Abstract
An unknown species of the diatom genus Ulnaria was identified during the study of freshwater environments in Mongolia. Monoclonal strains of the unknown species were obtained and subjected to molecular analysis. The strains were investigated through light and scanning electron microscopy, coupled with molecular analysis of SSU rRNA and rbcL gene sequences. The features of sexual reproduction of the cultured specimens were studied, and traits previously unknown to representatives of Ulnaria were demonstrated. Based on the results of morphological comparison against similar species from the Ulnaria ulna species complex and data from the two-gene molecular analysis, a description of Ulnaria williamsii sp. nov. was provided. Analysis of the features of sexual reproduction—gametogenesis, the mating system, and gamete movement—allowed us to discuss the species dispersion and evolution within the genus Ulnaria.
1. Introduction
The genus Ulnaria (Kützing) Compère was established in 2001 []. While this name is currently accepted taxonomically [], the taxonomic history of the genus is quite complex [,,,,] and faced several challenges over time. In particular, the species currently belonging to Ulnaria were historically assigned to the large heterogenous genera Fragilaria Lyngbye [] and Synedra Ehrenberg []. At the same time, when describing the latter genus, Ehrenberg [] did not select a generitype, thus initiating further development of different interpretations of the taxonomic entity of Synedra. The issue remained unsolved until the beginning of the XXI century, when Compère [] raised Synedra subgen. Ulnaria Kützing to the rank of genus and proposed Synedra ulna (Nitzsch) Ehrenberg syn. Ulnaria ulna (Nitzsch) Compère as its type species. Later, Lange-Bertalot & Ulrich [] made an attempt to study Ulnaria ulna but were unable to find the original material representing Bacillaria ulna Nitzsch. As the type locality of this taxon was lost [], the authors conducted an epitypification of the species [].
Diatoms of the genus Ulnaria are characterized by significant morphological diversity. The main diagnostic features of morphology are linear or linear–lanceolate valves (sometimes constricted in the middle portion), striae mostly uniseriate or rarely biseriate, apical pore fields (APFs) located at both apices, two rimoportulae at each valve, and two plastids appressed to the girdle. In several species, the valves are equipped with spines that are used to link adjacent cells together in a colony. The key morphological character of the genus, illustrated in multiple studies [,,,,,], is the presence of closed valvocopulae. This feature separates Ulnaria from Fragilaria, which bears open valvocopulae.
Regarding the geographical distribution, representatives of Ulnaria have been recorded worldwide, but some species are restricted to only one area []. Species from this genus are epiphytic (growing on hydrophyte plants) or epilithic, forming colonies and attaching to substratum with mucilage pads secreted by the APFs []. The genus is also found in phytoplankton. Its species prefer moderately alkaline environments with low levels of mineralization, with oligosaprobic and eutrophic conditions [,].
To date, 70 species have been acknowledged within Ulnaria []. New species are actively described from waterbodies in Asia. For instance, 2 new species have been discovered in Lake Baikal, Russia [], and 21 have been recorded in different regions of China since 2017 [,,,,]. This indicates the immense potential of Asian aquatic ecosystems for further analysis of diatom diversity. In addition, recent studies on the genus involve analyses of the type materials of several species of Ulnaria which were introduced back in the XIX century [,,,,].
From the perspective of molecular genetics, the genus is studied quite unevenly, i.e., molecular data is available on a limited number of species, such as U. ulna, U. acus (Kützing) Aboal, and Ulnaria danica (Kützing) Compère & Bukhtiyarova. Notably, rbcL-based phylogenetic analysis of the genus suggested that Ulnaria is closely related to Fragilaria s.s. but nevertheless belongs to an independent clade [,]. A subsequent study by Hwang et al. [], which incorporated a molecular analysis of rbcL and SSU rRNA, also demonstrated that Ulnaria is independent from the genera Fragilaria and Synedra. In addition, studies by Marchenkov et al. [,] presented the results of comparative analyses of cox1 gene fragments from different U. danica and U. ulna populations. Molecular studies on the genus continue, with the most recent involving investigations of physiological adaptations [].
The material for this study was collected in Mongolia, a region with a diatom research history of over 100 years [,,]. Some recent investigations in this part of Asia have made it possible to study the morphology, phylogeny, and distribution of already known taxa, as well as to propose descriptions of new species, e.g., Nupela matryoshka Kulikovskiy, Lange-Bertalot & Witkowski, Cymbopleura deviatkinii Kulikovskiy, Lange-Bertalot, Witkowski & Dorofeyuk, Pinnularia trifonovae Kulikovskiy, Lange-Bertalot, Witkowski & Dorofeyuk, and Paraplaconeis dorofeyukae Glushchenko, Mironov, Nergui & Kulikovskiy, and genera, e.g., Boreozonacola Lange-Bertalot, Kulikovskiy & Witkowski and Vladinikolaevia Kulikovskiy, Glushchenko, Y. Liu & Kociolek [,,,,,,,,,,,]. According to the latest data from the literature, 13 species and interspecific taxa of Ulnaria have been recorded for Mongolia, but not a single one of them has been described from the freshwater ecosystems of this country [].
It is also worth mentioning that modern studies on diatoms are typically based on an integrative approach. While the standard methodology combines morphological and molecular analyses, this two-pronged approach does not always resolve complex questions of species boundaries, especially within morphologically plastic genera like Ulnaria. In such cases, a third, more definitive line of evidence is required. Only a very limited number of studies on the genus have applied the biological species concept to species delimitation [], and data on reproductive biology have been acquired for only three species so far: U. ulna, U. danica, and U. acus [,,]. Nevertheless, the analysis of mating systems and reproductive compatibility provides a powerful tool for assessing evolutionary processes at the species level []. Based directly on the biological species concept, this approach, though laborious, may act as a definitive arbiter in cases where morphological and molecular data are inconclusive. Furthermore, studying the life cycle provides insights into the full size range of a given species and yields data on its critical points (e.g., readiness for size restoration and auxospore formation, maximal species-specific cell size), which serve as additional crucial parameters for species delimitation. Such detailed data are unattainable from field samples, necessitating laboratory-based life cycle studies.
We hereby propose a description of a new species from the genus Ulnaria—Ulnaria williamsii Glushchenko, Podunay, Davidovich, Mironov & Kulikovskiy sp. nov.—with the help of the integrative approach. Thus, the species is described on the basis of morphological data, molecular–phylogenetic analysis, and data from experiments on clonal mating. Within the latter part of this study, we demonstrate some formerly unknown features of sexual reproduction of Ulnaria. The evolutionary role of these features is discussed herein, too.
2. Materials and Methods
2.1. Sample Collection
For this study, a single sample from the northern shore of Lake Terkhiin-Tsagaan, Arkhangai, Mongolia, was analyzed. The sample collection method follows Mironov et al. [,]. The sample was collected on 31 July 2024, near the shore (48°10′53.5021″ N; 99°47′23.6915″ E). To obtain live material, small rocks with visible biofilms were scrubbed with toothbrushes, and silty sand was collected at a shallow depth with a plastic spatula. The water parameters at the sampling location were as follows: pH = 8.1; temperature = 17.5 °C; electrical conductivity = 272 µS (measured using a Hanna Combo HI 98129 device, Hanna Instruments Ltd., Bedfordshire, UK).
2.2. Cultivation, DNA Processing, and Molecular Analysis
Isolation of single cells was performed with the help of a Zeiss Axio Vert. A1 (ZEISS, Oberkochen, Germany) inverted microscope equipped with a ×20 objective. Monoclonal strains were obtained through micropipetting in sterile conditions. Established cultures were maintained in WC liquid medium [] under constant conditions: 23 °C, a 12:12 h light–dark photoperiod, 100 Lx. Cultures were visually inspected at weekly intervals and re-inoculated into fresh medium every two weeks to ensure purity. All cultures studied herein (CBM496mnp-YA, CBM496mnp-YB, CBM496mnp-YH, CBM496mnp-YM) originated from sample Mon294 (see Section 2.1) and were established on 23 August 2024. The cultures are stored in the Culture and Barcode Collection of Microalgae and Cyanobacteria “Algabank” (CBMC) in K.A. Timiryazev Institute of Plant Physiology, RAS, Moscow, Russia.
After four weeks of cultivation, DNA extraction procedures were carried out using the Chelex100 Chelating Resin in accordance with the 2.2. protocol (Bio-Rad Laboratories, Hercules, CA, USA). For amplification of the V4 region of SSU rDNA, the D512for and D978rev primers [] were utilized. rbcL sequences were amplified using rbcL dp7− [] and rbcL404+ [] primers. The specifications for PCR of V4 SSU rDNA were selected as follows: initial denaturation (95 °C, 5 min) followed by 35 cycles of denaturation (94 °C, 30 s), annealing (52 °C, 30 s), and elongation (72 °C, 50 s) with final extension (72 °C, 7 min) and maintenance (12 °C). For PCR of rbcL, were applied the following algorithm: (94 °C, 5 min), 44 cycles of denaturation (94 °C, 50 s), annealing (53 °C, 50 s), and elongation (72 °C, 80 s), finished by one-time extension (72 °C, 10 min) and maintenance (12 °C). All PCRs were processed with ScreenMix (Evrogen, Moscow, Russia), while the products were visualized through horizontal electrophoresis in 1.0% agarose gel. For this procedure, SYBRTM Safe stain (Life Technologies, Carlsbad, CA, USA) was utilized.
The acquired DNA material was sequenced by means of the Genetic Analyzer 3500 (Applied Biosystems, Waltham, MA, USA). Afterwards, the raw sequences were viewed and manually trimmed in Ridom TraceEdit (Ridom© GmbH, Münster, Germany). The newly obtained SSU rDNA and rbcL sequences were supplemented with reference sequences from GenBank in order to assemble the datasets for molecular analysis. Two separate datasets for SSU rDNA and rbcL were constructed and edited further in MEGA7.0.26 software (Pennsylvania State University, Pennsylvania, PA, USA []). The SSU rDNA dataset (392 nt) included 4 new and 63 reference sequences, while the rbcL dataset (919 nt) contained 4 new and 64 reference sequences. In each dataset, 3 strains of Cyclotella (Kützing) Brébisson spp. were chosen as the outgroup. Alignments were conducted separately as well, according to the G-INS-I algorithm, using Mafft ver.7 software (RIMD, Osaka, Japan []). Furthermore, unpaired regions were eliminated in MEGA7.0.26. A Bayesian inference (BI) analysis was carried out in BEAST ver.1.10.1 (BEAST Developers, Auckland, New Zealand []). The following parameters were chosen for BI: a yule process tree prior speciation model, a GTR + G + I substitution model, 10 MCMC analyses for 10 million generations (burn-in: 1000 million generations). Afterwards, the initial 10% of trees were removed in Tracer ver. 1.7.1 software (MCMC Trace Analysis Tool, Edinburgh, UK []). Rapid bootstrapping and maximum likelihood (RAxML) analysis was performed in raxmlGUI 2.0 software [] with 1000 replicas, a GTR substitution matrix, and agamma substitution rate. Both BI and RAxML were run separately for the SSU rDNA and rbcL matrices. Thus, the two best resulting trees from BI and RAxML were visualized in FigTree ver. 1.4.4 (University of Edinburgh, Edinburgh, UK) and edited in Adobe Photoshop CC ver.19.0 (Adobe, San Jose, CA, USA). The input and resulting data utilized for the current molecular analysis can be accessed in the Supplementary Materials.
2.3. Slide Preparation and Microscopy
The technique of sample preparation follows Kulikovskiy et al. []. To prepare the samples for morphological analysis, the obtained cultures were utilized. The cultures were treated with hydrochloric acid (10% concentration) for the removal of carbonates and washed in distilled water through centrifugation with a Liston C 2201 centrifuge (Liston, Obninsk, Russia) for 5 min, at 3600 rpm. Afterwards, the material was processed with chromic acid for 5 min, and the remaining content of acid was washed off through centrifugation, as described above. In addition, to preserve the delicate girdle band structure, live specimens were treated through calcination of the culture suspension for 1 h to remove the organic contents.
For light microscopy (LM), a Zeiss Axio Scope A1 (Carl Zeiss Microscopy GmbH, Göttingen, Germany) microscope was operated. The microscope was equipped with an oil immersion objective (plan-apochromatic ×100/n.a.1.4, Nomarski differential interference contrast, DIC) and a Zeiss Axio Cam ERc 5s camera (Carl Zeiss NTS Ltd., Oberkochen, Germany). For the preparation of permanent slides, Naphrax® (Brunel Microscopes Ltd., Chippenham, UK; refractive index = 1.73) was utilized.
Scanning electron microscopy (SEM) was carried out using a TESCAN Vega III (TESCAN, Brno, Czech Republic) SE microscope (operating voltage = 10 kV; working distance = 6 mm). To prepare the permanent SEM slides, we applied suspensions of the oxidized material onto aluminum stubs and left them to air-dry for 24 h. Afterwards, the stubs were sputter-coated with 50 nm Au, using the Eiko IB 3 (Eiko Engineering Co., Ltd., Hitachinaka, Japan) device.
The original sample (Mon294) and permanent slides made from the corresponding cultures (11386, 11387, 11388, 11389) are currently housed in the Diatom Herbarium (HD) at K.A. Timiryazev Institute of Plant Physiology, RAS, Moscow, Russia
2.4. Observing Sexual Reproduction
The clones used to study sexual reproduction were named according to Y.MMDD-X, where Y is the last digit of the year of isolation, MM is the month, DD is the day, and X is the short name of the clone. Clones were cultivated in glass Petri dishes (diameter = 5–9 cm; medium volume = 8–45 mL) using the Dm medium []. Cultures were maintained at a stable temperature (19–21 °C), under diffuse daylight from a northward-directed window. Every 4–6 days, the cultures were re-inoculated into fresh medium in order to prolong the exponential growth phase. They were inspected daily for signs of interclonal sexual reproduction, general vitality, and growth. Clones were periodically inoculated in pairs to stimulate interclonal reproduction. Besides the newly cultured clones, we analyzed previously obtained clones. These included U. ulna from the Moscow River (clones 1.0929-G and 1.0929-F), the Aduy River, Sverdlovsk Oblast (5.1124-A and 5.1124-B), and the Artyomovka River, Primorsky Krai (5.1111-B and 5.1111-C), Russia, as well as U. danica (5.0901-A and 5.0901-K) from Lake Khovsgol, Mongolia.
In the process of the experiments, regular observations were carried out using a Nib-100 inverted microscope (Biobase Biotech Co., Jinan, China) and a Biolar PI upright light microscope (PZO Ltd., Warsaw, Poland). The latter was equipped with an achromat 40×/0.75 water immersion objective (LOMO, Saint-Petersburg, Russia) and Pluta DIC and was adjusted for bright-field microscopy following the method by Keller []. Microphotographs of gametes and reproducing cells were captured using a Canon PowerShot A640 (Canon Inc., Tokyo, Japan) digital camera. Cell lengths (apical axis) were measured using an ocular ruler calibrated with a stage micrometer. The data are presented as the mean ± standard error; N represents the number of measurements.
2.5. Terminology
In the process of morphological analysis, the valve terminology follows Kulikovskiy et al. [] and Liu et al. []. The description of sexual reproduction adopts the terminology of Davidovich & Davidovich [].
3. Results
3.1. Molecular Analysis
The constructed phylograms (Figure 1 and Figure 2) represent the phylogeny of Ulnaria and related genera from the families Ulnariaceae E.J. Cox and Fragilaricaeae Kützing. In general, the phylograms are similar, with four strains of Ulnaria williamsii sp. nov. clustering together within the clade of Ulnaria spp. The support for this subclade, however, is available only from SSU rRNA analysis (likelihood bootstrap, LB = 65; posterior probability, PP = 0.97). It is difficult to assess the mutual molecular–phylogenetic relationships between other representatives of the genus, due to high degree of similarity of SSU rRNA and rbcL sequences. However, the overall Ulnaria clade is well supported by both BI and RAxML (SSU rRNA: LB = 51, PP = 1; rbcL: LB = 98, PP = 1). Regarding the SSU rRNA phylogram (Figure 1), Ulnaria spp. comprise the major clade, while the rest of the analyzed strains from a sister group (PP = 0.96). In particular, within this group, Hannaea R.M. Patrick is represented by five highly similar strains of Hannaea librata E.A. Hwang & B.H. Kim (LB = 81, PP = 1). Three strains of Tabularia (Kützing) D.M. Williams & Round are closely related to each other as well (LB = 62, PP = 0.98). The rbcL phylogram (Figure 2) clearly demonstrates that the clade of Ulnaria is sister to the clade of Hannaea and Fragilaria (LB = 99, PP = 1). The lower part of the clade includes a branch of Catacombas D.M. Wiliams & Round and Tabularia, with the latter genus demonstrated as paraphyletic. Lastly, strains of Punctastriata D.M. Wiliams & Round are grouped together with Synedra fragilarioides Hargraves & Guillard, Fragilaria rotundissima Hargraves & Guillard, and Fragilariforma virescens (Ralfs) D.M. Williams & Round (LB = 58, PP = 1).
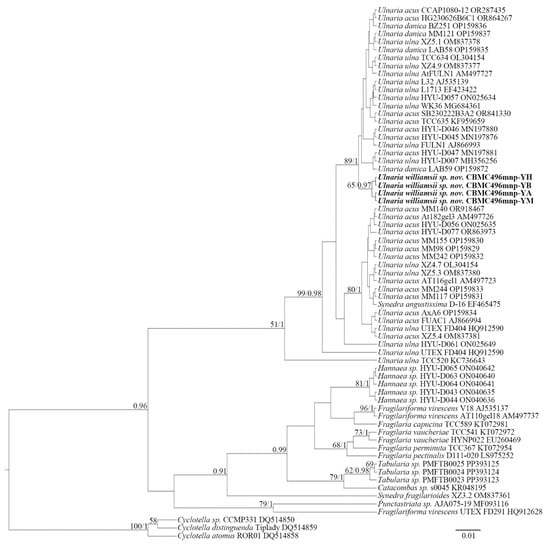
Figure 1.
Phylogeny of Ulnaria and allied taxa based on BI and RAxML analyses of the SSU rRNA gene sequences. Values of LB below 50 and PP below 0.90 are hidden. Strain numbers and GenBank accessions are indicated for all sequences.
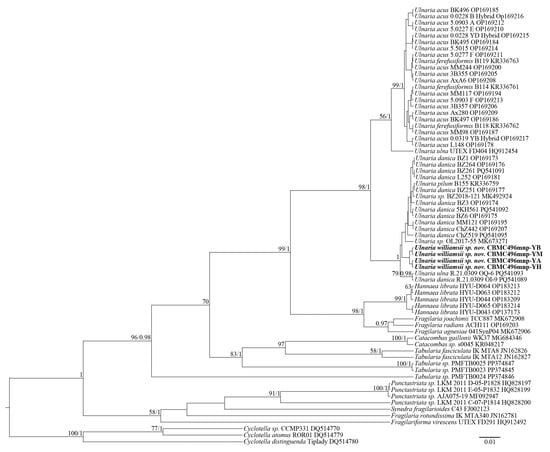
Figure 2.
Phylogeny of Ulnaria and allied taxa based on BI and RAxML analyses of the rbcL gene sequences. Values of LB below 50 and PP below 0.90 are hidden. Strain numbers and GenBank accessions are indicated for all sequences.
Thus, the SSU rDNA and rbcL phylograms show similar results. Most importantly, the representative strains of Ulnaria form an extensive group, which also includes new strains of Ulnaria williamsii sp. nov. Other species of the genus, i.e., Ulnaria acus, Ulnaria danica, and Ulnaria ulna, require further molecular studies to determine their phylogenetic relationships. Inter-connections between the rest of the araphid genera represented are hardly resolvable through our analysis, too, mostly because of the small size of the matrices and scarcity of representation of the largest genera, i.e., Fragilaria and Tabularia.
3.2. Description of the New Species
Ulnaria williamsii Glushchenko, Podunay, Davidovich, Mironov & Kulikovskiy sp. nov. (Figure 3, Figure 4, Figure 5, Figure 6 and Figures S1–S12)

Figure 3.
Ulnaria williamsii sp. nov. Slide 11386 from oxidized culture strain CBMC496mnp-YA. LM, DIC. (A–J) Size diminution series. (A) Holotype specimen. Scale bar = 10 μm.
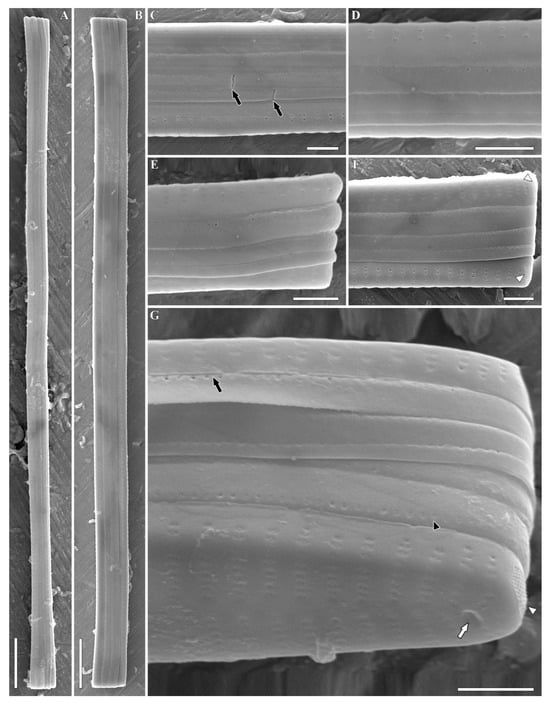
Figure 4.
Ulnaria williamsii sp. nov. Slide 11386 from oxidized culture strain CBMC496mnp-YA. SEM, girdle views. (A,B) Whole valve. (C,D) Central valve portion. Black arrows show the breaches in the copulae. (E–G) Valve pole. Black arrows show the perforation situated in the central part of the valvocopula continuing under the epivalve; white arrow shows the rimoportula; black arrowhead shows the row of poroids at the valvocopula; white arrowheads show the ocellulimbi. Scale bars = 10 µm (A,B), 2 µm (C–G).
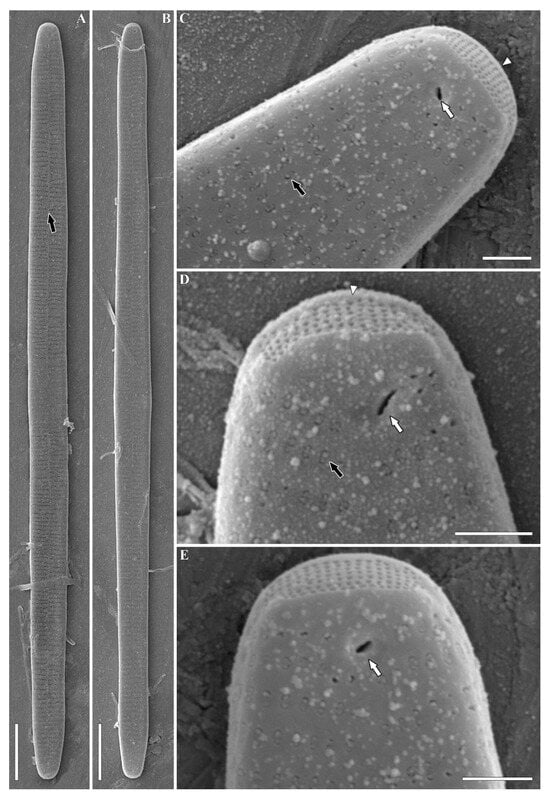
Figure 5.
Ulnaria williamsii sp. nov. Slide 11386 from oxidized culture strain CBMC496mnp-YA. SEM, external views. (A,B) Whole valve. The black arrow shows the sternum. (C–E) Valve pole. The black arrow shows the plaque covering the opening of areola; white arrows show the single openings of rimoportulae; the white arrowhead shows the ocellulimbus. Scale bars = 10 µm (A,B), 1 µm (C–E).
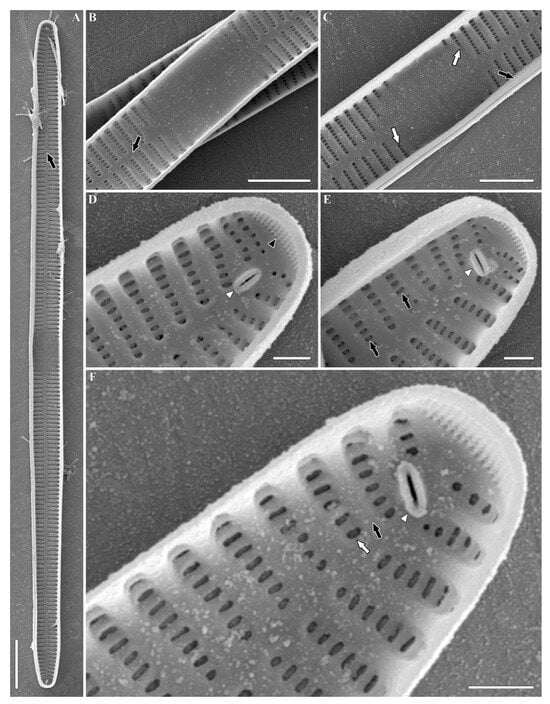
Figure 6.
Ulnaria williamsii sp. nov. Slide 11386 from oxidized culture strain CBMC496mnp-YA. SEM, internal views. (A) Whole valve. The black arrow shows the sternum. (B) The central portion of the valve. The black arrow shows the sternum. (C) The central portion of the valve. The black arrow shows the valvocopula; white arrows show the shortened striae. (D,E) Valve pole. Black arrows show the areolae with apically elongated openings; the black arrowhead shows the ocellulimbus; the white arrowhead shows the rimoportula. (F) Valve pole. The black arrow shows the virga; the white arrow shows the vimin; the white arrowhead shows the rimoportula. Scale bars = 10 µm (A), 5 µm (B,C), 1 µm (D–F).
Description. LM (Figure 3 and Figures S1–S3). Valves linear, with parallel margins, moderately tapering towards the apices. Valve ends wedge-shaped and broadly rounded to obtusely rostrate. Valve length 54–337 µm, width 5.5–7.3 µm in the central portion. Sternum well expressed, narrow, linear in shape. Central area variable, rectangular to elliptic, delimited by shortened striae on each side. Central area with clearly visible ghost striae. Striae 11–13/10 µm, located mostly parallel throughout the valve, becoming slightly radiate near the apices. Each valve end bears a single rimoportular opening.
Description. SEM, external view (Figure 4, Figure 5 and Figures S4–S9). Valves rectangular in girdle view, somewhat slightly expanded towards the ends (Figure 4A,B and Figures S4A,B, S5A–C and S6A). Copulae are not always visible at valve apices, mostly unseparated from each other (Figure 4C–F and Figures S4C–E, S5E and S6B,C). Each frustule is equipped with four closed copulae and two valvocopulae (Figure S4D). Usually, each copula is furnished with a single continuous row of poroids situated along the axial part of the copula. Where the copulae are closely appressed towards each other, the row of poroids is located at the border of external and internal parts of each copula (Figure S4C, black arrows) or of the valve and valvocopula (Figure 4G and Figure S5F, black arrows). The second row of poroids at the copula can be partially developed (Figure 4G, black arrowhead). Sometimes, copulae are irregular in shape, with breaches near the center of the frustule (Figure S4D, white arrows). Perforations are absent at the apices of valvocopulae (Figure S6C, black arrows).
Valve face is flat. Sternum narrow, linear (Figure 5A and Figures S7A,B, S8A–D and S9A–C, black arrows). Striae uniseriate or rarely biseriate in the apical valve region, near the margin (Figure 4E and Figure S9C, marked by white arrow at Figure S9C). Striae comprising 10–11 areolae near the central nodule, 4–6 towards the apices. Striae continue onto the valve margin with 2 to 5 areolae (Figures S4D, S5D and S6B, white arrows). Areolae densely arranged (40/10 µm). Areolar openings covered by plaques with struts attaching them to the walls of areolae (Figure 5C,D). Well-boiled material demonstrates slightly eroded areolae with round to apically elongated openings (Figure S7B–D). Interstriae (virgae) much wider than striae (Figures S4D, S5D, S8C and S9B, marked by black arrowheads at Figures S4D, S5D and S8C). Notably, one or two isolated areolae are located at valve margin, against the central area (Figures S4C and S5D, marked by white arrowheads at Figure S4C). Ocellulimbi evident, consisting of minute pores arranged into 6–10 transapical rows. They are located at valve poles (Figure 4F,G, Figure 5C–E and Figures S5E, S7C,D, S8D and S9C,D, white arrowhead). Two small horn-shaped outgrowths are sometimes situated near the ocellulimbi, above the valve margin (Figure S5E). Rimoportulae represented by single roundish or slit-like openings lying in a shallow depression (Figure 4G, Figure 5C–E and Figures S5F, S6C, S7C,D, S8D and S9D, white arrows). In the latter case, the opening is angled at approximately 45°.
SEM, internal view (Figure 6 and Figures S10–S12). Valvocopulae tightly pressed towards the inner valvar surface (Figure 6C, black arrow). Sternum narrow, linear (Figure 6A,B and Figures S10A,B, S11A,B and S12A,B, black arrows). Virgae (Figure 6F and Figures S10D, S11C and S12C, black arrows) raised above the narrower vimines (Figure 6F and Figures S10D, S11C and S12C, white arrows). Vimines slightly tapering towards the sternum. The outer siliceous plaques are visible through the openings of areolae (Figure 6E, black arrows). Internally, central area is furnished by shallow transapical depressions, which correspond to ghost striae in LM (Figure S11B, white arrows). Sometimes, the central area is bordered by shortened striae or isolated areolae (Figure 6C and Figure S10B, white arrows). Isolated areolae are also notable in the marginal area against the central nodule (Figure S12C, white arrowhead). Valve apices equipped with ocellulimbi (Figure 6D and Figures S10C,D and S12D–G, black arrowheads). Sometimes, the surface of ocellulimbi is hidden by the valve margin at the apex (Figure S11C,D, black arrowheads). Rimoportulae simple, well discerned, with inner aperture formed by two lips, typically located beside the sternum and angled (45–180°) in relation to the apical axis of the valve (Figure 6D–F and Figures S10C,D, S11C,D and S12D–G, white arrowheads).
Type. Slide no. 11386 (from sample Mon294), deposited into the Diatom Herbarium (HD) in K.A. Timiryazev Institute of Plant Physiology, Russian Academy of Science, Moscow, Russia.
Holotype. Illustrated in Figure 3A (deposited into the Diatom Herbarium (HD) in K.A. Timiryazev Institute of Plant Physiology, Russian Academy of Science, Moscow, Russia).
Reference strains. CBMC496mnp-YA, CBMC496mnp-YB, CBMC496mnp-YH, CBMC496mnp-YM isolated from sample Mon294. Strains were deposited into the Culture and Barcode Collection of Microalgae and Cyanobacteria “Algabank” (CBMC) at K.A. Timiryazev Institute of Plant Physiology, RAS, Moscow, Russia.
Type locality. Mongolia, Arkhangai Province, Tariat District, Lake Terkhiin-Tsagaan, biofilms on rocks and silty sand near the shore, leg. A. Mironov, collection date: 31 July 2024.
Etymology. The specific epithet “williamsii” is dedicated to Dr. David M. Williams, for his contribution to the study of araphid diatoms, namely Ulnaria.
Distribution. The new species is currently known only from its type locality.
3.3. Sexual Reproduction in Ulnaria williamsii sp. nov.
In the process of cultivation of U. williamsii sp. nov., different characteristics of sexual reproduction were registered—the mating types of clones, gametogenesis (with related alterations in protoplast), and the movement of gametes. It is worth mentioning that the clones (vegetative cells) of Ulnaria williamsii sp. nov. contained in the cultures demonstrated a wide range of apical sizes—from 92 µm in the shortest observed cells at the end of their life history to 337 µm in the longest initial cell resulting from sexual reproduction. The apical sizes of the cells in the cultures at different stages of the life cycle are presented in Figure 7.
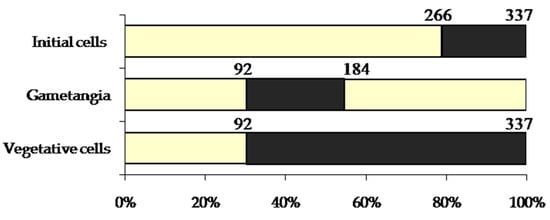
Figure 7.
A cardinal point diagram representing the stages in the life cycle of Ulnaria williamsii sp. nov. The values of valve length are given in µm. Ranges of valve length values recorded during the study are indicated in black. Percents correspond to the proportion of the maximal cell size recorded.
3.3.1. Mating System
Clones of U. williamsii sp. nov. readily underwent interclonal mating according to their mating types, conditionally designated as male (M) and female (F) (Table 1). In the present paper, we use the terms “male” and “female” instead of “mating type +” or “mating type –”, thus presuming that behavioral and morphological divergence corresponds not only to cross-compatibility but also to a diplogenotypic mode of inheritance of sex determinants. Interclonal reproduction was observed in one female and several male clones. In these clones, interclonal reproduction was presumably allogamous; we did not observe a paedogamous mode of reproduction. Intraclonal reproduction was always less intense than interclonal reproduction. Moreover, clones of U. williamsii sp. nov. actively crossed with each other but did not engage in sexual reproduction with the clones of U. ulna and U. danica included in this study (Table 1).

Table 1.
Mating compatibility of U. williamsii sp. nov., U. ulna, and U. danica clones.
3.3.2. Male and Female Gametogenesis
Each male and female gametangium produced two gametes. By the end of gametogenesis, the gametes were spherical and morphologically identical. However, the early stages of gametogenesis differed significantly between the sexes. In the male gametangium, the protoplast divided into two equal parts in the transapical plane (Figure 8A,B). Chloroplasts, which were arranged along the apical axis in vegetative cells, migrated to opposite ends of the gametangium. After the rearrangement of the chloroplasts was completed, a septum was formed, separating the gametes (Figure 8C,D). During the early stages, male gametes were rod-shaped with rounded ends. Later, the gametes sequentially rounded up, gradually became spherical, and—thereby pushing the gametangial valves apart—exited the gametangium (Figure 8F–H).
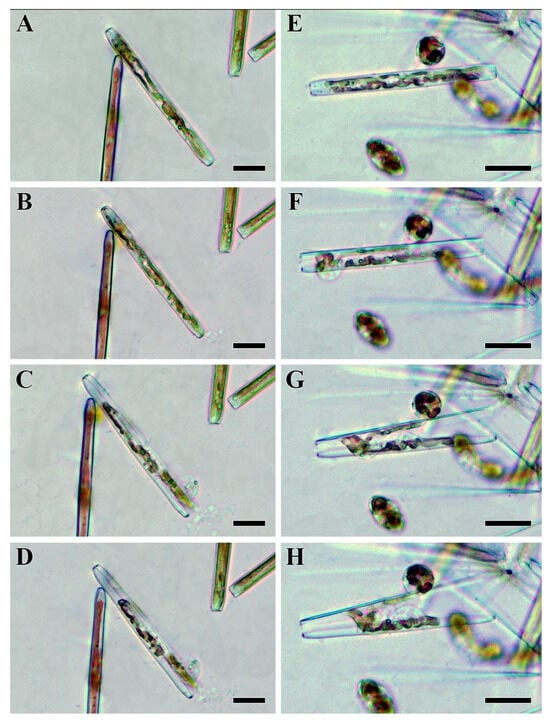
Figure 8.
Stages of male (A–D) and female (E–H) gametogenesis in Ulnaria williamsii sp. nov., live material, LM, DIC. (A,B) Chloroplast rearrangement in a male gametangium. (C,D) Division of the male protoplast in the transapical plane. (E) Division of the female protoplast along the apical axis. (F–H) Mature female gametes become spherical and move the gametangial valves apart. Scale bars = 30 µm.
In contrast to male gametangia, female gametangia divided along the apical plane (Figure 8E,F). Notably, chloroplast rearrangement was not observed. Initially, female gametes were attached to the valves of the mother frustule, opposite each other (Figure 8E). The development of the two gametes within the female gametangium was not completely synchronous; very often, one gamete developed faster. However, both female gametes eventually rounded and lost contact with the valves (Figure 8G,H). After being released from the gametangium, the female gametes also became spherical like male ones. The mature female gametes were often observed near the empty gametangial frustules.
Following physical contact, male and female gametes fused, giving rise to spherical zygotes (Figure 9A). Neither copulation tubes nor mucilage envelopes were observed. Approximately one to two hours later, the zygotes began to expand bipolarly, at which point they were considered auxospores. The growing auxospores did not maintain tight contact with the valves of the mother frustule and were located between or away from them (Figure 9B). Elongated auxospores were slightly curved (Figure 9C). No remnant zygote envelopes, such as polar caps, were visible. Inside the fully expanded auxospore, a primary frustule was deposited, giving rise to the initial cell (Figure 9D), which then resumed mitotic divisions.
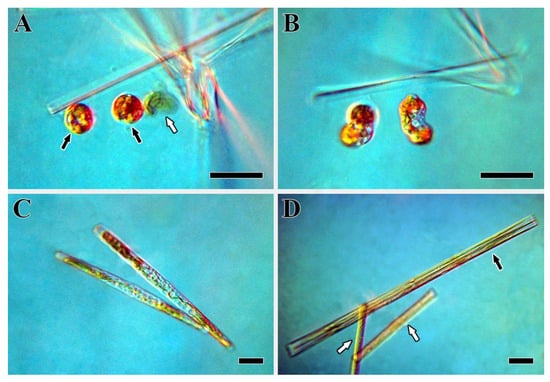
Figure 9.
Development of auxospores in Ulnaria williamsii sp. nov., live material, LM, DIC. (A) Zygotes. The black arrows point to the formed zygotes; the white arrow indicates an aborted gamete. (B) The early stage of auxospore development. (C) The late stage of auxospore development. (D) Post-initial cells. The black arrow points to a post-initial cell; the white arrows indicate the mother cells. Scale bars = 30 µm.
3.3.3. Gamete Movement
Gamete movement was associated with the formation of slender cytoplasmic projections on the gamete surface (Figure 10 and Figure 11). In the studied species, both male and female gametes were capable of forming these projections. The projections were transient structures that resembled pseudopodia to some extent. Pseudopodial activity was observed during the early stages of male gamete development, while they were still within the gametangial valves. Some female gametes began to form pseudopodia while still attached to the gametangial valves. After being released from the gametangium, mature gametes of both sexes were almost perfectly spherical and immobile. The gamete surface then became active, forming a few wide, amoeboid-like projections that distorted the spherical cell shape. Over time, one or two of these projections elongated into thin filaments, while the shorter, broader protrusions retracted. The long threads bore thickenings (knots), and the increasing distance between these knots and the cell body suggested that elongation occurred from the base. Upon reaching their maximum length, the projections became flexible and began to retract. The retracted projections appeared to coil around the gamete, causing it to rotate in the opposite direction akin to a “ball of yarn”. Finally, the projections were fully retracted, and the cell returned its spherical shape. This cycle was repeated every few minutes. Male gametes more frequently form cytoplasmic projections and tend to move for longer durations and at higher speeds. In contrast, not all female gametes initiated movement after detaching from the gametangial cell.
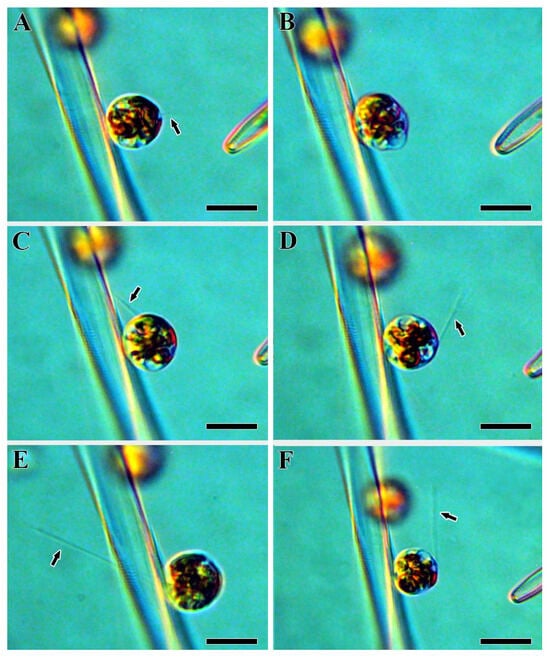
Figure 10.
The formation of cytoplasmic projections of male gametes in Ulnaria williamsii sp. nov., live material, LM, DIC. (A) The initial stage of gamete movement. (B) A gamete changing its shape during movement. (C–F) Male gametes with cytoplasmic projections. Black arrows indicate the cytoplasmic projections. Scale bars = 20 µm.
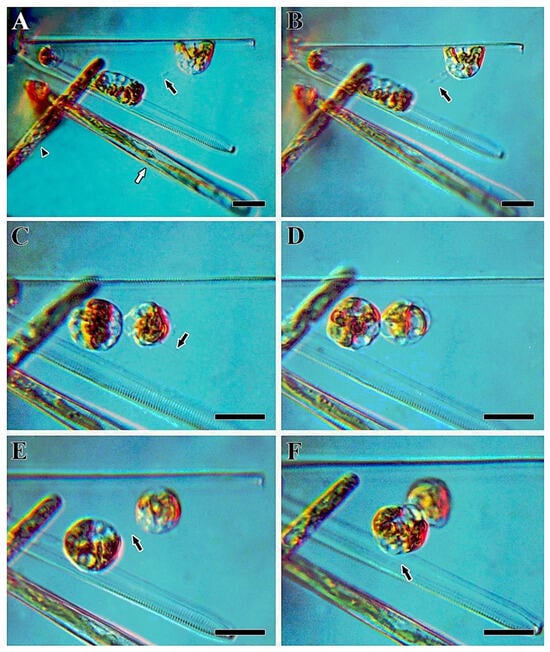
Figure 11.
The formation of cytoplasmic projections of female gametes in Ulnaria williamsii sp. nov., live material, LM, DIC. (A) Neighboring cells with ongoing male gametogenesis. The black arrow indicates a cytoplasmic projection; the white arrow indicates the beginning of male gametogenesis in a neighboring cell; the black arrowhead indicates a growing auxospore. (B–F) Female gametes with cytoplasmic projections. The black arrows indicate the cytoplasmic projections. Scale bars = 20 µm.
4. Discussion
4.1. Morphological Variability in the Acquired Strains
Individual measurements of valve length and width and striae density were conducted for each strain of Ulnaria williamsii sp. nov. obtained during this study (Table 2). Subsequently, this data was utilized for the morphological comparison of the new species with similar taxa.

Table 2.
Quantitative characteristics of the acquired strains.
The longest valves were detected for the specimens of strain CBMC496mnp-YA—139.6 µm. The shortest valves were typical for strain CBMC496mnp-YH—54.0 µm. At the same time, in strain CBMC496mnp-YA, the valve length range was smaller—6.1 µm—which was slightly less in comparison with another strain, CBMC496mnp-YB, where this parameter was 11.3 µm. In contrast, strains CBMC496mnp-YH and CBMC496mnp-YM were characterized by a greater difference between the smallest and the biggest valves: the size ranges were 64.7 µm and 40.7 µm, respectively. Regarding the overall morphology, constriction of the valves is more notable for strain CBMC496mnp-YM (Figure S3). We also registered numerous specimens of this culture with evenly constricting valves that resembled the specimens from other cultured material of U. williamsii sp. nov. The valve ultrastructure is identical among the four strains. However, in the strain CBMC496mnp-YM, the apical pore fields (ocellulimbi) extend to the valve face to a greater extent than we noted for other strains of the new species.
4.2. Morphological Comparison of Ulnaria williamsii sp. nov. with Similar Species
For the morphological analysis, we selected the species from the Ulnaria ulna species complex (Table 3). Undoubtedly, all taxa listed below resemble each other in the fine structure of the valves: they are quite similar in frustule organization, striae arrangement, and the structure of the ocellulimbi and rimoportulae. Thus, we hereby emphasize the morphological dissimilarities related to the valve outlines and quantitative features.

Table 3.
Comparison of Ulnaria williamsii sp. nov. with similar species of Ulnaria.
Ulnaria williamsii sp. nov. resembles U. ulna (epitype) in the following features: valve width (5.5–7.3 µm in U. williamsii sp. nov. vs. 6.3–7.7 µm in U. ulna) and overall valve shape in girdle view (see Table 3). At the same time, the dissimilarities between these two taxa are expressed in the type of valve outlines—linear in the new species but needle-shaped (distinctly narrowing towards the apices) in U. ulna (Ref. []: pl. 28). The valve apices are wedge-shaped and broadly rounded to more obtuse/rostrate in U. williamsii sp. nov., while in U. ulna, the apices are subcapitate, extremely thin, and thus sometimes hard to discern (see Table 3). Moreover, the striae in the new species from Mongolia can become biseriate at the margins, which is untypical for U. ulna (Ref. []: pl. 29). The striae density is 11–13/10 µm in U. williamsii sp. nov. vs. 9.2–10.4/10 µm in U. ulna (see Table 3). Areolae density is also different—40/10 µm in U. williamsii sp. nov. vs. only 28–33/10 µm in the epitype species. The most important difference between the two taxa, perhaps, is the presence of a well-developed central area in Ulnaria williamsii sp. nov. By contrast, this structure is inevitably absent in the epitype specimens of U. ulna, and a single shortened stria can be located in the central valve portion (Ref. []: pl. 28, 29).
Another species resembling Ulnaria williamsii sp. nov. is U. splendens. The two taxa resemble each other in the linear outline of the valves, the narrowly rectangular shape of the frustules in girdle view, and the overlapping values of valve width (5.5–7.3 µm in U. williamsii sp. nov. vs. 5–10 in U. splendens) and areolae density (40/10 µm in U. williamsii sp. nov. vs. 35–40/10 µm in U. splendens), as well as the presence of the central area (see Table 3). Dissimilarities can be found in the morphology of the apices—wedge-shaped to obtusely rostrate in the new species while weakly protracted and broadly rounded in U. splendens (Ref. []: Figures 1–7). Again, a partially biseriate arrangement of the striae is typical only for the new species (Ref. []: Figures 9–11, 17 and 18). In addition, the striae density in U. williamsii sp. nov. is 11–13/10 µm vs. 6–10/10 µm in U. splendens. The central area is bigger in U. williamsii sp. nov., more rectangular than in U. splendens, and delimited by striae of a greater length (Ref. []: Figures 1–7).
U. xieriverensis, a species described from Hunan province, China, demonstrates a set of similarities with the species described in this study. In both species, the valves are linear and of comparable widths (5.5–7.3 µm in U. williamsii sp. nov. vs. 6.0–8.6 µm in U. xieriverensis) and striae (11–13/10 µm in U. williamsii sp. nov. vs. 10.5–12.0 /10 µm in U. xieriverensis) and areolae densities (40/10 µm in both species) (see Table 3). Despite that, U. williamsii sp. nov. is characterized by broadly wedge-shaped or obtusely rostrate apices, while U. xieriverensis is characterized by broadly rostrate apices (Ref. []: Figures 73A–H). Biseriate striation is visible at the margins only in Ulnaria williamsii sp. nov., i.e., in U. xieriverensis, the striae are always uniseriate (Ref. []: Figures 74A–F and 75A–F). The central area is well expressed in Ulnaria williamsii sp. nov. but reduced in U. xieriverensis (Ref. []: Figures 73A–H, 74A–F and 75A–F).
Another taxon worth mentioning in the comparison is the material identified as (?) Synedra ulna sensu Metzeltin et al. (Ref. []: pl. 14, Figures 1–5). It is similar to the newly proposed species according to the following criteria: linear valve outlines, the shape of the apices (wedge-shaped to rostrate), and the morphology of the central area (see Table 3). Nevertheless, the two taxa should be treated as the representatives of the same species due to the differences in valve widths (5.5–7.3 µm in U. williamsii sp. nov. vs. 7.4–8.5 µm in (?)S. ulna sensu Metzeltin et al. []) and striae density (11–13/10 µm in U. williamsii sp. nov. vs. 9–10/10 µm in (?)S. ulna sensu Metzeltin et al. []). Moreover, the central area in (?)Synedra ulna sensu Metzeltin et al. [] is more irregular in its shape (rectangular to nearly elliptic) due to the different length of the shortened striae located beside the central nodule (Ref. []: pl. 14, Figures 1–5).
4.3. Breeding Behavior of Ulnaria williamsii sp. nov.
Gametogenesis in U. williamsii sp. nov. began with the formation of numerous vacuoles in the cell. Initially, female and male gametangia could be distinguished by the mode of gamete formation. This feature has also been noted for U. ulna and U. acus [,,]. We observed chloroplast rearrangement during male gametogenesis. Specifically, the septum separating the gametes formed after the chloroplasts had repositioned. A similar process was suggested in Tabularia fasciculata (C. Agardh) D.M. Williams []. Interestingly, in the araphid pennate Licmophora ehrenbergii (Kützing) Grunow, female gametes themselves never rearranged, whereas gametes in male gametangia rearranged or did not depending on the relative position of the mating gametangia []. In contrast to species of the genus Ulnaria, in T. fasciculata and T. tabulata (C. Agardh) Snoeijs, female gametes were more tightly associated with gametangial valves [,].
In the diatom life cycle, the upper size limit suitable for sexual reproduction may range from 30 to 75% of the maximal cell size, depending on the species, but in most diatoms, it corresponds to 45–55% []. U. williamsii sp. nov. has an upper critical size close to 55% of its maximal size (see Figure 7). For U. ulna, this value was 40% of the maximal size, and for U. acus, it was 54% [,].
Locomotion of the gametes results from the activity of the cell surface, manifested in the production of thin cytoplasmic projections and a weakly distinguishable amoeboid-like movement. The gametes of the studied species demonstrated a mode of gamete movement associated with the formation of pseudopodia-like structures. The movement of gametes of Ulnaria williamsii sp. nov. is similar to that observed in the closely related species U. ulna [], U. acus [], T. tabulate, and T. fasciculata []. Such pseudopodia-like structures are also characteristic of the polar centric diatom Synedrosphenia crystallina (C. Agardh) Lobban & Ashworth (=Ardissonea crystallina (C. Agardh) Grunow) []. The mechanism of locomotion is also similar to that of the araphid pennate Pseudostaurosira trainorii E. Morales (=Nanofrustulum trainorii (E. Morales) E. Morales) []. Thus, the formation of cytoplasmic projections in active male gametes has been described to date in only five species of araphid diatom and one species of polar centric diatom. Further study of gamete movement in other genera of immobile araphid diatoms is necessary to establish how common this mechanism is and to elucidate the ways these organisms overcome the problem of delivering gametes to the site of syngamy.
Unlike the above-mentioned species, in U. williamsii sp. nov., both male and female gametes form cytoplasmic projections and are capable of movement. The ability to form pseudopodia in both types of gametes not only significantly increases the probability of contact with gametes of the opposite sex but also promotes interclonal (homothallic) allogamous reproduction in both sexes. In the studied pennate diatoms, the ability for homothallic reproduction was previously observed predominantly in one sex (male), which was shown to be heterogametic [,].
The additional capacity for homothallic reproduction in both sexes of U. williamsii sp. nov. significantly expands the species’ dispersal capabilities. This is particularly relevant for freshwater diatoms that need to overcome a land barrier when moving from one water basin to another or an even greater challenge—the colonization of isolated island freshwater bodies. When a single male cell—which, by analogy with its closest relatives U. ulna [] and T. tabulata [], can be considered heterogametic—arrives in a new location, it is capable of not only multiplying through mitotic divisions but also establishing a new mixed-sex population. This is achieved through homothallic reproduction, which yields in heterogametic clones both male and female offspring [,,]. A single female cell (homogametic), having arrived in a new place, will also persist over generations, producing exclusively female offspring through homothallic reproduction. The resulting increase in the number of colonizers enhances the chances of subsequent interclonal crossbreeding.
The classification of sexual reproduction modes in diatoms most commonly used today was developed by L. Geitler, who divided them into four main categories []. This scheme remains a good generalization and covers most of what is known about the diversity of sexual reproduction patterns in pennate diatoms. However, as some authors have already noted [,,], Geitler’s classification requires refinement. In our case, the sexual process in the species we studied most closely corresponds to Type IA2 in Geitler’s system. However, further detailing does not align with the options proposed by Geitler: (a) with rearrangement and rounding-up of the gametes; (b) without rearrangement but with contraction of the gametes. As mentioned above, in U. williamsii sp. nov., as well as in the previously studied U. ulna and U. acus, gametogenesis proceeds differently in male and female clones, leading us to describe two distinct types of gametogenesis []. This scenario is absent from Geitler’s classification.
The description of Ulnaria williamsii sp. nov. exemplifies a comprehensive integrative approach, combining detailed morphology, molecular phylogenetics, and experimental data on the life cycle and reproductive biology. When viewed in the context of the genus, this study is a significant advancement. To date, the vast majority of the approximately 70 acknowledged Ulnaria species [] have been described based solely on morphological characteristics, often from field samples. Only a handful of recent descriptions, primarily from Lake Baikal [] and China [,,,,], have started to integrate molecular data with morphological observations. However, the inclusion of laboratory-based reproductive experiments as a core component of species delimitation remains exceptionally rare not only for Ulnaria but for diatom taxonomy in general. While the integrative approach is increasingly viewed as the gold standard for describing new diatom taxa, its application is still constrained by the significant challenges of establishing clonal cultures and conducting mating experiments. Therefore, this work on U. williamsii sp. nov. not only adds a new species to the genus but also serves as a model for a more rigorous, multi-faceted methodology that can resolve complex species boundaries and provide insights into diatom reproductive evolution.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/phycology5040065/s1. File S1: Alignment of the SSU rRNA genes used for phylogenetic analysis in this study; File S2: The Bayesian phylogenetic topology for the SSU rRNA tree; File S3: Alignment of the rbcL rRNA genes used for phylogenetic analysis in this study; File S4: The Bayesian phylogenetic topology for the rbcL tree; File S5: Compiled LM (Figures S1–S3) and SEM (Figures S4–S12) illustrations of strains CBMCmnp496-YB, CBMCmnp496-YH, and CBMCmnp496-YM with corresponding figure legends.
Author Contributions
Conceptualization: A.G., Y.P., N.D. and M.K.; methodology: E.K., N.D. and Y.M.; software, Y.M.; validation: A.G., A.M. and N.D.; formal analysis: A.G., Y.P. and A.M.; investigation: A.G., Y.P. and A.M.; resources: N.D., S.N. and M.K.; data curation: Y.M. and M.K.; writing—original draft preparation: A.G., Y.P. and A.M.; writing—review and editing: N.D., Y.M. and M.K.; visualization: A.G., Y.P. and A.M.; supervision: N.D. and M.K.; project administration: M.K.; funding acquisition: M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This publication is based on research carried out with financial support by the Russian Science Foundation (24-44-03001, https://rscf.ru/project/24-44-03001/ accessed on 14 August 2025) for sample collection and molecular analysis, the NSF of Mongolia (2024/206) for the work of S.N., percentage contribution is 90%. LM and SEM was performed with financial support by state assignment of the Ministry of Science and Higher Education of the Russian Federation (theme 122042700045-3), percentage contribution is 10%. Analysis of sexual reproduction was performed with financial support by state assignment of the Ministry of Science and Higher Education of the Russian Federation (theme 124030100100-0).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The samples collected during this study and prepared samples were deposited into the Diatom Herbarium (HD) at K. A. Timiryazev Institute of Plant Physiology, RAS, Moscow, Russia. The strains analyzed herein are housed at the Culture and Barcode Collection of Microalgae and Cyanobacteria “Algabank” (CBMC) at K. A. Timiryazev Institute of Plant Physiology, RAS, Moscow, Russia. The sequences obtained during the current study will be available in the NCBI SRA database upon the publication of this article. Until then, sequence data for the strains CBMC496mnp-YA, CBMC496mnp-YB, CBMC496mnp-YH, CBMC496mnp-YM should be requested from A. Mironov. The molecular datasets and results of the molecular analyses performed can be accessed in the Supplementary Files.
Acknowledgments
The facilities of the World Ocean Diatoms Collection (WODC) were used in the work. The authors express their gratitude to R.A. Rakitov (from the instrument analytics room of the Borissiak Paleontological Institute of the Russian Academy of Science, PIN RAS) for assistance in working with the scanning electron microscope.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| APF | Apical pore field |
| LM | Light microscopy |
| SEM | Scanning electron microscopy |
| rbcL | Gene-encoding large subunit of RuBisCO |
| RuBisCO | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| SUS rRNA | Small subunit of the rRNA |
| DIC | Differential interference contrast |
| PCR | Polymerase chain reaction |
| BI | Bayesian inference |
| RAxML | Randomized accelerated maximum likelihood |
| GTR | General time-reversible |
| LB | Likelihood bootstrap |
| PP | Posterior probability |
References
- Compère, P. Ulnaria (Kützing) Compère, a new genus name for Fragilaria subgen. Alterasynedra Lange-Bertalot with comments on the typification of Synedra Ehrenberg. In Lange-Bertalot-Festschrift: Studies on Diatoms. Dedicated to Prof. Dr. Dr. h.c. Horst Lange-Bertalot on the Occasion of His 65th Birthday; Jahn, R., Kociolek, J.P., Witkowski, A., Compère, P., Eds.; A.R.G. Gantner Verlag: Königstein, Germany, 2001; pp. 97–102. [Google Scholar]
- Van de Vijver, B.; Cocquyt, C. Four new diatom species from La Calera hot spring in the Peruvian Andes (Colca Canyon). Diatom Res. 2009, 24, 209–223. [Google Scholar] [CrossRef]
- Silva, P.; Hasle, G.R. Taxonomic and nomenclatural history of Fragilaria (Bacillariophyceae). Taxon 2006, 55, 200–202. [Google Scholar] [CrossRef]
- Williams, D.M. Synedra, Ulnaria: Definitions and descriptions—A partial resolution. Diatom Res. 2011, 26, 149–153. [Google Scholar] [CrossRef]
- Morales, E.A.; Rivera, S.F.; Wetzel, C.E.; Novais, M.H.; Hamilton, P.B.; Hoffmann, L.; Ector, L. New epiphytic araphid diatoms in the genus Ulnaria (Bacillariophyta) from Lake Titicaca, Bolivia. Diatom Res. 2013, 1, 41–54. [Google Scholar] [CrossRef]
- Lange-Bertalot, H.; Ulrich, S. Contributions to the taxonomy of needle-shaped Fragilaria and Ulnaria species. Lauterbornia 2014, 78, 1–73. [Google Scholar]
- Kulikovskiy, M.S.; Glushchenko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Identification Book of Diatoms from Russia; Filigran: Yaroslavl, Russia, 2016; pp. 1–804. [Google Scholar]
- Ehrenberg, C.G. Beiträge zur Kenntnis der Organisation der Infusorien und ihrer geographischen Verbreitung, besonders in Sibirien. Abh. Königlichen Akad. Wiss. Zu Berl. 1830, 1, 1–88. [Google Scholar] [CrossRef]
- Nitzsch, C.L. Beitrag zur Infusorienkunde oder Naturbeschreibung der Zerkarien und Bazillarien. Neue Schriften Naturforschenden Ges. Zu Halle 1817, 3, 1–128. [Google Scholar]
- Tuji, A. The transfer of two Japanese Synedra species (Bacillariophyceae) to the genus Ulnaria. Bull. Natl. Mus. Nat. Sci. Ser. Bot. 2009, 35, 11–16. [Google Scholar]
- Cantonati, M.; Lange-Bertalot, H.; Kelly, M.G.; Angeli, N. Taxonomic and ecological characterization of two Ulnaria species (Bacillariophyta) from streams in Cyprus. Phytotaxa 2018, 346, 78–92. [Google Scholar] [CrossRef]
- Williams, D.M.; Blanco, S. Studies on type material from Kützing’s diatom collection II: Synedra acus Kützing, Synedra arcus Kützing, their morphology, types and nomenclature. Diatom Res. 2020, 34, 237–250. [Google Scholar] [CrossRef]
- Liu, B. The diatom genus Ulnaria (Bacillariophyta) in China. PhytoKeys 2023, 228, 1–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Williams, D.M.; Tan, L. Three new species of Ulnaria (Bacillariophyta) from the Wuling Mountains Area, China. Phytotaxa 2017, 306, 241–258. [Google Scholar] [CrossRef]
- Zakharova, Y.R.; Bedoshvili, Y.D.; Petrova, D.P.; Marchenkov, A.M.; Volokitina, N.A.; Bashenkhaeva, M.V.; Kopyrina, L.I.; Grachev, M.A.; Likhoshway, Y.V. Morphological description and molecular phylogeny of two diatom clones from the genus Ulnaria (Kützing) Compère isolated from an ultraoligotrophic lake at the Pole of Cold in the Northern Hemisphere, Republic of Sakha (Yakutia), Russia. Cryptogam. Algol. 2020, 41, 37–45. Available online: http://cryptogamie.com/algologie/41/6 (accessed on 24 September 2025). [CrossRef]
- Ulnaria acus. Diatoms of North America. Available online: https://diatoms.org/species/51793/ulnaria_acus (accessed on 24 September 2025).
- AlgaeBase. World-Wide Electronic Publication, University of Galway. Available online: https://www.algaebase.org/ (accessed on 24 September 2025).
- Kulikovskiy, M.; Lange-Bertalot, H.; Annenkova, N.; Gusev, E.; Kociolek, J.P. Morphological and molecular evidence support description of two new diatom species from the genus Ulnaria in Lake Baikal. Fottea 2016, 16, 34–42. [Google Scholar] [CrossRef]
- Liu, B.; Williams, D.M.; Blanco, S.; Liu, Z.-X.; Liu, D. Three new species of Ulnaria (Kützing) Compère (Bacillariophyta) from China, with reference to the fine structure of their valvocopula. Cryptogam. Algol. 2019, 40, 119–139. [Google Scholar] [CrossRef]
- Liu, B.; Williams, D.M.; Liu, Q.; Yang, X. Three species of Ulnaria (Bacillariophyceae) from China, with reference to the valve central area and apices. Diatom Res. 2019, 34, 49–65. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, B.; Rioual, P.; Long, J.-Y.; Zhou, M. Ulnaria shun-biseriata sp. nov. (Bacillariophyta) from Shun River in Hunan Province, China. PhytoKeys 2024, 246, 315–327. [Google Scholar] [CrossRef]
- Williams, D.M. Studies on type material from Kützing’s diatom collection I: Synedra vítrea Kützing, with comments on Ulnaria fragilariaeformis (F.E. Fritsch and M.F. Rich) D.M. Williams, nov. stat. et nov. comb. and Ulnaria undulata (Rabenhorst) D.M. Williams, nov. stat. et nov. comb. Bot. Lett. 2019, 167, 70–85. [Google Scholar] [CrossRef]
- Williams, D.M.; Van de Vijver, B. Studies on type material from Kützing’s diatom collection III: Synedra splendens (Kütz.) Kütz., Synedra aequalis (Kütz.) Kütz. and a note on Synedra obtusa W.Sm. Fottea 2021, 21, 164–179. [Google Scholar] [CrossRef]
- Wetzel, C.E.; Potapova, M.; Williams, D.M. Synedra phantasma M.H. Hohn (Bacillariophyta, Fragilariaceae) from the Amazon river (South America): Its typification and transfer to the genus Fragilaria. Not. Algarum 2022, 252, 1–12. [Google Scholar]
- Williams, D.M.; Wetzel, C.E. A note on the two species of Ulnaria (Bacillariophyta) first described in the Erbario Crittogamico Italiano (1858–1885). Diatom Res. 2024, 39, 187–197. [Google Scholar] [CrossRef]
- Hwang, E.-A.; Kim, H.-K.; Cho, I.-H.; Yi, C.; Kim, B.-H. Morphological and Molecular Studies of Three New Diatom Species from Mountain Streams in South Korea. Diversity 2022, 14, 790. [Google Scholar] [CrossRef]
- Marchenkov, A.M.; Zakharova, Y.R.; Volokitina, N.A.; Davidovich, N.A.; Davidovich, O.I.; Podunay, Y.A.; Petrova, D.P. Genotypic diversity of Ulnaria acus (Kützing) Aboal from Eurasia. Limnol. Freshw. Biol. 2022, 6, 1705–1711. [Google Scholar] [CrossRef]
- Marchenkov, A.M.; Nalimova, M.A.; Zakharova, Y.R.; Davidovich, N.A.; Davidovich, O.I.; Podunay, Y.A.; Petrova, D.P. Genetic diversity of freshwater diatom algae populations Ulnaria danica (Kützing) Compère & Bukhtiyarova and Ulnaria ulna (Nitzsch) Compère. Limnol. Freshw. Biol. 2024, 6, 1471–1490. [Google Scholar] [CrossRef]
- Bayramova, E.; Petrova, D.; Marchenkov, A.; Morozov, A.; Galachyants, Y.; Zakharova, Y.; Bedoshvili, Y.; Likhoshway, Y. Differential Expression of Stress Adaptation Genes in a Diatom Ulnaria acus under Different Culture Conditions. Int. J. Mol. Sci. 2024, 25, 2314. [Google Scholar] [CrossRef]
- Edlund, M.; Shinneman, A.L.C.; Soninkhishig, N. Diatoms (Bacillariophyceae) from the Valley of the Great Lakes in Western Mongolia. Mong. J. Biol. Sci. 2010, 8, 17–26. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Dorofeyuk, N.I. New diatoms for the Mongolian flora. Nov. Sist. Nizshikh Rastenii 2010, 44, 69–80. [Google Scholar] [CrossRef]
- Dorofeyuk, N.I.; Kulikovskiy, M.S. Diatoms of Mongolia. Biological Resources and Natural Conditions of Mongolia. Proc. Jt. Russ.-Mong. Complex Biol. Exped. 2012, 59, 367. [Google Scholar]
- Kulikovskiy, M.; Lange-Bertalot, H.; Witkowski, A. Nupela matrioschka sp. nov., Nupela thurstonensis comb. nov. and Nupela neogracillima comb. & nom. nov. (Bacillariophyceae): Critical analysis of their morphology. Pol. Bot. J. 2009, 54, 13–20. [Google Scholar]
- Kulikovskiy, M.; Lange-Bertalot, H.; Witkowski, A.; Dorofeyuk, N. Morphology and taxonomy of selected cymbelloid diatoms from a Mongolian Sphagnum ecosystem with a description of three species new to science. Fottea 2009, 9, 223–232. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Witkowski, A.; Dorofeyuk, N.I.; Genkal, S.I. Diatom assemblages from Sphagnum bogs of the world. I. Nur bog in northern Mongolia. Bibl. Diatomol. 2010, 55, 1–326. [Google Scholar]
- Kulikovskiy, M.; Kociolek, J.P.; Liu, Y.; Kuznetsova, I.; Glushchenko, A. Vladinikolaevia, gen. nov.—A new enigmatic freshwater diatom genus (Cymbellaceae; Bacillariophyceae) from Mongolia. Fottea 2022, 22, 204–210. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Glushchenko, A.M.; Genkal, S.I.; Kuznetsova, I.V.; Maltsev, Y.I.; Kociolek, J.P. Is Sellaphora the New Navicula? Cymbosellaphora (Cymbellales), a New Genus Based on Taxa Previously Assigned to Sellaphora. Plants 2023, 12, 3890. [Google Scholar] [CrossRef]
- Edlund, M.; Shinneman, A.L.C.; Levkov, Z. Diatom biodiversity in Mongolia: A new amphoroid diatom from saline lakes in western Mongolia, Amphora soninkhishigae sp. nov. Acta Bot. Croat. 2009, 68, 251–262. [Google Scholar]
- Jovanovska, E.; Levkov, Z.; Edlund, M.B. The genus Diploneis Ehrenberg ex Cleve (Bacillariophyta) from Lake Hövsgöl, Mongolia. Phytotaxa 2015, 217, 201–248. [Google Scholar] [CrossRef]
- Glushchenko, A.; Genkal, S.; Kuznetsova, I.; Kulikovskiy, M. Taxonomy and Distribution of the Genus Sellaphora Mereschowsky (Bacillariophyceae: Sellaphoraceae) in Aquatic Ecosystems of Mongolia. Plants 2023, 12, 3611. [Google Scholar] [CrossRef]
- Mironov, A.; Glushchenko, A.; Maltsev, Y.; Nergui, S.; Chebotaryova, S.; Kulikovskiy, M. Paraplaconeis dorofeyukae sp. nov. (Cymbellales, Bacillariophyceae)—A new species from Mongolia, described based on molecular and morphological investigations. Phytotaxa 2025, 681, 277–289. [Google Scholar] [CrossRef]
- Mironov, A.; Glushchenko, A.; Genkal, S.; Iurmanov, A.; Kociolek, J.P.; Nergui, S.; Kulikovskiy, M. Two new species of Witkowskia (Bacillariophyceae, Cymbellales) from Mongolia and Russia. Nova Hedwig. 2025, 120, 79–94. [Google Scholar] [CrossRef]
- Tseplik, N.D.; Glushchenko, A.; Maltsev, Y.; Genkal, S.; Nergui, S.; Kulikovskiy, M. Molecular investigation of Nupela mongolica sp. nov. (Bacillariophyceae) with the description of the family Nupelaceae fam. nov. Phytotaxa 2025, 681, 186–198. [Google Scholar] [CrossRef]
- Amato, A. Species concepts and definitions: Reproductive isolation as a tool to reveal species boundaries. Int. J. Plant Reprod. Biol. 2010, 2, 114–126. [Google Scholar]
- Podunay, Y.A.; Davidovich, N.A.; Kulikovskiy, M.S.; Gusev, E.S. Features of Sexual Reproduction and Mating System of Ulnaria acus (Bacillariophyta). J. Sib. Fed. Univ. Biol. 2018, 11, 75–87. [Google Scholar] [CrossRef]
- Podunay, Y.A.; Davidovich, O.I.; Davidovich, N.A. Mating system and two types of gametogenesis in the fresh water diatom Ulnaria ulna (Bacillariophyta). Algologia 2014, 24, 3–19. [Google Scholar] [CrossRef]
- Zakharova, Y.; Marchenkov, A.; Petrova, D.; Bukin, Y.; Morozov, A.; Bedoshvili, Y.; Podunay, Y.; Davidovich, O.; Davidovich, N.; Bondar, A.; et al. Delimitation of some taxa of Ulnaria and Fragilaria (Bacillariophyceae) based on genetic, morphological data and mating compatibility. Diversity 2023, 15, 271. [Google Scholar] [CrossRef]
- Davidovich, N.A.; Davidovich, O.I. Reproductive Biology of Diatoms; IT “ARIAL”: Simferopol, Russia, 2022; pp. 1–196. [Google Scholar]
- Guillard, R.R.L.; Lorenzen, C.J. Yellow-green algae with chlorophyllide c1,2. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers. Evol. 2011, 11, 173–192. [Google Scholar] [CrossRef]
- Daugbjerg, N.; Andersen, R.A. A molecular phylogeny of the heterokont algae based on analyses of chloroplast-encoded rbcL sequence data. J. Phycol. 1997, 33, 1031–1041. [Google Scholar] [CrossRef]
- Ruck, E.C.; Theriot, E.C. Origin and evolution of the canal raphe system in diatoms. Protist 2011, 162, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Chepurnov, V.A.; Mann, D.G. Auxosporulation of Licmophora communis (Bacillariophyta) and a review of mating systems and sexual reproduction in araphid pennate diatoms. Phycol. Res. 2004, 52, 1–12. [Google Scholar] [CrossRef]
- Lacey, A.J. Light Microscopy in Biology: A Practical Approach; Oxford University Press: Oxford, UK, 1999; pp. 1–474. [Google Scholar] [CrossRef]
- Metzeltin, D.; Lange-Bertalot, H.; Soninkhishig, N. Diatoms in Mongolia. Iconogr. Diatomol. 2009, 20, 3–686. [Google Scholar]
- Kützing, F.T. Synopsis diatomearum oder Versuch einer systematischen Zusammenstellung der Diatomeen. Linnaea 1833, 8, 529–620. [Google Scholar] [CrossRef]
- Geitler, L. Die Auxosporenbildung von Synedra ulna. Berichte Dtsch. Ges. 1939, 57, 432–436. [Google Scholar] [CrossRef]
- Davidovich, N.A.; Kaczmarska, I.; Ehrman, J.M. Heterothallic and homothallic sexual reproduction in Tabularia fasciculata (Bacillariophyta). Fottea 2010, 10, 251–266. [Google Scholar] [CrossRef]
- Davidovich, N.A.; Davidovich, O.I. Sexual reproduction and mating system of the diatom Tabularia tabulata (C. Agardh) Snoeijs (Bacillariophyta). Int. J. Algae. 2011, 13, 18–36. [Google Scholar] [CrossRef]
- Davidovich, N.A. Species specific sizes and size range of sexual reproduction in diatoms. In Proceedings of the 16th International Diatom Symposium, Athens, Greece, 28 August–1 September 2000. [Google Scholar]
- Davidovich, N.A.; Kaczmarska, I.; Karpov, S.A.; Davidovich, O.I.; MacGillivary, M.L.; Mather, L. Mechanism of male gamete motility in araphid pennate diatoms from the genus Tabularia (Bacillariophyta). Protist 2012, 163, 480–494. [Google Scholar] [CrossRef]
- Davidovich, N.A.; Davidovich, O.I.; Podunay, Y.A.; Gastineau, R.; Kaczmarska, I.; Poulíčková, A.; Witkowski, A. Ardissonea crystallina has a type of sexual reproduction that is unusual for centric diatoms. Sci. Rep. 2017, 7, 14670. [Google Scholar] [CrossRef]
- Sato, S.; Beakes, G.; Idei, M.; Nagumo, T.; Mann, D.G. Novel sex cells and evidence for sex pheromones in diatoms. PLoS ONE 2011, 6, e26923. [Google Scholar] [CrossRef]
- Davidovich, N.A. Sexual heterogeneity of the clones of Nitzschia longissima (Bréb.) Ralfs (Bacillariophyta). Int. J. Algae 2002, 4, 104–116. [Google Scholar] [CrossRef]
- Geitler, L. Auxosporenbildung und Systematik bei pennaten Diatomeen und die Cytologie von Cocconeis-Sippen. Osterr. Bot. Z. 1973, 122, 299–321. [Google Scholar] [CrossRef]
- Stickle, A.J. Mastogloia smithii has a method of sexual reproduction hitherto unknown in raphid diatoms. Diatom Res. 1986, 1, 271–282. [Google Scholar] [CrossRef]
- Mizuno, M. Sexual reproduction and auxospore formation in Achnanthes javanica f. subconstricta. Diatom Res. 1994, 9, 133–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).