Volatiles Generated in the Pyrolysis of Greenhouse Vegetable Waste
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Biomass Characterization
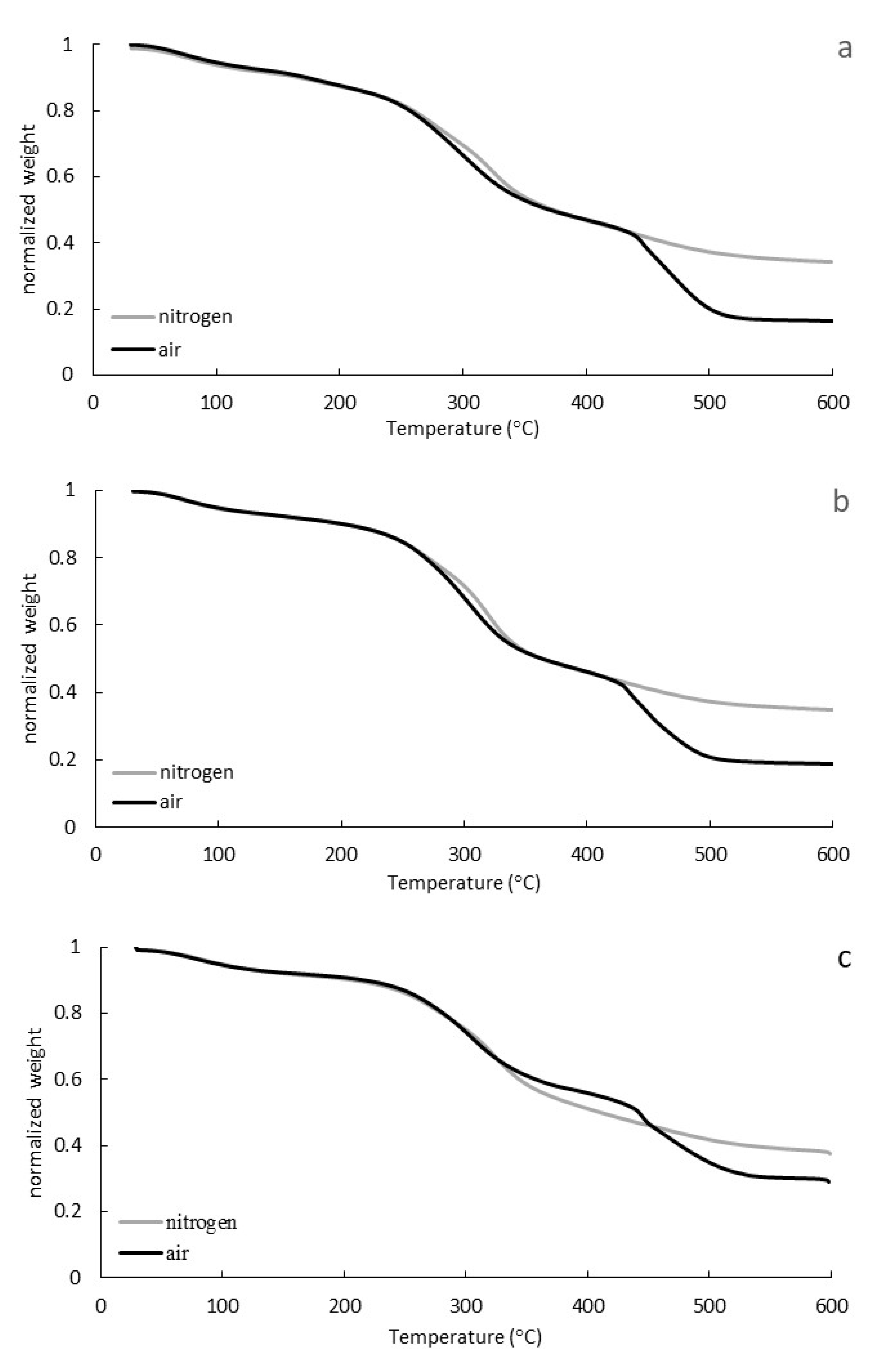
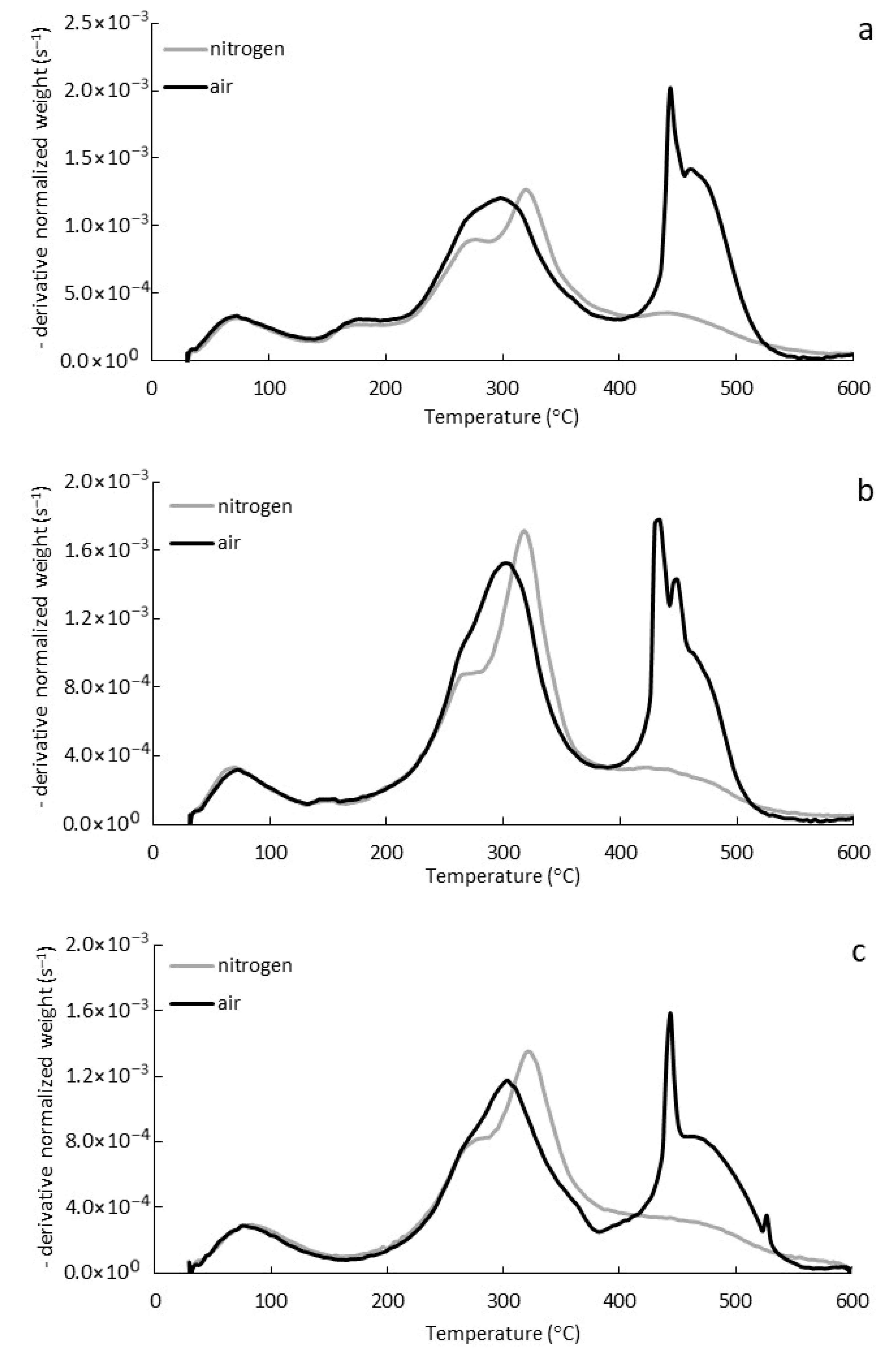
3.2. Thermogravimetric Analysis (TGA, DTG)
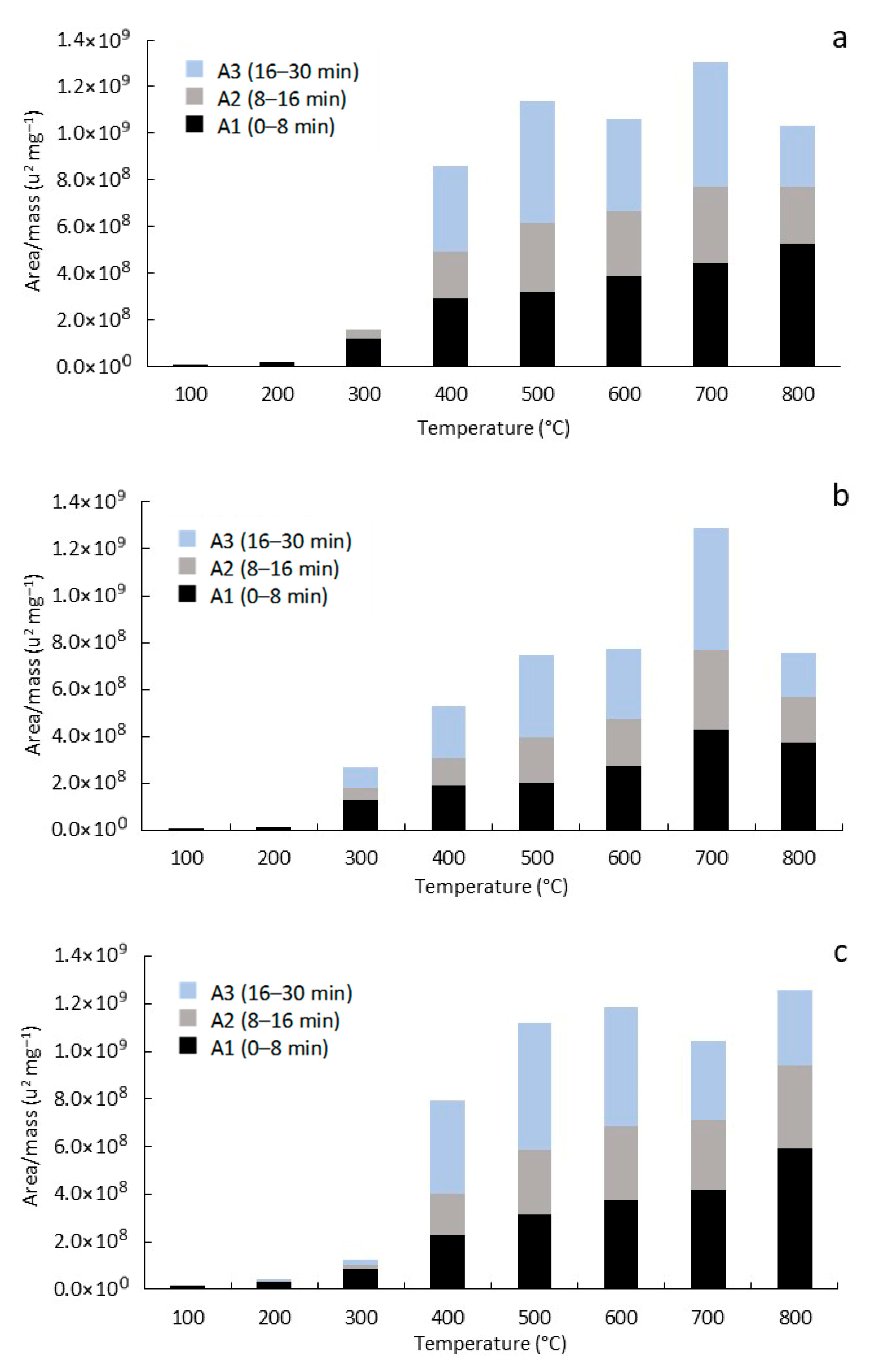
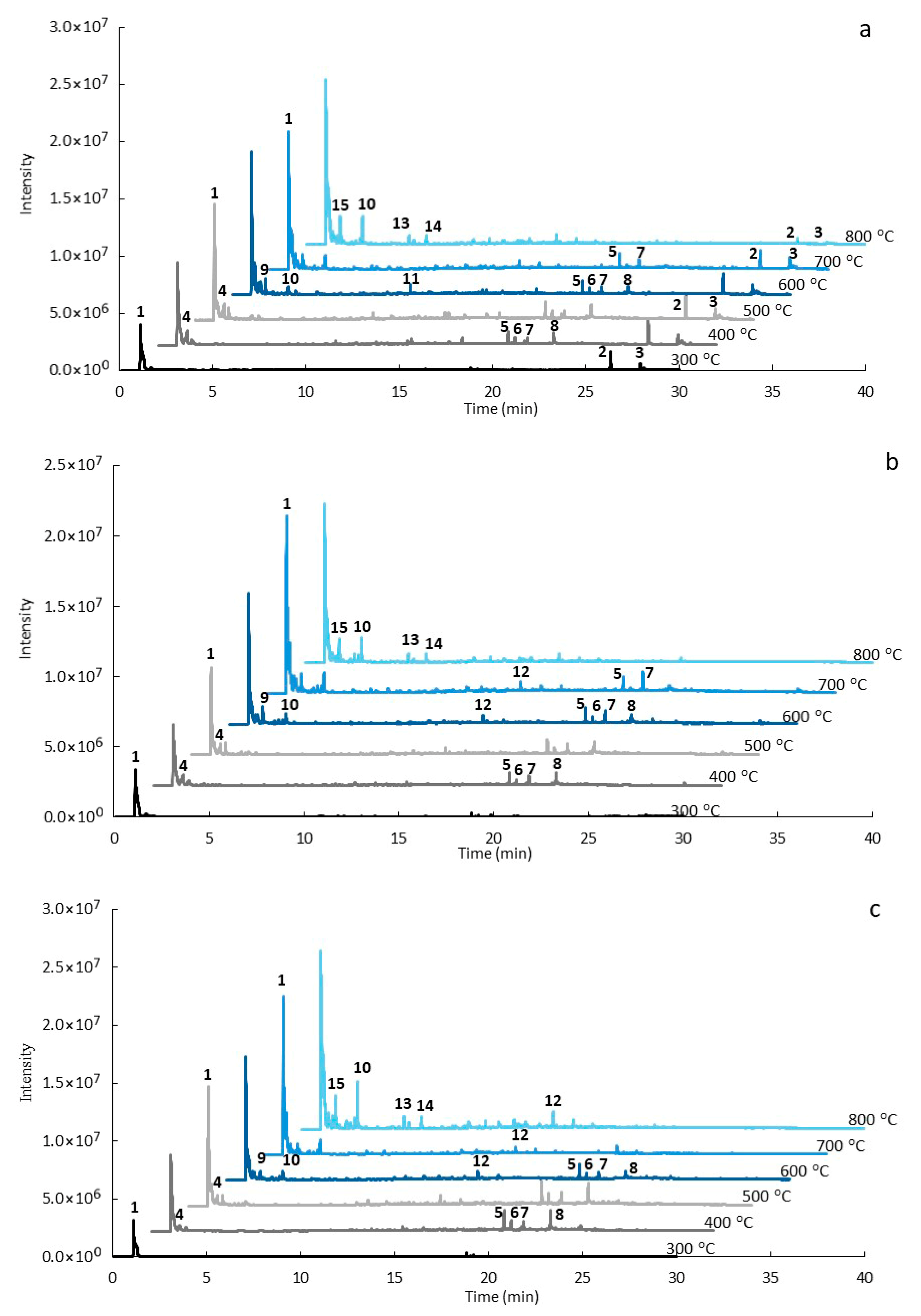
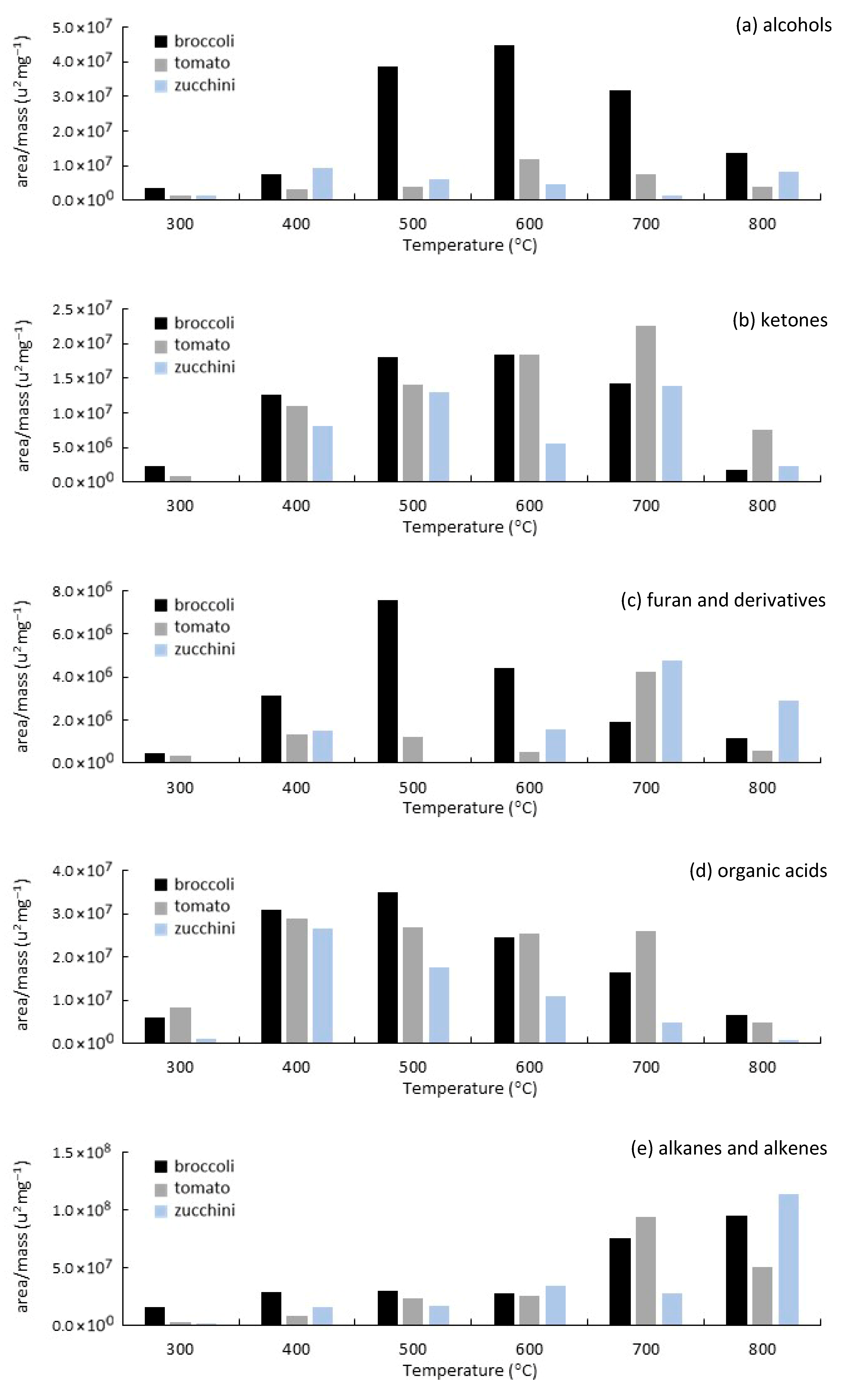
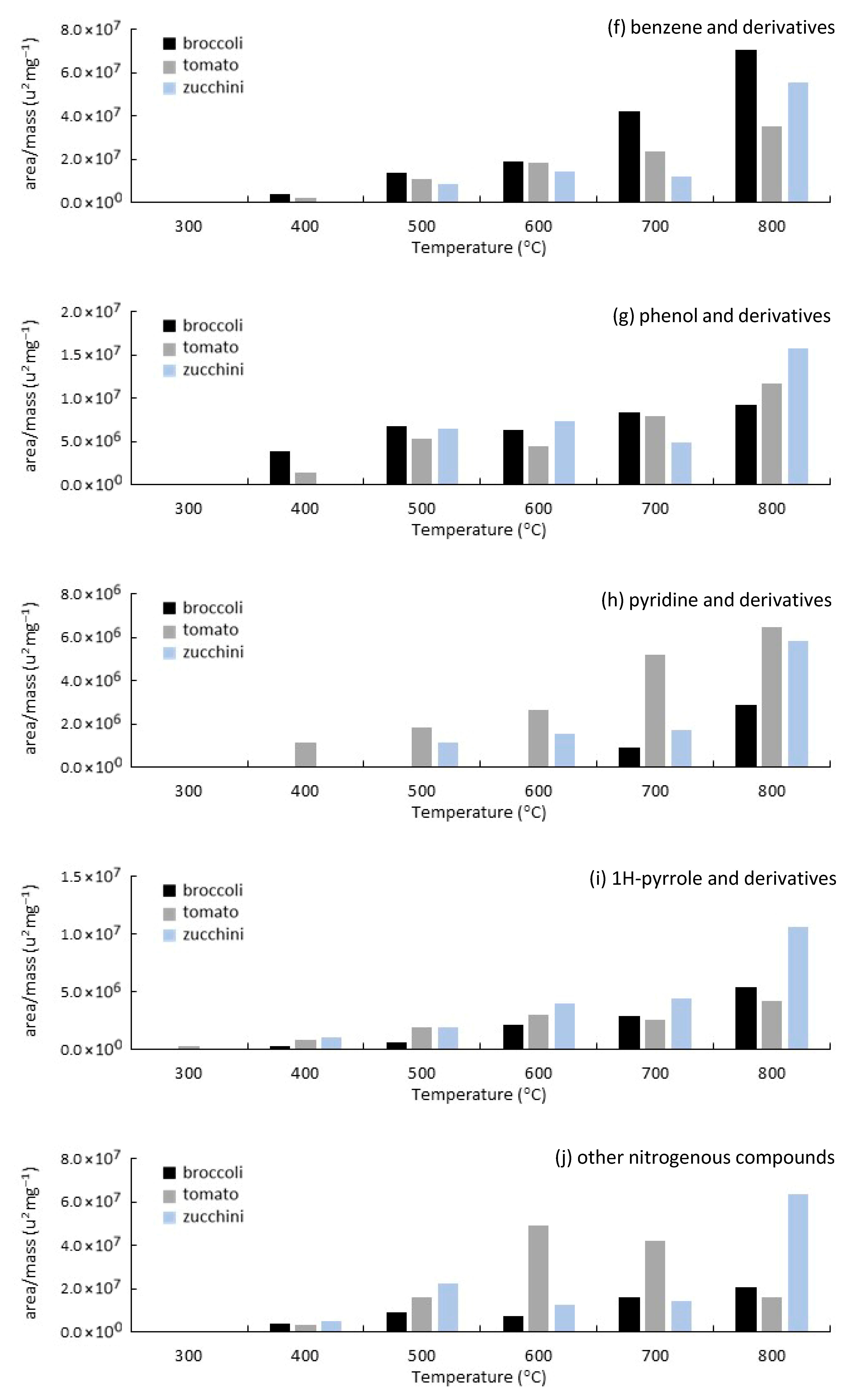
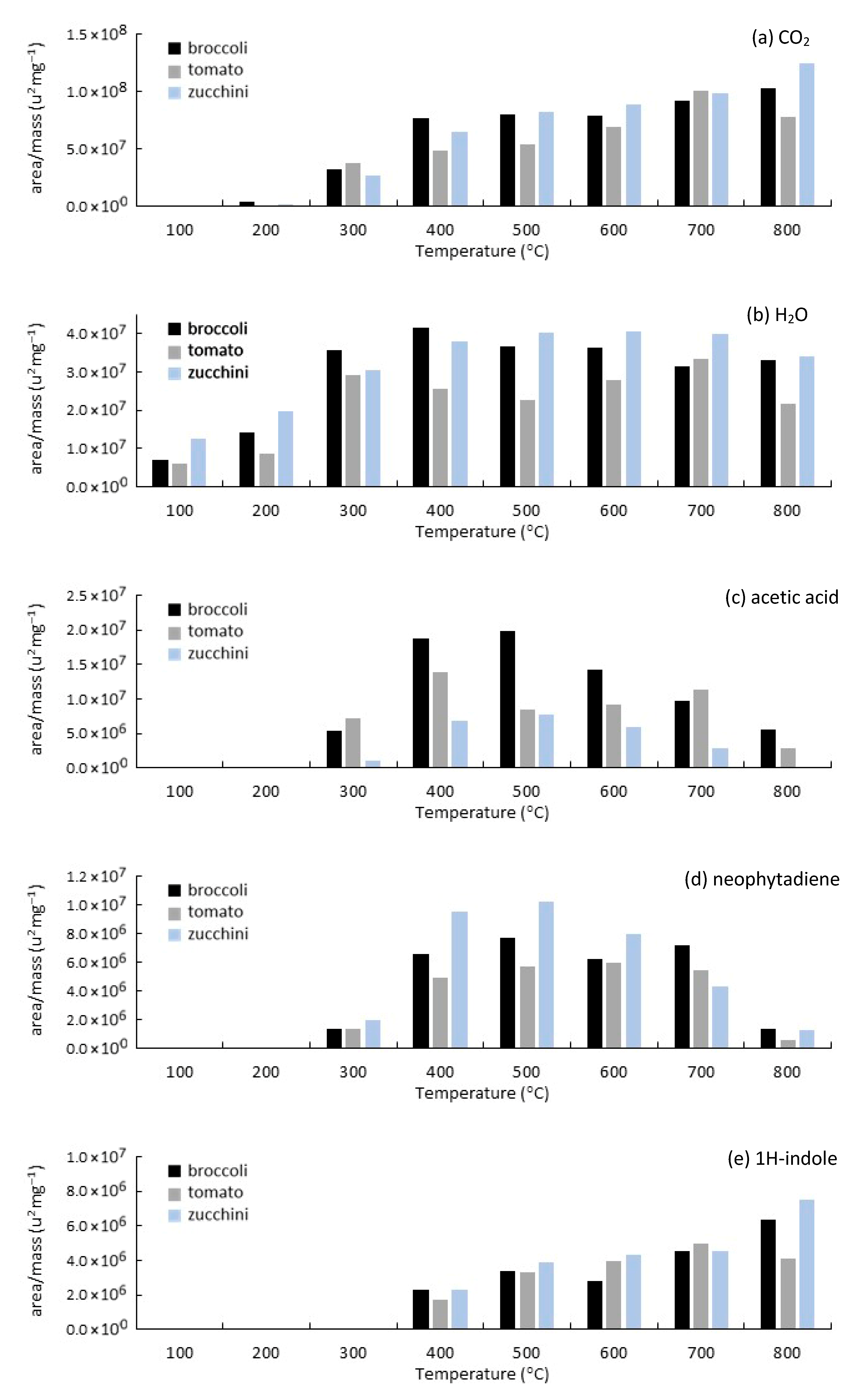
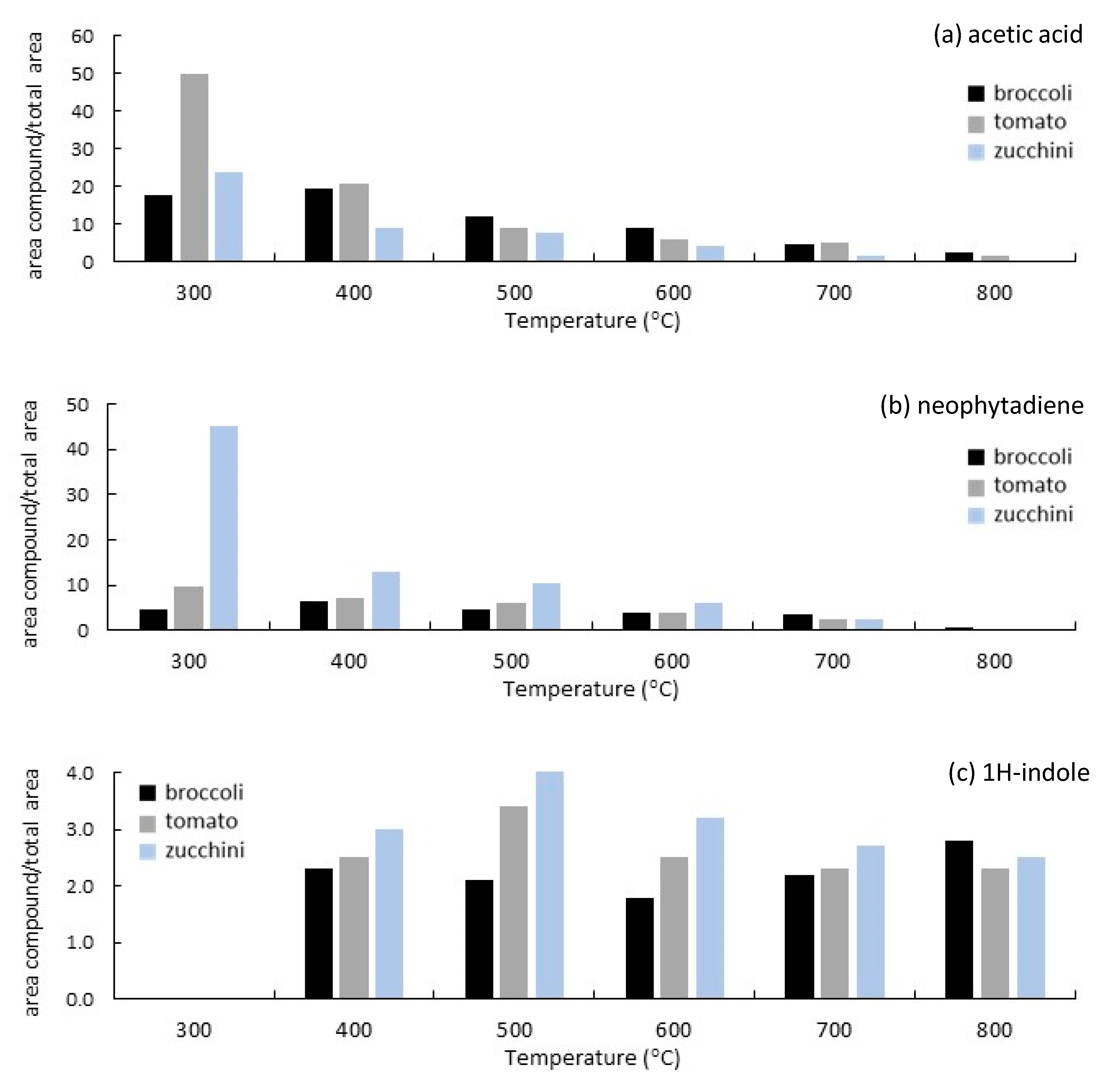
3.3. Analytical Pyrolysis (EGA/Py-GC/MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DTG | Derivative thermogravimetric |
| EGA/Py-GC/MS | Evolved gas analysis/Pyrolyzer—Gas chromatography/Mass spectrometry |
| HHV | Higher heating value |
| Py-GC/MS | Pyrolyzer—Gas chromatography/Mass spectrometry |
| TGA | Thermogravimetric analysis |
References
- Sathish Kumar, R.K.; Sasikumar, R.; Dhilipkumar, T. Exploiting agro-waste for cleaner production: A review focusing on biofuel generation, bio-composite production, and environmental considerations. J. Clean. Prod. 2024, 435, 140536. [Google Scholar] [CrossRef]
- Jerzak, W.; Acha, E.; Li, B. Comprehensive review of biomass pyrolysis: Conventional and advanced technologies, reactor designs, product compositions and yields, and techno-economic analysis. Energies 2024, 17, 5082. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Sikarwar, V.S.; Ahma, E.; Pant, K.K.; Murugavelh, S.; Pandey, A.; Bhattacharya, S.; Sarmah, A.; Leu, S.-Y. A critical review on biomass pyrolysis: Reaction mechanisms, process modeling and potential challenges. J. Energy Inst. 2023, 108, 101236. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A review of recent advances in biomass pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Xu, F.-X.; Zhang, X.; Zhang, F.; Jiang, L.-Q.; Zhao, Z.-L.; Li, H.-B. TG-FTIR for kinetic evaluation and evolved gas analysis of cellulose with different structures. Fuel 2020, 268, 117365. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, C.W.; Ong, H.C.; Show, P.L.; Hsieh, T.H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG-FTIR and Py-GC-FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef]
- Shen, D.; Xiao, R.; Gu, S.; Zhang, H. The Overview of Thermal Decomposition of Cellulose in Lignocellulosic Biomass. In Cellulose—Biomass Conversion; van der Ven, T., Kadla, J., Eds.; InTechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Font, R.; Moltó, J.; Gálvez, A.; Rey, M.D. Kinetic study of the pyrolysis and combustion of tomato plant. J. Anal. Appl. Pyrolysis 2009, 85, 268–275. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Martínez, G. Energetic use of the tomato plant waste. Fuel Process. Technol. 2008, 89, 1193–1200. [Google Scholar] [CrossRef]
- Llorach-Massana, P.; Lopez-Capel, E.; Peña, J.; Rieradevall, J.; Montero, J.I.; Puy, N. Technical feasibility and carbon footprint of biochar co-production with tomato plant residue. Waste Manag. 2017, 67, 121–130. [Google Scholar] [CrossRef]
- Wang, B.; Xu, F.; Zong, P.; Zhang, J.; Tian, Y.; Qiao, Y. Effects of heating rate on fast pyrolysis behavior and product distribution of Jerusalem artichoke stalk by using TG-FTIR and Py-GC/MS. Renew. Energy 2019, 132, 486–496. [Google Scholar] [CrossRef]
- Mohawesh, O.; Coolong, T.; Aliedeh, M.; Qaraleh, S. Greenhouse evaluation of biochar to enhance soil properties and plant growth performance under arid environment. Bulg. J. Agric. Sci. 2018, 24, 1012–1019. [Google Scholar]
- Hidayat, S.; Abu Bakar, M.S.; Yang, Y.; Phusunti, N.; Bridgwater, A.V. Characterisation and Py-GC/MS analysis of Imperata Cylindrica as potential biomass for bio-oil production in Brunei Darussalam. J. Anal. Appl. Pyrolysis 2018, 134, 510–519. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ye, G.; Luo, H.; Liu, C.; Malik, S.; Afzal, I.; Xu, J.; Ahmad, M.S. Pyrolysis and kinetic analyses of Camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour. Technol. 2017, 228, 18–24. [Google Scholar] [CrossRef]
- Medina, S.; Stahl, U.; Ruiz, W.; García, A.N.; Marcilla, A. Characterization of Biochar Produced from Greenhouse Vegetable Waste and Its Application in Agricultural Soil Amendment. AgriEngineering 2025, 7, 348. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Lo, S.-L. Predicting heating value of lignocellulosic biomass based on elemental analysis. Energy 2020, 191, 116501. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Rojas, A.; Flores, C. Valorización de residuos de frutas para combustión y pirólisis. Rev. Politec. 2019, 15, 42–53. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Zhang, M.; Resende, F.L.P.; Moutsoglou, A.; Raynie, D.E. Pyrolysis of lignin extracted from prairie cordgrass, aspen, and Kraft lignin by Py-GC/MS and TGA/FTIR. J. Anal. Appl. Pyrolysis 2012, 98, 65–71. [Google Scholar] [CrossRef]
- He, M.; Mourant, D.; Gunawan, R.; Lievens, C.; Wang, X.S.; Ling, K.; Bartle, J.; Li, C.Z. Yield and properties of bio-oil from the pyrolysis of mallee leaves in a fluidised-bed reactor. Fuel 2012, 102, 506–513. [Google Scholar] [CrossRef]
- MacLeod, A.J.; Panesar, S.S.; Gil, V. Thermal degradation of glucosinolates. Phytochemistry 1981, 20, 977–980. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Aguado, R.; Artetxe, M.; Bilbao, J.; Olazar, M. Kinetic study of lignocellulosic biomass oxidative pyrolysis. Fuel 2012, 95, 305–311. [Google Scholar] [CrossRef]
- Londoño-Larrea, P.; Villamarín-Barriga, E.; García, A.N.; Marcilla, A. Study of cocoa pod husks thermal decomposition. Appl. Sci. 2022, 12, 9318. [Google Scholar] [CrossRef]
- Marcilla, A.; Berenguer, D.; Martínez, I. Effect of the addition of zeolites and silicate compounds on the composition of the smoke generated in the decomposition of Heet tobacco under inert and oxidative atmospheres. J. Anal. Appl. Pyrolysis 2022, 164, 105532. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Ansari, K.B.; Arora, J.S.; Chew, J.W.; Dauenhauer, P.J.; Mushrif, S.H. Fast Pyrolysis of Cellulose, Hemicellulose, and Lignin: Effect of Operating Temperature on Bio-oil Yield and Composition and Insights into the Intrinsic Pyrolysis Chemistry. Ind. Eng. Chem. Res. 2019, 58, 15838–15852. [Google Scholar] [CrossRef]
- Vikram, S.; Rosha, P.; Kumar, S. Recent modeling approaches to biomass pyrolysis: A review. Energy Fuels 2021, 35, 7406–7433. [Google Scholar] [CrossRef]
- Shi, X.; Preisser, E.L.; Liu, B.; Pan, H.; Xiang, M.; Xie, W.; Wang, S.; Wu, Q.; Li, C.; Liu, Y.; et al. Variation in both host defense and prior herbivory can alter plant-vector-virus interactions. BMC Plant Biol. 2019, 19, 556. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Tomato | Broccoli | Zucchini |
|---|---|---|---|
| Elemental analysis: | |||
| C (%) | 37.7 | 33.6 | 34.6 |
| H (%) | 2.88 | 1.97 | 4.49 |
| N (%) | 3.95 | 2.25 | 4.41 |
| O 1 (%) | 26.2 | 36.7 | 27.6 |
| S (%) | 1.70 | 0.49 | 0.35 |
| Proximate analysis: | |||
| Moisture (%) | 6.6 | 7.8 | 7.0 |
| Volatiles (%) | 58.6 | 60.0 | 55.2 |
| Fixed carbon (%) | 13.8 | 15.0 | 16.2 |
| Ash (%) | 21.0 | 17.2 | 21.6 |
| Higher heating value 2 (MJ kg−1) | 13.5 | 9.7 | 14.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Medina, S.; Stahl, U.; Gómez, F.; García, A.N.; Marcilla, A. Volatiles Generated in the Pyrolysis of Greenhouse Vegetable Waste. Biomass 2026, 6, 2. https://doi.org/10.3390/biomass6010002
Medina S, Stahl U, Gómez F, García AN, Marcilla A. Volatiles Generated in the Pyrolysis of Greenhouse Vegetable Waste. Biomass. 2026; 6(1):2. https://doi.org/10.3390/biomass6010002
Chicago/Turabian StyleMedina, Sergio, Ullrich Stahl, Fernando Gómez, Angela N. García, and Antonio Marcilla. 2026. "Volatiles Generated in the Pyrolysis of Greenhouse Vegetable Waste" Biomass 6, no. 1: 2. https://doi.org/10.3390/biomass6010002
APA StyleMedina, S., Stahl, U., Gómez, F., García, A. N., & Marcilla, A. (2026). Volatiles Generated in the Pyrolysis of Greenhouse Vegetable Waste. Biomass, 6(1), 2. https://doi.org/10.3390/biomass6010002






