Abstract
Human pluripotent stem cells (hPSCs) have become a powerful tool to generate the various kinds of cell types comprising the human body. Recently, organoid technology has emerged as a platform to generate a physiologically relevant tissue-like structure from PSCs. Compared to an actual human organ, this structure more closely represents a three-dimensional microenvironment than the conventional monolayer culture system for transplantation, disease modeling, and drug development. Despite its advantages, however, the organoid culture system still has various problems related to culture methods, which have become a challenge for attempts to obtain similar physiological properties to their original tissue counterparts. Here, we discuss the current development of organoid culture methods, including the problems that may arise from the currently available culture systems, as well as a possible approach for overcoming their current limitations and improving their optimum utilization for translational application purposes.
1. Introduction
Animal models are a routinely used platform for understanding many biological processes related to human disease. Although they can partially represent human physiological and pathological conditions, these models cannot accurately provide similar features for human tissue physiology and disease mechanisms due to the interspecies genomic divergence which impacts various complex cellular activities [1,2]. In actual translational applications, this discrepancy can reduce accuracy, which potentially contributes to a low success rate in their utilization (for example, clinical trials of newly developed drugs) [3]. On the other hand, commonly used two-dimensional human cell cultures often do not completely represent actual human tissue function and architecture [4].
Organoids are three-dimensional miniature organ-like structures that recapitulate physiologically relevant tissue function through self-organization in vitro. The generation of a human pluripotent stem cells (hPSCs) derived organoid can facilitate the improvement of tissue culture by addressing current limitations in disease modeling or drug screening platforms, as well as providing new insights into their application in regenerative therapy [3].
The development of human-induced pluripotent stem cells (hiPSCs) allows for greater experimental accessibility with fewer ethical constraints than the usage of human embryonic stem cells (hESCs), which require a sacrificial human embryo. Moreover, PSCs can be generated either from a healthy or diseased individual. This can be useful for personalized medicine applications, such as disease modeling [5], drug screening [6], or even regenerative therapy [7].
Currently, hPSCs-derived organoid production mostly involves a complex multistep protocol during their differentiation and maturation process, which makes the production of organoids technically challenging. Various methods have been developed to produce organoids from PSCs. However, some important factors related to the organoid culture system need to be considered to achieve the optimum culture conditions for each specific translational purpose. In this review, we highlight the currently available methodology for generating organoids from hPSCs as well as the current challenges involved in and possible alternatives available for improving the generation of hPSCs-derived organoids.
2. The Main Features of hPSCs Derived-Organoids
Organoids have become a promising research tool for elucidating the mechanism of various diseases and for testing the effect of substance exposure, which provides a precise preclinical setting for research [8]. Recently, the concept of organoid transplantation has emerged as a potential method for obtaining insight into alternative partial tissue replacement for regeneration [9]. Nevertheless, possible improvements to the current culture methodologies still need to be explored to achieve better overall reliability for translational applications.
At present, numerous studies of hPSCs-derived organoids have succeeded in representing a broad spectrum of specific organ types for different applications (Table 1). A 3D organoid can enable crosstalk between different cells inside its structure, something which cannot be achieved by a conventional monolayer culture. Therefore, these 3D “miniaturized organs” possess decent potential to become physiologically relevant models and better predictive tools than monolayer cultures, which reflect the cellular interactions between different cells within the organ during their development, healthy or diseased conditions.

Table 1.
Selected study of hPSCs derived organoids and their targeted applications.
Generally, an organoid needs to meet some criteria which confirm the authenticity of the original organ [10]. First, the organoid should be a three-dimensional (3D) structure consisting of multiple cell types that retain the identity of the specific organ. Secondly, this 3D structure should be formed by self-organization according to the similar intrinsic organization principles of the organ. Thirdly, the organoid should recapitulate the key features that represent the functional capability of the actual organ (both in terms of structure and physiological activity) [11,12].
3. Culture Strategy to Generate hPSCs Derived-Organoids
Currently, several approaches have been developed to generate a miniaturized tissue-like organoid comprising the complex functional cellular components derived from hPSCs. These methods mostly involve a differentiation process via a stepwise treatment using specific combined factors to direct the hPSC cell fates.
This step can be improved by creating or adjusting the in vitro culture condition to stimulate subsequent organ-specific lineage differentiation using different available culture platforms. Depending on the particular application and expected organoid type, several factors need to be considered when choosing a culture strategy to obtain the best hPSCs derived-organoid production outcomes (Table 2).

Table 2.
A brief comparison of the common culture platform for organoid production.
3.1. Direct Organoid Differentiation from hPSCs EBs
Several methodologies were previously developed for generating hPSCs-derived organoids to represent miniaturized specific tissues or organs. The simplest strategy is to directly induce differentiation toward organoid formation from the three-dimensional structure of hPSCs (which are referred to as embryoid bodies (EBs)). This structure can be generated using the dynamic suspension culture, hanging drop techniques, or by using Matrigel (Figure 1). Subsequently, one can employ a stepwise treatment using a cocktail of biomolecule inducers, which mostly consists of lineage-specific signaling factors (e.g., small molecule and/or growth factors) to mimic the cellular signaling during organogenesis in embryo development. This biomolecule formulation differs between the organoid types, depending ultimately on which pathways that needed to direct the hPSCs lineage. For example, hPSCs differentiation into the hepatic lineage requires exposure to a high concentration of Activin A during the endodermal differentiation followed by BMP4 to further direct the endoderm cells into hepatic competent cells. Here, final liver organoid maturation can be induced by HGF and OSM in the differentiation medium [44].
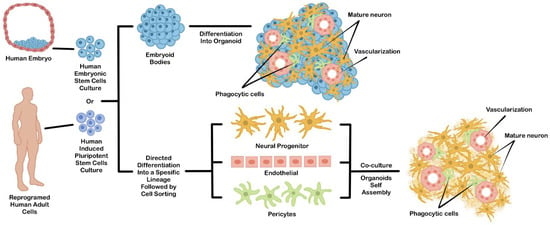
Figure 1.
An example of general strategies used to generate hPSCs-derived brain organoids. This organoid can be generated directly from differentiated EBs (upper chart) or by coculture from independently differentiated hPSCs-derived cell types (lower chart).
Direct EBs-based differentiation permits intrinsic self-organization during directed differentiation, which generates three-dimensional structures consisting of multiple cell types comprising a specific organ [52]. Due to their spontaneous morphogenesis during differentiation, this approach can be utilized for developing a model for the developmental study of a certain organ during embryogenesis. However, the emergence of undesirable cell types that did not complement the expected organ is more difficult to control during differentiation. This problem may reduce reliability in other translational applications, such as transplantation or drug testing.
3.2. Coculture of Multiple Differentiated Cells
The problem of cell heterogeneity coming from undesirable differentiated cell types often makes the reproducibility of the resulting organoid quite low. To solve this problem, hPSCs can be differentiated separately into specific cell types, sorted, and then mixed to form an organoid. This technique can be a solution for addressing the uncontrollable heterogeneous cell population problems. The hPSCs-derived cellular components can be achieved by performing an in vitro co-culture of multiple PSCs-derived cells or PSCs-derived progenitor cells (Figure 1). For example, the specific progenitor cell type can be individually differentiated from PSCs using a monolayer culture. Then, each cell component is purified and co-cultured together to induce their self-organization into a 3D structure. Since the organoid was assembled from an independent and specific hPSCs-derived cell type, the occurrence of other cells which are not normally part of the expected organs can be minimized. Additionally, the cellular diversity inside the organoids can be controlled by mixing each cell type based on the actual cellular composition ratio of the original organ [53]. This approach can be further improved by some engineering techniques, such as 3D cell bioprinting or scaffold based-template to partially direct the arrangement of each cellular component inside the organoid structure [54].
4. General Culture Platform to Generate the hPSCs Derived-Organoids
4.1. Matrigel Embedded Technique
As similar as in vivo organ development, extracellular matrix (ECM) remodeling plays an essential role in organoid morphogenesis. This component is very important (mainly for the branching formation that dictates the functional architecture of certain organs, such as the salivary glands, lungs, or kidneys) [55]. Matrigel is an extracellular matrix extracted from Engelbreth-Holm-Swarm mouse sarcomas which are routinely used in various cell culture applications [56]. During organoid differentiation, the Matrigel dome serves as structural support and provides a three-dimensional niche for the organoid assembly [56,57,58,59] (Figure 2a).
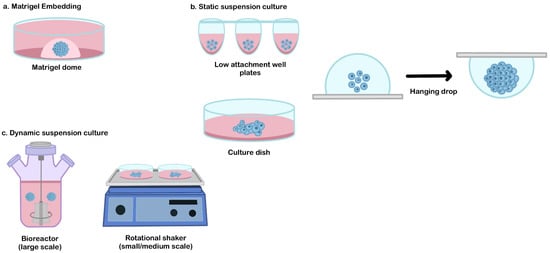
Figure 2.
The common culture platform for the hPSCs derived-organoid. (a) Matrigel embedding technique, (b) static suspension culture, and (c) dynamic suspension culture.
Despite its benefits in organoid culture, Matrigel also has some disadvantages. The variations in biochemical composition and mechanical variability of this sarcoma-derived-ECM complex may result in single- and batch-to-batch variability during the hPSCs-derived organoids culture [60]. Although this approach can be used for developmental study or disease modeling, this method may not be compatible with therapeutic applications. For example, xenogeneic materials need to be eliminated to achieve safe transplantable organoids. These animal-derived components such as Matrigel may potentially induce antigenicity and transmit pathogens such as viruses into the human recipient [61].
Recently, several efforts have been made to find synthetic alternatives for Matrigel [60,62,63]. These alternative materials may provide a chemically defined and xeno-free niche that can be independently tuned by adjusting various variables (such as their composition) and by using polymerization methods to produce expected mechanical and biological properties [60,62]. Moreover, by using a bioengineering approach, these synthetic matrices can be further utilized to provide a specific boundary guiding tissue morphogenesis [64].
Another challenge of the Matrigel embedded method is related to its application in organoid-based transplantation of large organs. Due to its technical difficulties in scaling up, this technique may be limited to small-scale drug screening and disease modeling.
4.2. Static Suspension Culture
A static suspension culture can be used as an alternative method to produce broad quantities of organoid production, from a small scale (e.g., patterned well plates or hanging drop method), medium-scale (e.g., low attachment culture dish [65,66]), or larger scale (e.g., oxygen-permeable static tissue culture bag [67]) (Figure 2b). This approach can be selected when the usage of ECM embedded is not required and when the interference of hydrodynamic conditions needs to be avoided to generate a specific lineage that is sensitive to shear stress (such as in hepatic differentiation) [68].
Traditionally, the formation of a 3D structure relies on spontaneous aggregation in the static suspension culture. This simple method has been adapted to generate hPSCs-derived organoids in medium or large-scale organoid productions (for example, using a low attachment culture dish [65,66] or culture bag [67]). The main drawback of this technique is the failure to control EBs aggregation. Due to the epithelial characteristic of hPSCs, spontaneous aggregation often occurs and results in a random size. These conditions may impact the variability of their 3D-differentiated organoid structure. Therefore, several alternative methods have been developed to improve aggregation control, such as the hanging drop method or patterned culture vessel (Figure 2b). The hanging drop method is a technique used to induce cellular assembly by utilizing gravitational forces. In this method, a cell suspension droplet is hung on the reversed surface, and the EBs are formed from the surface tension properties of the culture medium. This method is relatively simple during cell inoculation, but the medium replacement is very challenging due to the limited droplet volume that can be hung on the culture vessel surface [69]. Patterned well plates (e.g., microwell plates, U or V-bottom-well plates) can be used as a tool to force and control cell aggregation [14,70]. By adjusting the cell number, precise control of the EBs size can be enabled, which significantly reduces the variability of the resulting organoids. This culture system has potential for use in drug screening or disease modeling but is difficult to be applied for the therapeutic purposes of large organs that require a large number of organoids (due to the difficulties involved in their scalability).
4.3. Dynamic Suspension Culture
Dynamic suspension culture is a culture technique that is commonly used to produce a medium to a large number of organoids for various applications (such as transplantation of large organs) [61]. A dynamic culture environment can be achieved by using rotating or stirring mechanisms to promote the formation of a spherical 3D formation and control its initial size. The simple platform that is routinely used for organoid generation is by using the rotational shaker or stirred bioreactor (Figure 2c). These types of suspension cultures enable better control of the size and uniformity of organoids population in the culture system. The size control of initial EBs influences the dynamic profile of their pluripotency and lineage specification during differentiation [71]. The spheroid size and uniformity can be controlled by adjusting the inoculation density and manipulating the medium dynamics (such as rotation/stirring speed, fluidic flow, or medium volume) [61,72].
Hydrodynamic condition is an important biomechanical factor involved in dynamic suspension culture that may affect the differentiation process of hPSCs. Despite its importance in controlling agglomeration and medium mixing, the amount of hydrodynamic shear force needs to be carefully considered. Our previous comparison study showed that diverse hydrodynamic conditions coming from the shear force in various culture vessels which were set up (such as rotational cultures, ring-shaped culture vessels, or spinner flasks) may implicate the differentiation tendencies of hPSCs. The results indicated that higher shear stress may improve the hPSC EBs differentiation tendency toward an ectoderm and mesoderm lineage rather than an endoderm lineage [71]. A study conducted by Wolfe, et al. revealed that the shear force was potentially inducing early germ specification into ectodermal and mesodermal lineage on stress magnitude, ranging from 1.5 to 15 dynes cm−2 [73]. This result also corresponds well with another study conducted by Vosough et al. which reports several spontaneously differentiated-mesodermal populations from hepatic differentiation (which is presumably exposed by excessive shear stress in the stirred tank bioreactor) [68].
5. Other Limitations and Possible Improvements of PSCs Derived-Organoids Culture
5.1. Maturation
Currently, several methods for generating a broad spectrum ofhPSCs-derived organoids with specific tissue functionality have been reported, such as nephron filtration [47], beating cardiac organoid [74], insulin-secreting organoid [75], or organoids with drug metabolism capability [41]. However, developing efficient techniques for obtaining a mature and functional organoid remains challenging [48]. PSCs-derived cells are still relatively immature and exhibit the expression profile of fetal or progenitor cells rather than adult tissue cell types [47]. Moreover, these cells are still lacking in both architectural and functional characteristics compared to their original adult tissue counterparts. These maturation problems not only impact their reliability in disease modeling or drug screening but also importantly affect the graft immaturity and safety concerns when they are used in regenerative therapy [76].
A functional organ usually takes several years to be fully mature in vivo [77]. However, current studies in hPSCs-derived organoid generation have mostly been performed only within a couple of weeks or several months. Based on this understanding, extending the culture period using a differentiation induction medium may hypothetically improve their physiological and structural maturation. Giandomenico and his colleagues showed a good example of this approach which was used to achieve further maturation [78]. In the prolonged maturation period, they successfully generated cerebral organoids with the telencephalic identity of later stages of neural development, including axon outgrowth and neuronal maturation systems using micropatterned filaments [78]. Another study also showed a similar result in hPSC-derived-cardiac organoids [79]. The extended culture display (for up to 50 days) improved cardiac specification, survival, and maturation, as well as a good response to cardioactive drugs.
The signaling pathways play a vital role in regulating mature phenotypes during organ development and growth. This mechanism involved several biomolecules, such as cytokines, growth factors, hormones, inhibitory molecules, and other small molecules. By using the screening method, we may identify the specific regulator and optimize the in vitro organoid maturation using the same functional molecule compositions during their growth and development [80,81]. The experimental approach carried out by Huang et al. uncover the potential of this method [82]. A combination of biochemical factors, such as thyroid hormone, dexamethasone, and insulin-like growth factor-1 has been shown to improve the maturation of hiPSC-derived cardiac organoids in a micropatterned culture system [82]. In addition to using an exogenous source, these signaling molecules also can be provided by coculturing the hPSCs-derived cells with other cell types that existed in the original organs. These additional cell types may endogenously secrete molecule components required to promote further maturation of hPSC-derived-organoids. One example showed this by incorporating endothelial and mesenchymal stem cells which secrete a specific factor that plays a role in the functional maturation of hPSCs-derived kidney organoids [83].
Metabolism is a critical process that influences the self-renewal and cell fate specification of hPSCs [84,85,86]. Based on this knowledge, switching the energy source in the culture medium may direct the cellular metabolic adaptation towards a more mature and functional cell type. For example, the increasing concentration of glucose and an adjustment of palmitate concentration in the culture medium can significantly improve the hPSCs derived-cardiac organoid maturation [87].
The hPSCs-derived-organoids required a complex signal that partially can be mimicked in vitro to become fully mature. However, in vivo niches can provide better complex necessary signals similar to their developmental or maturation period. For instance, Dye et al. [88] and Miller, et al. [39] found that the hPSCs derived-lung organoids that were transplanted in vivo showed improved cellular differentiation of secretory lineages and resulted in airway-like structures that were remarkably similar to the native adult human lung.
5.2. High-Cost Production
Different from disease modeling and drug screening purposes, clinical/translational regenerative therapy applications often require a decent amount of cell numbers. For instance, a billion cells are necessary to regenerate both partial and total organs, such as the heart, pancreas, or liver [89,90,91]. Therefore, the scalability needs to be performed by a biomanufacturing process that involves a high-cost of production. Among all of the culture components, the high-cost consumption mainly comes from the requirement of expensive recombinant proteins, such as growth factors, that essentially require one to induce the differentiation of PSCs into specific and functional cell types.
Some effort has been made to minimize the requirements of these components, such as the development of growth factor-free differentiation protocols using small molecules [92]. However, differentiated cells often show less functional maturation compared to ones differentiated by the inclusion of growth factors. Since the usage of cytokine or growth factors is still becoming a necessity, possible alternative solutions have been developed to optimize the efficient usage of growth factors during differentiation. For example, researchers have used a specific molecular weight cutoff dialysis membrane barrier to recycle and accumulate high molecular weight endogenous and exogenous growth factors while continuously maintaining the exchange between the small molecular weight nutrition and toxic metabolic byproducts [44,93,94,95,96]. This approach may significantly improve the production efficiency per unit of hPSCs-derived organoids for various purposes such as drug screening or transplantation.
5.3. Tissue Complexity
As a model system, hPSCs-derived organoids still show incomplete in vivo features compared to the original human tissue, such as tissue architecture similarity, vascularization, innervation, functional immune regulation, and the representation of multi-organ interactions. Consequently, this miniaturized structure often fails to imitate the complex and integrated interplay between each organ system.
During hPSCs derived-organoids culture, several progenitor cells developed from hPSCs communicate via paracrine factors to arrange the tissue formation in vitro. Their self-organization was not only affected by the microenvironment and cellular interaction inside the structure but also by their external culture environment, which is normally difficult to control, resulting in variability within the same batch or between batches [97]. Several attempts have been made to precisely define the structural tissue-like geometry by niche modification [36], which also potentially reduces batch-to-batch variability during organoid construction. For example, a bioengineering approach called “guided self-organization” which utilized a fiber scaffold to control organoid elongation has been reported [19]. This technique showed improvements in increasing tissue complexity, enhancing differentiation, and creating better reproducibility for their applications such as drug screening and disease modeling.
Vascularization plays a vital role in the exchange of various necessary components for tissue homeostases such as nutrition, oxygen, and signaling molecules in actual human tissue [64]. The absence of this vasculature was becoming one of the major limitations in the current development of organoid technology. During the in vitro culture period, an organoid may excessively grow up to a millimeter in size, causing a diffusion gradient of nutrients, oxygen, signaling molecules, and toxic cellular byproducts inside the structure. The failure to control this spatiotemporal distribution of these molecules may lead to the development of necrotic tissue inside their three-dimensional structure [70,71]. Although neovascularization can be natively achieved during in vivo tissue engraftment [98,99], the generation of the in vitro vascular structure remained difficult. To address this problem, several techniques can be performed to generate vascularized organoids. A simple method to enable vascularization involves performing a co-culture between the pre-differentiated hPSCs or hPSC derived-progenitor cells with endothelial cells before or during the aggregation period to let them self-organize. The earlier development of this method was introduced by Takebe et al. [100,101] by introducing endothelial cells (HUVECs) and mesenchymal stem cells (MSCs) into hPSCs derived-multipotent hepatic endoderm spheroid to generate a vascularized liver buds organoids. A similar technique was also performed by Pettinato et al. [66] using interlaced human adipose microvascular endothelial cells (HAMEC) within the hiPSCs spheroids followed by hepatic differentiation. Alternatively, the mesoderm can be incorporated into the EBs, which will differentiate into endothelial cells to form a vascular network [102]
To further improve vascularization inside the organoid, a bioengineering approach can be applied using 3D fabrication techniques. A recent study by Skylar-Scott et al. [21] represents a bioengineering approach used to produce a vascularized organoid by controlling both its composition and structure using tissue bioprinting. In this technique, the hPSCs-derived progenitor cells were individually incorporated within a hydrogel and printed using a layer-by-layer deposition to form the expected structure [54,103,104]. Another strategy to enable vascularization involves utilizing a sacrificial networks method using a degradable- or removable biomaterial. The vascularization was formed by seeding endothelial cells or hPSC-derived-endothelial cells in the space created by the voids from the removal of sacrificial networks (making the specific pattern required inside the organoid) [104]. Although all of these 3D bio-fabrication techniques provide enormous potential to guide the 3D organoid assembly, several challenges still need to be addressed. In addition to the poor survival of hPSCs or hPSC-derived progenitor cells, they are very sensitive to environmental cues due to their embryonic-like nature in response to developmental signals. Moreover, they also tend to spontaneously agglomerate and form random clusters after being printed [54].
The other components that are often lacking in current hPSCs-derived organoids designed to mimic actual organs are the neuronal innervation complex and resident immune system. Neuronal innervation is an essential component that regulates various biological processes. For example, acetylcholine (ACh) is a neurotransmitter that triggers cholinergic signaling and plays a pivotal role in the regulation of the small intestine cells [105]. A study conducted by Workman et al. tried to provide a solution to this problem by introducing hPSCs-derived neural crest cells (NCC) to hPSCs-derived intestinal organoids in a static suspension [106]. Inside the organoid structure, the ECC demonstrated migration and formed a microglia structure. This complex structure showed the functional regulation of intestinal contraction and could be utilized to investigate the mechanisms involved in motility disorders of the human gastrointestinal tract [106].
To demonstrate immune regulation for developmental study and disease modeling applications, several important immune components need to exist inside the organoid or in the culture environment. Several attempts have been carried out to mimic the necessary conditions by adding some cytokines to partially imitate in vivo immune regulation [107]. However, this kind of approach cannot completely reflect the complete regulation by actual immune cells since they also secrete several cytokine complexes [108]. Moreover, immune cells also play a critical role during infection (for example, in phagocytosis) [109]. An experimental approach was conducted by Xu et al. to investigate the possibility of immune cell inclusion by incorporating hPSC-derived microglia into brain organoids in ultra-low-attachment 96-well plates [17]. The results showed that the hPSCs derived-microglia exhibit phagocytic activity and synaptic pruning function inside the cerebral organoid. Additionally, the secretion of neurotrophic factors and cytokines, such as insulin growth factor 1 (IGF1) and IL-1b, was able to modulate neurogenesis in organoid structures [17].
Most current studies have been concentrated on a single hPSCs-derived organoid type, which means that studies about the complex interactions between different organs have not yet been explored much. A few studies have constructed multi-organ organoids or assembloids via the fusing and interconnecting of multiple organoid types that exhibit a specific function (for example, hepato-biliary-pancreatic organoids [50,51] or retinoganglial organoids [110]). Although organ crosstalk can be simulated, this approach is still limited to the utilization of early progenitor organoid types which do not completely reflect fully mature and functional organs. Another problem that requires further investigation is the development of methods that can be used to precisely control the further maturation of individual organoid after fusion. This is necessary since each organoid type requires specific growth factors and a particular cocktail of small molecules, as well as different culture environment conditions, to maintain their characteristics.
6. Conclusions and Future Perspectives
Organoid culture represents a remarkable technology that may provide an ex vivo platform of miniaturized organs for various applications, such as in vitro models of organogenesis, disease modeling, drug screening, and possibly for regenerative therapy. To realize the best utilization of hiPSCs derived-organoids, the selection of the culture system needs to be carefully considered, depending ultimately on the types of application and the effect of the culture environment during the culture period. Despite their improvement through bioengineering approaches, several challenges still need to be addressed to ensure both their reliability to reconstitute actual complex organ functions and their safety for future regenerative therapy.
To achieve decent organoid production for a specific purpose, the mechanisms related to the culture condition during organoid forming and differentiation from hPSCs into each specific organoid type need to be optimized. By using these optimizations, a standardized methodology can be developed for generating organ-specific-hiPSCs-derived organoids.
A personalized hiPSCs-derived organoid can not only overcome individual variability problems in drug screening and disease modeling but also enable immune-compatible grafts for full- or partial organ transplantation when combined with xeno-free organoid production.
Author Contributions
Conceptualization, F.G.T.; writing—original draft preparation, Z.G., D.S.F., N.P.D.P.S. and S.T.N.; writing—review and editing, F.G.T.; scientific illustration, Z.G. and F.G.T.; supervision, F.G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardoso-Moreira, M.; Sarropoulos, I.; Velten, B.; Mort, M.; Cooper, D.N.; Huber, W.; Kaessmann, H. Developmental Gene Expression Differences between Humans and Mammalian Models. Cell Rep. 2020, 33, 108308. [Google Scholar] [CrossRef]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Ma, Z.; Davis, C.; Pope, B.D.; et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 2014, 515, 355–364. [Google Scholar] [CrossRef]
- Schutgens, F.; Clevers, H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 211–234. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Iefremova, V.; Manikakis, G.; Krefft, O.; Jabali, A.; Weynans, K.; Wilkens, R.; Marsoner, F.; Brändl, B.; Müller, F.J.; Koch, P.; et al. An Organoid-Based Model of Cortical Development Identifies Non-Cell-Autonomous Defects in Wnt Signaling Contributing to Miller-Dieker Syndrome. Cell Rep. 2017, 19, 50–59. [Google Scholar] [CrossRef]
- Mills, R.J.; Parker, B.L.; Quaife-Ryan, G.A.; Voges, H.K.; Needham, E.J.; Bornot, A.; Ding, M.; Andersson, H.; Polla, M.; Elliott, D.A.; et al. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell 2019, 24, 895–907.e6. [Google Scholar] [CrossRef]
- Willenbring, H.; Soto-Gutierrez, A. Transplantable liver organoids made from only three ingredients. Cell Stem Cell 2013, 13, 139–140. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef]
- Berishvili, E.; Casiraghi, F.; Amarelli, C.; Scholz, H.; Piemonti, L.; Berney, T.; Montserrat, N. Mini-organs forum: How to advance organoid technology to organ transplant community. Transpl. Int. 2021, 34, 1588–1593. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Sasai, Y. Next-generation regenerative medicine: Organogenesis from stem cells in 3D culture. Cell Stem Cell 2013, 12, 520–530. [Google Scholar] [CrossRef]
- Sasai, Y. Cytosystems dynamics in self-organization of tissue architecture. Nature 2013, 493, 318–326. [Google Scholar] [CrossRef]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Boris, A.; Kriegstein, A.R. Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell 2017, 20, 435–449. [Google Scholar] [CrossRef]
- Anastasaki, C.; Wilson, A.F.; Chen, A.S.; Wegscheid, M.L.; Gutmann, D.H. Generation of human induced pluripotent stem cell-derived cerebral organoids for cellular and molecular characterization. STAR Protoc. 2022, 3, 101173. [Google Scholar] [CrossRef]
- Anastasaki, C.; Wegscheid, M.L.; Hartigan, K.; Papke, J.B.; Kopp, N.D.; Chen, J.; Cobb, O.; Dougherty, J.D.; Gutmann, D.H. Human iPSC-Derived Neurons and Cerebral Organoids Establish Differential Effects of Germline NF1 Gene Mutations. Stem Cell Rep. 2020, 14, 541–550. [Google Scholar] [CrossRef]
- Wegscheid, M.L.; Anastasaki, C.; Hartigan, K.A.; Cobb, O.M.; Papke, J.B.; Traber, J.N.; Morris, S.M.; Gutmann, D.H. Patient-derived iPSC-cerebral organoid modeling of the 17q11.2 microdeletion syndrome establishes CRLF3 as a critical regulator of neurogenesis. Cell Rep. 2021, 36, 109315. [Google Scholar] [CrossRef]
- Xu, R.; Boreland, A.J.; Li, X.; Erickson, C.; Jin, M.; Atkins, C.; Pang, Z.P.; Daniels, B.P.; Jiang, P. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Rep. 2021, 16, 1923–1937. [Google Scholar] [CrossRef]
- Qian, X.; Jacob, F.; Song, M.M.; Nguyen, H.N.; Song, H.; Ming, G.L. Generation of human brain region–specific organoids using a miniaturized spinning bioreactor. Nat. Protoc. 2018, 13, 565–580. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.W.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar] [CrossRef]
- Mansour, A.A.; Gonçalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Huang, J.Y.; Lu, A.; Ng, A.H.M.; Duenki, T.; Liu, S.; Nam, L.L.; Damaraju, S.; Church, G.M.; Lewis, J.A. Orthogonally induced differentiation of stem cells for the programmatic patterning of vascularized organoids and bioprinted tissues. Nat. Biomed. Eng. 2022, 6, 449–462. [Google Scholar] [CrossRef]
- Watanabe, M.; Buth, J.E.; Vishlaghi, N.; de la Torre-Ubieta, L.; Taxidis, J.; Khakh, B.S.; Coppola, G.; Pearson, C.A.; Yamauchi, K.; Gong, D.; et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 2017, 21, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Tanaka, Y.; Patterson, B.; Kang, Y.J.; Govindaiah, G.; Roselaar, N.; Cakir, B.; Kim, K.Y.; Lombroso, A.P.; Hwang, S.M.; et al. Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell 2017, 21, 383–398.e7. [Google Scholar] [CrossRef]
- Trisno, S.L.; Philo, K.E.D.; McCracken, K.W.; Catá, E.M.; Ruiz-Torres, S.; Rankin, S.A.; Han, L.; Nasr, T.; Chaturvedi, P.; Rothenberg, M.E.; et al. Esophageal Organoids from Human Pluripotent Stem Cells Delineate Sox2 Functions during Esophageal Specification. Cell Stem Cell 2018, 23, 501–515. [Google Scholar] [CrossRef]
- Koide, T.; Koyanagi-Aoi, M.; Uehara, K.; Kakeji, Y.; Aoi, T. CDX2-induced intestinal metaplasia in human gastric organoids derived from induced pluripotent stem cells. Iscience 2022, 25, 104314. [Google Scholar] [CrossRef]
- McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef]
- McCracken, K.W.; Aihara, E.; Martin, B.; Crawford, C.M.; Broda, T.; Treguier, J.; Zhang, X.; Shannon, J.M.; Montrose, M.H.; Wells, J.M. Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature 2017, 541, 182–187. [Google Scholar] [CrossRef]
- Broda, T.R.; McCracken, K.W.; Wells, J.M. Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nat. Protoc. 2019, 14, 28–50. [Google Scholar] [CrossRef]
- Nadkarni, R.R.; Abed, S.; Cox, B.J.; Bhatia, S.; Lau, J.T.; Surette, M.G.; Draper, J.S. Functional Enterospheres Derived In Vitro from Human Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 897–912. [Google Scholar] [CrossRef] [Green Version]
- Mithal, A.; Capilla, A.; Heinze, D.; Berical, A.; Villacorta-Martin, C.; Vedaie, M.; Jacob, A.; Abo, K.; Szymaniak, A.; Peasley, M.; et al. Generation of mesenchyme free intestinal organoids from human induced pluripotent stem cells. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Romitti, M.; Fonseca, B.d.F.d.; Doumont, G.; Gillotay, P.; Tourneur, A.; Eski, S.E.; Van Simaeys, G.; Chomette, L.; Lasolle, H.; Monestier, O.; et al. Transplantable human thyroid organoids generated from embryonic stem cells to rescue hypothyroidism. BioRixv. 2021. BioRxiv 2021.12.01.470729. Available online: https://www.biorxiv.org/content/10.1101/2021.12.01.470729v1 (accessed on 2 February 2022).
- Montel-Hagen, A.; Seet, C.S.; Li, S.; Chick, B.; Zhu, Y.; Chang, P.; Tsai, S.; Sun, V.; Lopez, S.; Chen, H.C.; et al. Organoid-Induced Differentiation of Conventional T Cells from Human Pluripotent Stem Cells. Cell Stem Cell 2019, 24, 376–389. [Google Scholar] [CrossRef]
- Drakhlis, L.; Biswanath, S.; Farr, C.M.; Lupanow, V.; Teske, J.; Ritzenhoff, K.; Franke, A.; Manstein, F.; Bolesani, E.; Kempf, H.; et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 2021, 39, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Drakhlis, L.; Devadas, S.B.; Zweigerdt, R. Generation of heart-forming organoids from human pluripotent stem cells. Nat. Protoc. 2021, 16, 5652–5672. [Google Scholar] [CrossRef]
- Hoang, P.; Kowalczewski, A.; Sun, S.; Winston, T.S.; Archilla, A.M.; Lemus, S.M.; Ercan-Sencicek, A.G.; Gupta, A.R.; Liu, W.; Kontaridis, M.I.; et al. Engineering spatial-organized cardiac organoids for developmental toxicity testing. Stem Cell Rep. 2021, 16, 1228–1244. [Google Scholar] [CrossRef] [PubMed]
- Voges, H.K.; Mills, R.J.; Elliott, D.A.; Parton, R.G.; Porrello, E.R.; Hudson, J.E. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development 2017, 144, 1118–1127. [Google Scholar] [CrossRef]
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef]
- Miller, A.J.; Hill, D.R.; Nagy, M.S.; Aoki, Y.; Dye, B.R.; Chin, A.M.; Huang, S.; Zhu, F.; White, E.S.; Lama, V.; et al. In Vitro Induction and In Vivo Engraftment of Lung Bud Tip Progenitor Cells Derived from Human Pluripotent Stem Cells. Stem Cell Reports 2018, 10, 101–119. [Google Scholar] [CrossRef]
- Guan, Y.; Xu, D.; Garfin, P.M.; Ehmer, U.; Hurwitz, M.; Enns, G.; Michie, S.; Wu, M.; Zheng, M.; Nishimura, T.; et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight 2017, 2, e94954. [Google Scholar] [CrossRef]
- Mun, S.J.; Ryu, J.S.; Lee, M.O.; Son, Y.S.; Oh, S.J.; Cho, H.S.; Son, M.Y.; Kim, D.S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985. [Google Scholar] [CrossRef]
- Ramli, M.N.B.; Lim, Y.S.; Lim, C.T.; Demircioglu, D.; Tng, W.; Gonzales, K.A.U.; Tan, C.P.; Szczerbinska, I.; Liang, H.; Soe, E.L.; et al. Human Pluripotent Stem Cell-Derived Organoids as Models of Liver Disease. Gastroenterology 2020, 159, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshizawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Torizal, F.G.; Utami, T.; You, L.Q.; Inamura, K.; Nishikawa, M.; Sakai, Y. Generation of high-density human induced pluripotent stem cell derived-liver organoid by enabling growth factors accumulation in a simple dialysis medium refinement culture platform. Preprint 2022, 1–10. [Google Scholar] [CrossRef]
- Wiedenmann, S.; Breunig, M.; Merkle, J.; von Toerne, C.; Georgiev, T.; Moussus, M.; Schulte, L.; Seufferlein, T.; Sterr, M.; Lickert, H.; et al. Single-cell-resolved differentiation of human induced pluripotent stem cells into pancreatic duct-like organoids on a microwell chip. Nat. Biomed. Eng. 2021, 5, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hämmerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Przepiorski, A.; Sander, V.; Tran, T.; Hollywood, J.A.; Sorrenson, B.; Shih, J.H.; Wolvetang, E.J.; McMahon, A.P.; Holm, T.M.; Davidson, A.J. A Simple Bioreactor-Based Method to Generate Kidney Organoids from Pluripotent Stem Cells. Stem Cell Rep. 2018, 11, 470–484. [Google Scholar] [CrossRef]
- Sander, V.; Przepiorski, A.; Crunk, A.E.; Hukriede, N.A.; Holm, T.M.; Davidson, A.J. Protocol for Large-Scale Production of Kidney Organoids from Human Pluripotent Stem Cells. STAR Protoc. 2020, 1, 100150. [Google Scholar] [CrossRef]
- Zhang, R.R.; Koido, M.; Tadokoro, T.; Ouchi, R.; Matsuno, T.; Ueno, Y.; Sekine, K.; Takebe, T.; Taniguchi, H. Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem Cell Rep. 2018, 10, 780–793. [Google Scholar] [CrossRef]
- Koike, H.; Iwasawa, K.; Ouchi, R.; Maezawa, M.; Kimura, M.; Kodaka, A.; Nishii, S.; Thompson, W.L.; Takebe, T. Engineering human hepato-biliary-pancreatic organoids from pluripotent stem cells. Nat. Protoc. 2021, 16, 919–936. [Google Scholar] [CrossRef]
- Koike, H.; Iwasawa, K.; Ouchi, R.; Maezawa, M.; Giesbrecht, K.; Saiki, N.; Ferguson, A.; Kimura, M.; Thompson, W.L.; Wells, J.M.; et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut–midgut boundary. Nature 2019, 574, 112–116. [Google Scholar] [CrossRef]
- Takebe, T.; Wells, J.M. Organoids by design. Science 2019, 364, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, X.; Li, G.; Gokulnath, P.; Xiao, J. Generating 3D human cardiac constructs from pluripotent stem cells. eBioMedicine 2022, 76, 103813. [Google Scholar] [CrossRef] [PubMed]
- Salaris, F.; Rosa, A. Construction of 3D in vitro models by bioprinting human pluripotent stem cells: Challenges and opportunities. Brain Res. 2019, 1723, 146393. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Nelson, C.M. Extracellular matrix and cytoskeletal dynamics during branching morphogenesis. Organogenesis 2012, 8, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Garreta, E.; Kamm, R.D.; Chuva de Sousa Lopes, S.M.; Lancaster, M.A.; Weiss, R.; Trepat, X.; Hyun, I.; Montserrat, N. Rethinking organoid technology through bioengineering. Nat. Mater. 2021, 20, 145–155. [Google Scholar] [CrossRef]
- Mukhopadhyay, M. Recapitulating early cardiogenesis in vitro. Nat. Methods 2021, 18, 331. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Torizal, F.G.; Horiguchi, I.; Sakai, Y. Physiological Microenvironmental Conditions in Different Scalable Culture Systems for Pluripotent Stem Cell Expansion and Differentiation. Open Biomed. Eng. J. 2017, 13, 41–54. [Google Scholar] [CrossRef]
- Heo, J.H.; Kang, D.; Seo, S.J.; Jin, Y. Engineering the Extracellular Matrix for Organoid Culture. Int. J. Stem Cells 2022, 15, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Poudel, H.; Sanford, K.; Szwedo, P.K.; Pathak, R.; Ghosh, A. Synthetic Matrices for Intestinal Organoid Culture: Implications for Better Performance. ACS Omega 2022, 7, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, K.; Spee, B.; Costa, P.; Sachs, N.; Clevers, H.; Malda, J. Converging biofabrication and organoid technologies: The next frontier in hepatic and intestinal tissue engineering? Biofabrication 2017, 9, 013001. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, G.; Ramanathan, R.; Fisher, R.A.; Mangino, M.J. Scalable Differentiation of Human iPSCs in a Multicellular Spheroid- based 3D Culture into Hepatocyte- like Cells through Direct Wnt/β—Catenin Pathway Inhibition. Sci. Rep. 2016, 6, 32888. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, G.; Lehoux, S.; Ramanathan, R.; Salem, M.M.; He, L.X.; Muse, O.; Flaumenhaft, R.; Thompson, M.T.; Rouse, E.A.; Cummings, R.D.; et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci. Rep. 2019, 9, 8920. [Google Scholar] [CrossRef]
- Otsuji, T.G.; Bin, J.; Yoshimura, A.; Tomura, M.; Tateyama, D.; Minami, I.; Yoshikawa, Y.; Aiba, K.; Heuser, J.E.; Nishino, T.; et al. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Rep. 2014, 2, 734–745. [Google Scholar] [CrossRef]
- Vosough, M.; Omidinia, E.; Kadivar, M.; Shokrgozar, M.-A.; Pournasr, B.; Aghdami, N.; Baharvand, H. Generation of functional hepatocyte-like cells from human pluripotent stem cells in a scalable suspension culture. Stem Cells Dev. 2013, 22, 2693–2705. [Google Scholar] [CrossRef]
- Velasco, V.; Shariati, S.A.; Esfandyarpour, R. Microtechnology-based methods for organoid models. Microsyst. Nanoeng. 2020, 6, 76. [Google Scholar] [CrossRef]
- Torizal, F.G.; Kimura, K.; Horiguchi, I.; Sakai, Y. Size-dependent hepatic differentiation of human induced pluripotent stem cells spheroid in suspension culture. Regen. Ther. 2019, 16, 66–73. [Google Scholar] [CrossRef]
- Torizal, F.G.; Kim, S.M.; Horiguchi, I.; Inamura, K.; Suzuki, I.; Morimura, T.; Nishikawa, M.; Sakai, Y. Production of homogenous size-controlled human induced pluripotent stem cell aggregates using ring-shaped culture vessel. J. Tissue Eng. Regen. Med. 2022, 16, 254–266. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; De Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.P.; Leleux, J.; Nerem, R.M.; Ahsan, T. Effects of shear stress on germ lineage specification of embryonic stem cells. Integr. Biol. 2012, 4, 1263–1273. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.A.; Kim, S.J.; Lee, H.A. Improving Generation of Cardiac Organoids from Human Pluripotent Stem Cells Using the Aurora Kinase Inhibitor ZM447439. Biomedicines 2021, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.H.; Ko, U.H.; Oh, Y.; Lim, A.; Sohn, J.W.; Shin, J.H.; Kim, H.H.; Han, Y.M. Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo. Sci. Rep. 2016, 6, 35145. [Google Scholar] [CrossRef]
- Nam, S.A.; Seo, E.; Kim, J.W.; Kim, H.W.; Kim, H.L.; Kim, K.; Kim, T.M.; Ju, J.H.; Gomez, I.G.; Uchimura, K.; et al. Graft immaturity and safety concerns in transplanted human kidney organoids. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vreeker, A.; Van Stuijvenberg, L.; Hund, T.J.; Mohler, P.J.; Nikkels, P.G.J.; Van Veen, T.A.B. Assembly of the cardiac intercalated disk during preand postnatal development of the human heart. PLoS ONE 2014, 9, e94722. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Sutcliffe, M.; Lancaster, M.A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 2021, 16, 579–602. [Google Scholar] [CrossRef]
- Ergir, E.; Oliver-De La Cruz, J.; Fernandes, S.; Cassani, M.; Niro, F.; Sousa, D.; Vrbský, J.; Vinarský, V.; Perestrelo, A.R.; Debellis, D.; et al. Generation and Maturation of Human iPSC-derived Cardiac Organoids in Long Term Culture. BioRxiv 2022. BioRxiv 2022.03.07.483273. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mondragon-Gonzalez, R.; Xu, B.; Magli, A.; Kim, H.; Lainé, J.; Kiley, J.; McKee, H.; Rinaldi, F.; Aho, J.; et al. Screening identifies small molecules that enhance the maturation of human pluripotent stem cell-derived myotubes. Elife 2019, 8, e47970. [Google Scholar] [CrossRef] [PubMed]
- Hergenreder, E.; Zorina, Y.; Zhao, Z.; Munguba, H.; Calder, L.; Baggiolini, A.; Minotti, A.P.; Walsh, R.M.; Levitz, J.; Garippa, R.; et al. Combined small molecule treatment accelerates timing of maturation in human pluripotent stem cell-derived neurons. BioRxiv 2022. BioRxiv 2022.06.02.494616. [Google Scholar] [CrossRef]
- Huang, C.Y.; Peres Moreno Maia-Joca, R.; Ong, C.S.; Wilson, I.; DiSilvestre, D.; Tomaselli, G.F.; Reich, D.H. Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. J. Mol. Cell. Cardiol. 2020, 138, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khoshdel-Rad, N.; Zahmatkesh, E.; Moeinvaziri, F.; Haghparast, N.; Baharvand, H.; Aghdami, N.; Moghadasali, R. Promoting Maturation of Human Pluripotent Stem Cell-Derived Renal Microtissue by Incorporation of Endothelial and Mesenchymal Cells. Stem Cells Dev. 2021, 30, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Tsogtbaatar, E.; Landin, C.; Minter-Dykhouse, K.; Folmes, C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nuebel, E.; Daley, G.Q.; Koehler, C.M.; Teitell, M.A. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 2012, 11, 589–595. [Google Scholar] [CrossRef]
- Wu, J.; Ocampo, A.; Belmonte, J.C.I. Cellular Metabolism and Induced Pluripotency. Cell 2016, 166, 1371–1385. [Google Scholar] [CrossRef]
- Mills, R.J.; Titmarsh, D.M.; Koenig, X.; Parker, B.L.; Ryall, J.G.; Quaife-Ryan, G.A.; Voges, H.K.; Hodson, M.P.; Ferguson, C.; Drowley, L.; et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl. Acad. Sci. USA 2017, 114, E8372–E8381. [Google Scholar] [CrossRef]
- Dye, B.R.; Dedhia, P.H.; Miller, A.J.; Nagy, M.S.; White, E.S.; Shea, L.D.; Spence, J.R. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. Elife 2016, 5, e19732. [Google Scholar] [CrossRef]
- Kempf, H.; Andree, B.; Zweigerdt, R. Large-scale production of human pluripotent stem cell derived cardiomyocytes. Adv. Drug Deliv. Rev. 2016, 96, 18–30. [Google Scholar] [CrossRef]
- Zweigerdt, R. Large Scale Production of Stem Cells and Their Derivatives. Adv. Biochem. Eng. Biotechnol. 2009, 123, 127–141. [Google Scholar] [CrossRef]
- Iansante, V.; Chandrashekran, A.; Dhawan, A. Cell-based liver therapies: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170229. [Google Scholar] [CrossRef]
- Siller, R.; Greenhough, S.; Naumovska, E.; Sullivan, G.J. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Rep. 2015, 4, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Torizal, F.G.; Lau, Q.Y.; Ibuki, M.; Kawai, Y.; Horikawa, M.; Minami, M.; Michiue, T.; Horiguchi, I.; Nishikawa, M.; Sakai, Y. A miniature dialysis-culture device allows high-density human-induced pluripotent stem cells expansion from growth factor accumulation. Commun. Biol. 2021, 4, 1316. [Google Scholar] [CrossRef] [PubMed]
- Torizal, F.G.; Lau, Q.Y.; Ibuki, M.; Kawai, Y.; Horikawa, M.; Minami, M.; Horiguchi, I.; Nishikawa, M.; Sakai, Y. High-density hiPSCs expansion supported by growth factors accumulation in a simple dialysis- culture platform. Preprint 2020, 1–17. [Google Scholar] [CrossRef]
- Choi, H.; Torizal, F.G.; Shinohara, M.; Sakai, Y. Differentiation of Human Induced Pluripotent Stem Cells into Definitive Endoderm Using Simple Dialysis Culture Device. Methods Mol. Biol. 2021, 2158, 141–153. [Google Scholar] [CrossRef]
- Miki, T.; Ring, A.; Gerlach, J.; Ph, D.; Ring, A.; Ph, D.; Gerlach, J. Hepatic differentiation of human embryonic stem cells is promoted by three-dimensional dynamic perfusion culture conditions. Tissue Eng. Part C. Methods 2011, 17, 557–568. [Google Scholar] [CrossRef]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef]
- Daviaud, N.; Friedel, R.H.; Zou, H. Vascularization and engraftment of transplanted human cerebral organoids in mouse cortex. Eneuro 2018, 5, 0219-18. [Google Scholar] [CrossRef]
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Takebe, T.; Zhang, R.-R.; Koike, H.; Kimura, M.; Yoshizawa, E.; Enomura, M.; Koike, N.; Sekine, K.; Taniguchi, H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat. Protoc. 2014, 9, 396–409. [Google Scholar] [CrossRef]
- Wörsdörfer, P.; Dalda, N.; Kern, A.; Krüger, S.; Wagner, N.; Kwok, C.K.; Henke, E.; Ergün, S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 2019, 9, 15663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, L.; Yi, Q. Engineering the vasculature of stem-cell-derived liver organoids. Biomolecules 2021, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Grebenyuk, S.; Ranga, A. Engineering organoid vascularization. Front. Bioeng. Biotechnol. 2019, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Fujishima, K.; Kengaku, M. Modeling intestinal stem cell function with organoids. Int. J. Mol. Sci. 2021, 22, 10912. [Google Scholar] [CrossRef] [PubMed]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017, 23, 49–59. [Google Scholar] [CrossRef]
- Jung, K.B.; Lee, H.; Son, Y.S.; Lee, M.O.; Kim, Y.D.; Oh, S.J.; Kwon, O.; Cho, S.; Cho, H.S.; Kim, D.S.; et al. Interleukin-2 induces the in vitro maturation of human pluripotent stem cell-derived intestinal organoids. Nat. Commun. 2018, 9, 3039. [Google Scholar] [CrossRef]
- Stanley, A.C.; Lacy, P. Pathways for cytokine secretion. Physiology 2010, 25, 218–229. [Google Scholar] [CrossRef]
- Rosales, C.; Uribe-Querol, E. Phagocytosis: A Fundamental Process in Immunity. Biomed Res. Int. 2017, 2017, 9042851. [Google Scholar] [CrossRef]
- Fligor, C.M.; Lavekar, S.S.; Harkin, J.; Shields, P.K.; VanderWall, K.B.; Huang, K.C.; Gomes, C.; Meyer, J.S. Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids. Stem Cell Rep. 2021, 16, 2228–2241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).