Osmopriming Increases Seed Germination of Amaranthus cruentus (L.)
Abstract
1. Introduction
2. Materials and Methods
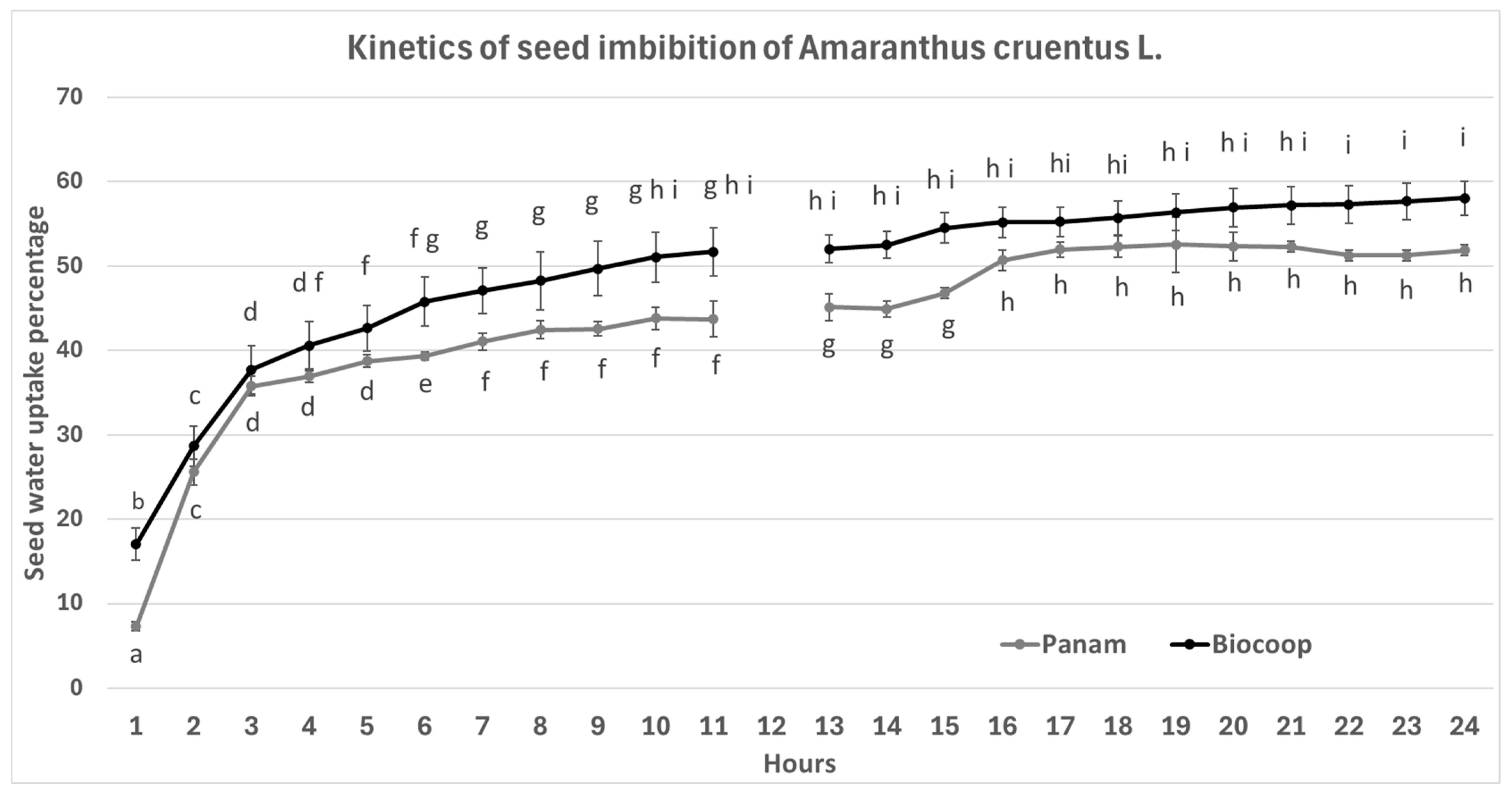
2.1. Kinetics of Imbibition Seed of Amaranthus Cruentus
2.2. Germination Test
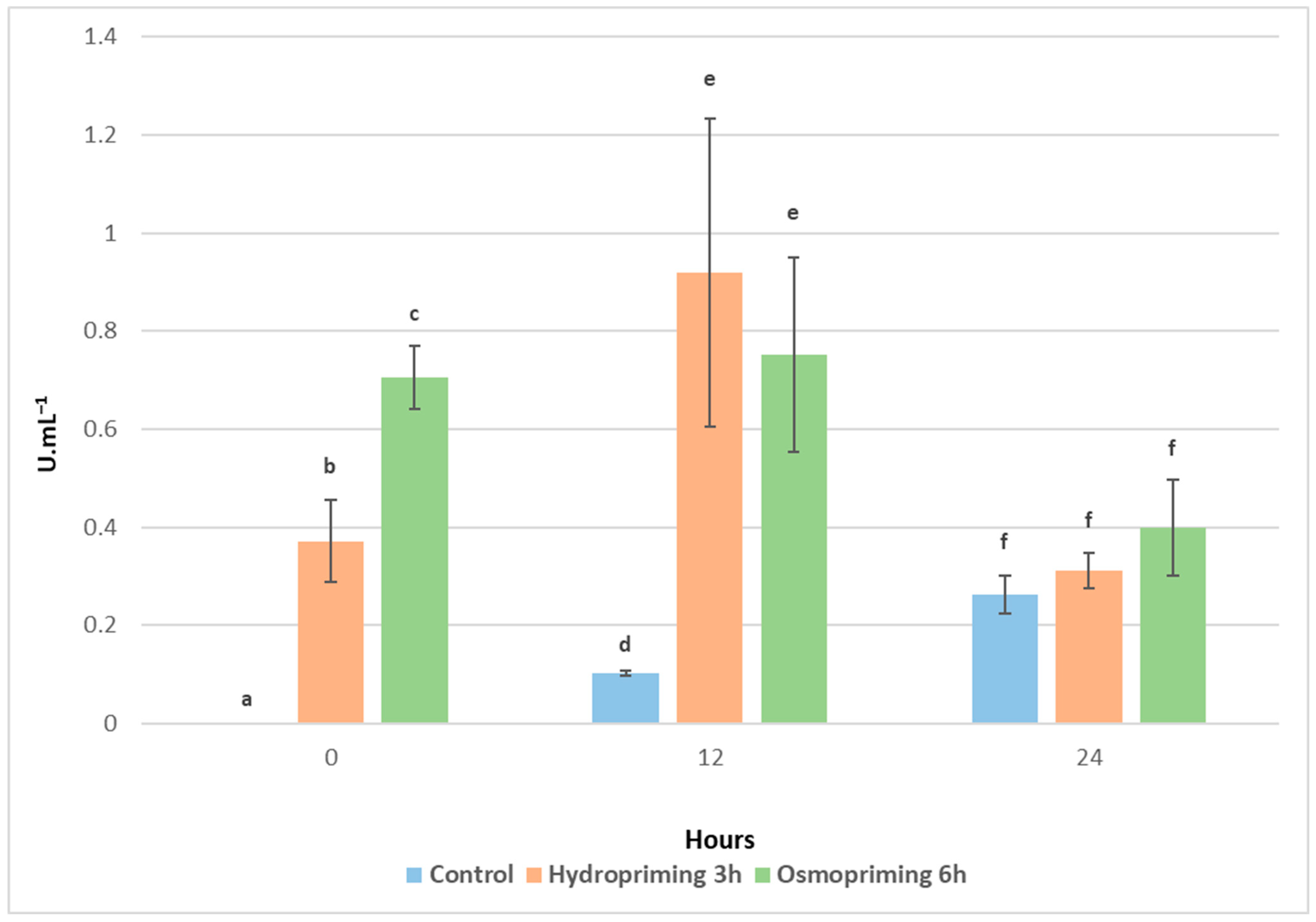
2.3. Determination of Polyphenol Oxidase Activity
2.3.1. PPO Extraction
2.3.2. Assay of PolyPhenol Oxidase (PPO) Activity
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sayed-Ahmad, B.; Urrutigoïty, M.; Hijazi, A.; Saad, Z.; Cerny, M.; Evon, P.; Merah, O. Amaranth oilseed composition and cosmetic applications. Separations 2022, 9, 181. [Google Scholar] [CrossRef]
- Baraniak, J.; Kania-Dobrowolska, M. The Dual Nature of Amaranth—Functional Food and Potential Medicine. Foods 2022, 11, 618. [Google Scholar] [CrossRef]
- Kaźmierczak, A.; Bolesławska, I.; Przysławski, J. Szarłat—Jego wykorzystanie w profilaktyce i leczeniu wybranych chorób cywilizacyjnych. Now. Lek. 2011, 80, 192–198. [Google Scholar]
- Obiedzinska, A.; Waszkiewicz-Robak, B. Oleje tłoczone na zimno jako żywność funkcjonalna. Żywność Nauka Technol. Jakość 2012, 1, 27–44. [Google Scholar]
- Azri, A.; Sassi-Aydi, S.; Aydi, S.; Debouba, M.; Bouajila, J.; Cerny, M.; Valentin, R.; Tricoulet, L.; Galaup, P.; Merah, O. Nutritional and Bioactive Lipid Composition of Amaranthus Seeds Grown in Varied Agro-Climatic Conditions in France. Agronomy 2025, 15, 672. [Google Scholar] [CrossRef]
- Berghoff, S.A.; Spieth, L.; Sun, T.; Hosang, L.; Schlaphoff, L.; Depp, C.; Düking, T.; Winchenbach, J.; Neuber, J.; Ewers, D. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci. 2021, 24, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant sources, extraction methods, and uses of squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef]
- Mendes, A.; Azevedo-Silva, J.; Fernandes, J.C. From Sharks to Yeasts: Squalene in the Development of Vaccine Adjuvants. Pharmaceuticals 2022, 15, 265. [Google Scholar] [CrossRef]
- Costea, M.; Weaver, S.E.; Tardif, F.J. The biology of Canadian weeds. 130. Amaranthus retroflexus L., A. powellii S. Watson and A. hybridus L. Can. J. Plant Sci. 2004, 84, 631–668. [Google Scholar] [CrossRef]
- Kauffman, C.S.; Weber, L.E. Grain amaranth. In Advances in New Crops; Timber Press: Portland, OR, USA, 1990; pp. 127–139. [Google Scholar]
- Yeshitila, M.; Gedebo, A.; Tesfaye, B.; Gobena Roro, A.; Demissie Degu, H.; Merah, O. Assessment of physio-morphological traits, genetic variability, and growth performance among amaranth (Amaranthus species) genotypes from Ethiopia. Ecol. Gen. Genom. 2024, 32, 100270. [Google Scholar] [CrossRef]
- Aufhammer, W.; Kaul, H.P.; Herz, P.; Nalborczyk, E.; Dalbiak, A.; Gontarczyk, M. Grain yield formation and nitrogen uptake of amaranth. Eur. J. Agr. 1995, 4, 379–386. [Google Scholar] [CrossRef]
- Aufhammer, W.; Czuczorova, D.; Kaul, H.P.; Kruse, M. Germination of grain amaranth (Amaranthus hypochondriacus × A. hybridus): Effects of seed quality, temperature, light, and pesticides. Eur. J. Agr. 1998, 8, 127–135. [Google Scholar] [CrossRef]
- Putnam, D.H.; Mohammad, S.; Herbert, S.J. Planting pattern and density effects on groundnut: Sorghum and groundnut: Sunflower intercrops. Crop Res. 1990, 3, 115–128. [Google Scholar]
- Hill, H.J. Recent developments in seed technology. J. New Seeds 1999, 1, 105–112. [Google Scholar] [CrossRef]
- Boucelha, L.; Djebbar, R. Influence of different pre-germination treatments of Vigna unguiculata (L.) Walp. seeds on germination performance and tolerance to water stress. Biotec. Agr. Soc. Environ. 2015, 19, 160–170. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20153242969 (accessed on 22 July 2025).
- Zulfiqar, F. Effect of seed priming on horticultural crops. Sci. Hort. 2021, 286, 110197. [Google Scholar] [CrossRef]
- Jarrar, H.; El-Keblawy, A.; Ghenai, C.; Abhilash, P.C.; Bundela, A.K.; Abideen, Z.; Sheteiwy, M.S. Seed enhancement technologies for sustainable dryland restoration: Coating and scarification. Sci. Total Environ. 2023, 904, 166150. [Google Scholar] [CrossRef]
- Muhie, S.H.; Akele, F.; Yeshiwas, T. Economic feasibility of carrot (Daucus carota L) production in response to different seed priming techniques under deficit irrigation. Sci. Hort. 2024, 325, 112662. [Google Scholar] [CrossRef]
- Sharma, A.D.; Rathore, S.V.S.; Srinivasan, K.; Tyagi, R.K. Comparison of various seed priming methods for seed germination, seedling vigour and fruit yield in okra (Abelmoschus esculentus L. Moench). Sci. Hort. 2014, 165, 75–81. [Google Scholar] [CrossRef]
- Kimura, E.; Fransen, S.C.; Collins, H.P.; Guy, S.O.; Johnston, W.J. Breaking seed dormancy of switchgrass (Panicum virgatum L.): A review. Biomass Bioenergy 2015, 80, 94–101. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, S.; Sharma, R.K.; Kumar, V.; Sharma, S.S. Influence of seed pre-treatment and storage on germination and physiological characteristics of seeds of common mullein (Verbascum thapsus L.). J. Appl. Res. Med. Arom. Plant 2024, 43, 100573. [Google Scholar] [CrossRef]
- Bessam, F.Z.; Mehdadi, Z.; Hassiba, M.B.; Marouf, A. Effets de quelques prétraitements physiques sur l’amélioration des performances germinatives de Stipa tenacissima L. et caractérisation des substances inhibitrices. Acta Bot. Gallica 2010, 157, 349–360. [Google Scholar] [CrossRef]
- Bera, A.; Mukhopadhyay, E.; Kar, C.S.; Kumar, M.; Bhandari, H.R. Efficacy of scarification treatments on release of seed coat-imposed dormancy in five wild species of genus Corchorus. S. Afr. J. Bot. 2020, 135, 144–147. [Google Scholar] [CrossRef]
- Cho, J.S.; Jang, B.K.; Lee, C.H. Breaking combinational dormancy of Rhus javanica L. seeds in South Korea: Effect of mechanical scarification and cold-moist stratification. S. Afr. J. Bot. 2020, 133, 174–177. [Google Scholar] [CrossRef]
- Labdelli, A.; Adda, A.; Rebiai, A.; Zebib, B.; Merah, O. Study of seed dormancy origins in three Atlas pistachio ecotypes. Appl. Ecol. Environ. Res. 2019, 17, 13555–13565. [Google Scholar] [CrossRef]
- Ellouzi, H.; Oueslati, S.; Hessini, K.; Rabhi, M.; Abdelly, C. Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Sci. Hort. 2021, 288, 110360. [Google Scholar] [CrossRef]
- Yan, N.; Cao, J.; Wang, J.; Zou, X.; Yu, X.; Zhang, X.; Si, T. Seed priming with graphene oxide improves salinity tolerance and increases productivity of peanut through modulating multiple physiological processes. J. Nanobiotechnol. 2024, 22, 565. [Google Scholar] [CrossRef]
- Moosavi, A.; Tavakkol-Afshari, R.; Sharif-Zadeh, F.; Aynehband, A. Effect of seed priming on germination characteristics, polyphenoloxidase, and peroxidase activities of four amaranth cultivars. J. Food Agric. Environ. 2009, 7, 353–358. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20093344642 (accessed on 22 July 2025).
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Patra, H.K.; Mishra, D. Pyrophosphatase, peroxidase and polyphenoloxidase activities during leaf development and senescence. Plant Physiol. 1979, 63, 318–323. [Google Scholar] [CrossRef]
- Mayer, A.M.; Harel, E.; Ben-Shaul, R. Assay of catechol oxidase—A critical comparison of methods. Phytochemistry 1996, 5, 783–789. [Google Scholar] [CrossRef]
- Avallone, S.; Guiraud, J.P.; Brillouet, J.M.; Teisson, C. Enzymatic browning and biochemical alterations in black spots of pineapple [Ananas comosus (L.) Merr.]. Curr. Microbiol. 2003, 47, 0113–0118. [Google Scholar] [CrossRef]
- Romuald, K. Caractérisation Biochimique des Enzymes liées aux Réactions de Défense de L’ananas et du Bananier en Réponse aux Stress. Ph.D. Thesis, Université de Poitiers, Poitiers, France, 2009. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef]
- Cabrera-Santos, D.; Ordoñez-Salanueva, C.A.; Sampayo-Maldonado, S.; Campos, J.E.; Orozco-Segovia, A.; Flores-Ortiz, C.M. Chia (Salvia hispanica L.) seed soaking, germination, and fatty acid behavior at different temperatures. Agriculture 2021, 11, 498. [Google Scholar] [CrossRef]
- Ghafoor, F.; Liaqat, S.; Iqbal, W. The hormonal seed priming in relation to carrot germination. J. Life Sci. 2020, 1, 1–5. [Google Scholar] [CrossRef]
- Lacroux, É.; Cerny, M.; Fabre, J.F.; Valentin, R.; Merah, O. Impact of chia seed germination on its lipid composition and on its processability in water by an integrated process designed for the release of oil bodies. LWT 2025, 223, 117802. [Google Scholar] [CrossRef]
- Caseiro, R.; Bennett, M.; Marcos-Filho, J. Comparison of three priming techniques for onion seed lots differing in initial seed quality. Seed Sci. Technol. 2004, 32, 356–375. [Google Scholar] [CrossRef]
- Heshmati, S.; Dehaghi, M.A.; Farooq, M.; Wojtyla, Ł.; Maleki, K.; Heshmati, S. Role of melatonin seed priming on antioxidant enzymes and biochemical responses of Carthamus tinctorius L. under drought stress conditions. Plant Stress 2021, 2, 100023. [Google Scholar] [CrossRef]
- Gour, T.; Sharma, A.; Lal, R.; Heikrujam, M.; Gupta, A.; Agarwal, L.K.; Chetri, S.P.K.; Kumar, R.; Sharma, K. Amelioration of the physio-biochemical responses to salinity stress and computing the primary germination index components in cauliflower on seed priming. Heliyon 2023, 9, e14403. [Google Scholar] [CrossRef]
- Chandel, N.S.; Tripathi, V.; Singh, H.B.; Vaishnav, A. Breaking seed dormancy for sustainable food production: Revisiting seed priming techniques and prospects. Biocat. Agric. Biotechnol. 2024, 55, 102976. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Asgari, A.; Pasalari, H. Comprehensive seed priming assessment of Hibiscus sabdariffa L. in germination and seedling growth stage under salt stress. Acta Agric. Slov. 2022, 118, 1–17. [Google Scholar] [CrossRef]
- Ibrahimi, K.; Ben Attia, K.; Amami, R.; Pinê Américo-Pinheiro, J.H.; Sher, F. Assessment of three decades treated wastewater impact on soil quality in semi-arid agroecosystem. J. Saudi Soc. Agri Sci. 2022, 21, 525–535. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-Priming Effects on Seed Germination and Field Performance of Faba Bean in Spring Sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef]
- Noorhosseini, S.A.; Jokar, N.K.; Damalas, C.A. Improving Seed Germination and Early Growth of Garden Cress (Lepidium sativum) and Basil (Ocimum basilicum) with Hydropriming. J. Plant Growth Regul. 2018, 37, 323–334. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.; Nisar, F.; Rasheed, A.; Shah, S.Z. Seed Priming as an Effective Technique for Enhancing Salinity Tolerance in Plants: Mechanistic Insights and Prospects for Saline Agriculture with a Special Emphasis on Halophytes. Seeds 2025, 4, 14. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Mohan, V.R. Chemical Seed Priming: Molecules and Mechanisms for Enhancing Plant Germination, Growth, and Stress Tolerance. Curr. Issues Mol. Biol. 2025, 47, 177. [Google Scholar] [CrossRef]
- Mahra, S.; Tripathi, S.; Tiwari, K.; Sharma, S.; Mathew, S.; Kumar, V.; Shivesh Sharma, S. Harnessing nanotechnology for sustainable agriculture: From seed priming to encapsulation. Plant Nano Biol. 2025, 11, 100124. [Google Scholar] [CrossRef]
- Alam, A.; Ullah, H.; Chaum, S.; Tisarum, R.; Datta, A. Effect of seed priming with potassium nitrate on growth, fruit yield, quality and water productivity of cantaloupe under water-deficit stress. Sci Hort. 2021, 288, 110354. [Google Scholar] [CrossRef]
- Kimura, E.; Islam, M.A. Seed scarification methods and their use in forage legumes. Res. J. Seed Sci. 2012, 5, 38–50. [Google Scholar] [CrossRef]
- Travlos, I.S.; Economou, G.; Karamanos, A.I. Germination and emergence of the hard seed coated Tylosema esculentum (Burch) A. Schreib in response to different pre-sowing seed treatments. J. Arid Environ. 2007, 68, 501–507. [Google Scholar] [CrossRef]
- Jones, T.A.; Johnson, D.A.; Bushman, B.S.; Connors, K.J.; Smith, R.C. Seed dormancy mechanisms in basalt milkvetch and western prairie clover. Rangel. Ecol. Manag. 2016, 69, 117–122. [Google Scholar] [CrossRef]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed priming: A feasible strategy to enhance drought tolerance in crop plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef]
- Brocklehurst, P.A.; Dearman, J. Interaction between seed priming treatments and nine seed lots of carrot, celery and onion II. Seedling emergence and plant growth. Ann. Appl. Biol. 2008, 102, 583–593. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Hussain, H.A.; Nie, L. Physiological and biochemical mechanisms of seed priming-Induced chilling tolerance in rice cultivars. Front. Plant Sci. 2016, 7, 116. [Google Scholar] [CrossRef]
- Demeke, T.; Chang, H.G.; Morris, C.F. Effect of germination, seed abrasion and seed size on polyphenol oxidase assay activity in wheat. Plant Breed. 2001, 120, 369–373. [Google Scholar] [CrossRef]
- Côme, D. Influence of refrigeration and freezing on the quality and germination capacity of seeds. Int. J. Refrig. 1982, 5, 333–336. [Google Scholar] [CrossRef]
- Côme, D.; Corbineau, F. Some aspects of metabolic regulation of seed germination and dormancy. In Recent Advances in the Development and Germination of Seeds; Springer: New York, NY, USA, 1989; pp. 165–179. [Google Scholar] [CrossRef]
- He, M.; Li, H.; Sun, Z.; Li, X.; Li, Q.; Cai, J.; Zhou, Q.; Zhong, Y.; Wang, X.; Jiang, D. Drought priming enhances young spike development in wheat under drought stress during stem elongation. J. Integr. Agric. 2025. [Google Scholar] [CrossRef]
- Brzozowska, J.; Hanower, P. Sur les composés phénoliques des végétaux et leur rapport avec un déficit hydrique chez des cotonniers. Ann Univ. Abidjan. Série C Sci. 1976, 12, 65–87. [Google Scholar]
- Maki, H.; Morohashi, Y. Development of polyphenol oxidase activity in the micropylar endosperm of tomato seeds. J. Plant Physiol. 2006, 163, 1–10. [Google Scholar] [CrossRef]
- Iseri, O.D.; Sahin, F.; Hberal, M. Sodium chloride priming improves salinity responses of tomato at seedling stage. J. Plant Nutr. 2014, 37, 374–392. [Google Scholar] [CrossRef]
- Sánchez-Pérez, D.M.; Márquez-Guerrero, S.Y.; Ramírez-Moreno, A.; Rodríguez-Sifuentes, L.; Galindo-Guzmán, M.; Flores-Loyola, E.; Marszalek, J.E. Impact of Biologically and Chemically Synthesized Zinc Oxide Nanoparticles on Seed Germination and Seedlings’ Growth. Horticulturae 2023, 9, 1201. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Lou, J. Optimization of Low-Temperature Plasma Inhibition of Potato Germination Using Response Surface Methodology. Appl. Sci. 2025, 15, 3233. [Google Scholar] [CrossRef]
- Hu, W.; Guan, Y.; Ji, Y.; Yang, X. Effect of cutting styles on quality, antioxidant activity, membrane lipid peroxidation, and browning in fresh-cut potatoes. Food Biosci. Part B 2021, 44, 101435. [Google Scholar] [CrossRef]
- Badawy, A.A.; Alshammari, W.K.; Salem, N.F.G.; Alshammari, W.S.; Hussein, H.-A.A. Arginine and Spermine Ameliorate Water Deficit Stress in Fenugreek (Trigonella foenum-graecum L.) by Enhancing Growth and Physio-Biochemical Processes. Antioxidants 2025, 14, 329. [Google Scholar] [CrossRef]
- Akram, W.; Waqar, S.; Hanif, S.; Anjum, T.; Aftab, Z.-e.-H.; Li, G.; Ali, B.; Rizwana, H.; Hassan, A.; Rehman, A.; et al. Comparative Effect of Seed Coating and Biopriming of Bacillus aryabhattai Z-48 on Seedling Growth, Growth Promotion, and Suppression of Fusarium Wilt Disease of Tomato Plants. Microorganisms 2024, 12, 792. [Google Scholar] [CrossRef]
- Heidarieh, Z.; Jafari, A.; Ebrahimi, H.R.; Haghighi, B.J.; Miri, H.R. Seed priming alleviates the adverse effects of drought stress on sesame genotypes by improving biochemical and physiological characteristics. S. Afr. J. Bot. 2024, 167, 256–269. [Google Scholar] [CrossRef]
- Shelar, A.; Singh, A.V.; Chaure, N.; Jagtap, P.; Chaudhari, P.; Shinde, M.; Nile, S.H.; Chaskar, M.; Rajendra, P. Nanoprimers in sustainable seed treatment: Molecular insights into abiotic-biotic stress tolerance mechanisms for enhancing germination and improved crop productivity. Sci. Total Environ. 2024, 951, 175118. [Google Scholar] [CrossRef]
- Hatcher, D.W.; Kruger, J.E. Distribution of polyphenoloxidase in our millstreams of Canadian common wheat classes milled to three extraction rates. Cereal Chem. 1993, 70, 51–55. [Google Scholar]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2015, 2, 67. [Google Scholar] [CrossRef]
- Gajewska, E.; Witusińska, A.; Kornaś, A.; Wielanek, M. Phenolics Profile and Phenol-Related Enzyme Activities in Cucumber Plants Under Ni Stress. Int. J. Mol. Sci. 2025, 26, 1237. [Google Scholar] [CrossRef]
- Elsherif, D.E.; Safhi, F.A.; Subudhi, P.K.; Shaban, A.S.; El-Esawy, M.A.; Khalifa, A.M. Phytochemical Profiling and Bioactive Potential of Grape Seed Extract in Enhancing Salinity Tolerance of Vicia faba. Plants 2024, 13, 1596. [Google Scholar] [CrossRef] [PubMed]
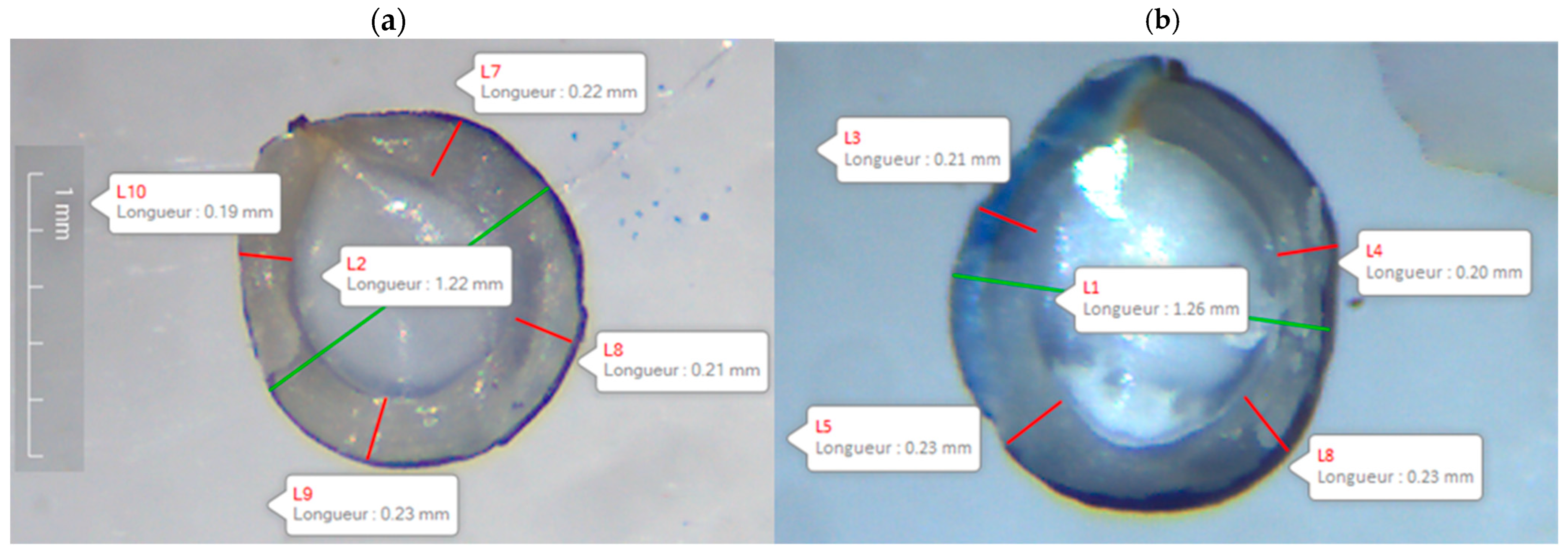
| Genotype | Initial Mass (mg) | Initial Seed Moisture (%) |
|---|---|---|
| Panam | 0.782 ± 0.018 a | 16.13 ± 0.012 a |
| Biocoop | 1.082 ± 0.016 b | 14.15 ± 0.009 b |
| Modality | Germination Speed T50 (hours) | Germination Rate at T50 (%) | Germination Rate (%) |
|---|---|---|---|
| Control seeds | 72 | 71 ± 5.85 b | 84 ± 5.85 b |
| Scarification | 72 | 71 ± 9.65 a | 84 ± 3.7 a |
| Scarification and hydropriming | 48 | 73 ± 9.25 a | 86 ± 9.65 a |
| Scarification with algal biostimulant | 72 | 68 ± 13.25 a | 81 ± 9.7 a |
| Control with chia extract | 48 | 57 ± 8.7 a | 80 ± 6.3 a |
| Control with algal biostimulant | 48 | 56 ± 11.1 a | 95 ± 5.45 b |
| Simple hydropriming 1 h | 72 | 62 ± 12.85 a | 75 ± 11.4 a,c |
| Simple hydropriming 3 h | 72 | 52 ± 7.45 a | 61 ± 3.7 c |
| Dual hydropriming 1 h | 48 | 63 ± 11.4 a | 79 ± 11.1 a |
| Dual hydropriming 3 h | 72 | 58 ± 5.05 a | 68 ± 12.45 c |
| Osmopriming 3 h | 24 | 63 ± 6.41 b | 97 ± 3.75 b |
| Osmopriming 6 h | 24 | 74 ± 8.74 a | 100 ± 2.45 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busquère, A.; Lefebvre, D.; Galaup, P.; Tricoulet, L.; Musset, C.; Lacroux, E.; Merah, O. Osmopriming Increases Seed Germination of Amaranthus cruentus (L.). Seeds 2025, 4, 37. https://doi.org/10.3390/seeds4030037
Busquère A, Lefebvre D, Galaup P, Tricoulet L, Musset C, Lacroux E, Merah O. Osmopriming Increases Seed Germination of Amaranthus cruentus (L.). Seeds. 2025; 4(3):37. https://doi.org/10.3390/seeds4030037
Chicago/Turabian StyleBusquère, Arnaud, Dominique Lefebvre, Patrice Galaup, Lucas Tricoulet, Charline Musset, Eric Lacroux, and Othmane Merah. 2025. "Osmopriming Increases Seed Germination of Amaranthus cruentus (L.)" Seeds 4, no. 3: 37. https://doi.org/10.3390/seeds4030037
APA StyleBusquère, A., Lefebvre, D., Galaup, P., Tricoulet, L., Musset, C., Lacroux, E., & Merah, O. (2025). Osmopriming Increases Seed Germination of Amaranthus cruentus (L.). Seeds, 4(3), 37. https://doi.org/10.3390/seeds4030037







