Abstract
Background/Objectives: Bolting in lettuce (Lactuca sativa L.) is highly sensitive to elevated temperatures, leading to premature flowering and reduced crop quality and yield. Although SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) is a well-known floral integrator in Arabidopsis, its role in heat-induced bolting in lettuce remains unclear. Methods: In this study, we generated CRISPR/Cas9-mediated LsSOC1 knockout (KO) lines and evaluated their phenotypes under high-temperature conditions. Results: LsSOC1-KO lines exhibited delayed bolting up to 18.6 days, and stem elongation was reduced by approximately 3.8 cm, which is equivalent to a 36.1% decrease compared to wild-type (WT) plants. Transcriptome analysis of leaf and bud tissues identified 32 up-regulated and 10 down-regulated genes common to leaf tissue (|log2FC| ≥ 1, adjusted p < 0.05). Among them, GA20-oxidase1 was significantly down-regulated in both tissues, which may have contributed to delayed floral transition and possibly to reduced stem elongation, although tissue-specific regulation of gibberellin metabolism warrants further investigation. In contrast, genes encoding heat shock proteins, ROS-detoxification enzymes, and flavonoid biosynthetic enzymes were up-regulated, suggesting a dual role of LsSOC1 in modulating thermotolerance and floral transition. qRT-PCR validated the sustained suppression of flowering-related genes in LsSOC1 KO plants under 37 °C heat stress. Conclusions: These findings demonstrate that LsSOC1 is a key integrator of developmental and thermal cues, orchestrating both bolting and stress-responsive transcriptional programs. Importantly, delayed bolting may extend the harvest window and improve postharvest quality in lettuce, highlighting LsSOC1 as a promising genetic target for breeding heat-resilient leafy vegetables.
1. Introduction
Lettuce (Lactuca sativa L.) is one of the most widely cultivated leafy vegetables worldwide, valued for its high nutritional content and consumer preference []. However, it is highly sensitive to elevated temperatures, and exposure to heat stress during the vegetative stage can trigger premature bolting, which results in early floral transition, reduced leaf quality, bitterness, and significant yield losses [,]. In temperate and subtropical regions, the rising frequency of extreme temperature events due to global climate change has made heat-induced bolting a major constraint for year-round lettuce production [,]. Bolting not only reduces marketable yield but also limits the commercial window for harvest, complicating cultivation cycles under field and greenhouse conditions []. Although progress has been made in understanding hormonal and environmental cues involved in bolting, the genetic and molecular mechanisms that control heat-induced floral transition in lettuce remain poorly defined [].
SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) is a MADS-box transcription factor that functions as a central integrator of flowering-time control in plants. In Arabidopsis thaliana, SOC1 acts downstream of multiple inductive pathways—including photoperiod, ambient temperature, vernalization, and gibberellin—and promotes floral transition by activating LEAFY (LFY), APETALA1 (AP1), and FRUITFULL (FUL), while repressing floral inhibitors such as AGL24 and SHORT VEGETATIVE PHASE (SVP) [,,]. Its central role is further supported by feedback and feedforward loops involving FLOWERING LOCUS T (FT) and CONSTANS (CO), as well as by its integration with hormonal signaling networks []. Orthologs of SOC1 in crops such as rice (OsSOC1), tomato, and Brassica have been shown to regulate flowering time, inflorescence development, and bud dormancy, supporting its evolutionary conservation across plant species [,,]. In lettuce, recent studies have begun to characterize LsSOC1, a functional ortholog of Arabidopsis SOC1. Transcriptomic data from laser-capture microdissected shoot apical meristems (SAMs) revealed up-regulation of LsSOC1 during heat-induced bolting, implicating this gene in the temperature-responsive floral transition []. Preliminary gene editing studies using protoplast-based CRISPR/Cas9 mutagenesis have further suggested that LsSOC1 disruption may delay bolting and suppress stem elongation []. However, most studies to date have focused on SOC1 expression within the SAM, providing limited understanding of its regulatory functions in upstream tissues that initially perceive environmental signals, such as leaves and young buds. Given that systemic floral inducers like FT are generated in peripheral organs—particularly photosynthetic and reproductive tissues—before being transported to the SAM [], exploring SOC1 activity in these tissues is essential to fully understand how thermal and developmental signals are integrated prior to floral transition. Bud tissue is a critical relay point where hormonal and metabolic cues converge, yet it remains understudied in the context of SOC1-mediated transcription. Studies in other species have shown that heat stress alters hormonal gene expression in young buds, including repression of gibberellin and auxin biosynthetic pathways [], both of which are major promotors of stem elongation and reproductive commitment. In addition to hormonal regulation, accumulating evidence indicates that secondary metabolites such as flavonoids and anthocyanins may modulate reproductive timing under heat stress. Recent studies have shown that flavonoids and anthocyanins accumulate under heat stress, enhancing heat tolerance and delaying flower bud formation through antioxidant and regulatory functions []. These compounds are known to be associated with enhanced heat tolerance, suppression of oxidative stress, and delayed flower bud formation, likely through a metabolite-mediated buffering mechanism that counteracts stress-induced reproductive stimulation. Despite these insights, how LsSOC1 contributes to transcriptional reprogramming in bud tissues under heat stress remains unclear. Therefore, in this study, we hypothesized that LsSOC1 integrates thermal, hormonal, and metabolic signals within floral bud tissue to regulate the timing of floral transition under high-temperature conditions. To test this, we examined the role of LsSOC1 in heat-induced bolting and tissue-specific transcriptional regulation in lettuce. By generating CRISPR/Cas9-mediated knockout (KO) lines and performing transcriptome analysis of floral buds exposed to elevated temperatures, we aimed to elucidate the molecular mechanisms by which LsSOC1 coordinates developmental and environmental signaling. These findings provide new insights into the upstream regulatory functions of SOC1 beyond the meristem and highlight its potential as a genetic target for developing thermotolerant leafy crops.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Lettuce (Lactuca sativa L. cv. Seonpung Plus; a Korean red romaine-type cultivar) seeds were obtained from Kwonnong Seed Co., Ltd. (Cheongju, Chungbuk, Republic of Korea). Seeds were surface-sterilized with 70% ethanol for 1 min, followed by 2% sodium hypochlorite for 10 min, and rinsed five times with sterile distilled water. Sterilized seeds were germinated on Murashige and Skoog (MS) basal medium and cultivated in a growth chamber at 22 °C under a 16 h light/8 h dark photoperiod (light intensity: 100 µmol·m−2·s−1) for 3 weeks. These growth conditions were selected based on established protocols for Lactuca sativa seedling development under controlled environments []. At 21 days after sowing, seedlings were subjected to heat treatment by transferring them to a growth chamber set at 37 °C for 10 days. This treatment condition was based on previous studies demonstrating that temperatures above 35 °C effectively induce heat-responsive bolting in lettuce []. A parallel control group was maintained at 22 °C throughout the experiment. Following the heat treatment period, plants were returned to 22 °C and monitored for bolting-related phenotypes.
2.2. CRISPR/Cas9-Mediated Editing of LsSOC1
Two sgRNAs targeting exon 1 of LsSOC1 were designed using CRISPR RGEN Tools (http://www.rgenome.net/, accessed on 8 September 2019). Candidate sgRNAs were selected based on GC content (30–40%), out-of-frame scores (>60), and absence of predicted off-target mismatches (Supplementary Table S1). Each sgRNA was cloned into the pKAtC vector under the control of the Arabidopsis U6 (AtU6) promoter using the Aar I restriction site (Supplementary Figure S1A). The T-DNA cassette contained the Streptococcus pyogenes Cas9 (SpCas9) coding sequence driven by the CaMV 35S promoter and a kanamycin resistance gene (NPTII) as the selection marker. Recombinant constructs were introduced into Agrobacterium tumefaciens strain EHA105 (Supplementary Table S2). Regenerated T0 plants (S1 and S2 series) were screened by PCR using NPTII-specific primers (Supplementary Figure S1B–D and Table S3). To identify target site mutations, PCR amplicons were subjected to paired-end sequencing using the MiniSeq platform (Illumina, San Diego, CA, USA), and the resulting reads were analyzed using Cas-Analyzer (http://www.rgenome.net/cas-analyzer/#!, accessed on 12 January 2020) [,].
2.3. Selection of Transgenic Lines and Isolation of Null-Segments
Among the 30 T0 plants generated per construct, representative LsSOC1 KO lines—S1 #1–11 and S2 #2–17—were selected based on indel mutations detected at the sgRNA target sites. The selected T0 mutants were self-pollinated to generate T1 progeny, from which homozygous mutants were identified through genotyping. These lines were subsequently advanced to the T2 generation, and null segregants lacking the transgene were isolated by PCR. Primer sequences used for genotyping are listed in Supplementary Table S3.
2.4. Phenotypic Characterization and Bolting Assay
Bolting phenotypes were evaluated in T2-generation LsSOC1 KO, null-segregant, and wild-type (WT) lettuce plants. To ensure stable genotypes and phenotypes, all bolting-related analyses were conducted using these lines under controlled chamber conditions (22 °C, 16 h light/8 h dark, 100 µmol·m−2·s−1). Bolting was defined as the emergence of the main stem visibly protruding ≥ 1.0 cm above the rosette, based on previously established criteria []. Flowering was defined as the appearance of the first visible floral bud. Phenotypic observations were conducted daily at a consistent time point (10 a.m.) to minimize diurnal variation. For each plant, bolting time and flowering time were recorded as the number of days from sowing to the first visible occurrence of each event. To assess thermal responsiveness, growing degree days (GDDs) were calculated using the following formula:
where the T_base was set to 4 °C for lettuce based on previous thermal response models [,]. GDDs were determined separately for bolting and flowering, representing the accumulated thermal units required to reach each stage. Stem length and internode length were measured at the time of bolting using a digital caliper. Stem length was recorded from the base of the plant to the apex of the primary inflorescence axis, and average internode length was calculated by dividing total stem length by the number of internodes []. A minimum of 12 biological replicates (n ≥ 12) per genotype were analyzed to ensure statistical robustness. A minimum of 12 biological replicates (n ≥ 12) per genotype were analyzed to ensure statistical robustness.
GDD = Σ [(T_day + T_night)/2 − T_base],
2.5. RNA-Sequencing
Fully expanded leaves and bud tissues were harvested from WT, S1 #1–11, and S2 #2–17 lettuce plants grown under standard growth conditions (22 °C, 16 h light/8 h dark, 100 µmol·m−2·s−1). Collected samples were immediately frozen in liquid nitrogen and sent to Seeders Co., Ltd. (Daejeon, Korea) for RNA extraction and transcriptome sequencing. RNA integrity was verified using an (Agilent Technologies, Santa Clara, CA, USA) and libraries were constructed using the TruSeq mRNA Sample Preparation Kit (Illumina, San Diego, CA, USA). Sequencing was performed on the Illumina NovaSeq 6000 platform to generate 150 bp paired-end reads. Three biological replicates per genotype and tissue type were analyzed. Raw sequence reads were quality-checked using FastQC (v0.11.9) and trimmed with Trimmomatic (v0.39) to remove low-quality bases and adapters. Cleaned reads were aligned to the lettuce reference genome (GCA_002870075.2) using HISAT2 (v2.2.1). Transcript abundance was quantified with StringTie (v2.1.7), and expression values were normalized as transcripts per million (TPM). Differentially expressed genes (DEGs) were identified using DESeq2 (v1.34.0) in R (v4.1.0), applying a false discovery rate (FDR) < 0.05 and a log2 fold-change ≥ 1 as thresholds. Gene Ontology (GO) enrichment analysis was performed using the agriGO v2.0 platform.
2.6. Anthocyanin Quantification
Leaf tissues were collected from WT and LsSOC1 KO plants at the onset of bolting, defined by stem elongation of ≥1.0 cm, under controlled chamber conditions (22 °C, 16 h light/8 h dark, 100 µmol·m−2·s−1). Samples were immediately frozen in liquid nitrogen and stored at −80 °C prior to extraction. Approximately 300 mg of frozen leaf tissue was ground in liquid nitrogen using a chilled mortar and pestle and extracted with 1.5 mL of acidified methanol (80% methanol containing 1% HCl, v/v) []. The extracts were vortexed and incubated in the dark at 4 °C for 16 h, followed by centrifugation at 12,000× g for 10 min at 4 °C. The absorbance of the supernatant was measured at 530 nm using a spectrophotometer. Total anthocyanin content was calculated using the molar extinction coefficient of cyanidin-3-glucoside (ε = 29,600 M−1 cm−1) [] and expressed as micrograms per gram of fresh weight (μg/g FW). Three biological replicates (n = 3) were analyzed per genotype.
2.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from fully expanded leaf and bud tissues using the FavorPrep Plant Total RNA Mini Kit (Favorgen Biotech Corp., Ping-Tung, Taiwan). First-strand cDNA was synthesized from 1 μg of total RNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). Quantitative real-time PCR (qRT-PCR) was performed on a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) using SYBR Green Master Mix (Bioneer Corporation, Daejeon, Republic of Korea). Gene-specific primers targeting selected differentially expressed genes (DEGs) were designed and are listed in Supplementary Table S3. LsActin was used as the internal reference gene for normalization. The qRT-PCR thermal cycling conditions were as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Relative gene expression levels were calculated using the 2−ΔΔCt method. Each biological sample was analyzed with three biological replicates and three technical replicates.
2.8. Statistical Analysis
All statistical analyses were performed using R software (v4.3.1). For phenotypic traits and gene expression assays, one-way ANOVA followed by Tukey’s Honestly Significant Difference (HSD) test was applied for multiple comparisons. Statistical significance was defined as p < 0.1 (*), p < 0.05 (**), and p < 0.01 (***). Data are presented as mean ± standard deviation (SD) unless stated otherwise.
3. Results
3.1. Generation of LeSOC1 KO Lines via CRISPR/Cas9
CRISPR/Cas9-mediated targeted mutagenesis of LsSOC1 was performed using two independent binary vectors, each harboring a single sgRNA targeting exon 2 or exon 4 of the gene. These constructs were introduced into Lactuca sativa L. via Agrobacterium tumefaciens-mediated transformation (Figure 1A,B). A total of 60 transgenic plants were regenerated under kanamycin selection, and transgene integration was confirmed by PCR using NPTII-specific primers (Supplementary Figure S1 and Table S3). Deep amplicon sequencing of 35 T0 lines revealed a diverse spectrum of indel mutations at the expected Cas9 cleavage sites (Supplementary Table S4). To obtain stable homozygous mutants, selected T0 plants were self-pollinated and advanced to the T1 generation. Two independent lines were chosen based on the complete absence of WT alleles and the presence of frameshift-inducing mutations. The S1 #1 line harbored a 1 bp deletion upstream of the K-box domain, leading to a premature stop codon, whereas the S2 #2 line contained a 2 bp insertion within the K-box domain, resulting in a frameshift and early protein truncation (Figure 1C; Supplementary Figure S2). In the T2 generation, null segregants lacking T-DNA sequences were identified by PCR and verified as transgene-free (Supplementary Figure S2). These lines displayed normal vegetative morphology and were selected for downstream phenotypic, transcriptomic, and metabolomic analyses.
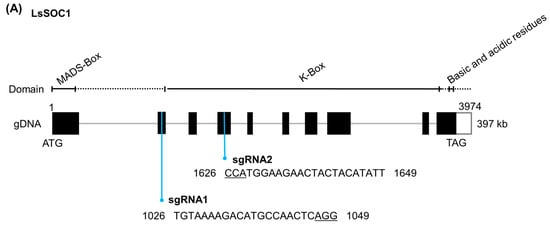
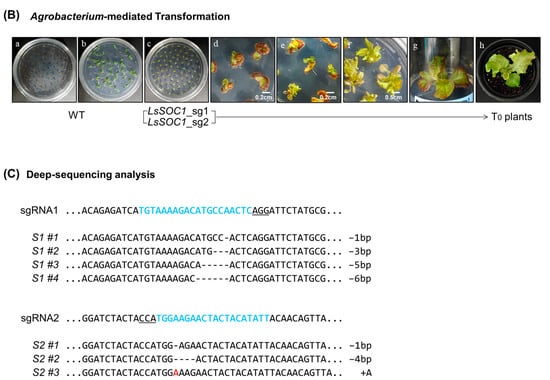
Figure 1.
CRISPR/Cas9-mediated generation of LsSOC1 KO lines in lettuce. (A) Structure of LsSOC1 with sgRNA target sites (blue arrows); exons and UTRs are shown as black and white boxes, respectively. PAM sequences are underlined. (B) Overview of Agrobacterium-mediated transformation and regeneration from cotyledon explants under kanamycin selection. (a); seed sowing, (b); germination after 3 days, (c); Agrobacterium infection and co-culture, (d); callus development, (e,f); shoot development, (g); root development, (h); acclimatization. (C) Deep sequencing results of target sites; deleted bases are shown as “–”, inserted bases in red. Each sgRNA site is marked in blue with corresponding PAMs underlined.
3.2. Delayed Bolting and Reduced Stem Elongation in LsSOC1 KO Lines
Homozygous LsSOC1 KO lines, S1 #1–11 and S2 #2–17, were selected from T2 transgene-free plants based on confirmed biallelic frameshift mutations and the absence of T-DNA insertions (Supplementary Figure S2). Bolting-related traits in earlier generations (T0 and T1) showed phenotypic variability, likely due to somatic chimerism or mosaicism. To ensure genotypic and phenotypic stability, all subsequent analyses were performed using T2 null lines. Under controlled growth chamber conditions (22 °C, 16 h light/8 h dark photoperiod, 100 µmol·m−2·s−1), both KO lines exhibited significantly delayed bolting compared to WT plants (Supplementary Figure S3). Bolting occurred at 125.33 ± 1.57 days in the S1 #1–11 line and 115.67 ± 1.52 days in the S2 #2–17 line, while WT plants bolted at 106.67 ± 0.57 days. This represents a developmental delay of approximately 18.7 days (17.4%) and 9.0 days (8.5%) for S1 and S2 lines, respectively. Flowering was also significantly delayed, with the S1 #1–11 and S2 #2–17 lines flowering at 139.67 ± 2.08 and 131.67 ± 1.53 days, respectively, compared to 121.67 ± 1.53 days in WT, corresponding to 14.8% and 8.2% delays, respectively. This delay was further supported by cumulative thermal time analysis: LsSOC1 KO lines required 2332.41 ± 15.51 °C (S1 #1–11) and 2145.81 ± 8.01 °C (S2 #2–17) to initiate bolting, while WT plants bolted at 1934.45 ± 8.96 °C (Table 1). These values represent an increase in thermal time requirement of 20.6% for S1 and 10.9% for S2, suggesting that LsSOC1 KO lines exhibit impaired responsiveness to heat accumulation during the transition to reproductive development.

Table 1.
Bolting and flowering traits of WT and LsSOC1 KO lettuce lines under controlled conditions.
Morphological analysis of stem architecture revealed that both LsSOC1 KO lines exhibited shorter and more closely spaced internodes compared to WT plants (Figure 2). The average internode length was 7.8 ± 1.4 cm in WT, while S1 #1–11 and S2 #2–17 showed reduced lengths of 4.5 ± 0.7 cm and 5.7 ± 1.1 cm, representing 42.3% and 26.9% reductions, respectively. These morphological differences were consistent with the delayed bolting phenotype. These results support a critical role for LsSOC1 as an integrator of temperature-dependent flowering signals and a coordinator of both developmental timing and stem architecture during the vegetative-to-reproductive phase transition.
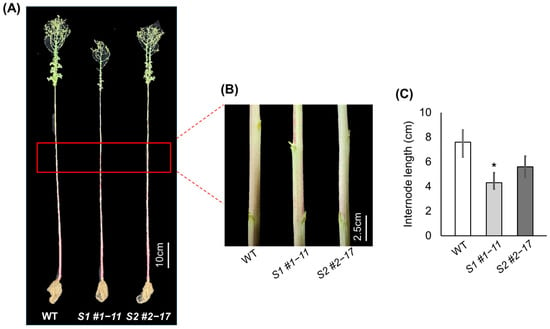
Figure 2.
Morphological comparison of internode length in WT and LsSOC1 KO lines. (A) Post-anthesis reproductive shoot architecture with rosette leaves removed, highlighting reduced stem elongation and altered inflorescence structure in KO lines. (B) Visual comparison of stem internodes at bolting, showing compressed internodal elongation in LsSOC1 KO lines. (C) Quantitative measurements of stem length. Data are presented as mean ± SD. Asterisks indicate statistically significant differences from WT: p < 0.1.
3.3. Transcriptome Analysis in LsSOC1 Knockout Lines
Transcriptome analysis revealed marked transcriptional reprogramming in LsSOC1 KO lines, particularly in leaf tissues. RNA-seq data from leaf and bud tissues of WT and LsSOC1 KO lines were mapped to the Lactuca sativa reference genome with high alignment rates (Supplementary Table S5), ensuring reliable downstream comparisons. Principal component analysis (PCA) showed distinct separation of mutant and WT leaves along PC1 and PC2, indicating strong genotype-dependent variation, while bud samples clustered closely across genotypes, suggesting relatively conserved expression profiles (Figure 3A). MA plots further supported these findings, with a greater number of differentially expressed genes (DEGs) identified in leaves (1422 in the S1 #1–11 line, 2540 in the S2 #2–17 line) than in buds (1319 in the S1 #1–11 line, 1228 in the S2 #2–17 line) (Supplementary Figure S4). The biological implications of these transcriptional changes were further explored through GO enrichment analysis of up- and down-regulated DEGs in each genotype–tissue comparison. GO terms significantly enriched in each group are listed in Supplementary Tables S6 and S7. In the leaves of the S1 #1–11 line, GO terms such as “photoperiodism,” “flowering,” and “circadian rhythm” were down-regulated, whereas in the leaves of the S2 #2–17 line, categories such as “defense response,” “response to oxygen-containing compound,” and “response to stress” were enriched among up-regulated genes. These functional categories likely contributed to the sample clustering observed in PCA and provide insight into the physiological shifts underlying the delayed bolting phenotype in LsSOC1 KO lines.
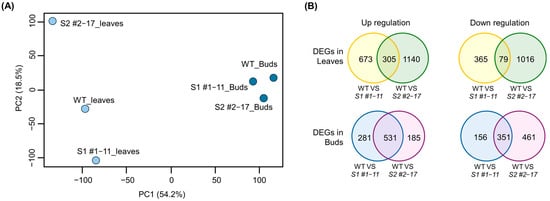
Figure 3.
Principal component analysis and DEG overlap in LsSOC1 KO lines. (A) PCA of transcriptome profiles from leaves and buds of the WT, S1 #1–11 and S2 #2–17 lines. (B) Venn diagrams showing the number of overlapping DEGs between the S1 #1–11 and S2 #2–17 lines in each tissue.
To identify robust genotype-dependent transcriptional changes, DEG overlaps between the S1 #1–11 line and the S2 #2–17 line were analyzed for each tissue (Figure 3B). Under a strict threshold (|log2FC| ≥ 2, adjusted p < 0.001), 59 up-regulated and 23 down-regulated genes were commonly regulated in leaves, and 309 up-regulated and 192 down-regulated genes were regulated in buds (Supplementary Tables S8 and S9). In the leaves, commonly up-regulated genes included chalcone synthase, flavonol synthase, WRKY and MYB transcription factors, and heat shock proteins, implicating stress adaptation and secondary metabolism. Conversely, GA20ox1, GA20ox1-B, aquaporins, and methyl-CpG-binding proteins were consistently down-regulated, indicating the repression of growth-promoting and epigenetic regulatory pathways. These findings suggest that LsSOC1 coordinates floral transition by integrating hormonal and environmental cues—promoting gibberellin-mediated bolting while suppressing stress-induced transcriptional programs under heat conditions.
3.4. Consistent Transcriptomic Reprogramming in LsSOC1 Knockout Lettuce Reveals Enhanced Stress Responses and Repressed Floral Induction
A core set of transcriptional changes was reproducibly detected across both leaf and shoot tissues in LsSOC1 knockout lines, regardless of line-specific variability. In total, 32 genes were consistently up-regulated, while 10 were significantly down-regulated across all four knockout sample groups (Figure 4, Supplementary Table S10). The up-regulated cluster prominently featured genes involved in flavonoid biosynthesis (chalcone synthase, dihydroflavonol 4-reductase, anthocyanidin synthase) and cellular detoxification (glutathione S-transferase F11, peroxidase 21), alongside a coordinated induction of molecular chaperones including heat shock protein 83, ClpB1, HSP70, and multiple small HSPs. This suggests that in the absence of LsSOC1, leaf tissues preferentially activate stress-responsive and secondary metabolic programs, potentially as a compensatory mechanism in response to developmental perturbation. By contrast, down-regulated genes included MADS-box SOC1 itself, validating the knockout event, along with flowering-related components such as GA20 oxidase 1, GH3.10, and 10-epi-juneol synthase, which are involved in gibberellin and auxin-mediated floral induction. The suppression of homeobox proteins and vicilin-type storage genes further implies a delay or repression of reproductive commitment. These findings highlight that LsSOC1 exerts a dual regulatory function, coordinating reproductive transition with environmental stress buffering. In its absence, lettuce tissues shift transcriptional priorities toward defense and metabolic stabilization rather than floral progression.
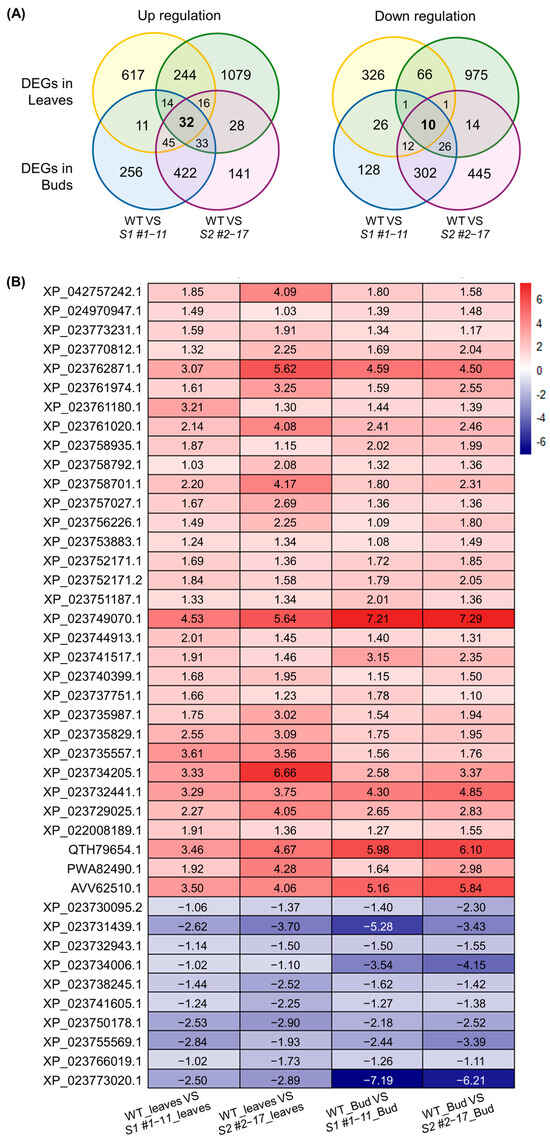
Figure 4.
Overlap and expression patterns of shared DEGs in leaves and buds of LsSOC1 KO lines (A) Venn diagrams showing the overlap of up-regulated and down-regulated genes between S1 #1–11 and S2 #2–17 in leaf and bud tissues. (B) Heatmap showing normalized log2 fold-change values of the 42 shared DEGs (32 up-regulated, 10 down-regulated) across four groups: S1 #1–11 leaves, S2 #2–17 leaves, S1 #1–11 buds, and S2 #2–17 buds.
3.5. Validation of Anthocyanin Biosynthesis, GA Metabolism, and Heat-Responsive Gene Expression in LsSOC1 KO Lines
The deletion of LsSOC1 triggered widespread transcriptional reprogramming across pathways related to flavonoid biosynthesis, gibberellin metabolism, and abiotic stress responses (Figure 5). Anthocyanin content was significantly increased in both KO lines, reaching 29.8 mg/100 g DW in the S1 #1–11 line and 25.6 mg/100 g DW in the S2 #2–17 line, compared with 17.8 mg/100 g DW in WT. This biochemical enhancement was accompanied by transcriptional up-regulation of flavonoid biosynthesis genes: CHS and DFR exhibited ~1.5–2.0-fold increases, while UFGT and F3H were elevated by ~1.3–1.7-fold, consistent with enhanced flux through the anthocyanin pathway. Expression of HSP70, a canonical heat-response gene, was also elevated in both mutants (1.7–1.9-fold) under non-stress conditions, indicating constitutive activation of basal stress-responsive transcription in the absence of external thermal cues. Genes involved in gibberellin biosynthesis, including GA20ox1 and GA3ox1, were significantly down-regulated in both leaf and bud tissues, consistent with reduced stem elongation in the KO lines. By contrast, expression of GID1, encoding the gibberellin receptor, was also reduced in leaves (0.25–0.64-fold) but remained unchanged in buds, indicating organ-specific attenuation of GA signal perception. Collectively, these results suggest that LsSOC1 functions as a dual regulator, repressing stress- and flavonoid-related transcription while promoting gibberellin-associated growth pathways. Its disruption leads to a transcriptional shift favoring metabolic adaptation over reproductive acceleration, thereby contributing to delayed bolting in lettuce.
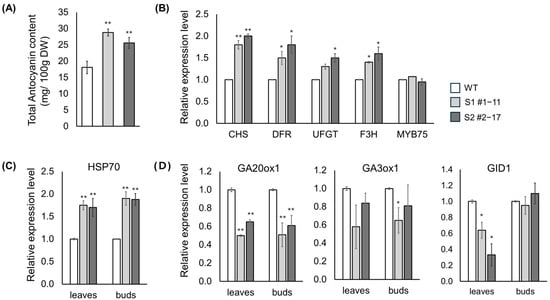
Figure 5.
Validation of anthocyanin accumulation and expression of stress- and gibberellin-related genes in LsSOC1 KO lines. (A) Total anthocyanin content measured in leaf tissues of the WT, S1 #1–11, and S2 #2–17 lines. (B) Relative expression levels of flavonoid biosynthetic genes (CHS, DFR, UFGT, F3H, and MYB75). (C) Expression of the heat-responsive gene HSP70. (D) Expression of gibberellin biosynthesis genes (GA20ox1, GA3ox1) and the GA receptor gene GID1. Error bar represents SD from three biological replicates. Asterisks indicate statistically significant differences from WT: * p < 0.1, ** p < 0.05.
3.6. Functional Confirmation of Bolting Delay in LsSOC1 KO Lines Under High-Temperature Conditions
High-temperature treatment (37 °C, 10 days) was applied to assess whether heat stimulation could rescue the delayed bolting phenotype observed in LsSOC1-deficient lettuce. Bolting was accelerated in both WT and KO lines; however, the extent of acceleration in the KO lines remained significantly lower than in WT, and the delayed phenotype was not restored to WT levels (Figure 6A, Supplementary Table S11). To investigate the transcriptional responsiveness underlying this partial recovery, qRT-PCR analysis was conducted on key flowering pathway genes (FT, LFY, FD, AGL24, APL1) in both leaf and shoot tissues (Figure 6B). In WT plants, all genes showed a marked increase in expression in response to heat, consistent with activation of the flowering pathway. In contrast, LsSOC1 KO lines exhibited impaired gene induction under the same conditions. While most flowering regulators remained unresponsive in mutants, LFY expression was notably elevated in the leaves of the S2 #2–17 line under heat stress, suggesting partial compensation through alternative regulatory inputs. Despite thermal stimulation, LsSOC1 KO lines retained transcriptional inertia in the floral induction pathway, failing to mount a sufficient response for timely reproductive transition. These findings underscore the critical role of LsSOC1 in transducing temperature cues into the activation of flowering genes, and demonstrate that LsSOC1 KO results in a persistent bolting delay even under inductive high-temperature conditions.
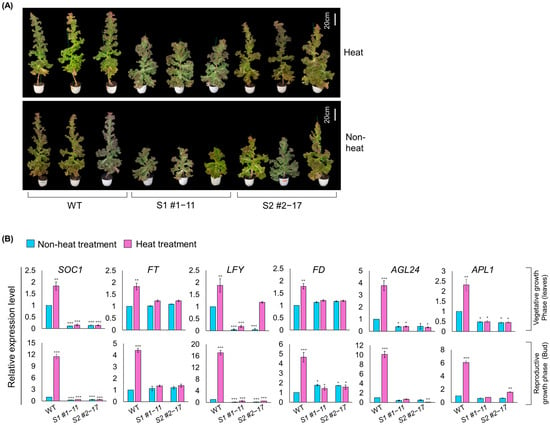
Figure 6.
Functional validation of bolting delay in LsSOC1 KO lines under high-temperature conditions. (A) Representative phenotype of WT and LsSOC1 KO lines grown under elevated temperature (37 °C) for 10 days. Bar = 20 cm. (B) qRT-PCR analysis of floral integrator and meristem identity genes in shoot tissues. Error bar represents SD from three biological replicates. Asterisks indicate statistically significant differences from WT: * p < 0.1, ** p < 0.05, and *** p < 0.01.
4. Discussion
SOC1 is a well-established floral integrator across various plant species, mediating the transition from vegetative to reproductive growth in response to both environmental and endogenous cues [,]. While its core function is largely conserved, functional divergence has been observed between monocotyledonous and dicotyledonous plants. For example, OsSOC1 in rice integrates Ehd1-dependent photoperiodic signals [], whereas AtSOC1 in Arabidopsis responds more strongly to vernalization and gibberellin (GA) []. As a dicot and cool-season crop, lettuce may present a distinct regulatory context for SOC1 function, particularly under heat stress. In this study, we functionally validated the role of LsSOC1 in lettuce by generating CRISPR/Cas9-induced KO lines and conducting comprehensive phenotypic, transcriptomic, and biochemical analyses. Our results demonstrate that LsSOC1 is essential for timely bolting and floral induction under both standard and elevated temperature conditions. Notably, LsSOC1 expression was moderately up-regulated under heat stress (Figure 6B), suggesting its direct involvement in thermal signal perception. The loss of LsSOC1 resulted in a stem-elongation-inhibited phenotype and delayed bolting in a restricted environment (Table 1, Figure 2) and resulted in extensive transcriptional reprogramming, establishing LsSOC1 as a key regulator of heat-responsive flowering in lettuce (Figure 3 and Figure 4, Supplementary Figure S4, Supplementary Tables S5–S10). Consistent with previous findings in Arabidopsis and rice—where SOC1 orthologs integrate vernalization, photoperiod, and GA signaling [,]—lettuce LsSOC1 KO lines exhibited a pronounced delay in bolting and flowering []. Remarkably, this delay persisted even under high-temperature conditions (37 °C), which typically promote bolting in leafy vegetables by accelerating floral pathway activation (Supplementary Table S11). These results suggest that LsSOC1 is a critical component for transducing thermal cues into the activation of floral integrators such as FT, FD, LFY, and APL1. Indeed, qRT-PCR data showed robust induction of these genes under heat stress in WT plants, while LsSOC1 KO lines failed to up-regulate key floral regulators (Figure 6), indicating a bottleneck in downstream signaling []. The transcriptomic landscape of LsSOC1 KO plants revealed a consistent shift from developmental progression toward a stress-responsive transcriptional state, particularly in leaf tissues. Transcriptome profiling indicated suppression of floral activators and concurrent enrichment of stress-related and secondary metabolic pathways. Up-regulated DEGs included canonical abiotic stress markers such as HSP70, ClpB1, and several glutathione S-transferases (GSTU19, GSTF8), suggesting activation of protective networks typically induced by heat or oxidative stress [,]. Transcription factors including WRKY33, MYB12, and DREB2A were also differentially expressed, implying that the loss of LsSOC1 triggers compensatory stress-response mechanisms. Among the most striking changes was the up-regulation of genes involved in flavonoid and anthocyanin biosynthesis—CHS, DFR, F3H, and UFGT. Notably, these transcriptomic patterns were accompanied by a significant increase in anthocyanin accumulation in LsSOC1 KO lines, as confirmed by quantification assays (Figure 5). The concurrent up-regulation of flavonoid biosynthesis genes and classical abiotic stress markers supports the notion that both metabolic and protective pathways are constitutively activated in the absence of LsSOC1 [,]. The strong correlation between gene expression and metabolite levels highlights a functional connection between LsSOC1 and the regulation of secondary metabolism. Previous studies have shown that flavonoids not only serve as ROS scavengers under thermal stress but may also inhibit floral induction, suggesting a metabolite-mediated buffering mechanism that contributes to the delayed bolting phenotype [,]. Similar metabolic shifts have been reported in other species where floral integrators are disrupted, often leading to increased secondary metabolite accumulation as a compensatory strategy [,,]. Interestingly, although gibberellin biosynthesis genes (GA20ox1, GA3ox1) were down-regulated in both leaves and buds of LsSOC1 KO plants, the expression of GID1—which encodes the GA receptor—was significantly reduced in leaf tissues. This decoupling of GA biosynthesis from perception suggests that LsSOC1 may influence hormonal responsiveness in addition to biosynthetic regulation. Reduced GA production and perception in vegetative tissues could hinder bolting activation, thereby contributing to the persistent late-flowering phenotype []. Such tissue-specific divergence between hormone biosynthesis and signal perception underscores the complexity of LsSOC1-mediated integration of environmental and hormonal pathways. Furthermore, the constitutive up-regulation of heat shock proteins and increased anthocyanin accumulation in LsSOC1 KO plants under non-stress conditions implies a pre-activated or “primed” stress state, even in the absence of external stimuli. This transcriptional profile mirrors phenotypes observed in SOC1-like mutants of other species, where delayed flowering is often accompanied by increased tolerance to abiotic stressors such as heat, oxidative stress, or drought [,,]. These findings indicate that LsSOC1 suppresses secondary metabolism under normal conditions, while its absence promotes a shift toward stress-buffering metabolic programs. The accumulation of flavonoids and anthocyanins under heat stress is known to delay reproductive development and protect meristematic tissues via ROS scavenging and hormonal modulation [,]. Altogether, our results support the hypothesis that LsSOC1 exerts dual regulatory roles: under favorable conditions, it promotes floral induction by activating flowering gene networks and enhancing GA responsiveness; under stress or in its absence, the plant defaults to a defense-prioritized transcriptional program that restricts reproductive investment. In conclusion, this study highlights the multifaceted role of LsSOC1 in integrating thermal signals, hormonal pathways, and stress responses to coordinate reproductive development in lettuce. These insights not only broaden our understanding of SOC1 function in a non-model crop, but also identify promising genetic targets for improving bolting control and thermal resilience in commercial lettuce breeding.
5. Conclusions
Our findings demonstrate that LsSOC1 plays an essential role in promoting thermally induced bolting and flowering in lettuce. CRISPR/Cas9-mediated knockout of LsSOC1 resulted in delayed reproductive transition, even under high-temperature conditions, highlighting a failure to translate environmental cues into floral activation. Transcriptomic profiling revealed that LsSOC1 deficiency triggered the up-regulation of stress-responsive and flavonoid biosynthesis pathways, while repressing gibberellin biosynthesis and flowering-related genes. This transcriptional shift was consistent across tissues and validated by qRT-PCR and metabolite data. Taken together, these results suggest that LsSOC1 acts as a key molecular hub integrating environmental and hormonal signals to coordinate bolting, and its loss redirects developmental programs toward stress adaptation. This study provides valuable insight into the genetic regulation of flowering in lettuce and offers potential targets for breeding cultivars with improved bolting control under climate stress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/dna5030040/s1, Figure S1: Generation and selection of LsSOC1 KO lettuce lines; Figure S2: Characterization of LsSOC1 mutations and selection of null segregants; Figure S3: Representative morphology of WT and LsSOC1 KO lines (S1 #1–11 and S2 #2–17) at the bolting stage; Figure S4: Transcriptome-wide differential gene expression in wild-type and LsSOC1 KO lettuce lines; Table S1: Designed sgRNA1,2 of LsSOC1 in lettuce genome using CRISPR RGEN tools (http://www.rgenome.net/); Table S2: Composition of media used for tissue culture; Table S3: List of primers used in this study; Table S4: LsSOC1 transgenic plants and genotype ratio generated using the CRISPR/Cas9 system; Table S5: Statistics of reads mapping to reference (GCA_002870075.4); Table S6: Top5 GO enrichment in WT vs. LsSOC1 KO line leaves; Table S7: Top5 GO enrichment in WT vs. LsSOC1 KO line buds; Table S8: List of common DEGs between WT and LsSOC1 KO lines in leaves. Stringent threshold (|log2FC| ≥ 2, adjusted p < 0.001) was applied; Table S9: List of common DEGs between WT and LsSOC1 KO lines in buds. Stringent threshold (|log2FC| ≥ 2, adjusted p < 0.001) was applied; Table S10: List of common DEGs between WT and LsSOC1 KO lines; Table S11: Bolting and flowering characteristics of WT and LsSOC1 KO lines under high temperature (37 °C) and non-heat conditions.
Author Contributions
Methodology, J.-Y.K., Y.-J.J. and T.-S.K.; formal analysis, J.-Y.K.; investigation, J.-Y.K. and Y.-H.J.; writing—original draft preparation, J.-Y.K. and Y.-J.J.; writing—review and editing, Y.-J.J. and K.-K.K.; supervision, Y.-J.J. and K.-K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the New Breeding Technologies Development Program (Project No. RS-2024-00322378), Republic of Korea.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ryder, E.J. Lettuce: A Garden Heritage. HortScience 1999, 34, 715–719. [Google Scholar]
- Zhao, X.; Carey, E.E.; Wang, W. Influence of temperature and photoperiod on bolting in lettuce. J. Am. Soc. Hortic. Sci. 2007, 132, 492–496. [Google Scholar]
- Dufresne, C.J.; Wallace, D.H. Temperature-induced bolting in lettuce and spinach: A review. J. Am. Soc. Hortic. Sci. 1997, 122, 230–235. [Google Scholar]
- Zhu, Y.; Thakur, P.; Bai, Z.; Liang, Y.; Wang, H. Responses of plants to heat stress: Physiology, biochemical and molecular mechanisms. Plant Cell Rep. 2022, 41, 501–518. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Han, Y.; Liu, X.; Benny, J.; Kim, H.J.; Park, E.J.; Kim, M.J.; Park, S.H.; Nou, I.S. High temperature accelerates lettuce bolting by inducing LsFT expression via LsELF3 degradation. Plant Physiol. 2021, 186, 1097–1110. [Google Scholar] [CrossRef]
- Bouché, F.; Lobet, G.; Tocquin, P.; Périlleux, C. Floral transition, gene networks and environmental interactions. Trends Plant Sci. 2016, 21, 378–388. [Google Scholar] [CrossRef]
- Moon, J.; Suh, S.S.; Lee, H.; Choi, K.; Hong, C.B.; Paek, N.-C.; Kim, S.-G.; Lee, I. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003, 15, 635–646. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, H.S.; Chung, K.S.; Pose, D.; Kim, S.; Schmid, M.; Ahn, J.H. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 2007, 21, 397–402. [Google Scholar] [CrossRef]
- Kim, D.H.; Doyle, M.R.; Sung, S.; Amasino, R.M. Vernalization: Winter and the timing of flowering in plants. Annu. Rev. Cell Dev. Biol. 2009, 25, 277–299. [Google Scholar] [CrossRef]
- Liu, C.; Teo, Z.W.N.; Bi, Y.; Song, S.; Xi, W.; Yang, X.; Yin, Z.; Yu, H. A conserved genetic pathway determines inflorescence architecture in Arabidopsis and rice. Dev. Cell 2013, 24, 612–622. [Google Scholar] [CrossRef]
- Joliffe, J.B.; Moser, C.; Pilati, S. The grapevine SOC1 homolog, VviMADS8/SOC1a, regulates floral organ specification in tomato. Fruit Res. 2024, 4, 703–715. [Google Scholar] [CrossRef]
- Tadege, M.; Sheldon, C.C.; Helliwell, C.A.; Stoutjesdijk, P.; Dennis, E.S.; Peacock, W.J. Control of flowering time by FLC orthologs in Brassica napus. Plant J. 2001, 28, 545–553. [Google Scholar] [CrossRef]
- Jung, C.; Müller, A.E. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009, 14, 563–573. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Ge, D.; Han, Y.; Ning, K.; Luo, C.; Wang, S.; Lui, R.; Zhang, X.; Wang, Q. LCM-seq reveals the crucial role of LsSOC1 in heat-promoted bolting of lettuce (Lactuca sativa L.). Plant J. 2018, 95, 516–528. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, W.S.; Jie, E.Y.; Cho, H.-S.; Kim, S.W. Development of late-bolting plants by CRISPR/Cas9-mediated genome editing from mesophyll protoplasts of lettuce. Plant Cell Rep. 2022, 41, 1627–1630. [Google Scholar] [CrossRef]
- Capovilla, G.; Pajoro, A.; Immink, R.G.H.; Angenent, G.C. The interplay of ambient temperature and flowering time. Trends Plant Sci. 2015, 20, 693–704. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of flavonoid biosynthesis products. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kang, K.K. Stable expression and characterization of brazzein, thaumatin and miraculin genes related to sweet protein in transgenic lettuce. J. Plant Biotechnol. 2018, 45, 257–265. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, H.J.; Bae, S.; Kim, J.H.; Kim, D.H.; Kim, H.K.; Nam, K.H.; Nogoy, F.M.; Cho, Y.G.; Kang, K.K. Acquisition of seed dormancy breaking in rice (Oryza sativa L.) via CRISPR/Cas9-targeted mutagenesis of OsVP1 gene. Plant Biotechnol. Rep. 2019, 13, 511–520. [Google Scholar] [CrossRef]
- Park, J.; Lim, K.; Kim, J.S.; Bae, S. Cas-analyzer: An online tool for assessing genome editing results using NGS data. Bioinformatics 2016, 33, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Ryder, E.J. Lettuce, Endive and Chicory; CABI Publishing: New York, NY, USA, 1999. [Google Scholar]
- Lafta, A.; Sandoya, G.; Mou, B. Genetic variation and genotype by environment interaction for heat tolerance in crisphead lettuce. HortScience 2021, 56, 126–135. [Google Scholar] [CrossRef]
- Lafta, A.; Sandoya, G.; Mou, B. Genetic variation and genotype by environment interaction for heat tolerance in crisphead lettuce. HortScience 2021, 56, 126–135. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by Uv-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wiley: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Lee, J.; Lee, I. Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 2010, 61, 2247–2254. [Google Scholar] [CrossRef]
- Moon, J.; Suh, S.S.; Lee, H.; Choi, K.R.; Hong, C.B.; Paek, N.C.; Kim, S.G.; Lee, I. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003, 35, 613–623. [Google Scholar] [CrossRef]
- Ryu, C.H.; Lee, S.; Cho, L.H.; Kim, S.L.; Lee, Y.S.; Choi, S.C.; Jeong, H.J.; Yi, J.; Park, S.H.; Han, C.D.; et al. OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)-dependent flowering in rice. Plant Cell Environ. 2009, 32, 1412–1427. [Google Scholar] [CrossRef] [PubMed]
- Komiya, R.; Ikegami, A.; Tamaki, S.; Yokoi, S.; Shimamoto, K. Hd3a and RFT1 are essential for flowering in rice under long-day conditions. Plant Cell 2008, 20, 2960–2971. [Google Scholar] [CrossRef]
- Liu, X.; Lv, S.; Liu, R.; Fan, S.; Liu, C.; Liu, R.; Han, Y. Transcriptomic analysis reveals the roles of gibberellin-regulated genes and transcription factors in regulating bolting in lettuce (Lactuca sativa L.). PLoS ONE 2018, 13, e0191518. [Google Scholar] [CrossRef] [PubMed]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1058. [Google Scholar] [CrossRef]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Dixon, D.P.; Lapthorn, A.; Edwards, R. Plant glutathione transferases. Genome Biol. 2002, 3, 3004.1. [Google Scholar] [CrossRef][Green Version]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Bandyopadhyay, A.; Blakeslee, J.J.; Makam, S.N.; Chen, R.J.; Masson, P.H.; Murphy, A.S. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 2004, 16, 1898–1911. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, W.; Zhang, X.; Wang, L.; Jia, S.; Zhao, S.; Li, W.; Lu, R.; Ren, A.; Zhang, S. Transcriptomics and metabolomics analyses of Rosa hybrida to identify heat stress response genes and metabolite pathways. BMC Plant Biol. 2024, 24, 874. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Ito, S.; Imaizumi, T. Flowering time regulation: Photoperiod- and temperature-sensing in leaves. Trends Plant Sci. 2013, 18, 575–583. [Google Scholar] [CrossRef]
- Bouzroud, S.; Gouiaa, S.; Hu, N.; Bernadac, A.; Mila, I.; Bendaou, N.; Smouni, A.; Bouzayen., M.; Zouine, M. Auxin response factors (ARFs) are potential mediators of auxin action in tomato response to biotic and abiotic stress (Solanum lycopersicum). PLoS ONE 2018, 13, e0193517. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.Y.; Hsing, Y.I.; Kitano, H.; Yamaguchi, I.; et al. Gibberellin Insensitive Dwarf1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef]
- Tao, Z.; Shen, L.; Liu, C.; Liu, L.; Yan, Y.; Yu, H. Genome-wide identification of SOC1 and SVP targets during the floral transition in Arabidopsis. Plant J. 2012, 70, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Shen, L.; Gu, X.; Wang, Y.; Yu, H.; He, Y. Embryonic epigenetic reprogramming by a pioneer transcription factor in plants. Nature 2017, 551, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Long, H.; Qiu, F.; Wang, Y.; Zhang, M.; Chao, Y.; Chen, L. MADS-box protein MtSOC1c regulates flowering and seed development in Medicago truncatula. Ind. Crops Prod. 2023, 193, 116125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).