1. Errors in Figure
In the original publication [1], there was a mistake in Figure 1 as published. The data in the Figure is incorrect. “From references (N = 14)” should not be bulleted and should be in alignment with Databases. “Records screened n = 668” should be “Records screened n = 671.” Additionally, “Records excluded n = 939” should be “Records excluded n = 642.” The corrected Figure 1 appears below.
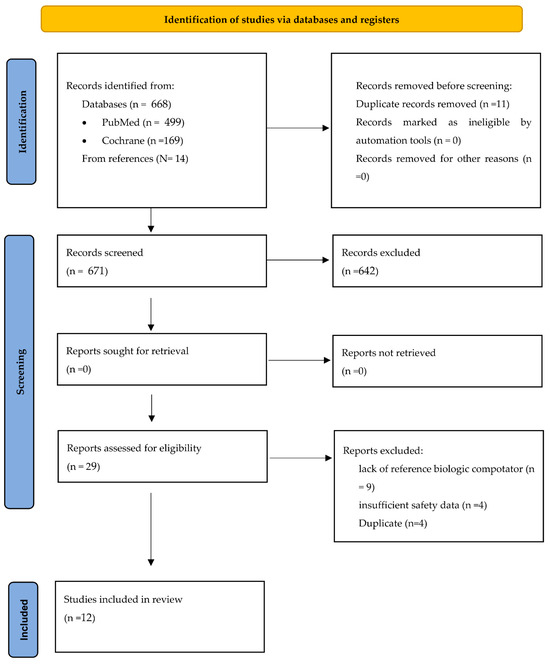
Figure 1.
Study Selection.
2. Errors in Table
There were three mistakes in Table 2 in the originally published paper [1]. The corrected Table 2 appears below.

Table 2.
Characteristics of included studies.
3. Text Correction
3.1. Changes to Section 3.1. Study Characteristics and Quality Assessment
In the original publication [1], there was an error in the second sentence of the first paragraph in the Results Section. The number 27 should be changed to 29 so the sentence should be “After the initial screening process, 29 studies were deemed eligible for full-text screening, of which 12 studies met the inclusion criteria [15,16,24–33] (Figure 1).”
The second paragraph of the Results section should be corrected to begin with “Of the 12 studies, 3 investigated adalimumab biosimilars, 3 focused on rituximab biosimilars, and 3 evaluated infliximab biosimilars, and one study each assessing natalizumab, ustekinumab and trastuzumab biosimilars. The sample sizes varied significantly, with some studies including 171 participants in each group, while others had fewer than 60 participants in one or both arms.”
3.2. Changes to Section 3.2. Total Adverse Events
The third sentence of the first paragraph should be corrected to “Heterogeneity was reported as I2 = 19% (p = 0.248) (Figure 2).”
3.3. Changes to Section 4. Discussion
The second sentence of the fifth paragraph should be corrected to “For instance, a review by Cohen et al. demonstrated a conclusion that switching between biologics and biosimilars did not lead to any significant increase in treatment-related adverse events [17].”
The authors state that the scientific conclusions are unaffected. This correction was approved by the Academic Editor. The original publication has also been updated.
Reference
- Aljahili, S.S.; Alshuwairikh, S.S.; AlKhaldi, A.; Althiban, A.; Hafiz, R.; Korayem, G.B.; Alkofide, H. Safety of Switching from a Reference Biologic to Its Biosimilar: A Systematic Review and Meta-Analysis. Biologics 2025, 5, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).