Cerebral Oxygenation and Cardiac Responses in Adult Women’s Rugby: A Season-Long Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Design
2.3. CAB
2.4. SCAT6
2.5. SCG
2.6. Game Data Capture
2.7. fNIRS Protocol
2.8. fNIRS Data Processing and Analysis
2.8.1. Data Acquisition and Preprocessing
2.8.2. Task-Specific Analytical Approaches
2.8.3. Wavelet Analysis for Squat–Stand Task
2.8.4. Statistical Analyses
3. Results
3.1. CAB
3.2. SCAT6
3.3. SCG and Cardiac Function Monitoring
3.4. fNIRS Global and Hemispheric Analysis
3.4.1. Global ‘Where’s Wally’ Task
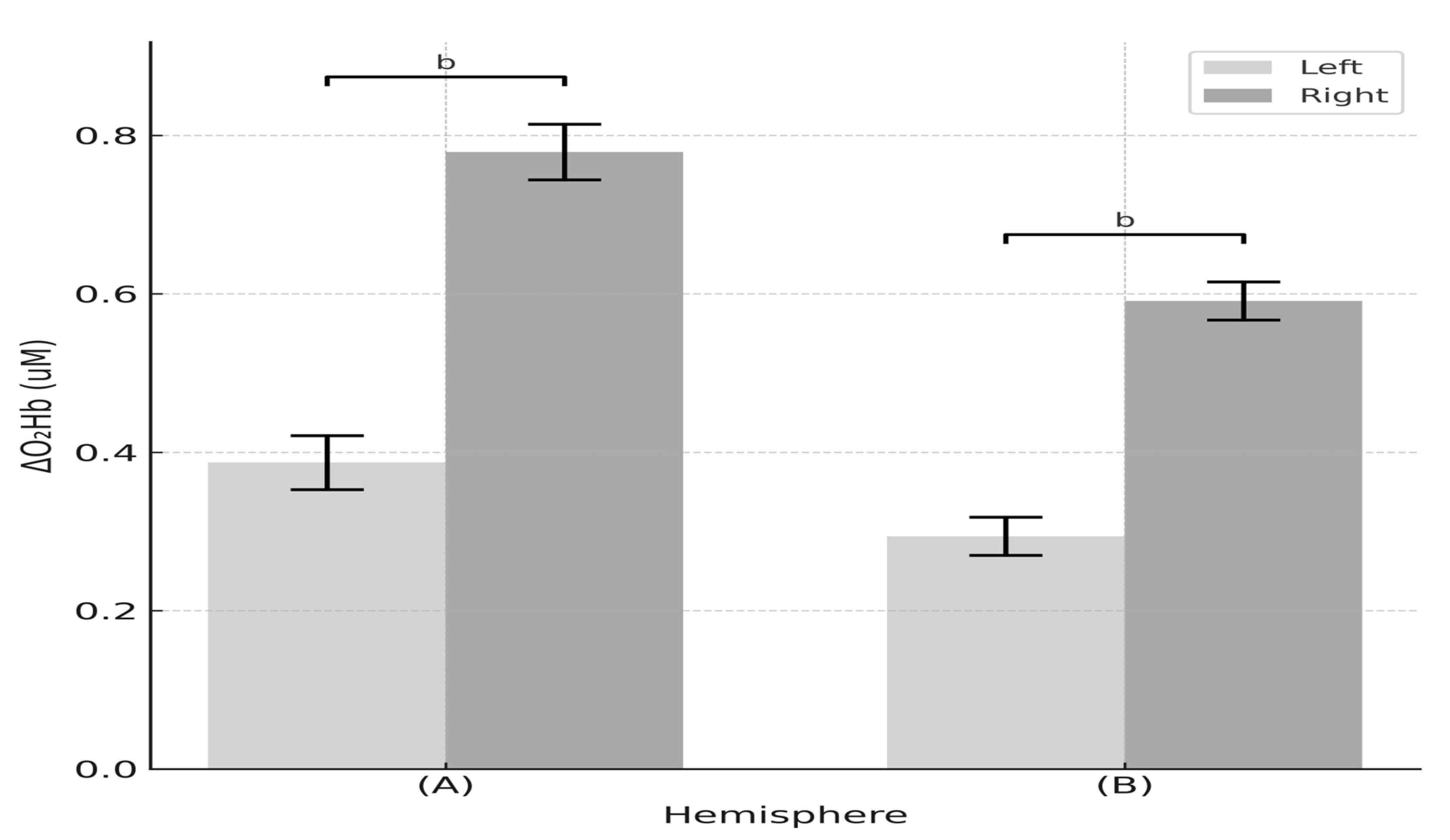
3.4.2. Hemispheric NVC ‘Where’s Wally’ Task
3.4.3. Global Analysis—Squat–Stand Task
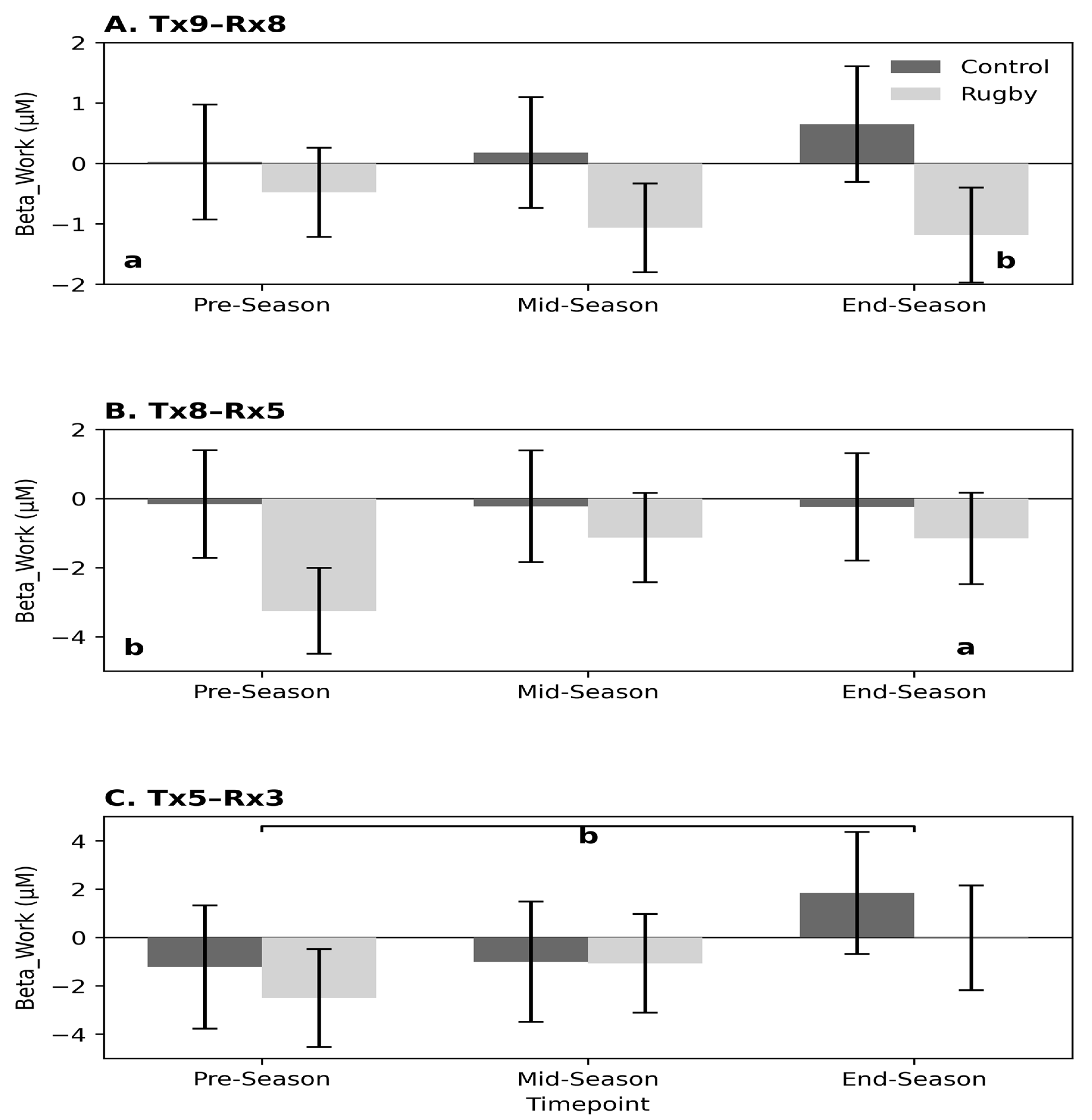
3.4.4. Hemispheric Analysis—Squat–Stand Task
3.5. GLM-SPM
3.6. Task Responses
3.7. Wavelet Analysis
3.8. Concussion Exploratory Case Study Analysis
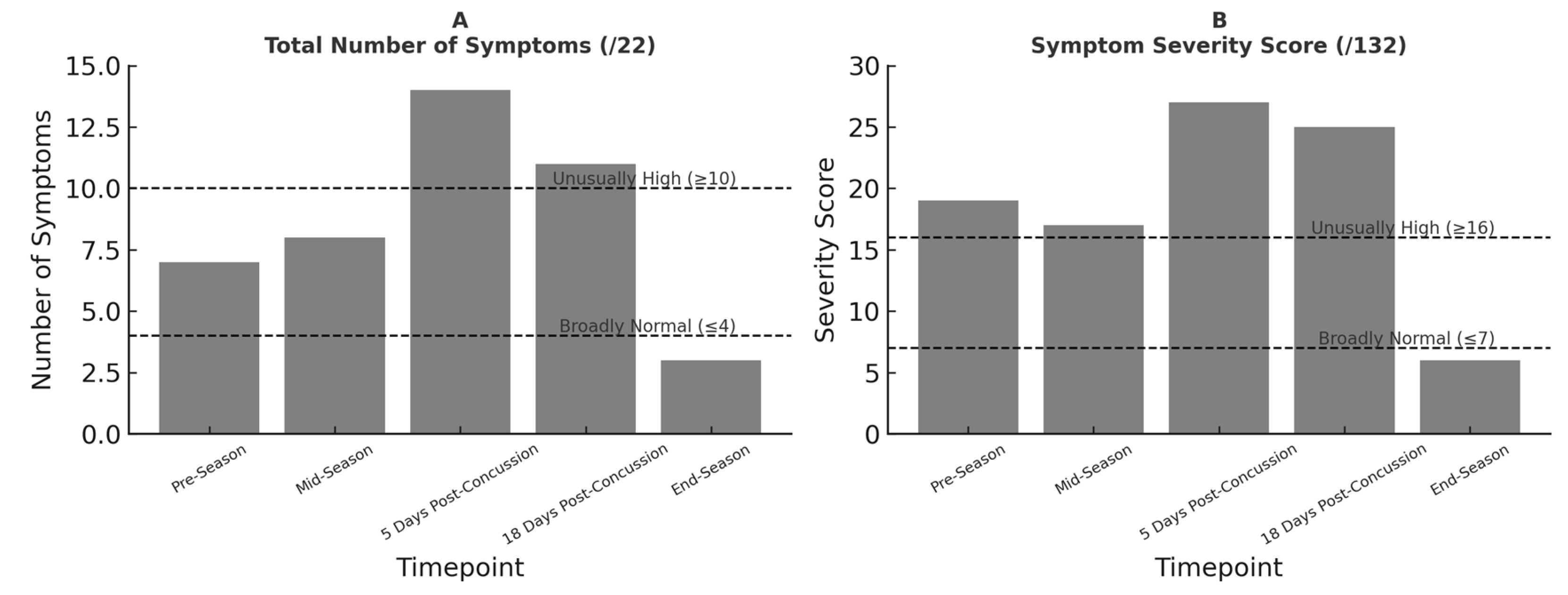
SCAT6
3.9. Cardiac Function Monitoring
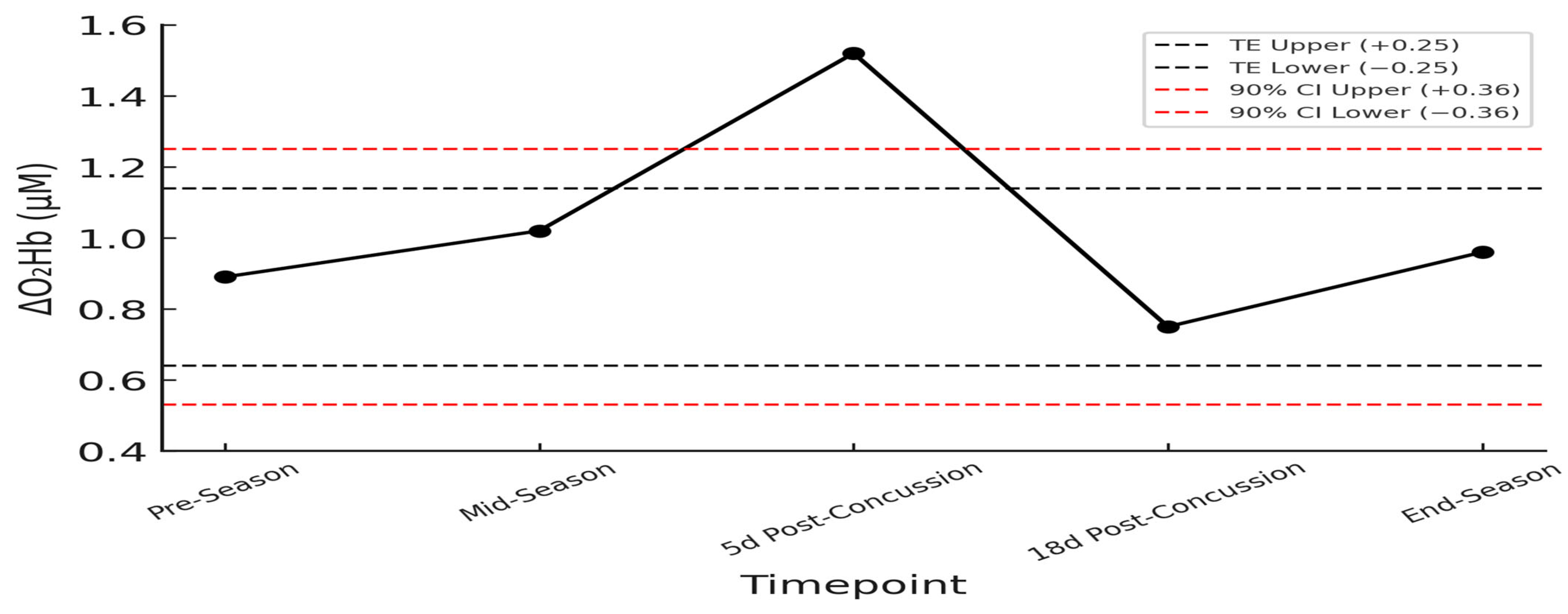
3.10. fNIRS Global and Hemispheric Analysis
3.11. GLM-SPM
3.12. Wavelet Analysis
4. Discussion
4.1. Seasonal Analysis
4.2. Cardiac Function (SCG)
4.3. fNIRS
4.4. SCAT6
4.5. Exploratory Concussion Case Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAB | Cognitive assessment battery |
| dCA | Dynamic cerebral autoregulation |
| fNIRS | Functional Near-infrared Spectroscopy |
| GLM | General Linear Model |
| NVC | Neurovascular coupling |
| O2Hb | Oxyhaemoglobin |
| PFC | Prefrontal cortex |
| SCG | Seismocardiography |
| SQI | Signal quality index |
| SCAT6 | Sport Concussion Assessment Tool version 6 |
| SPM | Statistical Parametric Mapping |
| TE | Typical Error |
References
- King, D.; Hume, P.A.; Brughelli, M.; Gissane, C. Instrumented mouthguard acceleration analyses for head impacts in amateur rugby union players over a season of matches. Am. J. Sports Med. 2015, 43, 614–624. [Google Scholar] [CrossRef]
- Sirant, L.W.; Singh, J.; Martin, S.; Gaul, C.A.; Stuart-Hill, L.; Candow, D.G.; Mang, C.; Neary, J.P. Long-term effects of multiple concussions on prefrontal cortex oxygenation during repeated squat-stands in retired contact sport athletes. Brain Inj. 2022, 36, 931–938. [Google Scholar] [CrossRef]
- Sirant, L.W.; Singh, J.; Martin, S.; Gaul, C.A.; Stuart-Hill, L.; Candow, D.G.; Mang, C.; Neary, J.P. Long-term effects of multiple concussions on prefrontal cortex oxygenation during neurovascular coupling activation in retired male contact sport athletes. Curr. Res. Physiol. 2022, 5, 421–428. [Google Scholar] [CrossRef]
- Batty, G.D.; Frank, P.; Kujala, U.M.; Sarna, S.J.; Valencia-Hernández, C.A.; Kaprio, J. Dementia and Alzheimer’s disease in former contact sports participants: Population-based cohort study, systematic review, and meta-analysis. eClinicalMedicine 2023, 61, 102056. [Google Scholar] [CrossRef]
- Kieffer, E.E.; Brolinson, P.G.; Maerlender, A.E.; Smith, E.P.; Rowson, S. In-Season Concussion Symptom Reporting in Male and Female Collegiate Rugby Athletes. Neurotrauma Rep. 2021, 2, 503–511. [Google Scholar] [CrossRef]
- Patricios, J.S.; Schneider, K.J.; Dvorak, J.; Ahmed, O.H.; Blauwet, C.; Cantu, R.C.; Davis, G.A.; Echemendia, R.J.; Makdissi, M.; McNamee, M.; et al. Consensus statement on concussion in sport: The 6th International Conference on Concussion in Sport-Amsterdam, October 2022. Br. J. Sports Med. 2023, 57, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.; Melinz, J.; King, D.; Sanctuary, C.; Murphy, A. Call to action: A collaborative framework to better support female rugby league players. Br. J. Sports Med. 2020, 54, 501–502. [Google Scholar] [CrossRef] [PubMed]
- D’Lauro, C.; Jones, E.R.; Swope, L.M.; Anderson, M.N.; Broglio, S.; Schmidt, J.D. Under-representation of female athletes in research informing influential concussion consensus and position statements: An evidence review and synthesis. Br. J. Sports Med. 2022, 56, 981–987. [Google Scholar] [CrossRef] [PubMed]
- World Rugby. World Rugby Year in Review 2018. Available online: https://publications.worldrugby.org/yearinreview2018/en/2-1/ (accessed on 16 October 2025).
- Flower, K.D.; Knight, C.J.; Rouquette, O.Y.; Waldron, M.; Barrell, D.; Mumford, E.; Love, T.D. Barriers and facilitators to participation in women’s and girls’ rugby: A mixed-methods study. J. Sports Sci. 2025, 43, 1907–1923. [Google Scholar] [CrossRef]
- Brown, N.; Williams, G.K.R.; Stodter, A.; McNarry, M.A.; Roldan-Reoyo, O.; Mackintosh, K.A.; Moore, I.S.; Williams, E.M.P. A Global Women’s Rugby Union Web-Based Survey. Int. J. Environ. Res. Public Health 2023, 20, 5475. [Google Scholar] [CrossRef]
- Covassin, T.; Moran, R.; Elbin, R.J. Sex Differences in Reported Concussion Injury Rates and Time Loss from Participation: An Update of the National Collegiate Athletic Association Injury Surveillance Program from 2004–2005 Through 2008-2009. J. Athl. Train. 2016, 51, 189–194. [Google Scholar] [CrossRef]
- Covassin, T.; Savage, J.L.; Bretzin, A.C.; Fox, M.E. Sex differences in sport-related concussion long-term outcomes. Int. J. Psychophysiol. 2018, 132, 9–13. [Google Scholar] [CrossRef]
- Snook, M.L.; Henry, L.C.; Sanfilippo, J.S.; Zeleznik, A.J.; Kontos, A.P. Association of Concussion With Abnormal Menstrual Patterns in Adolescent and Young Women. JAMA Pediatr. 2017, 171, 879–886. [Google Scholar] [CrossRef]
- Tierney, R.T.; Sitler, M.R.; Swanik, C.B.; Swanik, K.A.; Higgins, M.; Torg, J. Gender differences in head-neck segment dynamic stabilization during head acceleration. Med. Sci. Sports Exerc. 2005, 37, 272–279. [Google Scholar] [CrossRef]
- Walshe, A.; Daly, E.; Ryan, L. Epidemiology of sport-related concussion rates in female contact/collision sport: A systematic review. BMJ Open Sport Exerc. Med. 2022, 8, e001346. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, C.J.; Shafiq, M.A.; Ellingson, C.A.; Neary, J.P.; Dehghani, P.; Singh, J. Assessment of cardiovascular functioning following sport-related concussion: A physiological perspective. Auton. Neurosci. 2024, 252, 103160. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Ellingson, C.J.; Ellingson, C.A.; Scott, P.; Neary, J.P. Cardiac cycle timing intervals in university varsity athletes. Eur. J. Sport Sci. 2023, 23, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Taebi, A.; Solar, B.E.; Bomar, A.J.; Sandler, R.H.; Mansy, H.A. Recent Advances in Seismocardiography. Vibration 2019, 2, 64–86. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, C.J.; Singh, J.; Ellingson, C.A.; Sirant, L.W.; Krätzig, G.P.; Dorsch, K.D.; Piskorski, J.; Neary, J.P. Alterations in Baroreflex Sensitivity and Blood Pressure Variability Following Sport-Related Concussion. Life 2022, 12, 1400. [Google Scholar] [CrossRef]
- Singh, J.; Ellingson, C.J.; Ellingson, C.A.; Scott, P.; Neary, J.P. Cardiac cycle timing and contractility following acute sport-related concussion. Res. Sports Med. 2024, 32, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Graci, V.; Beam, M.E.; Ayaz, H.; Prosser, L.A.; Master, C.L.; McDonald, C.C.; Arbogast, K.B. Neurophysiological and gait outcomes during a dual-task gait assessment in concussed adolescents. Clin. Biomech. 2023, 109, 106090. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Hind, K.; Hume, P.; Singh, J.; Neary, J.P. Neurovascular Coupling by Functional Near Infra-Red Spectroscopy and Sport-Related Concussion in Retired Rugby Players: The UK Rugby Health Project. Front. Hum. Neurosci. 2020, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Huber, C.M.; Patton, D.A.; McDonald, C.C.; Wang, L.; Ayaz, H.; Master, C.L.; Arbogast, K.B. Use of functional near-infrared spectroscopy to quantify neurophysiological deficits after repetitive head impacts in adolescent athletes. Sports Biomech. 2023, 24, 1278–1292. [Google Scholar] [CrossRef]
- Clark, A. Changes in Cognitive Function and Cerebral Oxygenation Patterns in Rugby and Non-Contact Sportspersons over a 15-Week Season; Stellenbosch University: Stellenbosch, South Africa, 2018; Available online: https://hdl.handle.net/10019.1/103656 (accessed on 19 April 2024).
- Jones, B.; Jamalifard, M.; Waterworth, S.; Rogerson, M.; Andreu-Perez, J.; Perrett, J.; Hope, E.; Moran, J.; Adams, T.; Singh, J.; et al. Cerebral Haemodynamic Assessment Following Sport-related Concussion (Mild Traumatic Brain Injury) in Youth and Amateur Rugby Union Players. Sports Med. Open 2025, 11, 47. [Google Scholar] [CrossRef]
- Feinberg, C.; Mayes, K.D.; Portman, E.; Carr, C.; Mannix, R. Non-invasive fluid biomarkers in the diagnosis of mild traumatic brain injury (mTBI): A systematic review. J. Neurol. Neurosurg. Psychiatry 2024, 95, 184–192. [Google Scholar] [CrossRef]
- Tabor, J.B.; Penner, L.C.; Galarneau, J.-M.; Josafatow, N.; Cooper, J.; Ghodsi, M.; Huang, J.; Fraser, D.D.; Smirl, J.; Esser, M.J.; et al. Plasma Biomarkers of Traumatic Brain Injury in Adolescents with Sport-Related Concussion. JAMA Netw. Open 2024, 7, e2431959. [Google Scholar] [CrossRef]
- Howell, D.R.; Kirkwood, M.W.; Laker, S.; Laker, S.; Wilson, J.C. Collision and Contact Sport Participation and Quality of Life Among Adolescent Athletes. J. Athl. Train. 2020, 55, 1174–1180. [Google Scholar] [CrossRef]
- Irazoki, E.; Contreras-Somoza, L.M.; Toribio-Guzmán, J.M.; Jenaro-Río, C.; van der Roest, H.; Franco-Martín, M.A. Technologies for Cognitive Training and Cognitive Rehabilitation for People With Mild Cognitive Impairment and Dementia. A Systematic Review. Front. Psychol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Echemendia, R.J.; Brett, B.L.; Broglio, S.; Davis, G.A.; Giza, C.C.; Guskiewicz, K.M.; Harmon, K.G.; Herring, S.; Howell, D.R.; Master, C.L.; et al. Introducing the Sport Concussion Assessment Tool 6 (SCAT6). Br. J. Sports Med. 2023, 57, 619–621. [Google Scholar] [CrossRef]
- Iverson, G.L.; Howell, D.R.; Van Patten, R.; Bloomfield, P.; Gardner, A.J. Sport Concussion Assessment Tool-5th Edition (SCAT5): Normative Reference Values for the National Rugby League Women’s Premiership. Front. Sports Act. Living 2021, 3, 653743. [Google Scholar] [CrossRef] [PubMed]
- MacQuarrie, D.S.; Neary, J.P.; Sauchyn, R.D. Tri-Axial Seismocardiography Devices and Methods. WO2022090799A2. 5 May 2022. Available online: https://patents.google.com/patent/WO2022090799A2/en (accessed on 4 September 2025).
- Neary, J.P.; Singh, J.; Bishop, S.A.; Dech, R.T.; Butz, M.J.A.; Len, T.K. An Evidence-Based Objective Study Protocol for Evaluating Cardiovascular and Cerebrovascular Indices Following Concussion: The Neary Protocol. Methods Protoc. 2019, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Smirl, J.D.; Wright, A.D.; Bryk, K.; van Donkelaar, P. Where’s Waldo? The utility of a complicated visual search paradigm for transcranial Doppler-based assessments of neurovascular coupling. J. Neurosci. Methods 2016, 270, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Burma, J.S.; Van Roessel, R.K.; Oni, I.K.; Dunn, J.F.; Smirl, J.D. Neurovascular coupling on trial: How the number of trials completed impacts the accuracy and precision of temporally derived neurovascular coupling estimates. J. Cereb. Blood Flow Metab. 2022, 42, 1478–1492. [Google Scholar] [CrossRef]
- Claassen, J.A.H.R.; Levine, B.D.; Zhang, R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J. Appl. Physiol. 2009, 106, 153–160. [Google Scholar] [CrossRef]
- Duncan, A.; Meek, J.H.; Clemence, M.; Elwell, C.E.; Fallon, P.; Tyszczuk, L.; Cope, M.; Delpy, D.T. Measurement of Cranial Optical Path Length as a Function of Age Using Phase Resolved Near Infrared Spectroscopy. Pediatr. Res. 1996, 39, 889–894. [Google Scholar] [CrossRef]
- Abraham, A.; Pedregosa, F.; Eickenberg, M.; Gervais, P.; Mueller, A.; Kossaifi, J.; Gramfort, A.; Thirion, B.; Varoquaux, G. Machine learning for neuroimaging with scikit-learn. Front. Neuroinform 2014, 8, 14. [Google Scholar] [CrossRef]
- Sappia, M.S.; Hakimi, N.; Colier, W.N.J.M.; Horschig, J.M. Signal quality index: An algorithm for quantitative assessment of functional near infrared spectroscopy signal quality. Biomed. Opt. Express BOE 2020, 11, 6732–6754. [Google Scholar] [CrossRef]
- Delpy, D.T.; Cope, M.; van der Zee, P.; van der Arridge, S.; Wray, S.; Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433. [Google Scholar] [CrossRef]
- Naseer, N.; Hong, K.-S. fNIRS-based brain-computer interfaces: A review. Front. Hum. Neurosci. 2015, 9, 3. [Google Scholar] [CrossRef]
- Huppert, T.J. Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics 2016, 3, 010401. [Google Scholar] [CrossRef]
- Santosa, H.; Zhai, X.; Fishburn, F.; Huppert, T. The NIRS Brain AnalyzIR Toolbox. Algorithms 2018, 11, 73. [Google Scholar] [CrossRef]
- Glover, G.H. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage 1999, 9, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Addison, P.S. The Illustrated Wavelet Transform Handbook: Introductory Theory and Applications in Science, Engineering, Medicine and Finance, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zhang, Y.; Brooks, D.H.; Franceschini, M.A.; Boas, D.A. Eigenvector-based spatial filtering for reduction of physiological interference in diffuse optical imaging. J. Biomed. Opt. 2005, 10, 011014. [Google Scholar] [CrossRef] [PubMed]
- Kirilina, E.; Jelzow, A.; Heine, A.; Niessing, M.; Wabnitz, H.; Brühl, R.; Ittermann, B.; Jacobs, A.M.; Tachtsidis, I. The physiological origin of task-evoked systemic artefacts in functional near infrared spectroscopy. NeuroImage 2012, 61, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, B.; Fidell, L. Using Multivariate Statistics. In Using Multivariate Statistics; Pearson: New York, NY, USA, 2019; pp. 63–71. [Google Scholar]
- Swinton, P.A.; Hemingway, B.S.; Saunders, B.; Gualano, B.; Dolan, E. A Statistical Framework to Interpret Individual Response to Intervention: Paving the Way for Personalized Nutrition and Exercise Prescription. Front. Nutr. 2018, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Gardner, A.J.; Maietta, J.E.; Iverson, G.L.; Howell, D.R.; Bloomfield, P.; Fuller, G.W.; Jones, B.; Lakisa, D.R.; Ravulo, J.; Senituli, S.; et al. Sport Concussion Assessment Tool-5th Edition (SCAT5) normative reference values for professional men’s rugby league players. J. Sci. Med. Sport 2025, 28, 535–541. [Google Scholar] [CrossRef]
- Alhakak, A.S.; Olsen, F.J.; Skaarup, K.G.; Lassen, M.C.H.; Johansen, N.D.; Jørgensen, P.G.; Abildgaard, U.; Jensen, G.B.; Schnohr, P.; Søgaard, P.; et al. Age- and sex-based normal reference ranges of the cardiac time intervals: The Copenhagen City Heart Study. Clin. Res. Cardiol. 2025, 114, 430–442. [Google Scholar] [CrossRef]
- Biering-Sørensen, T.; Mogelvang, R.; de Knegt, M.C.; Olsen, F.J.; Galatius, S.; Jensen, J.S. Cardiac Time Intervals by Tissue Doppler Imaging M-Mode: Normal Values and Association with Established Echocardiographic and Invasive Measures of Systolic and Diastolic Function. PLoS ONE 2016, 11, e0153636. [Google Scholar] [CrossRef]
- Singh, J.; Carleton, R.N.; Kratzig, G.P.; Neary, J.P. Characterization of the cardiac cycle in Royal Canadian Mounted Police cadets. Appl. Physiol. Nutr. Metab. 2025, 50, 1–5. [Google Scholar] [CrossRef]
- Bishop, S.; Dech, R.; Baker, T.; Butz, M.; Aravinthan, K.; Neary, J.P. Parasympathetic baroreflexes and heart rate variability during acute stage of sport concussion recovery. Brain Inj. 2017, 31, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Pyndiura, K.L.; Di Battista, A.P.; Hutchison, M.G. A history of concussion is associated with minimal perturbations to heart rate variability in athletes. Brain Inj. 2020, 34, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Bhagaloo, L.; Sy, E.; Lavoie, A.J.; Dehghani, P.; Bardutz, H.A.; Mang, C.S.; Buttigieg, J.; Neary, J.P. Cardiac impairments in postacute COVID-19 with sustained symptoms: A review of the literature and proof of concept. Physiol. Rep. 2022, 10, e15430. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Sandmann, P.; Thorne, J.D.; Herrmann, C.S.; Debener, S. Association of Concurrent fNIRS and EEG Signatures in Response to Auditory and Visual Stimuli. Brain Topogr. 2015, 28, 710–725. [Google Scholar] [CrossRef]
- Burma, J.S.; Copeland, P.; Macaulay, A.; Khatra, O.; Wright, A.D.; Smirl, J.D. Dynamic cerebral autoregulation across the cardiac cycle during 8 hr of recovery from acute exercise. Physiol. Rep. 2020, 8, e14367. [Google Scholar] [CrossRef]
- Bartel, G.; Marko, M.; Rameses, I.; Lamm, C.; Riečanský, I. Left Prefrontal Cortex Supports the Recognition of Meaningful Patterns in Ambiguous Stimuli. Front. Neurosci. 2020, 14, 152. [Google Scholar] [CrossRef]
- Csipo, T.; Mukli, P.; Lipecz, A.; Tarantini, S.; Bahadli, D.; Abdulhussein, O.; Owens, C.; Kiss, T.; Balasubramanian, P.; Nyúl-Tóth, Á.; et al. Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience 2019, 41, 495–509. [Google Scholar] [CrossRef]
- Burma, J.S.; Bailey, D.M.; Johnson, N.E.; Griffiths, J.K.; Burkart, J.J.; Soligon, C.A.; Fletcher, E.K.S.; Javra, R.M.; Debert, C.T.; Schneider, K.J.; et al. Physiological influences on neurovascular coupling: A systematic review of multimodal imaging approaches and recommendations for future study designs. Exp. Physiol. 2025, 110, 23–41. [Google Scholar] [CrossRef]
- Hagen, A.C.; Tracy, B.L.; Stephens, J.A. Altered neural recruitment during single and dual tasks in athletes with repeat concussion. Front. Hum. Neurosci. 2024, 18, 1515514. [Google Scholar] [CrossRef]
- Wu, Z.; Mazzola, C.A.; Catania, L.; Owoeye, O.; Yaramothu, C.; Alvarez, T.; Gao, Y.; Li, X. Altered cortical activation and connectivity patterns for visual attention processing in young adults post-traumatic brain injury: A functional near infrared spectroscopy study. CNS Neurosci. Ther. 2018, 24, 539–548. [Google Scholar] [CrossRef]
- Singh, J.; Ellingson, C.J.; Ellingson, C.A.; Shafiq, M.A.; Dech, R.T.; Sirant, L.W.; Dorsch, K.D.; Gruszecki, M.; Kratzig, G.P.; Neary, J.P. Acute sport-related concussion alters cardiac contribution to cerebral oxygenation during repeated squat stands. J. Sports Sci. 2024, 42, 2474–2480. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Smirl, J.D.; Bryk, K.; Fraser, S.; Jakovac, M.; van Donkelaar, P. Sport-Related Concussion Alters Indices of Dynamic Cerebral Autoregulation. Front. Neurol. 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Schranz, A.L.; Manning, K.Y.; Dekaban, G.A.; Fischer, L.; Jevremovic, T.; Blackney, K.; Barreira, C.; Doherty, T.J.; Fraser, D.D.; Brown, A.; et al. Reduced brain glutamine in female varsity rugby athletes after concussion and in non-concussed athletes after a season of play. Hum. Brain Mapp. 2017, 39, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Eckner, J.T.; Wang, J.; Nelson, L.D.; Bancroft, R.; Pohorence, M.; He, X.; Broglio, S.P.; Giza, C.C.; Guskiewicz, K.M.; Kutcher, J.S.; et al. Effect of Routine Sport Participation on Short-Term Clinical Neurological Outcomes: A Comparison of Non-Contact, Contact, and Collision Sport Athletes. Sports Med. 2020, 50, 1027–1038. [Google Scholar] [CrossRef]
- Piantella, S.; McDonald, S.J.; Wright, B.J. Gender and Workplace Stress Affect the Association Between Concussion History and Depression Symptoms in Professional Jockeys. Arch. Clin. Neuropsychol. 2023, 38, 537–547. [Google Scholar] [CrossRef]
- Tucker, R.; Falvey, E.; Fuller, G.; Brown, J.C.; Raftery, M. Effect of a concussion on subsequent baseline SCAT performance in professional rugby players: A retrospective cohort study in global elite Rugby Union. BMJ Open 2020, 10, e036894. [Google Scholar] [CrossRef]
- Echemendia, R.J.; Burma, J.S.; Bruce, J.M.; Davis, G.A.; Giza, C.C.; Guskiewicz, K.M.; Naidu, D.; Black, A.M.; Broglio, S.; Kemp, S.; et al. Acute evaluation of sport-related concussion and implications for the Sport Concussion Assessment Tool (SCAT6) for adults, adolescents and children: A systematic review. Br. J. Sports Med. 2023, 57, 722–735. [Google Scholar] [CrossRef]
- Bishop, S.A.; Neary, J.P. Assessing prefrontal cortex oxygenation after sport concussion with near-infrared spectroscopy. Clin. Physiol. Funct. Imaging 2018, 38, 573–585. [Google Scholar] [CrossRef]
- Len, T.K.; Neary, J.P.; Asmundson, G.J.G.; Goodman, D.G.; Bjornson, B.; Bhambhani, Y.N. Cerebrovascular reactivity impairment after sport-induced concussion. Med. Sci. Sports Exerc. 2011, 43, 2241–2248. [Google Scholar] [CrossRef]
- Hecimovich, M.; Moriarty, T.; King, D.; Majewski-Schrage, T.; Hermsen, K. Measuring Brain Haemodynamic Activity and Afferent Visual Function: A Preliminary Study on the Relationship Between fNIRS, the King–Devick Test and Suspected Sport-Related Concussions. Physiologia 2025, 5, 4. [Google Scholar] [CrossRef]
- Pfeifer, M.D.; Scholkmann, F.; Labruyère, R. Signal Processing in Functional Near-Infrared Spectroscopy (fNIRS): Methodological Differences Lead to Different Statistical Results. Front. Hum. Neurosci. 2017, 11, 641. [Google Scholar] [CrossRef]
- Yücel, M.A.; Lühmann, A.V.; Scholkmann, F.; Gervain, J.; Dan, I.; Ayaz, H.; Boas, D.; Cooper, R.J.; Culver, J.; Elwell, C.E.; et al. Best practices for fNIRS publications. Neurophotonics 2021, 8, 012101. [Google Scholar] [CrossRef]
- Yücel, M.A.; Luke, R.; Mesquita, R.C.; von Lühmann, A.; Mehler, D.M.A.; Lührs, M.; Gemignani, J.; Abdalmalak, A.; Albrecht, F.; de Almeida Ivo, I.; et al. fNIRS reproducibility varies with data quality, analysis pipelines, and researcher experience. Commun. Biol. 2025, 8, 1149. [Google Scholar] [CrossRef]
- Burma, J.S.; Wassmuth, R.M.; Kennedy, C.M.; Miutz, L.N.; Newel, K.T.; Carere, J.; Smirl, J.D. Does task complexity impact the neurovascular coupling response similarly between males and females? Physiol. Rep. 2021, 9, e15020. [Google Scholar] [CrossRef]
- Macleod, H.; Smith, C.L.; Laycock, R. Using neuroimaging to identify sex differences in adults with sports-related concussion: A systematic review. Brain Imaging Behav. 2025, 19, 594–608. [Google Scholar] [CrossRef]






| Variable | Group | Mean ± SD/Median (IQR) | Test Statistic | p Value | Effect Size |
|---|---|---|---|---|---|
| Height (cm) | Rugby | 165.26 ± 6.46 | t(27) = −0.93 | 0.363 | d = −0.36 |
| Control | 167.98 ± 9.29 | ||||
| Body Mass (kg) | Rugby | 75.25 ± 11.42 | t(27) = 0.98 | 0.335 | d = 0.38 |
| Control | 70.15 ± 16.40 | ||||
| Age (years) | Rugby | 26 (24–30) | U = 73.50 | 0.334 | r = 0.18 |
| Control | 39.5 (23.5–44.75) | ||||
| BMI (kg·m−2) | Rugby | 27.2 (25.4–28.6) | U = 46.50, z = −2.23 | 0.024 * | r = 0.41 |
| Control | 24.9 (22.5–26.7) | ||||
| Physical Activity | Rugby | 4 (3–5) | U = 76.50, z = −0.86 | 0.403 | r = 0.16 |
| Control | 4 (3–5) | ||||
| Concussion History | Rugby | 2 (1–3) | U = 61.50, z = −1.88 | 0.126 | r = 0.35 |
| Control | 1 (0–2) | ||||
| Contraception Use | Rugby | 4/13 (30.8%) | χ2 (1, N = 20) = 0.66 | 0.417 | φ = 0.18 |
| Control | 1/7 (14.3%) | Fisher’s Exact p = 0.613 | |||
| Average Cycle Length (Days) | Rugby | 4.5 (0–6) | U = 35.00, z = −0.83 | 0.395 | r = 0.19 |
| Control | 5.0 (2–6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, B.; Jamalifard, M.; Rogerson, M.; Andreu-Perez, J.; Perrett, J.; Hope, E.; Carpenter, L.; Lewis, T.; Neary, J.P.; Cooper, C.E.; et al. Cerebral Oxygenation and Cardiac Responses in Adult Women’s Rugby: A Season-Long Study. Physiologia 2025, 5, 46. https://doi.org/10.3390/physiologia5040046
Jones B, Jamalifard M, Rogerson M, Andreu-Perez J, Perrett J, Hope E, Carpenter L, Lewis T, Neary JP, Cooper CE, et al. Cerebral Oxygenation and Cardiac Responses in Adult Women’s Rugby: A Season-Long Study. Physiologia. 2025; 5(4):46. https://doi.org/10.3390/physiologia5040046
Chicago/Turabian StyleJones, Ben, Mohammadreza Jamalifard, Mike Rogerson, Javier Andreu-Perez, Jay Perrett, Ed Hope, Lachlan Carpenter, Tracy Lewis, J. Patrick Neary, Chris E. Cooper, and et al. 2025. "Cerebral Oxygenation and Cardiac Responses in Adult Women’s Rugby: A Season-Long Study" Physiologia 5, no. 4: 46. https://doi.org/10.3390/physiologia5040046
APA StyleJones, B., Jamalifard, M., Rogerson, M., Andreu-Perez, J., Perrett, J., Hope, E., Carpenter, L., Lewis, T., Neary, J. P., Cooper, C. E., & Waterworth, S. (2025). Cerebral Oxygenation and Cardiac Responses in Adult Women’s Rugby: A Season-Long Study. Physiologia, 5(4), 46. https://doi.org/10.3390/physiologia5040046








