, C.; McNeill, G.P.; Padleyc, F.B.; Pierceb, J.H. Effects of Tocopherols, Ascorbyl Palmitate, and Lecithin on Autoxidation of Fish Oil. J. Am. Oil Chem. 1998, 75, 813–822. [Google Scholar] [CrossRef]
- Budilarto, E.S.; Kamal-Eldin, A. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1095–1137. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Abramovic, H.; Abram, V. Effect of added rosemary extract on oxidative stability of Camelina sativa oil. Acta Agric. Slov. 2006, 87, 255–261. [Google Scholar] [CrossRef]
- Taneva, S.; Momchilova, S. Effect of the extraction method on the lipid composition of purslane (Portulaca oleracea L.) seed oil. Discov. Food 2024, 4, 112. [Google Scholar] [CrossRef]
- Pashaei, H.; Farhoosh, R. A new insight into the weight gain method to monitor and evaluate lipid peroxidation. Foods 2025, 14, 700. [Google Scholar] [CrossRef] [PubMed]
- Yanishlieva, N.; Popov, A.; Marinova, E. Eine Modifizierte Jodometrische Methode zur Bestimmung der Peroxidzahl in kleinen Lipidproben. C. R. Acad. Bulg. Sci. 1978, 31, 869–871. [Google Scholar]
- Bondioli, P.; Folegatti, L.; Rovellini, P. Oils rich in alpha linolenic acid: Chemical composition of perilla (Perilla frutescens) seed oil. Oilseeds Fats Crops Lipids 2020, 27, 67. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Xin, H.-L. Portulaca oleracea L.: A Review of Phytochemistry and Pharmacological Effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef]
- Taneva, S.; Konakchiev, A.; Totzeva, I.; Kamenova-Nacheva, M.; Nikolova, Y.; Momchilova, S.; Dimitrov, V. Super-critical carbon dioxide extraction as an effective green technology for production of high quality rose hip oil. Bulg. Chem. Commun. 2017, 49, 126–131. Available online: http://www.bcc.bas.bg/bcc_volumes/Volume_49_Special_B_2017/BCC2017-49-SE-B-126-131.pdf (accessed on 6 June 2025).
- Abramovic, H.; Abram, V. Physico-Chemical Properties, Composition and Oxidative stability of Camelina sativa oil. Food Technol. Biotechnol. 2005, 43, 63–70. [Google Scholar]
- Veljković, V.B.; Kostić, M.D.; Stamenković, O.S. Camelina seed harvesting, storing, pretreating, and processing to recover oil: A review. Ind. Crops Prod. 2022, 178, 114539. [Google Scholar] [CrossRef]
- Amorati, R.; Fiammetta, F.; Lucarini, M.; Pedulli, G.F.; Valgimigli, L. A Quantitative Approach to the Recycling of α-Tocopherol by Coantioxidants. J. Org. Chem. 2002, 67, 9252–9303. [Google Scholar] [CrossRef]
- Doert, M.; Grebenteuch, S.; Kroh, L.W.; Rohn, S. A ternary system of α-tocopherol with phosphatidylethanolamine and l-ascorbyl palmitate in bulk oils provides antioxidant synergy through stabilization and regeneration of α-tocopherol. Food Chem. 2022, 391, 133084. [Google Scholar] [CrossRef]
- Marinova, E.; Toneva, A.; Yanishlieva, N. Synergistic antioxidant effect of α-tocopherol and myricetin on the autoxidation of triacylglycerols of sunflower oil. Food Chem. 2008, 106, 628–633. [Google Scholar] [CrossRef]
- Bayram, I.; Decker, E. Analysis of the mechanism of antioxidant synergism between α-tocopherol and myricetin in bulk oil. J. Am. Oil Chem. Soc. 2023, 101, 477–492. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.; Kancheva, V. Synergism between DL-alpha-tocopherol and ascorbyl palmitate at various ratios in binary antioxidant compositions. Riv. It Sost. Gr. 2018, 95, 75–87. [Google Scholar]
- Villeneuve, P.; Bourlieu-Lacanal, C.; Durand, E.; Lecomte, J.; McClements, D.J.; Decker, E.A. Lipid oxidation in emulsions and bulk oils: A review of the importance of micelles. Crit. Rev. Food Sci. Nutr. 2023, 60, 4687–4727. [Google Scholar] [CrossRef]

| Camelina Seed Oil (Control Sample *) | Mean ± SD |
|---|---|
| Conj. Dienes (K232) | 2.2 ± 0.1 ** |
| Conj. Trienes (K268) | 0.6 ± 0.01 |
| PV (meqO2/kg) | 2.8 ± 0.10 |
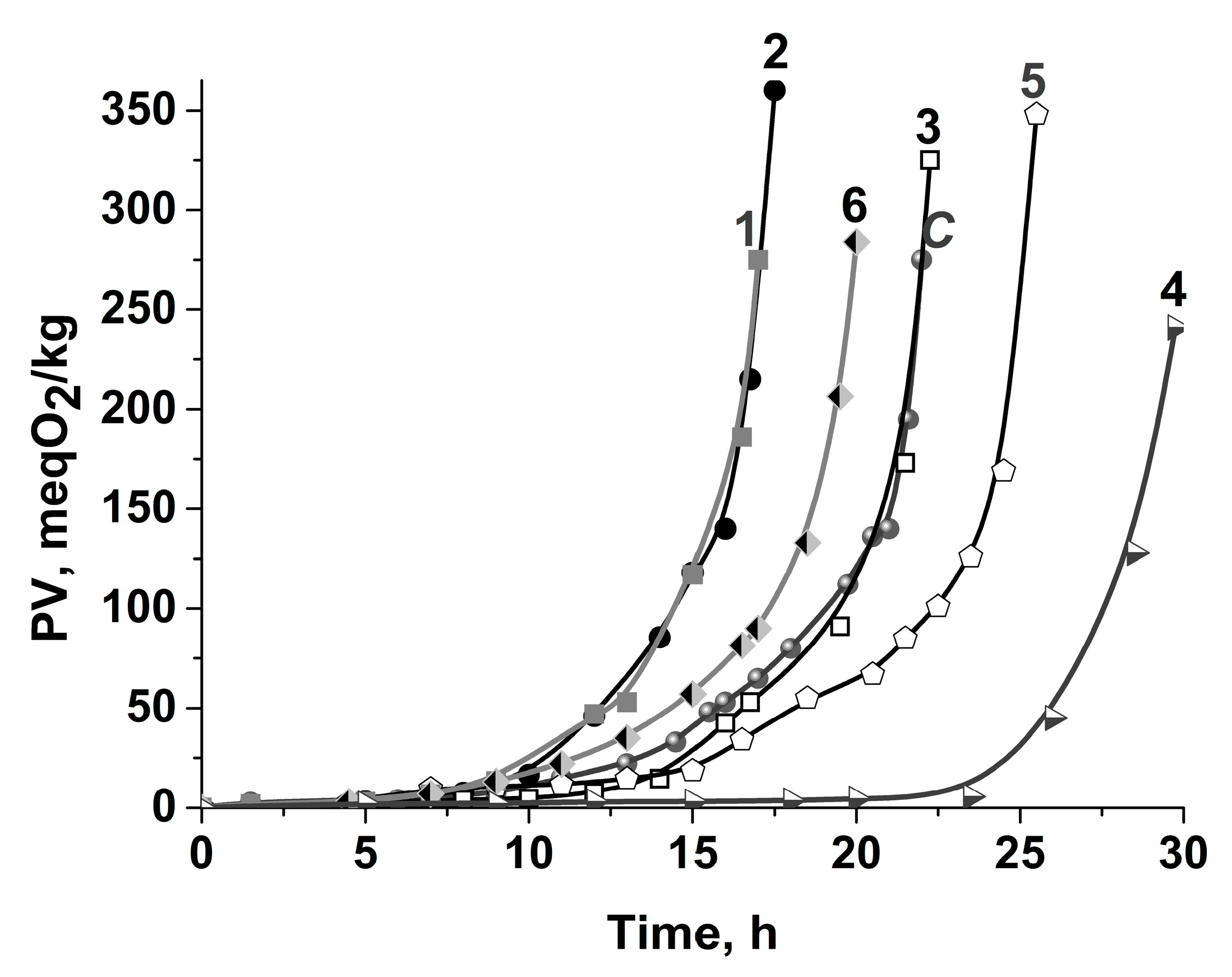
| Sample | Abbrev. | IPA, h | R, 10−7 Ms−1 |
|---|---|---|---|
| Control sample | C | 20 ± 2 * | 1.15 ± 0.05 |
| Samples enriched with: | |||
| 0.1 mM (0.004%) AscPH 0.2 mM (0.008%) AscPH 1.0 mM (0.041%) AscPH 2.0 mM (0.082%) AscPH | 1 | 15.6 ± 1.5 | 1.30 ± 0.05 |
| 2 | 16.0 ± 1.5 | 1.24 ± 0.06 | |
| 3 | 20.5 ± 1.5 | 0.69 ± 0.03 | |
| 4 | 28.0 ± 2.0 | 0.31 ± 0.02 | |
| 0.2 mM (0.007%) RA 0.2 mM AscPH:RA (1:1) | 5 | 24.0 ± 2.0 | 1.60 ± 0.05 |
| 6 | 18.0 ± 2.0 | 1.40 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavova-Kazakova, A.; Marcheva, M.; Taneva, S.; Momchilova, S. Oxidative Stability of the Oil from Camelina (Camelina sativa L.) Seeds: Effects of Ascorbyl Palmitate Concentrations. Seeds 2025, 4, 38. https://doi.org/10.3390/seeds4030038
Slavova-Kazakova A, Marcheva M, Taneva S, Momchilova S. Oxidative Stability of the Oil from Camelina (Camelina sativa L.) Seeds: Effects of Ascorbyl Palmitate Concentrations. Seeds. 2025; 4(3):38. https://doi.org/10.3390/seeds4030038
Chicago/Turabian StyleSlavova-Kazakova, Adriana, Marina Marcheva, Sabina Taneva, and Svetlana Momchilova. 2025. "Oxidative Stability of the Oil from Camelina (Camelina sativa L.) Seeds: Effects of Ascorbyl Palmitate Concentrations" Seeds 4, no. 3: 38. https://doi.org/10.3390/seeds4030038
APA StyleSlavova-Kazakova, A., Marcheva, M., Taneva, S., & Momchilova, S. (2025). Oxidative Stability of the Oil from Camelina (Camelina sativa L.) Seeds: Effects of Ascorbyl Palmitate Concentrations. Seeds, 4(3), 38. https://doi.org/10.3390/seeds4030038






