The Influence of Cannabinoids on Multiple Myeloma Cells: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
- Quantitative, original research;
- Was a peer-reviewed, full-text article;
- Written in English;
- Tests the effects of CB treatment/CB receptor upregulation in MM patients/MM cell lines.
3. Results
3.1. Searches and Included Articles
3.2. Study Characteristics
3.3. Effects of CB on MM cells
3.3.1. Cell Viability
3.3.2. Apoptotic Effects/Pathways
3.3.3. Combinatory Effects
3.3.4. Effects on Drug-Resistant Cells
3.4. Effects of CB on Normal Cells
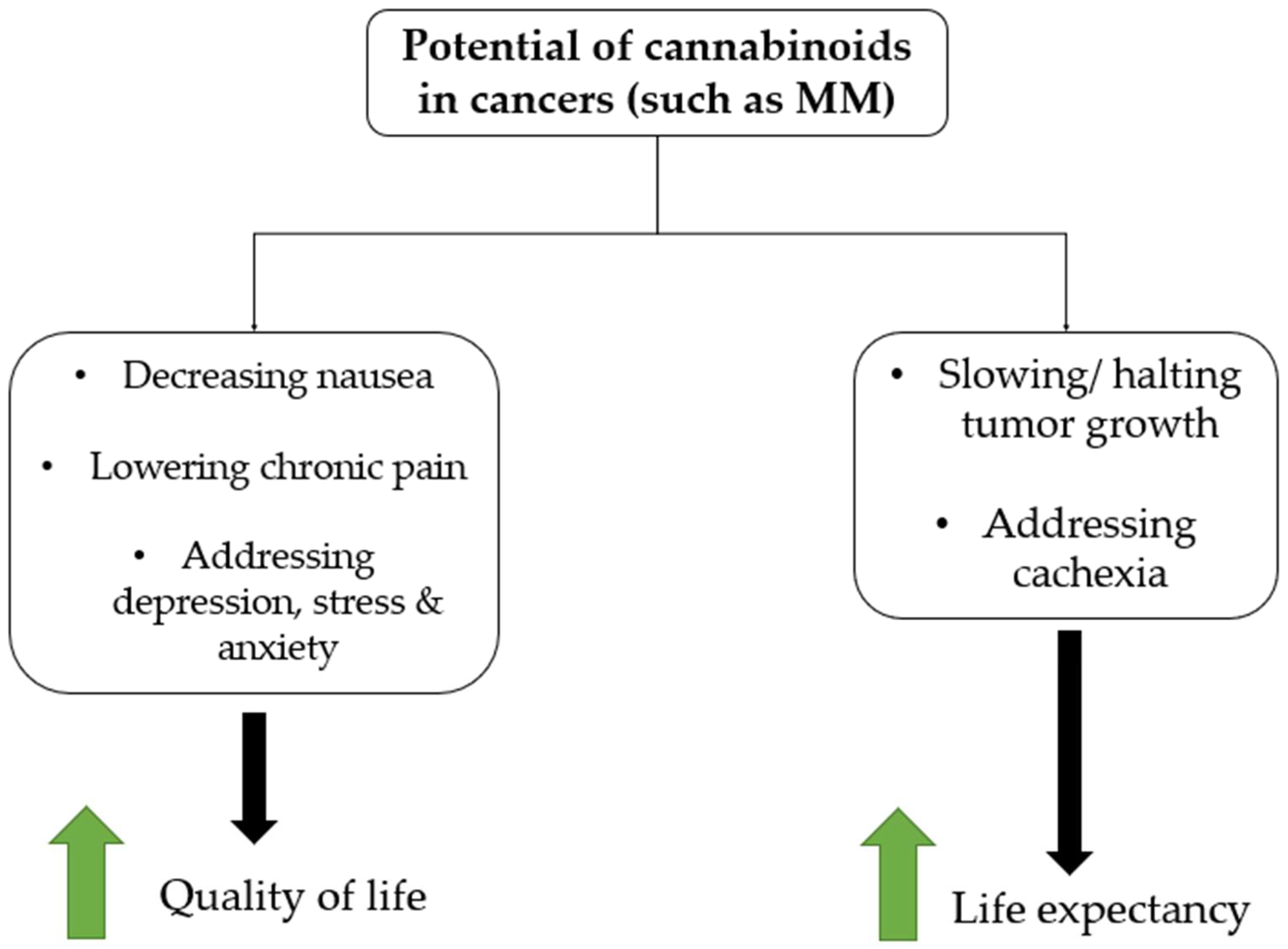
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, N.; Rizzoli, V.; Roodman, G.D. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood 2006, 108, 3992–3996. [Google Scholar] [CrossRef]
- Richardson, P.G.; Blood, E.; Mitsiades, C.S.; Jagannath, S.; Zeldenrust, S.R.; Alsina, M.; Schlossman, R.L.; Rajkumar, S.V.; Desikan, K.R.; Hideshima, T.; et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood 2006, 108, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Myeloma. CDC.gov. 2022. Available online: https://www.cdc.gov/cancer/myeloma/index.htm (accessed on 1 June 2022).
- American Cancer Society. Key Statistics About Multiple Myeloma. Cancer.org. 2022. Available online: https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html (accessed on 5 June 2022).
- SEER Lifetime Risk (Percent) of Being Diagnosed with Cancer by Site and Race/Ethnicity: Both Sexes, 18 SEER Areas, 2012–2014 (Table 1.15) National Cancer Institute: Bethesda, MD, USA. Available online: https://seer.cancer.gov/csr/1975_2014/results_merged/topic_lifetime_risk.pdf (accessed on 5 January 2018).
- Landgren, O.; Gridley, G.; Turesson, I.; Caporaso, N.E.; Goldin, L.R.; Baris, D.; Fears, T.R.; Hoover, R.N.; Linetl, M.S. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood 2006, 107, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Graubard, B.I.; Katzmann, J.A.; Kyle, R.A.; Ahmadizadeh, I.; Clark, R.; Kumar, S.K.; Dispenzieri, A.; Greenberg, A.; Therneau, T.M.; et al. Racial disparities in the prevalence of monoclonal gammopathies: A population-based study of 12,482 persons from the National Health and Nutritional Examination Survey. Leukemia 2014, 28, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI). SEER Cancer Statistics Review, 1975–2013; National Cancer Institute: Bethesda, MD, USA, 2016. Available online: http://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 15 June 2022).
- Multiple Myeloma Research Foundation. TheMMRF. Understanding survival statistics. N.d. Available online: https://themmrf.org/multiple-myeloma/prognosis/understanding-survival-statistics/ (accessed on 20 June 2022).
- Gregory, W.M.; Richards, M.; Malpas, J.S. Combination chemotherapy versus melphalan and prednisolone in the treatment of multiple myeloma: An overview of published trials. J. Clin. Oncol. 1992, 10, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Mehta, J.; Desikan, R.; Ayers, D.; Roberson, P.; Eddlemon, P.; Munshi, N.; Anaissie, E.; Wilson, C.; Dhodapkar, M.; et al. Antitumor Activity of Thalidomide in Refractory Multiple Myeloma. N. Engl. J. Med. 1999, 341, 1565–1571. [Google Scholar] [CrossRef]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Irwin, D.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.-L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2005, 352, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Hayman, S.R.; Lacy, M.Q.; Dispenzieri, A.; Geyer, S.M.; Kabat, B.; Zeldenrust, S.R.; Kumar, S.; Greipp, P.R.; Fonseca, R.; et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood 2005, 106, 4050–4053. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Jacobus, S.; Callander, N.S.; Fonseca, R.; Vesole, D.H.; Williams, M.; Abonour, R.; Siegel, D.S.; Katz, M.; Greipp, P.R. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010, 11, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Cavo, M.; Perrone, G.; Buttignol, S.; Calabrese, E.; Galli, M.; Bringhen, S.; Spadano, T.; Baldini, L.; Caravita, T.; Nozzoli, C.; et al. Bortezomib-thalidomide-dexamethasone compared with thalidomide-dexamethasone as induction and consolidation therapy before and after double autologous transplantation in newly diagnosed multiple myeloma: Results from a randomized phase 3 study. Blood 2010, 116, a42. [Google Scholar] [CrossRef]
- Kotla, V.; Goel, S.; Nischal, S.; Heuck, C.; Vivek, K.; Das, B.; Verma, A. Mechanism of action of lenalidomide in hematological malignancies. J. Hematol. Oncol. 2009, 2, 36. [Google Scholar] [CrossRef]
- Kane, R.C.; Bross, P.F.; Farrell, A.T.; Pazdur, R. Velcade®: U.S. FDA Approval for the Treatment of Multiple Myeloma Progressing on Prior Therapy. Oncologist 2003, 8, 508–513. [Google Scholar] [CrossRef]
- Richardson, P.G.; Weller, E.; Lonial, S.; Jakubowiak, A.J.; Jagannath, S.; Raje, N.S.; Avigan, D.E.; Xie, W.; Ghobrial, I.M.; Schlossman, R.L.; et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010, 116, 679–686. [Google Scholar] [CrossRef]
- Kumar, S.; Flinn, I.W.; Richardson, P.G.; Hari, P.; Callander, N.S.; Noga, S.J.; Stewart, A.K.; Glass, J.; Rifkin, R.M.; Wolf, J.L.; et al. Novel Three- and Four-Drug Combination Regimens of Bortezomib, Dexamethasone, Cyclophosphamide, and Lenalidomide, for Previously Untreated Multiple Myeloma: Results From the Multi-Center, Randomized, Phase 2 EVOLUTION Study. Blood 2010, 116, 621. [Google Scholar] [CrossRef]
- Kleber, M.; Ntanasis-Stathopoulos, I.; Terpos, E. BCMA in Multiple Myeloma—A Promising Key to Therapy. J. Clin. Med. 2021, 10, 4088. [Google Scholar] [CrossRef]
- Alhallak, K.; Sun, J.; Jeske, A.; Park, C.; Yavner, J.; Bash, H.; Lubben, B.; Adebayo, O.; Khaskiah, A.; Azab, A. Bispecific T Cell Engagers for the Treatment of Multiple Myeloma: Achievements and Challenges. Cancers 2021, 13, 2853. [Google Scholar] [CrossRef] [PubMed]
- ASH Clinical News. CAR T-Cell Therapies Predicted to Cost More Than $1 Million Per Patient. N.d. Ash Publications. Available online: https://ashpublications.org/ashclinicalnews/news/3469/CAR-T-Cell-Therapies-Predicted-to-Cost-More-Than-1 (accessed on 5 June 2022).
- Thorsteinsdottir, S.; Dickman, P.W.; Landgren, O.; Blimark, C.; Hultcrantz, M.; Turesson, I.; Björkholm, M.; Kristinsson, S.Y. Dramatically improved survival in multiple myeloma patients in the recent decade: Results from a Swedish population-based study. Haematologica 2018, 103, e412–e415. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Försti, A.; Hansson, M. Incidence, mortality and survival in multiple myeloma compared to other hematopoietic neoplasms in Sweden up to year 2016. Sci. Rep. 2021, 11, 17272. [Google Scholar] [CrossRef]
- Heider, C.G.; Itenberg, S.A.; Rao, J.; Ma, H.; Wu, X. Mechanisms of Cannabidiol (CBD) in Cancer Treatment: A Review. Biology 2022, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Likar, R.; Köstenberger, M.; Stultschnig, M.; Nahler, G. Concomitant Treatment of Malignant Brain Tumours With CBD–A Case Series and Review of the Literature. Anticancer Res. 2019, 39, 5797–5801. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Tong, M.; Pan, H.; Li, D. New Prospect for Cancer Cachexia: Medical Cannabinoid. J. Cancer 2019, 10, 716–720. [Google Scholar] [CrossRef]
- O’Brien, K. Cannabidiol (CBD) in Cancer Management. Cancers 2022, 14, 885. [Google Scholar] [CrossRef]
- Griffiths, C.; Aikins, J.; Warshal, D.; Ostrovsky, O. Can Cannabidiol Affect the Efficacy of Chemotherapy and Epigenetic Treatments in Cancer? Biomolecules 2021, 11, 766. [Google Scholar] [CrossRef]
- Hosami, F.; Ghadimkhah, M.H.; Salimi, V.; Ghorbanhosseini, S.S.; Tavakoli-Yaraki, M. The strengths and limits of cannabinoids and their receptors in cancer: Insights into the role of tumorigenesis-underlying mechanisms and therapeutic aspects. Biomed. Pharmacother. 2021, 144, 112279. [Google Scholar] [CrossRef]
- Zenone, M.; Snyder, J.; Caulfield, T. Crowdfunding Cannabidiol (CBD) for Cancer: Hype and Misinformation on GoFundMe. Am. J. Public Health 2020, 110, S294–S299. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Barbado, M.V.; Medrano, M.; Caballero-Velázquez, T.; Álvarez-Laderas, I.; Sánchez-Abarca, L.I.; García-Guerrero, E.; Martín-Sánchez, J.; Rosado, I.V.; Piruat, J.I.; Gonzalez-Naranjo, P.; et al. Cannabinoid derivatives exert a potent anti-myeloma activity both in vitro and in vivo. Int. J. Cancer 2017, 140, 674–685. [Google Scholar] [CrossRef]
- Feng, R.; Tong, Q.; Xie, Z.; Cheng, H.; Wang, L.; Lentzsch, S.; Roodman, G.D.; Xie, X.-Q. Targeting cannabinoid receptor-2 pathway by phenylacetylamide suppresses the proliferation of human myeloma cells through mitotic dysregulation and cytoskeleton disruption. Mol. Carcinog. 2015, 54, 1796–1806. [Google Scholar] [CrossRef] [Green Version]
- Garofano, F.; Schmidt-Wolf, I.G.H. High Expression of Cannabinoid Receptor 2 on Cytokine-Induced Killer Cells and Multiple Myeloma Cells. Int. J. Mol. Sci. 2020, 21, 3800. [Google Scholar] [CrossRef]
- Mannino, F.; Pallio, G.; Corsaro, R.; Minutoli, L.; Altavilla, D.; Vermiglio, G.; Allegra, A.; Eid, A.H.; Bitto, A.; Squadrito, F.; et al. Beta-Caryophyllene Exhibits Anti-Proliferative Effects through Apoptosis Induction and Cell Cycle Modulation in Multiple Myeloma Cells. Cancers 2021, 13, 5741. [Google Scholar] [CrossRef]
- Morelli, M.B.; Offidani, M.; Alesiani, F.; Discepoli, G.; Liberati, S.; Olivieri, A.; Santoni, M.; Santoni, G.; Leoni, P.; Nabissi, M. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int. J. Cancer 2014, 134, 2534–2546. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Offidani, M.; Amantini, C.; Gentili, S.; Soriani, A.; Cardinali, C.; Leoni, P.; Santoni, G. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016, 7, 77543. [Google Scholar] [CrossRef]
- Goodwin, J.A.; Coleman, E.A.; Sullivan, E.; Easley, R.; McNatt, P.K.; Chowdhury, N.; Stewart, C.B. Personal Financial Effects of Multiple Myeloma and Its Treatment. Cancer Nurs. 2013, 36, 301–308. [Google Scholar] [CrossRef]
- Katta, S.F. A Study of the Efficacy of Cannabidiol in Patients With Multiple Myeloma, Glioblastoma Multiforme, and GI Malignancies. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03607643 (accessed on 4 June 2022).
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef]

| Search terms: ((cannabinoids.mp) OR (Dronabinol.mp) OR (Cannabidiol.mp) OR (cannabis.mp) OR (Cannabaceae.mp) OR (THC) OR (delta-9-tetrahydrocannabinol) OR (cannabinoid) OR (canabinoid)) AND ((Multiple Myeloma.mp) OR (Myeloma Proteins.mp) OR (myelomas) OR (myeloma) OR (myelomatos) OR (Kahler disease)) | Result total: 18 |
| Author | Country | Aim | Drug | Cell Type/Receptor | Analyses | Main Findings |
|---|---|---|---|---|---|---|
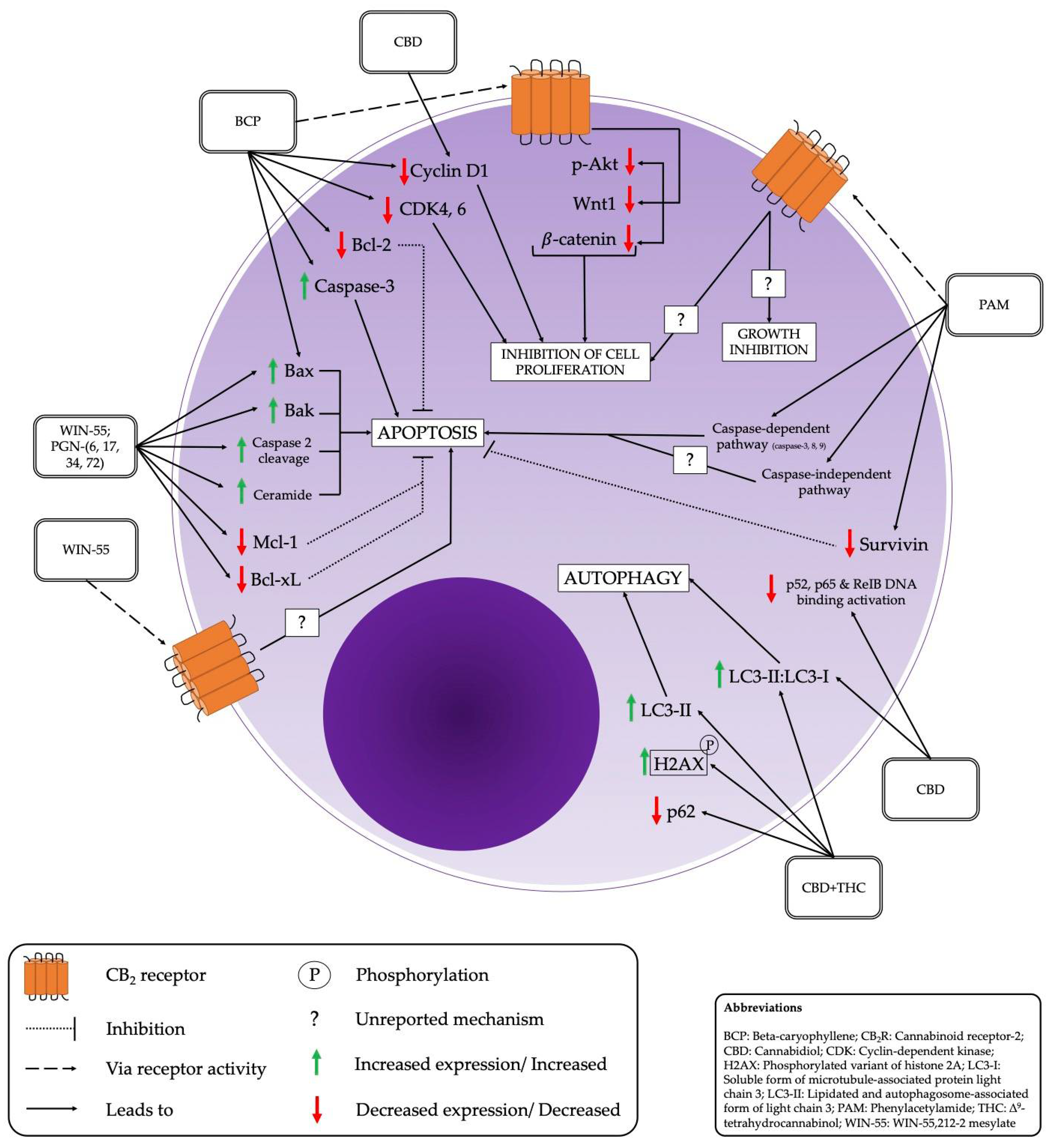
| Barbado et al. (2017) [35] | Spain | To study the effects of certain cannabinoids on proliferation and viability of myeloma plasma cells in vitro, as well as in vivo. | WIN-55, PGN-6, PGN-17, PGN-34, PGN-72 | CB2 | Assessed the effect of CB on cell viability using MTT assay and flow cytometry, Western blot analysis of CB-induced apoptotic mechanisms, protein expression and molecular pathways, confirmed the effects of CB mediated by CB2R, analyzed the effect of CB on other anti-myeloma agents using MTT assay and investigated the antitumor effect of CB in vivo using xenograft models. | PGN cannabinoids significantly MM cell viability. in pro-apoptotic proteins Bak and Bax, and expression of anti-apoptotic proteins Bcl-xL and Mcl-1. The apoptotic caspase-2 pathway was the most strongly activated. Akt is most strongly modulated, with a biphasic response. Ceramide was shown to have a major role in cannabinoid-induced apoptosis of MM cells. Slight, but sustained, in ER-stress protein markers such as CHOP, ATF-4, p-IRE1, and XBP-1 sec. WIN-55 has a synergistic effect in combination with dexamethasone and melphalan and overcame melphalan-resistance. CB administration tumor-volume. |
| Feng et al. (2015) [36] | USA | To provide insights regarding how CB2 ligands exert anti-MM effects and provide rationale for future in vivo investigations. | PAM | CB2 | Confirmation of significance of CB2R pathway in PAM-induced myeloma cell apoptosis with the use of gene silencing, H-thymidine incorporation assay, computer molecular modeling and docking studies, assessment of apoptotic cell death and cell viability, cell cycle analysis with flow cytometry, RT-PCR, and Western blotting. | PAM behaves as an inverse agonist of CB2R, and PAM may dock to the binding pocket of CB2 inverse agonist SR114528. PAM has antiproliferative and growth-inhibitory effects on myeloma cells. MM cells resistant to dexamethasone and melphalan exhibited either similar, or better, responses to PAM treatment, indicating that PAM may be able to overcome chemoresistance. PAM-induced apoptosis involves both caspase-independent and caspase-dependent pathways. It also survivin levels. PAM may exert its negative regulation of cell cycle of MM cells at different levels in the checkpoint. PAM is suggested to have an influence on cytoskeletal proteins via posttranslational modification mechanisms. |
| Garofano & Schmidt-Wolf (2020) [37] | Germany | To determine the antitumor effect of CIK cells combined with pure CBD in KMS-12 MM cells. | Pure CBD | CB2, CIK cells | Flow cytometry analysis of expression of CB2R and NKG2D in cells, analyzed the effect of CBD alone and in combination with CIK cells on MM cell viability and cytotoxicity using CCK8, LDH assay and flow-cytometry analysis, determined effect of CBD on NKT cell growth using flow cytometry. | CBD significantly LDH release in CIKs. CBD significantly LDH release in KMS-12 PE cells. CBD significantly cytotoxic activity of CIKs against MM cells at high concentrations. CBD significantly absolute number of alive CIK cells relative to control at 1 μM, 3 μM, 5 μM and 10 μM concentration and significant at 20 μM. CBD the percentage of NKT cells relative to controls. |
| Mannino et al. (2021) [38] | Qatar | To evaluate the antiproliferative and anticancer effects of BCP for MM.1R and MM.1S cells. | BCP | CB2 | After treating the cells, utilized FDA/PI staining for determining cell viability, MTT assay to evaluate cancer cell viability after BCP treatment, Trypan blue dye to quantify number of living and dead cells, ELISA to determine CDK4, CDK6, and Wnt1 levels, Western blot analysis to determine protein expression levels, and immunofluorescence staining. ANOVA with Tukey’s post hoc test for statistical analysis. | BCP selectively cell viability in MM cell lines but not healthy control populations. BCP significantly number of non-viable MM cells and number of viable MM cells. BCP induced apoptotic process in MM cells, by in expression of caspase-3, Bax, and Bcl-2 expression. BCP had anti-proliferative effects by significantly Wnt1 levels, p-AkT and -catenin protein expression. BCP significantly CDK4 and CDK6 levels and cyclin D1 protein expression. |
| Morelli et al. (2013) [39] | Italy | To evaluate the expression of TRPV2 in MM cells, and the TRPV-2 independent and dependent effects of CBD with BORT and CBD alone in MM cells. | CBD | CD138, CD34, TRPV2 | Cells were isolated, and compounds were obtained. The following analyses were conducted: FISH analysis, FACS analysis, Western blot analysis, flow cytometry analysis, colony forming assay, gene expression analysis, MTT assay, BrdU cell proliferation assay, cell cycle analysis, apoptosis assay, JC-1 staining, fluorescent probe DCFDA, DNA fragmentation assay, ELISA assay. ANOVA or Student’s t-test used for statistical analysis. | susceptibility to CBD in TRPV2+ MM cells compared to TRPV− MM cells. CBD and BORT combination treatment had a synergistic effect on MM cell viability with no effect on CD34+ cell growth. BORT and CBD combination treatment strongly cell proliferation and arrested cell cycle at G1. CBD and BORT significantly mitochondrial and ROS-dependent necrosis in MM cell lines. CBD and BORT synergized to pERK levels and inhibited/abrogated both ERK activation and AKT phosphorylation. CBD alone, and in combination with BORT, DNA binding activation of p52, p65, and RelB NF-kB subunits. |
| Nabissi et al. (2016) [40] | Italy | To evaluate the effects of THC alone, and in combination with CBD, on MM cell lines, and the effect of both of these in combination with CFZ. | THC, CBD | CB2, CXCR4 | MM cell lines, THC, and CBD compounds were obtained. Thereafter, the following analyses were conducted: MTT assay, cell cycle analysis, apoptosis assay, PI-staining, Western blot analysis, DNA fragmentation assay, RT-PCR analysis, cell migration assay. ANOVA or Student’s t-test were used for statistical analysis. | THC alone and in combination with CBD cytotoxicity of MM cells in a CB2R independent manner. THC-CBD combination was statistically more effective at arresting cells at G1 phase of cell cycle and cell accumulation in G1 and sub-G1 phases. THC-CBD combination induces autophagic cell death in MM cells. CBD alone LC3-II/LC3-I ratio, and THC-CBD greatly levels of LC3-II and LC3-II/LC3-I ratio; combination treatment also p62 levels. THC-CBD combination necrotic cell death and levels of H2AX compared to single treatments. THC-CBD the increased expression of β5i in MM cells and impaired expression of mature and precursor forms. THC-CBD synergizes with CFZ to significantly MM cell viability and induce cytotoxic effects. CFZ-THC-CBD cotreatment significantly CXCR4 and CD147 mRNA expression and SDF-1- and eCyPa-mediated chemotaxis in MM cells. |
| Compounds/Drugs | Apoptotic Pathways/Effects |
|---|---|
| WIN-55, PGN-(6, 17, 34, 72) [35] | Increase in pro-apoptotic proteins Bak and Bax. Decreased expression of anti-apoptotic proteins Bcl-xL and Mcl-1. The apoptotic caspase-2 pathway most strongly activated. Ceramide shown to have a major role in CB-induced apoptosis |
| PAM [36] | Both caspase-independent and caspase-dependent pathways activated. Lower survivin levels (anti-apoptotic protein). |
| CBD [37,39] | LDH release significantly higher than in controls [37]. Mitochondrial and ROS-dependent necrosis [39]. |
| BCP [38] | Induced an increase in caspase-3 and Bax (apoptotic), and decrease in Bcl-2 expression (anti-apoptotic). Showed a significant reduction in p-AkT, Wnt1, and B-catenin–thereby demonstrating an anti-proliferative effect. |
| THC and CBD [40] | Shown to induce a minor increase in LC3-II/LC3-I ratio. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varshney, K.; Ghosh, P.; Patel, A. The Influence of Cannabinoids on Multiple Myeloma Cells: A Scoping Review. Future Pharmacol. 2022, 2, 347-359. https://doi.org/10.3390/futurepharmacol2030024
Varshney K, Ghosh P, Patel A. The Influence of Cannabinoids on Multiple Myeloma Cells: A Scoping Review. Future Pharmacology. 2022; 2(3):347-359. https://doi.org/10.3390/futurepharmacol2030024
Chicago/Turabian StyleVarshney, Karan, Prerana Ghosh, and Akash Patel. 2022. "The Influence of Cannabinoids on Multiple Myeloma Cells: A Scoping Review" Future Pharmacology 2, no. 3: 347-359. https://doi.org/10.3390/futurepharmacol2030024
APA StyleVarshney, K., Ghosh, P., & Patel, A. (2022). The Influence of Cannabinoids on Multiple Myeloma Cells: A Scoping Review. Future Pharmacology, 2(3), 347-359. https://doi.org/10.3390/futurepharmacol2030024







