Assistive Intelligence: A Framework for AI-Powered Technologies Across the Dementia Continuum
Abstract
1. Introduction
2. Background and Current Gaps
2.1. Dementia Trajectory Across Stages: Preclinical, Mild, Moderate, Severe
2.2. Limitations of Conventional and Non-Personalized Assistive Technologies
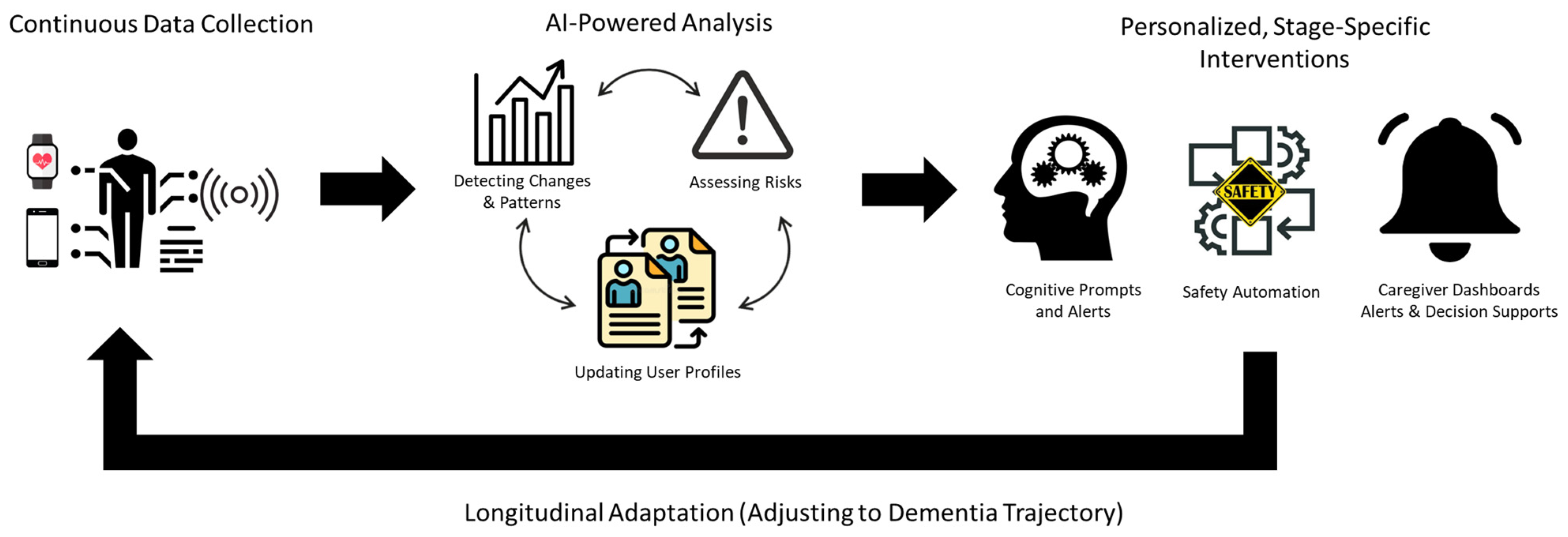
2.3. The Emerging Role of AI and GenAI in Dementia Care
2.4. Gaps in the Current Technological Landscape
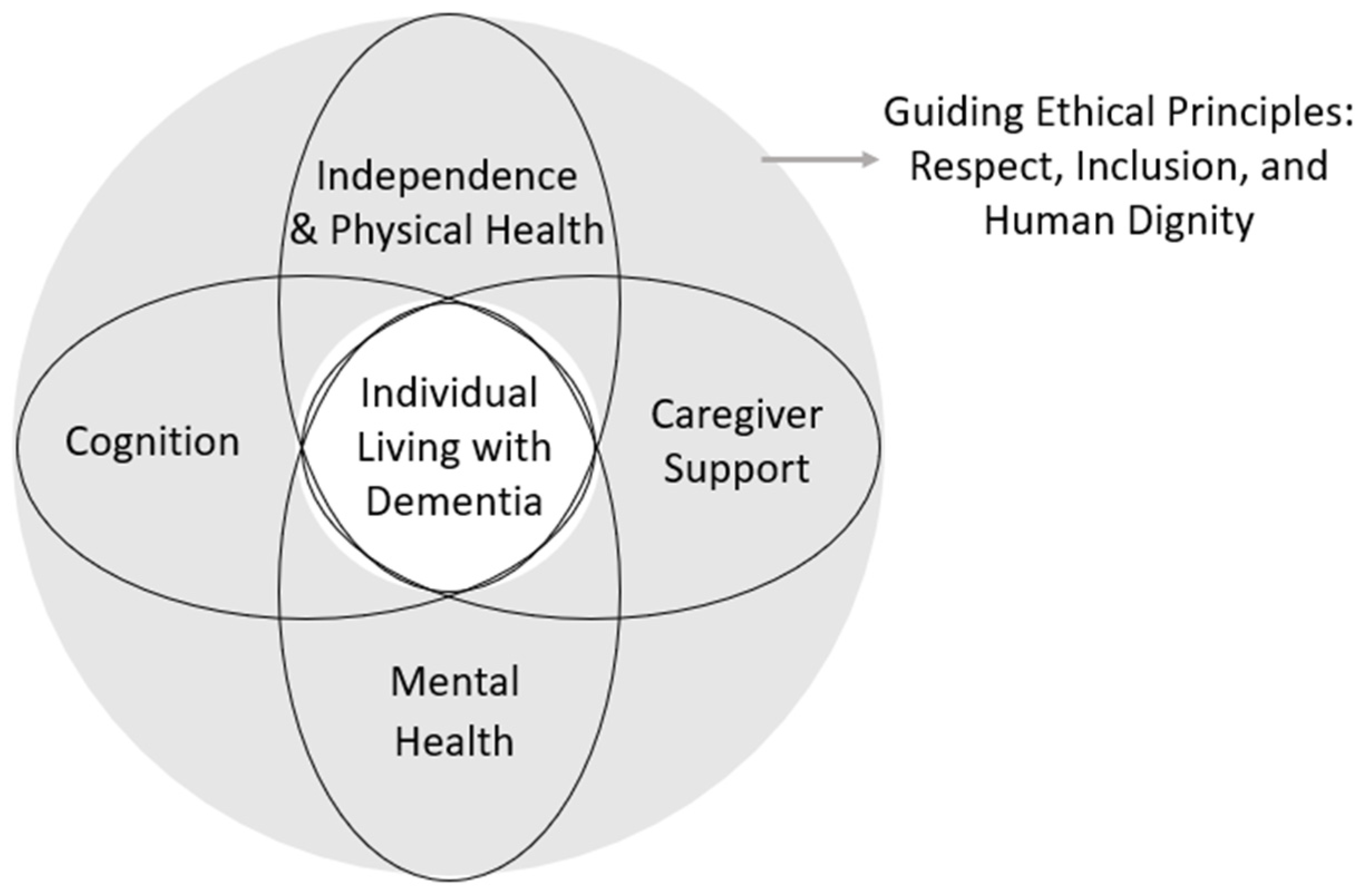
2.5. AI Domain Framework
- A.
- Cognition (Early Detection and Cognitive Stimulation)
- B.
- Mental Health (Emotion Detection and Social Support)
- C.
- Independence and Physical Health (ADLs, Safety, and Health Monitoring)
- D.
- Caregiver Support (Monitoring, Alerts, and Decision Support)
2.6. Ethical and Social Considerations
2.7. Privacy and Data Protection
2.8. Autonomy and Informed Consent
2.9. Fairness and Algorithmic Bias
2.10. Explainability and Trust
2.11. Other Considerations for Implementation of AI Powered Assistive Technologies for Dementia
2.12. Sensor Ecosystem Integration
2.13. Caregiver–AI Interface Co-Design
2.14. Usability and Accessibility for Older Adults
2.15. Cost, Scalability, and Economic Viability
2.16. Research and Innovation Roadmap
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 8, 131–168. [Google Scholar]
- Palmdorf, S.; Stark, A.L.; Nadolny, S.; Eliaß, G.; Karlheim, C.; Kreisel, S.H.; Gruschka, T.; Trompetter, E.; Dockweiler, C. Technology-assisted home care for people with dementia and their relatives: Scoping review. JMIR Aging 2021, 4, e25307. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.; Dickinson, C.; Brittain, K.; Robinson, L. The everyday use of assistive technology by people with dementia and their family carers: A qualitative study. BMC Geriatr. 2015, 15, 89. [Google Scholar] [CrossRef]
- Daly Lynn, J.; Rondón-Sulbarán, J.; Quinn, E.; Ryan, A.; McCormack, B.; Martin, S. A systematic review of electronic assistive technology within supporting living environments for people with dementia. Dementia 2019, 18, 2371–2435. [Google Scholar] [CrossRef]
- Kameyama, M.; Umeda-Kameyama, Y. Applications of artificial intelligence in dementia. Geriatr. Gerontol. Int. 2024, 24, 25–30. [Google Scholar] [CrossRef]
- de Arriba-Pérez, F.; García-Méndez, S.; González-Castaño, F.J.; Costa-Montenegro, E. Automatic detection of cognitive impairment in elderly people using an entertainment chatbot with Natural Language Processing capabilities. J. Ambient Intell. Humaniz. Comput. 2023, 14, 16283–16298. [Google Scholar] [CrossRef]
- Lyall, D.M.; Kormilitzin, A.; Lancaster, C.; Sousa, J.; Petermann-Rocha, F.; Buckley, C.; Harshfield, E.L.; Iveson, M.H.; Madan, C.R.; McArdle, R. Artificial intelligence for dementia—Applied models and digital health. Alzheimer’s Dement. 2023, 19, 5872–5884. [Google Scholar] [CrossRef]
- Davis, M.; O’Connell, T.; Johnson, S.; Cline, S.; Merikle, E.; Martenyi, F.; Simpson, K. Estimating Alzheimer’s disease progression rates from normal cognition through mild cognitive impairment and stages of dementia. Curr. Alzheimer Res. 2018, 15, 777–788. [Google Scholar] [CrossRef]
- Scharre, D.W. Preclinical, prodromal, and dementia stages of Alzheimer’s disease. Pr. Neurol. 2019, 15, 36–47. [Google Scholar]
- Goodall, G.; Taraldsen, K.; Granbo, R.; Serrano, J.A. Towards personalized dementia care through meaningful activities supported by technology: A multisite qualitative study with care professionals. BMC Geriatr. 2021, 21, 468. [Google Scholar] [CrossRef]
- Chien, S.-Y.; Zaslavsky, O.; Berridge, C. Technology usability for people living with dementia: Concept analysis. JMIR Aging 2024, 7, e51987. [Google Scholar] [CrossRef] [PubMed]
- Thordardottir, B.; Malmgren Fänge, A.; Lethin, C.; Rodriguez Gatta, D.; Chiatti, C. Acceptance and use of innovative assistive technologies among people with cognitive impairment and their caregivers: A systematic review. BioMed Res. Int. 2019, 2019, 9196729. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Nordberg, O.E.; Rongve, A.; Bachinski, M.; Fjeld, M. State-of-the-Art HCI for Dementia Care: A Scoping Review of Recent Technological Advances. J. Dement. Alzheimer’s Dis. 2025, 2, 41. [Google Scholar] [CrossRef]
- Brookman, R.; Parker, S.; Hoon, L.; Ono, A.; Fukayama, A.; Matsukawa, H.; Harris, C.B. Technology for dementia care: What would good technology look like and do, from carers’ perspectives? BMC Geriatr. 2023, 23, 867. [Google Scholar] [CrossRef]
- Vollmer Dahlke, D.; Ory, M.G. Emerging issues of intelligent assistive technology use among people with dementia and their caregivers: A US perspective. Front. Public Health 2020, 8, 191. [Google Scholar] [CrossRef]
- Boyle, L.D.; Husebo, B.S.; Vislapuu, M. Promotors and barriers to the implementation and adoption of assistive technology and telecare for people with dementia and their caregivers: A systematic review of the literature. BMC Health Serv. Res. 2022, 22, 1573. [Google Scholar] [CrossRef]
- Ghaiumy Anaraky, R.; Byrne, K.A.; Wisniewski, P.J.; Page, X.; Knijnenburg, B. To disclose or not to disclose: Examining the privacy decision-making processes of older vs. younger adults. In Proceedings of the 2021 CHI Conference on Human Factors in Computing Systems, Yokohama, Japan, 8–13 May 2021; pp. 1–14. [Google Scholar]
- Ghaiumy Anaraky, R.; Bulgurcu, B.; Byrne, K.; Li, Y.; Cho, H.; Knijnenburg, B. Would You Preserve Your Privacy or Enhance it? How to Best Frame Privacy Interventions for Older and Younger Users. In Proceedings of the 58th Hawaii International Conference on System Sciences, Hilton Waikoloa Village, HI, USA, 7–10 January 2025. [Google Scholar]
- Ghaiumy Anaraky, R.; Schuster, A.M.; Van Fossen, J.; Nov, O.; Cotten, S. Increased Use of Asocial Technologies Is Associated with Reduced Well-being Among Older Adults. In Proceedings of the 2025 CHI Conference on Human Factors in Computing Systems, Hilton Waikoloa Village, HI, USA, 7–10 January 2025; pp. 1–10. [Google Scholar]
- Graham, S.A.; Lee, E.E.; Jeste, D.V.; Van Patten, R.; Twamley, E.W.; Nebeker, C.; Yamada, Y.; Kim, H.-C.; Depp, C.A. Artificial intelligence approaches to predicting and detecting cognitive decline in older adults: A conceptual review. Psychiatry Res. 2020, 284, 112732. [Google Scholar] [CrossRef]
- Wahul, R.M.; Ambadekar, S.; Dhanvijay, D.M.; Dhanvijay, M.M.; Dudhedia, M.A.; Gaikwad, V.; Kanawade, B.; Pansare, J.; Bodkhe, B.; Gawande, S. Multimodal approaches and AI-driven innovations in dementia diagnosis: A systematic review. Discov. Artif. Intell. 2025, 5, 96. [Google Scholar] [CrossRef]
- Amini, S.; Hao, B.; Yang, J.; Karjadi, C.; Kolachalama, V.B.; Au, R.; Paschalidis, I.C. Prediction of Alzheimer’s disease progression within 6 years using speech: A novel approach leveraging language models. Alzheimer’s Dement. 2024, 20, 5262–5270. [Google Scholar] [CrossRef]
- Chi, L.; Sharma, A.; Gebhardt, A.; Colonel, J. Predicting Cognitive Decline: A Multimodal AI Approach to Dementia Screening from Speech. In Proceedings of the 2025 IEEE International Conference on AI and Data Analytics (ICAD), Medford, MA, USA, 24 June 2025; pp. 1–8. [Google Scholar]
- Steijger, D.; Christie, H.; Aarts, S.; IJselsteijn, W.; Verbeek, H.; de Vugt, M. Use of artificial intelligence to support quality of life of people with dementia: A scoping review. Ageing Res. Rev. 2025, 108, 102741. [Google Scholar] [CrossRef]
- Sun, F.; Jiang, L.; Chen, X.S.; Feng, Y. Interactive AI Technology for Dementia Caregivers: Needs and Implementation Evidence. J. Technol. Hum. Serv. 2025, 43, 91–116. [Google Scholar] [CrossRef]
- Hird, N.; Osaki, T.; Ghosh, S.; Palaniappan, S.K.; Maeda, K. Enabling personalization for digital cognitive stimulation to support communication with people with dementia: Pilot intervention study as a prelude to AI development. JMIR Form. Res. 2024, 8, e51732. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Somoza, L.; Irazoki, E.; Toribio-Guzmán, J.; de la Torre-Díez, I.; Diaz-Baquero, A.; Parra-Vidales, E.; Perea-Bartolomé, M.; Franco-Martín, M. Usability and user experience of cognitive intervention technologies for elderly people with MCI or dementia: A systematic review. Front. Psychol. 2021, 12, 636116. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Lawler, K.; Garg, S.; Bai, Q.; Alty, J. Applications of artificial intelligence to aid early detection of dementia: A scoping review on current capabilities and future directions. J. Biomed. Inform. 2022, 127, 104030. [Google Scholar] [CrossRef]
- Ghaiumy Anaraky, R.; Schuster, A.M.; Cotten, S.R. Can changes in older adults’ technology use patterns be used to detect cognitive decline? Gerontologist 2024, 64, gnad158. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Liang, T.; Hasan, W.U.; Zaman, K.T.; Du, Y.; Xie, B.; Tao, C. Promoting personalized reminiscence among cognitively intact older adults through an AI-driven interactive multimodal photo album: Development and usability study. JMIR Aging 2024, 7, e49415. [Google Scholar] [CrossRef]
- Huo, J.; Yu, Y.; Lin, W.; Hu, A.; Wu, C. Application of AI in multilevel pain assessment using facial images: Systematic review and meta-analysis. J. Med. Internet Res. 2024, 26, e51250. [Google Scholar] [CrossRef]
- Otaka, E.; Osawa, A.; Kato, K.; Obayashi, Y.; Uehara, S.; Kamiya, M.; Mizuno, K.; Hashide, S.; Kondo, I. Positive emotional responses to socially assistive robots in people with dementia: Pilot study. JMIR Aging 2024, 7, e52443. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Xiang, X.; An, R. AI applications to reduce loneliness among older adults: A systematic review of effectiveness and technologies. Healthcare 2025, 13, 446. [Google Scholar] [CrossRef]
- Bourgeois, M.; Fried-Oken, M.; Rowland, C. AAC strategies and tools for persons with dementia. ASHA Lead. 2010, 15, 8–11. [Google Scholar] [CrossRef]
- Arthanat, S.; Begum, M.; Gu, T.; LaRoche, D.P.; Xu, D.; Zhang, N. Caregiver perspectives on a smart home-based socially assistive robot for individuals with Alzheimer’s disease and related dementia. Disabil. Rehabil. Assist. Technol. 2020, 15, 789–798. [Google Scholar] [PubMed]
- Arthanat, S.; Wilcox, J.; LaRoche, D. Smart home automation technology to support caring of individuals with Alzheimer’s disease and related dementia: An early intervention framework. Disabil. Rehabil. Assist. Technol. 2024, 19, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Nithya, S.; Palanisamy, S.K.; Obaid, A.J.; Apinaya Prethi, K.; Alkhafaji, M.A. AI-Based Secure Software-Defined Controller to Assist Alzheimer’s Patients in Their Daily Routines. In Proceedings of the International Conference on Frontiers of Intelligent Computing: Theory and Applications, Cardiff, UK, 11–12 April 2023; Springer: Berlin/Heidelberg, Germany, 2023; pp. 453–463. [Google Scholar]
- Tian, Y.J.; Felber, N.A.; Pageau, F.; Schwab, D.R.; Wangmo, T. Benefits and barriers associated with the use of smart home health technologies in the care of older persons: A systematic review. BMC Geriatr. 2024, 24, 152. [Google Scholar] [CrossRef]
- Jia, S.; Si, Y.; Guo, C.; Wang, P.; Li, S.; Wang, J.; Wang, X. The prediction model of fall risk for the elderly based on gait analysis. BMC Public Health 2024, 24, 2206. [Google Scholar] [CrossRef]
- Puthusseryppady, V.; Morrissey, S.; Aung, M.H.; Coughlan, G.; Patel, M.; Hornberger, M. Using GPS tracking to investigate outdoor navigation patterns in patients with Alzheimer disease: Cross-sectional study. JMIR Aging 2022, 5, e28222. [Google Scholar] [CrossRef]
- Ramkumar, J.; Karthikeyan, C.; Vamsidhar, E.; Dattatraya, K.N. Automated pill dispenser application based on IoT for patient medication. In IoT and ICT for Healthcare Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 231–253. [Google Scholar]
- Gargioni, L.; Fogli, D.; Baroni, P. A systematic review on pill and medication dispensers from a human-centered perspective. J. Healthc. Inform. Res. 2024, 8, 244–285. [Google Scholar] [CrossRef]
- Menghi, R.; Gullà, F.; Germani, M. Assessment of a Smart Kitchen to Help People with Alzheimer’s Disease. In Proceedings of the International Conference on Smart Homes and Health Telematics, Singapore, 10–12 July 2018; Springer: Berlin/Heidelberg, Germany, 2018; pp. 304–309. [Google Scholar]
- Chen, H.; Soh, Y. A cooking assistance system for patients with Alzheimers disease using reinforcement learning. Int. J. Inf. Technol. 2017, 23, 1–12. [Google Scholar]
- Al-Naami, B.; Abu Owida, H.; Abu Mallouh, M.; Al-Naimat, F.; Agha, M.D.; Al-Hinnawi, A.-R. A new prototype of smart wearable monitoring system solution for alzheimer’s patients. Med. Devices Evid. Res. 2021, 14, 423–433. [Google Scholar] [CrossRef]
- Abedi, A.; Chu, C.H.; Khan, S.S. Early Prediction of Agitation in Community-Dwelling People with Dementia Using Multimodal Sensors and Machine Learning: Benchmarking of State-of-the-Art Techniques. In Proceedings of the International Joint Conference on Artificial Intelligence, Montreal, QC, Canada, 16–22 August 2025; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–16. [Google Scholar]
- Borna, S.; Maniaci, M.J.; Haider, C.R.; Gomez-Cabello, C.A.; Pressman, S.M.; Haider, S.A.; Demaerschalk, B.M.; Cowart, J.B.; Forte, A.J. Artificial intelligence support for informal patient caregivers: A systematic review. Bioengineering 2024, 11, 483. [Google Scholar] [CrossRef]
- Milella, F.; Russo, D.; Bandini, S. AI-powered solutions to support informal caregivers in their decision-making: A systematic review of the literature. OBM Geriatr. 2023, 7, 262. [Google Scholar] [CrossRef]
- Xie, B.; Tao, C.; Li, J.; Hilsabeck, R.C.; Aguirre, A. Artificial intelligence for caregivers of persons with Alzheimer’s disease and related dementias: Systematic literature review. JMIR Med. Inform. 2020, 8, e18189. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, T.G.; Lazarou, I.; Diaz, A.; Gove, D.; Georges, J.; Manyakov, N.V.; Pich, E.M.; Hinds, C.; Tsolaki, M.; Nikolopoulos, S. Wearable devices for assessing function in Alzheimer’s disease: A European public involvement activity about the features and preferences of patients and caregivers. Front. Aging Neurosci. 2021, 13, 643135. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Claudio, D. Alarm fatigue and its influence on staff performance. IIE Trans. Healthc. Syst. Eng. 2015, 5, 183–196. [Google Scholar] [CrossRef]
- Ruggiano, N.; Brown, E.L.; Clarke, P.J.; Hristidis, V.; Roberts, L.; Framil Suarez, C.V.; Allala, S.C.; Hurley, S.; Kopcsik, C.; Daquin, J. An Evidence-Based IT Program With Chatbot to Support Caregiving and Clinical Care for People with Dementia: The CareHeroes Development and Usability Pilot. JMIR Aging 2024, 7, e57308. [Google Scholar] [CrossRef]
- Ali, Z.; Zhong, J.; Sadarangani, T.R. A mixed-methods examination of the acceptability of, CareMOBI, a dementia-focused mhealth app, among primary care providers. Digit. Health 2024, 10, 20552076241287361. [Google Scholar] [CrossRef]
- Ali, Z.; Sadarangani, T. Likelihood of Adoption of Caremobi: Addressing Communication Gaps Between Adult Day Health Centers and Primary Care. Innov. Aging 2022, 6, 841. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Wu, W.; Najarian, C.; Aikens, J.; Bynum, J.; Vydiswaran, V.V. Identifying caregiver availability using medical notes with rule-based natural language processing: Retrospective cohort study. JMIR Aging 2022, 5, e40241. [Google Scholar] [CrossRef]
- World Health Organization. iSupport. 2025. Available online: https://www.who.int/teams/mental-health-and-substance-use/treatment-care/isupport (accessed on 10 September 2025).
- Bennett, B.; McDonald, F.; Beattie, E.; Carney, T.; Freckelton, I.; White, B.; Willmott, L. Assistive technologies for people with dementia: Ethical considerations. Bull. World Health Organ. 2017, 95, 749. [Google Scholar] [CrossRef]
- Ghaiumy Anaraky, R.; Lowens, B.; Li, Y.; Byrne, K.A.; Risius, M.; Wisniewski, P.; Soleimani, M.; Soltani, M.; Knijnenburg, B. Older and younger adults are influenced differently by dark pattern designs. arXiv 2023, arXiv:2310.03830. [Google Scholar] [CrossRef]
- Aly, H.; Liu, Y.; Khan, S.; Anaraky, R.G.; Byrne, K.; Knijnenburg, B. Digital privacy education: Customized interventions for US older and younger adults in rural and urban settings. Technol. Soc. 2025, 81, 102805. [Google Scholar] [CrossRef]
- Aly, H.; Liu, Y.; Anaraky, R.G.; Khan, S.; Namara, M.; Byrne, K.A.; Knijnenburg, B. Tailoring digital privacy education interventions for older adults: A comparative study on modality preferences and effectiveness. Proc. Priv. Enhancing Technol. 2024, 2024, 635–656. [Google Scholar] [CrossRef]
- Buhr, E.; Welsch, J.; Shaukat, M.S. Value preference profiles and ethical compliance quantification: A new approach for ethics by design in technology-assisted dementia care. AI Soc. 2025, 40, 1209–1225. [Google Scholar] [CrossRef]
- AboJabel, H.; Welsch, J.; Schicktanz, S. Cross-cultural perspectives on intelligent assistive technology in dementia care: Comparing Israeli and German experts’ attitudes. BMC Med. Ethics 2024, 25, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ghaiumy Anaraky, R.; Knijnenburg, B. How not to measure social network privacy: A cross-country investigation. Proc. ACM Hum. Comput. Interact. 2021, 5, 144. [Google Scholar] [CrossRef]
- Ghaiumy Anaraky, R.; Li, Y.; Knijnenburg, B. Difficulties of measuring culture in privacy studies. Proc. ACM Hum. Comput. Interact. 2021, 5, 378. [Google Scholar] [CrossRef]
- Kropczynski, J.; Ghaiumy Anaraky, R.; Akter, M.; Godfrey, A.J.; Lipford, H.; Wisniewski, P.J. Examining collaborative support for privacy and security in the broader context of tech caregiving. Proc. ACM Hum. Comput. Interact. 2021, 5, 1–23. [Google Scholar] [CrossRef]
- Yuan, C.; Linn, K.A.; Hubbard, R.A. Algorithmic fairness of machine learning models for Alzheimer disease progression. JAMA Netw. Open 2023, 6, e2342203. [Google Scholar] [CrossRef]
- Deiana, A. A Pre-Processing Framework for Mitigating Representation Bias in Machine Learning Classification Algorithms. Doctoral Dissertation, Politecnico di Torino, Torino, Italy, 2024. [Google Scholar]
- Gu, T.; Pan, W.; Yu, J.; Ji, G.; Meng, X.; Wang, Y.; Li, M. Mitigating bias in AI mortality predictions for minority populations: A transfer learning approach. BMC Med. Inform. Decis. Mak. 2025, 25, 30. [Google Scholar] [CrossRef]
- Nazer, L.H.; Zatarah, R.; Waldrip, S.; Ke, J.X.C.; Moukheiber, M.; Khanna, A.K.; Hicklen, R.S.; Moukheiber, L.; Moukheiber, D.; Ma, H. Bias in artificial intelligence algorithms and recommendations for mitigation. PLoS Digit. Health 2023, 2, e0000278. [Google Scholar] [CrossRef]
- ali Skaiky, A.; Ali, H.M.S.; Mohammed, A.; Mahdi, Z.A. Comprehensive Bias Mitigation in AI: Evaluating Pre-Processing, In-Processing, and Post-Processing Techniques for Fair Decision-Making. In Proceedings of the 2025 IEEE 4th International Conference on Computing and Machine Intelligence (ICMI), Mount Pleasant, MI, USA, 5–6 April 2025; pp. 1–5. [Google Scholar]
- Mackin, S.; Major, V.J.; Chunara, R.; Newton-Dame, R. Post-processing methods for mitigating algorithmic bias in healthcare classification models: An extended umbrella review. BMC Digit. Health 2025, 3, 26. [Google Scholar] [CrossRef]
- Mudiyanselage, U.B.; Jayprakash, B.; Lee, K.; Kwon, K.H. Disaggregated Health Data in LLMs: Evaluating Data Equity in the Context of Asian American Representation. In Proceedings of the AAAI/ACM Conference on AI, Ethics, and Society, Madrid, Spain, 20–22 October 2025; pp. 292–303. [Google Scholar]
- Markus, A.F.; Kors, J.A.; Rijnbeek, P.R. The role of explainability in creating trustworthy artificial intelligence for health care: A comprehensive survey of the terminology, design choices, and evaluation strategies. J. Biomed. Inform. 2021, 113, 103655. [Google Scholar] [CrossRef] [PubMed]
- Medani, M.; Elhessewi, G.M.S.; Alqahtani, M.; Asklany, S.A.; Alamro, S.; Albalawneh, D.a.; Alshammeri, M.; Assiri, M. Leveraging explainable artificial intelligence with ensemble of deep learning model for dementia prediction to enhance clinical decision support systems. Sci. Rep. 2025, 15, 16639. [Google Scholar] [CrossRef]
- Nap, H.H.; Stolwijk, N.E.; Ipakchian Askari, S.; Lukkien, D.R.; Hofstede, B.M.; Morresi, N.; Casaccia, S.; Amabili, G.; Bevilacqua, R.; Margaritini, A. The evaluation of a decision support system integrating assistive technology for people with dementia at home. Front. Dement. 2024, 3, 1400624. [Google Scholar] [CrossRef] [PubMed]
- Gazzarata, R.; Almeida, J.; Lindsköld, L.; Cangioli, G.; Gaeta, E.; Fico, G.; Chronaki, C.E. HL7 Fast Healthcare Interoperability Resources (HL7 FHIR) in digital healthcare ecosystems for chronic disease management: Scoping review. Int. J. Med. Inform. 2024, 189, 105507. [Google Scholar] [CrossRef] [PubMed]
- Swarnamugi, M.; Chinnaiyan, R. Smart and reliable transportation system based on message queuing telemetry transport protocol. In Proceedings of the 2019 International Conference on Intelligent Computing and Control Systems (ICCS), Madurai, India, 15–17 May 2019; pp. 918–922. [Google Scholar]
- Mun, D.-H.; Le Dinh, M.; Kwon, Y.-W. An assessment of internet of things protocols for resource-constrained applications. In Proceedings of the 2016 IEEE 40th Annual Computer Software and Applications Conference (COMPSAC), Atlanta, GA, USA, 10–14 June 2016; pp. 555–560. [Google Scholar]
- Wu, X.; Li, J. An AIoT-enabled autonomous dementia monitoring system. arXiv 2022, arXiv:2207.00804. [Google Scholar]
- D’Onofrio, G.; Fiorini, L.; Toccafondi, L.; Rovini, E.; Russo, S.; Ciccone, F.; Giuliani, F.; Sancarlo, D.; Cavallo, F. Pilots for healthy and active ageing (PHArA-ON) project: Definition of new technological solutions for older people in Italian pilot sites based on elicited user needs. Sensors 2021, 22, 163. [Google Scholar] [CrossRef]
- Karaberi, C.; Dafoulas, G.E.; Βallis, A.; Raptis, O. ACTIVAGE project: European Multi Centric Large Scale Pilot on Smart Living Environments. Case Study of the GLOCAL evaluation framework in Central Greece. Dialogues Clin. Neurosci. Ment. Health 2018, 1, 138–143. [Google Scholar]
- Gioulekas, F.; Pinaka, O.; Gounaris, K.; Tzikas, A.; Stamatiadis, E.; Loukatzikou, A.; Andreou, A.; Gonidis, F.; Karkaletsis, K.; Kratouni, M. Smart and Healthy Ageing Through People Engaging in Supportive Systems; European Union: Brussels, Belgium, 2023. [Google Scholar]
- Byrne, K.A.; Ghaiumy Anaraky, R. Strive to win or not to lose? Age-related differences in framing effects on effort-based decision-making. J. Gerontol. Ser. B 2020, 75, 2095–2105. [Google Scholar] [CrossRef]
- Schneider, C.; Nißen, M.; Kowatsch, T.; Vinay, R. Impact of digital assistive technologies on the quality of life for people with dementia: A scoping review. BMJ Open 2024, 14, e080545. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Kercher, V.M.; Jordan, E.J.; Savoy, A.; Hill, J.R.; Werner, N.; Owora, A.; Castelluccio, P.; Boustani, M.A.; Holden, R.J. Technology caregiver intervention for Alzheimer’s disease (I-CARE): Feasibility and preliminary efficacy of Brain CareNotes. J. Am. Geriatr. Soc. 2023, 71, 3836–3847. [Google Scholar] [CrossRef]
- Albala, S.A.; Kasteng, F.; Eide, A.H.; Kattel, R. Scoping review of economic evaluations of assistive technology globally. Assist. Technol. 2021, 33, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.E.; Cooper, J.; Scheibling, C.; Parikh, A. Economic evaluation of passive monitoring technology for seniors. Aging Clin. Exp. Res. 2020, 32, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Sumner, J.; Wang, Y.; Wenjun Yip, A. A systematic review of the impacts of remote patient monitoring (RPM) interventions on safety, adherence, quality-of-life and cost-related outcomes. NPJ Digit. Med. 2024, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Naunton Morgan, B.; Windle, G.; Lamers, C.; Brotherhood, E.; Crutch, S. Adaptation of an eHealth Intervention: iSupport for carers of people with rare dementias. Int. J. Environ. Res. Public Health 2023, 21, 47. [Google Scholar] [CrossRef]
- Clarke, A. AI Enhances Dementia Detection in Hospitals, Monash University Study Shows. 2025. Available online: https://insideageing.com.au/ai-enhances-dementia-detection-in-hospitals-monash-university-study-shows/ (accessed on 10 September 2025).
- Yuan, F.; Hasnaeen, N.; Zhang, R.; Bible, B.; Taylor, J.R.; Qi, H.; Yao, F.; Zhao, X. Integrating Reinforcement Learning and AI Agents for Adaptive Robotic Interaction and Assistance in Dementia Care. arXiv 2025, arXiv:2501.17206. [Google Scholar] [CrossRef]
| Supported Domains | Cognition | Mental Health | Independence and Physical Health | Caregiver Support | |
|---|---|---|---|---|---|
| Dementia Stage | |||||
| Preclinical |
|
|
|
| |
| Mild Dementia |
|
|
|
| |
| Moderate Dementia |
|
|
|
| |
| Severe Dementia |
|
|
|
| |
| Domain | Example Intervention | Status |
|---|---|---|
| Cognition | AI speech-based screening | Piloted, not widespread |
| Mental Health | Emotion recognition via facial AI | Prototype |
| Physical Health | Smart pill dispensers | Commercially available |
| Caregiver Support | CareHeroes app | Operational (pilot) |
| Communication | Voice-to-symbol systems | Conceptual/emerging |
| Platform | Target Population | Core Technologies | Integration Approach | Deployment Scope | Operational Challenges |
|---|---|---|---|---|---|
| PHArA-ON | Older adults with varying support needs | IoT, AI, robotics, wearables, cloud-based analytics | Modular open platform with pilot-specific extensions | Piloted in 6 EU countries with real user data | Complexity of customizing deployments for local needs |
| ACTIVAGE | Older adults in smart home settings | IoT networks, smart devices, semantic interoperability | Common data models with interoperable device frameworks | Deployed across 9 pilot sites with ~10,000 users | Usability issues with device interfaces among older adults |
| SHAPES | Older adults across health, social, and care systems | Smart sensors, mobile apps, data integration platforms | Unified platform with integrated pilot-specific services | Implemented in 14 pilot sites in Europe | Fragmented data standards, difficult cross-site evaluation |
| INTER-IoT | Cross-domain (mHealth, eHealth, smart cities) | Middleware interoperability, cloud gateways, IoT APIs | Interoperability layers (e.g., middleware and semantic adapters) | Demonstrated across smart city and health use cases | Semantic alignment across heterogeneous systems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Mohapatra, B.; Ghaiumy Anaraky, R. Assistive Intelligence: A Framework for AI-Powered Technologies Across the Dementia Continuum. J. Ageing Longev. 2026, 6, 8. https://doi.org/10.3390/jal6010008
Mohapatra B, Ghaiumy Anaraky R. Assistive Intelligence: A Framework for AI-Powered Technologies Across the Dementia Continuum. Journal of Ageing and Longevity. 2026; 6(1):8. https://doi.org/10.3390/jal6010008
Chicago/Turabian StyleMohapatra, Bijoyaa, and Reza Ghaiumy Anaraky. 2026. "Assistive Intelligence: A Framework for AI-Powered Technologies Across the Dementia Continuum" Journal of Ageing and Longevity 6, no. 1: 8. https://doi.org/10.3390/jal6010008
APA StyleMohapatra, B., & Ghaiumy Anaraky, R. (2026). Assistive Intelligence: A Framework for AI-Powered Technologies Across the Dementia Continuum. Journal of Ageing and Longevity, 6(1), 8. https://doi.org/10.3390/jal6010008






