Abstract
Multiple myeloma (MM) is one of the most common hematological malignancies. There is a clear need for research into new treatment options that can improve the life expectancy and quality of life for MM patients; this is particularly salient for those with relapsed/refractory disease. Cannabinoids (CB) have shown potential in treatment regimens for a number of cancers, but little is currently known about their effectiveness against MM. Hence, we conducted a scoping review regarding the usage of CB against MM cells. For our review, searches were conducted in PubMed, Web of Science, and OVID Medline. After screening, six articles were eligible for inclusion, all of which were laboratory studies. It was demonstrated that CB decrease MM cell viability, and this was consistently shown to occur alongside the activation of apoptotic pathways in MM cells. These effects were shown to continue to occur in dexamethasone-resistant MM cells. The effects of CB on MM cells were enhanced when used in combination with standard treatments for MM. Critically, these marked decreases in MM cell viability induced by CB did not occur in non-MM cells. Overall, these findings indicate a clear need for future clinical trials of the integration of CB into MM treatment regimens.
1. Introduction
Multiple myeloma (MM) is a hematological malignancy of B cells occurring when genetically mutated plasma cells fail to undergo cell apoptosis and accumulate in the bone marrow [1]. Generally, a diagnosis of MM consists of two components: the clinical presentation of anemia, hypercalcemia, renal disorder, and bone lesions; and a bone marrow biopsy confirming a plasma cell population of greater than or equal to 10% [2]. In contrast to normal bone remodeling, the coupling mechanism of osteoclasts and osteoblasts is lost in MM. Increased osteoclastic activity, resulting in bone resorption, and suppressed osteoblastic activity, leading to decreased/absent bone formation, are key factors in the development of bone destruction in MM [2].
MM is the second most common hematological malignancy, with nearly 35,000 cases projected to be diagnosed in 2022 across the United States; rates are higher in men, older individuals, and individuals of African descent [3,4,5,6,7,8]. Survival rates among patients with MM have been consistently improving over time since the 1990s. From 2000 to 2008, the five-year survival rate was found to be between 34.5% and 49.6% [9], and increased to 53.9% between 2010 to 2016 [10]. The explanation for this rise in survival rates among MM patients is the increased availability of new treatments for this disease.
Previously, melphalan-based treatment regimens were primarily utilized in MM treatment for roughly forty years. This was until the introduction of thalidomide, lenalidomide (immunomodulatory therapies), bortezomib (proteasome inhibitor), and stem cell transplantation in the 1990s [3,11,12,13,14,15,16,17,18]. While there currently are a number of different treatment regimens, the combination of bortezomib, lenalidomide, and dexamethasone has become the standard of treatment for patients with MM; this combination treatment has demonstrated very high response rates and has been shown to result in patients emerging from treatment with complete response/very good partial response [19,20].
While five-year survival rates have improved in recent years for MM patients, there is nonetheless a very clear need for the development of new treatments. Although MM is treatable, it remains an incurable disease. In cases of relapsed/refractory disease, treatment options remain fairly limited—one emerging form of treatment that has shown emerging potential in very recent times is B cell maturation antigen (BMCA)-targeted approaches, which may utilize options such as antibody–drug conjugates (ADCs), bispecific T cell engagers (BITEs), and chimeric antigen receptor (CAR) T cell therapy [21]. While promising, these forms of treatment have been shown to cost hundreds of thousands of dollars [22,23]. Treatment options also become limited in the case of drug-resistant disease. Furthermore, despite the progress in survival rates for MM, five-year and ten-year mortality rates are still notably higher, particularly in older individuals [24,25]. There is therefore a clear need for newer, cost-effective treatment options for MM.
One such treatment that may offer potential for MM patients is cannabinoids (CB). CB have been shown to have antitumor effects in a number of different cancers, such as colorectal cancer, as well as cancer of the lung, brain, prostate, and breast [26,27]. Notably, it has also been proposed that CB may have a positive health impact for those with cancer cachexia by improving low appetite, which is an issue for some MM patients [28]. CB may also have a role in improving a number of other aspects of quality of life for cancer patients by addressing issues such as anxiety, pain, vomiting, and other related issues [29,30]. Figure 1 summarizes the means by which CB have been proposed to improve quality of life and life expectancy for patients with cancers such as MM.
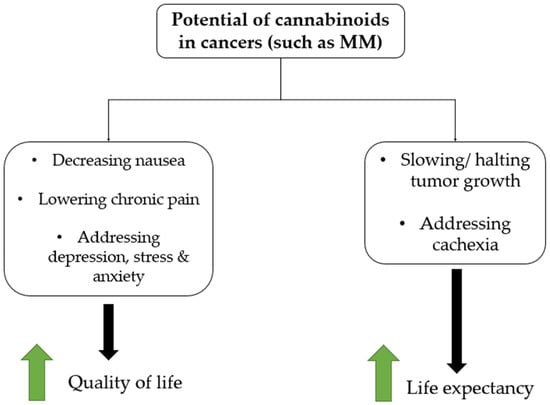
Figure 1.
Hypothesized therapeutic potential of cannabinoids in cancers such as MM.
While the potential of CB is increasingly being understood for a wide range of malignancies, many previous articles and reviews have focused very generally on the therapeutic role of CB in various cancers. For example, three reviews on the role of CB in the treatment of cancer placed minimal focus on any particular cancer and instead focused on a large array of different cancers [29,30,31]; as a result, little is currently known regarding the effects of CB on MM and the therapeutic potential of this compound for MM patients.
The limited understanding of the role of CB in MM treatment from previous reviews has important implications. First of all, the limited understanding of the risks and benefits may lead to clinicians withholding a recommendation of CB for MM patients or potentially making misinformed recommendations based on findings relating to other cancers. Second, it is an imperative to clear up misconceptions for cancers such as MM, as misinformation regarding CB treatment has been shown to be widespread in public platforms [32]. Therefore, in order to provide a clearer understanding of the role of CB in MM, the overall aim of this work is to provide a scoping review of the literature regarding the influence of CB on MM cells and MM patient outcomes.
2. Materials and Methods
This scoping review followed the ‘Preferred Items for Systematic Reviews and Meta-Analysis extension for Scoping Review’ (PRISMA-ScR) guidelines [33,34]. On 23 July 2022, searches were conducted in PubMed, OVID Medline, and Web of Science. Searches included terms for CB and MM, and MeSH terms, or their equivalent, were used. For the searches, no restrictions were placed based on date of publication. Table 1 lists the search terms used for OVID Medline.

Table 1.
Search terms for OVID Medline (conducted on 23 July 2022).
After retrieval from the respective databases, two experienced reviewers (KV and PG) screened articles independently. First, duplicate articles were removed. Next, all papers were screened by title/abstract. Thereafter, the full texts of all remaining articles were screened and analyzed in order to determine eligibility for inclusion in this review. The inclusion criteria were broad in order to include as many relevant articles as possible; articles were included if they met the following criteria:
- Quantitative, original research;
- Was a peer-reviewed, full-text article;
- Written in English;
- Tests the effects of CB treatment/CB receptor upregulation in MM patients/MM cell lines.
For this review, data were extracted regarding the study characteristics, the details of the study methods, and the key findings of the article. More precisely, the following data were extracted: year, country, aim, drug under evaluation, cell types/receptors being studied/influenced, analyses, and main findings. After the extraction of data was completed, relevant data were qualitatively synthesized, with patterns and trends being described.
3. Results
3.1. Searches and Included Articles
The searches produced a total of 58 results. After removal of 30 duplicates, 28 articles remained. Once articles were screened by title/abstract, a total of 9 articles remained for full-text analysis; 6 articles were ultimately eligible for inclusion in the review [35,36,37,38,39,40]. The full workflow for the screening of the articles are shown in Figure 2.
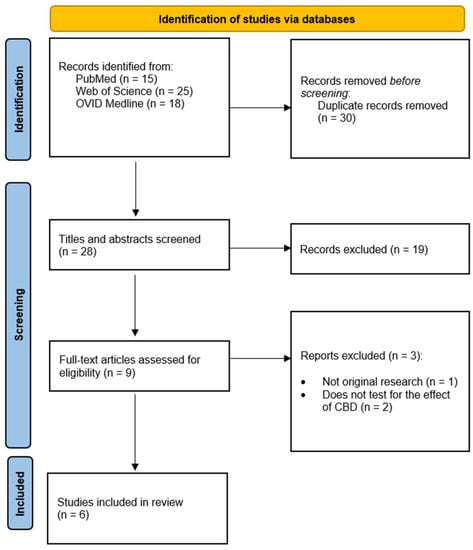
Figure 2.
Process of screening articles based on the PRISMA 2020 flow diagram [34].
3.2. Study Characteristics
The studies included in this review were conducted in the following countries: USA (n = 1), Germany (n = 1), Qatar (n = 1), Spain (n = 1), and Italy (n = 2). Studies were conducted between 2013 and 2021. There were no clinical trials eligible for this review; all included articles were laboratory studies. All six studies had in vitro/ex vivo analysis, although one article included both in vitro and in vivo analysis.
The following drug compounds were used in differing studies: WIN-55, PGN6, PGN17, PGN34, PGN72 [35], phenylacetamide (PAM) [36], pure cannabidiol (CBD) [37,39], B-caryophyllene (BCP) [38], and tetrahydrocannabinol (THC) [40]. Alongside these substances, the following treatments were used in combination with substances containing/activating CB: dexamethasone [35], melaphalan [35], bortezomib (BORT) [39], and carfilzomib (CFZ) [40]. CB2 was the most common receptor analyzed.
Complete study and drug characteristics for all included studies are listed in Table 2.

Table 2.
Study and drug characteristics from included studies.
3.3. Effects of CB on MM cells
The overall effects of CB on MM cells are summarized and depicted in Figure 3.
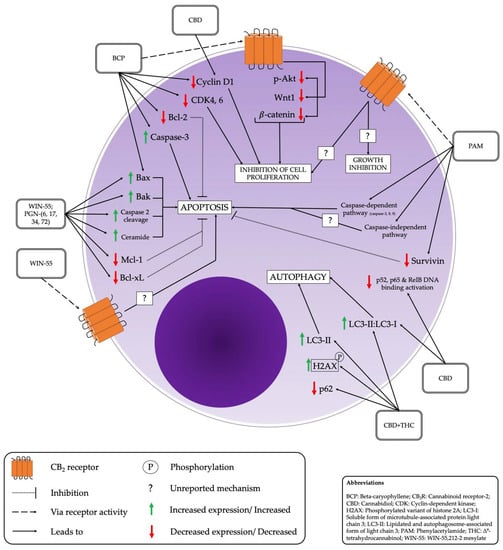
Figure 3.
Effects of cannabinoids on multiple myeloma cells and associated pathways.
3.3.1. Cell Viability
MM cell viability was decreased consistently across all studies after the administration of CB/CB-containing compounds. This effect was demonstrated to occur in a dose-dependent manner. For example, KMS-12 PE cells (MM cells) exposed to CBD at 1–20 μM for 24 h had significant decreases in cell viability compared to controls [37]. Similarly, BCP at 50 μM resulted in a significant reduction in the number of viable MM cells, alongside a significant increase in non-viable MM cells [38]. Notably, in the only in vivo analysis in these studies, CB was shown to result in a major, progressive decrease in tumor volume [35].
3.3.2. Apoptotic Effects/Pathways
Across studies, apoptotic effects were also seen consistently. PGN cannabinoids showed a selective pro-apoptotic effect in MM cells [35], as did CBD and BCP administration respectively [38,40]. A number of different apoptotic pathways were activated, depending on the type of drug utilized. Table 3 lists the apoptotic pathways/effects associated with respective compounds.

Table 3.
Compounds/drugs and corresponding apoptotic pathways/effects.
3.3.3. Combinatory Effects
It was consistently demonstrated that the combinatory effect of CB with other drugs that are part of the standard treatment for MM is stronger than the effect of using either drug alone. For example, WIN-55 in combination with dexamethasone and melphalan showed a stronger anti-MM effect than utilization of any of these substances alone [35]. Similarly, use of BORT and CBD had a synergistic effect in inhibiting MM cells than the use of BORT only or CBD only [39]. THC along with CBD had a stronger effect in combination, and the potency of this anti-MM effect was further amplified when CFZ was used alongside the THC–CBD combination [40].
3.3.4. Effects on Drug-Resistant Cells
Numerous studies demonstrated that the use of CB/activation of CB receptors was capable of overcoming drug-resistance in MM. PAM treatment on cells resistant to dexamethasone and melphalan exhibited effects that were comparable to drug-sensitive MM cells [36]. Treatment of 50 μM BCP was shown to reduce the viability of dexamethasone-resistant MM cells to 80%, and 100 μM treatment to 50% [38]. The combinatory effect of WIN-55 with dexamethasone and melphalan was shown to be capable of overcoming melphalan-resistance [35].
3.4. Effects of CB on Normal Cells
While the effect of CB was consistently shown to reduce the viability of MM cells, CB was shown to have either a minor effect or no detrimental effect on normal (non-MM) cells. While PAM usage did lead to a decrease in MM cell viability, the cytotoxic effects on normal mononuclear cells were minor [36]. PGN cannabinoids showed a pro-apoptotic effect that occurred in MM plasma cells, but this did not occur in normal cells, including hematopoietic stem cells [35]. Furthermore, the usage of BCP affected cancer cell viability, but did not have an effect on normal cells [38].
4. Discussion
Overall, the findings of this review show that there is consistent evidence that CB demonstrate a pro-apoptotic effect that reduces the viability of MM cells. This can occur by an array of different apoptotic pathways/effects, depending on the type of compound. These effects are amplified when used in combination with standard MM treatment drugs, such as dexamethasone, melphalan, and CFZ, and the effects show potential to overcoming MM drug resistance. Critically, these cytotoxic effects are not shown to occur in normal cells.
It must be emphasized that all the articles in this review were laboratory studies; due to the lack of clinical data regarding the usage of CB in MM treatment, clinical recommendations to offer CB cannot be made at this point. Nonetheless, the fact that CB showed an antitumor effect in vivo [35] highlights that these results offer enormous potential. Therefore, these findings demonstrate a clear need for preclinical and clinical trials to be conducted to test the effectiveness and safety of integrating CB in MM treatment regimens. This will be particularly valuable for MM patients with relapsed/refractory or drug-resistant disease who are frequently left with few treatment regimens that are affordable. Furthermore, this will provide far more insights into the other benefits and risks of CB for MM patients relating to appetite and quality of life. As MM patients have been shown to describe financial issues as a serious impact of MM and treatment [41], the low cost of CB offers additional promise for MM patients.
There are likely some ethical concerns regarding the usage of CB in such clinical trials. However, conducting clinical trials for MM patients with the integration of CB can occur in a safe manner. Control groups can be provided with standard treatment regimens, and experimental groups can be provided with the exact same treatment regimens along with the addition of CB in the form of WIN-55, PGN-6, PGN-17, PGN-34, PGN-72, PAM, or BCP. While one such trial was initially proposed back in 2018, recruitment for the trial never occurred [42]. The fact that CB were shown to have synergistic effects with standard MM treatment drugs, and that they have been shown to have minimal/no effects on normal cells, highlights that the integration of CB in trials can potentially be performed in a manner that is safe for patients.
The findings of this review also emphasize a clear need for further study on the tests of CB on MM, as well as for other cancers. The consistency of the effects shown in this review, and in studies on other cancers [26,27], demonstrates the importance of developing a deeper understanding regarding the pathways of biological effects that CB have on tumors. These findings also need to be conducted regarding cancer cachexia, which, to date, has very limited treatment options [43].
It is worthwhile to note that this review may be able to clear up misinformation regarding CB in MM—while some potential has been shown in the included laboratory studies, no definitive statements can be made at this time regarding the safety and effectiveness of the usage of CB in treatment regimens for MM patients. Such recommendations cannot be made until clinical trials are completed, again emphasizing the imperative for such trials to be conducted.
The findings of this review must be considered alongside the limitations. Only six studies were included, which is a relatively low number. It is possible that including more, and larger scale studies, may have revealed differing findings. Furthermore, as already alluded to, there were no clinical studies included. Therefore, clinical guidelines and recommendations cannot be made at this point regarding the effect of CB on MM patients. An additional limitation is that, while all the drugs/compounds included had CB/had effects on CB receptors, there was little consistency in the types of drugs/compounds studied. This was also true for the combinatory drugs. This limits the extent to which results can be generalized. Regardless of these limitations, it is imperative to denote that, across all six studies, CB showed very consistent effects on MM cell viability and consistent cytotoxic effects on MM cells. These findings will be vital in guiding future studies that can potentially lead to changes that can improve the quality of life of MM patients.
5. Conclusions
Our scoping review has shown that CB may be highly effective for MM treatment due to their antitumor effect. While there is a clear need to study these effects in far more detail and for large-scale clinical trials in the future, these findings provide important first steps in potentially improving the management of MM.
Author Contributions
Conceptualization: K.V., P.G., A.P.; methodology: K.V., P.G.; validation: K.V., P.G.; formal analysis: K.V., P.G.; data curation: K.V., P.G.; writing—original draft preparation: K.V., A.P.; writing—review and editing: K.V., P.G., A.P.; visualization: K.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors have no acknowledgements to be made.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, N.; Rizzoli, V.; Roodman, G.D. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood 2006, 108, 3992–3996. [Google Scholar] [CrossRef]
- Richardson, P.G.; Blood, E.; Mitsiades, C.S.; Jagannath, S.; Zeldenrust, S.R.; Alsina, M.; Schlossman, R.L.; Rajkumar, S.V.; Desikan, K.R.; Hideshima, T.; et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood 2006, 108, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Myeloma. CDC.gov. 2022. Available online: https://www.cdc.gov/cancer/myeloma/index.htm (accessed on 1 June 2022).
- American Cancer Society. Key Statistics About Multiple Myeloma. Cancer.org. 2022. Available online: https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html (accessed on 5 June 2022).
- SEER Lifetime Risk (Percent) of Being Diagnosed with Cancer by Site and Race/Ethnicity: Both Sexes, 18 SEER Areas, 2012–2014 (Table 1.15) National Cancer Institute: Bethesda, MD, USA. Available online: https://seer.cancer.gov/csr/1975_2014/results_merged/topic_lifetime_risk.pdf (accessed on 5 January 2018).
- Landgren, O.; Gridley, G.; Turesson, I.; Caporaso, N.E.; Goldin, L.R.; Baris, D.; Fears, T.R.; Hoover, R.N.; Linetl, M.S. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood 2006, 107, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Graubard, B.I.; Katzmann, J.A.; Kyle, R.A.; Ahmadizadeh, I.; Clark, R.; Kumar, S.K.; Dispenzieri, A.; Greenberg, A.; Therneau, T.M.; et al. Racial disparities in the prevalence of monoclonal gammopathies: A population-based study of 12,482 persons from the National Health and Nutritional Examination Survey. Leukemia 2014, 28, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI). SEER Cancer Statistics Review, 1975–2013; National Cancer Institute: Bethesda, MD, USA, 2016. Available online: http://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 15 June 2022).
- Multiple Myeloma Research Foundation. TheMMRF. Understanding survival statistics. N.d. Available online: https://themmrf.org/multiple-myeloma/prognosis/understanding-survival-statistics/ (accessed on 20 June 2022).
- Gregory, W.M.; Richards, M.; Malpas, J.S. Combination chemotherapy versus melphalan and prednisolone in the treatment of multiple myeloma: An overview of published trials. J. Clin. Oncol. 1992, 10, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Mehta, J.; Desikan, R.; Ayers, D.; Roberson, P.; Eddlemon, P.; Munshi, N.; Anaissie, E.; Wilson, C.; Dhodapkar, M.; et al. Antitumor Activity of Thalidomide in Refractory Multiple Myeloma. N. Engl. J. Med. 1999, 341, 1565–1571. [Google Scholar] [CrossRef]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Irwin, D.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.-L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2005, 352, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Hayman, S.R.; Lacy, M.Q.; Dispenzieri, A.; Geyer, S.M.; Kabat, B.; Zeldenrust, S.R.; Kumar, S.; Greipp, P.R.; Fonseca, R.; et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood 2005, 106, 4050–4053. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Jacobus, S.; Callander, N.S.; Fonseca, R.; Vesole, D.H.; Williams, M.; Abonour, R.; Siegel, D.S.; Katz, M.; Greipp, P.R. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010, 11, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Cavo, M.; Perrone, G.; Buttignol, S.; Calabrese, E.; Galli, M.; Bringhen, S.; Spadano, T.; Baldini, L.; Caravita, T.; Nozzoli, C.; et al. Bortezomib-thalidomide-dexamethasone compared with thalidomide-dexamethasone as induction and consolidation therapy before and after double autologous transplantation in newly diagnosed multiple myeloma: Results from a randomized phase 3 study. Blood 2010, 116, a42. [Google Scholar] [CrossRef]
- Kotla, V.; Goel, S.; Nischal, S.; Heuck, C.; Vivek, K.; Das, B.; Verma, A. Mechanism of action of lenalidomide in hematological malignancies. J. Hematol. Oncol. 2009, 2, 36. [Google Scholar] [CrossRef]
- Kane, R.C.; Bross, P.F.; Farrell, A.T.; Pazdur, R. Velcade®: U.S. FDA Approval for the Treatment of Multiple Myeloma Progressing on Prior Therapy. Oncologist 2003, 8, 508–513. [Google Scholar] [CrossRef]
- Richardson, P.G.; Weller, E.; Lonial, S.; Jakubowiak, A.J.; Jagannath, S.; Raje, N.S.; Avigan, D.E.; Xie, W.; Ghobrial, I.M.; Schlossman, R.L.; et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010, 116, 679–686. [Google Scholar] [CrossRef]
- Kumar, S.; Flinn, I.W.; Richardson, P.G.; Hari, P.; Callander, N.S.; Noga, S.J.; Stewart, A.K.; Glass, J.; Rifkin, R.M.; Wolf, J.L.; et al. Novel Three- and Four-Drug Combination Regimens of Bortezomib, Dexamethasone, Cyclophosphamide, and Lenalidomide, for Previously Untreated Multiple Myeloma: Results From the Multi-Center, Randomized, Phase 2 EVOLUTION Study. Blood 2010, 116, 621. [Google Scholar] [CrossRef]
- Kleber, M.; Ntanasis-Stathopoulos, I.; Terpos, E. BCMA in Multiple Myeloma—A Promising Key to Therapy. J. Clin. Med. 2021, 10, 4088. [Google Scholar] [CrossRef]
- Alhallak, K.; Sun, J.; Jeske, A.; Park, C.; Yavner, J.; Bash, H.; Lubben, B.; Adebayo, O.; Khaskiah, A.; Azab, A. Bispecific T Cell Engagers for the Treatment of Multiple Myeloma: Achievements and Challenges. Cancers 2021, 13, 2853. [Google Scholar] [CrossRef] [PubMed]
- ASH Clinical News. CAR T-Cell Therapies Predicted to Cost More Than $1 Million Per Patient. N.d. Ash Publications. Available online: https://ashpublications.org/ashclinicalnews/news/3469/CAR-T-Cell-Therapies-Predicted-to-Cost-More-Than-1 (accessed on 5 June 2022).
- Thorsteinsdottir, S.; Dickman, P.W.; Landgren, O.; Blimark, C.; Hultcrantz, M.; Turesson, I.; Björkholm, M.; Kristinsson, S.Y. Dramatically improved survival in multiple myeloma patients in the recent decade: Results from a Swedish population-based study. Haematologica 2018, 103, e412–e415. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Försti, A.; Hansson, M. Incidence, mortality and survival in multiple myeloma compared to other hematopoietic neoplasms in Sweden up to year 2016. Sci. Rep. 2021, 11, 17272. [Google Scholar] [CrossRef]
- Heider, C.G.; Itenberg, S.A.; Rao, J.; Ma, H.; Wu, X. Mechanisms of Cannabidiol (CBD) in Cancer Treatment: A Review. Biology 2022, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Likar, R.; Köstenberger, M.; Stultschnig, M.; Nahler, G. Concomitant Treatment of Malignant Brain Tumours With CBD–A Case Series and Review of the Literature. Anticancer Res. 2019, 39, 5797–5801. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Tong, M.; Pan, H.; Li, D. New Prospect for Cancer Cachexia: Medical Cannabinoid. J. Cancer 2019, 10, 716–720. [Google Scholar] [CrossRef]
- O’Brien, K. Cannabidiol (CBD) in Cancer Management. Cancers 2022, 14, 885. [Google Scholar] [CrossRef]
- Griffiths, C.; Aikins, J.; Warshal, D.; Ostrovsky, O. Can Cannabidiol Affect the Efficacy of Chemotherapy and Epigenetic Treatments in Cancer? Biomolecules 2021, 11, 766. [Google Scholar] [CrossRef]
- Hosami, F.; Ghadimkhah, M.H.; Salimi, V.; Ghorbanhosseini, S.S.; Tavakoli-Yaraki, M. The strengths and limits of cannabinoids and their receptors in cancer: Insights into the role of tumorigenesis-underlying mechanisms and therapeutic aspects. Biomed. Pharmacother. 2021, 144, 112279. [Google Scholar] [CrossRef]
- Zenone, M.; Snyder, J.; Caulfield, T. Crowdfunding Cannabidiol (CBD) for Cancer: Hype and Misinformation on GoFundMe. Am. J. Public Health 2020, 110, S294–S299. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Barbado, M.V.; Medrano, M.; Caballero-Velázquez, T.; Álvarez-Laderas, I.; Sánchez-Abarca, L.I.; García-Guerrero, E.; Martín-Sánchez, J.; Rosado, I.V.; Piruat, J.I.; Gonzalez-Naranjo, P.; et al. Cannabinoid derivatives exert a potent anti-myeloma activity both in vitro and in vivo. Int. J. Cancer 2017, 140, 674–685. [Google Scholar] [CrossRef]
- Feng, R.; Tong, Q.; Xie, Z.; Cheng, H.; Wang, L.; Lentzsch, S.; Roodman, G.D.; Xie, X.-Q. Targeting cannabinoid receptor-2 pathway by phenylacetylamide suppresses the proliferation of human myeloma cells through mitotic dysregulation and cytoskeleton disruption. Mol. Carcinog. 2015, 54, 1796–1806. [Google Scholar] [CrossRef] [Green Version]
- Garofano, F.; Schmidt-Wolf, I.G.H. High Expression of Cannabinoid Receptor 2 on Cytokine-Induced Killer Cells and Multiple Myeloma Cells. Int. J. Mol. Sci. 2020, 21, 3800. [Google Scholar] [CrossRef]
- Mannino, F.; Pallio, G.; Corsaro, R.; Minutoli, L.; Altavilla, D.; Vermiglio, G.; Allegra, A.; Eid, A.H.; Bitto, A.; Squadrito, F.; et al. Beta-Caryophyllene Exhibits Anti-Proliferative Effects through Apoptosis Induction and Cell Cycle Modulation in Multiple Myeloma Cells. Cancers 2021, 13, 5741. [Google Scholar] [CrossRef]
- Morelli, M.B.; Offidani, M.; Alesiani, F.; Discepoli, G.; Liberati, S.; Olivieri, A.; Santoni, M.; Santoni, G.; Leoni, P.; Nabissi, M. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int. J. Cancer 2014, 134, 2534–2546. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Offidani, M.; Amantini, C.; Gentili, S.; Soriani, A.; Cardinali, C.; Leoni, P.; Santoni, G. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016, 7, 77543. [Google Scholar] [CrossRef]
- Goodwin, J.A.; Coleman, E.A.; Sullivan, E.; Easley, R.; McNatt, P.K.; Chowdhury, N.; Stewart, C.B. Personal Financial Effects of Multiple Myeloma and Its Treatment. Cancer Nurs. 2013, 36, 301–308. [Google Scholar] [CrossRef]
- Katta, S.F. A Study of the Efficacy of Cannabidiol in Patients With Multiple Myeloma, Glioblastoma Multiforme, and GI Malignancies. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03607643 (accessed on 4 June 2022).
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).