Abstract
Background/Objectives: Tinnitus lateralization, a perceptual characteristic often neglected in clinical assessment, may reflect underlying auditory plasticity. This study aimed to investigate whether changes in tinnitus lateralization following a multimodal neuromodulation protocol are associated with improved clinical outcomes, particularly regarding tinnitus severity and discomfort. Methods: A retrospective interventional study was conducted with 104 adults diagnosed with chronic tinnitus. All participants underwent a combined protocol involving transcutaneous auricular vagus nerve stimulation (taVNS), cervical transcutaneous electrical nerve stimulation (TENS), and photobiomodulation (PBM) targeting auditory pathways. Clinical assessments included the Tinnitus Handicap Inventory (THI), Visual Analog Scales (VAS) for loudness and discomfort, and lateralization reports before and after treatment. Lateralization patterns were categorized and compared using ANOVA and Kruskal–Wallis tests. Linear models explored demographic and clinical predictors of symptom change. Results: Substantial changes in lateralization were observed post-treatment. Participants who shifted from bilateral to unilateral tinnitus or from unilateral to non-perception showed the greatest symptom reductions (p < 0.001). The Bilateral → Unilateral group presented the most marked THI reduction (−20.82 ± 7.12), while minimal changes were observed in the Bilateral → Bilateral group. Loudness and discomfort improvements followed similar trends. No significant influence of age or sex on clinical response was identified, whereas longer tinnitus duration showed a modest positive association with symptom improvement. Conclusions: Perceptual reorganization of tinnitus lateralization may serve as a clinical marker of response to neuromodulation. Tracking lateralization could provide a simple, cost-effective adjunct to outcome monitoring in tinnitus management.
1. Introduction
Tinnitus is a highly prevalent auditory phantom perception that affects approximately 10–15% of the adult population, with significant heterogeneity in its phenomenology, severity, and underlying mechanisms [1,2]. Among the various subtypes, somatosensory tinnitus (ST) represents a clinically distinct entity in which auditory perception can be modulated by inputs from the cervical or trigeminal systems [3,4,5]. This cross-modal influence reflects the anatomical and functional convergence between auditory and somatosensory pathways, particularly within the dorsal cochlear nucleus (DCN), where inputs from the cochlea, upper cervical nerves (C1–C3), and the trigeminal system interact [6,7,8]. In such cases, alterations in neck or jaw muscle tone, postural asymmetry, or temporomandibular dysfunction can modify tinnitus loudness, pitch, or even its spatial perception.
Over the past decade, several interventions have aimed to target this somatosensory–auditory coupling through physical therapy, orofacial rehabilitation, or non-invasive neuromodulatory techniques [9,10,11,12,13]. These approaches seek to restore normal afferent balance and suppress the maladaptive hyperexcitability within the DCN and auditory cortices that perpetuate tinnitus [14,15,16]. Among the non-invasive modalities, the auricular vagus nerve stimulation (aVNS) and the transcutaneous electrical nerve stimulation (TENS) of cervical branches have attracted particular interest due to their ability to modulate ascending and descending inhibitory circuits. When delivered concurrently, these interventions may act synergistically, facilitating cortical reorganization through Hebbian-like mechanisms of associative plasticity [5,17,18]. Adjunctive techniques, such as transmeatal photobiomodulation, have also been proposed to reduce local oxidative stress and create a permissive environment for neural recovery [19,20,21,22].
Despite advances in multimodal therapy, most tinnitus studies still focus on global symptom outcomes—such as reductions in the Tinnitus Handicap Inventory (THI) or subjective loudness—without addressing how perceptual characteristics of tinnitus, particularly lateralization, evolve during treatment. Lateralization refers to the spatial localization of tinnitus within the auditory scene (right, left, bilateral, or diffuse perception) and reflects the balance of activity between bilateral auditory and somatosensory networks [14,23]. Cross-sectional neuroimaging studies have documented asymmetries in cortical and subcortical activity between patients with unilateral versus bilateral tinnitus [24,25,26], yet longitudinal changes in tinnitus lateralization have never been systematically examined as a therapeutic outcome.
Understanding whether perceptual reorganization—specifically, a shift in tinnitus lateralization—accompanies or predicts clinical improvement may provide valuable insights into the mechanisms of neuromodulation. If such spatial transformations mirror the normalization of neural synchrony across auditory–somatosensory circuits, they could serve as non-invasive behavioral markers of underlying plastic change. Furthermore, exploring how patient factors such as tinnitus chronicity influence this process may elucidate why some individuals respond more robustly to multimodal interventions than others.
Therefore, the present study aimed to investigate whether changes in tinnitus lateralization following multimodal stimulation protocol (aVNS combined with cervical TENS and transmeatal laser stimulation) are associated with differential clinical outcomes in patients with somatosensory tinnitus. We hypothesized that a shift from bilateral to unilateral or undefined tinnitus perception would correspond to greater improvements in tinnitus handicap, loudness, and discomfort, reflecting adaptive neuroplastic reorganization within the auditory–somatosensory network.
2. Results
The total number of participants included in this analysis was 104, representing the subset with complete data for pre- and post-treatment lateralization and outcome measures. A total of six distinct tinnitus lateralization change patterns, each including at least five participants, were retained for comparative analysis. These included: Bilateral → Bilateral (n = 29; 24.2%), Bilateral → Left (n = 25; 20.8%), Bilateral → Right (n = 7; 5.8%), Bilateral → Unilateral/Not specified (n = 11; 9.2%), Left → Left (n = 15; 12.5%), and Right → Right (n = 17; 14.2%). Table 1 summarizes the clinical outcomes according to tinnitus lateralization.

Table 1.
Summary of clinical outcomes according to tinnitus lateralization change pattern.
Descriptive statistics revealed meaningful variability in clinical outcomes across change patterns. The Bilateral → Unilateral/Not specified group exhibited the greatest mean reduction in THI (−48.18 ± 22.42), followed by Bilateral → Right (−44.86 ± 13.26), Bilateral → Left (−42.44 ± 14.46), and Left → Left (−38.67 ± 19.22). The Right → Right group showed a mean reduction of (−34.12 ± 15.74), whereas the Bilateral → Bilateral group demonstrated the smallest reduction in THI (−27.93 ± 13.71).
A similar pattern of change was observed in both loudness and discomfort outcomes across lateralization transition groups. Regarding loudness, the greatest reduction in tinnitus loudness scores was observed in the Bilateral → Unilateral/Not specified group (mean change = −5.82, SD = 2.89), followed closely by the Bilateral → Right group (mean = −5.43, SD = 1.27). In contrast, the smallest improvement occurred in the Bilateral → Bilateral group (mean = −3.28, SD = 1.93). Intermediate levels of improvement were observed in the Right → Bilateral group (mean = −4.47, SD = 2.75), the Unilateral/Not specified → Right group (mean = −4.29, SD = 3.45), and the Right → Right group (mean = −4.06, SD = 2.40). Discomfort scores exhibited a similar distribution pattern. The largest reductions were again seen in the Bilateral → Unilateral/Not specified (mean = −4.91, SD = 3.13) and Bilateral → Right (mean = −5.04, SD = 1.93) groups, whereas the Bilateral → Bilateral group had the smallest reduction (mean = −3.32, SD = 2.50). The other groups showed moderate reductions: Right → Bilateral (mean = −4.00, SD = 2.65), Right → Right (mean = −3.94, SD = 2.61), and Unilateral/Not specified → Right (mean = −4.18, SD = 3.04). These consistent trends reinforce the association between changes in tinnitus lateralization and the degree of symptom alleviation as perceived by patients.
Statistical comparisons using one-way ANOVA confirmed significant group effects for all three outcomes: ΔTHI (F5,98 = 4.27, p = 0.0015), ΔLoudness (F5,98 = 4.11, p = 0.0020), and ΔDiscomfort (F5,98 = 3.15, p = 0.0111). Assumption checks indicated deviations from normality and homogeneity of variances in at least one outcome variable. As a result, Kruskal–Wallis tests were also performed, which corroborated the findings: ΔTHI (H5 = 19.26, p = 0.0017), ΔLoudness (H5 = 17.84, p = 0.0031), and ΔDiscomfort (H5 = 14.06, p = 0.0152).
To explore whether other patient characteristics contributed to the magnitude of symptom improvement, multivariate linear regression models were constructed including age and tinnitus duration as covariates, alongside the change pattern. Age was not significantly associated with any of the outcome variables (all p > 0.21). In contrast, tinnitus duration showed a statistically significant or borderline association with ΔTHI (p = 0.038), ΔLoudness (p = 0.021), and ΔDiscomfort (p = 0.051), particularly in participants with tinnitus longer than five years. These findings suggest that longer tinnitus chronicity may be associated with greater symptom relief, independently of the perceptual change in lateralization.
Together, these results highlight that a change in the spatial perception of tinnitus—particularly from bilateral to unilateral or undefined patterns—is associated with greater clinical benefit, and that tinnitus chronicity may further modulate treatment responsiveness, possibly through mechanisms of cumulative neuroplastic adaptation.
3. Discussion
The present study demonstrated that changes in tinnitus lateralization following a multimodal neuromodulatory intervention were significantly associated with clinical improvement. Participants who exhibited a shift from bilateral to unilateral or unspecified lateralization patterns experienced the greatest reductions in tinnitus handicap, subjective loudness, and tinnitus-related discomfort. Statistical analyses confirmed robust group differences across all outcome measures, corroborated by both parametric and non-parametric tests. Furthermore, while age was not a significant predictor of clinical response, tinnitus duration showed a modest but consistent association with greater symptom relief, suggesting that individuals with longer-standing tinnitus may exhibit enhanced responsiveness to neuromodulatory treatment. These findings support the hypothesis that perceptual reorganization of tinnitus lateralization could serve as a potential marker of neuromodulation-induced clinical improvement.
From a neurobiological perspective, tinnitus lateralization is shaped by asymmetric patterns of hyperexcitability across the dorsal cochlear nucleus (DCN), inferior colliculi, and auditory cortices [14,16]. The DCN is a major site of convergence between auditory input and somatosensory afferents from the trigeminal and upper cervical nerves (particularly C2) [15]. In somatosensory tinnitus, aberrant somatic input enhances spontaneous firing rates and synchrony within the DCN, leading to a pathological coupling between neck/jaw proprioceptive signals and auditory perception [5]. This abnormal cross-modal integration can result in bilateral or shifting tinnitus percepts. When applied concurrently, taVNS and C2 TENS provide temporally synchronous afferent drive from both vagal and somatosensory sources [12]. This coactivation enhances Hebbian-like plasticity within the DCN and auditory cortex, promoting suppression of maladaptive bilateral synchrony [3,27,28]. Consequently, one hemisphere or pathway may normalize faster, leading to a perceptual shift from bilateral to unilateral tinnitus [12,14,17,29,30,31]. This asymmetry in cortical reorganization corresponds to a reduction in global network hyperconnectivity and, therefore, to symptom improvement.
An intriguing and counterintuitive observation was that participants with longer tinnitus duration exhibited greater clinical improvements, independently of lateralization pattern. Rather than reflecting resistance to change, long-standing tinnitus may represent a dynamically unstable system with expanded and redundant neural representations [3,5,6,11,32,33]. Chronic maladaptive plasticity, while initially pathogenic, also provides a broader substrate for corrective reorganization when appropriately stimulated [12,20,28,30,34]. Multimodal neuromodulation may exploit this latent plastic potential, driving large-scale network recalibration that manifests as perceptual and symptomatic improvement [15,16,19,22,35]. This interpretation aligns with observations in chronic pain and depression, where neuromodulatory interventions induce more marked effects in long-duration cases due to higher baseline excitability and compensatory circuit recruitment. Although chronic stress and long-standing tinnitus are typically considered more resistant to treatment, our results revealed the opposite pattern—greater improvement in participants with longer tinnitus duration. This divergence from prior studies may reflect the engagement of compensatory or latent neuroplastic mechanisms in chronic cases, in which prolonged maladaptive excitability provides a broader substrate for reorganization when multimodal stimulation is applied. This hypothesis aligns with literature demonstrating that high baseline neural hyperactivity can enhance responsiveness to coordinated neuromodulatory interventions.
Clinically, these findings suggest that a shift in tinnitus spatial perception—particularly from bilateral to unilateral or undefined forms—may be interpreted as an adaptive marker of neural reorganization, not as a negative or unstable outcome. Monitoring such perceptual transitions could provide valuable feedback for titrating neuromodulation protocols, serving as a noninvasive, patient-reported indicator of effective plastic change. The observed hierarchy of response (Bilateral → Unilateral/Undefined > Bilateral → Unilateral > Unilateral stable > Bilateral stable) highlights that perceptual consolidation into a single locus of tinnitus may correspond to the restoration of asymmetric, functionally balanced network dynamics [34].
To our knowledge, this is the first clinical study to treat pre-to-post treatment shifts in tinnitus lateralization as a primary marker and to show that bilateral → unilateral/undefined transitions are independently associated with larger improvements in THI, loudness, and discomfort. However, previous investigations on somatosensory tinnitus have primarily characterized within-session modulation phenomena, in which somatic maneuvers—such as jaw clenching, head rotation, or pressure over cervical trigger points—produce transient changes in tinnitus loudness, pitch, or localization [2,3,33,36]. These studies established that the tinnitus percept is dynamically linked to somatosensory input, but they did not longitudinally quantify stable shifts in lateralization following therapeutic intervention. Most clinical trials evaluating physical therapy [2], orofacial treatment [12,22], or bimodal neuromodulation [37] have focused on symptom reduction (THI, VAS) without examining whether perceptual reorganization occurred over time.
Furthermore, existing neurophysiological research has demonstrated asymmetric patterns of activity in the dorsal cochlear nucleus and auditory cortex among patients with unilateral tinnitus [14,16,25]. However, these asymmetries have been interpreted as static correlates of tinnitus side, not as dynamic markers of recovery. Functional imaging studies revealed lateralized alterations in auditory and limbic connectivity [33,34,38], but none tracked perceptual migration from bilateral to unilateral tinnitus during or after treatment. As such, although lateralization is a recognized attribute of tinnitus perception, its transformation through neuromodulation has remained undocumented.
The present findings therefore extend prior literature by introducing tinnitus lateralization change as a quantifiable, behaviorally accessible marker of neural reorganization. Unlike prior works that assessed modulation within a single session, this study captured pre-to-post treatment transitions across a large clinical sample, linking them statistically to improvements in tinnitus handicap, loudness, and discomfort. The observation that shifts from bilateral to unilateral or undefined perception predicted greater benefit supports the notion that spatial consolidation of the tinnitus percept reflects normalization of interhemispheric excitability and suppression of maladaptive bilateral synchrony [5,6,24]. This interpretation bridges the gap between neurophysiological theories of tinnitus asymmetry and clinically observable outcomes.
Additionally, no previous clinical trial has concurrently employed multimodal peripheral neuromodulation—combining auricular vagus nerve stimulation, cervical TENS, and transmeatal laser stimulation—to investigate perceptual reorganization. Prior bimodal paradigms have typically paired vagal stimulation with acoustic tones [39] or used somatosensory input alone [9]. By demonstrating that synchronized vagal and cervical inputs can drive both symptom improvement and spatial reorganization, the present study suggests a broader principle of network-level entrainment, in which coordinated peripheral stimulation fosters hemispheric rebalancing within auditory–somatosensory circuits.
While earlier research has often associated tinnitus chronicity with treatment resistance [20,21,29,32,34], our findings revealed the opposite trend—greater improvement among individuals with longer-standing tinnitus. This discrepancy may arise from methodological differences: prior studies employed single-modality interventions or focused on early tinnitus stages, whereas the current multimodal approach may have leveraged the enhanced plastic potential inherent in chronically hyperactive networks [40]. Consequently, our results suggest that chronicity does not preclude responsiveness; rather, it may amplify susceptibility to corrective plasticity when stimulation is multidimensional and temporally coordinated. Although the specific combination of taVNS, cervical TENS, and transmeatal photobiomodulation represents a novel multimodal approach, the individual contribution of each component remains undetermined. Future randomized controlled studies should systematically compare single-modality versus combined interventions to delineate synergistic versus independent effects on tinnitus lateralization and clinical outcomes.
Although the present findings are robust and statistically supported, the study design was observational and limited to perceptual and clinical measures. Future research should combine neurophysiological assessments—such as EEG coherence, brainstem auditory evoked potentials, or functional near-infrared spectroscopy (fNIRS)—to directly quantify the neural correlates of lateralization changes. Additionally, incorporating temporal tracking of lateralization during treatment could clarify whether early shifts predict long-term improvement. Replication in randomized controlled designs and comparison across different neuromodulation modalities (e.g., taVNS alone versus multimodal stimulation) would help delineate the specific contribution of each component. The retrospective and observational nature of this study imposes intrinsic limitations, as it precludes causal inference and relies on pre-existing clinical records. Consequently, the temporal dynamics of perceptual reorganization and its mechanistic underpinnings cannot be fully established. Prospective and randomized controlled designs are warranted to validate these findings and isolate the causal contribution of each stimulation component. Another limitation is the absence of objective neurophysiological measures (e.g., EEG, auditory evoked potentials, or functional near-infrared spectroscopy) that could validate the neural correlates of perceptual reorganization. These techniques were not part of the routine clinical protocol at the time of data collection. Future studies should incorporate such tools to directly assess neural synchronization and connectivity changes underlying tinnitus lateralization shifts. Furthermore, long-term follow-up data were unavailable, preventing assessment of the persistence or relapse of lateralization changes and symptom improvement over time. Future prospective studies should include extended post-intervention monitoring to determine whether perceptual reorganization is stable or subject to reversion.
4. Materials and Methods
4.1. Participants
This quasi-experimental retrospective investigation was based on the clinical records of 104 individuals diagnosed with somatosensory tinnitus (Table 2). All participants had received treatment at the Cabugueira Health Center, Rio de Janeiro, Brazil. A post hoc two-tailed power analysis indicated an achieved statistical power greater than 0.99, considering a sample size of 104, an alpha level of 0.05, and an observed effect size of 2.01. Patients presenting with additional otologic conditions, such as otosclerosis or otitis media, were excluded to ensure diagnostic specificity. Exclusion criteria also encompassed individuals with severe psychiatric disorders, significant cardiovascular or cerebrovascular disease, inability to complete study procedures, or incomplete clinical records. Participants’ characteristics are described in Table 2. As this was a retrospective clinical analysis, neurophysiological or neuroimaging data such as EEG or brain MRI were not available in patient records and therefore could not be included in the present analysis.

Table 2.
Participants’ characteristics.
All participants were fully informed about the study objectives and procedures before inclusion, and written informed consent was obtained from each subject. The research protocol received prior approval from the Ethics Committee of the Federal University of Juiz de Fora (CAAE: 85801925.2.0000.5147).
4.2. Outcome Assessment
Each patient record included data on audiometric thresholds, tinnitus duration, and tinnitus lateralization (left, right, or bilateral). Tinnitus intensity (“How loud is your tinnitus?”) was rated on a 10-point Visual Analogue Scale (VAS), ranging from 0 (“no tinnitus”) to 10 (“as loud as imaginable”). The VAS consisted of a 10 cm horizontal line anchored at both extremes, where participants indicated their perceived loudness. The same scale was applied to assess tinnitus-related discomfort. According to established clinical criteria, VAS scores were classified as mild (0–3), moderate (4–7), or severe (8–10) [7].
Functional impact was further evaluated using the THI, developed by Newman and Jacobson [41]. The validated Portuguese version of the THI was administered to assess functional, emotional, and catastrophic dimensions of tinnitus-related distress [42]. The total THI score was categorized into five severity grades: Grade 1 (0–16): Slight—perceived only in quiet environments, Grade 2 (18–36): Mild—easily masked by environmental sounds and forgotten with distraction, Grade 3 (38–56): Moderate—noticeable in background noise but compatible with normal activities, Grade 4 (58–76): Severe—frequently perceived, interferes with sleep and daily tasks, Grade 5 (78–100): Catastrophic—constantly perceived, causes sleep disturbance and functional impairment. All assessments were performed both before and after the multimodal intervention.
4.3. Intervention
Each patient underwent a standardized course of multimodal therapy consisting of ten sessions, administered twice weekly over five weeks, following a uniform stimulation protocol. Figure 1 illustrates the intervention procedures. Auricular stimulation was conducted using a point-finder device (EL 11, NKL, Brusque, Brazil) connected to a reference electrode to identify low-impedance sites on the left cymba conchae and the internal surface of the tragus—areas predominantly innervated by the auricular branch of the vagus nerve. With the patient positioned in lateral decubitus, the auricular surface was disinfected with 70% alcohol, and disposable acupuncture needles (Dux, 0.25 × 15 mm, Porto Alegre, Brazil) were inserted approximately 2 mm into the identified points.
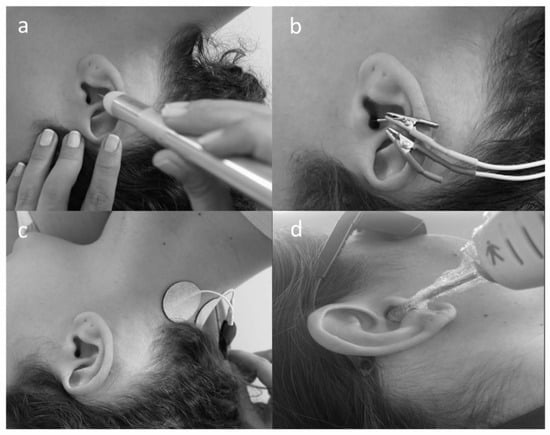
Figure 1.
Intervention procedures: (a) point finder device locating the low-level impedance points; (b) electrical stimulation using the percutaneous vagus nerve stimulation; (c) TENS protocol: C2 electrodes positioning; (d) photobiomodulation protocol: transmeatal laser irradiation.
Electrical stimulation was applied via alligator-clip electrodes attached to the needles and delivered through a two-channel TENS device (Neurodyn Portable, Ibramed, Amparo, Brazil) for 15 min, generating biphasic square-wave pulses (pulse width: 250 μs, frequency: 20 Hz; resistance: ~4–7 kΩ). When necessary due to discomfort, stimulation parameters were adjusted to 15 Hz and 12 min. The intensity was individually adjusted to each patient’s subjective tolerance threshold.
For cervical TENS, the C2 vertebra was located by palpation, and two 3-cm reusable circular electrodes (Valutrode, Arktus, Santa Teresa do Oeste, Brazil) were placed bilaterally over the C2 dermatomes. Stimulation was delivered through the same TENS unit for 30 min using a biphasic rectangular waveform (pulse width: 150 μs). The first 10 min employed a frequency of 40 Hz, the next 10 min 6 Hz, and the final 10 min a burst mode (burst rate: 2 Hz; within-burst frequency: 250 Hz). Intensity was set according to the maximum comfortable sensory level. Both aVNS and cervical TENS were administered simultaneously.
Photobiomodulation therapy was applied using an AlGaAs diode laser (808 nm wavelength, Class II, 100 mW output; Therapy EC, DMC, São Carlos, Brazil). The laser probe was positioned in the external auditory canal, at the level of its second curvature, for 180 s, delivering approximately 18 J of energy. A second irradiation was performed on the mastoid region for 90 s, delivering an additional 9 J at the same wavelength.
4.4. Statistical Procedures
All statistical analyses were conducted using Python (v3.11), employing the pandas, scipy.stats, and statsmodels libraries for data preprocessing, assumption testing, and inferential statistics. Pre- and post-treatment tinnitus lateralization was recorded using structured descriptors and standardized into three main categories: Right, Left, and Bilateral. Descriptions such as “unilateral” or “not specified/unilateral” were classified as Unilateral/Not specified, based on internal clinical criteria. A derived variable, Change Pattern, was created by concatenating pre- and post-intervention lateralization categories (e.g., “Bilateral → Left”).
All patients with available data for lateralization at both timepoints and complete pre/post scores for clinical outcomes were included. Clinical response was measured using three outcome variables: The THI, subjective loudness VAS, and tinnitus-related discomfort VAS. For each, a delta score (post-treatment minus pre-treatment) was computed. Descriptive statistics (mean, standard deviation, and count) were calculated for each Change Pattern group. For group comparisons, only change patterns that included five or more participants were retained to ensure sufficient statistical power and to meet the assumptions of parametric testing. Six change patterns met this criterion: Bilateral → Bilateral (n = 29), Bilateral → Left (n = 25), Bilateral → Right (n = 7), Bilateral → Unilateral/Not specified (n = 11), Left → Left (n = 15), and Right → Right (n = 17), totaling 104 participants.
Normality of residuals within each group was assessed using Shapiro–Wilk tests and visual diagnostics (histograms, Q–Q plots). Homogeneity of variances across groups was evaluated using Levene’s test. Based on these assessments, a one-way ANOVA was employed to compare means across groups for each clinical outcome (ΔTHI, ΔLoudness, and ΔDiscomfort). Given mild violations of parametric assumptions in some groups, a non-parametric Kruskal–Wallis H test was also performed for each outcome as a confirmatory procedure. All computations were conducted using reproducible Python scripts, ensuring methodological transparency.
To evaluate the potential influence of additional clinical factors on treatment response, multiple linear regression models were constructed for each outcome variable (ΔTHI, ΔLoudness, and ΔDiscomfort), including age and tinnitus duration as covariates alongside the categorical variable Change Pattern. Tinnitus duration was treated as a categorical variable, reflecting the clinical recording structure. The significance of each predictor was assessed while controlling for the others. This analysis aimed to determine whether these patient characteristics independently contributed to clinical improvement beyond the observed lateralization pattern. The significance level was set at α = 0.05 for all inferential tests.
5. Conclusions
In summary, the perceptual reorganization of tinnitus lateralization following multimodal neuromodulation—particularly transitions from bilateral to unilateral perception—appears to reflect underlying normalization of auditory–somatosensory interactions within the dorsal cochlear and cortical networks. This phenomenon may serve as a clinically observable correlate of beneficial neuroplastic adaptation, with potential utility as a biomarker of treatment efficacy in somatosensory tinnitus.
Author Contributions
Conceptualization, B.C. and A.C.B.; methodology, B.C. and B.R.B.; software, K.R.F.; validation, K.R.F., T.C.O. and B.R.B.; formal analysis, K.R.F., M.A.B. and G.L.G.; investigation, B.C., B.R.B. and T.C.O.; resources, A.C.B., G.L.G. and M.A.B.; data curation, B.R.B., K.R.F. and T.C.O.; writing—original draft preparation, B.R.B. and K.R.F.; writing—review and editing, A.C.B., G.L.G. and M.A.B.; visualization, B.C.; supervision, A.C.B., G.L.G. and M.A.B.; project administration, A.C.B. and G.L.G.; funding acquisition, A.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Foundation of the State of Minas Gerais (FAPEMIG), research grant APQ 02040-18, with contribution from Coordination for the Improvement of Higher Education Personnel (CAPES), code 001.
Institutional Review Board Statement
Ethics Committee of the Federal University of Juiz de Fora (number CAAE: 85801925.2.0000.5147); date: 28 March 2025.
Informed Consent Statement
Written informed consent was obtained from each subject. Written informed consent has been obtained from all participants to publish this paper.
Data Availability Statement
Raw data is fully online available through the https://doi.org/10.17632/87hy5gmjh9.1.
Acknowledgments
The authors acknowledge the Department of Physical Therapy of the Federal University of Juiz de Fora for supporting the research group. During the preparation of this manuscript/study, the authors used the OpenAI chatGPT, version 5, for the purposes of English editing. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jarach, C.M.; Lugo, A.; Scala, M.; van den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global Prevalence and Incidence of Tinnitus. JAMA Neurol. 2022, 79, 888. [Google Scholar] [CrossRef]
- Haider, H.F.; Hoare, D.J.; Costa, R.F.P.; Potgieter, I.; Kikidis, D.; Lapira, A.; Nikitas, C.; Caria, H.; Cunha, N.T.; Paço, J.C. Pathophysiology, Diagnosis and Treatment of Somatosensory Tinnitus: A Scoping Review. Front. Neurosci. 2017, 11, 207. [Google Scholar] [CrossRef]
- Ralli, M.; Greco, A.; Turchetta, R.; Altissimi, G.; de Vincentiis, M.; Cianfrone, G. Somatosensory Tinnitus: Current Evidence and Future Perspectives. J. Int. Med. Res. 2017, 45, 933–947. [Google Scholar] [CrossRef]
- Butt, M.F.; Albusoda, A.; Farmer, A.D.; Aziz, Q. The Anatomical Basis for Transcutaneous Auricular Vagus Nerve Stimulation. J. Anat. 2020, 236, 588–611. [Google Scholar] [CrossRef]
- Shore, S.E.; Roberts, L.E.; Langguth, B. Maladaptive Plasticity in Tinnitus—Triggers, Mechanisms and Treatment. Nat. Rev. Neurol. 2016, 12, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.F.; Bojić, T.; Ribeiro, S.F.; Paço, J.; Hall, D.A.; Szczepek, A.J. Pathophysiology of Subjective Tinnitus: Triggers and Maintenance. Front. Neurosci. 2018, 12, 866. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, L.S.; de Moraes Marchiori, L.L.; de Almeida Soares Ciquinato, D.; de Castro Teixeira, D.; de Moraes Marchiori, G.; Branco, B.H.M.; Poli-Frederico, R.C. Inflammatory Biomarkers and Tinnitus in Older Adults. Noise Health 2024, 26, 535–542. [Google Scholar] [CrossRef] [PubMed]
- da Costa, W.S.M.; de Araújo, L.B.; de Paula Bedaque, H.; Ferreira, L.M.d.B.M.; de Figueiredo, K.M.O.B. Impact of the Somatosensory Influence on Annoyance and Quality of Life of Individuals with Tinnitus: A Cross-Sectional Study. Braz. J. Otorhinolaryngol. 2025, 91, 101542. [Google Scholar] [CrossRef]
- van der Wal, A.; Michiels, S.; Van de Heyning, P.; Braem, M.; Visscher, C.M.; Topsakal, V.; Gilles, A.; Jacquemin, L.; Van Rompaey, V.; De Hertogh, W. Treatment of Somatosensory Tinnitus: A Randomized Controlled Trial Studying the Effect of Orofacial Treatment as Part of a Multidisciplinary Program. J. Clin. Med. 2020, 9, 705. [Google Scholar] [CrossRef]
- Nikookam, Y.; Zia, N.; Lotfallah, A.; Muzaffar, J.; Davis-Manders, J.; Kullar, P.; Smith, M.E.; Bale, G.; Boyle, P.; Irving, R.; et al. The Effect of Photobiomodulation on Tinnitus: A Systematic Review. J. Laryngol. Otol. 2024, 138, 710–731. [Google Scholar] [CrossRef]
- Kleinjung, T.; Peter, N.; Schecklmann, M.; Langguth, B. The Current State of Tinnitus Diagnosis and Treatment: A Multidisciplinary Expert Perspective. J. Assoc. Res. Otolaryngol. 2024, 25, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Jain, S.; Patil, N.; Jungade, S. Cervicogenic Somatic Tinnitus: A Narrative Review Exploring Non-Otologic Causes. Cureus 2024, 16, e65476. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernando, D.; Fernández-de-las-Peñas, C.; Pareja-Grande, J.A.; García-Esteo, F.J.; Mesa-Jiménez, J.A. Management of Auricular Transcutaneous Neuromodulation and Electro-Acupuncture of the Vagus Nerve for Chronic Migraine: A Systematic Review. Front. Neurosci. 2023, 17, 1151892. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, T.; Ciralli, B.; Hilscher, M.M.; Leao, R.N.; Leao, K.E. Decreasing Dorsal Cochlear Nucleus Activity Ameliorates Noise-Induced Tinnitus Perception in Mice. BMC Biol. 2022, 20, 102. [Google Scholar] [CrossRef]
- Vanneste, S.; Plazier, M.; Van de Heyning, P.; De Ridder, D. Transcutaneous Electrical Nerve Stimulation (TENS) of Upper Cervical Nerve (C2) for the Treatment of Somatic Tinnitus. Exp. Brain Res. 2010, 204, 283–287. [Google Scholar] [CrossRef]
- Bolandi, M.; Javanbakht, M.; Shaabani, M.; Bakhshi, E. Effectiveness of Bimodal Stimulation of the Auditory-Somatosensory System in the Treatment of Tonal Tinnitus. Am. J. Otolaryngol. 2024, 45, 104449. [Google Scholar] [CrossRef]
- Keute, M.; Gharabaghi, A. Brain Plasticity and Vagus Nerve Stimulation. Auton. Neurosci. Basic Clin. 2021, 236, 102876. [Google Scholar] [CrossRef]
- Engineer, N.D.; Riley, J.R.; Seale, J.D.; Vrana, W.A.; Shetake, J.A.; Sudanagunta, S.P.; Borland, M.S.; Kilgard, M.P. Reversing Pathological Neural Activity Using Targeted Plasticity. Nature 2011, 470, 101–104. [Google Scholar] [CrossRef]
- Siedentopf, C.M.; Ischebeck, A.; Haala, I.A.; Mottaghy, F.M.; Schikora, D.; Verius, M.; Koppelstaetter, F.; Buchberger, W.; Schlager, A.; Felber, S.R.; et al. Neural Correlates of Transmeatal Cochlear Laser (TCL) Stimulation in Healthy Human Subjects. Neurosci. Lett. 2007, 411, 189–193. [Google Scholar] [CrossRef]
- Teggi, R.; Bellini, C.; Piccioni, L.O.; Palonta, F.; Bussi, M. Transmeatal Low-Level Laser Therapy for Chronic Tinnitus with Cochlear Dysfunction. Audiol. Neurotol. 2009, 14, 115–120. [Google Scholar] [CrossRef]
- Gungor, A.; Dogru, S.; Cincik, H.; Erkul, E.; Poyrazoglu, E. Effectiveness of Transmeatal Low Power Laser Irradiation for Chronic Tinnitus. J. Laryngol. Otol. 2008, 122, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Demirkol, N.; Usumez, A.; Demirkol, M.; Sari, F.; Akcaboy, C. Efficacy of Low-Level Laser Therapy in Subjective Tinnitus Patients with Temporomandibular Disorders. Photomed. Laser Surg. 2017, 35, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Mun, S.; Sohn, S.; Piao, X.; Park, I.; Chang, M. Axonal Sprouting in the Dorsal Cochlear Nucleus Affects Gap-prepulse Inhibition Following Noise Exposure. Int. J. Mol. Med. 2019, 44, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Martel, D.T.; Shore, S.E. Increased Synchrony and Bursting of Dorsal Cochlear Nucleus Fusiform Cells Correlate with Tinnitus. J. Neurosci. 2016, 36, 2068–2073. [Google Scholar] [CrossRef]
- Shore, S.E.; Koehler, S.; Oldakowski, M.; Hughes, L.F.; Syed, S. Dorsal Cochlear Nucleus Responses to Somatosensory Stimulation Are Enhanced after Noise-induced Hearing Loss. Eur. J. Neurosci. 2008, 27, 155–168. [Google Scholar] [CrossRef]
- Li, J.; Zou, Y.; Kong, X.; Leng, Y.; Yang, F.; Zhou, G.; Liu, B.; Fan, W. Exploring Functional Connectivity Alterations in Sudden Sensorineural Hearing Loss: A Multilevel Analysis. Brain Res. 2024, 1824, 148677. [Google Scholar] [CrossRef]
- Kim, A.Y.; Marduy, A.; de Melo, P.S.; Gianlorenco, A.C.; Kim, C.K.; Choi, H.; Song, J.-J.J.; Fregni, F. Safety of Transcutaneous Auricular Vagus Nerve Stimulation (TaVNS): A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 22055. [Google Scholar] [CrossRef]
- Stegeman, I.; Velde, H.M.; Robe, P.A.J.T.; Stokroos, R.J.; Smit, A.L. Tinnitus Treatment by Vagus Nerve Stimulation: A Systematic Review. PLoS ONE 2021, 16, e0247221. [Google Scholar] [CrossRef]
- De Ridder, D.; Langguth, B.; Vanneste, S. Vagus Nerve Stimulation for Tinnitus: A Review and Perspective. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 262, pp. 451–467. ISBN 9780128223758. [Google Scholar]
- Phillips, J.S.; McFerran, D.J.; Hall, D.A.; Hoare, D.J. The Natural History of Subjective Tinnitus in Adults: A Systematic Review and Meta-analysis of No-intervention Periods in Controlled Trials. Laryngoscope 2018, 128, 217–227. [Google Scholar] [CrossRef]
- De Ridder, D.; Adhia, D.; Langguth, B. Tinnitus and Brain Stimulation. In Current Topics in Behavioral Neurosciences; Springer: Cham, Switzerland, 2021; Volume 51, pp. 249–293. [Google Scholar]
- Spisila, T.; Fontana, L.C.; Hamerschmidt, R.; de Cássia Cassou Guimarães, R.; Hilgenberg-Sydney, P.B. Phenotyping of Somatosensory Tinnitus and Its Associations: An Observational Cross-sectional Study. J. Oral Rehabil. 2024, 51, 2008–2018. [Google Scholar] [CrossRef]
- Hu, J.; Cui, J.; Xu, J.-J.; Yin, X.; Wu, Y.; Qi, J. The Neural Mechanisms of Tinnitus: A Perspective From Functional Magnetic Resonance Imaging. Front. Neurosci. 2021, 15, 621145. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, W.; Yu, C.; Wei, W.; Bai, Y.; Shen, Y.; Yue, X.; Wang, X.; Zhang, X.; Shen, G.; et al. Abnormal Static and Dynamic Brain Network Connectivity Associated with Chronic Tinnitus. Neuroscience 2024, 554, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tutar, B.; Atar, S.; Berkiten, G.; Üstün, O.; Kumral, T.L.; Uyar, Y. The Effect of Transcutaneous Electrical Nerve Stimulation (TENS) on Chronic Subjective Tinnitus. Am. J. Otolaryngol. 2020, 41, 102326. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, M.; Michalska, J.; Polatyńska, K.; Olszewski, J. An Increase in Alpha Band Frequency in Resting State EEG after Electrical Stimulation of the Ear in Tinnitus Patients—A Pilot Study. Front. Neurosci. 2016, 10, 453. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, J.; Han, D.; Qian, L.; Hu, H.; Gao, H. Current Status of Transcutaneous Auricular Vagus Nerve Stimulation for Tinnitus: A Narrative Review of Modern Research. Front. Neurosci. 2024, 18, 1405310. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Weisz, N.; Londero, A.; Schlee, W.; Elgoyhen, A.B.; Langguth, B. An Integrative Model of Auditory Phantom Perception: Tinnitus as a Unified Percept of Interacting Separable Subnetworks. Neurosci. Biobehav. Rev. 2014, 44, 16–32. [Google Scholar] [CrossRef]
- Tyler, R.; Cacace, A.; Stocking, C.; Tarver, B.; Engineer, N.; Martin, J.; Deshpande, A.; Stecker, N.; Pereira, M.; Kilgard, M.; et al. Vagus Nerve Stimulation Paired with Tones for the Treatment of Tinnitus: A Prospective Randomized Double-Blind Controlled Pilot Study in Humans. Sci. Rep. 2017, 7, 11960. [Google Scholar] [CrossRef]
- To, W.T.; De Ridder, D.; Hart, J., Jr.; Vanneste, S. Changing Brain Networks Through Non-Invasive Neuromodulation. Front. Hum. Neurosci. 2018, 12, 128. [Google Scholar] [CrossRef]
- Newman, C.W.; Jacobson, G.P.; Spitzer, J.B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol.-Head Neck Surg. 1996, 122, 143–148. [Google Scholar] [CrossRef]
- Paula Erika Alves, F.; Cunha, F.; Onishi, E.T.; Branco-Barreiro, F.C.A.; Ganança, F.F. Tinnitus Handicap Inventory: Cross-Cultural Adaptation to Brazilian Portuguese. Pró-Fono Rev. De Atualização Científica 2005, 17, 303–310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).