Abstract
Fungal infections are a major but often neglected global health challenge, affecting both human health and agricultural productivity. Current treatments are limited by few drug classes and increasing multidrug resistance, exacerbated by the widespread use of antifungal agents in clinical and agricultural settings. This study investigates the antifungal potential of a novel 8-hydroxyquinoline derivative with a triazole core at the 5-position, synthesized to improve both efficacy and mechanistic understanding as a fluorescent chemical probe. Biological assays demonstrated significant antifungal activity of compound 10 against a range of pathogens, which was active against all Candida species, dermatophytes, and Fusarium solani with MIC values ranging from 0.5 to 4 µg/mL. Confocal fluorescence microscopy of treated fungal cells was conducted and showed a high accumulation of compound 10 at the cell edge. To further investigate the mode of action, results from a sorbitol protection assay suggested a possible cell wall action, and scanning electron microscopy (SEM) revealed cell wall disruption, such as cell shrinkage and surface roughness, in treated fungal cells. These findings highlight the 8-hydroxyquinoline-triazole scaffold as a promising antifungal agent with cell wall damage properties, providing a basis for future therapeutic development against human and plant fungal pathogens.
1. Introduction
Fungal infections are widely recognized as a major global health concern. Among other factors, the epidemiology of these infections is affected by geographic location, cultural habits, climate characteristics, and economic status of the country [,,,]. In addition to human infections, fungi can colonize plants and soil causing low-quality crops and mycotoxin contamination. Both infections bring about serious economic impacts such as medical costs or food waste [,,,,,]. Treatment of fungal infections has become increasingly challenging due to the limited classes of antifungal drugs available, the rise of multidrug-resistant strains, and the widespread prophylactic use of these drugs in clinical settings. The uncontrolled use of agricultural fungicides also contributes to the resistance problem [,,,,,,,].
In recent years, drug discovery has been focused on privileged structures, such as the quinoline core []. The quinoline nucleus has already shown great pharmacological potential depending on its substitution pattern, and some quinoline derivatives have been reported as antiviral, antioxidant, antibacterial, or antifungal compounds [,,,,,,,,,,,,]. Among the quinoline derivatives, the 8-hydroxyquinoline class could be highlighted due to its potent antimicrobial activity, which seems to be related to the nature of the substituents at the 5- or 7-position [,,,,]. Previous publications from our research group were focused on exploring the 5-position modifications to evaluate the antifungal activity of novel derivatives against important medical strains, including Candida species and dermatophyte species. Joaquim et al. [] synthesized a novel series of 8-hydroxyquinoline-5-sulfonamide derivatives and evaluated their antifungal potential. The derivatives showed high potency and encouraged us to further explore the 5-position modifications. In a second work, Silva et al. [] synthesized 8-hydroxyquinoline bearing a substituted triazole ring at the 5-position. The triazole ring was chosen for its versatility, stability, and a broad spectrum of biological activities, including antifungal activity [,]. Silva’s work resulted in 5-triazole-8-hydroxy-quinoline derivatives with a MIC range of 1–16 μg/mL against Candida and dermatophyte species but showed no activity against Fusarium species.
Despite the potent antifungal activity demonstrated by the 8-hydroxyquinoline derivatives, few studies have explored the mechanism of action of this class, which is an essential step for the further development of these compounds. Preliminary results from our research group suggest a potential effect on the fungal cell wall. In a pioneering study, Pippi et al. [] used scanning electron microscopy (SEM) and sorbitol assays to show cell wall damage caused by clioquinol, a well-known commercial 8-hydroxyquinoline derivative with potent antifungal properties. Subsequent studies by Pippi et al. and Silva et al. on 8-hydroxyquinoline-5-sulfonamide and 5-triazole-8-hydroxyquinoline derivatives reported similar results [,]. Taken together, these results highlight the 8-hydroxyquinoline scaffold as a promising basis for the development of new antifungal agents.
To elucidate the cellular action of this class, the main goal of this study was to design and synthesize a potent and fluorescent chemical probe of an 8-hydroxyquinoline compound containing a triazole core at the 5-position. The derivative was designed to enable cell studies using confocal fluorescence microscopy while also serving as a tool for the identification of novel antifungals as a hit molecule. Given the critical role of antifungals in both human and plant health, experiments were conducted with strains relevant to both fields, broadening the impact of this work.
2. Materials and Methods
2.1. Chemistry
Reactants were obtained from commercial suppliers and used without further purification. Column chromatography was performed on silica gel Fluka (Sigma-Aldrich, St. Louis, MO, USA) 0.035–0.070 mm. Solvents were distilled, when necessary, before use. The 1H and 13C NMR spectra were obtained on a Bruker 400 nuclear magnetic resonance spectrometer (Billerica, MA, USA). Proton and carbon shifts (δ) are given concerning TMS (tetramethylsilane). The compound’s purity was determined using high-performance liquid chromatography (HPLC) with a Shimazdu equipped with a UV DAD detection (Kyoto, Japan) and Phenomenex C8 100A column (250 mm × 4.6 mm, 5 μm). Detection was performed at 254 nm wavelength. The characterization data of intermediates and final compound are available in the Supplementary Information in Figure S1 (1H NMR of 5-nitro-8-hydroxyquinoline), Figure S2 (zoom of the 1H NMR of 5-nitro-8-hydroxyquinoline spectrum), Figure S3 (13C NMR of 5-nitro-8-hydroxyquinoline), Figure S4 (HPLC trace of 5-nitro-8-hydroxyquinoline), Figure S5 (IR spectrum of 5-nitro-8-hydroxyquinoline), Figure S6 (HRMS spectrum of 5-nitro-8-hydroxyquinoline), Figure S7 (1H NMR of 5-azide-8-hydroxyquinoline spectrum), Figure S8 (zoom of the 1H NMR of 5-azide-8-hydroxyquinoline), Figure S9 (13C NMR of 5-azide-8-hydroxyquinoline spectrum), Figure S10 (HPLC trace of 5-azide-8-hydroxyquinoline), Figure S11 (IR spectrum of 5-azide-8-hydroxyquinoline), Figure S12 (HRMS spectrum of 5-azide-8-hydroxyquinoline), Figure S13 (1H NMR of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol spectrum), Figure S14 (zoom of the 1H NMR of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol spectrum), Figure S15 (13C NMR of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol spectrum), Figure S16 (HPLC trace of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol), Figure S17 (IR spectrum of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol) and Figure S18 (HRMS spectrum of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol).
2.1.1. Synthesis of 5-Nitro-8-Hydroxyquinoline (2)
It was obtained as previously described by Mazumder et al. []. Bright yellow crystal; 53% yield; mp: 179–181 °C; 1H NMR (400 MHz, CDCl3) δ (ppm): 9.34 (dd, 1H, J = 1.4 Hz, 8.8 Hz), 8.89 (dd, 1H, J = 1.4 Hz, 4.4 Hz), 8.60 (d, 1H, J = 8.6 Hz), 7.74 (dd, 1H, J = 4.4 Hz, 8.8 Hz), 7.20 (d, 1H, J = 8.6 Hz). 13C NMR (100 MHz, CDCl3) δ (ppm): 157.68, 148.76, 137.04, 136.10, 133.74, 129.06, 125.21, 122.53, 108.12. IR (ῡ/cm−1): 3113, 1571, 1510, 1471, 1418, 1401. HRMS (m/s): [MH+] calc for C9H7N2O3, 191.0451; found, 191.0453.
2.1.2. Synthesis of 5-Azido-8-Hydroxyquinoline (4)
To a stirred solution of 5-nitro-8-hydroxyquinoline (3.45 g, 18.40 mmol) in isopropanol (175 mL), palladium-on-carbon 10% (440 mg) and hydrazine solution (16% w/v, 13.10 mL, 64.40 mmol) were added. The mixture was heated to 82 °C and refluxed for 3 h. Then, the mixture was hot filtered and washed with hot isopropanol, and the filtrate was concentrated at reduced pressure to give 5-amino-8-hydroxyquinoline (2.85 g) as a black solid used in the next step without further purification, with 74% yield. The 5-amino-8-hydroxyquinoline (2.85 g, 18.73 mmol) was dissolved in concentrated hydrochloric acid (1.66 mL) and water (20.7 mL), cooled to −3 °C in an ice bath, and stirred for 10 min. Then, a solution of sodium nitrite (2.07 g, 29.97 mmol) in cold water (20.7 mL) was added dropwise to the mixture. After 20 min, a solution of sodium azide (2.44 g, 37.46 mmol) in water (89.10 mL) was added dropwise to the mixture and stirred at 0 °C for 1.5 h. The reaction was kept at room temperature for 24 h in the dark while stirring. After 24 h, ethyl acetate (100 mL) was added to the resulting mixture, filtered, and extracted. The organic layer was washed out with water, dried over with Na2SO4, filtered, and concentrated under reduced pressure to give 5-azido-8-hydroxyquinoline (2.45 g) as a brown solid used in the next step without further purification; 74% yield; mp: 130–134 °C; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 9.92 (bs, 1H), 8.91 (dd, 1H, J = 1.6 Hz, 4.2 Hz), 8.34 (dd, 1H, J = 1.6 Hz, 8.4 Hz), 7.60 (dd, 1H, J = 4.2 Hz, 8.4 Hz), 7.37 (d, 1H, J = 8.4), 7.13 (d, 1H, J = 8.4 Hz). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.74, 150.02, 139.51, 131.74, 126.20, 122.92, 122.47, 117.10, 112.27. IR (ῡ/cm−1): 3309, 2144, 2113, 1591, 1578, 1511, 1473, 1411. HRMS (m/s): [MH+] calc for C9H7N4O, 187.0614; found, 187.0613.
2.1.3. Synthesis of 1-Chloro-4-Ethynylbenzene (8)
It was obtained as previously described by Zhao et al. [], and all the NMR data are in conformity with the previous literature [,,,].
2.1.4. Synthesis of 5-(4-(4-Chlorophenyl)-1H-1,2,3-Triazol-1-yl)Uinoline-8-ol (10)
First, the 5-azido-8-hydroxyquinoline (0.105 g, 0.81 mmol) was solubilized in acetic anhydride (114.20 μL, 1.21 mmol), and pyridine (39.15 μL, 0.486 mmol) was added to the solution at room temperature. After 1 h, EtOAc was added (10 mL) to the mixture, and acetic acid (1M) and sodium acetate were used to adjust the pH to 7. The organic layer was washed with water (3 × 10 mL), dried over Na2SO4, filtered, and concentrated under reduced pressure to give 5-azidoquinolin-8-yl acetate as a brown solid, which was used in the next step without purification. Next, to a solution of 5-azidoquinolin-8-yl acetate (5) (1.0 eq.) in t-BuOH at room temperature, in order, 1-chloro-4-ethynylbenzene (8) (1.5 eq.), CuSO4.5H2O (0.4 eq.), and an aqueous solution of ascorbic acid (0.6 eq.) and NaHCO3 (0.6 eq.) were added. After 24 h, EtOAc was added, and the organic layer was washed out with water dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude was purified by silica gel column chromatography (cyclohexane/ethyl acetate). Due to the instable nature of the acetylated derivative, it was not possible to characterize it by NMR techniques. After the purification, the derivative was hydrolyzed to give the hydroxyl group at 8-position. To a stirred solution of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)uinoline-8-yl acetate (9) (1.0 eq.) in absolute ethanol at room temperature, KOH (2.0 eq.) was added. After 1 h, the mixture was concentrated under pressure, water was added, and acetic acid (1 M) was used to adjust to pH 7. EtOAc was added, and the organic layer was washed out with water, dried over Na2SO4, filtered, and concentrated under reduced pressure to give the final compound as a white solid; 65% yield (two steps); mp: 140–143 °C; 1H NMR (400 MHz, CDCl3) δ (ppm): 8.89 (dd, 1H, J = 1.4 Hz, 4.4 Hz), 8.15 (dd, 1H, J = 1.4 Hz, 8.6 Hz), 8.09 (s, 1H), 7.88 (d, 2H, J = 8.6 Hz), 7.62 (d, 1H, J = 8.2 Hz), 7.55 (dd, 1H, J = 4.4 Hz, 8.8 Hz), 7.45 (d, 2H, J = 8.8 Hz), 7.28 (d, 1H, J = 8.2 Hz). 13C NMR (100 MHz, CDCl3) δ (ppm): 153.9, 148.9, 146.8, 137.8, 134.3, 131.9, 129.2, 128.6, 127.1, 125.0, 124.4, 124.2, 123.5, 122.1, 108.8. IR (ῡ/cm−1): 3084, 1621, 1580, 1520, 1479, 1438, 1420, 1408. HRMS (m/s): [MH+] calc for C17H12ClN4O, 323.0694; found, 323.0693.
2.2. Fungal Strains
All microorganisms selected for this study are deposited in the library of the Applied Mycology Research Group laboratory at UFRGS. The sets of strains include Candida albicans: CA10; C. glabrata: CGM28; C. parapsilosis: CP10; C. tropicalis: CT04; Trycophyton mentagrophytes: TMEATCC; T. rubrum: TRU51; Microsporum canis: MCA01; Nannizia gypsea: MGYATCC; Fusarium solani: ATCC36031 and F. oxysporum: HCF46. The ATCC strains were acquired from the American Type Culture Collection (USA). The other strains were isolated from patients at HCPA (Hospital de Clínicas de Porto Alegre). All strains were chosen based on their relevance to this study.
Susceptibility Tests
The protocols of the Clinical Laboratory Standards Institute (CLSI) were applied to assess the susceptibility of the 8-hydroxyquinoline derivative against fungal species. The protocol M27-A3 was applied to yeast strains and the protocol M38-A2 to filamentous fungi [,]. All micro dilutions were performed in 96-well polystyrene microplates at inoculum concentration of 5 × 103 CFU/mL for Candida species and 6 × 103 CFU/mL for dermatophytes and Fusarium species.
The compound was solubilized in dimethyl sulfoxide and diluted in RPMI. The maximum DMSO concentration in the assay was limited to 2%. The experiments were conducted in triplicate and incubated for 48 h (30 °C for Fusarium spp. and 35 °C for yeasts) and 120 h (30 °C for dermatophytes). The minimum inhibitory concentration (MIC) was defined as the lowest concentration of compound 10 and other antifungal agents in which microorganisms did not grow. The concentration range evaluated for the novel derivative was 64 μg/mL–0.015 μg/mL. The positive controls were evaluated at 32 μg/mL–0.062 μg/mL (fluconazole), 64 μg/mL–0.125 μg/mL (voriconazole), and 8 μg/mL–0.015 μg/mL (ciclopirox olamine).
2.3. Cellular Action Studies
2.3.1. Sorbitol Protection Assays
A sorbitol protection assay was conducted to clarify the cellular action of the synthesized molecules on fungi cell walls. The MIC value of 10 was determined by microdilution in broth (CLSI M27-A3 for yeasts and CLSI M38-A2 for filamentous fungi) in the absence and presence of 0.8 M sorbitol (Sigma-Aldrich, St. Louis, MO, USA). The experiments were carried out in 96-well polystyrene microplates in duplicate. Anidulafungin (Pfizer®) was chosen for the positive control due to its action on the cellular wall. MICs were determined in two separate readings: after 2 and 7 days for the yeasts (35 °C) and after 5 and 7 days for the dermatophytes (30 °C) [].
2.3.2. Ergosterol Effect Assay
An ergosterol effect assay was conducted to try to elucidate the effects of 10 on the cell membrane. Both 10 and the positive control MIC values were determined via broth microdilution. The micro dilutions were conducted twice—with and without exogenous ergosterol (Sigma-Aldrich, St. Louis, MO, USA). Ergosterol was added at varying concentrations: 50 μg/mL, 100 μg/mL, 150 μg/mL, and 200 μg/mL. Commercial-grade ergosterol was dissolved in DMF (Sigma-Aldrich, St. Louis, MO, USA) and diluted in RPMI 1640 culture medium to a final concentration of 0.1%. Duplicate 96-well polystyrene microplates were used for the experiment. Amphotericin B (União Química, São Paulo, Brazil) was used as the positive control due to its affinity for ergosterol and its action on the cell membrane. The MIC values were determined after 2 days at 35 °C for the yeasts and 5 days at 30 °C for the dermatophytes [].
2.3.3. Confocal Fluorescence Microscopy of Fungal Strains Treated with Compound 10
A qualitative assay was carried out using confocal fluorescence microscopy to explore the action of derivative 10 on fungal cells. Monocultures of Candida albicans, Fusarium solani, and Trichophyton rubrum at 106 CFU/mL were treated with sub-inhibitory concentrations of 10. The fungal inoculum was prepared under the same standards for the evaluation of the susceptibility profile. The microscopy reading was performed up to 30 min after the administration of compound 10 in the inoculum.
2.3.4. Confocal Fluorescence Microscopy with Calcofluor White (CFW)
To gain deeper insight into the cellular effects of compound 10, a qualitative assay using Calcofluor White (CFW), a chitin-specific stain, was performed to assess potential changes in fungal cell wall chitin density following treatment. Candida albicans ATCC 24433 was selected for this assay. First, the MIC value of 10 was determined by broth microdilution (CLSI M27-A3), and the fungal culture was incubated for 24 h at 35 °C. Cells were then treated with 0.12 and 0.06 µg/mL of 10 (MIC/2 and MIC/4, respectively) to facilitate microscopic evaluation. Following treatment, cells were pelleted, stained with CFW, washed, and resuspended in PBS. DMSO 2% was used as a control.
2.3.5. Scanning Electron Microscopy
The effect of derivative 10 on the morphology of Candida albicans CA10 and Trichophyton rubrum TRU51 strains was evaluated by scanning electron microscopy (SEM). Inoculums of C. albicans and T. rubrum were incubated for 48 h at 35 °C and 96 h at 30 °C in sub-inhibitory concentration of derivatives. After the incubation period, treated and control cells were washed three times with PBS, followed by centrifugation. C. albicans cells were fixed in 1 mL of modified Karnovsky’s fixative composed of 2.5% glutaraldehyde and 0.1 M sodium cacodylate buffer for 1 h. T. rubrum cells were fixed in 1mL of the same solution supplemented with 4% paraformaldehyde for 1.5 h. After fixation, cells were washed three times in 1 mL of 0.1 M sodium cacodylate solution buffered at pH 7.2 containing 0.2 M sucrose and 2 mM MgCl2 and adhered in 18 mm round coverslips previously functionalized with poly-L-lysine. The adhered cells were dehydrated in solutions of increasing concentrations of absolute ethyl alcohol for analysis EMSURE® (30, 50, 70, 90, 95, and 100% for 10 min and 100% again for 20 min) and then subjected to critical point drying (EM CPD 300, Leica, Wetzlar, Germany). Samples were mounted on metallic stubs and sputter-coated with a 15–20 nm gold layer (Q150R Plus, Quorum Tech, Sacramento, CA, USA) and visualized in a scanning electron microscope (Carl Zeiss EVO® MA10 Carl, Oberkochen, Germany) operating at 10 kV.
3. Results
3.1. Chemistry
The synthesis route was based on the Ramirez–Corey–Fuchs reaction for the preparation of the appropriate terminal alkyne for the new 1,2,3-triazole derivative (Scheme 1). The first step was to synthesize 1,1-dibromoolefin 7 from 4-chlorobenzaldehyde. The 1,1-dibromoolefin were then converted to the terminal alkyne. Several bases such as n-BuLi, carbonates, or triethylamine could be used to eliminate the bromine hydrogen, which provided the desired terminal alkyne [,,,].
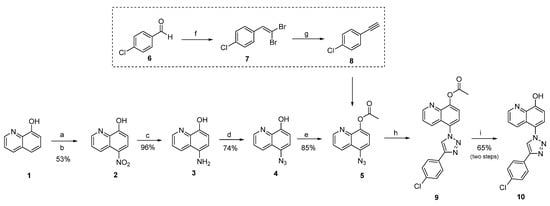
Scheme 1.
Synthesis of compound 10. Reactants and conditions: (a) NaNO3/HCl, 0 °C, 15 min; (b) HNO3/H2O, 17 °C, 1 h 15min; (c) Pd/C, 16% hydrazine aqueous solution, isopropyl alcohol, 82 °C, 3 h; (d) NaNO3, NaN3, HCl, −3 °C then 0 °C for 1.5 h, rt for 24 h; (e) acetic anhydride, pyridine, rt, 1 h; (f) CBr4, PPh3, 0 °C, 3 h; (g) Cs2CO3, 110 °C, overnight; (h) alkyne 8, CuSO4.5H2O, ascorbic acid, NaHCO3, t-BuOH/H2O, rt, 24 h; (i) KOH, EtOH, rt, 1 h.
After the synthesis and elucidation of the desired terminal alkyne, the synthesis of 1,2,3-triazole derivative 10 was carried out. Initially, 5-nitro-8-hydroxyquinoline was prepared as previously described by Mazumder et al. []. Briefly, commercially available 8-hydroxyquinoline was dissolved in HCl (37%) and treated with NaNO2 to give 5-nitroso-8-hydroxyquinoline, which was oxidized with HNO3 to give the nitro intermediate. Palladium on carbon 10% catalyzed the reduction of 5-nitro-8-hydroxyquinoline with hydrazine solution (16%) to give 5-amino-8-hydroxyquinoline 3. Then, 5-azido-8-hydroxyquinoline was formed by diazotization of 3 with NaNO2 and concentrated HCl, followed by treatment with sodium azide. The triazole derivative was prepared by click chemistry, as previously described by Silva []. The cycloaddition of 5-azido-8-hydroxyquinoline and alkyne could not be carried out using the 8-hydroxyquinoline intermediate. Previous studies have shown that 8-hydroxyquinolines are bidentate chelators, preferentially binding to copper (II) and zinc (II) via the phenolic oxygen and pyridine nitrogen. Based on these literature reports, it was decided to introduce an acetyl group at OH-8 prior to cycloaddition to prevent copper chelation and a low-yielding reaction. The 5-azido-8-hydroxyquinoline was then treated with acetic anhydride and pyridine to give 5-azidoquinolin-8-yl acetate.
After acetylation of the hydroxyl group, a copper (I) catalyzed azide-alkyne cycloaddition reaction was conducted, giving the formation of 1,4–disubstituted 1,2,3–triazole by coupling a terminal alkyne and organic azide. Ascorbic acid and NaHCO3 in a water medium gave sodium ascorbate, which was used as a reducing agent to generate copper (I) in situ from copper (II) sulfate (CuSO4.5H2O). A nitrogen atmosphere was not required despite the instability of the oxidation state of copper (I) in the presence of oxygen. After deprotection, the derivative was confirmed by 1H and 13C NMR spectra (Supplementary Materials). The hydrogen signal of the 1,4-disubstituted triazole group was detected as a singlet, confirming the synthesis of compound 10.
3.2. Biological Assays
3.2.1. Fungal Susceptibility Test
Compound 10 was evaluated against a variety of fungal strains using available commercial drugs as positive controls: fluconazole, voriconazole, and ciclopirox olamine (Table 1).

Table 1.
MIC results of compound 10 and antifungal control against fungal species.
The MIC values indicate the great potential of this compound as it showed activity against clinical yeast and dermatophyte strains.
Evaluation against Candida species showed low MIC values against all strains tested. In particular, compound 10 exhibited promising activity against C. glabrata and C. krusei—important pathogens with intrinsic resistance to fluconazole. Given the increasing prevalence of these fungi in immunocompromised patients, it is important to find alternatives to fluconazole-based treatments.
In addition, compound 10 showed potent activity against dermatophyte species, with MIC values ranging from 0.5 to 16 µg/mL. Furthermore, clinical infections caused by Fusarium species pose a significant therapeutic challenge. Remarkably, compound 10 showed activity against Fusarium species, successfully inhibiting the growth of F. solani at half the concentration required by the positive control, voriconazole.
3.2.2. Cellular Action Assays
Sorbitol Protection Assay
If the MIC value of a compound increases in the presence of sorbitol in the culture medium, this suggests that the compound may be acting on the fungal cell wall. As shown in Table 2, there was a slight increase in MIC values in the presence of sorbitol against C. albicans. On the other hand, the dermatophyte tests showed an increase in MIC values only after four days of treatment.

Table 2.
Minimum inhibitory concentration (µg/mL) for 10 and Micafungin obtained by sorbitol protection assay without and with sorbitol addition.
Exogenous Ergosterol Effect Assay
Compound 10 showed no change in MIC values with the addition of exogenous ergosterol against the three selected strains (Table 3).

Table 3.
Minimum inhibitory concentration (µg/mL) obtained by ergosterol binding assay for 10 and Amphotericin B before and after adding ergosterol.
It is expected that compounds acting on the fungal cell membrane may show higher MIC values in the presence of higher concentrations of ergosterol.
Confocal Fluorescence Microscopy
Confocal fluorescence microscopy of three different fungal species treated with compound 10 showed that the compound fluorescence appears to be concentrated at the edge of the fungal cells (Figure 1). A control image (Figure S19) of the fungal cell alone (without compound 10) showed very little fluorescence, confirming that the fluorescence was due to the presence of compound 10. These results are consistent with the sorbitol protection assay.
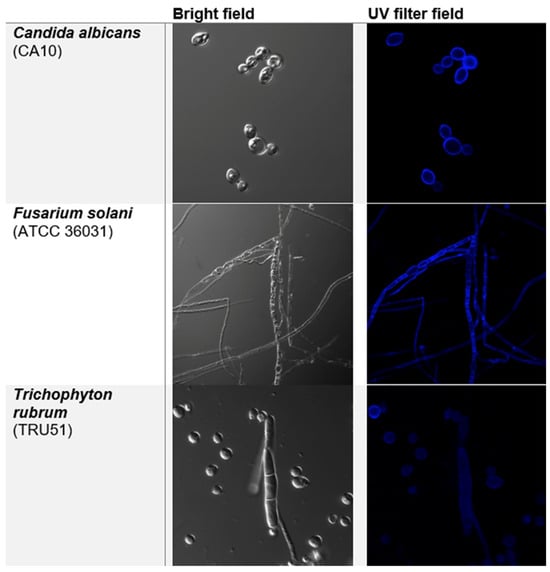
Figure 1.
Fluorescence microscopy of Candida albicans, Fusarium solani, and Trichophyton rubrum cells treated with compound 10 in MIC/2 concentrations in bright field (left) and UV filter (right).
Interestingly, the same behavior can be observed in both yeast and filamentous fungi, corroborating with the previous studies of 8-hydroxyquinoline-5-substituted class.
Confocal Fluorescence Microscopy with Calcofluor White (CFW)
The Calcofluor White (CFW) staining assay was performed to evaluate the effect of compound 10 on the chitin in the Candida albicans cell wall (Figure 2). In the control group, CFW staining clearly highlighted the cell edges, marking the chitin-rich regions of the cell wall. Similarly, in cells treated with compound 10, the fluorescence was also concentrated along the cell edges, showing no noticeable difference in chitin density compared to the control.
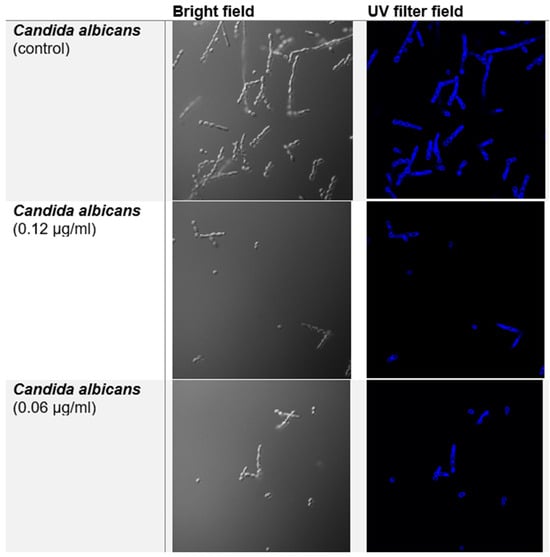
Figure 2.
Fluorescence microscopy of Candida albicans treated with CFW and compound 10 in MIC/2 and MIC/4 concentrations, respectively, in bright field (left) and UV filter (right).
These results indicate that treatment with compound 10 does not appear to affect the density of chitin in the fungal cell wall despite the morphological changes, suggesting that its antifungal activity may not involve direct interference with chitin synthesis or deposition.
Scanning Electron Microscopy
Several morphological changes were observed when C. albicans and T. rubrum strains were exposed to sub-inhibitory concentrations of compound 10 by analyzing the SEM images (Figure 3).
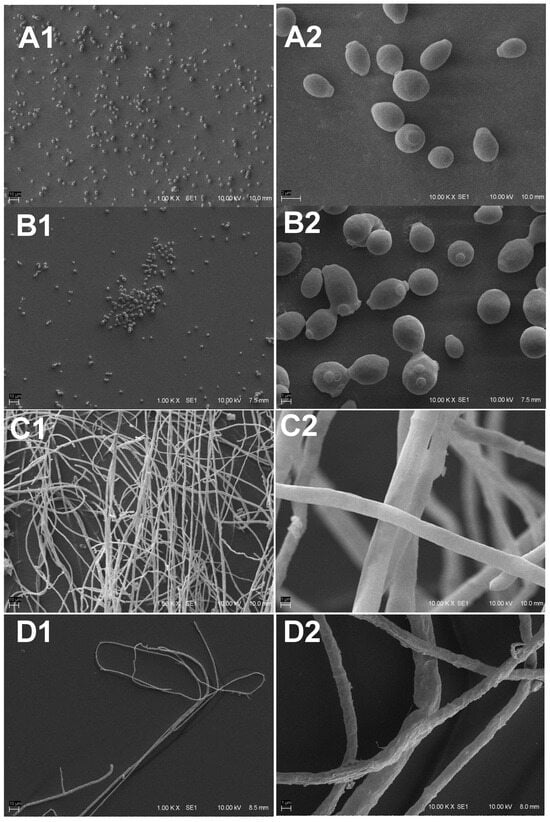
Figure 3.
Scanning electron microscopy of Candida albicans and Trichophyton rubrum treated with a sub-inhibitory concentration of 10 and untreated cells. (A) Untreated cells of C. albicans; (A1) (Bar = 10 μm): large number of regular cells; (A2) (Bar = 2 μm): same field of (A1), showing the oval-shaped cells. (B) C. albicans treated with 10; (B1) (Bar = 10 μm): fewer cells with cluster formation; (B2) (Bar = 2 μm): loss of natural shape and presence of large round cells. (C) Untreated T. rubrum; (C1) (Bar = 10 μm): intense formation of hyphae; (C2) (Bar = 2 μm): regular and intact hyphae. (D) Hyphal cells treated with 10; (D1) (Bar = 10 μm); (D2) (Bar = 2 μm): irregular hyphae, showing rough cell walls with grooves, pitting, and tears.
In C. albicans-treated cells, it was possible to observe the presence of cell clusters and changes in cell morphology, such as shrinkage and loss of smooth oval shape. In T. rubrum, treated hyphae showed roughness, grooves, cell wall damage, and changes in hypha width.
4. Discussion
Susceptibility tests were conducted against eleven fungal species capable of infecting humans or plants. Overall, compound 10 showed remarkable potency, being active against 10 of the 11 strains at low concentrations. In addition, compound 10 showed significant antifungal activity against Fusarium solani, with a MIC value lower than voriconazole. Based on its potency and fluorescence emission, compound 10 proved to be promising for further studies.
Previous studies by our research group have suggested that 8-hydroxyquinoline derivatives may damage the fungal cell wall. When compound 10 was tested in sorbitol protection and ergosterol assays, the results indicated that it likely affects the cell wall rather than the plasma membrane, as expected. These findings are consistent with those reported in the literature [,,,]. The planar structure and π-conjugated system of these aromatic compounds offer a promising approach to elucidate their cellular action through a qualitative assay, using the inherent fluorescence of the derivatives as chemical probes. To advance the study of the 8-hydroxyquinoline class, confocal fluorescence microscopy was proposed as it allows visualization of compound localization within fungal cells due to fluorescence accumulation. Confocal microscopy images of treated cells revealed a fluorescence accumulation along hyphae and at cell edges, while in untreated cells, only weak or no fluorescence was observed, showing that the intense fluorescence in treated cells is due to compound 10. The concentration of this derivative at the cell surface corroborates with the previously proposed effect of compound 10 on the fungal cell wall, consistent with sorbitol assay results and previous studies on non-fluorescent 8-hydroxyquinolines from our group. The Calcofluor White assay showed no significant difference in chitin density between treated and untreated Candida albicans cells, as indicated by similar staining patterns along the cell edges—this finding may suggest that the antifungal activity may be based on another component of the fungal cell wall.
To further investigate the morphological changes in fungal cells after treatment with compound 10, scanning electron microscopy (SEM) was used. SEM images showed remarkable structural changes in C. albicans and T. rubrum compared to untreated controls. In C. albicans, extensive cellular damage was observed in the blastoconidia, including significant changes in size and shape, multiple non-polar bud scars, and loss of the typical oval shape, indicating potential problems in the division process and signs of endopolyploidy. Altered cell shapes, suggesting changes in cell permeability and cell wall damage, were also noted. SEM images of T. rubrum showed severe hyphal damage compared to untreated cells, with irregularities, roughness, and visible cracks and pits on the hyphal surface. The rate of hyphae was also significantly reduced, which correlated with the low MIC value of compound 10 against T. rubrum.
Another important point to emphasize is that the three major classes of antifungals currently available (polyenes, echinocandins, and azoles) have significant limitations. Amphotericin (the major polyene agent) and echinocandins are natural or derivatives of natural products with limited physicochemical properties, mainly due to their higher molar mass (compared to most synthetic drugs) and the higher presence of hydrogen bond donor groups—such as hydroxyl. They are also expensive drugs limited to only hospital use. Echinocandins have a narrow spectrum, while amphotericin can cause severe side effects due to off-target binding of host membranes, resulting from its ability to form membrane pores []. On the other hand, the major class to control fungal infection, the azole derivatives, is associated with the development of fungal resistance due to the extensive use of this class in clinical and agricultural fields []. Compound 10 is a low-molecular-weight synthetic compound with relevant MIC values against 10 of the 11 strains tested, suggesting broad-spectrum activity. Its promising efficacy highlights its potential as an alternative to currently available drugs. In addition, cellular studies indicated an effect on the cell wall, which is a microorganism-specific structure, further increasing interest in this derivative.
Other results reported in the literature support the findings of this study. 8-hydroxyquinoline-5-sulfonamides have previously been evaluated against fungi responsible for dermatomycoses in the presence and absence of sorbitol []. Similar to the results found in this study, 8HQ derivatives containing sulfonamide substituents at the 5-position were less active in the presence of sorbitol, indicating a cellular effect on the fungal wall, which was also supported by the SEM assay. Although sulfonamides and triazoles are different functional groups, triazoles can be considered bioisosteres of sulfonamides, and similar results are, therefore, a good indication of validation of the results. In another relevant study, Silva et al. showed that 8HQ derivatives containing other triazole substituents at the 5-position gave similar results in the sorbitol and SEM assays [].
However, the results reported in this article are groundbreaking because, to the best of our knowledge, this is the first time that compound 10 has been reported in the literature, and, in general, the derivative was the most potent antifungal among the other 5-triazole-8-hydroxyquinolines already described. In addition, the accumulation of an 8HQ at the cell edge was described for the first time by confocal fluorescence microscopy, confirming previous results. It was also shown for the first time that the effect of 8HQ is not related to chitin synthesis. All these results contribute to a better understanding of the antifungal action of 5-triazole-8-hydroxyquinoline and allow us to advance the state of the art.
5. Conclusions
Overall, compound 10 was successfully synthesized and characterized, and it showed significant activity against several fungal strains. The new compound has great potential as a hit molecule, especially due to its low MIC values against T. rubrum, C. albicans, and F. solani. In addition, we successfully designed and synthesized a fluorescent chemical probe, which allowed a better understanding of the cellular action of the 8-hydroxyquinoline core. The novel derivative 10 showed strong potential, suggesting an action on the fungal cell edge due to fluorescence accumulation observed in confocal microscopy and corroborated by structural cell wall damage evident in SEM images.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/applmicrobiol5020038/s1, Figure S1: 1H NMR of 5-nitro-8-hydroxyquinoline; Figure S2: Zoom of the 1H NMR of 5-nitro-8-hydroxyquinoline spectrum; Figure S3: 13C NMR of 5-nitro-8-hydroxyquinoline; Figure S4: HPLC trace of 5-nitro-8-hydroxyquinoline; Figure S5: IR spectrum of 5-nitro-8-hydroxyquinoline; Figure S6: HRMS spectrum of 5-nitro-8-hydroxyquinoline; Figure S7: 1H NMR of 5-azide-8-hydroxyquinoline spectrum; Figure S8: Zoom of the 1H NMR of 5-azide-8-hydroxyquinoline; Figure S9: 13C NMR of 5-azide-8-hydroxyquinoline spectrum; Figure S10: HPLC trace of 5-azide-8-hydroxyquinoline; Figure S11: IR spectrum of 5-azide-8-hydroxyquinoline; Figure S12: HRMS spectrum of 5-azide-8-hydroxyquinoline; Figure S13: 1H NMR of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol spectrum; Figure S14: Zoom of the 1H NMR of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol spectrum; Figure S15: 13C NMR of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol spectrum; Figure S16: HPLC trace of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol; Figure S17: IR spectrum of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol; Figure S18: HRMS spectrum of 5-(4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)quinoline-8-ol; Figure S19: Fluorescence microscopy of Candida albicans, Fusarium solani and Trichophyton rubrum cells untreated in bright field (left) and UV filter (right).
Author Contributions
C.d.B.G. designed and performed experiments, collected data, analyzed the results, and wrote and reviewed the manuscript. M.S.L. designed and performed experiments, collected data, analyzed the results, and reviewed the manuscript. P.M.Q. performed experiments, collected data, and wrote the manuscript. M.P.G. performed experiments and collected data. M.A.d.C. performed experiments and collected data. A.P.P. performed experiments and collected data. W.L. designed and performed the experiments, collected data, analyzed the results, and reviewed the manuscript. A.M.F. designed the experiments, provided resources, analyzed the results, and reviewed the manuscript. M.H.V. designed the experiments, provided resources, analyzed the results, and reviewed the manuscript. S.F.d.A. designed the experiments, provided resources, analyzed the results, and wrote and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Chamada CNPq/MCTI/FNDCT No. 18/2021-Faixa A-Grupos Emergentes, grant 406112/2021-5; CNPq, Apoio a projetos de pesquisa científica, tecnológica e de inovação-Bolsas de Doutorado, grant 140293/2021-3) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS-EDITAL 05/2019—PROGRAMA PESQUISADOR GAÚCHO-PQG-19 grant 2551-0001972-6; EDITAL FAPERGS 06/2021—PROGRAMA DE REDES INOVADORAS DE TECNOLOGIAS ESTRATÉGICAS DO RIO GRANDE DO SUL—RITEs-RS, 22/2551-0000396-6) by financial support and research fellowships. This study was financed, in part, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giacobbe, D.R.; Maraolo, A.E.; Simeon, V.; Magnè, F.; Pace, M.C.; Gentile, I.; Chiodini, P.; Viscoli, C.; Sanguinetti, M.; Mikulska, M.; et al. Changes in the relative prevalence of candidaemia due to non-albicans Candida species in adult in-patients: A systematic review, meta-analysis and meta-regression. Mycoses 2020, 63, 334–342. [Google Scholar] [CrossRef]
- de Albuquerque Maranhão, F.C.; Oliveira-Júnior, J.B.; Araújo, M.A.D.S.; Silva, D.M.W. Mycoses in northeastern Brazil: Epidemiology and prevalence of fungal species in 8 years of retrospective analysis in Alagoas. Braz. J. Microbiol. 2019, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida parapsilosis: From Genes to the Bedside. Clin. Microbiol. Rev. 2019, 32, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Faure-Cognet, O.; Fricker-Hidalgo, H.; Pelloux, H.; Leccia, M.T. Superficial Fungal Infections in a French Teaching Hospital in Grenoble Area: Retrospective Study on 5470 Samples from 2001 to 2011. Mycopathologia 2016, 181, 59–66. [Google Scholar] [CrossRef]
- Xie, F.; Peng, F. Anti-Prostate Cancer Activity of 8-Hydroxyquinoline-2-Carboxaldehyde–Thiosemicarbazide Copper Complexes by Fluorescent Microscopic Imaging. J. Fluoresc. 2017, 27, 1937–1941. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.M.; Li, S.S.; Liu, D.M.; Lv, X.H.; Sun, X.L. Synthesis of Electron-Deficient Borinic Acid Polymers with Multiresponsive Properties and Their Application in the Fluorescence Detection of Alizarin Red S and Electron-Rich 8-Hydroxyquinoline and Fluoride Ion: Substituent Effects. Macromolecules 2017, 50, 6872–6879. [Google Scholar] [CrossRef]
- Rosa, P.D.; Ramirez-Castrillon, M.; Valente, P.; Fuentefria, A.M.; Van Diepeningen, A.D.; Goldani, L.Z. Fusarium riograndense sp. nov. a new species in the Fusarium solani species complex causing fungal rhinosinusitis. J. Mycol. Med. 2018, 28, 29–35. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Zhang, J.; Chen, R.; Zhong, X.; Wu, X.; Zheng, L.; Zhao, J. In vitro evaluation of photodynamic effects against biofilms of dermatophytes involved in onychomycosis. Front. Microbiol. 2019, 10, 1228. [Google Scholar] [CrossRef]
- Gauthier, G.M.; Keller, N.P. Crossover fungal pathogens: The biology and pathogenesis of fungi capable of crossing kingdoms to infect plants and humans. Fungal Genet. Biol. 2013, 61, 146–157. [Google Scholar] [CrossRef]
- Herkert, P.F.; Al-Hatmi, A.M.S.; De Oliveira Salvador, G.L.; Muro, M.D.; Pinheiro, R.L.; Nucci, M.; Queiroz-Telles, F.; De Hoog, G.S.; Meis, J.F. Molecular Characterization and Antifungal Susceptibility of Clinical Fusarium Species From Brazil. Front. Microbiol. 2019, 10, 737. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, T.; Shu, D.; Zhang, W.; Luan, F.; Shi, L.; Guo, D. Synthesis and luminescence properties of novel 8-hydroxyquinoline derivatives and their Eu(III) complexes. Luminescence 2018, 33, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.D.; Sheid, K.; Locatelli, C.; Marinho, D.; Goldani, L. Fusarium solani keratitis: Role of antifungal susceptibility testing and identification to the species level for proper management. Braz. J. Infect. Dis. 2019, 23, 197–199. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Bitencourt, T.A.; Peres, N.T.A.; Lang, E.A.S.; Gomes, E.V.; Quaresemin, N.R.; Martins, M.P.; Lopes, L.; Rossi, A. Dermatophyte resistance to antifungal drugs: Mechanisms and prospectus. Front. Microbiol. 2018, 9, 1108. [Google Scholar] [CrossRef]
- Scheel, C.M.; Hurst, S.F.; Barreiros, G.; Akiti, T.; Nucci, M.; Balajee, S.A. Molecular analyses of Fusarium isolates recovered from a cluster of invasive mold infections in a Brazilian hospital. BMC Infect. Dis. 2013, 13, 49. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Van Den Ende, A.H.G.G.; Stielow, J.B.; Van Diepeningen, A.D.; Seifert, K.A.; McCormick, W.; Assabgui, R.; Gräfenhan, T.; De Hoog, G.S.; Levesque, C.A. Evaluation of two novel barcodes for species recognition of opportunistic pathogens in Fusarium. Fungal Biol. 2016, 120, 231–245. [Google Scholar] [CrossRef]
- De Aguiar Peres, N.T.; Maranhão, F.C.A.; Rossi, A.; Martinez-Rossi, N.M. Dermatophytes: Host-pathogen interaction and antifungal resistance. An. Bras. Dermatol. 2010, 85, 657–667. [Google Scholar] [CrossRef]
- Patel, D.; Castelo-Soccio, L.A.; Rubin, A.I.; Streicher, J.L. Laboratory Monitoring During Systemic Terbinafine Therapy for Pediatric Onychomycosis. JAMA Dermatol. 2017, 153, 1326–1327. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Pastuch-Gawolek, G.; Mrozek-Wilczkiewicz, A.; Kuczak, M.; Skonieczna, M.; Musiol, R. Synthesis of 8-hydroxyquinoline glycoconjugates and preliminary assay of their β1,4-GalT inhibitory and anti-cancer properties. Bioorg. Chem. 2019, 84, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Cherdtrakulkiat, R.; Boonpangrak, S.; Sinthupoom, N.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Derivatives (halogen, nitro and amino) of 8-hydroxyquinoline with highly potent antimicrobial and antioxidant activities. Biochem. Biophys. Rep. 2016, 6, 135–141. [Google Scholar] [CrossRef]
- Kadri, D.; Crater, A.K.; Lee, H.; Solomon, V.R.; Ananvoranich, S. The potential of quinoline derivatives for the treatment of Toxoplasma gondii infection. Exp. Parasitol. 2014, 145, 135–144. [Google Scholar] [CrossRef]
- Kassem, E.M.; El-Sawy, E.R.; Abd-Alla, H.I.; Mandour, A.H.; Abdel-Mogeed, D.; El-Safty, M.M. Synthesis, antimicrobial, and antiviral activities of some new 5-sulphonamido-8-hydroxyquinoline derivatives. Arch. Pharm. Res. 2012, 35, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, J.; Li, W.; Sun, J.; Peng, M. Direct root penetration and rhizome vascular colonization by Fusarium oxysporum f. sp. cubense are the key steps in the successful infection of Brazil cavendish. Plant Dis. 2017, 101, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, V.; Vecchio, G. 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur. J. Med. Chem. 2016, 120, 252–274. [Google Scholar] [CrossRef]
- Zuo, R.; Garrison, A.T.; Basak, A.; Zhang, P.; Huigens, R.W.; Ding, Y. In vitro antifungal and antibiofilm activities of halogenated quinoline analogues against Candida albicans and Cryptococcus neoformans. Int. J. Antimicrob. Agents 2016, 48, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Joaquim, A.R.; Gionbelli, M.P.; Gosmann, G.; Fuentefria, A.M.; Lopes, M.S.; de Andrade, S.F. Novel Antimicrobial 8-Hydroxyquinoline-Based Agents: Current Development, Structure–Activity Relationships, and Perspectives. J. Med. Chem. 2021, 64, 16349–16379. [Google Scholar] [CrossRef]
- Joaquim, A.R.; Pippi, B.; de Cesare, M.A.; Rocha, D.A.; Boff, R.T.; Staudt, K.J.; Ruaro, T.C.; Zimmer, A.R.; de Araújo, B.V.; Silveira, G.P.; et al. Rapid tools to gain insights into the interaction dynamics of new 8-hydroxyquinolines with few fungal lines. Chem. Biol. Drug Des. 2019, 93, 1186–1196. [Google Scholar] [CrossRef]
- Fuentefria, A.M.; Pippi, B.; Lana, D.F.D.; Donato, K.K.; de Andrade, S.F. Antifungals discovery: An insight into new strategies to combat antifungal resistance. Lett. Appl. Microbiol. 2018, 66, 2–13. [Google Scholar] [CrossRef]
- Pippi, B.; Reginatto, P.; Machado, G.D.R.M.; Bergamo, V.Z.; Lana, D.F.D.; Teixeira, M.L.; Franco, L.L.; Alves, R.J.; Andrade, S.F.; Fuentefria, A.M. Evaluation of 8-Hydroxyquinoline Derivatives as Hits for Antifungal Drug Design. Med. Mycol. 2017, 55, 763–773. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb. Perspect. Med. 2014, 5, a019752. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef]
- Gershon, H.; Clarke, D.D.; Gershon, M. Preparation and Fungitoxicity of Some Trichloro-, Tribromo-, Tetrachloro-, and Tetrabromo-8-Quinolinols. Monatsh. Chem. 2001, 132, 1075–1080. [Google Scholar] [CrossRef]
- da Silva, N.M.; Gentz, C.d.B.; Reginatto, P.; Fernandes, T.H.M.; Kaminski, T.F.A.; Lopes, W.; Quatrin, P.M.; Vainstein, M.H.; Abegg, M.A.; Lopes, M.S.; et al. 8-Hydroxyquinoline 1,2,3-triazole derivatives with promising and selective antifungal activity. Med. Mycol. 2020, 59, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Ezabadi, I.R.; Camoutsis, C.; Zoumpoulakis, P.; Geronikaki, A.; Soković, M.; Glamočilija, J.; Ćirić, A. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg. Med. Chem. 2008, 16, 1150–1161. [Google Scholar] [CrossRef]
- Pippi, B.; Lopes, W.; Reginatto, P.; Silva, F.É.K.; Joaquim, A.R.; Alves, R.J.; Silveira, G.P.; Vainstein, M.H.; Andrade, S.F.; Fuentefria, A.M. New insights into the mechanism of antifungal action of 8-hydroxyquinolines. Saudi Pharm. J. 2019, 27, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Pippi, B.; Joaquim, A.R.; Lopes, W.; Machado, G.R.M.; Bergamo, V.Z.; Giuliani, L.M.; Abegg, M.A.; Cruz, L.; Vainstein, M.H.; Fuentefria, A.M.; et al. 8-Hydroxyquinoline-5-sulfonamides are promising antifungal candidates for the topical treatment of dermatomycosis. J. Appl. Microbiol. 2020, 128, 1038–1049. [Google Scholar] [CrossRef]
- Mazumder, U.K.; Gupta, M.; Karki, S.S.; Bhattacharya, S.; Rathinasamy, S.; Thangavel, S. Synthesis, anticancer and antibacterial activity of some novel mononuclear Ru(II) complexes. Chem. Pharm. Bull. 2004, 52, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Kuang, C.; Yang, Q.; Cheng, X. Cs2CO3-mediated synthesis of terminal alkynes from 1,1-dibromo-1-alkenes. Tetrahedron. Lett. 2011, 52, 992–994. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Hu, Y.; Yuan, L.; Chen, S.; Wu, P.; Wang, W.; Zhang, S.; Zhang, W. An Efficient Method for the Production of Terminal Alkynes from 1,1-Dibromo-1-alkenes and its Application in the Total Synthesis of Natural Product Dihydroxerulin. Adv. Synth. Catal. 2015, 357, 553–560. [Google Scholar] [CrossRef]
- Shen, T.; Xu, Y.; Jiang, C.; Lai, Y.; Liu, S.; Zhang, L.; Qian, C.; Zhou, S. Electrochemical Synthesis of Aryl Chlorides Using HCl as the Chlorine Source. ACS Sustain. Chem. Eng. 2024, 12, 3289–3297. [Google Scholar] [CrossRef]
- Singh, R.M.; Nandini, D.; Bharadwaj, K.C.; Gupta, T.; Singh, R.P. Na2S-mediated synthesis of terminal alkynes from gem-dibromoalkenes. Org. Biomol. Chem. 2017, 15, 9979–9982. [Google Scholar] [CrossRef]
- M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous fungi. Approved Standard-Second Edition. Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2008.
- M27-A3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard-Third Edition. Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2008.
- Escalante, A.; Gattuso, M.; Pérez, P.; Zacchino, S. Evidence for the Mechanism of Action of the Antifungal Phytolaccoside B Isolated from Phytolacca tetramera Hauman. J. Nat. Prod. 2008, 71, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Pianalto, K.M.; Alspaugh, J.A. New Horizons in Antifungal Therapy. J. Fungi 2016, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cheng, L.W.; Land, K.M. Advances in Antifungal Development: Discovery of New Drugs and Drug Repurposing. Pharmaceuticals 2022, 15, 787. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).