Abstract
Background/Objectives: Fungal keratitis (FK) is a current challenge in ophthalmology due to its association with severe visual impairment and the limitations of current antifungal therapies. We aim to evaluate the antifungal activity of essential oils (EOs) from the aromatic and medicinal plants Cymbopogon citratus and Lavandula pedunculata against selected FK pathogens collected from FK patients in two Portuguese hospitals. Methods: The antifungal activity of the EOs was tested at concentrations of 25%, 50%, 75%, and 100% for up to 7 days using the solid-phase disk diffusion in vitro assay. Results: Candida albicans was the most prevalent pathogen (28.6%), followed by Candida parapsilosis (21.4%) and Dicyma olivacea (14.2%). The other identified species were Aspergillus fumigatus and Scedosporium boydii (7.1%). Clinical diagnostic methodologies showed agreement with the molecular identification. Cymbopogon citratus EO showed higher antifungal activity than Lavandula pedunculata EO. The highest antifungal activity was observed against Aspergillus fumigatus and Scedosporium boydii (inhibition zone diameter, IZD = 90.0 mm) after 7 (Cymbopogon citratus EO) or 3 days of incubation (Lavandula pedunculata EO). While the antifungal activity of Cymbopogon citratus EO was maintained during the study (for Aspergillus fumigatus, Candida albicans, and Scedosporium boydii), the antifungal activity of Lavandula pedunculata EO decreased with time. Conclusions: Cymbopogon citratus EO and Lavandula pedunculata EO showed optimal antifungal activity against molds (Aspergillus fumigatus and Scedosporium boydii) after 3 days of incubation. Against yeasts (Candida albicans and Candida parapsilosis), the EOs showed lower activity. Our study sheds light on the development of new pharmacological strategies for FK based on EOs extracted from aromatic and medicinal plants.
1. Introduction
Fungal keratitis (FK) is a severe sight-threatening condition that often results in unilateral blindness and eye loss. It is typically caused by Aspergillus spp., Candida spp., and several species of the genus Fusarium [1]. The annual global incidence of FK is not known, but the highest incidences are in Asia (34 per 100,000 people) and Africa (14 per 100,000 people) [2]. However, in developed countries, prolonged contact lens use, ocular surface diseases (e.g., dry eye), and the widespread use of corticosteroids and other immunosuppressants, antibiotics, and chemotherapeutic drugs have increased the incidence of FK over the past decade [3,4,5]. The current therapeutic approach involves both topical and systemic antifungal therapies and surgical intervention when necessary. Concerning topical antifungal therapy, voriconazole and natamycin are the most used drugs, with the latter being shown to be more effective clinically [6]. Systemic antifungal therapy is used in severe cases of FK, including those with deep corneal involvement, impending corneal perforation, or endophthalmitis. Oral voriconazole and itraconazole are the most used systemic antifungal agents for FK, with voriconazole being preferred because of its better corneal penetration and broad-spectrum antifungal activity [7]. In cases where medical therapy fails or in the presence of complications such as corneal perforation, surgical intervention may be necessary. This may involve therapeutic penetrating keratoplasty (PK) and conjunctival flaps in cases where PK is not feasible or in patients with poor visual potential. Nevertheless, these surgical procedures may have complications, such as recurrence of infection, endophthalmitis, and graft rejection [8]. Therefore, FK is still a major challenge to treat, and alternative strategies to overcome the limited efficacy of currently available antifungal agents are urgently needed.
Essential oils (EOs) are complex mixtures of volatile compounds obtained through distillation or mechanical pressing from aromatic plants. They are characterized by their diverse chemical profiles, which may include terpenes, phenols, aldehydes, and other bioactive constituents. Each EO possesses a unique composition of chemicals, influencing its spectrum of biological activities [9]. The medical relevance of EOs has gained considerable attention in recent years, with a focus on their potential roles in therapeutics, disease prevention, and overall health promotion. In recent years, EOs have been explored as potential antifungal agents. Eucalyptus globulus EO was shown to have antifungal efficacy against a range of pathogenic fungi, including Candida, Aspergillus, and Fusarium spp. [10]. Also, cinnamon leaf EO was found to significantly inhibit the fungal growth [11]. The antifungal effect of EOs is believed to be due to their ability to disrupt the cellular integrity of fungi, leading to their death. This is thought to occur through a variety of mechanisms, including the disruption of the plasma membrane, leading to the leakage of cellular contents, and the disruption of mitochondrial structure, leading to cell death [12]. Additionally, EOs have shown promising results in circumventing common resistance mechanisms related to conventional antifungal agents [13]. This ability to overcome resistance highlights the importance of further exploring EOs as an alternative therapeutic approach against FK. In addition to their antifungal activity, EOs have also been found to have synergistic effects with conventional antifungal agents [14].
In the present study, we tested EOs extracted from Cymbopogon citratus (DC.) Stapf and Lavandula pedunculata (Mill.) Cav. for antifungal activity against selected fungal pathogens isolated from FK patients. Cymbopogon citratus EO is known to possess significant antifungal and antibacterial activities. Its main component, geranial, is believed to disrupt the cell membrane of fungi and bacteria, leading to cell death. Studies have demonstrated its effectiveness against a several pathogens, including Candida spp. [15] and Fusarium spp. [16]. Lavandula pedunculata EO has documented antifungal properties against a range of dermatophytes, including those responsible for skin infections [17,18]. Moreover, both Cymbopogon citratus and Lavandula pedunculata EOs are known for their safety and tolerability when used topically, making them suitable candidates for therapeutic applications. Their relatively low toxicity, compared with synthetic antifungal agents, reduces the risk of adverse effects in patients [19].
Although EOs have been demonstrated to exhibit antifungal activity, studies in clinical isolates are very scarce [20,21]. The use of clinical isolates provides more clinically relevant results and thus contributes to addressing the current challenges in the treatment of FK. Here, we hypothesize that Cymbopogon citratus EO and Lavandula pedunculata EO extracted from aromatic and medicinal plants may be effective against fungal pathogens associated with FK. We collected corneal samples from FK patients in two Portuguese hospitals—the University Hospital Center of São João (Porto) and Coimbra University Hospital (Coimbra)—and identified the pathogens using molecular methods. To assess the antifungal activity of Cymbopogon citratus EO and Lavandula pedunculata EO, we evaluated their in vitro activity against selected fungal pathogens (Aspergillus fumigatus, Candida albicans, Candida parapsilosis, and Scedosporium boydii) using the disk diffusion method. Ultimately, our study aims to support the validation of EOs as effective, safe, and sustainable therapeutic agents for FK, offering a potential alternative to current antifungal treatments.
2. Materials and Methods
2.1. Patients and Ethical Issues
This hospital-based cross-sectional study was carried out between February and September 2023 in two Portuguese hospitals: the University Hospital Center of São João based in Porto (northern region of Portugal) and Coimbra University Hospital based in Coimbra (central region of Portugal). All procedures were conducted following the tenets of the Declaration of Helsinki. The study was approved by the Ethics Committee of the Faculty of Medicine of the University of Coimbra, Coimbra, Portugal (approval No. CE-128/2023). For each patient with a clinical diagnosis of FK, data were recorded, including the age, sex, predisposing risk factors, prior ocular diseases, and visual acuity at presentation and after treatment.
2.2. Collection and Processing of Ocular Samples
Following the ocular examination of each patient with suspected infectious keratitis, the cornea was anesthetized with topical oxybuprocaine 4 mg/mL (Anestocil®, EDOL, Linda-a-Velha, Portugal), and corneal specimens were collected under sterile conditions in accordance with established safety protocols.
The processing of ocular samples and identification of the fungal pathogens were performed according to the protocol implemented in each hospital microbiology laboratory. At Coimbra University Hospital (Coimbra, Portugal), a portion of each specimen was carefully placed on a sterile glass slide for immediate microscopic examination while the remaining material was sent to the microbiology laboratory and inoculated onto Sabouraud dextrose agar (SDA) supplemented with chloramphenicol and gentamicin. The inoculated SDA media were then incubated at 30 °C until fungal growth was observed (maximum of two weeks). At the University Hospital Center of São João (Porto, Portugal), ocular samples were sent in culture medium to the microbiology laboratory where they were cultured in blood agar (BA), chocolate agar (CA), and SDA supplemented with chloramphenicol and gentamicin culture media. BA and CA cultures were incubated at 35 °C in a capnophilic atmosphere for five days, whereas SDA cultures were incubated at 25 °C for two weeks.
2.3. Molecular Identification of Fungal Pathogens
Fourteen ocular samples from fourteen patients underwent molecular testing to identify the fungal pathogens. Each clinical sample was inoculated in 5 plates of Potato Dextrose Agar (PDA) medium (CM139, Oxoid, Basingstoke, UK) supplemented with streptomycin sulfate (20 μL/L) [22] and incubated at 24 °C for 12 days in the dark. The media were regularly monitored for the emergence of fungal colonies. These emerging colonies were further clinically isolated onto PDA to form axenic cultures and incubated under the same conditions, and their morphologic characteristics, including color, texture, and overall colony appearance, were observed.
For molecular identification, the DNA of the axenic cultures were extracted and tested using the REDExtract-N-Amp™ polymerase chain reaction (PCR) ReadyMix™ Kit (Sigma-Aldrich, St. Louis, MO, USA) with modifications [22]. The molecular identification involved the amplification of the internal transcribed spacer (ITS)-rDNA region by PCR, using the commonly used primer pair for fungal identification (ITS1-F and ITS4). When the ITS region proved to be insufficient for conclusive identification, alternative genetic markers were used to increase the accuracy and specificity of the identification. The nucleotide sequences for all primers used in this study are listed in Table S1. The obtained amplicons were purified with the EXO/SAP Go PCR Purification Kit (GRISP, Porto, Portugal) following the manufacturer’s recommendations and sequenced using an ABI 3730xl DNA Analyzer system (96 capillary instruments) at STABVIDA, Caparica, Portugal. Similarity searches were performed using the National Center for Biotechnology Information nucleotide database (NCBI’s) online Basic Local Alignment Search Tool (BLAST), using the somewhat similar sequences (blastn) algorithm of BLAST “https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 7 November 2023)” [23]. Species identification was confirmed using macroscopic and microscopic analysis of taxonomic traits.
2.4. Preparation of Essential Oils
The extraction of EOs from Cymbopogon citratus and Lavandula pedunculata was performed by hydrodistillation for 3 h using a Clevenger-type apparatus according to the protocol described in the European Pharmacopeia [24]. Following the extraction process, the EOs were separated from the aqueous distillate and immediately transferred to amber glass vials and stored at 4 °C. The extracted EOs were chemically characterized using gas chromatography coupled to mass spectrometry (GC/MS) using the protocol that was previously published by our team [25]. The EOs used in this study were obtained from the same extraction batch to ensure homogeneity in terms of chemical composition, namely, the presence of compounds with antifungal activity.
2.5. Evaluation of Antifungal Activity of Essential Oils
The antifungal activity of the Cymbopogon citratus EO and Lavandula pedunculata EO was assessed against selected yeasts and molds from our study samples, namely Aspergillus fumigatus, Candida albicans, Candida parapsilosis, and Scedosporium boydii, using the solid-phase disk diffusion in vitro assay [26,27]. The EOs were prepared at four concentrations (25%, 50%, 75%, and 100% (v/v%)) by diluting it in pure dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA). Sterile paper disks, with a diameter of 8 mm, were impregnated with 25 μL of the corresponding EO dilution, ensuring uniform saturation of the disk. Fungal suspensions were prepared and calibrated to contain 105 spores/mL and 105 CFU/mL for filamentous fungi and yeasts, respectively. From each suspension, 100 μL was uniformly spread onto 90 mm PDA Petri dishes and left to dry. An impregnated disk was placed onto the center of each culture plate. The cultures were incubated at 30 °C to allow for the growth of each tested pathogen. On the 3rd, 5th, and 7th days of incubation, the inhibition zone diameter (IZD) was manually measured at its widest point using a ruler, with each measurement performed in duplicate. Experiments were performed in triplicate, using three plates for each condition. The final IZD was determined by averaging the values for the three experiments. Paper disks infused with pure DMSO without EOs and without DMSO or EOs were used as positive and negative controls, respectively.
2.6. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 30.0.0.0 [28]. The demographic and clinical characteristics of the patients enrolled in the study are presented as means ± standard deviations (SDs), ranges, and percentages. To evaluate the antifungal activity of the EOs, three independent experiments were performed for each condition (incubation time and EO concentration). The obtained IZD values are presented as means ± SDs. An independent t-test was performed to compare the antifungal activity of the EOs. One-way ANOVA followed by the Tukey post hoc test was performed to compare the antifungal sensitivity of the pathogens. Repeated-measures ANOVA with a Greenhouse–Geisser correction followed by post hoc Bonferroni analysis was performed to compare the antifungal activity of the EOs during the study (i.e., on the 3rd, 5th, and 7th day of incubation). The difference was considered significant if the p-value was less than 0.05.
3. Results
3.1. Characterization of Fungal Keratitis Patients
A total of fourteen ocular fungal infection samples were successfully collected from fourteen patients in two Portuguese university hospitals over an 8-month period (from February to September 2023), ensuring a representative sampling of the FK cases in northern and central Portugal. The demographic and clinical characteristics of the donor patients are described in Table 1. Molds and yeasts were both identified in the collected samples. The FK patients’ mean age was 63 ± 17 years (range: 20–84), with 57.1% (n = 8) being male. Molds were more frequently identified in younger patients (56 ± 20 years) and men (71.4%, n = 5). Yeasts were more frequently identified in older patients (70 ± 10 years) and women (57.1%, n = 4). The main risk factors identified were ocular surface disease (28.6%, n = 4) and previous keratoplasty (28.6%, n = 4). Mold infections were mostly found in those who use contact lenses (21.4%, n = 3), whereas previous keratoplasty was mainly associated with yeast infections (42.9%, n = 3). Most samples were collected in spring (42.9%, n = 6) and summer (42.9%, n = 6). Although mold infections were observed primarily in summer (57.1%, n = 4), yeast infections occurred throughout the year, from winter to summer. The mean visual acuity (VA) (logMAR) was 1.15 ± 1.04 units at initial presentation and worsened to 1.54 ± 1.06 units by the end of the treatment. Patients diagnosed with mold infection presented with better VA than those diagnosed with yeast infections. The VA changed from 0.33 ± 0.40 units to 0.87 ± 0.63 units in the mold group and from 1.74 ± 0.96 units to 2.03 ± 1.07 units in the yeast group. The individual characteristics of the patients are presented in Table S2.

Table 1.
Demographic and clinical characteristics of patients.
3.2. Phylogenetic Identification of Fungal Pathogens
The DNA sequences of the clinical isolates were compared with those in the NCBI BLAST database to determine the best match and the similarity percentages (Table 2). A graphical representation of the distribution and prevalence of different fungal species in the collected samples is presented in Table 3. Candida spp., particularly Candida albicans and Candida parapsilosis, were found to be the most prevalent, representing 28.6% (n = 4) and 21.4% (n = 3), respectively, of the total sample. Other clinical isolates were identified as Beauveria bassiana, Scedosporium boydii, and Aspergillus fumigatus (7.1%, n = 1). We also identified unusual strains, namely, Dicyma olivacea (14.2%, n = 2), Epicoccum nigrum, and Penicillium tealii (7.1%, n = 1).

Table 2.
Identification of fungal pathogens from ocular clinical isolates.

Table 3.
Distribution and prevalence of fungal pathogens from ocular clinical isolates.
3.3. In Vitro Evaluation of Antifungal Activity of Essential Oils
The antifungal efficacy of the Cymbopogon citratus and Lavandula pedunculata EOs against four selected fungal pathogens associated with FK—Aspergillus fumigatus (clinical isolate number 1), Candida albicans (clinical isolate sample number 6), Candida parapsilosis (clinical isolate sample number 9), and Scedosporium boydii (clinical isolate sample number 14)—is presented in Figure 1. Figure 1A shows representative images of the solid-phase disk diffusion in vitro assay. The IZD values are plotted in Figure 1B. Detailed values are listed in Table S3. Positive and negative control conditions are presented in Figure S1.
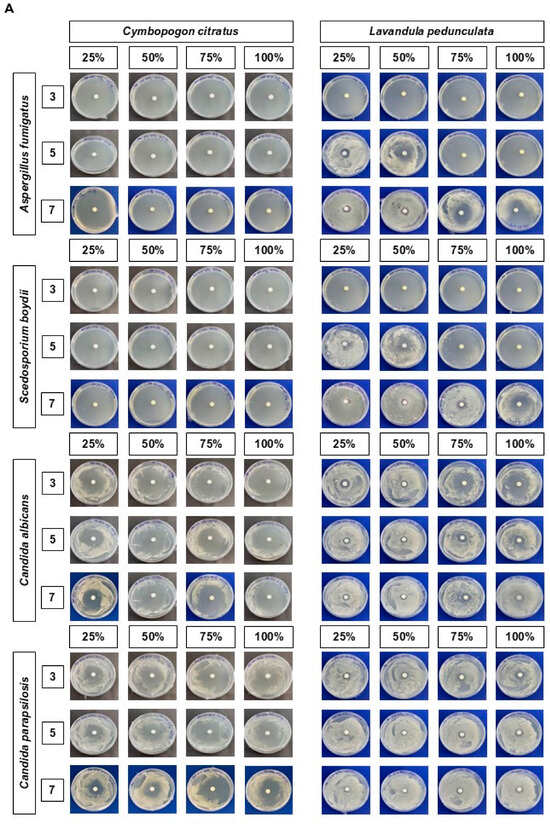
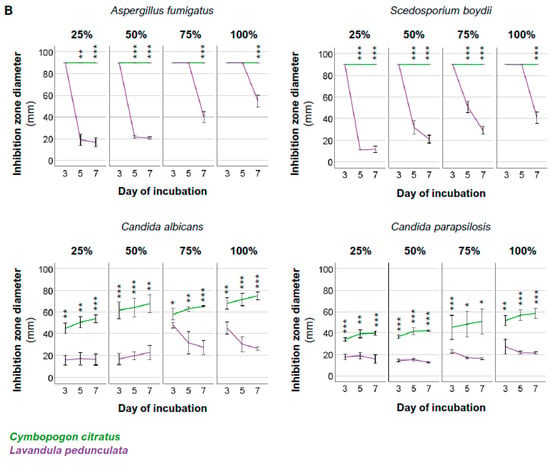
Figure 1.
Antifungal activity of Cymbopogon citratus EO and Lavandula pedunculata EO at concentrations of 25%, 50%, 75%, and 100% against selected FK pathogens after 3, 5, and 7 days of incubation. (A) Representative images of solid-phase disk diffusion in vitro assay showing the effect of the EOs on mycelial growth. (B) Inhibition zone diameter (in mm) values. Data are presented as the mean ± SD of three independent experiments. Statistical analysis was performed to compare mean values using an independent t-test at p < 0.05. Cymbopogon citratus EO versus Lavandula pedunculata EO: * p < 0.05; ** p < 0.01; *** p < 0.001.
We found that the antifungal activity of the Cymbopogon citratus EO was the same or statistically significantly higher than that of the Lavandula pedunculata EO against all clinical isolates tested at the different concentrations and incubation times.
We analyzed the antifungal activity of the Cymbopogon citratus EO and Lavandula pedunculata EO against selected clinical isolates (Figure S2). We found that the Cymbopogon citratus EO had the highest antifungal activity (IZD = 90 mm) against Aspergillus fumigatus and Scedosporium boydii, regardless of the concentration and the incubation time. Statistically lower IZD values were measured for Candida albicans, which varied between 45.0 ± 5.0 mm and 75.0 ± 3.6 mm, and for Candida parapsilosis, which varied between 34.0 ± 1.7 mm and 58.3 ± 4.9 mm, throughout the incubation time with different concentrations of the EOs. The IZD values for Candida albicans were statistically significantly higher than for Candida parapsilosis, except for the concentration of 75%. Concerning the Lavandula pedunculata EO, its antifungal activity against the different pathogens changed with the incubation time. Against Aspergillus fumigatus, the IZD values ranged from 90.0 mm to 16.7 ± 4.2 mm, whereas, against Scedosporium boydii, the IZD values ranged from 90.0 mm to 11.0 mm. Candida albicans and Candida parapsilosis presented IZD values ranging from 48.0 ± 2.6 mm and 16.3 ± 5.0 mm and from 27.3 ± 6.8 mm and 12.7 ± 0.6 mm, respectively. At a concentration of 25%, the Lavandula pedunculata EO presented statistically significantly higher antifungal activity against Aspergillus fumigatus and Scedosporium boydii than against Candida albicans and Candida parapsilosis, but only on the 3rd day of incubation. On the 5th and 7th days of incubation, the IZD values decreased, and no statistically significant difference in antifungal activity against the tested pathogens was observed. At a concentration of 50%, a similar trend was observed except that the Lavandula pedunculata EO showed statistically significantly higher antifungal activity against Scedosporium boydii on the 5th day of incubation. At concentrations of 75% and 100%, the Lavandula pedunculata EO showed statistically significantly higher antifungal activity against Aspergillus fumigatus and Scedosporium boydii than against Candida albicans and Candida parapsilosis, except on the 7th day of incubation. Regarding antifungal activity against Candida albicans and Candida parapsilosis, no statistically significant differences were observed, except on the 3rd day of incubation.
We evaluated the effect of different concentrations of the Cymbopogon citratus EO and Lavandula pedunculata EO on antifungal activity (Figure S3A,B). We found no statistically significant difference between the concentrations of the Cymbopogon citratus EO in general. The antifungal activity against Candida albicans was statistically significantly higher for the concentration of 100% compared to the concentration of 25% at all incubation time points; moreover, the antifungal activity was statistically significantly higher for the concentration of 50% compared to the concentration of 25% on the 3rd and 7th days of incubation. The Lavandula pedunculata EO presented the highest antifungal activity against Aspergillus fumigatus regardless of the concentration on the 3rd day of incubation; on the 5th day of incubation, the IZD values decreased significantly for the concentrations of 25% and 50% but remained the same for the concentrations of 100% and 75%; on the 7th day of incubation, the IZD values for the concentrations of 25% and 50% were statistically significantly lower compared to the concentrations of 75% and 100%. Concerning the antifungal activity against Candida albicans, differences were only observed on the 3rd day of incubation when the IZD values for the concentrations of 25% and 50% were statistically significantly lower compared to those for the concentrations of 75% and 100%. The antifungal activity against Candida parapsilosis was similar, regardless of the concentration, at all the incubation time points. The one exception was that the IZD values for the concentration of 50% were statistically significantly lower compared to those for the concentration of 100%. The Lavandula pedunculata EO presented the highest antifungal activity against Scedosporium boydii, regardless of the concentration, on the 3rd day of incubation; on the 5th day of incubation, the IZD values for the concentration of 75% were statistically significantly higher compared to those for the concentration of 50%, as well as between the values for the concentrations 25% and 50%, while the IZD values for the concentration of 100% remained the highest; on the 7th day of incubation, the IZD values for the concentration of 100% were statistically significantly higher compared to those for the concentrations of 75%, 50%, and 25%.
We also evaluated the antifungal activity of the Cymbopogon citratus EO and Lavandula pedunculata EO over time (Figure S3A,C). We found no statistically significant differences in the antifungal activity of the Cymbopogon citratus EO against Aspergillus fumigatus, Candida albicans, and Scedosporium boydii during the incubation time at any concentration tested. Concerning Candida parapsilosis, the IZD values significantly increased from the 3rd to the 7th day for the concentrations of 25%, 50%, and 75%, and from the 5th to the 7th day for the concentration of 75%. Conversely, the antifungal activity of the Lavandula pedunculata EO against Aspergillus fumigatus, Candida albicans, and Scedosporium boydii decreased with time. Regarding Aspergillus fumigatus, a statistically significant decrease was observed from the 3rd to the 5th day when using concentrations of 25% and 50%, while for the concentrations of 75% and 100%, a statistically significant decrease was observed from the 5th to the 7th day. In the case of Scedosporium boydii, the IZD values statistically significantly decreased from the 3rd to the 5th day when using concentrations of 25% and 50%, and from the 5th to the 7th day when using a concentration of 100%; for the concentration of 75%, the IZD values significantly decreased during the study. Concerning Candida albicans, there was a statistically significant decrease from the 3rd to the 5th day when using the concentration of 100%. No statistically significant differences in the antifungal activity of the Lavandula pedunculata EO against Candida parapsilosis throughout the experiment were observed.
4. Discussion
FK is characterized by the infection of the cornea by fungal species. It was a condition with a high incidence in agrarian communities, which was attributed to occupational exposure to vegetative matter. Nowadays, the widespread use of contact lenses, corticosteroids, antibiotics, immunosuppressants, and chemotherapeutic drugs has increased the incidence of FK [4,5]. The WHO has recently identified fungal infections as one of the major threats to human health, namely due to the lack of efficacy of current antifungal agents [29]. There is a pressing need to focus research on strengthening the global response to fungal infections. The complexity of FK etiology, which involves a wide range of fungal pathogens with diverse susceptibilities and resistance profiles, underscores the necessity for broad-spectrum and effective treatment strategies. Current therapeutic strategies, which are predominantly reliant on antifungal medications, are hampered by issues such as drug resistance, limited ocular penetration, and toxicity, which collectively compromise their effectiveness.
Herein, we explored the in vitro antifungal activity of an Cymbopogon citratus EO and Lavandula pedunculata EO, which were derived from well-known aromatic and medicinal plants, against fungal pathogens obtained from corneal samples collected from FK patients in two Portuguese university hospitals and are representative of the current scenario of FK. Since FK is primarily caused by Aspergillus spp., Candida spp., and Fusarium spp., we selected Aspergillus fumigatus, Candida albicans, and Candida parapsilosis for our study. As for Scedosporium boydii, it was selected because it has now been recognized as an important emerging FK pathogen [30,31].
This study aimed to be the first step in the validation of these EOs as an alternative treatment for FK. The selection of Cymbopogon citratus EO and Lavandula pedunculata EO was based on their known bioactive compounds, which exhibit broad-spectrum antimicrobial efficacy, including antifungal activities. A previous chemical characterization of Cymbopogon citratus EO and Lavandula pedunculata EO [25] showed the presence of compounds with proven antifungal properties, providing a scientific basis for testing their efficacy against FK pathogens. Here, the antifungal assays demonstrated significant inhibitory activity of the Cymbopogon citratus EO and Lavandula pedunculata EO against Aspergillus fumigatus, Candida albicans, Candida parapsilosis, and Scedosporium boydii, key fungal species associated with FK, suggesting that these EOs may be effective therapeutic agents for FK. Considering the antifungal assay results for the Cymbopogon citratus EO and Lavandula pedunculata EO, it is imperative to further explore the implications of these findings for the development of antifungal treatments for FK. The efficacy of the Cymbopogon citratus EO and Lavandula pedunculata EO—particularly against filamentous fungi, which are often more challenging to treat due to their complex structures and resistance mechanisms—highlights their potential as a key agent in FK therapy. Regarding their apparently lower efficacy against the tested yeasts, more studies are needed, namely using other methodologies like the standardized dilution method and the use of more yeast species. We hypothesize that the observed decrease in the antifungal activity of the Lavandula pedunculata EO over time may be attributable to the relatively higher volatility or shorter persistence of its bioactive compounds in the culture medium. A faster adaptation of fungi to these compounds may also have occurred.
Importantly, our findings draw attention to the potential mechanisms through which EOs exert their antifungal effects. The multi-targeted action of EOs—including disrupting fungal cell membranes, inhibition of spore germination, and interference with fungal metabolism—offers a notable advantage over conventional antifungals, which typically target a single cellular pathway and thus, fungi can more easily develop resistance. Given that EOs are a complex mixture of bioactive compounds, it is essential to consider their inherent volatility. This characteristic suggests that part of the observed antifungal activity may be due to the volatile fraction of the EOs, rather than solely their diffusion through the culture medium. Consequently, the activity of EOs may not be confined to direct application of the solutions but can also encompass airborne antifungal and fungistatic effects. Recently, the co-authors of this study showed that the volatilized fraction of Cymbopogon citratus and Lavandula angustifolia EOs strongly inhibited fungal growth, fully suppressing several fungi contaminating museum artifacts, even at low concentrations [27]. Future research will involve the comprehensive analytical separation and characterization of the EO constituents to identify the specific bioactive compounds responsible for their antifungal activity. Such an approach would allow for a detailed comparison between effects mediated by direct diffusion through the culture medium and those arising from the volatile fraction of the EOs. By integrating advanced fractionation methods with mass spectrometry and chromatography analyses, researchers could identify specific compounds that contribute to the observed biological activities and differentiate between the contact-dependent and volatile-mediated antifungal actions of the EOs.
In addition to further elucidating the mechanisms underlying the antifungal effects of Cymbopogon citratus EO and Lavandula pedunculata EO, future research should also focus on optimizing formulations for enhanced ocular delivery and retention, as well as evaluating their safety and efficacy in vivo. Moreover, the potential for synergistic interactions with conventional antifungal agents warrants investigation, as such combinations may improve therapeutic efficacy, reduce drug resistance, and broaden the treatment options available for FK. The translation of these findings into clinical practice, however, requires a cautious approach considering the safety and pharmacokinetic profiles of the EOs. The potential for toxicity, irritation, or allergic reactions, especially in the sensitive ocular environment, is a critical consideration. Therefore, future studies should include detailed toxicological evaluations, formulation development to improve ocular delivery and retention, and ultimately, clinical trials to establish efficacy, safety, and dosing guidelines for EO-based treatments for FK.
Importantly, our study highlights the value of biodiversity, as these medicinal plants constitute an invaluable reservoir of bioactive compounds with potential therapeutic applications. This study underscores the need for a multidisciplinary approach integrating ethnobotany, phytochemistry, microbiology, pharmacology, and medicine to fully explore the potential of natural products in addressing contemporary health challenges.
The implications of these findings extend beyond the direct treatment of FK. The demonstrated antifungal activity of Cymbopogon citratus EO and Lavandula pedunculata EO reinforces the value of natural products in pharmaceutical development, providing a rich source of bioactive compounds for drug discovery. Moreover, the exploration of EOs in antifungal therapy highlights the value of integrating traditional knowledge with modern scientific methodologies to unlock novel therapeutic potentials. This approach is particularly pertinent in the face of escalating antimicrobial resistance, which poses a global health threat.
5. Conclusions
The focus on EOs is not only innovative but also timely considering the urgent need for novel treatments for FK that can overcome the limitations of the existing strategies. The in vitro antifungal assays showed the efficacy of Cymbopogon citratus EO against selected FK pathogens, particularly molds, demonstrating its viability as an effective antifungal agent. Concerning Lavandula pedunculata EO, while it exhibited a significant initial antifungal effect, its efficacy decreased over time, indicating a need for further optimization to explore its full therapeutic potential. This study highlights the potential of these natural products to address the limitations of current FK therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/futurepharmacol5040073/s1, Figure S1: Antifungal activity of Cymbopogon citratus EO and Lavandula pedunculata EO against selected FK pathogens, after 3, 5, and 7 days of incubation. Representative images of solid-phase disk diffusion in vitro assay showing the mycelial growth positive (C+) and negative (C−) controls (paper disks infused with pure DMSO without EOs and without DMSO or EOs, respectively); Figure S2. Comparison of the antifungal activity of Cymbopogon citratus EO and Lavandula pedunculata EO against Aspergillus fumigatus, Candida albicans, Candida parapsilosis, and Scedosporium boydii. EOs were used at concentrations of 25%, 50%, 75%, and 100%, during 3, 5 and 7 days of incubation. A. Inhibition zone diameter (in mm) values are presented as mean ± SD (n = 3). B. Statistical analysis was performed to evaluate the differences between fungal pathogens using one-way ANOVA followed by Tukey post hoc test at p < 0.05. AF, Aspergillus fumigatus versus Candida albicans versus Candida parapsilosis versus Scedosporium boydii: ns = no statistically significant difference; * p < 0.05; ** p < 0.01; *** p < 0.001. AF, Aspergillus fumigatus; CA, Candida albicans; CP, Candida parapsilosis; SB, Scedosporium boydii; Conc., concentration in %. Figure S3. Comparison of the antifungal activity of Cymbopogon citratus EO and Lavandula pedunculata EO at concentrations of 25%, 50%, 75%, and 100%, against Aspergillus fumigatus, Candida albicans, Candida parapsilosis, and Scedosporium boydii. during 3, 5, and 7 days of incubation. A. Inhibition zone diameter (in mm) values are presented as mean ± SD (n = 3). B. Statistical analysis was performed to evaluate the differences between essential oils concentrations using one-way ANOVA followed by Tukey post hoc test at p < 0.05. AF, 25% versus 50% versus 75% versus 100%: ns = no statistically significant difference; * p < 0.05; ** p < 0.01; *** p < 0.001. AF, Aspergillus fumigatus; CA, Candida albicans; CP, Candida parapsilosis; SB, Scedosporium boydii. C. Statistical analysis was performed to evaluate the differences between fungal pathogens using repeated measures ANOVA with a Greenhouse-Geisser correction followed by post hoc Bonferroni analysis at p < 0.05. Day of incubation 3 versus day of incubation 5 versus day of incubation 7: ns = no statistically significant difference; * p < 0.05; ** p < 0.01; *** p < 0.001. Conc., concentration in %. Table S1. The sequencing primers used in this study for the identification of fungal pathogens. Table S2. Details on the demographic and clinical characteristics of the patients. Table S3. Mean and standard deviation (SD) values of the inhibition zone diameter (IZD), in mm, obtained for each experimental condition, performed in triplicated (n = 3).
Author Contributions
Conceptualization, N.M., C.C., A.M.R. and E.J.C.; Methodology, N.M., C.C., A.M.R. and E.J.C.; Formal Analysis, E.A., N.M., C.C., E.P., L.F., A.d.C., R.T., D.P., J.P.-C., A.M.R. and E.J.C.; Investigation, E.A., E.P., L.F., A.d.C., R.T., D.P. and J.P.-C.; Resources, N.M.; Writing—Original Draft Preparation, E.A.; Writing—Review and Editing, N.M., C.C., A.d.C., D.P., J.P.-C., A.M.R. and E.J.C.; Supervision, N.M., A.M.R. and E.J.C.; Project Administration, A.M.R. and E.J.C.; Funding Acquisition, A.M.R. and E.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the Portuguese Society of Ophthalmology (SPO) through a 2022Clinical Research Support Grant. EJC is financially supported by Foundation for Science and Technology through the Institutional Scientific Employment program, 2nd edition (CEECINST/00038/2021/CP2781/CT005).
Informed Consent Statement
All procedures were conducted following the tenets of the Declaration of Helsinki. The study was approved by Ethics Committee of the Faculty of Medicine of the University of Coimbra, Portugal (approval No. CE-128/2023; approval date: 23 November 2023). Informed consent for participation was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
During the revision of this manuscript, the authors used a free online grammar-checking tool, and enhancements to fluency and clarity were made with the assistance of an AI language model (ChatGPT 5.1). The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Abbreviations
BA: blood agar; BLAST: NCBI Basic Local Alignment Search Tool; CA: chocolate agar; DMSO: dimethyl sulfoxide; EOs: essential oils; FK: fungal keratitis; GC/MS: gas chromatography–mass spectrometry; ITS: internal transcribed spacer; IZD: inhibition zone diameter; PK: keratoplasty; PCR: polymerase chain reaction; PDA: Potato Dextrose Agar; SD: standard deviation; SDA: Sabouraud dextrose agar.
References
- Trovato, L.; Marino, A.; Pizzo, G.; Oliveri, S. Case Report: Molecular Diagnosis of Fungal Keratitis Associated with Contact Lenses Caused by Fusarium solani. Front. Med. 2021, 8, 579516. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef]
- Silva, B.T. Agreement Between In Vitro Fungal Drugs Sensitivity Tests and Clinical Response to Treatment in Fungal Keratitis. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2020. [Google Scholar]
- Cunha, A.M.; Loja, J.T.; Torrão, L.; Moreira, R.; Pinheiro, D.; Falcão-Reis, F.; Pinheiro-Costa, J. A 10-Year Retrospective Clinical Analysis of Fungal Keratitis in a Portuguese Tertiary Centre. Clin. Ophthalmol. 2020, 14, 3833–3839. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Galal, M.; Kulkarni, B.; Elalfy, M.S.; Lake, D.; Hamada, S.; Said, D.G.; Dua, H.S. Clinical Characteristics and Outcomes of Fungal Keratitis in the United Kingdom 2011-2020: A 10-Year Study. J. Fungi 2021, 7, 966. [Google Scholar] [CrossRef]
- Sharma, N.; Bagga, B.; Singhal, D.; Nagpal, R.; Kate, A.; Saluja, G.; Maharana, P.K. Fungal keratitis: A review of clinical presentations, treatment strategies and outcomes. Ocul. Surf. 2022, 24, 22–30. [Google Scholar] [CrossRef]
- Cai, Y.; Song, S.; Chen, Y.; Xu, X.; Zou, W. Oral voriconazole monotherapy for fungal keratitis: Efficacy, safety, and factors associated with outcomes. Front. Med. 2023, 10, 1174264. [Google Scholar] [CrossRef]
- Awad, R.; Ghaith, A.A.; Awad, K.; Mamdouh Saad, M.; Elmassry, A.A. Fungal Keratitis: Diagnosis, Management, and Recent Advances. Clin. Ophthalmol. 2024, 18, 85–106. [Google Scholar] [CrossRef]
- Pezantes-Orellana, C.; German Bermúdez, F.; Matías De la Cruz, C.; Montalvo, J.L.; Orellana-Manzano, A. Essential oils: A systematic review on revolutionizing health, nutrition, and omics for optimal well-being. Front. Med. 2024, 11, 1337785. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011, 126, 228–235. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.F.; Thakur, K.; Hu, F.; Li, X.L.; Zhang, Y.S.; Zhang, J.G.; Wei, Z.J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Cortés-Zavaleta, O.; López-Malo, A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021, 3, 44. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Rolta, R.; Dev, K.; Sourirajan, A. Synergistic potential of essential oils with antibiotics to combat fungal pathogens: Present status and future perspectives. Phytother. Res. 2021, 35, 6089–6100. [Google Scholar] [CrossRef]
- Sahal, G.; Woerdenbag, H.J.; Hinrichs, W.L.J.; Visser, A.; Tepper, P.G.; Quax, W.J.; van der Mei, H.C.; Bilkay, I.S. Antifungal and biofilm inhibitory effect of Cymbopogon citratus (lemongrass) essential oil on biofilm forming by Candida tropicalis isolates; an in vitro study. J. Ethnopharmacol. 2020, 246, 112188. [Google Scholar] [CrossRef]
- Zhang, Z.; Mo, Z.; Zhang, X.; Wang, J.; Li, J.; Shi, H.; Wang, P.; Lin, Z. The Antifungal Activity and Action Mechanism of Lemongrass (Cymbopogon flexuosus) Essential Oil Against Fusarium avenaceum. J. Essent. Oil Bear. Plants 2022, 25, 536–547. [Google Scholar]
- Chroho, M.; El Karkouri, J.; Hadi, N.; Elmoumen, B.; Zair, T.; Bouissane, L. Chemical composition, Antibacterial and Antioxidant Activities of the Essential Oil of Lavandula Pedunculata from Khenifra Morocco. IOP Conf. Ser. Earth Environ. Sci. 2022, 1090, 012022. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Dinis, A.M.; Canhoto, J.M.; Salgueiro, L.R. Chemical composition and antifungal activity of the essential oils of Lavandula pedunculata (Miller) Cav. Chem. Biodivers. 2009, 6, 1283–1292. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants: From Farm to Pharmacy; Joshee, N., Dhekney, S.A., Parajuli, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar]
- Homa, M.; Fekete, I.P.; Böszörményi, A.; Singh, Y.R.; Selvam, K.P.; Shobana, C.S.; Manikandan, P.; Kredics, L.; Vágvölgyi, C.; Galgóczy, L. Antifungal Effect of Essential Oils against Fusarium Keratitis Isolates. Planta Medica 2015, 81, 1277–1284. [Google Scholar] [CrossRef]
- Manganyi, M.C.; Regnier, T.; Olivier, E.I. Antimicrobial activities of selected essential oils against Fusarium oxysporum isolates and their biofilms. S. Afr. J. Bot. 2015, 99, 115–121. [Google Scholar] [CrossRef]
- Paiva, D.S.; Trovão, J.; Fernandes, L.; Mesquita, N.; Tiago, I.; Portugal, A. Expanding the Microcolonial Black Fungi Aeminiaceae Family: Saxispiralis lemnorum gen. et sp. nov. (Mycosphaerellales), Isolated from Deteriorated Limestone in the Lemos Pantheon, Portugal. J. Fungi 2023, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Consejo de Europa. European Directorate for the Quality of Medicines & HealthCare. In The European Pharmacopoeia (Ph. Eur.), 9th ed.; Consejo de Europa: Strasbourg, France, 2016. [Google Scholar]
- Marques, M.P.; Neves, B.G.; Varela, C.; Zuzarte, M.; Gonçalves, A.C.; Dias, M.I.; Amaral, J.S.; Barros, L.; Magalhães, M.; Cabral, C. Essential Oils from Côa Valley Lamiaceae Species: Cytotoxicity and Antiproliferative Effect on Glioblastoma Cells. Pharmaceutics 2023, 15, 341. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, e00069-19. [Google Scholar] [CrossRef]
- Fernandes, L.; Paiva, D.S.; Pereira, E.; Rufino, A.C.; Landim, E.; Marques, M.P.; Cabral, C.; Portugal, A.; Mesquita, N. Evaluating the Antifungal Activity of Volatilized Essential Oils on Fungi Contaminating Artifacts from a Museum Collection. Appl. Sci. 2025, 15, 2378. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, version 26.0; IBM Corp: Armonk, NY, USA, 2019.
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 29 September 2025).
- Turner, M.L.; Nguyen, M.; Schallhorn, J.; Seitzman, G.D. Ocular scedosporiosis: A case series. Am. J. Ophthalmol. Case Rep. 2024, 36, 102190. [Google Scholar] [CrossRef] [PubMed]
- Fathima, L.; Annapurneswari, L.; Rao, P.; Mendonca, T. Scedosporium-induced keratitis: Insights from a case study. J. Ophthalmic Inflamm. Infect. 2025, 15, 72. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).