The Charge and Phase State of Liposomes Dramatically Affects the Binding of Mannosylated Chitosan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liposome Preparation
2.3. Complex Preparation
2.4. Moxifloxacin Liposomal Form Preparation
2.5. DLS Measurements
2.6. NTA Measurements
2.7. ATR-FTIR Spectroscopy
2.8. Kdis Evaluation
2.9. Determination of Moxifloxacin Encapsulation Efficacy via UV-Vis Spectroscopy
2.10. Fluorescence Analysis
2.11. Moxifloxacin Release Kinetics Study
2.12. Studies of the Interaction of the Complex Liposomes—ChitMan with Concanavalin A
3. Results and Discussion
3.1. Objects of Study
3.2. Effect of Complex Formation on the Size and ζ-Potential of Complexes
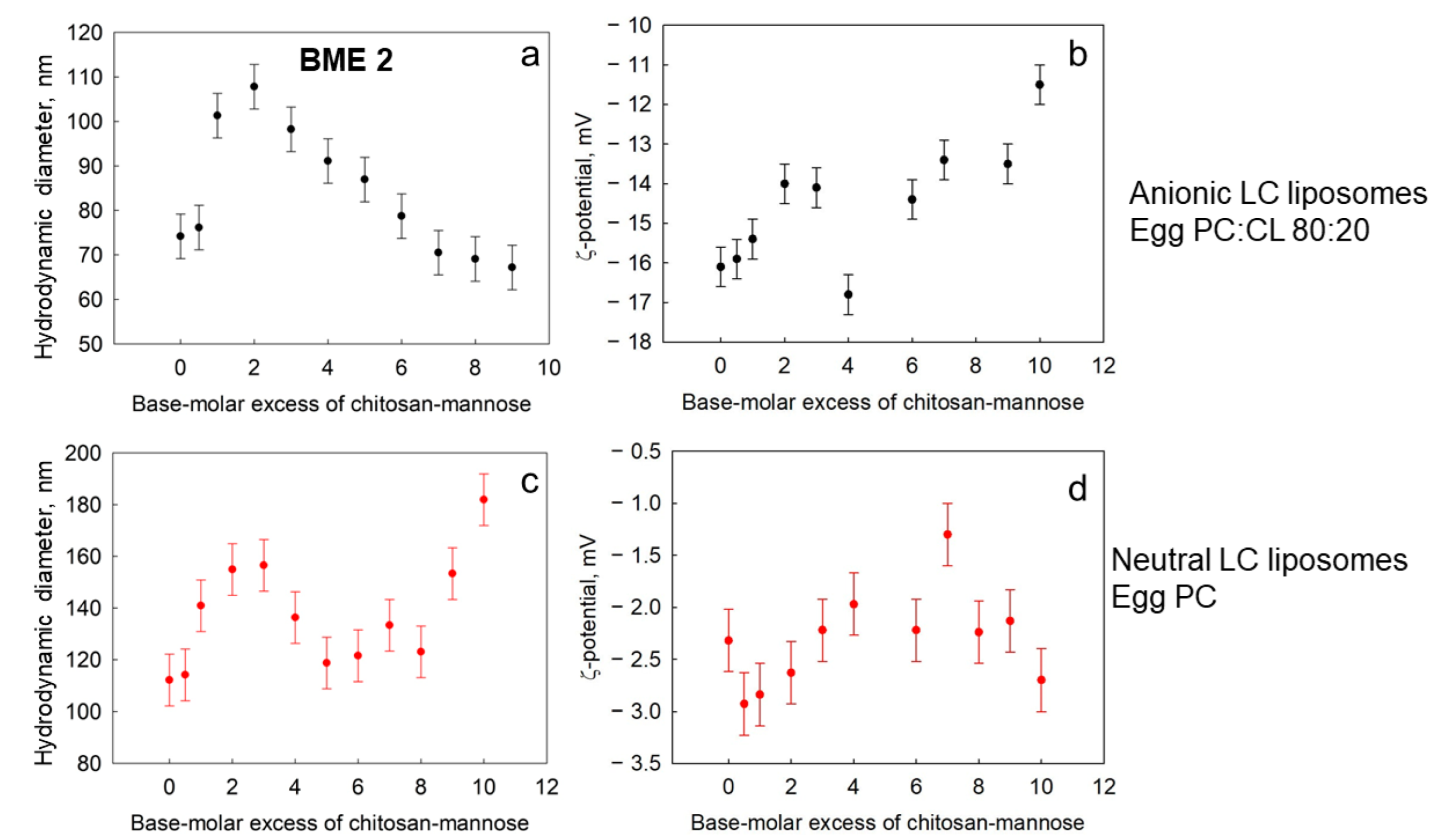
3.2.1. Liquid-Crystalline Liposomes: Role of Cardiolipin in Complex Formation
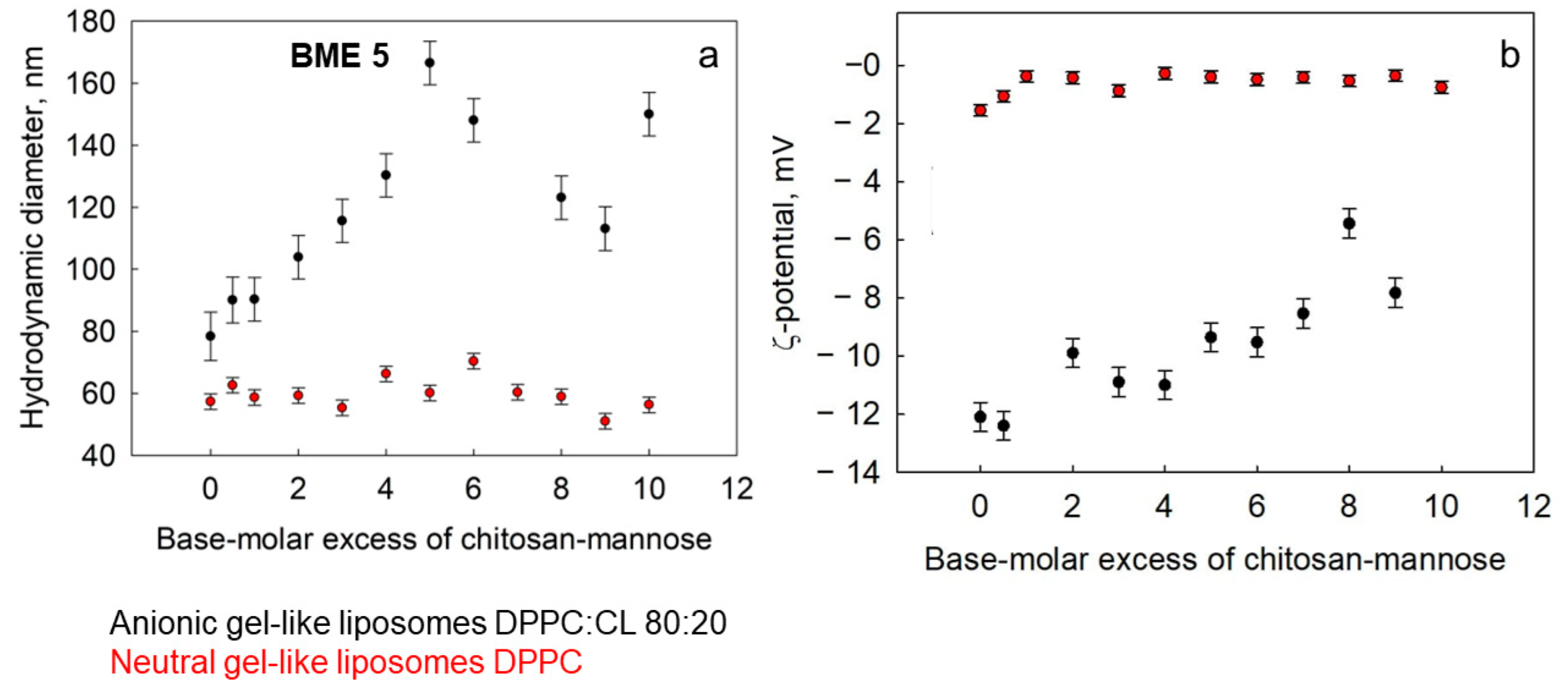
3.2.2. Gel-like Liposomes: The Role of Cardiolipin in Complex Formation
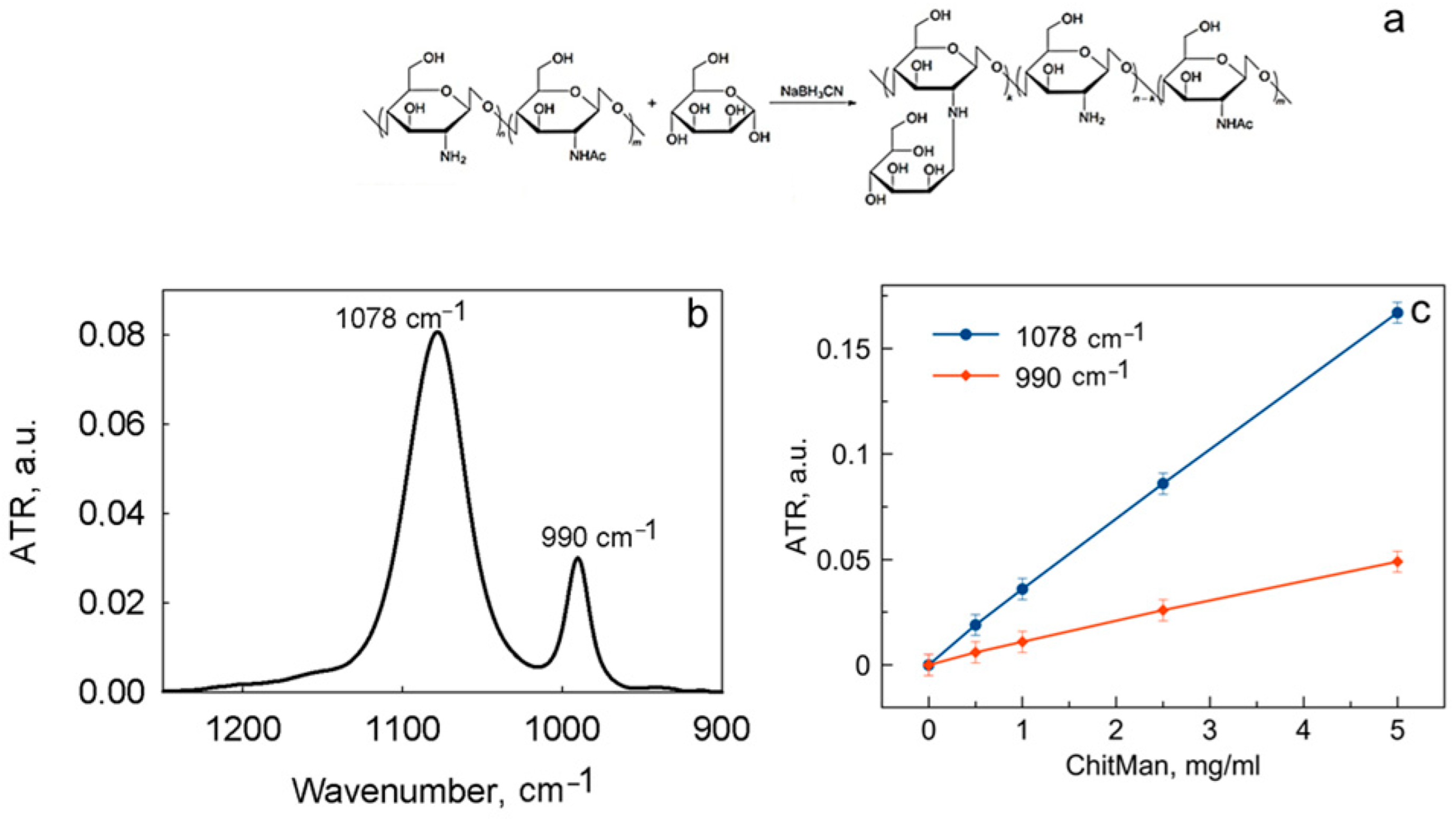
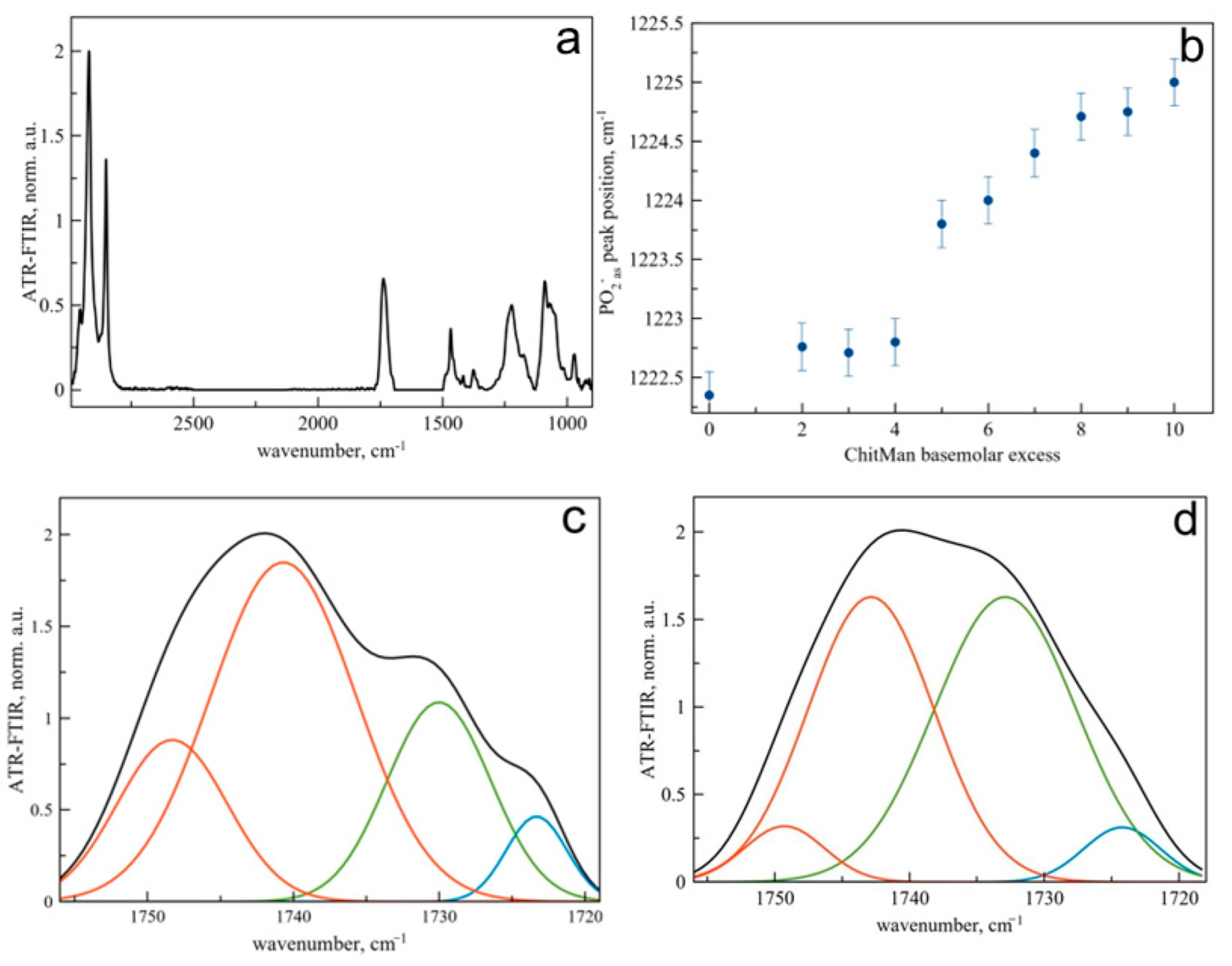
3.3. Discovering the Main Binding Sites of ChitMan via ATR-FTIR Spectroscopy
3.4. Influence of Preheating on the Complexation of Gel-like Liposomes with ChitMan
3.5. Kdis Evaluation of Liposome Complexes with ChitMan
3.6. Complex DPPC:CL Liposomes with ChitMan Binds with Model Mannose Receptor Concanavalin A
3.7. Moxifloxacin Release Kinetics Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sheikhpour, M.; Delorme, V.; Kasaeian, A.; Amiri, V.; Masoumi, M.; Sadeghinia, M.; Ebrahimzadeh, N.; Maleki, M.; Pourazar, S. An effective nano drug delivery and combination therapy for the treatment of Tuberculosis. Sci. Rep. 2022, 12, 9591. [Google Scholar] [CrossRef] [PubMed]
- Feliu, C.; Konecki, C.; Candau, T.; Vautier, D.; Haudecoeur, C.; Gozalo, C.; Cazaubon, Y.; Djerada, Z. Quantification of 15 antibiotics widely used in the critical care unit with a lc-ms/ms system: An easy method to perform a daily therapeutic drug monitoring. Pharmaceuticals 2021, 14, 1214. [Google Scholar] [CrossRef] [PubMed]
- Filatova, L.Y.; Klyachko, N.L.; Kudryashova, E. Targeted delivery of anti-tuberculosis drugs to macrophages: Targeting mannose receptors. Russ. Chem. Rev. 2018, 87, 374–391. [Google Scholar] [CrossRef]
- Galdopórpora, J.M.; Martinena, C.; Bernabeu, E.; Riedel, J.; Palmas, L.; Castangia, I.; Manca, M.L.; Garcés, M.; Lázaro-Martinez, J.; Salgueiro, M.J.; et al. Inhalable Mannosylated Rifampicin–Curcumin Co-Loaded Nanomicelles with Enhanced In Vitro Antimicrobial Efficacy for an Optimized Pulmonary Tuberculosis Therapy. Pharmaceutics 2022, 14, 959. [Google Scholar] [CrossRef]
- Peng, J.; Cai, Z.; Wang, Q.; Zhou, J.; Xu, J.; Pan, D.; Chen, T.; Zhang, G.; Tao, L.; Chen, Y.; et al. Carboxymethyl Chitosan Modified Oxymatrine Liposomes for the Alleviation of Emphysema in Mice via Pulmonary Administration. Molecules 2022, 27, 3610. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Mamaeva, P.V.; Skuredina, A.A.; Kudryashova, E.V. A Spectral Approach to Study Interaction between Chitosan Modified with Mannose and Concavalin A for the Creation of Address Delivery Systems of Antituberculosis Drugs. Mosc. Univ. Chem. Bull. 2020, 75, 213–217. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Methacrylated chitosan as a polymer with enhanced mucoadhesive properties for transmucosal drug delivery. Int. J. Pharm. 2018, 550, 123–129. [Google Scholar] [CrossRef]
- Garg, T.; Rath, G.; Goyal, A.K. Inhalable chitosan nanoparticles as antitubercular drug carriers for an effective treatment of tuberculosis. Artif. Cells Nanomed. Biotechnol. 2016, 44, 997–1001. [Google Scholar] [CrossRef]
- Abnoos, M.; Mohseni, M.; Mousavi, S.A.J.; Ashtari, K.; Ilka, R.; Mehravi, B. Chitosan-alginate nano-carrier for transdermal delivery of pirfenidone in idiopathic pulmonary fibrosis. Int. J. Biol. Macromol. 2018, 118, 1319–1325. [Google Scholar] [CrossRef]
- Efimova, A.A.; Abramova, T.A.; Popov, A.S. Complexes of Negatively Charged Liposomes with Chitosan: Effect of Phase State of the Lipid Bilayer. Russ. J. Gen. Chem. 2021, 91, 2079–2085. [Google Scholar] [CrossRef]
- Deygen, I.M.; Seidl, C.; Kolmel, D.K.; Bednarek, C.; Heissler, S.; Kudryashova, E.V.; Braese, S. Novel Prodrug of Doxorubicin Modified by Stearoylspermine encapsulated into PEG-Chitosan-Stabilized Liposomes. Langmuir 2016, 32, 10861–10869. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Niidome, T.; Lee, R. Glycol Chitosan-Docosahexaenoic Acid Liposomes for Drug Delivery: Synergistic Effect of Doxorubicin-Rapamycin in Drug-Resistant Breast Cancer. Mar. Drugs 2019, 17, 581. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Kudryashova, E.V. Computer simulation of the Receptor–Ligand Interactions of Mannose Receptor CD206 in Comparison with the Lectin Concanavalin A Model. Biochemistry 2022, 87, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Le-Deygen, I.M.; Skuredina, A.A.; Safronova, A.S.; Yakimov, I.D.; Kolmogorov, I.M.; Deygen, D.M.; Burova, V.T.; Grinberg, N.V.; Grinberg, V.Y.; Kudryashova, V.E. Moxifloxacin interacts with lipid bilayer, causing dramatic changes in its structure and phase transitions. Chem. Phys. Lipids 2020, 228, 104891. [Google Scholar] [CrossRef] [PubMed]
- Deygen, I.M.; Kudryashova, E.V. Structure and stability of anionic liposomes complexes with PEG-chitosan branched copolymer. Russ. J. Bioorg. Chem. 2014, 40, 547–557. [Google Scholar] [CrossRef]
- Deygen, I.M.; Kudryashova, E.V. New versatile approach for analysis of PEG content in conjugates and complexes with biomacromolecules based on FTIR spectroscopy. Colloids Surf. B Biointerfaces 2016, 141, 36–43. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Toskas, G.; Heinemann, S.; Heinemann, C.; Cherif, C.; Hund, R.D.; Roussis, V.; Hanke, T. Ulvan and ulvan/chitosan polyelectrolyte nanofibrous membranes as a potential substrate material for the cultivation of osteoblasts. Carbohydr. Polym. 2012, 89, 997–1002. [Google Scholar] [CrossRef]
- Kim, A.; Ng, W.B.; Bernt, W.; Cho, N.J. Validation of Size Estimation of Nanoparticle Tracking Analysis on Polydisperse Macromolecule Assembly. Sci. Rep. 2019, 9, 2639. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Rakhnyanskaya, A.A.; Yaroslavova, E.G.; Efimova, A.A.; Menger, F.M. Polyelectrolyte-coated liposomes: Stabilization of the interfacial complexes. Adv. Colloid. Interface Sci. 2008, 142, 43–52. [Google Scholar] [CrossRef]
- Wytrwal, M.; Bednar, J.; Nowakowska, M.; Wydro, P.; Kepczynski, M. Interactions of serum with polyelectrolyte-stabilized liposomes: Cryo-TEM studies. Colloids Surf. B Biointerfaces 2014, 120, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, E.A.A.M.; Kettenes-Van den Bosch, J.J.; Crommelin, D.J.A. Fourier transform infrared spectroscopic determination of the hydrolysis of poly(ethylene glycol)-phosphatidylethanolamine-containing liposomes. Langmuir 2002, 18, 3466–3470. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Raussens, V.; Ruysschaert, J.M. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim. Biophys. Acta 1999, 1422, 105–185. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Moreno, M.M.; Garidel, P.; Suwalsky, M.; Howe, J.; Brandenburg, K. The membrane-activity of Ibuprofen, Diclofenac, and Naproxen: A physico-chemical study with lecithin phospholipids. Biochim. Biophys. Acta 2009, 1788, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Moreno, M.; Howe, J.; Suwalsky, M.; Garidel, P.; Brandenburg, K. Physicochemical Interaction Study of Non-Steroidal Anti-Inflammatory Drugs with Dimyristoylphosphatidylethanolamine Liposomes. Lett. Drug Des. Discov. 2009, 7, 50–56. [Google Scholar] [CrossRef]
- Disalvo, E.A.; Frias, M.A. Water state and carbonyl distribution populations in confined regions of lipid bilayers observed by FTIR spectroscopy. Langmuir 2013, 29, 6969–6974. [Google Scholar] [CrossRef] [PubMed]
- Arsov, Z.; Quaroni, L. Direct interaction between cholesterol and phosphatidylcholines in hydrated membranes revealed by ATR-FTIR spectroscopy. Chem. Phys. Lipids 2007, 150, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Tamasi, G.; Donati, A.; Leone, G.; Consumi, M.; Cangeloni, L.; Volpi, V.; Magnani, A.; Cappelli, A.; Rossi, C. Physicochemical characterization of hyaluronic acid and chitosan liposome coatings. Appl. Sci. 2021, 11, 12071. [Google Scholar] [CrossRef]
- Varga, Z.; Mihály, J.; Berényi, S.; Bóta, A. Structural characterization of the poly(ethylene glycol) layer of sterically stabilized liposomes by means of FTIR spectroscopy. Eur. Polym. J. 2013, 49, 2415–2421. [Google Scholar] [CrossRef]
- Mady, M.M.; Darwish, M.M.; Khalil, S.; Khalil, W.M. Biophysical studies on chitosan-coated liposomes. Eur. Biophys. J. 2009, 38, 1127–1133. [Google Scholar] [CrossRef]
- Deygen, I.M.; Kudryashova, E.V. Effect of glycol chitosan on functional and structural properties of anionic liposomes. Mosc. Univ. Chem. Bull. 2016, 71, 154–159. [Google Scholar] [CrossRef]
- Bartucci, R.; Gulfo, N.; Sportelli, L. Effect of high electrolyte concentration on the phase transition behaviour of DPPC vesicles: A spin label study. Biomembranes 1990, 1025, 117–121. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Melik-Nubarov, N.S.; Menger, F.M. Polymer-induced flip-flop in biomembranes. Acc. Chem. Res. 2006, 39, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Mucha, M.; Pawlak, A. Thermal analysis of chitosan and its blends. Thermochim. Acta 2005, 427, 69–76. [Google Scholar] [CrossRef]
- van Landschoot, A.; Loontiens, F.G.; de Bruyne, C.K. Binding of Manno-oligosaccharides to Concanavalin A: Substitution Titration with a Fluorescent-Indicator Ligand. Eur. J. Biochem. 1980, 103, 307–312. [Google Scholar] [CrossRef]
- Mandal, D.K.; Kishore, N.; Brewer, C.F. Thermodynamics of Lectin-Carbohydrate Interactions Titration Microcalorimetry Measurements of the Binding of N-Linked Carbohydrates and Ovalbumin to Concanavalin A. Biochemistry 1994, 33, 1149–1156. [Google Scholar] [CrossRef]
- Glassford, S.E.; Byrne, B.; Kazarian, S.G. Recent applications of ATR FTIR spectroscopy and imaging to proteins. Biochim. Biophys. Acta 2013, 1834, 2849–2858. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Cabiaux, V.; Ruysschaert, M. Determination of Soluble and Membrane Protein Structure by Fourier Transform Infrared Spectroscopy III Secondary Structures. In Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 1994; pp. 405–450. [Google Scholar]
- Efremova, M.V.; Veselov, M.M.; Barulin, A.V.; Gribanovsky, S.L.; Le-Deygen, I.M.; Uporov, I.V.; Kudryashova, E.V.; Sokolsky-Papkov, M.; Majouga, A.G.; Golovin, Y.I.; et al. In situ Observation of Chymotrypsin Catalytic Activity Change Actuated by Non-Heating Low- Frequency Magnetic Field. ACS Nano 2018, 12, 3190–3199. [Google Scholar] [CrossRef]
- van de Weert, M.; Haris, P.I.; Hennink, W.E.; Crommelin, D.J.A. Fourier transform infrared spectrometric analysis of protein conformation: Effect of sampling method and stress factors. Anal. Biochem. 2001, 297, 160–169. [Google Scholar] [CrossRef]
- Sandoval-Altamirano, C.; Sanchez, S.A.; Ferreyra, N.F.; Gunther, G. Understanding the interaction of concanavalin a with mannosyl glycoliposomes: A surface plasmon resonance and fluorescence study. Colloids Surf. B Biointerfaces 2017, 158, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Roux, E.; Lafleur, M.; Lataste, É.; Moreau, P.; Leroux, J.C. On the characterization of pH-sensitive liposome/polymer complexes. Biomacromolecules 2003, 4, 240–248. [Google Scholar] [CrossRef] [PubMed]


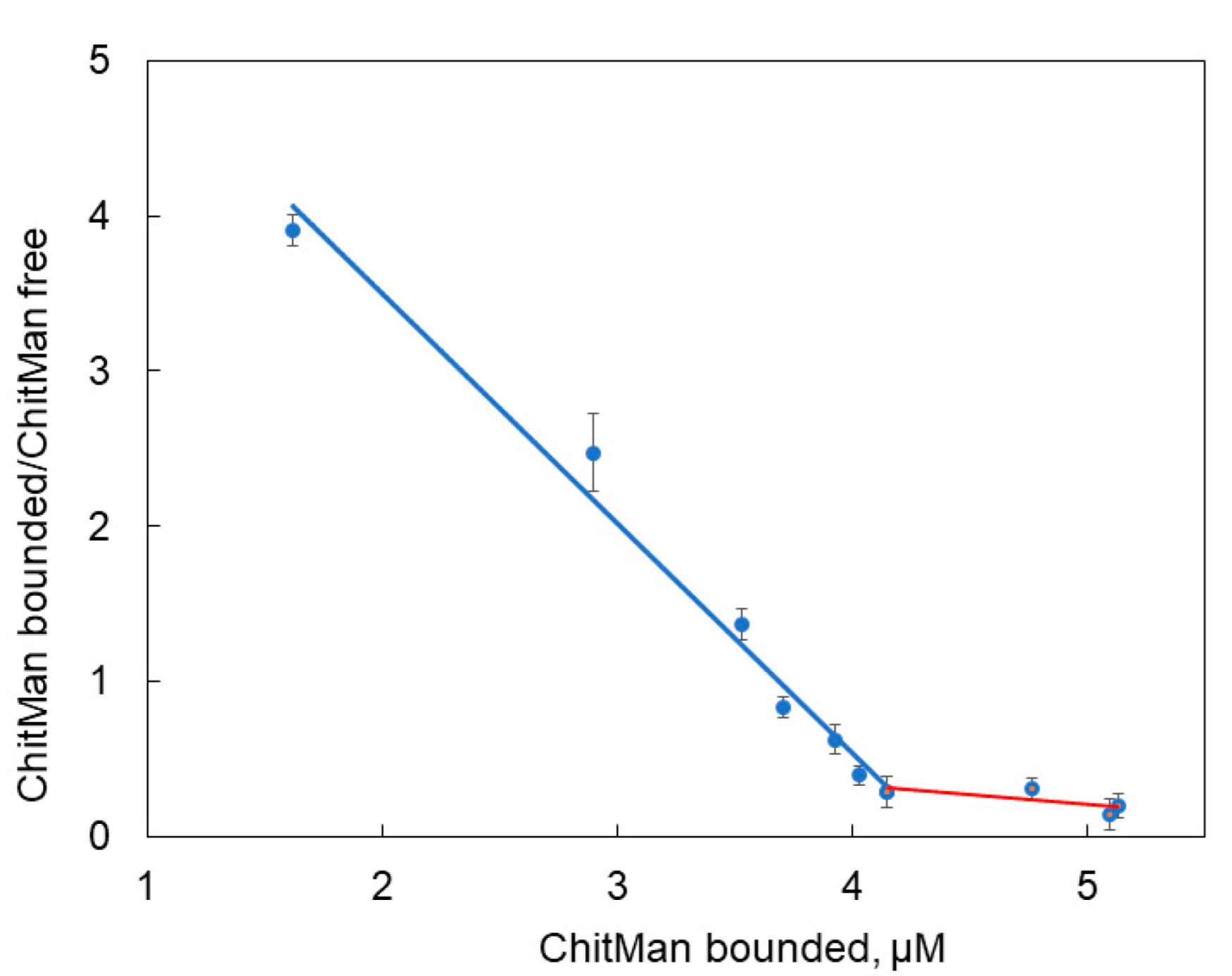
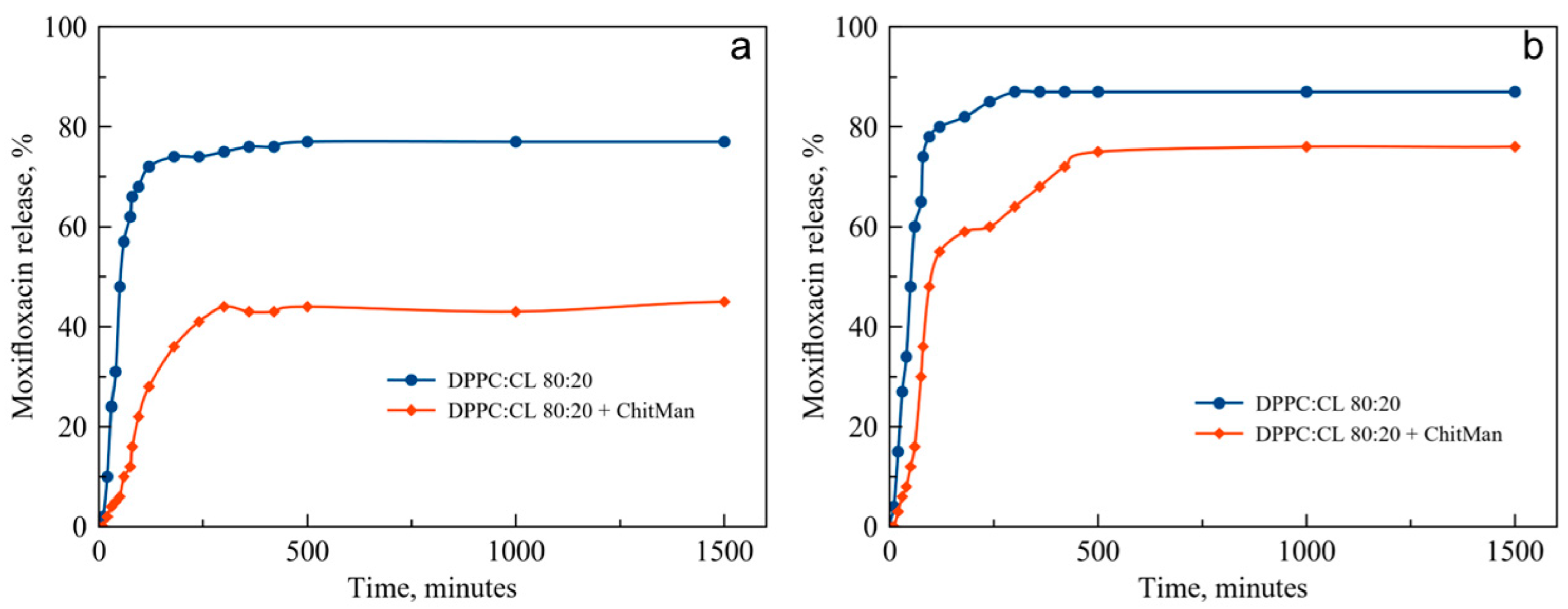
| Lipid Composition | Phase | Dh, nm (DLS) | Dh, nm (NTA) | ζ-Potential, mV |
|---|---|---|---|---|
| DPPC | Gel-like | 57 ± 2 | 70 ± 3 | −1.5 ± 0.5 |
| DPPC:CL 80:20 | Gel-like | 80 ± 3 | 89 ± 5 | −12 ± 1 |
| Egg PC | Liquid crystal | 112 ± 4 | 115 ± 3 | −2.5 ± 1 |
| Egg PC:CL 80:20 | Liquid crystal | 74 ± 5 | 80 ± 4 | −16.0 ± 1.5 |
| Lipid Composition | Preheating 37 °C | Kdis, M |
|---|---|---|
| DPPC | 0 min | (3.8 ± 0.1) × 104 |
| DPPC:CL 80:20 | 0 min | (1.2 ± 0.1) × 104 |
| DPPC:CL 80:20 | 15 min | (9.3 ± 0.1) × 105 |
| DPPC:CL 80:20 | 30 min | (9.5 ± 0.1) × 105 |
| DPPC:CL 80:20 | 45 min | (6.4 ± 0.1) × 105 |
| DPPC:CL 80:20 | 60 min | (3.5 ± 0.1) × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le-Deygen, I.M.; Rokosovina, V.V.; Skuredina, A.A.; Yakimov, I.D.; Kudryashova, E.V. The Charge and Phase State of Liposomes Dramatically Affects the Binding of Mannosylated Chitosan. Future Pharmacol. 2022, 2, 330-346. https://doi.org/10.3390/futurepharmacol2030023
Le-Deygen IM, Rokosovina VV, Skuredina AA, Yakimov ID, Kudryashova EV. The Charge and Phase State of Liposomes Dramatically Affects the Binding of Mannosylated Chitosan. Future Pharmacology. 2022; 2(3):330-346. https://doi.org/10.3390/futurepharmacol2030023
Chicago/Turabian StyleLe-Deygen, Irina M., Viktoria V. Rokosovina, Anna A. Skuredina, Ivan D. Yakimov, and Elena V. Kudryashova. 2022. "The Charge and Phase State of Liposomes Dramatically Affects the Binding of Mannosylated Chitosan" Future Pharmacology 2, no. 3: 330-346. https://doi.org/10.3390/futurepharmacol2030023
APA StyleLe-Deygen, I. M., Rokosovina, V. V., Skuredina, A. A., Yakimov, I. D., & Kudryashova, E. V. (2022). The Charge and Phase State of Liposomes Dramatically Affects the Binding of Mannosylated Chitosan. Future Pharmacology, 2(3), 330-346. https://doi.org/10.3390/futurepharmacol2030023








